- 1Department of Gastroenterology, Jiangyin Hospital affiliated to Nantong University, Jiangyin, China
- 2Department of Interventional Radiology, Jiangsu Hospital of Huocheng County, Huocheng, China
- 3Department of Interventional Medicine, Binzhou People’s Hospital, Binzhou, China
- 4Department of Interventional Radiology, Jiangyin Hospital affiliated to Nantong University, Jiangyin, China
Background: Currently, inoperable hepatocellular carcinoma (HCC) is treated by both transarterial radioembolization (TARE) and transarterial chemoembolization (TACE). However, their relative efficacy and outcomes remain unclear. This meta-analysis aimed to compare TARE and TACE to evaluate their safety and efficacy in treating inoperable HCC patients.
Methods: Relevant studies were identified by searching the Web of Science, PubMed, and Wanfang databases. Pooled analyses were used to compare treatment response rates, complications, and overall survival (OS) outcomes between the TARE and TACE groups.
Results: This analysis selected 8 studies comprising 1026 and 358 patients that respectively underwent TACE and TARE treatment. The results revealed that the TARE group had significantly higher pooled total response, disease control, and 1-year OS rates compared to the TACE group (P = 0.04, 0.003, and 0.02, respectively), with a corresponding increase in OS (P = 0.0002). Furthermore, rates of complications including fever and abdominal pain were also reduced in the TARE group (P = 0.006 and 0.02, respectively). Moreover, there were no significant differences in the pooled analyses of complete response rates, fatigue, nausea/vomiting, 3-year OS, or 5-year OS between these groups (P = 0.24, 0.69, 0.15, 0.73, and 0.38, respectively). Significant heterogeneity was detected for endpoints including fatigue, nausea/vomiting, fever, abdominal pain, OS duration, and 3-year OS (I2 = 89%, 82%, 72%, 90%, 96%, and 66%, respectively). All endpoints exhibited no significant risk of publication bias.
Conclusions: This study revealed that relative to TACE, TARE performed using 90Y can yield significantly higher treatment response rates and prolong HCC patient survival with fewer treatment-related side effects.
The PRISMA guidelines were used to guide the execution and publication of this meta-analysis. The study is registered at INPLASY.COM (No. INPLASY202380017).
Systematic review registration: INPLASY.COM, identifier INPLASY202380017.
Introduction
Hepatocellular carcinoma (HCC) is among the most common cancers with a high mortality rate globally (1, 2). Although curative surgical tumor resection is associated with a good HCC patient prognosis, most patients are diagnosed when the disease is relatively advanced, when liver reserve capacity is limited, or when liver transplantation is unavailable; therefore, only < 30% of patients undergo surgical treatment (3–5). For patients who cannot undergo curative surgery, various locoregional or systemic treatment strategies are instead used to prolong survival and improve quality of life (1, 2, 6).
The locoregional or systemic treatment strategies are usually chosen by the Barcelona Clinic Liver Cancer (BCLC) stages (3, 5). For BCLC stage C HCC, systematic therapy is regarded as the initial course of treatment (1, 2). Recently, combined systemic treatment based on tyrosine kinase inhibitors plus immune checkpoint inhibitors has become the most commonly used systematic treatment for HCC (3).
Locoregional approaches to inoperable HCC management include transarterial chemoembolization (TACE), percutaneous ablation (PA), and the insertion of 125I seeds under computed tomography (CT) guidance (5, 7), with TACE being the most commonly implemented strategy. Furthermore, TACE can be used as a standard therapeutic intervention in BCLC stage A or B HCC patients (8), and has also been employed as a baseline treatment for HCC patients undergoing additional PA and 125I seed insertion procedures (5, 7).
Recently, the transarterial radioembolization (TARE) technique has emerged as an alternative to TACE and utilizes 90Y integrated into resin or glass matrix microspheres. Relative to TACE, TARE has many benefits including better patient quality of life, longer time-to-progression, higher antitumor activity of portal vein invasion cases, and the potential for neoadjuvant application before tumor resection (6, 9). However, the efficacy of TARE in treating HCC patients remains elusive.
Therefore, this meta-analysis aimed to compare the efficacy and safety of TARE and TACE in the management of inoperable HCC.
Materials and methods
Study selection
The PRISMA guidelines were used to guide the execution and publication of this meta-analysis. The study is registered at INPLASY.COM (No. INPLASY202380017).
To identify relevant studies, the Web of Science, PubMed, and Wanfang databases were searched for articles published from July 2023 with the following strategy: (((transarterial chemoembolization) OR (TACE)) AND ((transarterial radioembolization) OR (TARE))) AND ((hepatocellular carcinoma) OR (HCC)).
Studies eligible for inclusion:
a. Study types: comparative analyses;
b. Diseases: inoperable HCC patients;
c. Intervention types: TARE vs. TACE;
d. Languages: not limited.
Excluded studies included:
a. single-arm studies;
b. studies of patients undergoing TACE/TARE as a bridging procedure before surgery;
c. studies comparing drug-eluting bead (DEB)-TACE and TARE procedures;
d. reviews, letters, and case reports.
