- Department of Oncology, Bishan Hospital of Chongqing Medical University, Chongqing, China
Background: Several head-to-head meta-analyses have compared the efficacy and safety of different first-line treatments in patients with EGFR mutation-positive (M+) advanced or metastatic non-squamous non-small cell lung cancer (nsq-NSCLC). However, there is a lack of comprehensive evaluation encompassing multiple treatment strategies. Our objective is to conduct a network meta-analysis that includes various treatment modalities, enabling both direct and indirect comparisons for a more thorough assessment.
Methods: We conducted a search of PubMed, Embase, Cochrane Library, and Web of Science databases from inception until May 8, 2024, to identify eligible randomized controlled trials (RCTs). The primary endpoints were progression-free survival (PFS) and overall survival (OS), while secondary outcomes included objective response rate (ORR) and grade 3 or higher adverse events (≥3AEs). Stata 15.0 and R 4.3.2 software were utilized for the network meta-analysis.
Results: A total of 30 RCTs, comprising 8654 participants, were included. The study encompassed the following 19 treatments: Chemotherapy; Afatinib; Afatinib + Cetuximab; Apatinib + Gefitinib; Befotertinib; Cetuximab + Chemotherapy; Erlotinib; Erlotinib + Bevacizumab; Erlotinib + Chemotherapy; Gefitinib; Gefitinib + Chemotherapy; Gefitinib + Olaparib; Icotinib; Icotinib + Chemotherapy; Lazertinib; Naquotinib; Osimertinib; Osimertinib + Bevacizumab; Osimertinib + Chemotherapy. The network meta-analysis results indicated that, in terms of PFS, Osimertinib + Chemotherapy (SUCRAs: 93.4%) and Osimertinib (SUCRAs: 84.61%) were the most effective. Regarding OS, Lazertinib (SUCRAs: 89.72%), Gefitinib (SUCRAs: 72.07%), and Osimertinib + Chemotherapy (SUCRAs: 70.74%) emerged as the top three options. Afatinib (SUCRAs: 92.27%) was associated with the best ORR improvement. For ≥3AEs, Afatinib (SUCRAs: 74.93%) and Osimertinib (SUCRAs: 69.42%) were likely the best choices.
Conclusion: Current evidence suggests that, considering both survival and safety, Osimertinib stands out as the preferred first-line treatment for untreated EGFR M + advanced or metastatic nsq-NSCLC. Notably, the combination of Osimertinib with chemotherapy demonstrated superior survival benefits. However, due to the limitations in the number and quality of included studies, these conclusions await further validation through more high-quality research.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024562981, identifier CRD42024562981.
1 Introduction
Lung cancer is a global health issue, with non-small cell lung cancer (NSCLC) being the most common type, accounting for approximately 80%-85% of cases. Most patients are diagnosed at an advanced stage (1, 2). About 70% of NSCLC cases are diagnosed at a late stage, characterized by poor prognosis, with a 5-year survival rate of only 26% (3). NSCLC can be further subdivided into two main histological subtypes: squamous NSCLC and non-squamous NSCLC, with the latter being the major subtype, accounting for 70%-75% of all NSCLC cases (4). In the progression of NSCLC, activating mutations in the epidermal growth factor receptor (EGFR) play a central role, particularly exon 19 deletions (ex19del) and exon 21 L858R mutations. Globally, the prevalence of EGFR mutations is approximately 32%, with a significantly higher prevalence in Asians (40%-60%) compared to Western NSCLC populations (10%-15%). Additionally, the mutation rate is higher in non-squamous NSCLC patients compared to squamous NSCLC patients (5, 6). The discovery of EGFR mutations has ushered in the era of targeted therapy for lung cancer, making tumor cells more sensitive to tyrosine kinase inhibitors (TKIs).
