
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 14 February 2025
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1443890
This article is part of the Research Topic Follicular Helper T Cells in Immunity and Autoimmunity - Volume II View all 6 articles
 Hao Lei1†
Hao Lei1† Jin Hu2†
Jin Hu2† Junpeng Zhu3†
Junpeng Zhu3† Runze Li3
Runze Li3 Yu Zhao3
Yu Zhao3 Yaqi Zhao3
Yaqi Zhao3 Guisheng He1
Guisheng He1 Tao Song1
Tao Song1 Chong Lu3
Chong Lu3 Wuping Zheng1*
Wuping Zheng1* Lei Li3*
Lei Li3* Chunping Liu3*
Chunping Liu3* Hengyu Chen1*
Hengyu Chen1*Background: T follicular helper (TFH) cells, a subset of CD4+Th cells, play a critical role in B cell activation, proliferation, and differentiation primarily within B follicles in secondary lymphoid organs, essential processes for effective antibody responses. TFH cells are also implicated in various conditions, including autoimmune diseases, cancer, infectious diseases, allergies, and vaccine reactions. Despite their broad impact, a review of the literature on TFH cells and tumors has not been conducted. We aimed to fill this gap by providing a detailed analysis of the research landscape concerning TFH cells and tumors.
Method: We conducted a bibliometric analysis of literature on TFH cells and tumors from 2012 to 2024 using the Web of Science Core Collection (WoSCC). For an analysis of the global research landscape, we employed VOSviewer (version 1.6.20), CiteSpace 6.2.R6 software, and the “bibliometric” package in R language (version 4.3.2) to evaluate data on countries/regions, authors and cited authors, institutions, journals, references, and keywords. We also conducted a systematic review to summarize the global research trends, prospects, and hotspots in this field.
Results: Our analysis included contributions from 60 countries/regions, 7,864 authors, 35,853 cited authors, 1,756 institutions, 385 academic journals, 50883 references, 222 keywords, and 1,181published papers. Over the past decade, the volume of research on TFH cells and tumors had consistently increased. China published the most papers, more than double that of the United States. The top 2 authors ranked by publication volume were Gaulard, Philippe (14 articles, 379 citations), and De leval, Laurence (12 articles, 236 citations) Notably, 9 of the top 10 most published institutions were from China. Frontiers in Immunology and Immunity were the leading journals in publications and citations. A cluster analysis revealed a shift in research focus from “expression”,”B cells” and “survival” to “tumor microenvironment”, “tumor infiltrating immune cells” and “immune infiltration” in recent years.
Conclusion: This bibliometric analysis suggests that TFH cells hold significant research value and potential clinical applications in tumor immunotherapy. Moreover, the bibliometric analysis offers valuable references and guidance for related research endeavors. It also points out the prevailing issues and challenges in TFH cell research, and underscores the need for further basic and clinical research to advance the related fields.
Follicular helper (TFH) cells, a distinct subset of T cells characterized by unique transcriptional profiles and functions, has emerged as a novel cell type over the last decade. At present, TFH cells are defined as CD4+ CXCR5+ PD-1+T cells, including three subgroups: TFH1 (CD3 + CD4 + CD45RA- CXCR5 + CXCR3 + CCR6-), TFH2 (CD3+ CD4+ CD45RA- CXCR5+ CXCR3- CCR6-), and TFH17 (CD3+ CD4+ CD45RA- CXCR5+ CXCR3- CCR6+) (1). First identified in human tonsils in 2000 and 2001, TFH cells are noted for their high expression of CXCR5 (2), and depend on the transcription factor Bcl6 for their function (3–6). The interaction between TFH cells and B cells, promotes B cell proliferation, class switching and affinity maturation. Class switching recombination (CSR) is a process that occurs after B cell activation, allowing B cells to change the type of antibodies they produce, such as converting from IgM to IgG, IgA, or IgE. The cytokines produced by TFH cells, such as IL-4 and IL-21, are crucial for CSR. In particular, TFH1 and TFH2 cells play a crucial role in isotype conversion, with TFH1 cell deficiency leading to reduced IgG2c and IgG2a conversion, while sustained IL-4 production by TFH2 cells can drive IgE conversion (7). Somatic hypermutation (SHM) is another process that B cells undergo in the germinal center (GC), involving mutations in the mutated region of the B cell receptor (BCR) gene, which can increase the affinity of BCR for antigens. TFH cells are crucial for SHM by providing co stimulatory signals and cytokines such as IL-21. IL-21 is absolutely essential for plasma cell formation and SHM (8). And gene conversion is another mechanism by which B cells generate diversity, involving the insertion of additional DNA fragments after V (D) J recombination, which can further increase antibody diversity. Although the role of gene conversion in B cell development is not as widely studied as in SHM and CSR, TFH cells may indirectly affect this process through the cytokines they produce.Recent research by Li Hanjie’s team has revealed that inhibiting the formation of tertiary lymphoid structures (TLS) through TFH or B cell depletion during the invasion of lung adenocarcinoma (LUAD) can promote tumor growth in mouse models. The anti-tumor effect of TFH dependent TLS is mediated through interleukin 21 (IL-21) - IL-21 receptor signaling (9). TFH cells play a crucial role in germinal center formation, influencing the differentiation of germinal center B cells into plasma cells and memory B cells (3, 6, 10). TFH cells also exhibit significant expression of IL-21 (10, 11). While the important role of TFH cells in infection and vaccination has been elucidated, their involvement in cancer is a burgeoning area of research. In malignancies originating from TFH cells or associated with B cells, an elevated TFH cell count often correlates with poor prognosis. Conversely, their presence in various non-lymphocytic solid tumors is frequently linked to a more favorable prognosis (12).