Data extraction
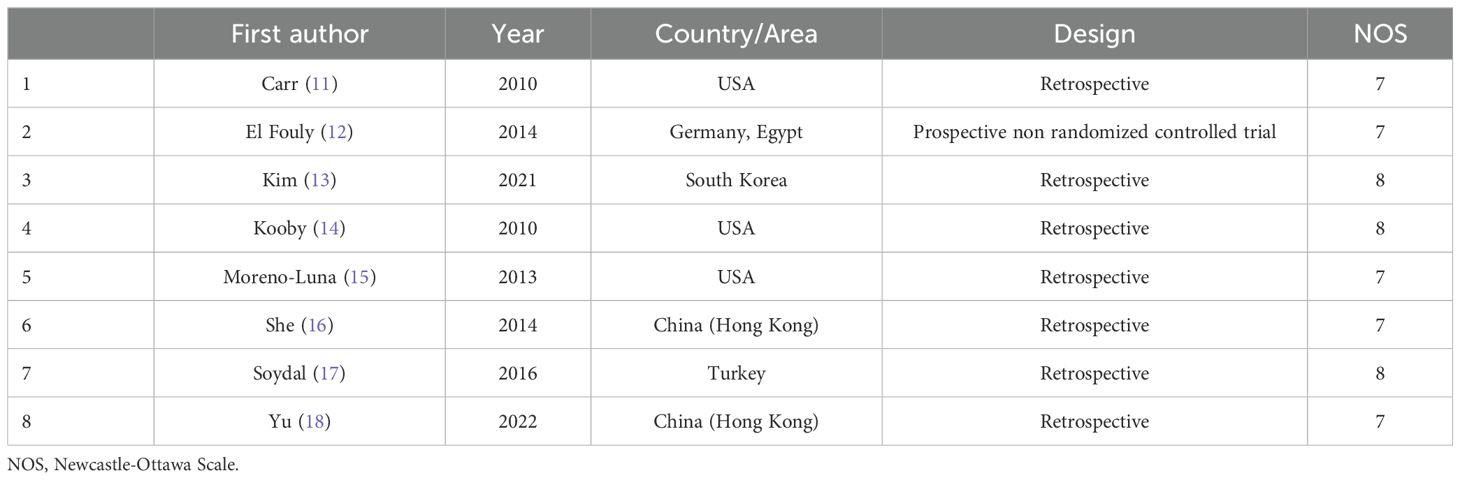
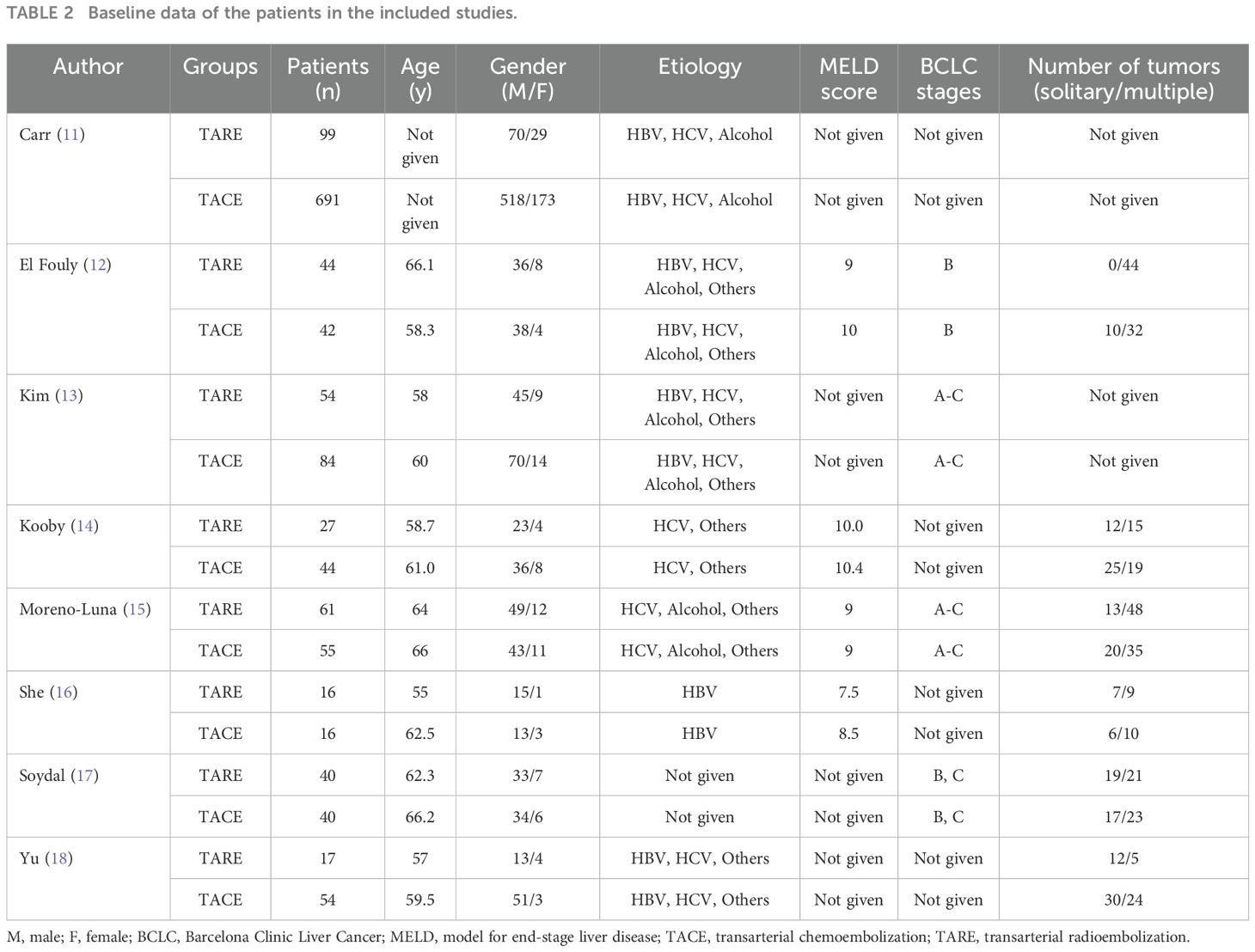
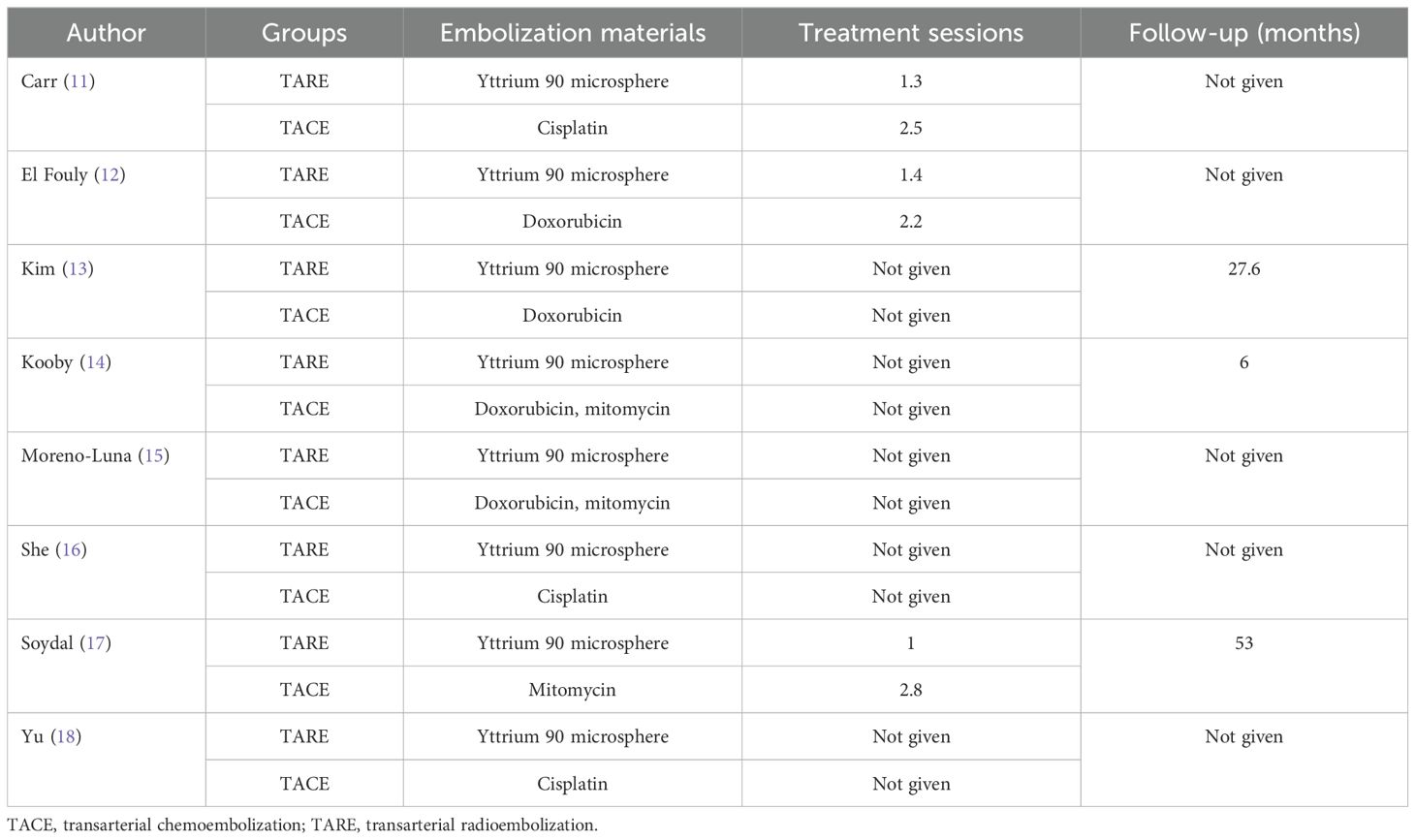
Two investigators (3-years and 5-years’ experience in conducting meta-analysis) independently extracted relevant data from these studies, and any inconsistencies were resolved by discussion with a third investigator (8-years’ experience in conducting meta-analysis). The inconsistencies mainly occurred in the treatment-related data. Study baseline data (Table 1), patient baseline data (Table 2), and treatment-related data (Table 3), as were results pertaining to treatment response rates, complications, and patient overall survival (OS), were extracted from all the studies.
