
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 29 November 2024
Sec. Cancer Molecular Targets and Therapeutics
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1506849
This article is part of the Research Topic Novel Molecular Targets in Cancer Therapy View all 18 articles
Lactate, which was traditionally viewed as a metabolic byproduct of anaerobic glycolysis, has emerged as a significant signaling molecule involved in the development of tumors. Current studies highlight its dual function, where it not only fuels tumor development but also modulates immune responses. Lactate has an effect on various tumor-associated immune cells, promoting immunosuppressive conditions that facilitate tumor growth and immune evasion. This phenomenon is strongly associated with the Warburg effect, a metabolic shift observed in many cancers that favors glycolysis over oxidative phosphorylation, resulting in elevated lactate production. Exploring the complex interplay between lactate metabolism and tumor immunity provides a novel understanding regarding the mechanisms of tumor immune evasion and resistance to therapies. This review discusses the unique biology of lactate in the TME, its impact on immune cell dynamics, and its potential as a tumor treatment target.
The recognition of lactate as a metabolic waste with harmful effects produced by cells under hypoxic conditions has evolved in recent years (1). A century ago, Otto Warburg first proposed the aerobic glycolysis phenomenon: tumor cells rapidly produce energy by glycolysis instead of oxidative phosphorylation (OXPHOS), even when there is an ample supply of oxygen. The phenomenon, which later acquired recognition as the “Warburg effect (2), has transformed lactate from a mere byproduct of metabolism to a signaling molecule that regulates metabolism, immune response, and intercellular communication (3). Both in vitro and in vivo experiments have demonstrated that the addition of lactate promotes tumor progression and treatment resistance (4). Moreover, metabolites such as lactate can act as acylase substrates or cofactors for epigenetic modifications (5). A 2019 study demonstrated the crucial role of lactate in promoting histone lysine residue modification. Resembling other posttranslational modifications (PTMs), lactylation regulates gene transcription and plays a significant part in inflammation and cancer (6). There is growing evidence that lactate has a profound impact on the growth progression, resistance to treatment, and immune evasion of tumors.
Tumors do not merely consist of abnormally proliferating cells but rather exhibit a highly structured system. The various components that make up a tumor are jointly known as the tumor microenvironment (TME) (7). In the context of TME, every element comprising the immune system is collectively referred to as the tumor immune microenvironment (TIME) due to their intricate interplay and crucial roles in tumor biology (8–10). Immune checkpoints (ICPs) are employed by cancer cells to evade immune system attacks (11). Now, immune checkpoint inhibitors (ICIs), like anti-cellular toxicity T lymphocyte-associated protein 4 (CTLA-4), anti-programmed death protein (PD-1), and anti-PD-1 ligand (PD-L1), have shown great promise in numerous cancer immunotherapies (12–14). Increasing evidence indicates that TIME exerts a more pivotal role in tumor immunity compared with ICPs (15–17). As the fundamental constituents of TIME, immune cells make crucial contributions to tumor immune responses. Distinct subsets of immune cells exhibit diverse functionalities and characteristics, making it essential to explore the functions of various immune cells in order to conquer cancers.
Tumor metabolic reprogramming, such as enhanced nutrient utilization, heightened oxygen uptake, and the reproduction of reactive nitrogen and oxygen species, can have a profound impact on immune responses (1–3). Furthermore, numerous metabolites present in the TME can influence the development and functional roles of immune cells (4, 5). Given the Warburg effect, it is reasonable to expect that the significantly elevated lactate concentration in the TME greatly influences the immune cells. Lactate can serve as a metabolic bridge between tumor cells and immune cells, facilitating the tumor’s enhanced adaptation to the microenvironment and evasion of immune surveillance. This review focuses on lactate within the TME and summarizes the distinct lactate metabolism observed in tumors. We analyze the effects of lactate on tumor-associated immune cells and investigate its clinical significance in the TME. like prognostic markers of tumors and potential drugs targeting lactate generation, transport, and lactylation for tumor immunotherapy.
Pyruvate dehydrogenase (PDH) catalyzes the conversion of pyruvate from glucose into acetyl-CoA in the mitochondria during aerobic respiration (18, 19). As a result, acetyl-CoA enters the cycle of tricarboxylic acid (TCA) for OXPHOS. Through this process, each glucose molecule can generate 36 molecules of adenosine triphosphate (ATP) (20, 21). In periods of intense exercise and infection, when cells have an inadequate oxygen supply, pyruvate molecules do not enter the TCA cycle, but instead, cytoplasmic lactate dehydrogenase (LDH) catalyzes them to lactic acid. This metabolic pathway is commonly referred to as glycolysis (22, 23). Glycolysis functions as the principal pathway for the production of lactate, yet it exhibits lower efficiency in terms of energy generation compared to OXPHOS, resulting in a yield of only 2 ATP molecules per glucose molecule (24). Therefore, in aerobic conditions, normal cells tend to opt for OXPHOS, which yields higher energy production. Only under hypoxic conditions do they resort to the inefficient glycolysis. Lactate accumulation in the human body poses a significant risk due to its potential to cause lactic acidosis. Therefore, it is crucial to rapidly remove lactate from tissues and the circulatory system (25). Lactate converts to pyruvate before entering the mitochondria, where PDH facilitates its metabolization via the TCA cycle. Additionally, hepatic and muscular tissues can activate gluconeogenesis in response to lactate accumulation, converting it into glucose and releasing it into circulation for enhanced glucose utilization during energy expenditure (26).
Malignant cells, as opposed to normal cells, exhibit a propensity for rapid energy generation through glycolysis despite the presence of sufficient oxygen (Figure 1). It is believed that tumor cells require this metabolic reprogramming to fulfill their energy requirements for growth and differentiation. Although strong glycolysis has been widely observed in tumor cells, the specific reasons and mechanisms behind it remain incompletely understood. However, research indicates that hypoxic cancer cells frequently demonstrate the activation of c-Myc and HIF-1α, resulting in enhanced anaerobic oxidation and increased lactate production (27–30). Pyruvate is transformed into lactate within the cell through LDH catalysis. The LDH protein is a heteromeric complex consisting of LDHA and LDHB, which exist in five isoforms. LDH-5 (A4) exhibits higher attraction for pyruvate than for lactate. Conversely, LDH-1 (B4) demonstrates a greater preference for lactate over pyruvate. Both c-Myc and HIF-1α upregulate LDH-5 activity and downregulate LDH-1 expression, thereby promoting lactate production (31). In summary, aerobic glycolysis can promote lactate production and lead to elevated concentrations of lactate in the TME.
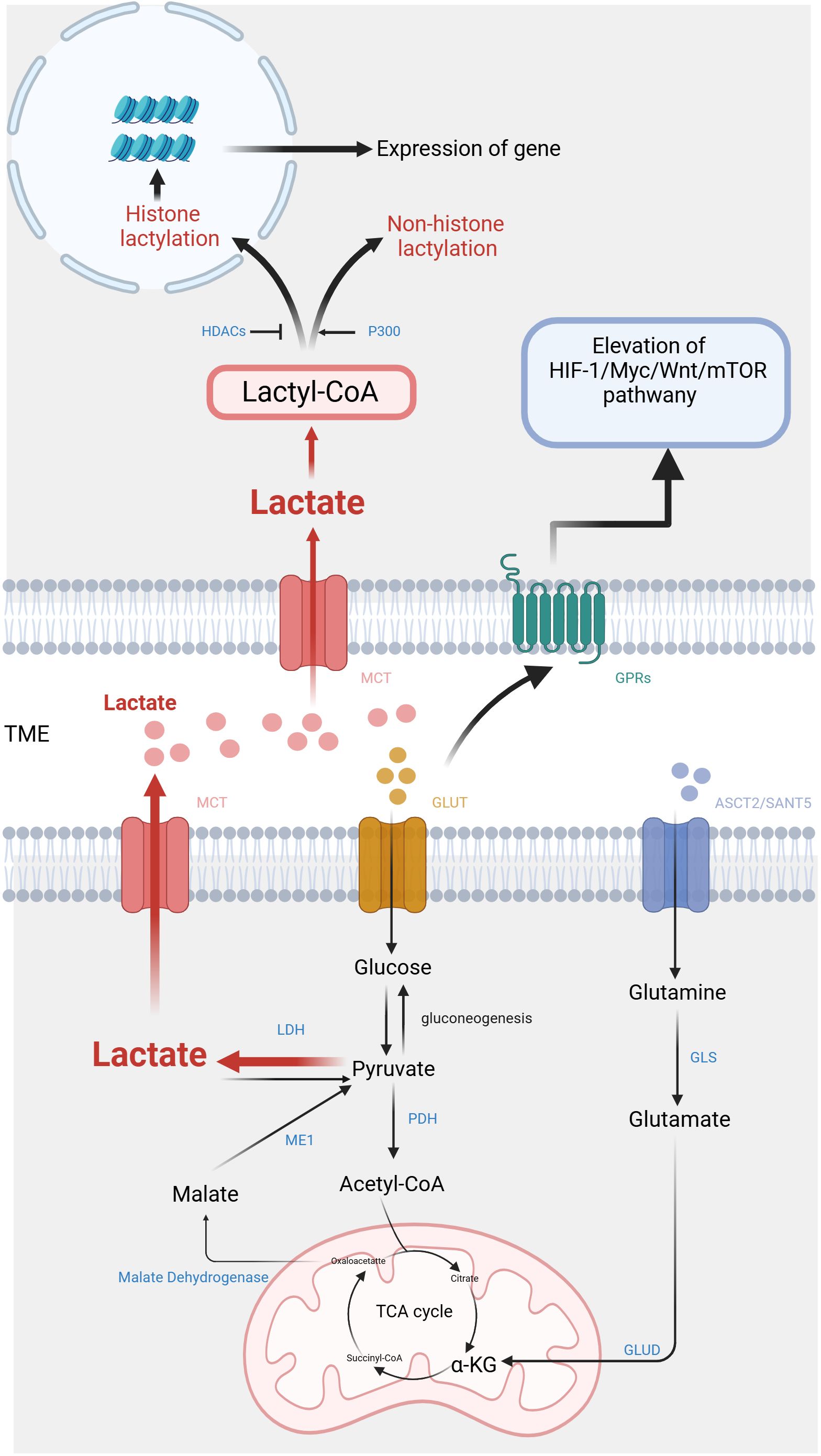
Figure 1. Metabolic pathways and signaling mechanisms of lactate in the tumor microenvironment. On one hand, lactate is produced by tumor cells through anaerobic glycolysis and is secreted into the extracellular space, where it contributes to the acidic microenvironment, promoting tumor progression and immune evasion. On the other hand, lactate also activates key signaling pathways, including the activation of HIF-1α, mTOR, and various inflammatory cytokines, which further influence tumor growth and metastasis. (Created with BioRender.com).