Bibliometric analysis is a quantitative method to delineate the knowledge structure and developmental trends within a specific field, assessing research output, productivity, and impact. This method, unlike other major review methodologies, is particularly well-suited for an evaluation of entire disciplines, encompassing thousands of publications. It offers a robust framework for evaluating the impact of scientific publications through mathematical and statistical techniques, thus identifying research gaps and areas that require further investigation (13). Despite the growing body of literature on the relationship between TFH cells and tumors in recent years, bibliometric studies on this topic remain scarce. Our study aimed to address this gap by conducting a bibliometric network analysis to assess the structural framework, current landscape, and future trajectories of research on TFH cells and tumors.
This bibliometric analysis focuses on papers concerning TFH cells and tumors published from January 1, 2012 to December 31, 2024, resulting in the retrieval of 1,181 articles. A bibliometric analysis was then performed to pinpoint the current research trends.
The Web of Science (WoS) database is widely acknowledged for its reliability and comprehensive coverage of academic information, making it the preferred choice for bibliometric analysis. A database for bibliometric analysis was established by retrieving relevant literature from the WoS Core Collection database. To ensure data accuracy and consistency, especially considering potential database upgrades, we conducted a thorough search and data export on January 9, 2025, encompassing published articles on TFH cells and tumors from 2012 to 2024. The search strategy included the terms: (TS = (T follicular Helper Cells) OR TS = (follicular B Helper T Cells) OR TS = (follicular Helper T Cells) OR TS=(TFH Cell) OR TS = (TFH Cells) AND (TS = (Tumor) OR TS = (Neoplasms) OR TS = (Neoplasia) OR TS = (Cancer)). To minimize potential biases, specific refinement criteria were applied: (1) only articles and reviews were included; (2) language was restricted to English; (3) the timeframe was set from January 1, 2012, to December 31, 2024. A total of 1,181 articles were retrieved for detailed analysis. The details of literature screening are summarized in Figure 1.
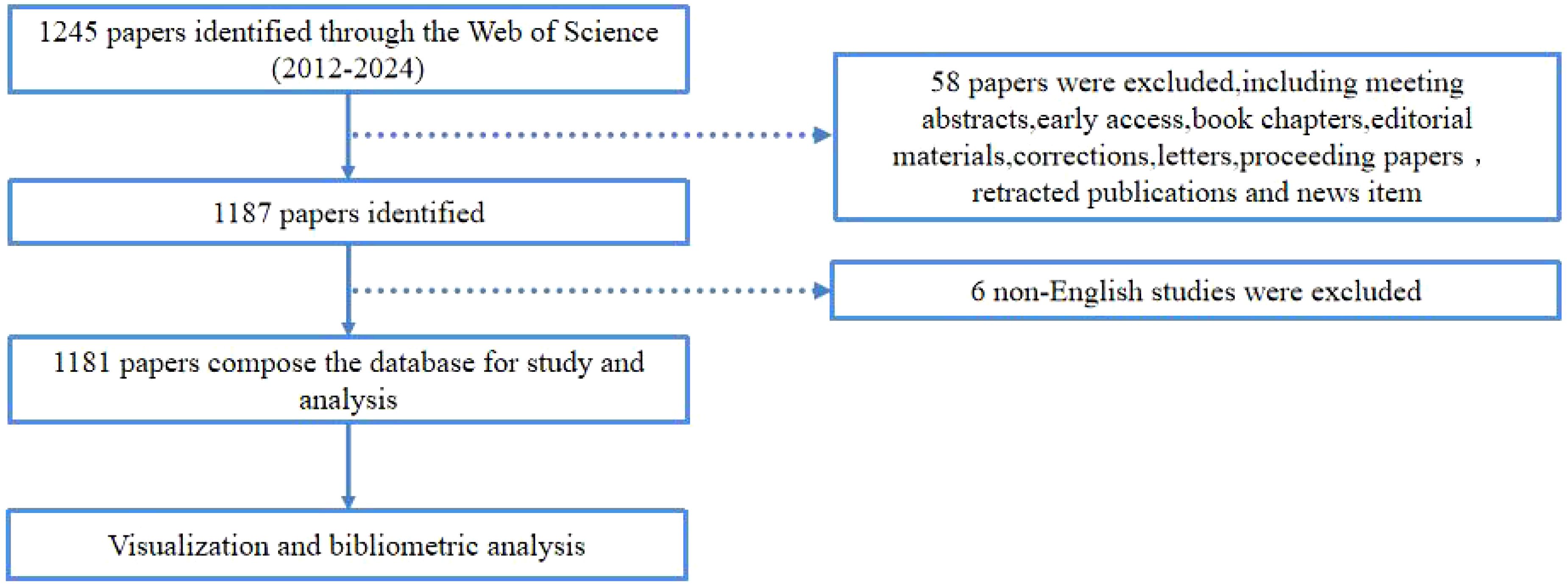
Figure 1. The flowchart illustrating the search strategy and selection process in TFH cell and tumor.
In this study, we utilized the “bibliometric” package within R (version 4.2.3) for in-depth scientific mapping analysis (8). We employed two distinct software tools for bibliometric analysis: CiteSpace [6.2.R6] and VOSviewer (version 1.6.20). CiteSpace, a Java-based citation visualization software developed by Chaomei Chen, facilitates statistical analysis and transforms raw data into visual representations of literature networks (14). Through CiteSpace, we examined keywords and references with significant citation bursts. VOSviewer, a robust bibliometric surveying and mapping tool developed by Nees Jan van Eck and LudoWaltman in 2009 (15), was used to visualize authors, cited authors, and institutions. Our study undertook a thorough and systematic evaluation of the research field by integrating these two software tools.
A total of 1,181 papers on TFH cell and tumor were reviewed, comprising 986 articles and 195 reviews. Figure 2 illustrates a fluctuating increasing trend in publications since 2012, indicating a growing research interest in this field. Notably, in the last five years, the number of publications had more than doubled the total publications of previous years. The year with the lowest publication count was 2012 (14 articles, 1.19%), and the year with the highest was 2022 (217 articles, 18.37%).