Evaluation of study quality
For randomized controlled trials (RCTs), study quality was evaluated using the Cochrane risk-of-bias tool. Furthermore, each item (performance, attrition, detection, selection, reporting, and other biases) was judged to exhibit a high, low, or unclear risk of bias.
For non-RCTs, the Newcastle-Ottawa scale (NOS) was employed to score studies based on the criteria of selection (4 points), exposure (3 points), and comparability (2 points). A score of ≥ 7 indicated a high-quality study.
Endpoints
For pooled analyses, the primary endpoint was the total response rate. Whereas secondary endpoints included disease control rates, complete response rates, complications, OS duration, and 1-, 3-, and 5-year OS rates. The mRECIST criteria were used to evaluate treatment response rates (10). The detailed classification of complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) are provided in Supplementary Table S1. The total response rate was computed by summing CR and PR rates, while the disease control rate was determined by summing the CR, PR, and SD rates.
Statistical analyses
RevMan v5.3 and Stata v12.0 were used to perform these analyses. Pooled analyses of OS were performed by calculating hazard ratio (HR) values, while pooled odds ratios (ORs) and 95% confidence intervals (CIs) were determined when comparing categorical variables. Heterogeneity was evaluated via the Q test and the I2 statistic. Random-effects models were used in cases of significant heterogeneity (I2 > 50%), otherwise, fixed-effects models were employed. The causes of heterogeneity were assessed via sensitivity analyses in which articles were individually excluded from pooled analyses. Funnel plots were generated to gauge the potential for publication bias, and this risk was considered low when all studies fell within the established plots. Egger’s test was performed to assess publication bias in cases where funnel plots could not exclude potential biases. P < 0.05 was set as the cut-off to define significance.
Results
Study selection
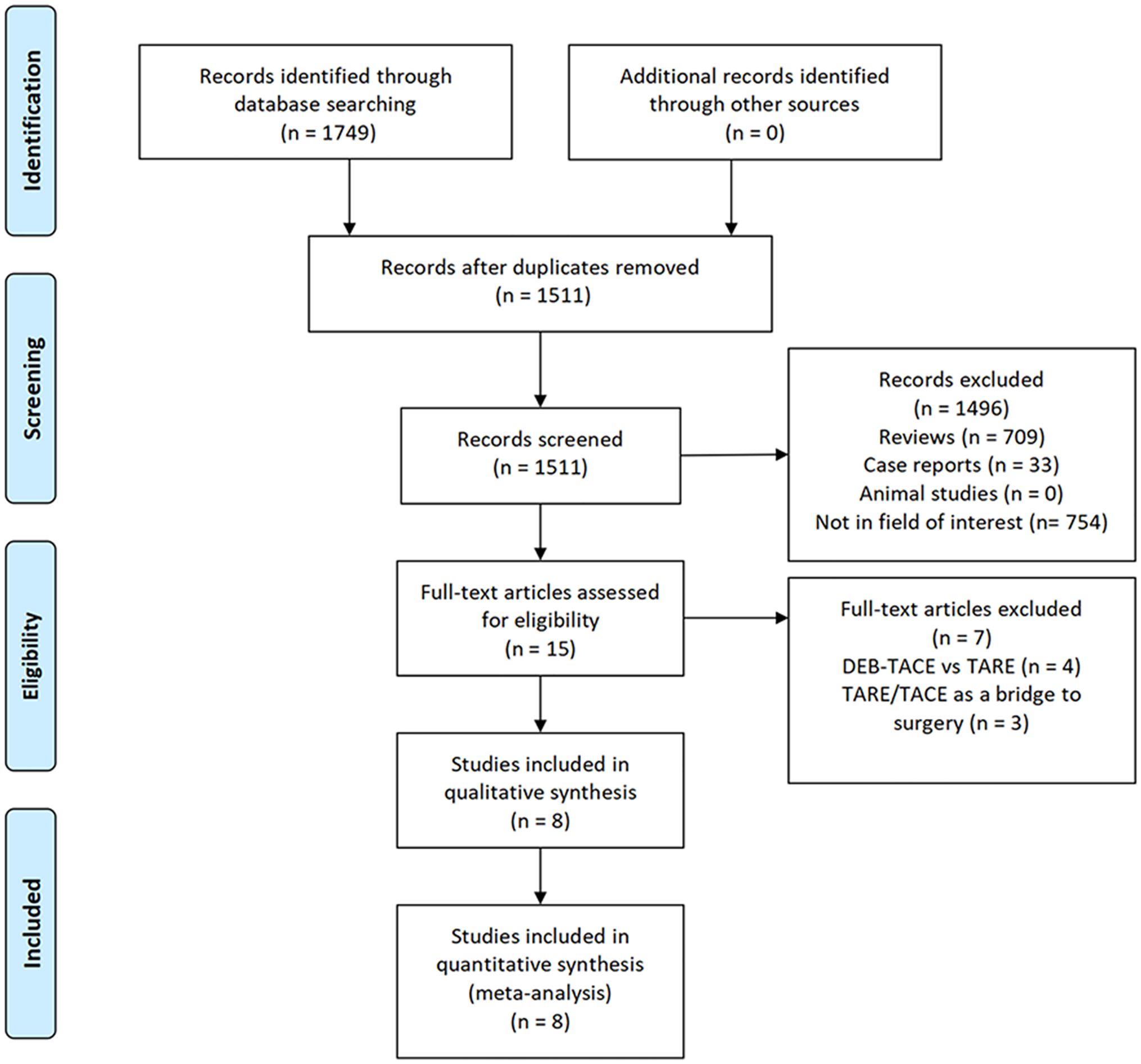
An initial literature search identified 1,749 potentially relevant studies, however, based on the defined criteria, 8 studies (11–18) were enrolled in the final meta-analysis (Figure 1). These 8 publications included 7 retrospective studies (11, 13–18) and 1 prospective non-RCT (12). These articles were published between 2010 and 2022 by research teams in Asia, Europe, North America, and Africa. All articles were of high quality with NOS scores from 7-8 (Supplementary Table S2). The patient populations in these articles included 358 and 1,026 HCC patients who respectively underwent TARE and TACE procedures. 90Y microspheres were used to perform all TARE procedures, while TACE procedures were performed using combinations of lipiodol with mitomycin, cisplatin, or doxorubicin.