Except for glycolysis, glutamine catabolism constitutes an alternative metabolic pathway employed by cancer cells for the lactate generation (32). Glutamine serves as a carbon framework for lactate production within the cancer cells (Figure 1). Glutamine is transported into the cell under the regulation of c-Myc, utilizing amino acid transporter protein type 2 (ASCT2) and sodium-coupled neutral amino acid transporter protein (SNAT5). Inside the cell, glutamine is converted to glutamate through the action of glutaminase (GLS). Subsequently, glutamate is transformed into α-ketoglutarate (α-KG) by glutamate dehydrogenase (GLUD) or various transaminases, including glutamate-oxaloacetate transaminase (GOT), glutamate-pyruvate transaminase (GPT), and phosphoserine aminotransferase (PSAT). α-KG then enters the TCA cycle. Within this cycle, carbon derived from glutamine is converted to oxaloacetate, which then exits the mitochondria to be transformed into malate. In the cytoplasm, malate undergoes further conversion into NADPH and pyruvate through the activity of malic enzyme 1 (ME1) (33). NADPH serves as essential during the biosynthesis of lipids and steroids, while pyruvate serves as a precursor for lactate. Reinforcing c-Myc activation can stimulate the glutamine metabolism, resulting in lactate production. This process establishes a positive loop that contributes to the accumulation of lactate (34).
Lactate shuttle denotes the complete process of transmembrane lactic acid migration (6), which serves as the primary mechanism of lactate entering and exiting tumor cells (Figure 1). Lactate shuttle primarily relies on monocarboxylate transporter proteins (MCTs) (7). Out of the acknowledged MCTs, MCT1-4 can be observed in different organs, contributing toprotons bonding and the bidirectional transportation of monocarboxylic acids (8). Following the attachment of the liberated proton with the MCT, lactate promptly associates with the MCT. Within the transport protein, lactate undergoes a structural change and is released along with protons from the opposite side of the membrane (9).
The combined function of MCT1-4 facilitates lactate transport between cells, being crucial for sustaining lactate homeostasis in various tissues (10). For typical tissues, MCT1 contributes a vital part in maintaining lactic acid balance by facilitating lactate transfer across the membrane in accordance with the membrane-based concentration gradient. In contrast, cells with elevated intracellular lactate levels, such as tumor cells, depend on the transporter MCT4 for the movement of lactic acid. Tumor cells regulate the expression of MCTs to maintain intracellular lactate homeostasis in order to benefit themselves and avoid harm. Certain tumor cells activate MCT4 to make use of lactate as a source of energy (11). Lactate also serves as a signaling molecule that modulates MCT expression. Lactate activates the GPR81/mTOR/HIF-1α/STAT3 pathway in pancreatic ductal adenocarcinoma cell lines, affecting the gene expression of MCT1 and MCT4 (12). In addition, glutamine can stimulate HIF-1α, thereby promoting the expression of MCT4 (13). The MCT-mediated lactate shuttle establishes intercellular connections and contributes to the cooperative metabolic interactions among various cancer cells, thereby promoting tumor initiation and progression. Lactate flowing out of tumor cells can prevent the intracellular environment from becoming more acidic, but it can cause acidification of the tumor microenvironment.
The function of lactate relies on specific G protein-coupled receptors (GPRs) (14), which are located on the cell surface. They can detect extracellular molecules and trigger cellular responses (15). Classic metabolites, including lactate, possess the ability to initiate direct signal transduction via GPRs (16) (Figure 1). The research indicates that lactate can act as a signaling molecule via GPR81 and GPR132, which are receptors sensitive to protons (17). Among them, GPR81 exhibits high expression in various tissues such as adipose tissue, kidney, bone, and heart, mediating the influence of lactic acid on energy metabolism, lipid metabolism, inflammation, and other biological processes (35–37).
Lactate and the activation of the GPR81 signaling pathway through lactate have important implications in various aspects of tumor advancement. Research has demonstrated that GPR81 is upregulated within cancer cells in response to lactic acid signals. This indicates lactate produced by cancer cells induces GPR81, promoting a carcinogenic phenotype development (12). In addition, lactate can promote tumor growth by paracrine secretion through activating GPR81 in non-tumor cells within the TME (38). On the other hand, GPR132 is expressed in the respiratory system, digestive system and immune cells, with a particular emphasis on macrophages (39), where its expression positively correlates with M2-type macrophage presence and transition (18).
In 2019, Zhang et al. employed high-performance liquid chromatography (HPLC)-tandem mass spectrometry (MS/MS) to detect core histones in MCF7 cells. This study discovered the mass shift observed on the lysine residues of three protein hydrolysates corresponds to the addition of a lactyl group to the ϵ-amino group of lysine (19). This research validates a novel epigenetic modification mechanism, referred to as histone lysine lactylation (Kla), that depends upon the presence of lactic acid. Histone Kla is observed to accumulate on gene promoters exposed to hypoxia, bacterial stimulation, interferon-γ (INF-γ), or lipopolysaccharide (LPS), thereby exerting an influence on gene expression. The circXRN2-Hippo pathway acts as an upstream regulator in human bladder cancer, exerting further control over tumor progression by suppressing H3K18 acetylation and inhibiting LCN2 expression (20). Instead, the oncogene BRAFV600E in undifferentiated thyroid carcinoma promotes tumor cell glycolysis, resulting in the H4K12la. This leads to dysregulation of gene transcription and the cell cycle (21). The subsequent investigations have revealed that lactylation is a prevalent PTM, occurring in both histones and non-histone proteins (22) (Figure 1).
It is known that p300/CREB-binding protein (CBP), which are classical histone acetyltransferases (HATs), are capable of catalyzing various acylation modifications (23, 24). An ex vivo cell-free experiment demonstrates that p300 is also capable of catalyzing the Kla reaction chemically. Multiple studies have provided evidence supporting the significant involvement of p300/CBP in regulating histone lactylation within induced pluripotent stem cells (iPSCs) and macrophages (19, 25, 26). Delactylation modification is an enzymatic process driven by histone deacetylases (HDACs): the mechanism of deacetylation was analyzed in detail through in vitro experiments on core histones and 18 recombinant HDACs. These findings showed that HDAC1-3 can remove Kla from histones, with HDAC3 exhibiting the most efficient erasing activity (27). Hence P300 and HDAC play a role in diverse protein modifications, thereby establishing a connection between lactylation and other PTMs. Similar to many other PTMs, Kla is regulated by the addition and removal of lactyl groups in histones theoretically (28, 29). However, the current understanding of the biochemical process of lactylation suggests that it depends on two metabolic mechanisms. Within these mechanisms, lactyl-CoA is strongly linked to enzymatic lactylation, whereas nonenzymatic lactylation involves the participation of lactyl-glutathione (LGSH) (30).
