
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 24 December 2024
Sec. Head and Neck Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1506840
 Gunnar Wichmann1,2,3†
Gunnar Wichmann1,2,3† Theresa Wald1,2*†
Theresa Wald1,2*† Veit Zebralla1,2
Veit Zebralla1,2 Matthaeus Stoehr1,2
Matthaeus Stoehr1,2 Markus Pirlich1,2,3
Markus Pirlich1,2,3 Susanne Wiegand4
Susanne Wiegand4 Viktor Kunz1,2‡
Viktor Kunz1,2‡ Andreas Dietz1,2,3‡
Andreas Dietz1,2,3‡Introduction: The larynx organ preservation (LOP) trial DeLOS-II enrolled n = 173 patients with advanced laryngeal/hypopharyngeal squamous cell carcinoma (LHSCC) amenable (only curatively resectable) through total laryngectomy (TL) to receive induction chemotherapy (IC) with TPF [docetaxel (T), cisplatin (P), and 5-fluorouracil (F)] (arm A, 85 patients) or additional cetuximab (E) weekly (arm B, 88 patients). Responders with endoscopic estimated tumor surface shrinkage (ETSS) ≥30% after 1 cycle IC (IC-1) received a further two cycles of IC followed by radiotherapy (RT), whereas TL was recommended for non-responders. Arm B failed to show superior 24-month laryngectomy-free survival (LFS) and overall survival (OS), the protocol-specified primary and secondary endpoints. Ten years after the last per-protocol visit, we are interested in the long-term outcome of our clinic’s DeLOS-II patients.
Methods: Our cohort of 52 DeLOS-II patients accrued between 2007 and 2012 included 27 and 25 patients randomized to arms A and B, respectively. F was omitted because of severe toxicity with amendment 2 of the DeLOS-II protocol, leading to 21 and 31 patients receiving TPF and TP IC backbone, respectively. Follow-up data were collected using electronic health records and information from the German Centre for Cancer Registry Data to evaluate long-term LFS and OS in treatment groups.
Results: According to ETSS ≥ 30%, 42 patients (80.8%; 21 and 21 corresponding to 77.8% and 84.0% in arms A and B, respectively) were responders to IC-1 and underwent the LOP attempt. Recommending early TL to non-responders (ETSS < 30%), eight patients (five and three in A and B, respectively) underwent early TL. At 125 months, 22 (eight and 14) patients were alive: 17 (six and 11) with a functioning larynx and five (two and three) without a larynx. Arm B had superior OS (p = 0.023). Disease-specific survival (DSS) and tumor-specific survival were not different, whereas non-cancer-related survival (NCRS) was impaired in arm A (p = 0.018). Receiving TP or TPF IC did not significantly influence survival. Pairwise comparing OS of patients receiving TP, TPF, TPE, and TPFE revealed a benefit from cetuximab in TPE vs. TP (p = 0.020).
Conclusion: While the per-protocol DeLOS-II results earlier reported comparable 24-month LFS and OS in arms A and B, our subcohort’s long-term follow-up data demonstrate a superior 125-month outcome in arm B.
The treatment of locally advanced (LA) laryngeal/hypopharyngeal squamous cell carcinoma (LHSCC) is challenging concerning survival and functional outcomes, including talking, swallowing, and breathing. Many tumors are solely completely (R0)–resectable via total laryngectomy (TL) and neck dissection (ND), potentially followed by adjuvant therapy [i.e., postoperative radiotherapy without (PORT) or with concomitant platinum-based chemotherapy (PORCT)]. Concurrent chemoradiation (CRT) with cisplatin (1, 2) and induction chemotherapy with PF (cisplatin and 5-fluorouracil) followed by radiotherapy (3) or TPF (docetaxel or another taxane combined with PF) followed by radiotherapy (4–7) are well-established alternatives to TL + ND + POR(C)T, at least for selected patients with rather small cT3 LHSCC and limited locoregional spread, preferably cN0 or cN1 (8, 9). Induction chemotherapy (IC) + RT and CRT offer the possibility of larynx organ preservation (LOP) by avoiding ablative surgery (1–10). Both IC + RT and CRT are accepted as alternative LOP approaches and are capable of preserving the larynx and its function in a substantial proportion of patients (1–10). In cT4a tumors, however, a surgical approach is recommended, as CRT was found to be associated with decreased survival and high rates of severe late toxicity (11, 12).
Several LOP trials explored IC in patients amenable to surgical treatment with TL (3–7). Inspired by the results of the Bonner trial that demonstrated a benefit from adding cetuximab (Erbitux®, E) to radiotherapy (13), the German LOP trial DeLOS-II (14) investigated E added to IC + RT. The objective of DeLOS-II was to evaluate the effect of adding cetuximab (loading dose 400 mg/m2 followed by 250 mg/m2 weekly for up to 6 months) to IC with docetaxel (T; 75 mg/m2 on day 1), cisplatin (P; 75 mg/m2 on day 1), and 5-fluorouracil (5-FU; 750 mg/m2 on days 1–5; later omitted from the protocol because of severe toxicity). In DeLOS-II, 173 patients were treated with one cycle IC followed by early response evaluation (ERE) using the newly established criterion of endoscopic estimated tumor surface shrinkage (ETSS) ≥30% to discriminate responders and patients with insufficient response (non-responders). Responders with ETSS ≥ 30% additionally received two cycles of IC followed by radiotherapy (RT). Non-responders received the recommendation for TL + ND followed by adjuvant treatment according to the recommendation of the local multidisciplinary tumor board (MDTB). The primary endpoint was 24-month laryngectomy-free survival (LFS), and the secondary endpoints were 24-month overall survival (OS) and 6- and 24-month functional LFS (fLFS), early response after IC-1, toxicity, and complications during and after salvage surgery (14). The primary objective (24-month LFS significantly above 35%) was equally met by both treatment arms (with and without cetuximab). The 24-month OS did not differ significantly between arms (14, 15). This, however, suggested the absence of significantly beneficial effects of additional cetuximab. Within an earlier subgroup analysis conducted after approximately 5 years of follow-up, we also reported slightly different outcomes between patients of arm A vs. arm B favoring (15). Now, 12 years after enrolling the last LA-LHSCC patient in DeLOS-II, we evaluated long-term follow-up data of the subgroup of n = 52 treated in our University Hospital and compared their outcomes with those achieved by each of the three alternative guideline-conform treatments, TL + ND + PORT, TL + ND + PORCT, and cisplatin-based CRT (16). We found DeLOS-II to be superior regarding the achieved outcome in general and could provide evidence for this superiority based on a comparison of propensity score (PS)-matched cases (16). Here, we provide additional data about the outcome differences among n = 52 DeLOS-II patients according to IC backbone, TPF vs. TP, the efficacy of cetuximab (arm B vs. arm A), and the different outcomes achieved by the four treatment regimens: TPF vs. TPFE vs. TP vs. TPE.
Originally, 173 patients were treated according to the DeLOS-II protocol at 22 study centers with the University Hospital Leipzig recruiting 52 patients, the highest number per study center. Detailed information concerning the study protocol is published elsewhere (14).
In brief, the study protocol of DeLOS-II utilized the first cycle of IC (IC-1) consisting of docetaxel (75 mg/m2; T) and cisplatin (75 mg/m2; P) on day 1 and 5-fluorouracil (750 mg/m2; F) on days 1–5 for response evaluation. F was later omitted from the protocol because of severe toxicity. Three weeks after IC-1, an endoscopy under general anesthesia was performed aiming for an estimation of the ETSS. Patients achieving ETSS ≥ 30% were considered responders and received a further two cycles of IC, whereas to poorly responding LHSCC patients with ETSS < 30% or progressing disease, early TL was recommended. Patients randomized into arm B received the identical treatment but additionally E [400 mg/m2 loading dose (day 1) followed by 250 mg/m2 weekly over 16 weeks in total].