In recent years, first-line treatment options for patients with EGFR mutation-positive non-squamous NSCLC have undergone significant advancements. First-generation EGFR-TKIs (such as gefitinib, erlotinib, and icotinib) and second-generation EGFR-TKIs (such as afatinib and dacomitinib) have established their superiority over platinum-based chemotherapy in the clinical practice for untreated advanced EGFR-mutant NSCLC patients (7–10). However, most patients develop acquired resistance to these treatments within approximately one year, with the most common mechanism being the EGFR T790M mutation (11). To overcome T790M-mediated resistance, third-generation EGFR-TKIs, such as osimertinib and lorlatinib, have been developed. Osimertinib is effective not only against common EGFR-sensitive mutations but also selectively inhibits the T790M resistance mutation, demonstrating significant clinical efficacy and manageable safety (12–14). Furthermore, combination therapies are being explored in addition to monotherapy. For example, combinations of EGFR-TKIs with anti-angiogenic agents such as bevacizumab, immune checkpoint inhibitors, or platinum-based chemotherapy are being investigated to further improve objective response rates (ORR) and prolong progression-free survival (PFS) (15). However, there is ongoing debate on whether combination therapy should be established as the standard first-line treatment.
Despite a few head-to-head meta-analyses comparing the efficacy and safety of different first-line treatment options in patients with EGFR-mutant advanced non-squamous NSCLC, these analyses focus on pairwise comparisons and lack a comprehensive consideration of multiple treatment strategies (16, 17). Our aim is to conduct a network meta-analysis (NMA) that simultaneously includes multiple treatment modalities for direct and indirect comparisons, providing a more comprehensive efficacy evaluation to better support clinical decision-making.
2 Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting systematic reviews and meta-analyses, and their requirements for NMA (18). The study protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42024562981).
2.1 Search strategy
The search strategy combined subject terms and free terms, searching four electronic databases: PubMed, Web of Science, Cochrane Library, and Embase. The search covered the period from the establishment of the databases until May 8, 2024, with the language limited to English. Additionally, to minimize the risk of omission, we cross-referenced review articles and meta-analyses. The specific search strategy can be found in Supplementary Text 1 in Supplementary Data Sheet 3.
2.2 Inclusion and exclusion criteria
Studies meeting the following criteria were included in this research: (1) Subjects: Patients who were untreated and had activating EGFR mutations (commonly exon 19 deletions or exon 21 L858R mutations), histologically confirmed as advanced or metastatic (stage III-IV) non-squamous NSCLC, regardless of the presence of T790M or other rare EGFR mutations associated with lower sensitivity or resistance; (2) Interventions: Targeted therapies for EGFR mutations, immunotherapies, anti-angiogenic agents used alone or in combination; Comparators: comparisons between chemotherapy alone or other therapies; (3) Study Type: Randomized controlled trials (RCTs); (4) Outcomes: The primary outcomes were progression-free survival (PFS) and overall survival (OS); the secondary outcomes were objective response rate (ORR) and the incidence of grade 3 or higher adverse events (≥3AEs).
Studies were excluded if they met the following criteria: (1) Animal or cell experiments, case reports, scientific study protocols, reviews, letters, editorials, conference papers, etc.; (2) Studies with missing or severely flawed data; (3) Duplicate publications; (4) Full text not available.
Two reviewers (SL and ZMY) independently assessed the titles and abstracts according to these criteria and retrieved relevant full-text articles to screen eligible studies. Disagreements during the screening process were resolved through discussion or by consulting a third reviewer (LXQ).
2.3 Data extraction
Two reviewers (SL and ZMY) independently extracted data from the final included studies, including the following aspects: (1) Basic information: first author, year of publication, country; (2) Study characteristics: cancer stage, histological characteristics, race, sample size, age, sex, type of EGFR mutation, interventions, and comparators; (3) Reported outcome measures.
2.4 Quality assessment
The quality of the included studies was assessed using the Cochrane Risk of Bias tool (RoB2) (19), evaluating five domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result. Each study was independently assessed by two researchers, who categorized each domain as "low risk," "high risk," or "some concerns." Disagreements were resolved through discussion or by consulting a third researcher. The assessment results were presented in a risk of bias summary figure.