It should be emphasized that the association between lactylation modification and RNA modification is noteworthy. In the case of ocular melanoma, YTHDF2 expression is increased by Kla. This protein aids tumor progression by enhancing destruction of m6A-modified PER1 and TP53 mRNA, which it detects specifically (31). In the TIME, lactic acid increases METTL3 expression, a methyltransferase-like protein in tumor-infiltrating myeloid cells (TIM), by H3K18 modification. Meanwhile, METTL3 can be directly influenced by lactate and control the pathway via METTL3-jak1-stat3 to amplify METTL3 binding. This process makes it easier to modify target RNA with m6A, thus boosting the subsequent molecules with immunosuppressive effect production (32).
One of the key regulating mechanisms of the TIME is lactate within the TME, which has abilities to affect various tumor-associated immune cells to exert immune suppression (Table 1). First, lactate is able to exert an influence on the metabolism and cellular respiration of immune cells themselves. Furthermore, the acidifying effect of lactate can lead to a reduction in immune cell function or modulation of downstream signal transduction pathways (Figure 2). Additionally, lactate has the ability to interfere with the identification and lethal activities of immune cells by either suppressing or enhancing the expression of ligands or receptors on both immune and tumor cells (Figure 2).
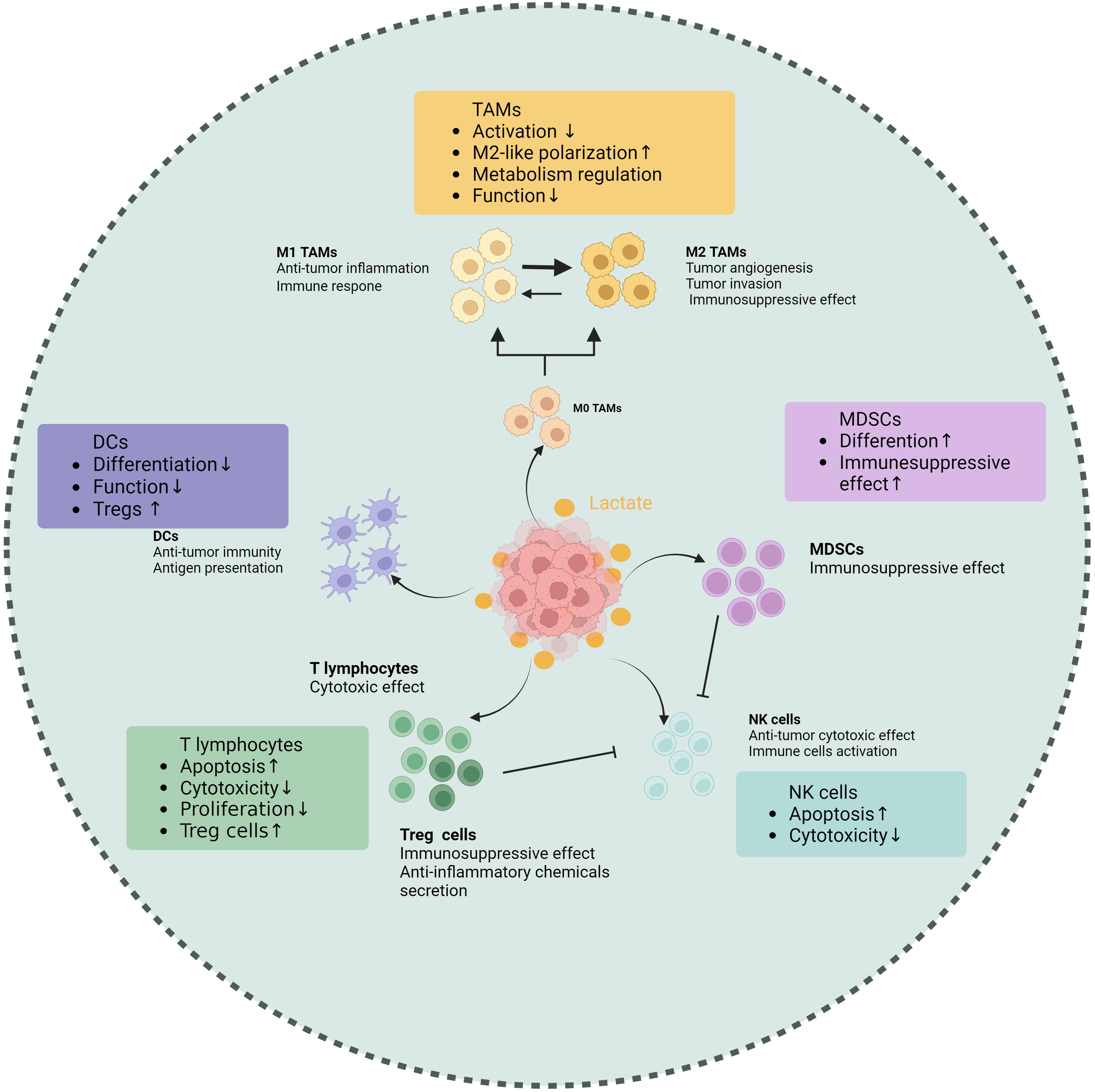
Figure 2. Effects of lactate on tumor-associated immune cells. Lactate makes different impacts on various immune cell populations within the TME, including tumor-associated macrophages (TAMs), T cells, natural killer (NK) cells, dendritic cells (DCs), and myeloid-derived suppressor cells (MDSCs). Elevated lactate levels are shown to promote the polarization of TAMs towards an immunosuppressive phenotype, enhancing their ability to support tumor growth. In T cells, lactate impairs proliferation and cytokine production, leading to reduced antitumor activity. NK cell function is also inhibited by lactate, which diminishes their cytotoxic potential. Conversely, lactate enhances the immunosuppressive properties of MDSCs, facilitating their accumulation in the tumor microenvironment. Dendritic cells exhibit altered maturation and function in the presence of lactate, impacting their ability to activate T cells. These findings underscore the role of lactate as a critical metabolic regulator of immune responses in tumors, highlighting its potential as a therapeutic target. (Created with BioRender.com).
It is not surprising that lactate can influence tumor-associated macrophages (TAMs), which are the most abundant immune cell population found in TIME. In terms of its mechanism, the GPR81a binds to lactate and exerts inhibitory effects on the activation of Yes1-associated transcription factor and NF-κB, thereby effectively suppressing macrophage activation (33). The macrophages in TME can generally be classified into M1 type (classically activated macrophages) and M2 type (alternatively activated macrophages). The presence of M1 macrophages in the TME inhibits tumor growth and is linked to a better prognosis in various cancers. In contrast, the M2 macrophages promote tumor initiation and progression (34, 40, 41). As a biological process, “macrophage polarization” is controlled by certain microenvironmental cues that govern the transition between M1 and M2 macrophages. Elevated lactate concentration is a key driving factor for TAM polarization (42). Initiation of STAT3 and ERK1/2 pathways in the tumor microenvironment (TME) has been shown in earlier research to induce M2 polarization in macrophages. Research that followed identified lactic acid as an ERK/STAT3 pathway promoter (43). Lactate enhances the stability of HIF-1α and MCT to increase the expression of Vascular Endothelial Growth Factors (VEGF) and arginine in TAMs, ultimately promoting their polarization towards an M2 type (44). The presence of lactate may potentially cause a rise in intracellular ROS levels, thereby activating Nrf2 in macrophages and promoting their transition into the M2 macrophage (45). In the breast cancer and Lewis lung cancer models, the lactate signal within the TME can activate GPR132, thereby promoting the M2 phenotype (46–48). The following discovery of histone lactylation revealed a novel mechanism whereby lactylation of histone arginine residues can regulate TAM polarization by increasing the ARG1 expression or other TAM-related genes (19). In summary, lactate-induced M2-type TAMs within the TME undergo polarization and facilitate immune evasion, thereby contributing to the sustenance of tumor progression and viability.
Additionally, lactate has the ability to control TAM metabolism, switching them from OXPHOS to glycolysis. In the long run, this helps tumor growth by increasing the release of lactate (49). Lactate has the potential to influence macrophages themselves function. The killing action of macrophages on tumors is effectively inhibited when CD47 on tumor cells binds with SIRP-α on macrophages. In colorectal cancer, lactate induces activation of the Ap-α/Elk-1 pathway in TAMs, which raises the expression level of SIRP-α, further suppressing tumor immune response (50). Additionally, lactate can upregulate the PD-L1 expression in TAMs, thereby facilitating immune evasion within the TME (51). Recent investigations have demonstrated that damage-associated molecular patterns (DAMPs), including high mobility group box protein 1 (HMGB1), which is associated with lactate and lactylatio (52), exert pivotal roles in triggering and perpetuating inflammation, thereby compromising immune cells and fostering tumorigenesis (53, 54).