Long-term follow-up data of these 52 patients were collected using electronic health records and information provided by the German Centre for Cancer Registry Data to evaluate 10-year survival including OS, tumor-specific survival (TSS), non-cancer-related survival (NCRS), and LFS. For statistical analyses, SPSS (SPSS version 29, IBM Corporation, Armonk, NY, USA) was used. Univariate analyses of categorical data included Pearson’s chi-squared tests and Fisher’s exact test, while cardinal-metric data were compared using homo- or heteroscedastic Student’s t-tests. The Kaplan–Meier cumulative survival plots (17), log-rank tests (18), Cox proportional hazards regression (19) utilizing the conditional logistic regression forward method, and bootstrapping (20) were used to analyze time-to-event data, LFS, OS, disease-specific survival (DSS), TSS, and NCRS. All time-to-event data were calculated from the time of randomization to the event (14, 15). Events were considered death from all causes (OS), death caused by head and neck squamous cell carcinoma (HNSCC) (DSS), death caused by any malignant tumor (HNSCC or cancer of other histology; TSS), or death caused by non-cancer-specific (other than cancer-related) reasons (NCRS), whereas patients alive or without the specified event were right-censored at time of last follow-up. Gaining more importance within the course of follow-up duration, NCRS was reported. Laryngectomy-free survival was calculated from the time of diagnosis to TL. Two-sided p < 0.05 was considered significant.
The characteristics of the 52 DeLOS-II patients treated at the University Hospital Leipzig are shown in Table 1, representing 30.1% of the whole study population with 173 patients (14). According to stratification factors in randomization (T4 vs. other, N0/N1 vs. N2/N3, and larynx vs. hypopharynx), arms A and B are not different regarding these covariates and IC backbone TPF vs. TP (all p ≥ 0.404) and also with respect to age categories, sex, smoking, alcohol consumption, and comorbidities when applying the Charlson score (all p ≥ 0.220).
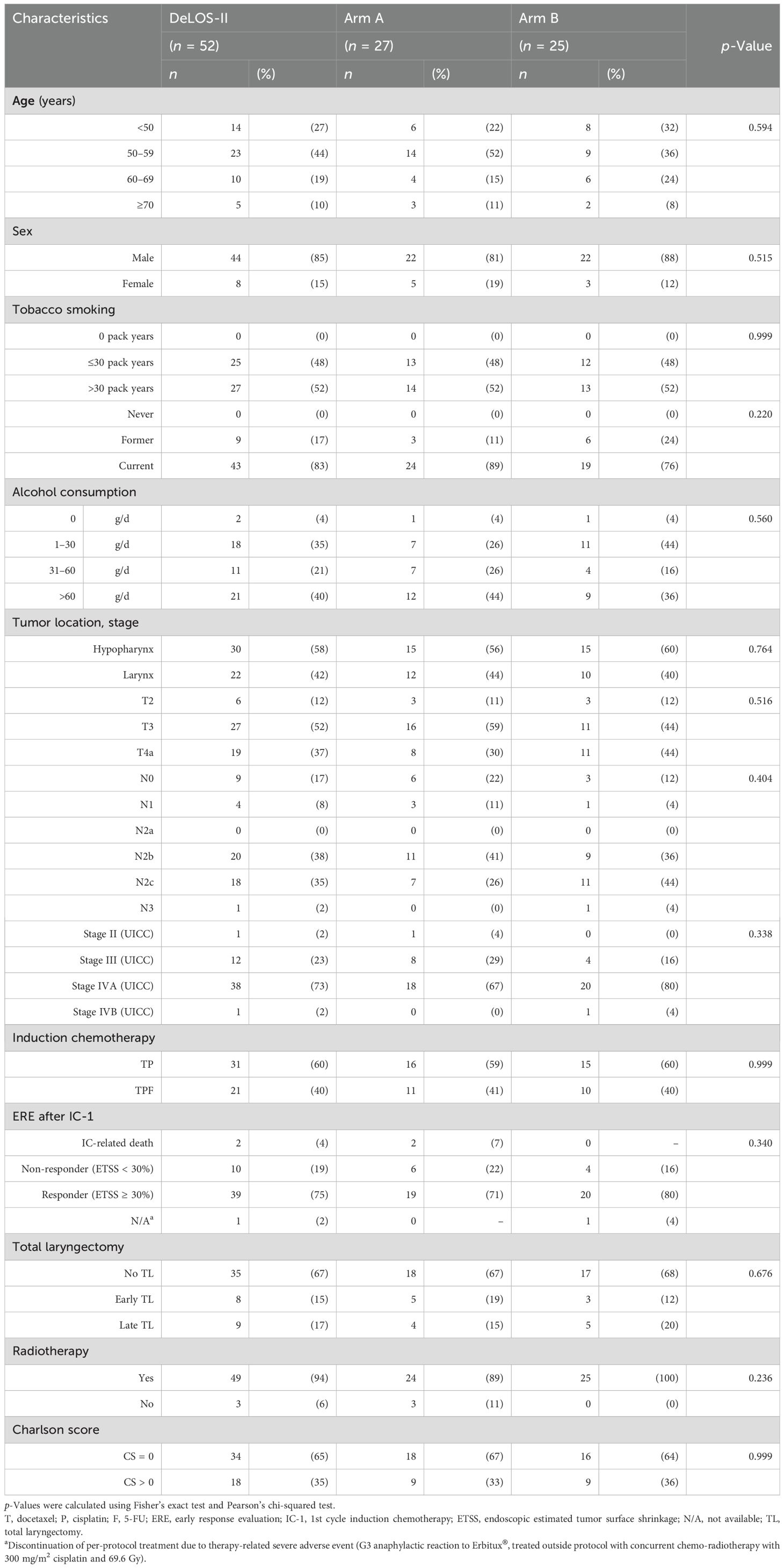
Table 1. Characteristics of the 52 DeLOS-II patients treated at the University Hospital Leipzig from 2007 to 2012 in arms A and B of the DeLOS-II trial.
Overall, the common risk factors including the Charlson comorbidity index were equally distributed among arms A and B, allowing for a reliable comparison of outcome in arm A vs. arm B.
Of 52 patients, 25 patients were randomized to the study arm (arm B) with the addition of cetuximab weekly to the regular treatment with TP(F) IC and 27 into arm A (control). A total of 39 patients were considered to be responders to IC-1 according to ETSS ≥ 30% in early response evaluation and consequently selected for the LOP attempt and further IC cycles. Recommending early TL to non-responders with ETSS < 30% after IC-1 (n = 6 in arm A and n = 4 in arm B), five patients in arm A and three patients in arm B underwent early TL. In five cases, TL within 12 months (i.e., salvage TL) was performed. In total, n = 9 patients received late TL within 24 months of follow-up time.
Within 3,769 months of follow-up (mean 72.5, median 63.2, SD 49.8, range 1.6–148 months), 36 patients died (61.5%): 11 patients died from cancer-associated reasons, 21 from non-cancer-related reasons, and four died from another tumor entity.
Patients receiving cetuximab (arm B) had improved 125-month OS compared to those without (p = 0.023; Figure 1). The mean OS in arm A was 64.2 (95% CI 46.1–82.3) months, while the median OS was 56.9 (95% CI 26.1–87.7) months; the mean OS in arm B was 92.2 (95% CI 75.7–108.7) months, while the median OS was not reached. DSS and TSS did not differ significantly between arms. However, NCRS was inferior in arm A (without E; p = 0.018). The Kaplan–Meier curves for OS, DSS, TSS, and NCRS for arm A vs. arm B are shown in Figure 1.