2.5 Statistical analysis
Network meta-analysis was conducted using the R software (version 4.3.2) with the gemtc package (version 1.0-1) and JAGS software, employing the Markov Chain Monte Carlo (MCMC) method within a Bayesian framework (20–22). Four Markov chains were simulated, with an initial value of 2.5, a thinning interval of 1, and 5,000 burn-in iterations to ensure proper annealing. The model was further iterated 20,000 times to achieve convergence. The Deviance Information Criterion (DIC) was used to compare model fit and global consistency (if the absolute difference between the DIC of the consistency and inconsistency models was less than 3, the consistency model was adopted) (23). For networks containing closed loops, the node-splitting method was employed to assess local consistency (24).
Survival outcomes were expressed as hazard ratios (HR), while binary outcomes were presented as relative risk ratios (RR), each accompanied by the corresponding 95% confidence intervals (CIs). A statistically significant difference was considered to be present if the 95% CI did not include the value of 1. A Bayesian random-effects model was used to analyze the efficacy of all treatment options simultaneously. The analysis results included network relationship diagrams, cumulative probability ranking plots, and league tables (25). The Surface Under the Cumulative Ranking Curve (SUCRA) was used as an indicator of cumulative ranking probability, with higher SUCRA values (closer to 100%) indicating better interventions (26). All processes of this network meta-analysis were conducted using Stata 15.0 and R software (version 4.3.2). We conducted a subgroup network meta-analysis (NMA) focusing on the primary survival outcome, progression-free survival (PFS). Subgroup network meta-analyses were performed based on baseline characteristics or subgroup data reported in individual trials, stratified by patient gender, age, and EGFR mutation type. This approach aims to further explore optimal treatment strategies tailored for specific populations.
3 Results
3.1 Literature search and screening process
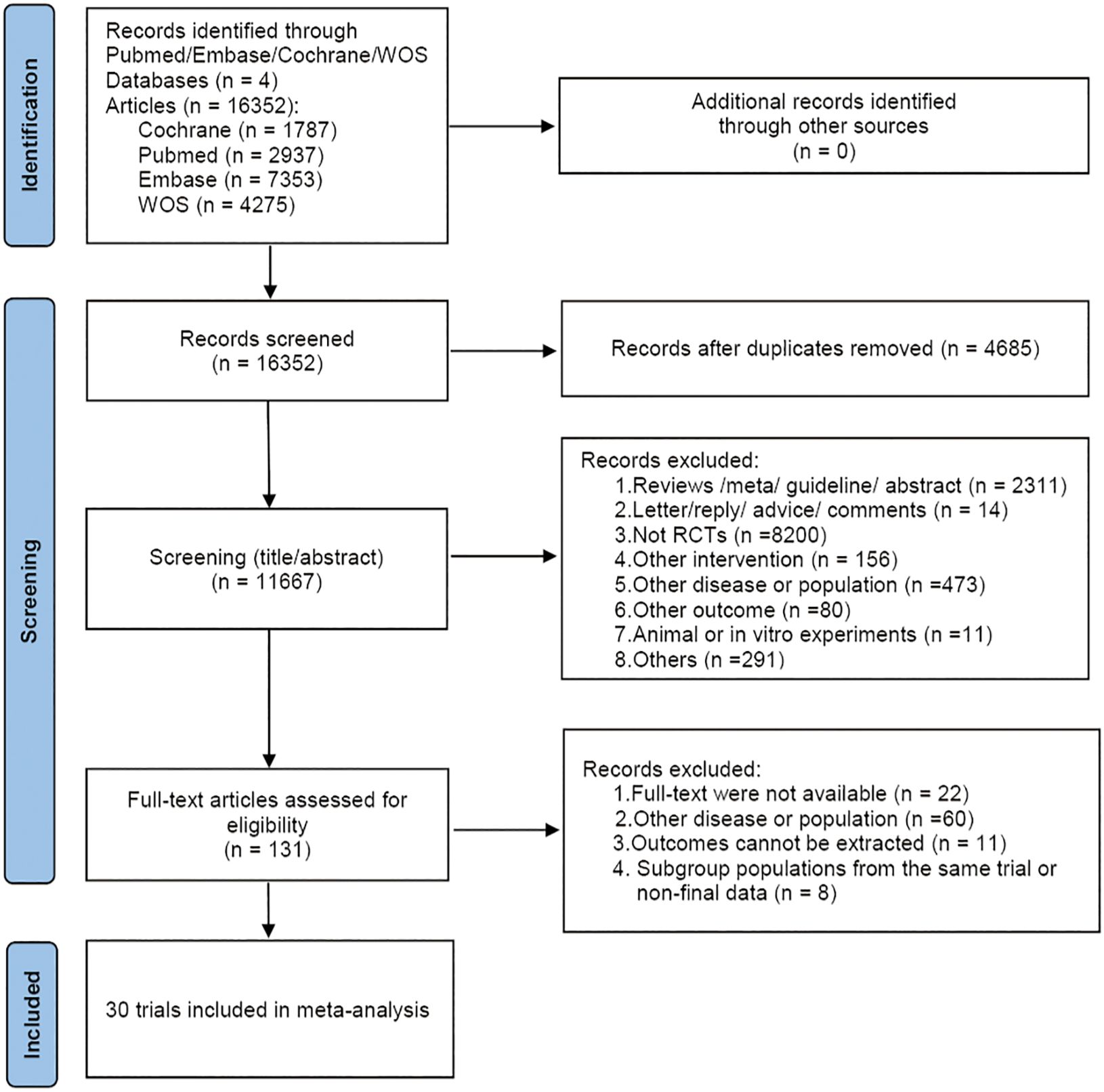
A total of 16,352 articles were retrieved. After removing 4,685 duplicates, 11,356 articles were excluded based on the preliminary reading of titles and abstracts. Full-text review was conducted on the remaining 131 articles, and strict inclusion and exclusion criteria were applied, resulting in the inclusion of 30 articles. The specific screening process was illustrated in Figure 1.
3.2 Basic characteristics of included studies
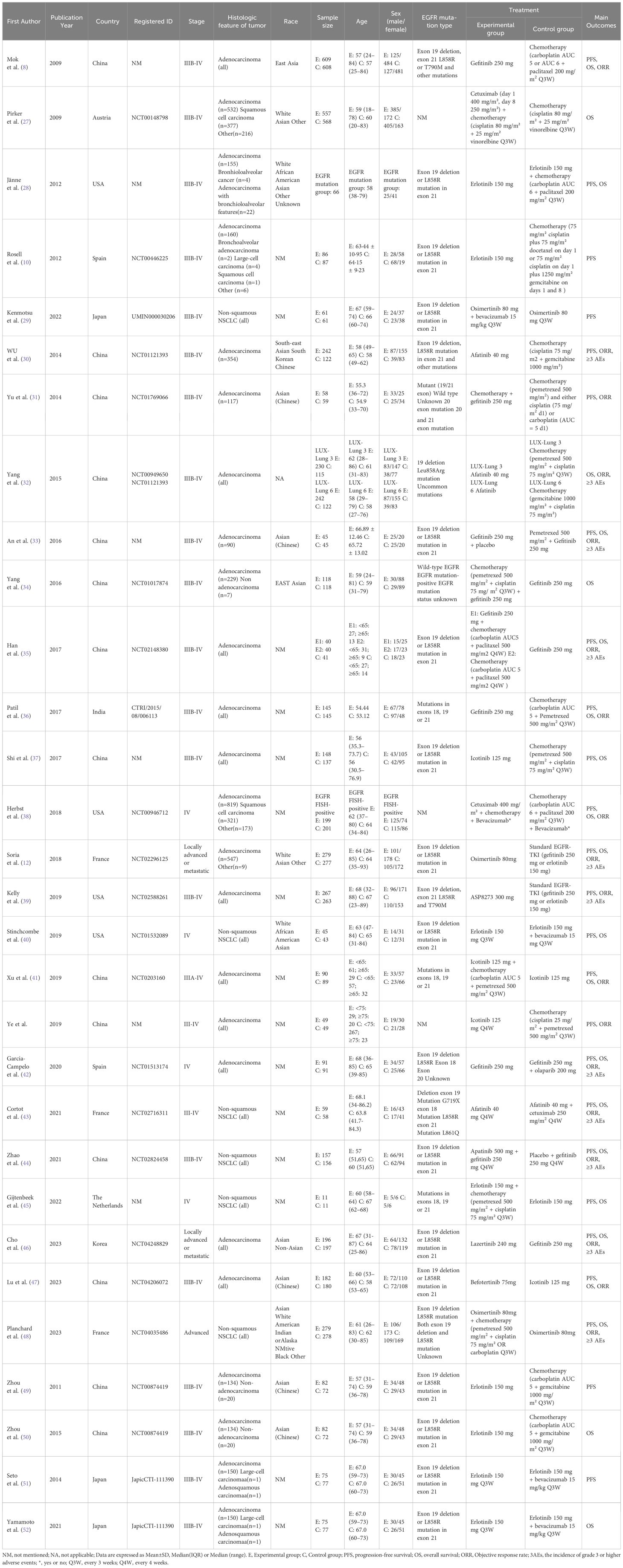
The 30 included studies (8, 10, 12, 27–52) originated from nine countries: Austria (n=1), China (n=14), France (n=3), India (n=1), Japan (n=3), Korea (n=1), Spain (n=2), The Netherlands (n=1), USA (n=4). These studies involved a total of 8,654 patients, including 3,547 males and 5,107 females, with an age range of 18-87 