
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 10 February 2025
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1395129
This article is part of the Research Topic Safety consideration in the development of anti-tumor monoclonal antibodies during drug development View all 7 articles
In clinical use, bevacizumab has improved the overall survival (OS) and progression-free survival (PFS) of malignant solid tumors, and the safe use of bevacizumab has gradually become the mainstream direction of clinical, nursing and pharmaceutical research. Bevacizumab is a humanized anti-VEGF drug. By binding to VEGF, it loses the opportunity to activate VEGFR and then plays an anti-angiogenic role. In addition, more and more studies have emphasized the efficacy and safety of bevacizumab in the treatment of brain metastases from solid tumors. Bevacizumab has its advantages in terms of crossing the blood-brain barrier, increasing radiosensitivity, and reducing radiation-induced brain edema. However, VEGF can maintain the normal function of vascular endothelial cells. Blocking the VEGF pathway can lead to endothelial dysfunction, and the anti-VEGF mechanism of bevacizumab will inevitably lead to a series of thrombotic events and bleeding events. This study reported six cases of cerebrovascular accidents, including ischemic stroke and cerebral hemorrhage, after bevacizumab use. At the same time, this study reviewed the related studies of cardiovascular and cerebrovascular accidents caused by bevacizumab, and analyzed the management of such bevacizumab-related adverse events. We put forward our own views on the early identification and long-term management of adverse events, as well as the subsequent reuse of bevacizumab, hoping to provide more basis for the management of clinical bevacizumab application.
Bevacizumab is A humanized IgG1 monoclonal antibody that specifically binds to human vascular endothelial growth factor A (VEGFA). By neutralizing VEGF and preventing the activation of VEGF tyrosine kinase receptors VEGFR1 and VEGFR2 on endothelial cells, it attenuates VEGFA-dependent tumor blood vessel formation, promotes tumor vascular normalization and promotes tumor cell apoptosis (1) (Figure 1). With the continuous expansion of indications for bevacizumab, more and more patients with solid tumors such as lung cancer, colorectal cancer and cervical cancer have been treated with bevacizumab and their survival has been significantly prolonged (2). Clinical studies have confirmed