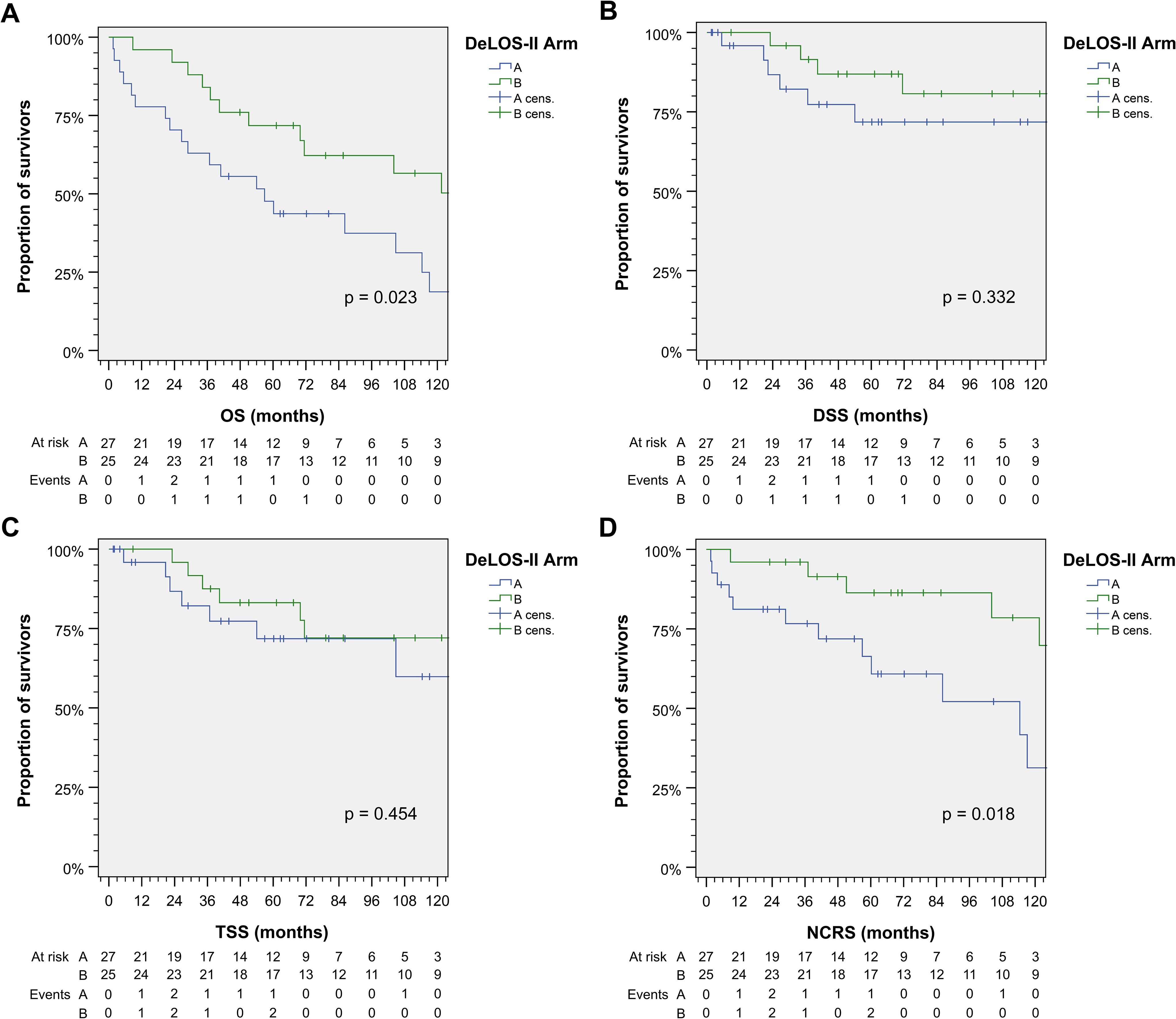
Figure 1. Outcome of DeLOS-II patients according to the treatment arm A vs. arm B. Kaplan–Meier cumulative survival plots for (A) overall survival (OS), (B) disease-specific survival (DSS), (C) tumor-specific survival (TSS), and (D) non-cancer-related survival (NCRS) among 52 DeLOS-II patients including patients at risk and number of events are shown together with p-values from two-sided log-rank tests.
In contrast to significant effects from cetuximab, receiving TP or TPF as chemotherapy backbone did not influence survival significantly (Figure 2). As 5-FU caused severe acute toxicity and even therapy-related deaths, it was omitted from the study protocol during the course of the trial. There obviously were differences between TPF and TP according to slight superiority in TSS and DSS, whereas NCRS (based on the attribution of the early deaths as treatment-related) was inferior. Overall, there was no survival benefit detectable from TPF vs. TP.
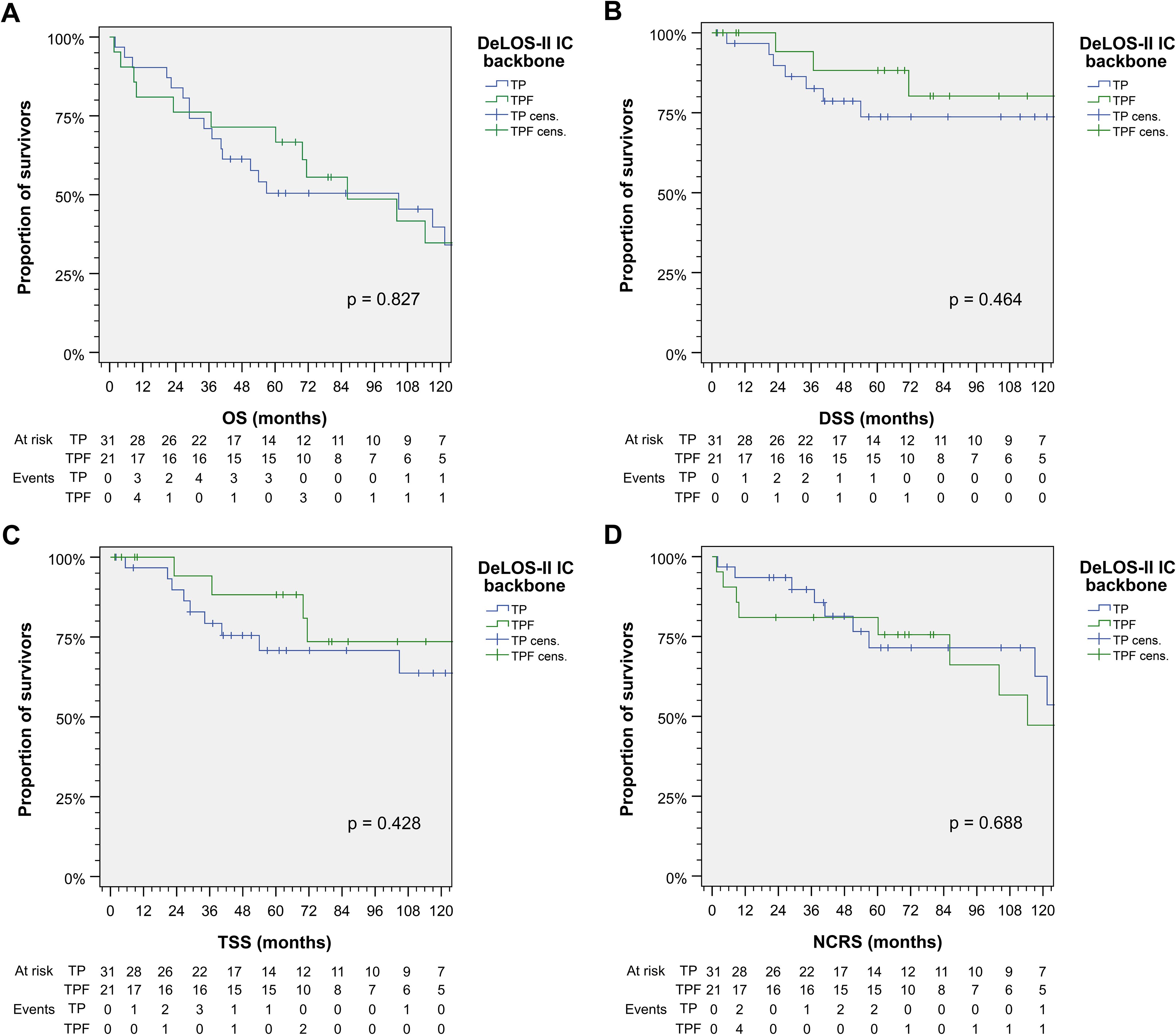
Figure 2. Outcome of DeLOS-II patients according to the induction-chemotherapy backbone TPF vs. TP. Kaplan–Meier cumulative survival plots for (A) overall survival (OS), (B) disease-specific survival (DSS), (C) tumor-specific survival (TSS), and (D) non-cancer-related survival (NCRS) among 52 DeLOS-II patients including patients at risk and number of events are shown together with p-values from two-sided log-rank tests. T, docetaxel; P, cisplatin; F, 5-fluorouracil.