Papers on TFH cells and tumors had been published across 60 countries or regions globally. The top 10 countries in terms of publication volume are presented in Table 1. Leading in publication volume were China (624 articles, 52.84%), the United States (304 articles, 25.74%), and France (71 articles, 6.01%). Together, China and the United States accounted for nearly 70% of global publications, with China alone accounting for almost half of these publications. However, the United States has a citation count of 15,137 and an H-index value of 60, which is higher than that of China. A cross-border cooperation map illustrated the collaboration density between countries, with notable close collaboration between China and the United States, which had partnered in 42 times (Figure 3). The United States not only published a substantial volume of articles but had also engaged in extensive international cooperation. It had forged deep collaborative relationships with countries such as China, Germany, the United Kingdom, Japan, France, and Italy. This approach set a valuable role model for other nations to follow.
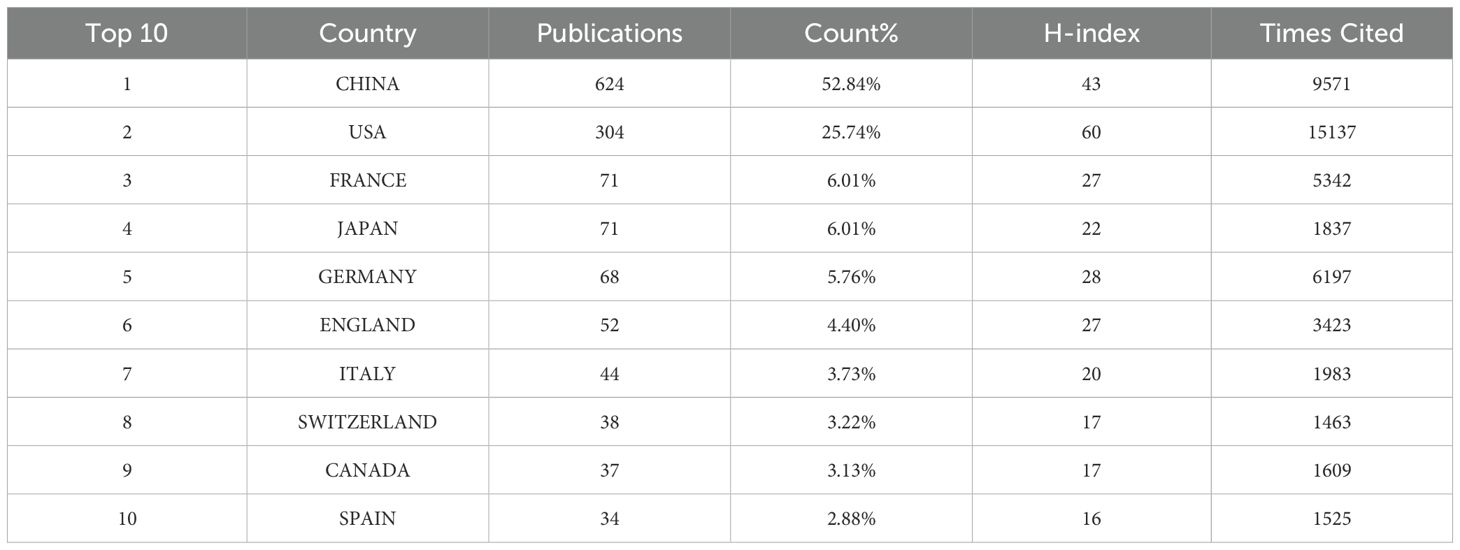
Table 1. Metrics of publications from the top 10 countries in the area of TFH cells and tumor research.
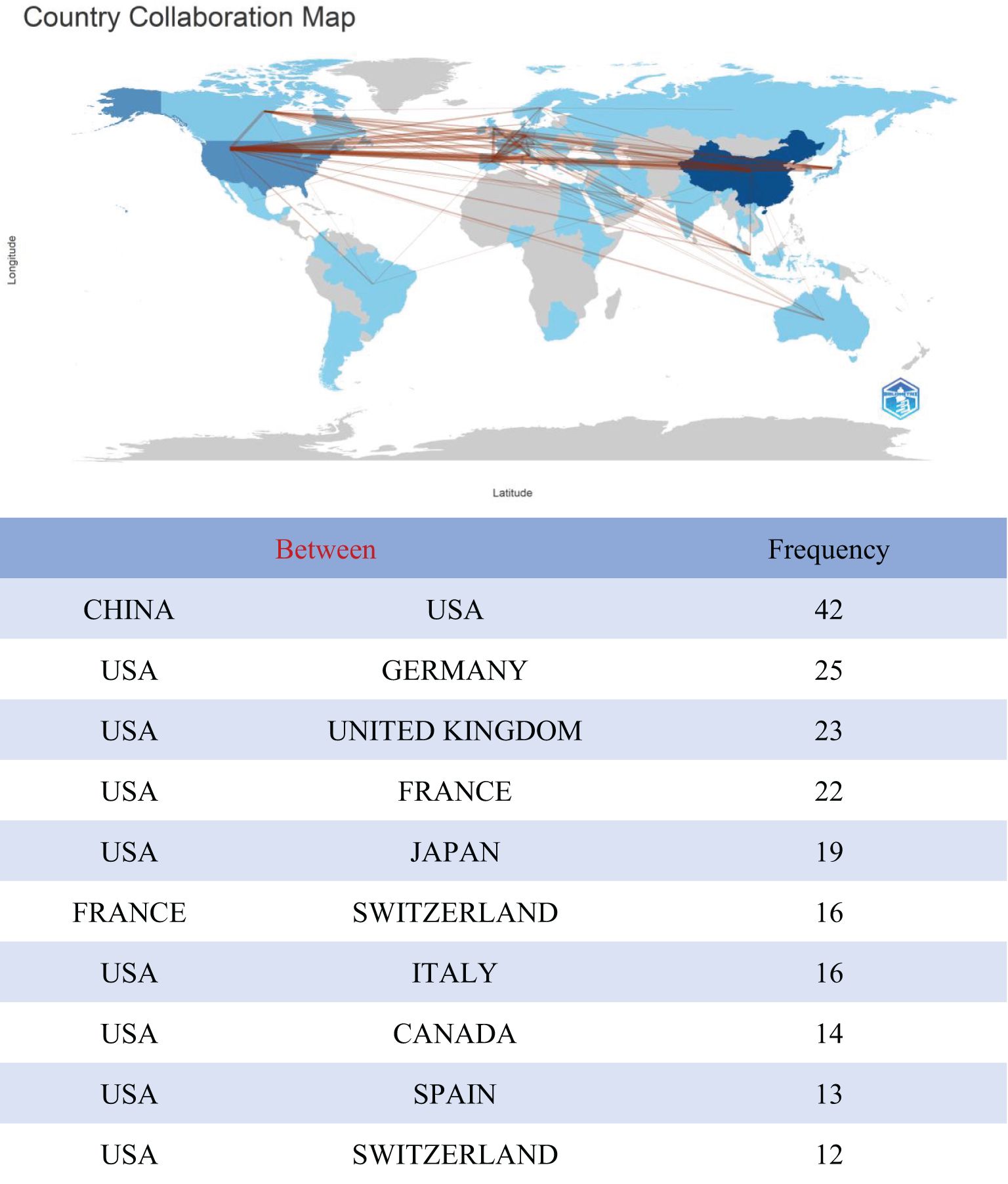
Figure 3. Analysis of the collaborative network between TFH cells and tumor related literature sources in different countries.