T lymphocytes serve as the main force in the immune system by exerting their cytotoxic effects on tumor cells. The high level of lactate has been demonstrated to negatively impact almost every aspect of T lymphocyte function (55). First, activated T cells require glycolytic metabolism and must secrete endogenous lactic acid to prevent cellular acidification. However, lactate accumulation inhibits T cells from releasing lactate, leading to disruptions in their metabolism and function. In addition, lactate can change the balance of the TCA cycle, finally impairing the cytotoxic T lymphocyte (CTL) function (56). Lactate accumulation within the TME may block p38 and JNK/c-Jun signal transduction to impair CTL function (57). Lactate suppresses nuclear factor of activated T cells (NFAT) expression in T cells and natural killer (NK) cells, leading to a decreased level of IFN-γ. This hinders the capacity of T cells and NK cells to conduct immune surveillance (58). Regarding proliferation, lactate regulates the NAD(H) oxidation-reduction state to limit T cell proliferation. Lactic acid can reduce NAD+ to NADH and alter NAD+-dependent enzyme reactions, thereby reducing the generation of intermediate glycolysis products vital to T cell proliferation and achieving limitation on their proliferation (59).
Regulatory T (Treg) cells, a specific subgroup of CD4+ T cells, contribute to tumor immune tolerance by inhibiting the proliferation and promotion of immune cells and secreting anti-inflammatory chemicals. Compared to other T cells, Treg cells exhibit different characteristics in the acidic TME. As a specific molecule of Tregs, FoxP3 maintains Tregs’ OXPHOS metabolism and provides metabolic advantages by inhibiting the c-Myc signaling pathway in a high glycolytic microenvironment (60). Lactate can enhance Treg infiltration in tumors along with upregulate CD25 expression, a surface marker associated with Treg activation (61). From a mechanistic standpoint, lactate may regulate Treg cell activity by facilitating MOESIN lactylation and modulating TGF-β signaling transduction, thereby contributing to their maturation and differentiation processes (62). Furthermore, Tregs enhance the functionality of PD-1 by actively absorbing lactate through the MCT1, promoting NFAT1 translocation into the cell nucleus (63).
Lactic acid not only affects common types of T cells but also promotes apoptosis in immature T cells and decreases cholesterol synthesis and IFN-γ release in invariant natural killer T (iNKT) cells, thereby influencing tumor immunity (64, 65).
NK cells serve as the primary defense by releasing molecules, including cytokines and granule enzymes, which facilitate the elimination of cancer cells. Moreover, they possess the ability to activate additional immune cells, thus augmenting the overall immune reaction.
Study indicates that the acidic tumor microenvironment negatively affects the functionality of NK cells (66, 67). Further experiments have revealed that the spontaneous release of LDH, a key enzyme in lactate production, was found to be associated with NK cell dysfunction (68). The lactate derived from tumors can inhibit NKp46 expression, resulting in suppression of cytotoxicity in NK cells. This decline in NK cell cytotoxicity is often associated with decreased perforin and granzyme levels (69). In addition, lactate can impair NKT cell function by interfering with the mTOR signaling pathway (70). In terms of cell homeostasis, colorectal cancer cells that metastasize to the liver produce a large amount of lactate when faced with NK cells with strong cytotoxicity in the liver. This lactate production lowers the pH value in the TME, thereby preventing NK cells from effectively removing lactate from the cytoplasm through concentration gradients. As a result, mitochondrial stress and cell apoptosis occur (71).
Dendritic cells (DCs) play a crucial role in anti-tumor immunity through their function in antigen presentation. Research indicates that lactic acid in the TME can affect DC and block its differentiation (72). Subsequently, Gottfried et al. developed multicellular tumor spheroids (MCTS) to facilitate the infiltration of monocytes and immune cells into MCTS comprising multiple tumor cells derived from diverse origins. They found that tumor-derived lactate was a potent modulator of human monocytes. Lactate can not only inhibit monocytes differentiating into DCs, thereby impairing antigen presentation, but also interfere with the migration of monocytes into MCTS (73). The phenotype of DCs is significantly influenced by lactate derived from tumors. The addition of lactate in vitro can induce alterations in antigen expression and a decrease in IL-12 secretion, similar to the phenotype change of tumor-associated DCs observed in co-cultures of melanoma cancer, and reducing lactate level can restore the normal phenotype of DCs (74). In lung cancer, lactate changes the adaptability of DCs by making it harder for cross-presenting to happen and making it easier for antigens to disappear (75). According to a breast cancer report, lactate activates GPR81 on DCs, which hinders the display of tumor-specific antigens to additional tumor-associated immune cells (38). The presence of lactate can impede the activation of IFN-α and IFN-γ in plasmacytoid dendritic cells (pDCs), which constitute a subset of dendritic cells and are recognized as the most effective producers of IFN-α in humans. thereby compromising the effectiveness of the immune response against tumors. Additionally, lactic acid promotes the metabolism of tryptophan and L-kynurenine generation in pDCs, facilitating the development of significant immunosuppressive immune cell groups in the tumor microenvironment, specifically FoxP3+CD4+Tregs (76).
Myeloid-derived suppressor cells (MDSCs) refer to a diverse group of cells originating from the bone marrow that possess an extraordinary capacity to effectively inhibit immune reactions.
After the activation of the Notch/RBP-J pathway, the downstream molecule HES1 reduces the expression of MCT2, ultimately lowering the intracellular lactic acid concentration, which in turn affects the differentiation of MDSCs and the maturation of TAMs (77). As early as 2013, it was discovered that lactic acid derived from tumors increases the frequency of MDSCs (69). Subsequent research indicates that lactate attracts MDSCs and controls their maturation, thus influencing tumor immunity and the advancement of tumors (77, 78).
Another study on pancreatic cancer has shown that lactate activates MDSCs through the GPR81/mTOR/HIF-1α/STAT3 pathway, thereby enhancing the immunosuppressive effects (79). Lactic acid has the potential to enhance the immunosuppressive capabilities of TIMs through epigenetic mechanisms. METTL3’s zinc finger domain contains two lactylation modification sites, which are associated with RNA m6A modification within MDSCs (32).
In the in-depth study of the unique metabolic patterns and immune response in tumors, scientists have discovered numerous therapeutic strategies targeting tumor metabolism and immunity. Lactate suppresses immune cell activity, diminishing their capacity to attack malignant cells and creating favorable conditions for tumor growth and spread. Currently, numerous clinical studies are being conducted to explore the significance of lactate in clinical practice as a specific metabolite. Therefore, intervening in lactate metabolism pathways holds promise for altering the tumor microenvironment and enhancing immunotherapy effectiveness.
Under normal circumstances, serum lactic acid concentration typically ranges from 1.5-3.0 mM (80). Due to the Warburg effect, lactate concentration in tumors can reach a range of 10-30 mM and even grow up to 50 mM within the necrotic core of the tumor (81). Indeed, elevated lactic acid levels are considered unfavorable prognostic indicators for various cancers, including cervical cancer (80), breast cancer (82), head and neck cancer (83), and non-small cell lung cancer (84). Hence, the assessment of patient prognosis and treatment selection can be facilitated by examining lactate and related metabolite levels (Table 2). New advancements in technology, such as Magnetic Resonance Spectroscopy (MRS) and Hyperpolarized 13C-MRI (HP 13C-MRI),are reforming the measurement approach for lactic acid and enhancing the practicality of utilizing the level as a cancer diagnostic tool. Current investigations into lactate imaging as a diagnostic biomarker for tumors encompass such as NCT01881386 (observing alterations in lactate concentration following treatment through magnetic MRS), NCT04584827 (examining the impact of lactate concentration death and morbidity in patients undergoing intracranial tumor surgery under general anesthesia), NCT03129776 (using HP 13C-MRI to determine radiation-resistant areas in neck tumors and guide radiotherapy), and NCT03531307 (investigating the association between lactic acid levels and tumor proliferation marker Ki67 in brain tumor patients).
Instead of measuring lactate level directly, specific proteins associated with lactate metabolism can also be utilized as indicators for tumor progression. For example, increased levels of LDH have been linked to the presence of aggressive clinical pathological characteristics in pancreatic cancer and an unfavorable prognosis in mesothelioma and lung cancer (85). MCTs can serve as biomarkers and potential therapeutic targets for colorectal cancer (86). Additionally, the level of lactylation in gastric cancer tissue can serve as an indicator of tumor immune evasion and advancement (87).