Figure 3 shows cumulative survival plots for the exact treatment (TP vs. TPE vs. TPF vs. TPFE) according to OS, TSS, DSS, and NCRS. Patients receiving one of the four treatment combinations were found to have only insignificantly deviating outcomes regarding DSS, TSS, and NCRS (all p ≥ 0.129), whereas OS tended to be different (p = 0.096). Indeed, pairwise comparisons revealed that receiving TP resulted in an impaired OS compared to TPE (p = 0.020; Table 2).
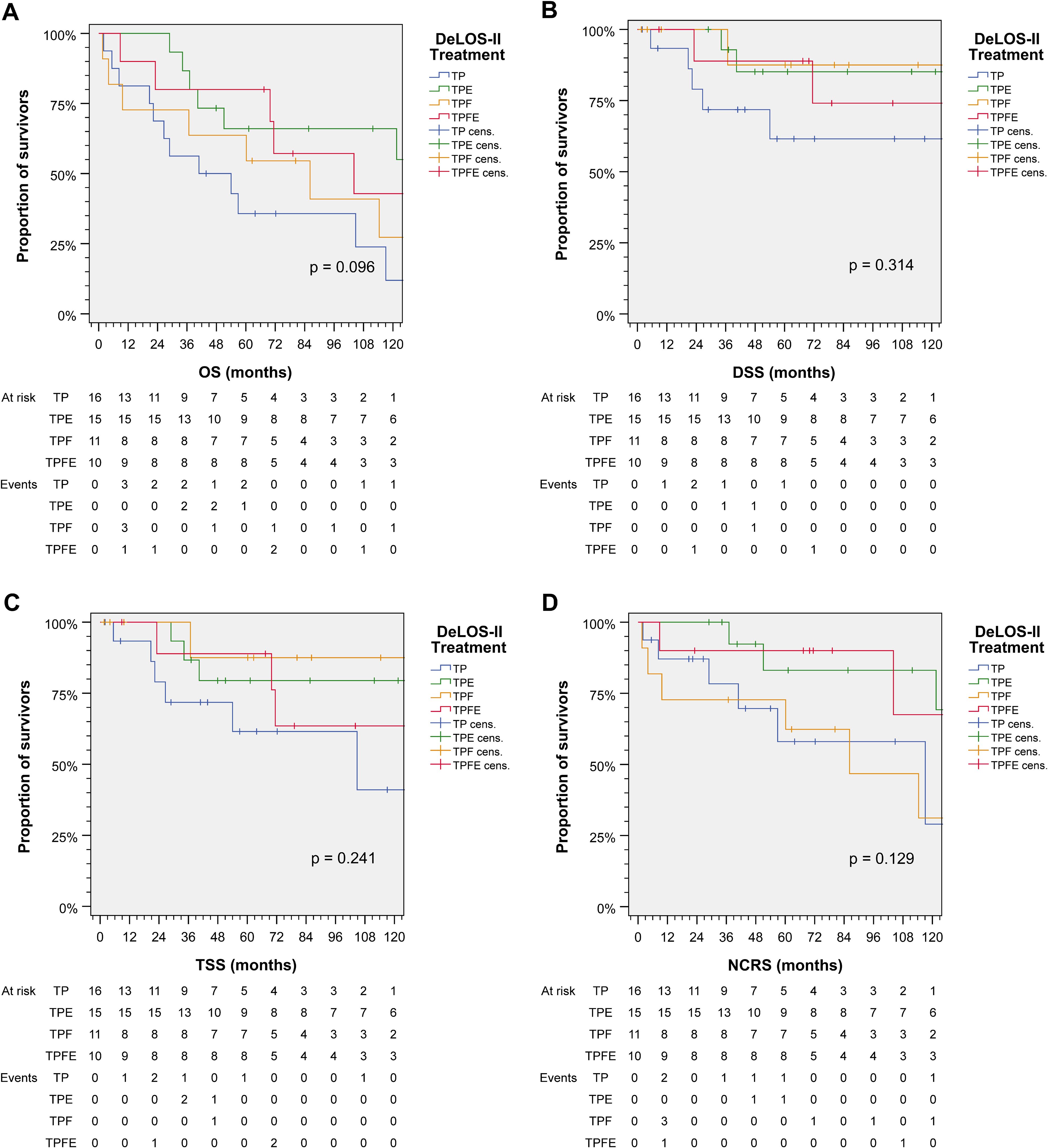
Figure 3. Outcome of DeLOS-II patients according to the exact treatment applied according to treatment arm A (without cetuximab) vs. B (receiving cetuximab) and induction-chemotherapy backbone TPF (before amendment 2) vs. TP (omission of 5-FU after amendment 2) resulting in TP, TPE, TPF, and TPFE. Kaplan–Meier cumulative survival plots for (A) overall survival (OS), (B) disease-specific survival (DSS), (C) tumor-specific survival (TSS), and (D) non-cancer-related survival (NCRS) among 52 DeLOS-II patients including patients at risk and number of events are shown together with p-values from two-sided log-rank tests. T, docetaxel; P, cisplatin; F, 5-fluorouracil; E, cetuximab.