Since 2012, a total of 7,864 authors and 35,853 cited authors had contributed to the field of TFH cells and tumors. The author visualization diagram (Figure 4A) illustrates collaboration among co-authors and the publication output of each author. A threshold of at least 2 articles per author was applied, resulting in the inclusion of 767 authors in the network. The top 3 authors based on publication count were Gaulard, Philippe (14 articles, 379 citations), De Leval, Laurence (12 articles, 236 citations), and Tarte, Karin (10 articles, 465 citations). In the co-citation analysis (Figure 4B), a minimum citation threshold of 12 per author was applied, leading to the inclusion of 734 authors in the network. The most frequently cited authors were Crotty, S (380 citations), Newman, Am (192 citations), and Gu-Trantien, C (186 citations). These results suggested a notable interest among these authors in the study of TFH cells and tumors.

Figure 4. (A) Author visualization: Node size represents the number of articles. (B) Co-citation author analysis chart, where the size of nodes represents the number of repetitions.
A total of 1,756 institutions had engaged in research on TFH cells and tumors. Among them, the top 10 institutions collectively published 265 articles, representing22.4% of the total publications (Table 2). Leading in publication volume were Shanghai Jiao Tong University (34 articles, Citations:887), Sun Yat Sen University (34 articles, Citations:454), and Fudan University (29 articles, Citations:567). Notably, The top 10 institutions are all located in China, underscoring the country’s prominent position in this field. Then, we used VOSviewer to visualize the density map of the extended network and inter-institutional collaborations (Figure 5). The node sizes reflected the number of articles per institution, and the curve thickness indicated the strength of collaboration. Different colors on the map represented distinct collaboration groups. Notably, Sun Yat-sen University, Shanghai Jiao Tong University, and Fudan University exhibited extensive connections with other institutions and were positioned at the core of the density map.
A total of 385 journals had published articles on TFH cells and tumors, with Table 3 listing the top 10 most prolific journals in this field. Notably, In the JCR (2023) partition, all these journals are in Q2 or above, underscoring their substantial academic influence. Together, these top 10 journals accounted for 24.8% of all publications on this topic. FRONTIERS IN IMMUNOLOGY had published 96 articles, making it the journal with the largest circulation. It was followed by FRONTIERS IN ONCOLOGY with 41 articles, and FRONTIERS IN GENETICS with 28 articles. The top 3 journals with the highest citations were IMMUNITY (cited 3,346 times), J IMMUNOL (cited 3,322 times), and BLOOD (cited 3,081 times), as detailed in Table 4.
In this study, among the 50,883 retrieved references, the top 10 most cited papers collectively received 10,513 citations. Table 5 presents these references in descending order of citation frequency, each having garnered over 200 citations. The top 3 most cited articles were:Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer (cited 3,721 times); T follicular helper cell differentiation, function, and roles in disease (cited 1,842 times); and T Follicular Helper Cell Biology: A Decade of Discovery and Diseases (cited 1,362 times). An analysis of these highly cited articles (Figure 6) provided insights into the latest advancements in the field of TFH cells and tumors. Notably, the article “Follicle helper CD4 T cells (TFH)” published in Annu Rev Immunol had the highest citation explosion value from 2012 to 2016, at 22.82.
Keyword co-occurrence analysis is valuable for uncovering semantic relationships between keywords, extracting latent information from text data, and enhancing comprehension of text content and associated themes. Employing CiteSpace to visualize the keyword co-occurrence network (Figure 7). The most frequently appearing keywords were “expression” (366 occurrences), “cancer” (162 occurrences), “survival” (142 occurrences), “B cells” (119 occurrences), and “differentiation” (105 occurrences). Subsequent cluster analysis (Figure 8) revealed 15 primary categories focused on research areas such as TFH cells and tumors. These categories included topics like “follicular lymphoma,” “colorectal cancer patient,” “bioinformatics analysis,” and “hepatocellular carcinoma.” To visually illustrate the temporal dynamics of keywords, we generated a keyword timeline chart (Figure 9) and a keyword emergence chart (Figure 10). The evolution of these keywords reflected the shifting priorities over time, indicating both continuity and variability in research focus. Recent trends showed a transition towards topics like “tumor microenvironment”, “tumor infiltrating immune cells”, and “immune infiltration”. This transition indicated the ongoing development and maturation in the field. Initially, research emphasis was on “expression,” “B cells,” and “survival,” with a gradual shift towards emerging areas of interest in recent years.