Given the crucial involvement of lactate in tumor progression, interventions aimed at lactate could potentially impede tumor proliferation and hinder metastasis (Table 3). Due to the increased expression of LDHA in cancer and its primary role in lactate production position, LDHA is a potential candidate for cancer treatment. In reality, numerous studies have shown that inhibiting LDHA can effectively impede the proliferation and metastasis (88–91). Various compounds, such as Gossypol (also referred to as AT-101) and its derivative FX-11, along with galloflavin, have been identified as potential inhibitors of LDHA with promising anti-tumor properties (92). Due to its ability to inhibit LDHA, vitamin C may be considered an effective therapy for stress-related breast cancer (93). Although LDHA has shown potential in cancer therapy, its inhibition can cause a variety of off-target effects. For instance, inhibition of LDHA leads to an increased intracellular pyruvate and NADH level, which drives extracellular matrix (ECM) remodeling and ultimately strengthens collagen protein durability and promotes the progression of breast cancer (94). Targeting LDHB could also offer a potential approach for cancer therapy. LDHB is essential for enhancing lysosomal function and tumor autophagy. When LDHB is silenced, selective inhibitory effects on cancer proliferation have been confirmed (95).
Except for the previously mentioned LDH, other glycolytic enzymes such as PDH, hexokinase II (HK2), and pyruvate kinase isozyme M2 (PKM2) can also be targeted to decrease lactate production (96). Additionally, disrupting lactate-producing signaling pathways like the PI3K/AKT/mTOR could be a potential approach (97). Direct consumption of lactate is an alternative approach. Lactic acid oxidase (LOX) can oxidize the lactate secreted by tumors into pyruvate and hydrogen peroxide (H2O2), thereby addressing lactate-induced drug resistance (98).
For lactate transporters, one possible antitumor approach could involve the inhibition of MCT1 and MCT4 activity or the reduction of their presence (99). Syrosingopine has been demonstrated as a potent dual inhibitor of MCT1 and MCT4 (100) making tumor cells more susceptible to metformin, thereby boosting the anticancer effects of metformin. These results highlight the possibility of syrosingopine as a supplementary treatment in upcoming clinical agents (101). The cell surface localization of MCT1 and MCT4 is dependent on the CD147 (102). For this reason, CD147 was presented as a prospective option to increase the efficacy of cancer treatment and intervention in MCT membrane integration. However, MCTs may also serve as transport proteins for specific medicine, like the potential anticancer agent 3-bromopyruvate (3-BP) (103). Consequently, if used in combination, there might be potential impediments to the pharmacological effects.
The observation of lactylation has greatly broadened the paradigm of lactic acid-related therapies beyond traditional targets. Several studies have been conducted focusing on histone lactylation. Demethylzellal (DML) has been shown to efficiently increase the effectiveness of chemotherapy treatments by preventing H3Kla in stem cells from liver cancer (104). Royal jelly acid (RJA) has been found to inhibit the progression of hepatocellular carcinoma (HCC) by suppressing H3K9la and H3K14la (105). Clinical studies are now under progress to assess the effectiveness of p300 inhibitors, including EP3160 and CCS1477, in directly inhibiting the lactylation process.
Due to the strong correlation between lactate and tumor immunity, a potential strategy for tumor treatment could involve combining lactate-targeted cancer therapy with immunotherapy. For example, by reducing lactate production while simultaneously administering PD-1 inhibitors, treatment efficacy may be enhanced.
In this review, we convey a summary of the unique lactate metabolism mechanism in tumors and the formation of acidic TME, as well as summarize the signaling pathways related to lactate. We emphasize the impact that lactic acid has on serval tumor-associated immune cells. Furthermore, we debate the prospective application of lactate as a tumor biomarker and clinical anticancer target.
Lactate is classified into three types according to the arrangement of carbon atoms, including D-lactate, L-lactate, and racemic DL-lactate. Present studies primarily focus on L-lactate in the realm of lactate research. In contrast, D-lactate, as an enantiomer of L-lactate, is typically present in negligible amounts within human tissues and thus remains undetectable in the bloodstream under normal physiological circumstances (106). Research has shown the regulatory function of D-lactate in cellular metabolism, antioxidant capacity, and energy generation, along with its correlation to disease (107–109). Moreover, D-lactate has been associated with the control of tumor growth and immunity, contributing to the development of various cancers such as esophageal cancer, prostate cancer, hepatocellular carcinoma and renal cell carcinoma (110–113). However, there are still significant gaps in comprehension involving the role of D-lactate and its impact on tumors. Therefore, further investigation in this field is necessary and meaningful.
Exploring lactylation is expected to emerge as a promising avenue for investigating the regulation of cell function in the TME and understanding mechanisms underlying tumor progression. Additionally, identifying enzymes or genes associated with lactylation modification could offer novel therapeutic targets. Nevertheless, there still exist a multitude of unresolved enigmas in this domain that merit further investigation. First, besides histone lactylation, what impact do other non-histone lactylations associated with KLA sites have on tumor angiogenesis, progression, invasion, and immune response? Secondly, what are the main factors of specialization and discrimination in the p300/CBP function concerning the function selectivity of acyl modifications? All in all, how does an indiscriminate HAT particularly facilitate lactylation? Research regarding Kla has identified additional forms of acylation occurring concurrently with lactylation on identical lysine residues of the same proteins, indicating the possibility of interplay between these modifications (114). So, there is still an unanswered question regarding the connections between lactylation and other PTMs. Additionally, it is crucial to determine the specific mechanism by which histone modifications interact with RNA modifications. By addressing these inquiries, we can enhance our comprehension of lactylation’s contribution to tumor development and subsequently propose more precise and promising treatment alternatives.
In relation to lactate and tumor immunity, it is commonly believed that lactate may potentially compromise the effectiveness of tumor immunity and facilitate immune evasion. However, lactate has a dual effect on T cells; in addition to inhibiting the function of T cells, the research conducted by Ing Wen revealed the potential of sodium lactate therapy in suppressing tumor development in living organisms, relying on the presence of T cells (115). Furthermore, CD8+ T cells have the capability to utilize lactate as a supplier for power and vital components, which improves their metabolic function and promotes an immune response against tumors (116). These offer a fresh outlook on the interactions between tumor cells and immune cells facilitated via lactic acid.
KS: Writing – original draft. YS: Writing – review & editing. XX: Writing – review & editing. HX: Writing – review & editing. ML: Project administration, Supervision, Writing – review & editing, Funding acquisition. QZ: Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Jiangsu Province (Project acceptance number: SBK2024021831).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. (2020) 2:132–41. doi: 10.1038/s42255-020-0174-0