CR rates
CR rates were reported in 3 studies (12, 13, 15) comprising 327 patients (TARE: 155, TACE: 172). Pooled CR rates were similar in both of these groups (26.5% vs. 27.9%, P = 0.24, Figure 2A), and no heterogeneity was detected (I2 = 0%).
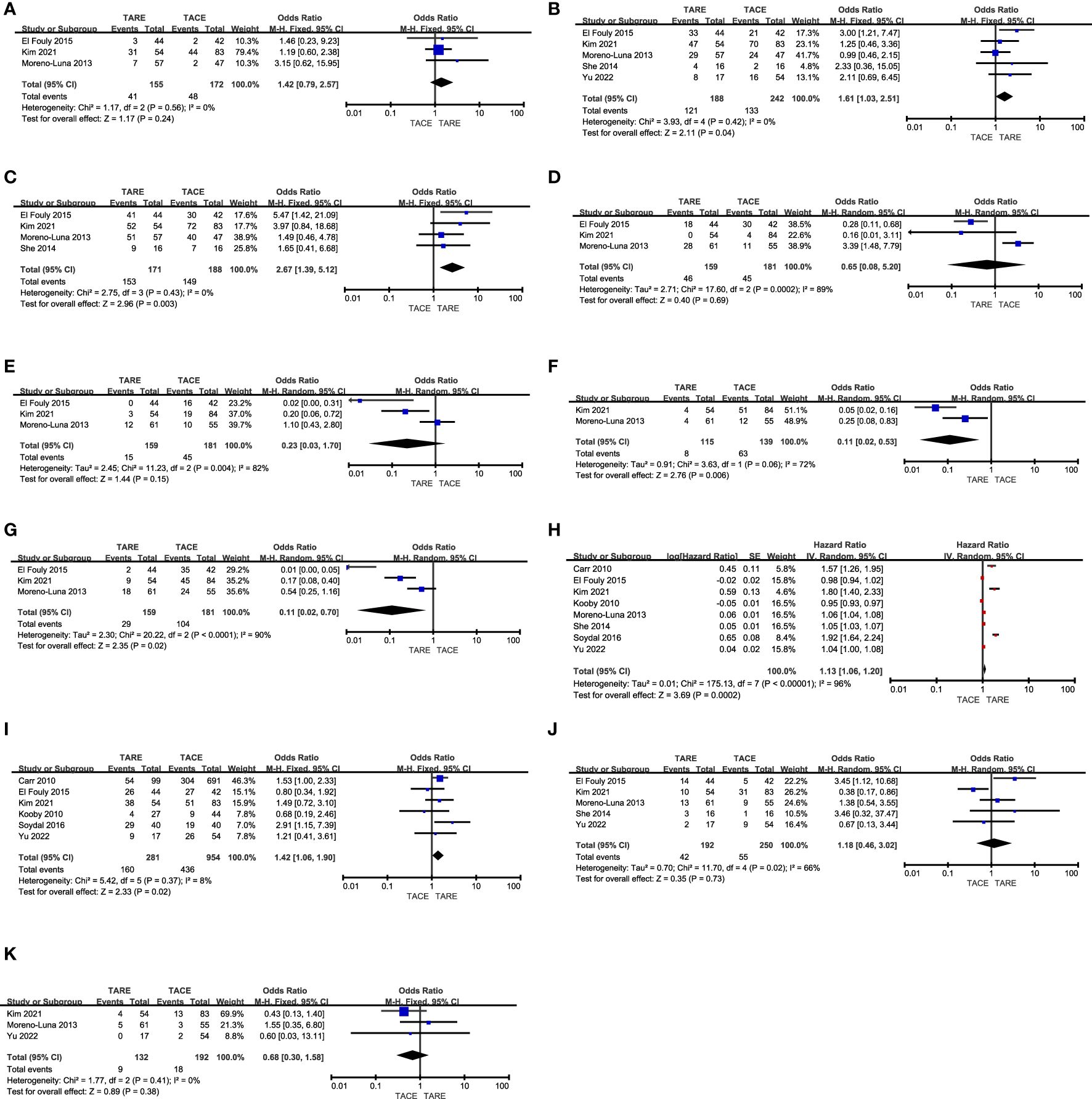
Figure 2. The comparative results of (A) CR rates, (B) total response rates, (C) disease control rates, (D) fatigue rates, (E) nausea/vomiting rates, (F) fever rates, (G) abdominal pain rates, (H) OS, (I) 1-year OS rates, (J) 3-year OS rates, (K) 5-year OS rates.