Theme analysis examined author’s keywords and their connections to identify central theme. In a thematic network, nodes with more connections were considered more central and pivotal. The cohesion among nodes, reflecting the density of the research field, indicated their capacity for growth and sustainability. We presented a thematic map of TFH cells and tumor research, where the theme “immunotherapy” in the first quadrant (upper right corner) indicated both significant and robust development (Figure 11).
On July 26, 2023, doctors and scientists from Stanford University published a groundbreaking study in Nature. This study unveiled a new compound capable of modulating the function of the BCL6 protein, switching its role from blocking gene expression to activating gene expression (16). This discovery opens up new possibilities for developing anticancer drugs targeting BCL6-associated cancers, such as diffuse large B-cell lymphoma. BCL6 plays a crucial role in the differentiation of follicular helper T cells (TFH) and B cells. It is also essential for various processes, such as the formation of BCL6 in the germinal center, maturation of B cell affinity, production of high affinity antibodies, differentiation of plasma cells, and generation of memory B cells. The intricate relationship between follicular helper T cells and tumors warrants further investigation. The number of publications in this field had been steadily increasing since 2012, with a significant surge in recent years. In the past five years, the number of publications had more than doubled compared to previous years, indicating a growing interest among researchers. China led in the number of publications, accounting for nearly half of the global publication volume. The year with the highest number of publications was 2022, with 217 articles (18.37%). Notable authors in this field were Gaulard, Philippe (France), de Léval, Laurence (Switzerland), and Tarte, Karin (France). The most cited authors were Crotty, S (USA), Newman, Am (USA), and Gu Trantien, C (Belgium). Although China had the highest number of publications in the field of TFH cells and tumors, the authors with the most publications and citations were mainly from the United States and France, indicating their high-quality research and leading position in the field. Surprisingly, The top 10 institutions with the highest number of published papers are all located in China. The journal with the most publications was FRONTIERS IN IMMUNOLOGY, covering basic, translational, and clinical immunology. The most cited journal was IMMUNITY. Research on TFH cells and tumor immunity was a current hot topic, reflected in the top 10 cited references. Analysis of keywords revealed ongoing hotspots and research trends, shifting towards “tumor microenvironment”, “tumor infiltrating immune cells”, and “immune infiltration”.
TFH cells are crucial mediators in tumor progression and anti-tumor immunotherapy. Previous studies have linked the presence of TFH cells to prognosis in specific cancer types. High levels of TFH cell infiltration have been shown to correlate with enhanced survival rates. Additionally, the efficacy of immune checkpoint inhibitors is influenced by TFH cell activity. Understanding the role and regulation of TFH cells in tumors could pave the way for more effective immunotherapies, ultimately improving survival rates and treatment outcomes for cancer patients. TFH cells express high levels of PD-1 and other co stimulatory and inhibitory receptors. Therefore, treatment targeting CTLA-4 or PD-1 and their ligand PD-L1 may significantly affect TFH cell function in patients receiving immune checkpoint inhibitors(ICIs) therapy, providing a link between ICI therapy and the development of secondary autoimmunity (17). Fudan University’s team led by Xiao Fei has revealed that MCRS1 can enhance the sensitivity of tumor cells to T-cell killing by upregulating MHC-I molecule expression in solid tumors, while improving the therapeutic effect of PD-1 blockade therapy (18). TFH cells have been known to play a crucial role in defending against infectious diseases. For instance, the IgG response to cowpox virus infection is significantly diminished by 98% in the absence of TFH cells (19). Additionally, TFH cells can be targeted and infected by HIV (20). TFH cells are also implicated in autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). The role of TFH cells in human vaccines for annual influenza immunization has been extensively investigated. The role of TFH cells has extended to antibody-mediated allergies. A study in 2012 showed that TFH cell-derived IL-4 is a key factor for inducing IgE production in a mouse model of worm infection (21). Additionally, their role in promoting atherosclerosis has been noted (22). In the context of organ transplantation, TFH cells and GCs present are associated with both acute and chronic rejection reactions in human kidney transplants (23, 24). The last ten years have seen remarkable discoveries linking TFH cells to a broad spectrum of human diseases, particularly cancer. In malignant tumors, the presence of infiltrating immune cells could impact tumor growth, cancer advancement, and patient outcomes (25).
TFH related cells have demonstrated diverse impacts on the long-term survival of patients with different cancer types. For example, in patients with breast cancer or colorectal cancer, TFH cells were positively correlated with survival (26, 27). However, in a mouse model of hepatocellular carcinoma, an inverse correlation between TFH cells and survival was observed (28). Further analysis has revealed that the immune infiltrating components in tumors changed throughout the tumor’s progression, significantly influencing patient survival. Specifically, the density of T follicular helper cells and intrinsic cells tend to increase as the tumor progress, while the density of most T cells decreased. Overall, TFH cells are increasingly recognized for their critical roles across various fields, especially in oncology. TFR (T follicular regulatory cells) are a relatively less mentioned population that may correspond to TFH cells and participate in regulating immune responses, particularly in autoimmune diseases and immune tolerance. The ratio of TFR cells to TFH cells may change in certain disease states, such as in systemic lupus erythematosus (SLE), where low-dose IL-2 therapy can inhibit TFH cells and expand TFR cells, thereby regulating pathogenic humoral immunity (29).