2. Terry S, Engelsen AST, Buart S, Elsayed WS, Venkatesh GH, Chouaib S. Hypoxia-driven intratumor heterogeneity and immune evasion. Cancer Lett. (2020) 492:1–10. doi: 10.1016/j.canlet.2020.07.004
3. Chen B, Gao A, Tu B, Wang Y, Yu X, Wang Y, et al. Metabolic modulation via mTOR pathway and anti-angiogenesis remodels tumor microenvironment using PD-L1-targeting codelivery. Biomaterials. (2020) 255:120187. doi: 10.1016/j.biomaterials.2020.120187
4. Yan Y, Chang L, Tian H, Wang L, Zhang Y, Yang T, et al. 1-Pyrroline-5-carboxylate released by prostate Cancer cell inhibit T cell proliferation and function by targeting SHP1/cytochrome c oxidoreductase/ROS Axis. J Immunother Cancer. (2018) 6:148. doi: 10.1186/s40425-018-0466-z
5. Karayama M, Masuda J, Mori K, Yasui H, Hozumi H, Suzuki Y, et al. Comprehensive assessment of multiple tryptophan metabolites as potential biomarkers for immune checkpoint inhibitors in patients with non-small cell lung cancer. Clin Trans Oncol. (2021) 23:418–23. doi: 10.1007/s12094-020-02421-8
6. Brooks GA. The science and translation of lactate shuttle theory. Cell Metab. (2018) 27:757–85. doi: 10.1016/j.cmet.2018.03.008
7. Felmlee MA, Jones RS, Rodriguez-Cruz V, Follman KE, Morris ME. Monocarboxylate transporters (SLC16): function, regulation, and role in health and disease. Pharmacol Rev. (2020) 72:466–85. doi: 10.1124/pr.119.018762
8. Halestrap AP. The SLC16 gene family - structure, role and regulation in health and disease. Mol Aspects Med. (2013) 34:337–49. doi: 10.1016/j.mam.2012.05.003
9. Offermans K, Jenniskens JC, Simons CC, Samarska I, Fazzi GE, Smits KM, et al. Expression of proteins associated with the Warburg-effect and survival in colorectal cancer. J Pathol Clin Res. (2022) 8:169–80. doi: 10.1002/cjp2.v8.2
10. Bröer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, et al. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J Biol Chem. (1997) 272:30096–102. doi: 10.1074/jbc.272.48.30096
11. Rabinowitz JD, Enerbäck S. Lactate: the ugly duckling of energy metabolism. Nat Metab. (2020) 2:566–71. doi: 10.1038/s42255-020-0243-4
12. Roland CL, Arumugam T, Deng D, Liu SH, Philip B, Gomez S, et al. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. (2014) 74:5301–10. doi: 10.1158/0008-5472.CAN-14-0319
13. Cacace A, Sboarina M, Vazeille T, Sonveaux P. Glutamine activates STAT3 to control cancer cell proliferation independently of glutamine metabolism. Oncogene. (2017) 36:2074–84. doi: 10.1038/onc.2016.364
14. Brown TP, Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther. (2020) 206:107451. doi: 10.1016/j.pharmthera.2019.107451
15. Trzaskowski B, Latek D, Yuan S, Ghoshdastider U, Debinski A, Filipek S. Action of molecular switches in GPCRs–theoretical and experimental studies. Curr Med Chem. (2012) 19:1090–109. doi: 10.2174/092986712799320556
16. Williams JA. Using PDX for preclinical cancer drug discovery: the evolving field. J Clin Med. (2018) 7(3):41. doi: 10.3390/jcm7030041
17. Pertega-Gomes N, Felisbino S, Massie CE, Vizcaino JR, Coelho R, Sandi C, et al. A glycolytic phenotype is associated with prostate cancer progression and aggressiveness: a role for monocarboxylate transporters as metabolic targets for therapy. J Pathol. (2015) 236:517–30. doi: 10.1002/path.2015.236.issue-4
18. Vadevoo SMP, Gunassekaran GR, Lee C, Lee N, Lee J, Chae S, et al. The macrophage odorant receptor Olfr78 mediates the lactate-induced M2 phenotype of tumor-associated macrophages. Proc Natl Acad Sci U.S.A. (2021) 118(37):e2102434118. doi: 10.1073/pnas.2102434118
19. Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature. (2019) 574:575–80. doi: 10.1038/s41586-019-1678-1
20. Yang Z, Yan C, Ma J, Peng P, Ren X, Cai S, et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat Metab. (2023) 5:61–79. doi: 10.1038/s42255-022-00710-w
21. Wang X, Ying T, Yuan J, Wang Y, Su X, Chen S, et al. BRAFV600E restructures cellular lactylation to promote anaplastic thyroid cancer proliferation. Endocrine-related Cancer. (2023) 30(8):e220344. doi: 10.1530/ERC-22-0344
22. Wan N, Wang N, Yu S, Zhang H, Tang S, Wang D, et al. Cyclic immonium ion of lactyllysine reveals widespread lactylation in the human proteome. Nat Methods. (2022) 19:854–64. doi: 10.1038/s41592-022-01523-1
23. Manickavinayaham S, Vélez-Cruz R, Biswas AK, Bedford E, Klein BJ, Kutateladze TG, et al. E2F1 acetylation directs p300/CBP-mediated histone acetylation at DNA double-strand breaks to facilitate repair. Nat Commun. (2019) 10:4951. doi: 10.1038/s41467-019-12861-8
24. Xu X, Zhang C, Xu H, Wu L, Hu M, Song L. Autophagic feedback-mediated degradation of IKKα requires CHK1- and p300/CBP-dependent acetylation of p53. J Cell Sci. (2020) 133(22):jcs246868. doi: 10.1242/jcs.246868
25. Cui H, Xie N, Banerjee S, Ge J, Jiang D, Dey T, et al. Lung myofibroblasts promote macrophage profibrotic activity through lactate-induced histone lactylation. Am J Respir Cell Mol Biol. (2021) 64:115–25. doi: 10.1165/rcmb.2020-0360OC
26. Li L, Chen K, Wang T, Wu Y, Xing G, Chen M, et al. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat Metab. (2020) 2:882–92. doi: 10.1038/s42255-020-0267-9
27. Moreno-Yruela C, Zhang D, Wei W, Bæk M, Liu W, Gao J, et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci Adv. (2022) 8:eabi6696. doi: 10.1126/sciadv.abi6696
28. Mews P, Donahue G, Drake AM, Luczak V, Abel T, Berger SL. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature. (2017) 546:381–6. doi: 10.1038/nature22405
29. Zhou W, Zhao T, Du J, Ji G, Li X, Ji S, et al. TIGAR promotes neural stem cell differentiation through acetyl-CoA-mediated histone acetylation. Cell Death Dis. (2019) 10:198. doi: 10.1038/s41419-019-1434-3
30. Gaffney DO, Jennings EQ, Anderson CC, Marentette JO, Shi T, Schou Oxvig AM, et al. Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell Chem Biol. (2020) 27:206–213.e206. doi: 10.1016/j.chembiol.2019.11.005
31. Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X, et al. Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. (2021) 22:85. doi: 10.1186/s13059-021-02308-z
32. Xiong J, He J, Zhu J, Pan J, Liao W, Ye H, et al. Lactylation-driven METTL3-mediated RNA m(6)A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol Cell. (2022) 82:1660–1677.e1610. doi: 10.1016/j.molcel.2022.02.033
33. Yang K, Xu J, Fan M, Tu F, Wang X, Ha T, et al. Lactate suppresses macrophage pro-inflammatory response to LPS stimulation by inhibition of YAP and NF-κB activation via GPR81-mediated signaling. Front Immunol. (2020) 11:587913. doi: 10.3389/fimmu.2020.587913
34. Chorro L, Geissmann F. Development and homeostasis of ‘resident’ myeloid cells: the case of the Langerhans cell. Trends Immunol. (2010) 31:438–45. doi: 10.1016/j.it.2010.09.003
35. Sun Z, Han Y, Song S, Chen T, Han Y, Liu Y. Activation of GPR81 by lactate inhibits oscillatory shear stress-induced endothelial inflammation by activating the expression of KLF2. IUBMB Life. (2019) 71:2010–9. doi: 10.1002/iub.v71.12
36. Madaan A, Nadeau-Vallée M, Rivera JC, Obari D, Hou X, Sierra EM, et al. Lactate produced during labor modulates uterine inflammation via GPR81 (HCA(1)). Am J Obstetr Gynecol. (2017) 216:60.e61–17. doi: 10.1016/j.ajog.2016.09.072
37. Wu G, Dai Y, Yan Y, Zheng X, Zhang H, Li H, et al. The lactate receptor GPR81 mediates hepatic lipid metabolism and the therapeutic effect of metformin on experimental NAFLDs. Eur J Pharmacol. (2022) 924:174959. doi: 10.1016/j.ejphar.2022.174959
38. Brown TP, Bhattacharjee P, Ramachandran S, Sivaprakasam S, Ristic B, Sikder MOF, et al. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene. (2020) 39:3292–304. doi: 10.1038/s41388-020-1216-5
39. Cheng WY, Huynh H, Chen P, Peña-Llopis S, Wan Y. Macrophage PPARγ inhibits Gpr132 to mediate the anti-tumor effects of rosiglitazone. eLife. (2016) 5:e18501. doi: 10.7554/eLife.18501
40. Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human Malignant tumors. Cancer Sci. (2014) 105:1–8. doi: 10.1111/cas.2014.105.issue-1
41. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. (2004) 4:71–8. doi: 10.1038/nrc1256
42. Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. (2010) 120:2699–714. doi: 10.1172/JCI39506
43. Mu X, Shi W, Xu Y, Xu C, Zhao T, Geng B, et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle (Georgetown Tex.). (2018) 17:428–38. doi: 10.1080/15384101.2018.1444305
44. Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. (2014) 513:559–63. doi: 10.1038/nature13490
45. Feng R, Morine Y, Ikemoto T, Imura S, Iwahashi S, Saito Y, et al. Nrf2 activation drive macrophages polarization and cancer cell epithelial-mesenchymal transition during interaction. Cell Communication Signaling: CCS. (2018) 16:54. doi: 10.1186/s12964-018-0262-x
46. Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q, Saghatelian A, et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U.S.A. (2017) 114:580–5. doi: 10.1073/pnas.1614035114
47. Nii T, Prabhu VV, Ruvolo V, Madhukar N, Zhao R, Mu H, et al. Imipridone ONC212 activates orphan G protein-coupled receptor GPR132 and integrated stress response in acute myeloid leukemia. Leukemia. (2019) 33:2805–16. doi: 10.1038/s41375-019-0491-z
48. Lahvic JL, Ammerman M, Li P, Blair MC, Stillman ER, Fast EM, et al. Specific oxylipins enhance vertebrate hematopoiesis via the receptor GPR132. Proc Natl Acad Sci U.S.A. (2018) 115:9252–7. doi: 10.1073/pnas.1806077115
49. Arts RJ, Plantinga TS, Tuit S, Ulas T, Heinhuis B, Tesselaar M, et al. Transcriptional and metabolic reprogramming induce an inflammatory phenotype in non-medullary thyroid carcinoma-induced macrophages. Oncoimmunology. (2016) 5:e1229725. doi: 10.1080/2162402X.2016.1229725
50. Wang X, Luo X, Chen C, Tang Y, Li L, Mo B, et al. The Ap-2α/Elk-1 axis regulates Sirpα-dependent tumor phagocytosis by tumor-associated macrophages in colorectal cancer. Signal Transduct Target Ther. (2020) 5:35. doi: 10.1038/s41392-020-0124-z
51. Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. (2021) 33:2040–2058.e2010. doi: 10.1016/j.cmet.2021.09.002
52. Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W, et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun. (2014) 5:4436. doi: 10.1038/ncomms5436
53. Wang S, Zhang Y. HMGB1 in inflammation and cancer. J Hematol Oncol. (2020) 13:116. doi: 10.1186/s13045-020-00950-x
54. Zhang QY, Wu LQ, Zhang T, Han YF, Lin X. Autophagy-mediated HMGB1 release promotes gastric cancer cell survival via RAGE activation of extracellular signal-regulated kinases 1/2. Oncol Rep. (2015) 33:1630–8. doi: 10.3892/or.2015.3782
55. Nakagawa Y, Negishi Y, Shimizu M, Takahashi M, Ichikawa M, Takahashi H. Effects of extracellular pH and hypoxia on the function and development of antigen-specific cytotoxic T lymphocytes. Immunol Lett. (2015) 167:72–86. doi: 10.1016/j.imlet.2015.07.003
56. Elia I, Rowe JH, Johnson S, Joshi S, Notarangelo G, Kurmi K, et al. Tumor cells dictate anti-tumor immune responses by altering pyruvate utilization and succinate signaling in CD8(+) T cells. Cell Metab. (2022) 34:1137–1150.e1136. doi: 10.1016/j.cmet.2022.06.008
57. Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int J Cancer. (2012) 131:633–40. doi: 10.1002/ijc.v131.3
58. Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. (2016) 24:657–71. doi: 10.1016/j.cmet.2016.08.011
59. Quinn WJ, Jiao 3J, TeSlaa T, Stadanlick J, Wang Z, Wang L, et al. Lactate limits T cell proliferation via the NAD(H) redox state. Cell Rep. (2020) 33:108500. doi: 10.1016/j.celrep.2020.108500
60. Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. (2017) 25:1282–1293.e1287. doi: 10.1016/j.cmet.2016.12.018
61. Chirasani SR, Leukel P, Gottfried E, Hochrein J, Stadler K, Neumann B, et al. Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine glioma model. Int J Cancer. (2013) 132:843–53. doi: 10.1002/ijc.v132.4
62. Gu J, Zhou J, Chen Q, Xu X, Gao J, Li X, et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-β signaling in regulatory T cells. Cell Rep. (2022) 39:110986. doi: 10.1016/j.celrep.2022.110986
63. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. (2022) 40:201–218.e209. doi: 10.1016/j.ccell.2022.01.001
64. Fu S, He K, Tian C, Sun H, Zhu C, Bai S, et al. Impaired lipid biosynthesis hinders anti-tumor efficacy of intratumoral iNKT cells. Nat Commun. (2020) 11:438. doi: 10.1038/s41467-020-14332-x
65. Xia H, Wang W, Crespo J, Kryczek I, Li W, Wei S, et al. Suppression of FIP200 and autophagy by tumor-derived lactate promotes naïve T cell apoptosis and affects tumor immunity. Sci Immunol. (2017) 2(17):eaan4631. doi: 10.1126/sciimmunol.aan4631
66. Piñeiro Fernández J, Luddy KA, Harmon C, O’Farrelly C. Hepatic tumor microenvironments and effects on NK cell phenotype and function. Int J Mol Sci. (2019) 20(17):4131. doi: 10.3390/ijms20174131
67. Pötzl J, Roser D, Bankel L, Hömberg N, Geishauser A, Brenner CD, et al. Reversal of tumor acidosis by systemic buffering reactivates NK cells to express IFN-γ and induces NK cell-dependent lymphoma control without other immunotherapies. Int J Cancer. (2017) 140:2125–33. doi: 10.1002/ijc.30646
68. Konjević G, Jurisić V, Banićevic B, Spuzić I. The difference in NK-cell activity between patients with non-Hodgkin’s lymphomas and Hodgkin’s disease. Br J Haematol. (1999) 104:144–51. doi: 10.1046/j.1365-2141.1999.01129.x
69. Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol (Baltimore Md.: 1950). (2013) 191:1486–95. doi: 10.4049/jimmunol.1202702
70. Xie D, Zhu S, Bai L. Lactic acid in tumor microenvironments causes dysfunction of NKT cells by interfering with mTOR signaling, Science China. Life Sci. (2016) 59:1290–6. doi: 10.1007/s11427-016-0348-7
71. Harmon C, Robinson MW, Hand F, Almuaili D, Mentor K, Houlihan DD, et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol Res. (2019) 7:335–46. doi: 10.1158/2326-6066.CIR-18-0481
72. Puig-Kröger A, Pello OM, Muñiz-Pello O, Selgas R, Criado G, Bajo MA, et al. Peritoneal dialysis solutions inhibit the differentiation and maturation of human monocyte-derived dendritic cells: effect of lactate and glucose-degradation products. J Leukocyte Biol. (2003) 73:482–92. doi: 10.1189/jlb.0902451
73. Gottfried E, Kunz-Schughart LA, Andreesen R, Kreutz M. Brave little world: spheroids as an in vitro model to study tumor-immune-cell interactions. Cell Cycle (Georgetown Tex.). (2006) 5:691–5. doi: 10.4161/cc.5.7.2624
74. Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. (2006) 107:2013–21. doi: 10.1182/blood-2005-05-1795
75. Caronni N, Simoncello F, Stafetta F, Guarnaccia C, Ruiz-Moreno JS, Opitz B, et al. Downregulation of membrane trafficking proteins and lactate conditioning determine loss of dendritic cell function in lung cancer. Cancer Res. (2018) 78:1685–99. doi: 10.1158/0008-5472.CAN-17-1307
76. Raychaudhuri D, Bhattacharya R, Sinha BP, Liu CSC, Ghosh AR, Rahaman O, et al. Lactate induces pro-tumor reprogramming in intratumoral plasmacytoid dendritic cells. Front Immunol. (2019) 10:1878. doi: 10.3389/fimmu.2019.01878
77. Zhao JL, Ye YC, Gao CC, Wang L, Ren KX, Jiang R, et al. Notch-mediated lactate metabolism regulates MDSC development through the Hes1/MCT2/c-Jun axis. Cell Rep. (2022) 38:110451. doi: 10.1016/j.celrep.2022.110451
78. Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu K, et al. Aerobic glycolysis controls myeloid-derived suppressor cells and tumor immunity via a specific CEBPB isoform in triple-negative breast cancer. Cell Metab. (2018) 28:87–103.e106. doi: 10.1016/j.cmet.2018.04.022
79. Yang X, Lu Y, Hang J, Zhang J, Zhang T, Huo Y, et al. Lactate-modulated immunosuppression of myeloid-derived suppressor cells contributes to the radioresistance of pancreatic cancer. Cancer Immunol Res. (2020) 8:1440–51. doi: 10.1158/2326-6066.CIR-20-0111
80. Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. (2000) 60:916–21.
81. Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. (2001) 51:349–53. doi: 10.1016/S0360-3016(01)01630-3
82. Cheung SM, Husain E, Masannat Y, Miller ID, Wahle K, Heys SD, et al. Lactate concentration in breast cancer using advanced magnetic resonance spectroscopy. Br J Cancer. (2020) 123:261–7. doi: 10.1038/s41416-020-0886-7
83. Blatt S, Voelxen N, Sagheb K, Pabst AM, Walenta S, Schroeder T, et al. Lactate as a predictive marker for tumor recurrence in patients with head and neck squamous cell carcinoma (HNSCC) post radiation: a prospective study over 15 years. Clin Oral Investigat. (2016) 20:2097–104. doi: 10.1007/s00784-015-1699-6
84. Yokota H, Guo J, Matoba M, Higashi K, Tonami H, Nagao Y. Lactate, choline, and creatine levels measured by vitro 1H-MRS as prognostic parameters in patients with non-small-cell lung cancer. J Magnetic Resonance Imaging: JMRI. (2007) 25:992–9. doi: 10.1002/jmri.20902
85. Comandatore A, Franczak M, Smolenski RT, Morelli L, Peters GJ, Giovannetti E. Lactate Dehydrogenase and its clinical significance in pancreatic and thoracic cancers. Semin Cancer Biol. (2022) 86:93–100. doi: 10.1016/j.semcancer.2022.09.001
86. Martins SF, Amorim R, Viana-Pereira M, Pinheiro C, Costa RF, Silva P, et al. Significance of glycolytic metabolism-related protein expression in colorectal cancer, lymph node and hepatic metastasis. BMC Cancer. (2016) 16:535. doi: 10.1186/s12885-016-2566-9
87. Yang H, Zou X, Yang S, Zhang A, Li N, Ma Z. Identification of lactylation related model to predict prognostic, tumor infiltrating immunocytes and response of immunotherapy in gastric cancer. Front Immunol. (2023) 14:1149989. doi: 10.3389/fimmu.2023.1149989
88. Hou X, Shi X, Zhang W, Li D, Hu L, Yang J, et al. LDHA induces EMT gene transcription and regulates autophagy to promote the metastasis and tumorigenesis of papillary thyroid carcinoma. Cell Death Dis. (2021) 12:347. doi: 10.1038/s41419-021-03641-8
89. Jin L, Chun J, Pan C, Alesi GN, Li D, Magliocca KR, et al. Phosphorylation-mediated activation of LDHA promotes cancer cell invasion and tumour metastasis. Oncogene. (2017) 36:3797–806. doi: 10.1038/onc.2017.6
90. Wu H, Wang Y, Ying M, Jin C, Li J, Hu X. Lactate dehydrogenases amplify reactive oxygen species in cancer cells in response to oxidative stimuli. Signal Transduct Target Ther. (2021) 6:242. doi: 10.1038/s41392-021-00595-3
91. Zhang W, Wang C, Hu X, Lian Y, Ding C, Ming L. Inhibition of LDHA suppresses cell proliferation and increases mitochondrial apoptosis via the JNK signaling pathway in cervical cancer cells. Oncol Rep. (2022) 47(4):77. doi: 10.3892/or.2022.8288
92. Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. (2013) 123:3685–92. doi: 10.1172/JCI69741
93. Vettraino M, Manerba M, Govoni M, Di Stefano G. Galloflavin suppresses lactate dehydrogenase activity and causes MYC downregulation in Burkitt lymphoma cells through NAD/NADH-dependent inhibition of sirtuin-1. Anti-cancer Drugs. (2013) 24:862–70. doi: 10.1097/CAD.0b013e328363ae50
94. Elia I, Rossi M, Stegen S, Broekaert D, Doglioni G, van Gorsel M, et al. Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature. (2019) 568:117–21. doi: 10.1038/s41586-019-0977-x
95. Brisson L, Bański P, Sboarina M, Dethier C, Danhier P, Fontenille MJ, et al. Lactate dehydrogenase B controls lysosome activity and autophagy in cancer. Cancer Cell. (2016) 30:418–31. doi: 10.1016/j.ccell.2016.08.005
96. Lin J, Liu G, Chen L, Kwok HF, Lin Y. Targeting lactate-related cell cycle activities for cancer therapy. Semin Cancer Biol. (2022) 86:1231–43. doi: 10.1016/j.semcancer.2022.10.009
97. Heuser C, Renner K, Kreutz M, Gattinoni L. Targeting lactate metabolism for cancer immunotherapy - a matter of precision. Semin Cancer Biol. (2023) 88:32–45. doi: 10.1016/j.semcancer.2022.12.001
98. Liao ZX, Kempson IM, Hsieh CC, Tseng SJ, Yang PC. Potential therapeutics using tumor-secreted lactate in nonsmall cell lung cancer. Drug Discovery Today. (2021) 26:2508–14. doi: 10.1016/j.drudis.2021.07.014
99. Huang HK, Lee SY, Huang SF, Lin YS, Chao SC, Huang SF, et al. Isoorientin decreases cell migration via decreasing functional activity and molecular expression of proton-linked monocarboxylate transporters in human lung cancer cells. Am J Chin Med. (2020) 48:201–22. doi: 10.1142/S0192415X20500111
100. Buyse C, Joudiou N, Warscotte A, Richiardone E, Mignion L, Corbet C, et al. Evaluation of syrosingopine, an MCT inhibitor, as potential modulator of tumor metabolism and extracellular acidification. Metabolites. (2022) 12(6):557. doi: 10.3390/metabo12060557
101. Benjamin D, Colombi M, Hindupur SK, Betz C, Lane HA, El-Shemerly MY, et al. Syrosingopine sensitizes cancer cells to killing by metformin. Sci Adv. (2016) 2:e1601756. doi: 10.1126/sciadv.1601756
102. Dana P, Saisomboon S, Kariya R, Okada S, Obchoei S, Sawanyawisuth K, et al. CD147 augmented monocarboxylate transporter-1/4 expression through modulation of the Akt-FoxO3-NF-κB pathway promotes cholangiocarcinoma migration and invasion. Cell Oncol (Dordrecht). (2020) 43:211–22. doi: 10.1007/s13402-019-00479-3
103. Sprowl-Tanio S, Habowski AN, Pate KT, McQuade MM, Wang K, Edwards RA, et al. Lactate/pyruvate transporter MCT-1 is a direct Wnt target that confers sensitivity to 3-bromopyruvate in colon cancer. Cancer Metab. (2016) 4:20. doi: 10.1186/s40170-016-0159-3
104. Pan L, Feng F, Wu J, Fan S, Han J, Wang S, et al. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol Res. (2022) 181:106270. doi: 10.1016/j.phrs.2022.106270
105. Xu H, Li L, Wang S, Wang Z, Qu L, Wang C, et al. Royal jelly acid suppresses hepatocellular carcinoma tumorigenicity by inhibiting H3 histone lactylation at H3K9la and H3K14la sites. Phytomedicine. (2023) 118:154940. doi: 10.1016/j.phymed.2023.154940
106. Talasniemi JP, Pennanen S, Savolainen H, Niskanen L, Liesivuori J. Analytical investigation: assay of D-lactate in diabetic plasma and urine. Clin Biochem. (2008) 41:1099–103. doi: 10.1016/j.clinbiochem.2008.06.011
107. de Bari L, Atlante A, Armeni T, Kalapos MP. Synthesis and metabolism of methylglyoxal, S-D-lactoylglutathione and D-lactate in cancer and Alzheimer’s disease. Exploring the crossroad of eternal youth and premature aging. Ageing Res Rev. (2019) 53:100915. doi: 10.1016/j.arr.2019.100915
108. Drabkin M, Yogev Y, Zeller L, Zarivach R, Zalk R, Halperin D, et al. Hyperuricemia and gout caused by missense mutation in d-lactate dehydrogenase. J Clin Invest. (2019) 129:5163–8. doi: 10.1172/JCI129057
109. McDonald B, Zucoloto AZ, Yu IL, Burkhard R, Brown K, Geuking MB, et al. Programing of an Intravascular Immune Firewall by the Gut Microbiota Protects against Pathogen Dissemination during Infection. Cell Host Microbe. (2020) 28:660–668.e664. doi: 10.1016/j.chom.2020.07.014
110. de Bari L, Moro L, Passarella S. Prostate cancer cells metabolize d-lactate inside mitochondria via a D-lactate dehydrogenase which is more active and highly expressed than in normal cells. FEBS Lett. (2013) 587:467–73. doi: 10.1016/j.febslet.2013.01.011
111. Han S, Bao X, Zou Y, Wang L, Li Y, Yang L, et al. d-lactate modulates M2 tumor-associated macrophages and remodels immunosuppressive tumor microenvironment for hepatocellular carcinoma. Sci Adv. (2023) 9:eadg2697. doi: 10.1126/sciadv.adg2697
112. Lv M, Gong Y, Liu X, Wang Y, Wu Q, Chen J, et al. CDK7-YAP-LDHD axis promotes D-lactate elimination and ferroptosis defense to support cancer stem cell-like properties. Signal Transduct Target Ther. (2023) 8:302. doi: 10.1038/s41392-023-01555-9
113. Wang Y, Li G, Wan F, Dai B, Ye D. Prognostic value of D-lactate dehydrogenase in patients with clear cell renal cell carcinoma. Oncol Lett. (2018) 16:866–74. doi: 10.3892/ol.2018.8782
114. Meng X, Baine JM, Yan T, Wang S. Comprehensive analysis of lysine lactylation in rice (Oryza sativa) grains. J Agric Food Chem. (2021) 69:8287–97. doi: 10.1021/acs.jafc.1c00760
115. Wen J, Cheng S, Zhang Y, Wang R, Xu J, Ling Z, et al. Lactate anions participate in T cell cytokine production and function, Science China. Life Sci. (2021) 64:1895–905. doi: 10.1007/s11427-020-1887-7
Keywords: lactate, tumor microenvironment, Warburg effect, immunosuppression, cancer immunotherapy
Citation: Sun K, Shen Y, Xiao X, Xu H, Zhang Q and Li M (2024) Crosstalk between lactate and tumor-associated immune cells: clinical relevance and insight. Front. Oncol. 14:1506849. doi: 10.3389/fonc.2024.1506849
Received: 06 October 2024; Accepted: 06 November 2024;
Published: 29 November 2024.
Edited by:
Sandip Patil, Shenzhen Children’s Hospital, ChinaReviewed by:
Johann Matschke, Essen University Hospital, GermanyCopyright © 2024 Sun, Shen, Xiao, Xu, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Li, bWluZ2xpQG5qbXUuZWR1LmNu; Quanli Zhang, MTU5NTEwODI4MTZAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.