Total response rates
In total 5 studies (12, 13, 15, 16, 18) with 430 patients (TARE: 188, TACE: 242) reported total response rates. The TARE group patients indicated a significantly higher pooled total response rate (64.4% vs. 55.0%, P = 0.04, Figure 2B), and no heterogeneity was detected (I2 = 0%).
Disease control rates
Disease control rates were reported in 4 studies (12, 13, 15, 16) analyzing 359 patients (TARE: 171, TACE: 188). A significantly higher pooled disease control rate was observed in the TARE group as compared to the TACE group (89.5% vs. 79.3%, P = 0.003, Figure 2C), and no heterogeneity was detected (I2 = 0%).
Fatigue
Rates of patient fatigue were reported in 3 studies (12, 13, 15), containing 159 TARE and 181 TACE group patients, respectively. These two groups indicated comparable pooled fatigue rates (28.9% vs. 24.9%, P = 0.69, Figure 2D). Significant heterogeneity was observed (I2 = 89%), however, omitting Moreno-Luna et al. study eliminated this heterogeneity (I2 = 0%) (15). When this study was not included in the pooled analysis, TARE patients indicated a significantly lower pooled fatigue rate than TACE patients (P = 0.002).
Nausea and vomiting
Nausea and vomiting rates were reported in 3 studies (12, 13, 15), analyzing 159 TARE and 181 TACE groups patients. Furthermore, both the groups indicated similar pooled nausea and vomiting rates (9.4% vs. 24.9%, P = 0.15, Figure 2E). While significant heterogeneity was detected (I2 = 82%), sensitivity analyses could not identify its source.
Fever
Fever rates were reported in 2 studies (13, 15), enrolling 115 and 139 patients who underwent TARE and TACE, respectively. A significantly lower pooled fever rate was detected for patients who underwent TARE relative to TACE (7.0% vs. 45.3%, P = 0.006, Figure 2F). Moreover, significant heterogeneity was detected (I2 = 72%). There were insufficient studies to perform a sensitivity analysis.
Abdominal pain
The incidence of abdominal pain was reported in 3 studies (12, 13, 15), comprising 159 and 181 patients in the TARE and TACE groups, respectively. Patients who underwent TARE had a significantly lower pooled abdominal pain rate relative to TACE (18.2% vs. 57.5%, P = 0.02, Figure 2G). Moreover, there was significant heterogeneity was detected (I2 = 90%) and sensitivity analyses could not identify its source.
OS
The OS duration for enrolled patients was reported in all studies, and the forest plots revealed a significantly longer pooled OS for patients who underwent TARE than those who underwent TACE (HR: 1.13, 95% CI: 1.06-1.20, P = 0.0002, Figure 2H). There was significant heterogeneity between the groups (I2 = 96%), sensitivity analyses could not identify its source.
1-year OS
Patient 1-year OS rates were reported in 6 studies (11–14, 17, 18), containing 281 and 951 patients in the TARE and TACE groups, respectively. TARE patients indicated a significantly higher pooled 1-year OS rate than TACE patients (56.9% vs. 45.7%, P = 0.02, Figure 2I), and no heterogeneity was detected (I2 = 8%).
3-year OS rate
Patient 3-year OS rates were reported in 5 studies (12, 13, 15, 16, 18), comprising 192 and 250 patients in the TARE and TACE groups, respectively. The pooled 3-year OS rates were comparable in both groups (21.9% vs. 22.0%, P = 0.73, Figure 2J), and there was significant heterogeneity (I2 = 66%), which was reduced (I2 = 8%) by omitting the Kim et al. study (13). Pooled analyses without this study revealed that the 3-year OS rates for patients in the TACE and TARE group patients were similar (P = 0.10).
5-year OS rates
Patient 5-year OS rates were reported in 3 studies (13, 15, 18), analyzing 132 and 192 patients in the TARE and TACE groups, respectively. The pooled 5-year OS rates were comparable in both groups (6.8% vs. 9.4%, P = 0.38, Figure 2K), and no heterogeneity was detected (I2 = 0%).