In recent years, research on TFH cells and tumors has shifted towards a deeper understanding of the “tumor microenvironment”, “tumor infiltrating immune cells”, and “immune infiltration”. The field of cancer treatment has significantly evolved over the past decade, moving away from traditional chemotherapies that target a broad spectrum of tumors to new treatment strategies that target cells within the tumor microenvironment. Immune checkpoint blockade therapies, which target immune cells expressing CTLA4,CD28,ICOS and PD1 in the tumor microenvironment, represents a first-generation antibody-based therapy for cancer (30). These inhibitors maintain T cell attack on tumors by inhibiting PD-1 function. We are familiar with PD-1 as an inhibitory receptor expressed on the surface of T cells, which interacts with PD-L1 and PD-L2 (B7-H1 and B7-DC) of the B7 family. PD-1 recruits phosphatases SHP1 and SHP2 through its intracellular tyrosine motif, thereby inhibiting T cell activation. In the tumor immune system, upregulation of PD-1 leads to T cell exhaustion, which is a mechanism of tumor immune escape (31). In addition, CD28 is a co stimulatory molecule belonging to the immunoglobulin superfamily, mainly expressed on all mouse T cells and most human CD4+T cells. CD28 provides the second signal required for T cell activation by binding to CD80 and CD86 (B7 family molecules) on antigen-presenting cells (APCs). The activation of CD28 leads to T cell proliferation and differentiation into various effector cell types. Without co stimulatory signals, T cells may become unresponsive to further stimuli (known as incompetence) or even undergo apoptosis (32). CTLA-4 is a homologous molecule of CD28, primarily expressed on regulatory T cells (Tregs) and upregulated upon activation of conventional T cells. CTLA-4 has a strong inhibitory effect on T cell function, and mice lacking CTLA-4 exhibit lymphoproliferative disorders. CTLA-4 competes with CD28 for CD80/86 on the surface of APCs, thereby controlling T cell activation (32). ICOS is another co stimulatory molecule of the CD28 family, and its ligand is ICOS-L (B7-H2), belonging to the B7 family. The expression of ICOS on the surface of T cells is rapidly upregulated after TCR cross-linking and/or CD28 co stimulation. ICOS-L is expressed on APCs and binding to ICOS triggers intracellular signaling, promoting T cell activation and differentiation (33).
Many researchers now recognize the tumor microenvironment as an active promoter in cancer progression, rather than merely a passive observer. From 2021 to 2022, studies have increasingly focused on how TFH cells interact with the tumor microenvironment and tumor infiltrating immune cells. TFH cells, also known as follicular helper T cells, play a crucial role in the tumor microenvironment and can impact the response to immune therapy and tumor prognosis. Different subpopulations of B cells identified in the tumor microenvironment may exhibit diverse roles, either promoting tumor growth or combating it (34–36). Studies have shown that tumor neoantigens can regulate their fate by promoting the interaction between tumor specific CD4 T cells and tumor specific B cells, thereby enhancing the effector function of CD8 T cells to promote anti-tumor immunity (37). In human colorectal tumors, Overacre Delgoffe et al. investigated the effect of immunogenic bacterium Helicobacter pylori (Hhep) on the immune response to colorectal cancer. Introducing Hhep into a mouse model of colorectal cancer (CRC) increased tumor infiltration of cytotoxic lymphocytes and inhibited tumor growth. Therefore, the introduction of immunogenic intestinal bacteria can promote TFH related anti-tumor immunity in the colon (38). suggesting that TFH cells might enhance the anti-tumor immune response. However, certain tumors may impair TFH cells functions by modifying the tumor microenvironment to evade immune defenses. Laurence Zitvogel et al. proposed a novel coordinated participant that provides humoral and cellular immune responses, operable to restore sensitivity to immune checkpoint inhibition. This operation leads to effective TFH and B cell dialogue in the mesenteric lymph nodes, ultimately resulting in tumor specific memory CD8 T cell responses and preservation of normal epithelium (39). Thus, modulating the function of TFH cells may boost the efficacy of immunotherapies against tumors.
Malignancies can be influenced by the presence of TFH cells, which can either support malignant B cells or provide assistance in combating solid tumors. In tumors with TLS, increasing the number or function of TFH cells may help boost anti-tumor immune responses. Tumors are often infiltrated by various immune cells, such as lymphocytes, macrophages, and mast cells. While lymphocytes can influence cancer outcomes, factors produced by mast cells can promote tumor growth through chronic inflammation (40). Recent research from 2023 to 2024 has focused on the concept of “immune infiltration” involving TFH cells and tumors. TFH cells, a subset of T lymphocytes, play a crucial role in lymphoid tissue and invasive diseases. Their presence in tumor immune infiltration is essential for regulating the immune response of T and B lymphocytes, which ultimately impacts the efficacy of immunotherapies and the prognosis of tumors. Some early studies have mentioned the presence of lymphoid structures in tumors, including breast cancer (41). However, the prognostic value of these structures was demonstrated in non-small cell lung cancer, where the number of mature dendritic cells served as an indicator of tumor lymphoid structures (42). However, the prognostic value of these structures was demonstrated in a study on non-small cell lung cancer, where the number of mature dendritic cells was used as an indicator of tumor lymphoid structures (41, 43). Despite this, their immunological significance in these patients remains unclear. The immune system may struggle to inhibit tumor growth, yet the presence of T follicular helper cells that produce CXCL13 is linked to organized immune structures near the tumor site (44). This association is thought to contribute to sustained and effective long-term anti-tumor immunity. The research group led by Liu Guangwei from the School of Life Sciences at Beijing Normal University has found that the NAD+- dependent deacetylase SIRT3 in mitochondria can regulate the differentiation and function of TFH cells, and play a key regulatory role in anti-tumor immunity. This study provides new experimental evidence for the research of targeted T-cell subpopulation tumor immunotherapy strategies (45). Nowadays, more and more new therapies are being discovered, including dietary fiber promoting cancer immunotherapy by maintaining the microbiota (46);Immune checkpoint inhibitors (ICI) that activate T cells may also lead to AID called rheumatoid immune related adverse events (Rh irAEs) (47); Treat responsive melanoma with ICI (immune checkpoint inhibitors) or MAPK pathway inhibitors (MAPKi) (48); Combination therapy between IL-21 and TFH cells and immune checkpoint in immunotherapy for non-small cell lung cancer (NSCLC) (49); And chimeric antigen receptor (CAR) T-cell therapy for rare cancer such as angioimmunoblastic T-cell lymphoma (AITL) (50). The presence of T follicular helper cells is associated with favorable immune infiltration and tumor prognosis, suggesting a key role in regulating tumor immune response. Enhancing the function of T follicular helper cells could potentially promote immune cell infiltration and elimination of tumors, thereby enhancing tumor immune response.