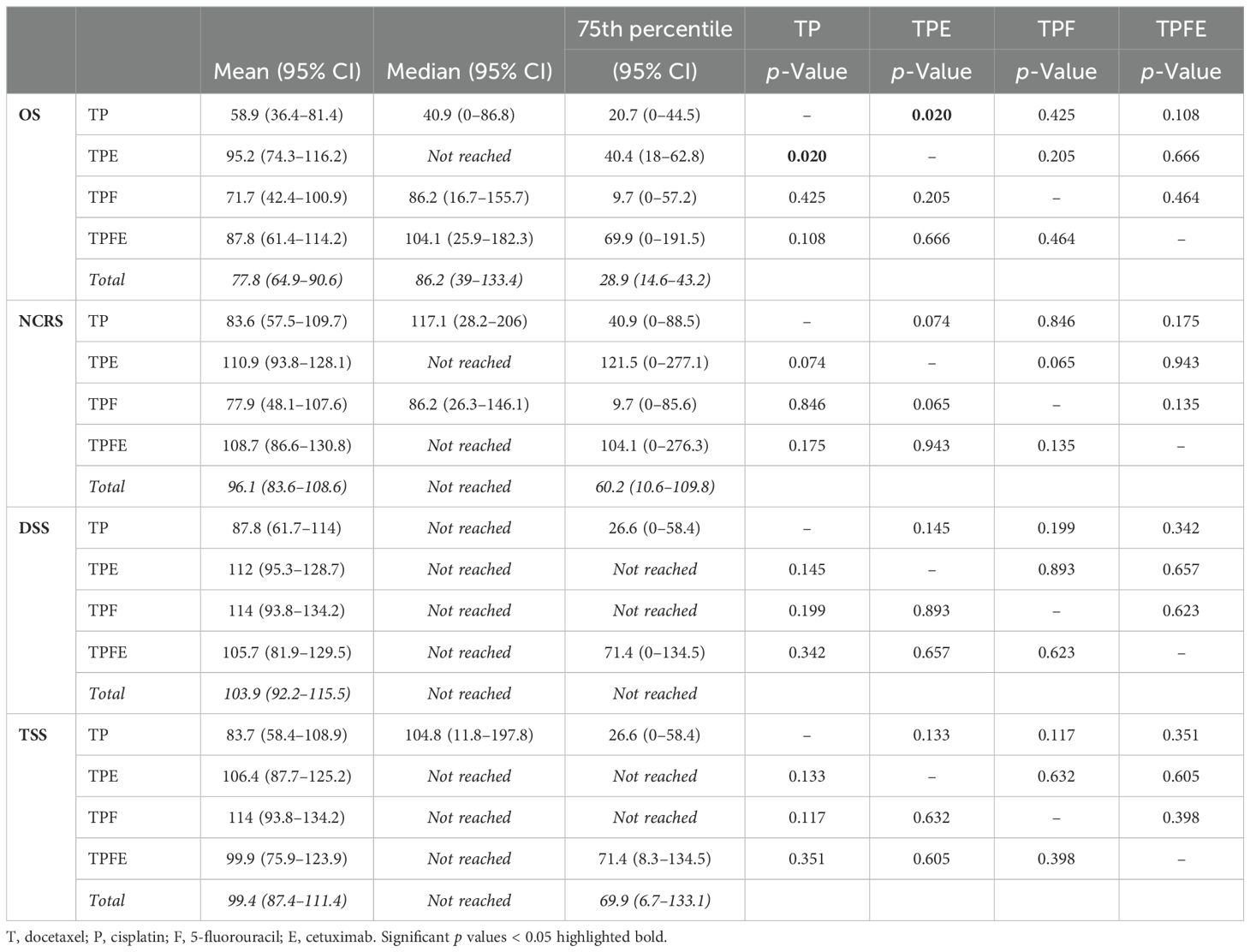
Table 2. Comparison of mean [95% confidence interval (95% CI)], median (95% CI), and 75th percentile (95% CI) of overall survival (OS), non-cancer-related survival (NCRS), disease-specific survival (DSS), and tumor-specific survival (TSS) in DeLOS-II patients according to treatment arm and induction-chemotherapy backbone and two-sided p-values from log-rank tests for pairwise comparison of Kaplan–Meier cumulative survival curves.
Comparing the mean and median of the four survival parameters OS, DSS, TSS, and NCRS, only TPE emerged as significantly superior to TP. However, we consistently detected numerically longer mean, median, and 75th percentiles for OS in TPFE and TPE (Table 2).
Comparing numbers and percentage of patients alive with a functioning larynx, alive after TL, or deceased with a larynx at place or after TL and summarizing data for either LOP or being alive after 125 months (Table 3), a consistent superiority of patients treated in arm B is obvious. Despite the low numbers of patients per treatment, a doubled frequency of patients alive at 125 months with a functioning larynx (44%) and 56% survivors in arm B compared to 22% and 30% in arm A point to a benefit gained through addition of cetuximab to IC with TP(F) followed by RT.
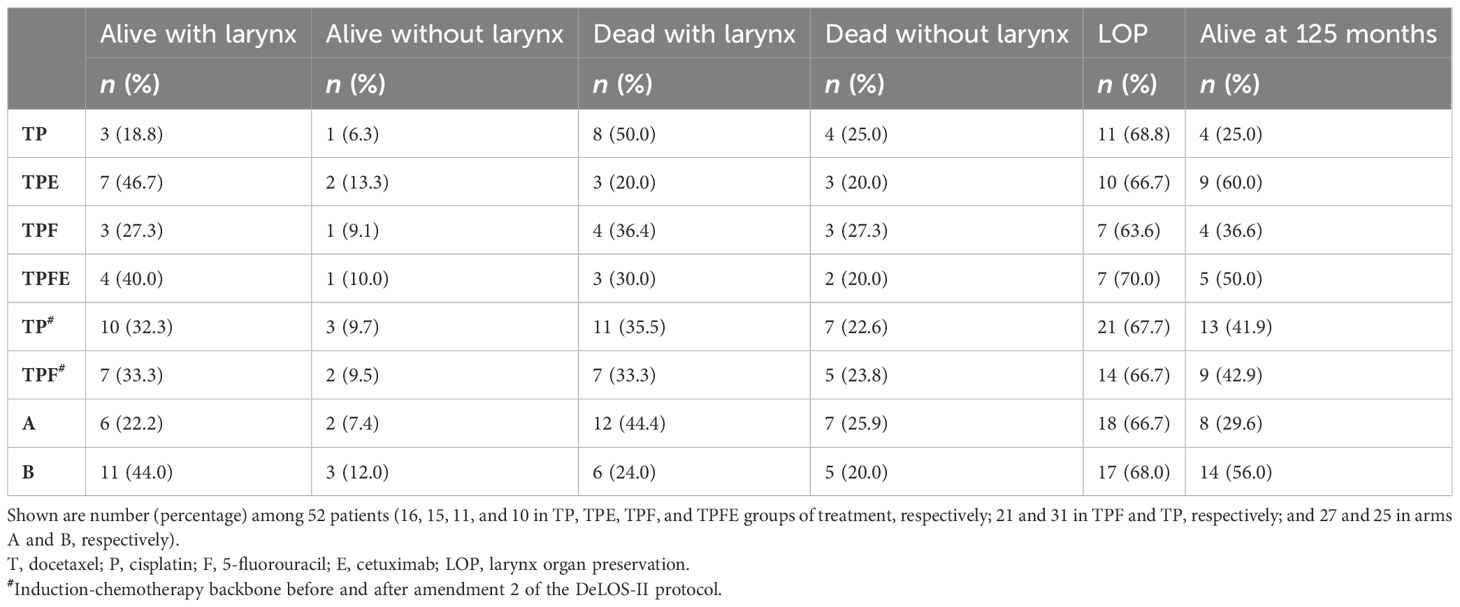
Table 3. Comparison of outcome of DeLOS-II patients 125 months after treatment indicated including larynx organ preservation and survival status at 125 months.
Now, available long-term follow-up data of 52 consecutively accrued DeLOS-II patients demonstrate superior OS of patients treated in arm B (p = 0.023) predominantly linked to improved NCRS (p = 0.018) due to reduced frequency of deaths from other causes, whereas DSS was numerically superior without reaching statistical significance. There were no significant differences in OS, TSS, DSS, and NCRS (all p ≥ 0.428) related to the IC backbone, TPF versus TP. However, we detected significant survival differences in the orthogonal analysis of arm vs. IC backbone with, for example, superior OS achieved by TPE compared to TP (Tables 2, 3).
Altogether, patients presenting with LA LHSCC have an unfavorable outcome. In many cases, TL is the only surgical technique to achieve a complete tumor resection. Because of the need for a tracheostomy, the loss of the natural voice function after TL, and the presumably negative impact on quality of life, LOP attempts are of great interest to ear, nose and throat (ENT) surgeons and oncologists as well as their patients. CRT is a comparable alternative to TL followed by adjuvant therapy, except for patients with cT4a LHSCC (11, 12), as they seem to experience higher recurrence rates when treated with CRT instead of TL + adjuvant therapy. In contrast, within analyses of the DeLOS-II trial data, survival and LOP rates were not inferior for all patients with T4 tumors (14, 15), provided an ETSS ≥ 30% in ERE in week 4 after IC-1 is achieved, and can be recommended also for T4 hypopharynx cancer. As prospective comparisons between the different treatment protocols are rare [except for the Radiation Therapy Oncology Group (RTOG) 91-11 study (1, 2, 21)], a propensity score-matched analysis of patients treated with Op + PORT, Op + PORCT, CRT, or the DeLOS-II protocol showed the non-inferiority (by showing superiority of DELOS-II regarding OS, TSS, and NCRS) of the LOP protocol according to DeLOS-II (16), questioning the non-inferiority of CRT compared to which TL once more, as discussed by Licitra et al. (21, 22).