Publication bias
Funnel plots revealed no evidence of significant publication bias for the CR rate, total response rate, disease control rate, fever, 1-year OS, or 5-year OS endpoints (Supplementary Figures S1A-K). The Egger’s test evaluated the remaining endpoints and revealed no significant publication bias for the fatigue, nausea/vomiting, abdominal point, OS, or 3-year OS endpoints (P = 0.76, 0.252, 0.213, 0.099, and 0.45).
Discussion
This meta-analysis was designed to evaluate the safety and clinical efficacy of TARE and TACE as treatment strategies to manage inoperable HCC based on treatment responses, complications, and survival outcomes for affected patients.
Treatment response rates are a key determinant of HCC patient prognosis, serving as an important short-term outcome for evaluating a therapeutic strategy. Pooled CR rates in the present meta-analysis were similar in the TARE and TACE patient groups, although the total response and disease control rates in the TARE group were higher than those in the TACE group. This suggests that TARE procedures performed using 90Y can more readily take advantage of the vascular nature of HCC tumors to induce necrotic cell death upon ablation (19). These benefits may be attributed to the high dose of absorbed radiation, the state of the liver, the goals of each therapeutic approach, and the target volume for treated patients. The delivery of high radiation doses to the tumor capillary bed can more significantly induce necrosis following TARE than after TACE treatment (20, 21).
Compared to external radiation therapy, brachytherapy has various advantages due to the direct contact between the radiation source and internal tumor regions, enabling the persistent delivery of radiotherapy while minimizing off-target damage (21). Here, CR, total response, and disease control rates indicated low heterogeneity (I2 = 0%), suggesting the credibility of these findings. Furthermore, the 89.5% pooled disease control role for the TARE group was also similar to the 91.1% rate reported previously in a meta-analysis comparing TACE and CT-guided 125I seed insertion in HCC patients (22). Although CT-guided 125I seed insertion has been employed as a brachytherapy-based approach to HCC patient treatment (7, 22), this technique carries a risk of hematoma and pneumothorax (22). TARE, however, is performed within tumor-feeding arteries and thus avoids the potential for such complications (16). TARE procedures can be implemented more easily than TACE or CT-guided 125I seed insertion.
The present analyses revealed that TARE treatment was associated with lower pooled rates of fever and abdominal pain than TACE. TARE entails the injection of radioactive particles into a target artery in the liver without occluding that artery (23). Furthermore, this approach does not induce VEGF or HIF-1a overexpression, which can cause fever and pain in patients (12). This study also observed no differences in nausea/vomiting or fatigue between these groups, suggesting that TARE cannot abrogate gastrointestinal or hepatic toxicity associated with this locoregional treatment.
There were substantial variations in outcomes comparing OS durations were observed among studies, however, the pooled data suggested that TARE is associated with the significant prolongation of OS relative to TACE. The 1-year survival rate in the TARE group was also superior to that in the TACE group, which might be associated with the higher treatment response rate in this group. Whereas no significant differences were observed in pooled 3-year (21.9% vs. 22.0%) or 5-year (6.8% vs. 9.4%) OS rates between these groups. The low 3 and 5-year OS rates in both groups highlight the limitations of TARE and TACE to control HCC over extended periods.
There are some limitations to the present study. For one, this meta-analysis did not include any RCTs and the results are subject to a high risk of bias. In the future, appropriately designed prospective RCTs should be performed to validate the present results. Moreover, these studies did not employ uniform TACE protocols for the medication types or dosages, potentially contributing to further bias. Third, the numbers of patients in the TARE and TACE groups were not balanced, and there were variations in the etiological basis for HCC across the included studies. All of these factors may have further contributed to bias with respect to these results.
Conclusion
In summary, this meta-analysis showed that relative to TACE, TARE performed using 90Y can achieve better treatment response rates and OS benefits in inoperable HCC patients while causing fewer side effects. However, this meta-analysis was conducted mainly based on the retrospective study. Therefore, the treatment effectiveness (such as treatment response, survival function, and safety) of TARE for inoperable HCC should be corroborated by future randomized controlled trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
WL: Data curation, Formal analysis, Methodology, Writing – original draft. TZ: Data curation, Methodology, Writing – original draft. FX: Data curation, Methodology, Writing – review & editing. XH: Formal analysis, Methodology, Writing – review & editing. FG: Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the general project of Jiangyin Health Committee (No. S202104).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1511210/full#supplementary-material
Supplementary Figure 1 | The funnel plots of (A) CR rates, (B) total response rates, (C) disease control rates, (D) fatigue rates, (E) nausea/vomiting rates, (F) fever rates, (G) abdominal pain rates, (H) OS, (I) 1-year OS rates, (J) 3-year OS rates, (K) 5-year OS rates.