that on the basis of traditional chemotherapy regimens for a variety of solid tumors, adding bevacizumab not only prolongs progression-free survival (PFS), but also prolongs overall survival (OS), and its treatment has also been promoted from first-line to second-line or even third-line or maintenance therapy, which has become an important weapon in the tumor treatment “toolbox” for the clinicians (3, 4).
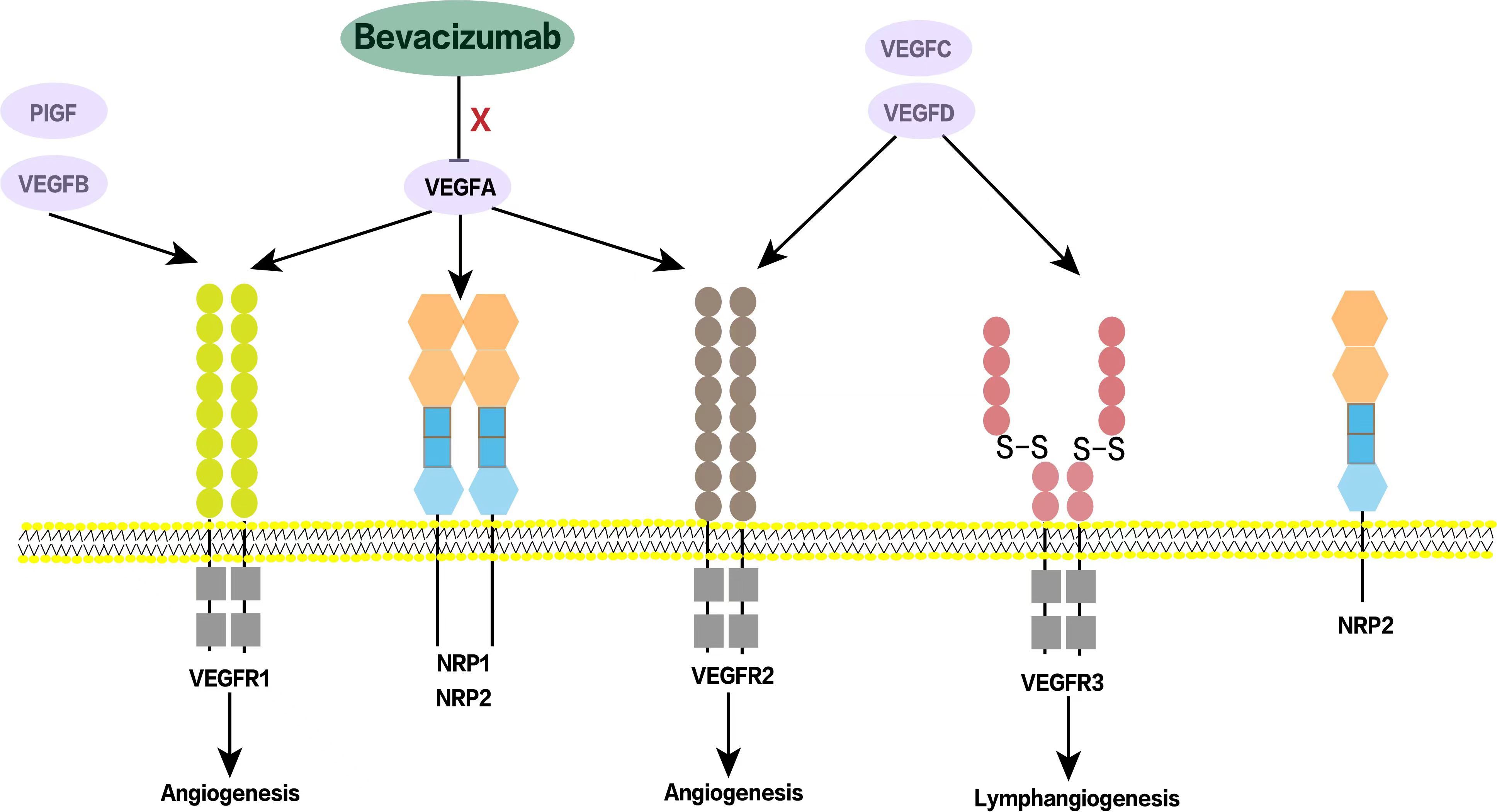
Figure 1. Schematic diagram of bevacizumab acting on VEGF receptors. VEGF family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D and other related factors. Bevacizumab binds to VEGF-A, prevents its interaction with VEGF receptors, plays an anti-angiogenesis role, and then inhibits tumor growth. NRP1/NRP2 are co-receptors of VEGF and also play an important role in tumor angiogenesis. PIGF, Placental growth factor; NRP, Neuropilin.
Bevacizumab has a half-life of approximately 20 days (2). This provides a time window for the combination of bevacizumab with chemoradiotherapy, but also lays the groundwork for adverse reactions. With the extension of patient survival, clinicians should also pay attention to and manage the adverse reactions of bevacizumab. The four representative adverse reactions of bevacizumab include hypertension, bleeding, proteinuria, and thromboembolism (5).Bevacizumab is an inhibitor of VEGF, which has the function of maintaining normal vascular endothelial cells, so blocking the VEGF pathway can lead to endothelial dysfunction (6).Vascular endothelial cell apoptosis or inhibition of endothelial cell regeneration can destroy the integrity of endothelial cells. The exposure of procoagulant phospholipids and the aggregation of various cytokines can promote thrombosis. In addition, bevacizumab-induced reductions in nitric oxide and prostacyclin levels also lead to platelet aggregation and thrombosis (7). In current bevacizuab-related clinical trials, the overall incidence of arterial thromboembolism in the bevacizumab group has been reported from 0.7% to 8.7%, venous embolism events have been reported from 0.5% to 10.1%, and cerebral hemorrhage events have been reported from 0.0% to 3.8% (Supplementary Table S1) (8–14).The clinical trial AVF2192 g included patients with metastatic colorectal cancer who were ineligible for irinotecan. Arterial thromboembolism was observed in 11% (11/100) of patients in the bevacizumab group and in 5.8% (6/104) of patients in the chemotherapy control group in this trial (15).