This study conducted a bibliometric analysis of TFH cells and tumors using the Web of Scientific Core Collection (WoSCC). The study identified current research hotspots and trends from various perspectives. However, the study has limitations. Firstly, it included only English-language publications, which may introduce selection bias by excluding literature published in other languages or non-English journals. Secondly, the focus was restricted to articles and review articles, which may lead to incomplete data. In addition, the analysis relies solely on the Web of Science, potentially omitting relevant studies from other databases.
Overall, our bibliometric analysis reveals a rapid growth in research on TFH cells and tumors over the past decade. Since 2012, there has been a surge in interest, with China, the United States, and France emerging as the main contributing countries. Leading the pack in contributions are Shanghai Jiao Tong University, Sun Yat Sen University, and Fudan University. In recent years, “tumor microenvironment”, “tumor infiltrating immune cells”, and “immune infiltration” have emerged as popular research topics that warrant further exploration and attention.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
HL: Data curation, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. JH: Funding acquisition, Writing – review & editing. JZ: Formal analysis, Resources, Writing – review & editing. RL: Conceptualization, Resources, Writing – review & editing. YZ: Resources, Writing – review & editing. YQZ: Resources, Writing – review & editing. GH: Resources, Writing – review & editing. TS: Resources, Writing – review & editing. HC: Funding acquisition, Resources, Writing – review & editing. CL: Resources, Writing – review & editing. LL: Funding acquisition, Supervision, Writing – review & editing. CPL: Funding acquisition, Supervision, Writing – review & editing. WZ: Funding acquisition, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant No. 82203813, 82203147, 82373055, 82373144, 82303861, 82460493), the High-level Talents Program of Hainan Province Natural Science Foundation (823RC594), the Hubei Provincial Key Research and Development Program (No. 2023BCB011,2024BE020).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T cells. Annu Rev Immunol. (2016) 34:335–68. doi: 10.1146/annurev-immunol-041015-055605
2. Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. (2011) 29:621–63. doi: 10.1146/annurev-immunol-031210-101400
3. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. (2009) 325:1006–10. doi: 10.1126/science.1175870
4. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. (2009) 325:1001–5. doi: 10.1126/science.1176676
5. Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. (2009) 31:457–68. doi: 10.1016/j.immuni.2009.07.002
6. Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, et al. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med. (2015) 212:539–53. doi: 10.1084/jem.20141380
7. Kim YJ, Choi J, Choi YS. Transcriptional regulation of Tfh dynamics and the formation of immunological synapses. Exp Mol Med. (2024) 56:1365–72. doi: 10.1038/s12276-024-01254-7
8. Olatunde AC, Hale JS, Lamb TJ. Cytokine-skewed Tfh cells: functional consequences for B cell help. Trends Immunol. (2021) 42:536–50. doi: 10.1016/j.it.2021.04.006
9. Liu W, You W, Lan Z, Ren Y, Gao S, Li S, et al. An immune cell map of human lung adenocarcinoma development reveals an anti-tumoral role of the Tfh-dependent tertiary lymphoid structure. Cell Rep Med. (2024) 5:101448. doi: 10.1016/j.xcrm.2024.101448
10. Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. (2008) 29:138–49. doi: 10.1016/j.immuni.2008.05.009
11. Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. (2010) 207:155–71. doi: 10.1084/jem.20091706
12. Gutiérrez-Melo N, Baumjohann D. T follicular helper cells in cancer. Trends Cancer. (2023) 9:309–25. doi: 10.1016/j.trecan.2022.12.007
13. Song B, Lin Z, Feng C, Zhao X, Teng W. Global research landscape and trends of papillary thyroid cancer therapy: a bibliometric analysis. Front Endocrinol (Lausanne). (2023) 14:1252389. doi: 10.3389/fendo.2023.1252389
14. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U.S.A. (2004) 101 Suppl 1:5303–10. doi: 10.1073/pnas.0307513100
15. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
16. Gourisankar S, Krokhotin A, Ji W, Liu X, Chang CY, Kim SH, et al. Rewiring cancer drivers to activate apoptosis. Nature. (2023) 620:417–25. doi: 10.1038/s41586-023-06348-2
17. Baumjohann D, Brossart P. T follicular helper cells: linking cancer immunotherapy and immune-related adverse events. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-002588
18. Li X, Yi H, Jin Z, Jiang K, Xue K, Wang J, et al. MCRS1 sensitizes T cell-dependent immunotherapy by augmenting MHC-I expression in solid tumors. J Exp Med. (2024) 221. doi: 10.1084/jem.20240959
19. Xiao N, Eto D, Elly C, Peng G, Crotty S, Liu YC. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat Immunol. (2014) 15:657–66. doi: 10.1038/ni.2912
20. Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, et al. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med. (2016) 22:754–61. doi: 10.1038/nm.4113
21. Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. (2011) 13:58–66. doi: 10.1038/ni.2182
22. Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun. (2018) 9:1095. doi: 10.1038/s41467-018-03493-5