References
1. Sacco R, Faggioni L, Bargellini I, Ginanni B, Battaglia V, Romano A, et al. Assessment of response to sorafenib in advanced hepatocellular carcinoma using perfusion computed tomography: results of a pilot study. Dig Liver Dis. (2013) 45:776–81. doi: 10.1016/j.dld.2013.03.004
2. Galle PR, Dufour JF, Peck-Radosavljevic M, Trojan J, Vogel A. Systemic therapy of advanced hepatocellular carcinoma. Future Oncol. (2021) 17:1237–51. doi: 10.2217/fon-2020-0758
3. Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: A phase III, randomized clinical trial (LAUNCH). J Clin Oncol. (2023) 41:117–27. doi: 10.1200/JCO.22.00392
4. Sheta E, El-Kalla F, El-Gharib M, Kobtan A, Elhendawy M, Abd-Elsalam S, et al. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: a randomized-controlled study. Eur J Gastroenterol Hepatol. (2016) 28:1198–203. doi: 10.1097/MEG.0000000000000688
5. Ren Y, Cao Y, Ma H, Kan X, Zhou C, Liu J, et al. Improved clinical outcome using transarterial chemoembolization combined with radiofrequency ablation for patients in Barcelona clinic liver cancer stage A or B hepatocellular carcinoma regardless of tumor size: results of a single-center retrospective case control study. BMC Cancer. (2019) 19:983. doi: 10.1186/s12885-019-6237-5
6. Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. (2016) 151:1155–1163.e2. doi: 10.1053/j.gastro.2016.08.029
7. Gao FL, Wang Y, Huang XZ, Pan TF, Guo JH. I-125 seeds brachytherapy with transcatheter arterial chemoembolization for subcapsular hepatocellular carcinoma. BMC Gastroenterol. (2022) 22:273. doi: 10.1186/s12876-022-02356-0
8. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. (2022) 76:681–93. doi: 10.1016/j.jhep.2021.11.018
9. Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. (2011) 140:497–507.e2. doi: 10.1053/j.gastro.2010.10.049
10. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. (2010) 30:52–60. doi: 10.1055/s-0030-1247132
11. Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. (2010) 116:1305–14. doi: 10.1002/cncr.24884
12. El Fouly A, Ertle J, El Dorry A, Shaker MK, Dechêne A, Abdella H, et al. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. (2015) 35:627–35. doi: 10.1111/liv.12637
13. Kim MA, Jang H, Choi NR, Nam JY, Lee YB, Cho EJ, et al. Yttrium-90 radioembolization is associated with better clinical outcomes in patients with hepatocellular carcinoma compared with conventional chemoembolization: A propensity score-matched study. J Hepatocell Carcinoma. (2021) 8:1565–77. doi: 10.2147/JHC.S335879
14. Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. (2010) 21:224–30. doi: 10.1016/j.jvir.2009.10.013
15. Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. (2013) 36:714–23. doi: 10.1007/s00270-012-0481-2
16. She WH, Cheung TT, Yau TC, Chan AC, Chok KS, Chu FS, et al. Survival analysis of transarterial radioembolization with yttrium-90 for hepatocellular carcinoma patients with HBV infection. Hepatobiliary Surg Nutr. (2014) 3:185–93. doi: 10.3978/j.issn.2304-3881.2014.07.09
17. Soydal C, Arslan MF, Kucuk ON, Idilman R, Bilgic S. Comparison of survival, safety, and efficacy after transarterial chemoembolization and radioembolization of Barcelona Clinic Liver Cancer stage B-C hepatocellular cancer patients. Nucl Med Commun. (2016) 37:646–9. doi: 10.1097/MNM.0000000000000486
18. Yu SCH, Hui JW, Li L, Cho CC, Hui EP, Chan SL, et al. Comparison of chemoembolization, radioembolization, and transarterial ethanol ablation for huge hepatocellular carcinoma (≥ 10 cm) in tumour response and long-term survival outcome. Cardiovasc Intervent Radiol. (2022) 45:172–81. doi: 10.1007/s00270-021-02777-6
19. Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. (2011) 53:1020–2. doi: 10.1002/hep.24199
20. Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver Malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. (2006) 17:1251–78. doi: 10.1097/01.RVI.0000233785.75257.9A
21. Lee IJ, Seong J. The optimal selection of radiotherapy treatment for hepatocellular carcinoma. Gut Liver. (2012) 6:139–48. doi: 10.5009/gnl.2012.6.2.139
22. Hou JP, Shi YB, Fu YF, Li H. I-125 seeds insertion with TACE for advanced HCC: a meta-analysis of randomized controlled trials. Minim Invasive Ther Allied Technol. (2022) 31:848–55. doi: 10.1080/13645706.2022.2033786
Keywords: hepatocellular carcinoma, transarterial chemoembolization, transarterial radioembolization, yttrium-90, meta-analysis
Citation: Lu W, Zhang T, Xia F, Huang X and Gao F (2025) Transarterial radioembolization versus chemoembolization for hepatocellular carcinoma: a meta-analysis. Front. Oncol. 14:1511210. doi: 10.3389/fonc.2024.1511210
Received: 14 October 2024; Accepted: 27 December 2024;
Published: 17 January 2025.
Edited by:
Adina Emilia Croitoru, Fundeni Clinical Institute, RomaniaReviewed by:
Lorenzo Faggioni, University of Pisa, ItalyGeorge Pappas-Gogos, Democritus University of Thrace, Greece
Copyright © 2025 Lu, Zhang, Xia, Huang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fulei Gao, bGVpZnVnYW9AMTI2LmNvbQ==
 Wenxiao Lu1
Wenxiao Lu1 Fengfei Xia
Fengfei Xia Fulei Gao
Fulei Gao