Although the adverse reactions of bevacizumab are well known in clinical practice, there is a lack of specific analysis of cerebrovascular events caused by bevacizumab in the real world. Cerebrovascular events reported in the literature refer to transient ischemic attacks, cerebral hemorrhage, and ischemic stroke that occur during treatment with bevacizumab. Theoretically, it seems that once such adverse events occur, the drug should be stopped permanently. However, it is difficult to balance the risk of disease progression associated with discontinuation with the risk of thrombosis/bleeding associated with continued treatment. At present, there are few research data to guide the clinical use of anticoagulant drugs during the use of bevacizumab, and the above problems need to be further explored in clinical practice. “Here, we report six cases of cerebrovascular events after the administration of bevacizumab at doses ranging from 5 to 7.5 mg/kg.
This study was a retrospective study, and the enrollment criteria were: (1) clear diagnosis of malignant tumor; (3) Cerebrovascular accidents occurred during the course of bevacizumab treatment, including transient ischemic attack, cerebral hemorrhage and ischemic stroke, which were specifically diagnosed with brain CT or brain MRI imaging support; (4) Complete case data.
Exclusion criteria were as follows: (1) The interval between bevacizumab use and cerebrovascular accident was more than 6 half-lives of bevacizumab or before bevacizumab use was judged to be unrelated to bevacizumab use. (2) Incomplete case data; (3)The patients had basic cerebrovascular malformations or previous cerebrovascular accidents;(4)Underlying uncontrolled severe hypertension.
We reviewed all patients with malignant tumors treated at Zhejiang Provincial People’s Hospital from January 2018 to December 2023. From January 2018 to December 2023, there were 5,584 patients with malignant tumors in the unit, and 2,157 patients received bevacizumab. The medical data intelligent platform was used for case information retrieval in our unit. The search keywords included “medical order content - bevacizumab”, “diagnostic name - cerebral infarction or cerebral hemorrhage or stroke”, “diagnostic name - malignant tumor”. A total of 39 cases were retrieved, of which 24 cases with cerebral infarction, cerebral hemorrhage or stroke diagnosis before the use of bevacizumab were excluded. In humans, the final half-life of bevacizumab is 17-21 days. Five patients were treated with bevacizumab only once during the treatment, and their cerebral infarction, cerebral hemorrhage or stroke occurred at an interval of more than 1 year after the treatment (far exceeding 6 half-lives of bevacizumab), which was judged to be irrelevant to bevacizumab and excluded. Four patients with incomplete clinical information were excluded (follow-up information and tumor evaluation information), and 6 patients were finally included for analysis. The specific retrieval process is shown in Figure 2.
The median overall survival (OS) of all patients was 54 months (12-66 months), and the median bevacizumab-related PFS was 5 months (3-28 months). The basic information of all patients, history of underlying diseases, dose of bevacizumab, concomitant treatments, and treatment for cerebrovascular accidents are summarized in Table 1. The six patients, whose tumors included lung, rectal, and endometrial cancers, ranged in age from 46 to 69 years and had no history of cerebrovascular disease. Two had a history of hypertension but were well controlled on medication. The dose of bevacizumab was 5.0-7.5mg/kg. One patient had a cerebrovascular accident and stopped taking bevacizumab (P02) after one dose, and the duration of bevacizumab used in the remaining patients ranged from 3 to 28 months. P01-P05 had cerebral infarction and were treated with anticoagulation. The patient P06 suffered from cerebral hemorrhage, and was treated with blood pressure control and supportive treatment. Representative images of ischemic stroke/cerebral hemorrhage in 6 patients are shown in Supplementary Figure S1.
These 6 patients included 4 cases of lung cancer (P01, P02,P03 and P05), 1 case of rectal cancer (P04) and 1 case of cervical cancer (P06). Four lung cancer patients had brain metastases and all of them were treated with gamma knife. Patients P02 and P03 had brain edema after gamma knife treatment. Three patients with lung cancer were treated with bevacizumab after gamma knife treatment (P01,P02 and P03) and subsequently had cerebrovascular events at various time points (1 day to 28 months). P01 and P03 continued bevacizumab in subsequent treatment after symptom recovery, while P02 left severe hemiplegia. Bevacizumab was permanently discontinued. Patient P04, a rectal cancer patient, had a cerebral infarction during the crossover of bevacizumab, and bevacizumab was permanently discontinued in the subsequent treatment. Patient P06 was a cervical cancer patient who developed cerebral hemorrhage during the use of bevacizumab and subsequently discontinued bevacizumab. The treatment timeline for patients is shown in Figure 3.
