
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Oncol. , 31 January 2025
Sec. Cancer Molecular Targets and Therapeutics
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1544394
This article is a correction to:
First-in-Human Phase 1 Dose-Escalation Results With Livmoniplimab, an Antibody Targeting the GARP:TGF-ß1 Complex, as Monotherapy and in Combination With the Anti-PD-1 Antibody Budigalimab in Patients With Advanced Solid Tumors
 Toshio Shimizu1,2*
Toshio Shimizu1,2* John Powderly3
John Powderly3 Albiruni Abdul Razak4
Albiruni Abdul Razak4 Patricia LoRusso5
Patricia LoRusso5 Kathy D. Miller6
Kathy D. Miller6 Steven Kao7
Steven Kao7 Sarah Kongpachith8
Sarah Kongpachith8 Catherine Tribouley8
Catherine Tribouley8 Michelle Graham8
Michelle Graham8 Brian Stoll8
Brian Stoll8 Maulik Patel8
Maulik Patel8 Mohammad Sahtout8
Mohammad Sahtout8 Martha Blaney8
Martha Blaney8 Rachel Leibman8
Rachel Leibman8 Talia Golan9,10
Talia Golan9,10 Anthony Tolcher11
Anthony Tolcher11By Shimizu T, Powderly J, Abdul Razak A, LoRusso P, Miller KD, Kao S, Kongpachith S, Tribouley C, Graham M, Stoll B, Patel M, Sahtout M, Blaney M, Leibman R, Golan T and Tolcher A (2024) Front. Oncol. 14:1376551. doi: 10.3389/fonc.2024.1376551
In the published article, there were errors in Figure 4 as published. The graph included incorrect labeling of livmoniplimab doses for a few patients, including for the patient with deepest response (corrected from livmoniplimab 100mg to livmoniplimab 1500mg) in Figure 4B. The corrected Figure 4 and its caption appear below.
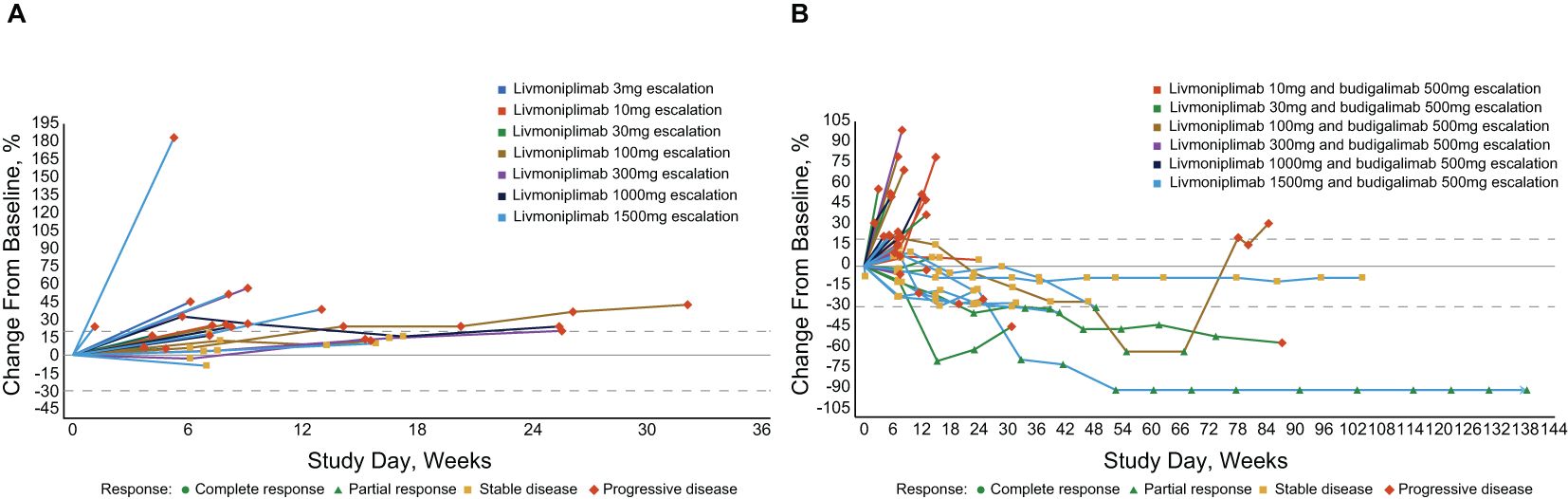
Figure 4. Percentage change in target lesion sum diameter measurements from baseline over time per investigator assessment in response-evaluable set (efficacy-evaluable patients defined as patients who have received at least 1 dose of study drug and have either had at least 1 postdose tumor assessment or discontinued treatment due to AE, progressive disease, or death); per RECIST v1.1 and iRECIST. (A) Livmoniplimab monotherapy (Q2W) cohorts (N=22). (B) Livmoniplimab (Q2W) and budigalimab combination therapy cohorts (N=34). → Denotes patients still on treatment. One patient did not have on-study tumor measurement data due to early death. AE, adverse event; iRECIST, modified RECIST v1.1 criteria for immune-based therapeutics; Q2W, once every 2 weeks; RECIST, Response Evaluation Criteria in Solid Tumors.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: advanced solid tumors, TGF-ß1, GARP, immunotherapy, anti-PD-1 antibody, combination drug therapy, investigational therapies, tumor microenvironment (TME)
Citation: Shimizu T, Powderly J, Abdul Razak A, LoRusso P, Miller KD, Kao S, Kongpachith S, Tribouley C, Graham M, Stoll B, Patel M, Sahtout M, Blaney M, Leibman R, Golan T and Tolcher A (2025) Corrigendum: First-in-human phase 1 dose-escalation results with livmoniplimab, an antibody targeting the GARP: TGF-ß1 complex, as monotherapy and in combination with the anti–PD-1 antibody budigalimab in patients with advanced solid tumors. Front. Oncol. 14:1544394. doi: 10.3389/fonc.2024.1544394
Received: 12 December 2024; Accepted: 19 December 2024;
Published: 31 January 2025.
Edited and Reviewed by:
Cory L. Brooks, California State University, Fresno, United StatesCopyright © 2025 Shimizu, Powderly, Abdul Razak, LoRusso, Miller, Kao, Kongpachith, Tribouley, Graham, Stoll, Patel, Sahtout, Blaney, Leibman, Golan and Tolcher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshio Shimizu, dG9zc2hpbWlAbmNjLmdvLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.