Within the DeLOS-II trial aiming at LOP, IC followed by RT in responders after response evaluation after IC-1 and TL followed by adjuvant therapy in non-responders were recommended to patients with LA LHSCC. While ERE and achieving ETSS ≥ 30% were highly predictive for cure and LOP (14, 15), even survival of non-responders treated with early TL after IC-1 was superior to that of CRT or TL + PORT and equal to that of TL + PORCT (16). Moreover, ERE after IC-1 helps prevent prolonged administration of an ineffective treatment to the patient and helps prevent salvage surgery with its well-known high complication rates and impossibility of curatively resecting the cancer in inappropriately high frequency (23).
As reported for the per-protocol defined outcome analysis of the whole DeLOS-II trial after 24 months, the study arm with patients receiving cetuximab failed to be superior to the standard arm without cetuximab. The primary endpoint 24-month LFS [both arms with LFS > 35%, arm A (40/85, 47.1%) and arm B (41/88, 46.6%)] and the secondary endpoint 24-month OS (arm A 68.2% and arm B 69.3%) were not different. This, however, could be related to the per-protocol defined 24-month follow-up that could be too short to identify outcome differences and even more long-term outcomes of patients.
In the present study, we provide long-term follow-up data of the patients treated at the University Hospital Leipzig participating in the DeLOS-II study. Surprisingly, after 60 months and especially after 125 months, patients who received cetuximab had significantly better OS than those without. There was no difference between IC with TP and IC with TPF. Analyzing DSS and TSS, patients benefitted from receiving E without statistical significance. In line with this finding, the frequency of relapse, distant metastasis and occurence of other malignancies demonstrate the same (Supplementary Table S1, available online). In the long-term, death from other, non-cancer-related causes becomes more impactful and differs significantly in our cohort with benefits for the patients who received cetuximab. In analyses of survival measures, competing risk factors have to be considered. Paying attention to this fact, we analyzed OS by censoring cancer-related deaths in addition to the commonly accepted right-censoring of patients alive at the last follow-up. As age- and senescence-related differences between arms or exact treatment were found to be without significance, the significant differences between arms regarding non-cancer-related survival by only calculating log-rank tests considering death from other causes (not related to cancer) as event appear to be important. Possible confounding of comorbidities was excluded here, as severe comorbidities were an exclusion criterion for DeLOS-II participation (14). Also retrospectively assessing the presence of any comorbidities with a known impact on survival by applying the Charlson score (24, 25), there was no difference between arms (Table 1). The causality of the observed association between NCRS and cetuximab remains unclear. Cetuximab enhances the antitumor immune response through the activation of NK cells and facilitates their interaction with tumor cells. Promising, but yet without approval, is the combination of cetuximab and pembrolizumab to overcome cetuximab resistance as shown in ex vivo experiments (26). Approved for HNSCC, the anti-epidermal growth factor receptor (anti-EGFR) monoclonal antibody cetuximab (E) is used for system treatment of recurrent or metastatic (R/M) HNSCC alone or in combination with PF [EXTREME (27–29)] or TP [TPExtreme (30) within the TREMPLIN-II study (31)], IC with TPF followed by radiochemotherapy with three more cycles P or bioradiotherapy with E weekly showed no significant differences in LOP and OS. However, because of a high dropout rate of 24% and thus, high selection bias, comparison with other LOP trials is questionable. TREMPLIN-II was conducted to choose an experimental arm to compare to the GORTEC 2000-01 trial (6, 32); as none of the arms was superior to the other, no further study in this regard was attempted.
Several studies on LOP using IC report similar OS rates. Mattei et al. (9) reported in a retrospective study with patients suffering from LA hypopharyngeal cancer only resectable via TL administered with TPF IC followed by RT with or without cisplatin or cetuximab a 5-year OS of 54%. In this study, the T4 category was a negative predictor for OS.
The milestone trial RTOG 91-11 (1, 2) prospectively randomized patients into different treatment regimens consisting of IC + RT, CRT, or RT alone between 1992 and 2000. They report a 5-year OS of 58.1% and a 10-year OS of 38.8% (2). LOP was 67.5% after 10-year follow-up (FU), matching exactly the above-reported LOP data in arms A and B in Table 3.
Pointreau et al. (6, 32) found 60-month OS of 51% and 120-month OS of 30% comparing survival rates of 213 patients administered with three cycles of IC with either TPF or PF followed by radio(chemo)therapy for responders or laryngectomy for non-responders. The authors recommend IC with TPF, as there were higher rates of LOP compared to PF only. This is in line with the findings of Vermorken et al., who described a survival benefit when applying TPF instead of PF only in patients with unresectable LHSCC (33).
However, the 60-month OS rate in the cohort of 52 DeLOS-II patients with 71.8% in arm B administered with TPE or TPFE was clearly superior. After 125-month FU, the OS rate was still 56.0%, differing significantly from the 29.6% in the TP/TPF group (arm A; Table 3) and according to the log-rank test (p = 0.023; Figure 1A), clearly surpassing the survival data of comparable studies mentioned above (1, 2, 22, 23, 31, 33).
The long-term outcomes of the complete cohort of DeLOS-II patients would have been desirable but unfortunately could not be obtained. Reflecting 30.1% (52/173) of the complete study cohort, our results still suggest a potential additional benefit when adding cetuximab to the IC, the RT, and 6 months in total to the DeLOS-II protocol. Further studies are needed, preferably randomized controlled trials (RCTs), to find out and evaluate the best treatment protocol for LOP attempts only via TL R0-resectable LHSCC patients. Other agents promising a treatment benefit should be examined. For instance, pembrolizumab is known and approved for the treatment of R/M HNSCC because of its antitumor effect that is enriched in HNSCC patients with a certain level of PD-L1 expression in their tumor. The tumor proportion score (TPS) is calculated as the percentage of cells staining positive for PD-L1 in immunohistochemistry among tumor cells, and the combined positive score (CPS) also considers immune cells (34); after KEYNOTE-048 (35), pembrolizumab is approved for R/M HNSCC with CPS ≥ 1 (which of course includes TPS ≥ 50%). KEYNOTE-689 investigates in LA HNSCC with CPS ≥ 1 the neoadjuvant treatment with two cycles of pembrolizumab upfront surgery followed by a further 15 cycles of pembrolizumab during adjuvant radiotherapy ± cisplatin (36). The European Larynx Organ Preservation Study [ELOS (37)] investigates the effect of pembrolizumab when added to IC with TP followed by RT according to the DeLOS-II protocol. It remains to be shown if replacing cetuximab with pembrolizumab enables higher LOP and OS rates.