years. The 8,654 patients in the trials received the following 19 treatments: Chemotherapy; Afatinib; Afatinib + Cetuximab; Apatinib + Gefitinib; Befotertinib; Cetuximab + Chemotherapy; Erlotinib; Erlotinib + Bevacizumab; Erlotinib + Chemotherapy; Gefitinib; Gefitinib + Chemotherapy; Gefitinib + Olaparib; Icotinib; Icotinib + Chemotherapy; Lazertinib; Naquotinib; Osimertinib; Osimertinib + Bevacizumab; Osimertinib + Chemotherapy. Detailed characteristics of the included studies were provided in Table 1.
3.3 Methodological quality assessment of included studies
The results of the risk of bias assessment for the 30 included studies were shown in Figure 2. In terms of bias arising from the randomization process, 27 studies were assessed as potentially having a risk due to the lack of random allocation or lack of allocation concealment, while the remaining 3 studies were deemed low risk. Regarding bias due to deviations from intended interventions, 22 studies were assessed as potentially having a risk because they were open-label and blinding was not feasible, while the remaining 8 studies were assessed as low risk. Except for one study assessed as potentially having a risk due to missing outcome data, the remaining 29 studies were assessed as low risk for both missing outcome data and measurement outcomes. No selective reporting was detected in any of the studies, and this was assessed as low risk. Overall, the risk of bias in the included literature was moderate.