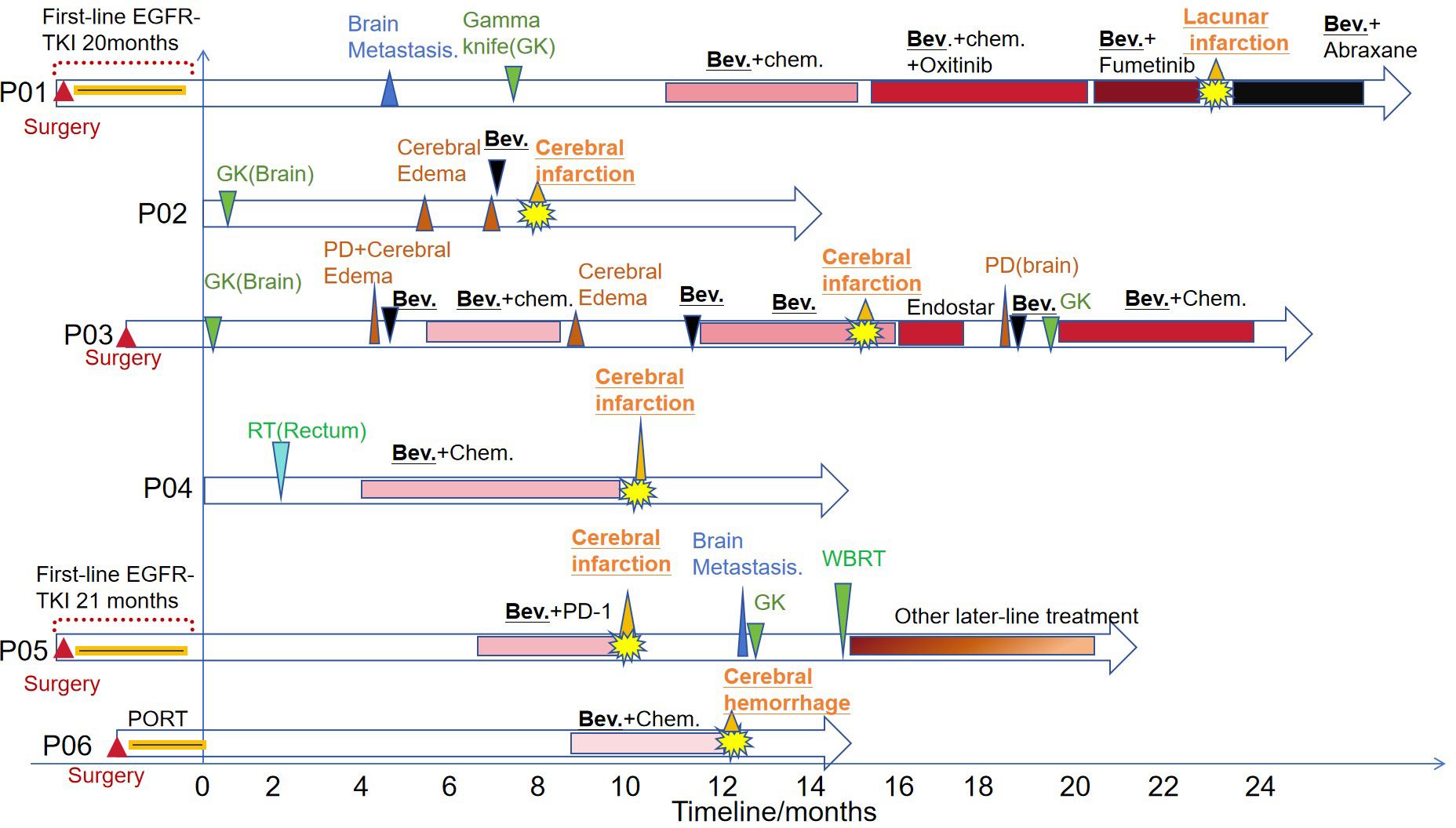
Figure 3. The treatment timeline of the six patients. PORT, Postoperative Radiotherapy; TKI, tyrosine kinase inhibitor; RT, radiotherapy; Chem., Chemotherapy; GK, Gamma knife; Bev., Bevacizumab; PD, progressive disease; WBRT, whole brain radiation therapy.