One strength of our study is the high number of participants per subgroup accrued and treated within a prospectively designed RCT at a single University Hospital. This led to 1) homogeneity in decision-making for participation in the LOP trial according to uniform per-protocol defined inclusion and exclusion criteria, 2) random assignment to pre-defined treatment groups while stratifying according to characteristics/covariates with the highest impact on outcome, 3) response evaluation according to the protocol-defined ERE criteria by a well-trained single ENT surgeon familiar with LOP, 4) regular follow-up of patients according to the National Comprehensive Cancer Network (NCCN) and national guidelines, 5) regular monitoring of organ function and health-related quality of life utilizing patient-reported outcome measures (PROM) using OncoFunction (38, 39) to detect early signs of functional impairment, and 6) high quality of documentation attributed to study patients. In addition to these strengths, there are limitations that have to be considered. Our results were achieved in (according to inclusion and exclusion criteria) highly selected patients suffering from a rare disease. Despite allowing for the detection of significant differences between groups, the number of patients in each of the treatment groups was rather low with <30 patients in each subgroup analyzed. This increases the possibility of random effects and, in particular, increases the false-positive reporting probability (FPRP) of (additionally in tendency rather to high) effect estimates, as small samples favor the overrepresentation of extreme distributions. Rather few patients per treatment may have caused a positive reporting bias that we cannot exclude. An increased FPRP could even more be expected, as the exploratory analyses of outcome measures were not pre-planned/scheduled within the DeLOS-II protocol and especially were not considered in the power calculation for the DeLOS-II trial. Because of the rather low accrual of patients in the other 22 DeLOS-II study centers, we accrued the highest number of DeLOS-II patients treated in our center and had the unique opportunity to assess long-term outcomes with sufficiently high numbers of patients per treatment in a unique environment. Our patients were treated in a certified tertiary tumor center of excellence, the Comprehensive Cancer Center Central Germany (CCCG). The CCCG has an infrastructure allowing for high-quality patient-centered workflows with professionalized interaction of all disciplines involved in diagnostic, treatment, and follow-up monitoring of head and neck cancer patients, patients who are always a special group of LA LHSCC patients with special and often unmet needs. Assessing these special needs (38, 39) during prolonged follow-up and addressing them adequately may have contributed to the overall good outcome. Building such infrastructure requires time and dedicated professionals in each of the clinics and institutes involved but especially dedicated consultation hours regularly utilizing the adequate tools (38, 39). Such an environment could hardly be immediately established in any clinic. Therefore, the results reported here may not be representative of results that can be easily achieved anywhere by ad hoc starting the LOP approach for every patient with LA LHSCC amenable for TL by applying (potentially without the availability of the infrastructure required) the treatment according to the DeLOS-II protocol utilizing TPE IC. The ERE after IC-1 assessing ETSS for decision-making for treatment with either TL or further IC followed by radiotherapy requires familiarity with the approach and hence an adequate training.
However, the superior survival in DeLOS-II patients treated with cetuximab and especially TPE points to a so far not reported benefit from cetuximab in LOP approaches, which deserves further investigation, for instance, in a LOP trial combining TPE IC with blockade of the PD-1:PD-L1 immune checkpoint by, for example, pembrolizumab or another monoclonal anti-PD-1 antibody. The LOP approach may additionally benefit from molecular and genetic analyses (40–42). Even a tailored approach according to a comprehensive predictive classifier integrating clinical, molecular, and multiomics data for personalized treatment decisions enhancing response to IC, to achieve LOP in a further increased frequency of patients (43), has the potential to result in long-term survival with good health-related quality of life, altogether the ultimate goals of preventive, predictive, and personalized medicine.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the Medical Faculty of the University Leipzig (votes 166-07-12072006, 201–10-12072010, and 202-10-12072010). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
GW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TW: Data curation, Formal analysis, Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. VZ: Writing – review & editing. MS: Writing – review & editing. MP: Writing – review & editing. SW: Resources, Supervision, Validation, Writing – review & editing. VK: Supervision, Validation, Writing – review & editing. AD: Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Open Access Publishing Fund of Leipzig University.
We thank all patients and their families who participated in the investigation. We especially thank all contributing physicians for providing clinical data and the entire technical staff, all nurses, and physicians in the involved departments. We acknowledge support from Leipzig University for Open Access Publishing.
The authors GW, SW, and AD declare that financial support was received for the research, authorship, and/or publication of this article. DeLOS-II was an investigator-initiated larynx organ preservation, phase 2, randomized trial sponsored by Leipzig University and supported by unrestricted educational grants from Merck, Darmstadt, Germany, and Sanofi, Berlin, Germany. No grant number is applicable. Merck and Sanofi had no influence on the design of the study and collection, analysis, and interpretation of data and in writing this manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1506840/full#supplementary-material
1. Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. (2003) 349:2091–8. doi: 10.1056/NEJMoa031317
2. Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. (2013) 31:845–52. doi: 10.1200/JCO.2012.43.6097
3. Wolf GT, Fisher SG, Hong WK, Hillman R, Spaulding M, Laramore GE, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. (1991) 324:1685–90. doi: 10.1056/NEJM199106133242402
4. Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. (2007) 357:1695–704. doi: 10.1056/NEJMoa071028
5. Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. (2007) 357:1705–15. doi: 10.1056/NEJMoa070956
6. Pointreau Y, Garaud P, Chapet S, Sire C, Tuchais C, Tortochaux J, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. (2009) 101:498–506. doi: 10.1093/jnci/djp007
7. Dietz A, Rudat V, Dreyhaupt J, Pritsch M, Hoppe F, Hagen R, et al. Induction chemotherapy with paclitaxel and cisplatin followed by radiotherapy for larynx organ preservation in advanced laryngeal and hypopharyngeal cancer offers moderate late toxicity outcome (DeLOS-I-trial). Eur Arch Otorhinolaryngol. (2009) 266:1291–300. doi: 10.1007/s00405-008-0846-y
8. Pfister DG, Laurie SA, Weinstein GS, Mendenhall WM, Adelstein DJ, Ang KK, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. (2006) 24:3693–704. doi: 10.1200/JCO.2006.07.4559
9. Mattei P, Gal J, Chamorey E, Dassonville O, Poissonnet G, Aloi D, et al. Docetaxel-cisplatin-fluorouracil induction chemotherapy for larynx preservation in patients with locally advanced hypopharyngeal cancer: predictive factors of oncologic and functional outcomes. J Clin Med. (2023) 12. doi: 10.3390/jcm12031131
10. Lefebvre J-L, Ang KK. Larynx preservation clinical trial design: Key issues and recommendations–a consensus panel summary. Head Neck. (2009) 31:429–41. doi: 10.1002/hed.21081
11. Grover S, Swisher-McClure S, Mitra N, Li J, Cohen RB, Ahn PH, et al. Total laryngectomy versus larynx preservation for T4a larynx cancer: patterns of care and survival outcomes. Int J Radiat Oncol Biol Phys. (2015) 92:594–601. doi: 10.1016/j.ijrobp.2015.03.004
12. Rosenthal DI, Mohamed ASR, Weber RS, Garden AS, Sevak PR, Kies MS, et al. Long-term outcomes after surgical or nonsurgical initial therapy for patients with T4 squamous cell carcinoma of the larynx: A 3-decade survey. Cancer. (2015) 121:1608–19. doi: 10.1002/cncr.29241
13. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. (2006) 354:567–78. doi: 10.1056/NEJMoa053422
14. Dietz A, Wichmann G, Kuhnt T, Pfreundner L, Hagen R, Scheich M, et al. Induction chemotherapy (IC) followed by radiotherapy (RT) versus cetuximab plus IC and RT in advanced laryngeal/hypopharyngeal cancer resectable only by total laryngectomy-final results of the larynx organ preservation trial DeLOS-II. Ann Oncol. (2018) 29:2105–14. doi: 10.1093/annonc/mdy332
15. Wichmann G, Krüger A, Boehm A, Kolb M, Hofer M, Fischer M, et al. Induction chemotherapy followed by radiotherapy for larynx preservation in advanced laryngeal and hypopharyngeal cancer: Outcome prediction after one cycle induction chemotherapy by a score based on clinical evaluation, computed tomography-based volumetry and (18)F-FDG-PET/CT. Eur J Cancer. (2017) 72:144–55. doi: 10.1016/j.ejca.2016.11.013
16. Wichmann G, Wald T, Pirlich M, Stoehr M, Zebralla V, Kuhnt T, et al. Improved survival of locoregional-advanced larynx and hypopharynx cancer patients treated according to the DeLOS-II protocol. Front Oncol. (2024) 14:1394691. doi: 10.3389/fonc.2024.1394691
17. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. (1958) 53:457–81. doi: 10.1080/01621459.1958.10501452
18. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. (1966) 50:163–70.