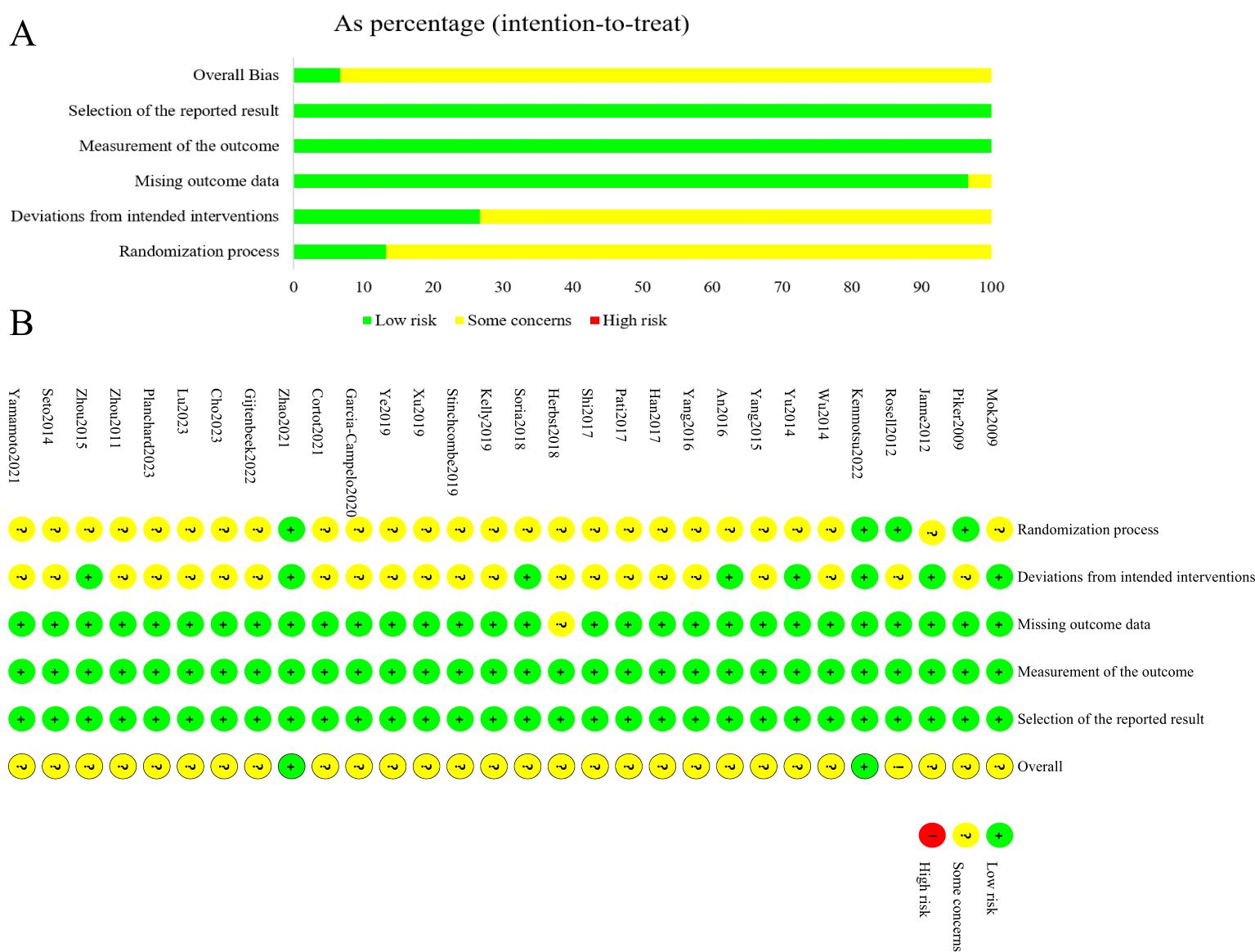
Figure 2. Summary results on risk of bias (using RoB2) of including RCTs. (A), Percent of studies with categories for risk of bias; (B), Summary for the risk of bias in each study.
3.4 Network meta-analysis results
3.4.1 Network evidence diagram
The 30 included studies covered 19 different interventions: Chemotherapy; Afatinib; Afatinib + Cetuximab; Apatinib + Gefitinib; Befotertinib; Cetuximab + Chemotherapy; Erlotinib; Erlotinib + Bevacizumab; Erlotinib + Chemotherapy; Gefitinib; Gefitinib + Chemotherapy; Gefitinib + Olaparib; Icotinib; Icotinib + Chemotherapy; Lazertinib; Naquotinib; Osimertinib; Osimertinib + Bevacizumab; Osimertinib + Chemotherapy. The network structure of different interventions for each outcome indicator was shown in Figure 3. In the diagram, the thickness of the lines is proportional to the number of studies comparing the two interventions, and the diameter of the circles is proportional to the number of participants included for each intervention.