Angiogenesis is a fundamental activity in the process of tumor growth and metastasis (16).Therefore, in the field of cancer research, there is great interest in studying the molecular mechanisms of tumor angiogenesis. The vascular endothelial growth factor (VEGF) pathway is a key regulator of this process. The role of VEGF in promoting tumor angiogenesis, which promotes tumor generation, development, and migration (17). Therefore, it is necessary to design and develop inhibitors targeting this pathway. Bevacizumab binds VEGF and prevents its binding to receptors on the surface of vascular endothelial cells, thereby inhibiting angiogenesis in malignant tumors (18). In contrast to chemotherapeutic agents, bevacizumab does not produce typical cytotoxic effects and does not increase chemotherapy-related toxicity when combined with chemotherapy. However, the use of bevacizumab as an antiangiogenic therapy may be associated with vascular-related adverse events, including embolization and bleeding.Bevacizumab is thought to be associated with an increased risk of arterial thromboembolic events (19), and its susceptibility to venous thrombosis in the vein remains controversial.The incidence of VTE in patients treated with bevacizumab reported in the literature ranged from 3% to 19.4% (20, 21).A meta-analysis showed that the use of bevacizumab significantly increased the risk of venous thromboembolism in cancer patients (RR=1.33) (22). Bleeds associated with bevacizumab were mostly minor epistaxis and other self-limiting mucosal bleeds. But there have also been reports of serious (≧̸ grade 3) bleeding events (23).In addition to pulmonary hemorrhage, it also included severe gastrointestinal (GI) hemorrhage, urogenital tract hemorrhage, and central nervous system (CNS) hemorrhage (24, 25).Vascular events in the CNS include intracranial hemorrhage and ischemic stroke (26).
Mechanistically, bevacizumab can damage the integrity of vascular endothelial cells, inhibit the expression of prostaglandin and nitric oxide, and promote platelet aggregation, thereby increasing the risk of ischemic cerebrovascular events. In addition, bevacizumab can also inhibit the proliferation and migration of endothelial cells, which may lead to damage to vascular integrity, resulting in endothelial dysfunction and bleeding (27).
In our report, three patients with lung cancer were treated with bevacizumab after gamma knife treatment (P01,P02, and P03), and cerebral edema developed in two patients. Through our conversations with the clinicians, we learned that bevacizumab was administered largely for the treatment of radiation-induced cerebral edema, and then the patient unfortunately had a cerebrovascular accident in the subsequent course of the disease. Some studies have suggested that bevacizumab can normalize blood vessels in brain metastases, reduce vascular endothelial permeability, and improve peritumoral edema, thereby alleviating symptoms in patients with brain metastases (28–30).In 2009, the PASSPORT study established the safety of bevacizumab in NSCLC patients who had been treated with either radiation or surgery for brain metastases (31).This study showed that bevacizumab combined with chemotherapy or erlotinib for NSCLC patients with brain metastases was safe and had a low incidence of cerebral hemorrhage. Retrospective analyses from both U.S. and European databases have shown that the incidence of symptomatic intracranial hemorrhage in patients with NSCLC and brain metastases is comparable, at less than 5%, with or without bevacizumab. Therefore, on March 25, 2009, the European Union removed brain metastases as a contraindication for bevacizumab treatment. According to the 2022 Chinese expert consensus on anti-angiogenic drugs for advanced non-small cell lung cancer, bevacizumab can significantly relieve the symptoms caused by peritumoral edema and reduce the degree of edema on imaging in advanced NSCLC patients with symptomatic brain metastases such as brain edema or brain necrosis (Grade II recommendation, Class 2A evidence). However, the specific dosage has not been clearly prescribed, and the commonly used dose is 5-10 mg/kg, once every 2-3 weeks, 2-6 courses of treatment (32, 33). Although a number of clinical studies and expert consensus have recommended the application of bevacizumab in patients with brain metastases, and bevacizumab has the advantage of crossing the blood-brain barrier, the risk of vascular-related adverse events still cannot be ignored. Although bevacizumab has largely prolonged survival in such patients, cerebrovascular events can occur as soon as 1 day after treatment, and the most severe patients are left with permanent impairment of physical activity. Early identification, prevention, and education of vascular events remain important in the clinical use of bevacizumab.