19. Cox DR. Regression models and life-tables. Royal statistical society. J Ser B: Methodological. (1972) 34:187–202. doi: 10.1111/j.2517-6161.1972.tb00899.x
20. Moons KGM, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. (2015) 162:W1–73. doi: 10.7326/M14-0698
21. Dietz A, Wiegand S, Kuhnt T, Wichmann G. Laryngeal preservation approaches: considerations for new selection criteria based on the deLOS-II trial. Front Oncol. (2019) 9:625. doi: 10.3389/fonc.2019.00625
22. Licitra L, Bonomo P, Sanguineti G, Bacigalupo A, Baldi GG, Valerini S, et al. Different view on larynx preservation evidence-based treatment recommendations. J Clin Oncol. (2018) 36:1376–7. doi: 10.1200/JCO.2018.77.8001
23. van der Putten L, Bree R, Doornaert PA, Buter J, Eerenstein SE, Rietveld DH, et al. Salvage surgery in post-chemoradiation laryngeal and hypopharyngeal carcinoma: outcome and review. Acta Otorhinolaryngol Ital. (2015) 35:162–72.
24. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. (1994) 47:1245–51. doi: 10.1016/0895-4356(94)90129-5
25. Wald T, Birnbaum K, Wiegand S, Dietz A, Zebralla V, Wichmann G. Automatic calculation and visualization of comorbidity scores for decision-making in tumor boards. Laryngorhinootologie. (2020) 99:31–6. doi: 10.1055/a-1058-0171
26. Berszin M, Michaelides I, Siemert J, Röhl L, Wellhausen J, Wald T, et al. Cytokine profiles of head and neck squamous cell carcinoma undergoing dual immunotherapy with cetuximab and pembrolizumab identify interferon gamma-induced protein 10 as novel biomarker. Front Oncol. (2022) 12:795277. doi: 10.3389/fonc.2022.795277
27. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. (2008) 359:1116–27. doi: 10.1056/NEJMoa0802656
28. Taberna M, Oliva M, Mesía R. Cetuximab-containing combinations in locally advanced and recurrent or metastatic head and neck squamous cell carcinoma. Front Oncol. (2019) 9:383. doi: 10.3389/fonc.2019.00383
29. Lübbers K, Pavlychenko M, Wald T, Wiegand S, Dietz A, Zebralla V, et al. Choosing the right treatment option for the right R/M HNSCC patient: should we adhere to PFE for first-line therapy? Front Oncol. (2021) 11:715297. doi: 10.3389/fonc.2021.715297
30. Guigay J, Aupérin A, Fayette J, Saada-Bouzid E, Lafond C, Taberna M, et al. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. (2021) 22:463–75. doi: 10.1016/S1470-2045(20)30755-5
31. Lefebvre JL, Pointreau Y, Rolland F, Alfonsi M, Baudoux A, Sire C, et al. Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: the TREMPLIN randomized phase II study. J Clin Oncol. (2013) 31:853–9. doi: 10.1200/JCO.2012.42.3988
32. Janoray G, Pointreau Y, Garaud P, Chapet S, Alfonsi M, Sire C, et al. Long-term results of a multicenter randomized phase III trial of induction chemotherapy with cisplatin, 5-fluorouracil, ± Docetaxel for larynx preservation. J Natl Cancer Inst. (2016) 108. doi: 10.1093/jnci/djv368
33. Remenar E, van Herpen C, Germa Lluch J, Stewart S, Gorlia T, Degardin M, et al. A randomized phase III multicenter trial of neoadjuvant docetaxel plus cisplatin and 5-fluorouracil (TPF) versus neoadjuvant PF in patients with locally advanced unresectable squamous cell carcinoma of the head and neck (SCCHN). Final analysis of EORTC 24971. J Clin Oncol. (2006) 24:5516. doi: 10.1200/jco.2006.24.18_suppl.5516
34. Emancipator K, Huang L, Aurora-Garg D, Bal T, Cohen EE, Harrington K, et al. Comparing programmed death ligand 1 scores for predicting pembrolizumab efficacy in head and neck cancer. Mod Pathol. (2021) 34:532–41. doi: 10.1038/s41379-020-00710-9
35. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, Castro de G JR, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. (2019) 394:1915–28. doi: 10.1016/S0140-6736(19)32591-7
36. Uppaluri R, Campbell KM, Egloff AM, Zolkind P, Skidmore ZL, Nussenbaum B, et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-unrelated head and neck cancer: A multicenter, phase II trial. Clin Cancer Res. (2020) 26:5140–52. doi: 10.1158/1078-0432.CCR-20-1695
37. Wichmann G, Wald T, Pirlich M, Napp J, Münter I, Asendorf T, et al. The european larynx organ preservation study MK-3475-C44. Front Oncol. (2024) 14:1433238. doi: 10.3389/fonc.2024.1433238
38. Zebralla V, Müller J, Wald T, Boehm A, Wichmann G, Berger T, et al. Obtaining patient-reported outcomes electronically with "OncoFunction" in head and neck cancer patients during aftercare. Front Oncol. (2020) 10:549915. doi: 10.3389/fonc.2020.549915
39. Zebralla V, Wiegand S, Dietz A, Wichmann G, Neumuth T, Mehnert-Theuerkauf A, et al. Course of self-reported dysphagia, voice impairment and pain in head and neck cancer survivors. Biol (Basel). (2021) 10. doi: 10.3390/biology10020144
40. Wichmann G, Herchenhahn C, Boehm A, Mozet C, Hofer M, Fischer M, et al. HLA traits linked to development of head and neck squamous cell carcinoma affect the progression-free survival of patients. Oral Oncol. (2017) 69:115–27. doi: 10.1016/j.oraloncology.2017.04.017
41. Wichmann G, Lehmann C, Herchenhahn C, Kolb M, Hofer M, Wiegand S, et al. Development of a human leukocyte antigen score to predict progression-free survival in head and neck squamous cell carcinoma patients. Front Oncol. (2018) 8:168. doi: 10.3389/fonc.2018.00168
42. Wiegand S, Wichmann G, Dietz A. Perspectives of induction with chemo and/or immune check point inhibition in head and neck organ preservation treatment. Front Oncol. (2019) 9:191. doi: 10.3389/fonc.2019.00191
Keywords: head neck squamous cell carcinoma (HNSCC), locoregional advanced head and neck cancer (LA HNC), larynx cancer, hypopharynx cancer, larynx organ preservation, overall survival, neoadjuvant chemotherapy, cetuximab
Citation: Wichmann G, Wald T, Zebralla V, Stoehr M, Pirlich M, Wiegand S, Kunz V and Dietz A (2024) Superior 125-month outcome through cetuximab in the larynx organ preservation trial DeLOS-II: a single study center’s experience. Front. Oncol. 14:1506840. doi: 10.3389/fonc.2024.1506840
Received: 06 October 2024; Accepted: 04 December 2024;
Published: 24 December 2024.
Edited by:
Panagiotis Balermpas, University Hospital Zürich, SwitzerlandReviewed by:
Ali-Farid Safi, Craniologicum - Center for Craniomaxillofacial Surgery, SwitzerlandCopyright © 2024 Wichmann, Wald, Zebralla, Stoehr, Pirlich, Wiegand, Kunz and Dietz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Theresa Wald, dGhlcmVzYS53YWxkQG1lZGl6aW4udW5pLWxlaXB6aWcuZGU=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.