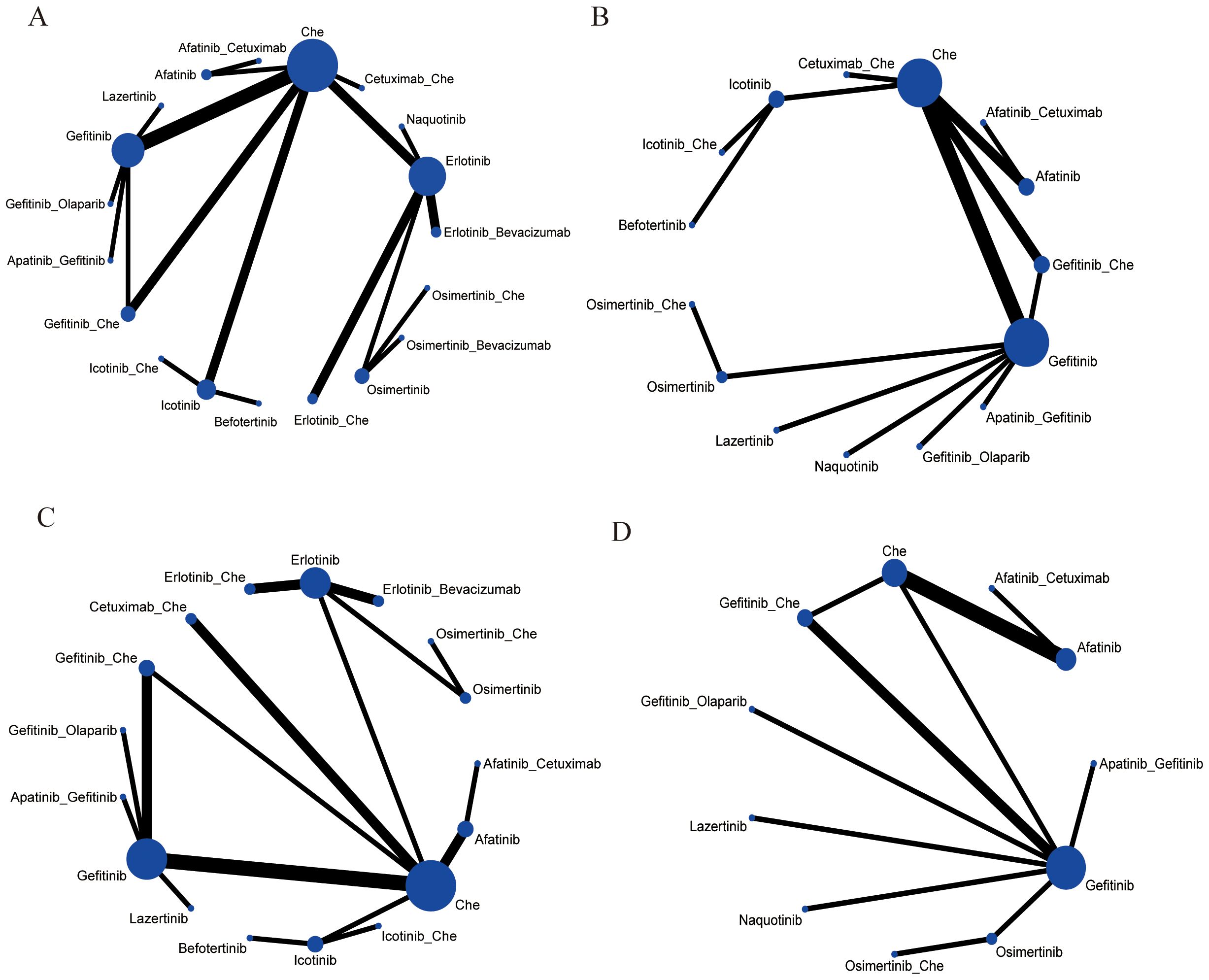
Figure 3. Network meta-analysis map concerning the efficacy and safety of first-line treatments in patients with EGFR mutation-positive advanced or metastatic non-squamous non-small cell lung cancer on (A) PFS, (B) OS, (C) ORR, (D) ≥3 AEs. The size of the nodes relates to the number of participants in that intervention type, and the thickness of lines between the interventions relates to the number of studies for that comparison. Che, Chemotherapy.