There is some controversy about whether bevacizumab can be continued after the occurrence of vascular adverse events. It is currently believed that in the event of a serious thrombotic event, bevacizumab should be discontinued and anticoagulant therapy should be given. Once an arterial thromboembolic event (ATE) occurs, bevacizumab therapy should be discontinued in the acute phase. Patients with a recent ATE should not be treated with bevacizumab for at least 6 months after the onset of ATE, and the patient should be determined to be stable or asymptomatic before initiating bevacizumab therapy (27). Ischemic stroke can be considered a serious arterial thromboembolic event, and the two patients in this case who continued bevacizumab after recovery from stroke had good disease control and did not worsen ATE during long-term follow-up. In the real world, anti-angiogenic therapy is the cornerstone of many solid tumors, including lung cancer and intestinal cancer. Continuous anti-angiogenic therapy effectively prolongs the survival of patients throughout the treatment. In our center, The number of patients using beva in this center is about 2350 a year. Therefore, through close follow-up and observation, evaluating the risk of patients, weighing the pros and cons of re-using bevacizumab, and effective management of side effects, we can better play the efficacy of bevacizumab and bring more valuable treatment hope for cancer patients.
In real-world clinical practice, bevacizumab is widely used in patients with solid tumors, especially brain metastases. However, vascular accidents caused by bevacizumab, especially cerebrovascular accidents, deserve clinical attention, and serious cases can leave irreversible sequelae. As a macromolecular drug, the efficacy of bevacizumab in the treatment of intracranial lesions has been controversial. Whether the risk of cerebrovascular accident is higher in patients with intracranial lesions is worthy of further discussion. Our study included cases of cerebrovascular accident following bevacizumab treatment, including patients with brain metastases, and fully evaluated the association between bevacizumab use and cerebrovascular accident. This study further reviewed previous studies to discuss the occurrence and management of bevacizumab related cerebrovascular accidents, so as to provide a basis for clinical practice.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the ethics committee of the Zhejiang Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
KC: Data curation, Investigation, Methodology, Writing – original draft. BJ: Data curation, Software, Writing – original draft. HY: Methodology, Software, Supervision, Writing – original draft. LY: Validation, Visualization, Writing – review & editing. ZC: Conceptualization, Data curation, Formal analysis, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1395129/full#supplementary-material
Supplementary Figure 1 | The cranial imaging of cerebralvascular accidents. (A) P01, acute lacunar cerebral infarction in the left hemioval region. (B) P02, acute cerebral infarction was considered in the right paraventricular basal ganglia. (C) P03, multiple abnormal signal foci around the left ventricle and basal ganglia were considered acute infarction. (D) P04, large cerebral infarction was observed in the left temporal lobe, frontal lobe, Broca area, and lateral ventricle. (E) P05, cerebral infarction in the left parietal lobe. (F) P06, cerebral hemorrhage in the left basal ganglia.
1. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discovery. (2004) 3:391–400. doi: 10.1038/nrd1381
2. Kazazi-Hyseni F, Beijnen JH, Schellens JH. Bevacizumab. Oncologist. (2010) 15:819–25. doi: 10.1634/theoncologist.2009-0317
3. Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin(R)) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. (2020) 86:102017. doi: 10.1016/j.ctrv.2020.102017
4. Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discovery. (2016) 15:385–403. doi: 10.1038/nrd.2015.17
5. Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. (2008) 62:779–86. doi: 10.1007/s00280-007-0664-8
6. Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. (2003) 107:1359–65. doi: 10.1161/01.CIR.0000061911.47710.8A
7. Keefe D, Bowen J, Gibson R, Tan T, Okera M, Stringer A. Noncardiac vascular toxicities of vascular endothelial growth factor inhibitors in advanced cancer: a review. Oncologist. (2011) 16:432–44. doi: 10.1634/theoncologist.2010-0271
8. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. (2014) 370:709–22. doi: 10.1056/NEJMoa1308345
9. Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. (2009) 27:4733–40. doi: 10.1200/JCO.2008.19.8721
10. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. (2007) 357:2666–76. doi: 10.1056/NEJMoa072113
11. Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol. (2016) 27:1539–46. doi: 10.1093/annonc/mdw206
12. Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. (2014) 370:734–43. doi: 10.1056/NEJMoa1309748
13. Tewari KS, Burger RA, Enserro D, Norquist BM, Swisher EM, Brady MF, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol. (2019) 37:2317–28. doi: 10.1200/JCO.19.01009
14. Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. (2011) 365:2484–96. doi: 10.1056/NEJMoa1103799
15. Hurwitz HI, Tebbutt NC, Kabbinavar F, Giantonio BJ, Guan ZZ, Mitchell L, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist. (2013) 18:1004–12. doi: 10.1634/theoncologist.2013-0107
16. Gordon MS, Mendelson DS, Kato G. Tumor angiogenesis and novel antiangiogenic strategies. Int J Cancer. (2010) 126:1777–87. doi: 10.1002/ijc.v126:8
17. Qi S, Deng S, Lian Z, Yu K. Novel Drugs with High Efficacy against Tumor Angiogenesis. Int J Mol Sci. (2022) 23(13):6934. doi: 10.3390/ijms23136934
18. Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. (2020) 77:1745–70. doi: 10.1007/s00018-019-03351-7
19. Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. (2007) 99:1232–9. doi: 10.1093/jnci/djm086
20. Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. (2007) 370:2103–11. doi: 10.1016/S0140-6736(07)61904-7
21. Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. (2003) 21:60–5. doi: 10.1200/JCO.2003.10.066
22. Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. (2008) 300:2277–85. doi: 10.1001/jama.2008.656
23. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. (2009) 27:1227–34. doi: 10.1200/JCO.2007.14.5466
24. Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. (2004) 22:2184–91. doi: 10.1200/JCO.2004.11.022
25. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. (2006) 355:2542–50. doi: 10.1056/NEJMoa061884
26. Hang XF, Xu WS, Wang JX, Wang L, Xin HG, Zhang RQ, et al. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. (2011) 67:613–23. doi: 10.1007/s00228-010-0988-x
27. Brandes AA, Bartolotti M, Tosoni A, Poggi R, Franceschi E. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist. (2015) 20:166–75. doi: 10.1634/theoncologist.2014-0330
28. Wang Z, Chen H, Chen Q, Zhu Y, Bai Z, Li M, et al. Efficacy and safety of a "sandwich therapy" based on staged stereotactic radiosurgery and bevacizumab for large brainstem metastases. Clin Neurol Neurosurg. (2023) 233:107911. doi: 10.1016/j.clineuro.2023.107911
29. Chen TW, Dai MS, Tseng LM, Chen SC, Chao TY, Chao TC, et al. Whole-brain radiotherapy alone vs preceded by bevacizumab, etoposide, and cisplatin for untreated brain metastases from breast cancer: A randomized clinical trial. JAMA Oncol. (2023) 10(3):325–34. doi: 10.1001/jamaoncol.2023.5456
30. Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer. (2019) 18:21. doi: 10.1186/s12943-019-0950-1
31. Socinski MA, Langer CJ, Huang JE, Kolb MM, Compton P, Wang L, et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol. (2009) 27:5255–61. doi: 10.1200/JCO.2009.22.0616
32. Hua YC, Gao DZ, Wang KY, Ding XS, Xu WR, Li YB, et al. Bevacizumab reduces peritumoral brain edema in lung cancer brain metastases after radiotherapy. Thorac Cancer. (2023) 14:3133–9. doi: 10.1111/1759-7714.15106
33. Expert Committee on Vessel Targeted Therapy of Chinese Society of Clinical Oncology, Expert Committee on Non-small Cell Lung Cancer of Chinese Society of Clinical Oncology, Expert Group on Antiangiogenic Drugs for Non-small Cell Lung Cancer of Chinese Society of Clinical Oncology. Chinese expert consensus on antiangiogenic drugs for advanced non-small cell lung cancer (2020 Edition). Zhonghua Zhong Liu Za Zhi. (2020) 42:1063–77. doi: 10.3760/cma.j.cn112152-20200918-00836
Keywords: bevacizumab, adverts effects, VEGF, cerebrovascular accident, cancer, case report
Citation: Chen K, Jiang B, Yan H, Yang L and Chen Z (2025) Case report: Bevacizumab-induced cerebrovascular events: a case series report and literature review. Front. Oncol. 15:1395129. doi: 10.3389/fonc.2025.1395129
Received: 03 March 2024; Accepted: 20 January 2025;
Published: 10 February 2025.
Edited by:
Shaad Essa Abedin, Immunocore, United KingdomReviewed by:
Wen Cai, Zhejiang University, ChinaCopyright © 2025 Chen, Jiang, Yan, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheling Chen, MzgzOTc0OTAzQHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.