23. Chenouard A, Chesneau M, Bui Nguyen L, Le Bot S, Cadoux M, Dugast E, et al. Renal operational tolerance is associated with a defect of blood Tfh cells that exhibit impaired B cell help. Am J Transplant. (2017) 17:1490–501. doi: 10.1111/ajt.14142
24. de Graav GN, Dieterich M, Hesselink DA, Boer K, Clahsen-van Groningen MC, Kraaijeveld R, et al. Follicular T helper cells and humoral reactivity in kidney transplant patients. Clin Exp Immunol. (2015) 180:329–40. doi: 10.1111/cei.12576
25. Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. (2015) 12:453–7. doi: 10.1038/nmeth.3337
26. Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. (2013) 39:782–95. doi: 10.1016/j.immuni.2013.10.003
27. Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. (2013) 123:2873–92. doi: 10.1172/JCI67428
28. Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. (2017) 551:340–5. doi: 10.1038/nature24302
29. Yu D, Walker LSK, Liu Z, Linterman MA, Li Z. Targeting T(FH) cells in human diseases and vaccination: rationale and practice. Nat Immunol. (2022) 23:1157–68. doi: 10.1038/s41590-022-01253-8
30. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. (2020) 30:R921–r925. doi: 10.1016/j.cub.2020.06.081
31. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. (2020) 20:651–68. doi: 10.1038/s41577-020-0306-5
32. Beyersdorf N, Kerkau T, Hünig T. CD28 co-stimulation in T-cell homeostasis: a recent perspective. Immunotargets Ther. (2015) 4:111–22. doi: 10.2147/ITT.S61647
33. Rujas E, Cui H, Sicard T, Semesi A, Julien JP. Structural characterization of the ICOS/ICOS-L immune complex reveals high molecular mimicry by therapeutic antibodies. Nat Commun. (2020) 11:5066. doi: 10.1038/s41467-020-18828-4
34. DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. (2010) 29:309–16. doi: 10.1007/s10555-010-9223-6
35. Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. (2010) 10:236–47. doi: 10.1038/nri2729
36. Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. (2011) 331:1203–7. doi: 10.1126/science.1201730
37. Cui C, Wang J, Fagerberg E, Chen PM, Connolly KA, Damo M, et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell. (2021) 184:6101–6118.e13. doi: 10.1016/j.cell.2021.11.007
38. King C. Tfh cells set the stage for tumor control. Immunity. (2021) 54:2690–2. doi: 10.1016/j.immuni.2021.11.013
39. Fidelle M, Yonekura S, Picard M, Cogdill A, Hollebecque A, Roberti MP, et al. Resolving the paradox of colon cancer through the integration of genetics, immunology, and the microbiota. Front Immunol. (2020) 11:600886. doi: 10.3389/fimmu.2020.600886
40. Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. (2010) 29:1093–102. doi: 10.1038/onc.2009.416
41. Coronella JA, Spier C, Welch M, Trevor KT, Stopeck AT, Villar H, et al. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J Immunol. (2002) 169:1829–36. doi: 10.4049/jimmunol.169.4.1829
42. Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. (2008) 26:4410–7. doi: 10.1200/JCO.2007.15.0284
43. Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. (2011) 71:5678–87. doi: 10.1158/0008-5472.CAN-11-0431
44. Goubet AG, Lordello L, Alves Costa Silva C, Peguillet I, Gazzano M, Mbogning-Fonkou MD, et al. Escherichia coli-Specific CXCL13-Producing TFH Are Associated with Clinical Efficacy of Neoadjuvant PD-1 Blockade against Muscle-Invasive Bladder Cancer. Cancer Discovery. (2022) 12:2280–307. doi: 10.1158/2159-8290.CD-22-0201
45. Hou Y, Cao Y, He Y, Dong L, Zhao L, Dong Y, et al. SIRT3 negatively regulates TFH-cell differentiation in cancer. Cancer Immunol Res. (2024) 12:891–904. doi: 10.1158/2326-6066.CIR-23-0786
46. Arifuzzaman M, Collins N, Guo CJ, Artis D. Nutritional regulation of microbiota-derived metabolites: Implications for immunity and inflammation. Immunity. (2024) 57:14–27. doi: 10.1016/j.immuni.2023.12.009
47. Pan M, Zhao H, Jin R, Leung PSC, Shuai Z. Targeting immune checkpoints in anti-neutrophil cytoplasmic antibodies associated vasculitis: the potential therapeutic targets in the future. Front Immunol. (2023) 14:1156212. doi: 10.3389/fimmu.2023.1156212
48. Ding L, Sun L, Bu MT, Zhang Y, Scott LN, Prins RM, et al. Antigen presentation by clonally diverse CXCR5+ B cells to CD4 and CD8 T cells is associated with durable response to immune checkpoint inhibitors. Front Immunol. (2023) 14:1176994. doi: 10.3389/fimmu.2023.1176994
49. Liu X, Zhang Y, Zhang X, He G, Cai W. Progress of IL-21 and Tfh mediated immunotherapy in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. (2024) 27:550–8. doi: 10.3389/fimmu.2023.1176994
Keywords: Tfh cells, tumor, immune, bibliometrics, CD4+Th cells
Citation: Lei H, Hu J, Zhu J, Li R, Zhao Y, Zhao Y, He G, Song T, Lu C, Zheng W, Li L, Liu C and Chen H (2025) Global research prospects and trends in TFH cells and tumors: a bibliometric analysis. Front. Oncol. 15:1443890. doi: 10.3389/fonc.2025.1443890
Received: 10 July 2024; Accepted: 24 January 2025;
Published: 14 February 2025.
Edited by:
Shahram Salek-Ardakani, Inhibrx, United StatesReviewed by:
Yoshiko Matsuda, National Center for Child Health and Development (NCCHD), JapanCopyright © 2025 Lei, Hu, Zhu, Li, Zhao, Zhao, He, Song, Lu, Zheng, Li, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wuping Zheng, aG56d3AyMDAwQDE2My5jb20=; Lei Li, bGVpbGkyMDA4QGh1c3QuZWR1LmNu; Chunping Liu, bGNwMTkxQDE2My5jb20=; Hengyu Chen, Y2hlbmh5OTAxMkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.