3.4.2 Progression-free survival
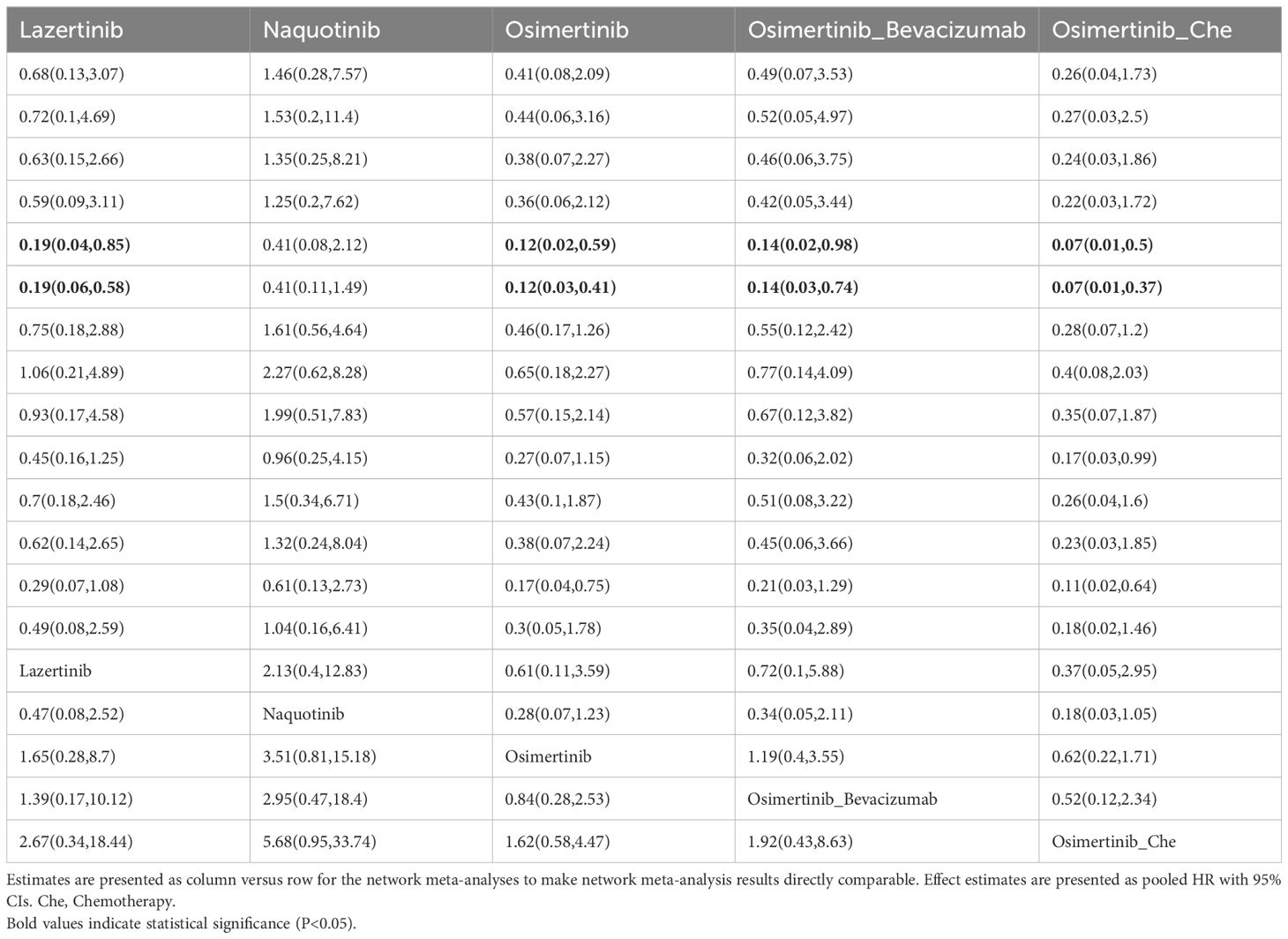
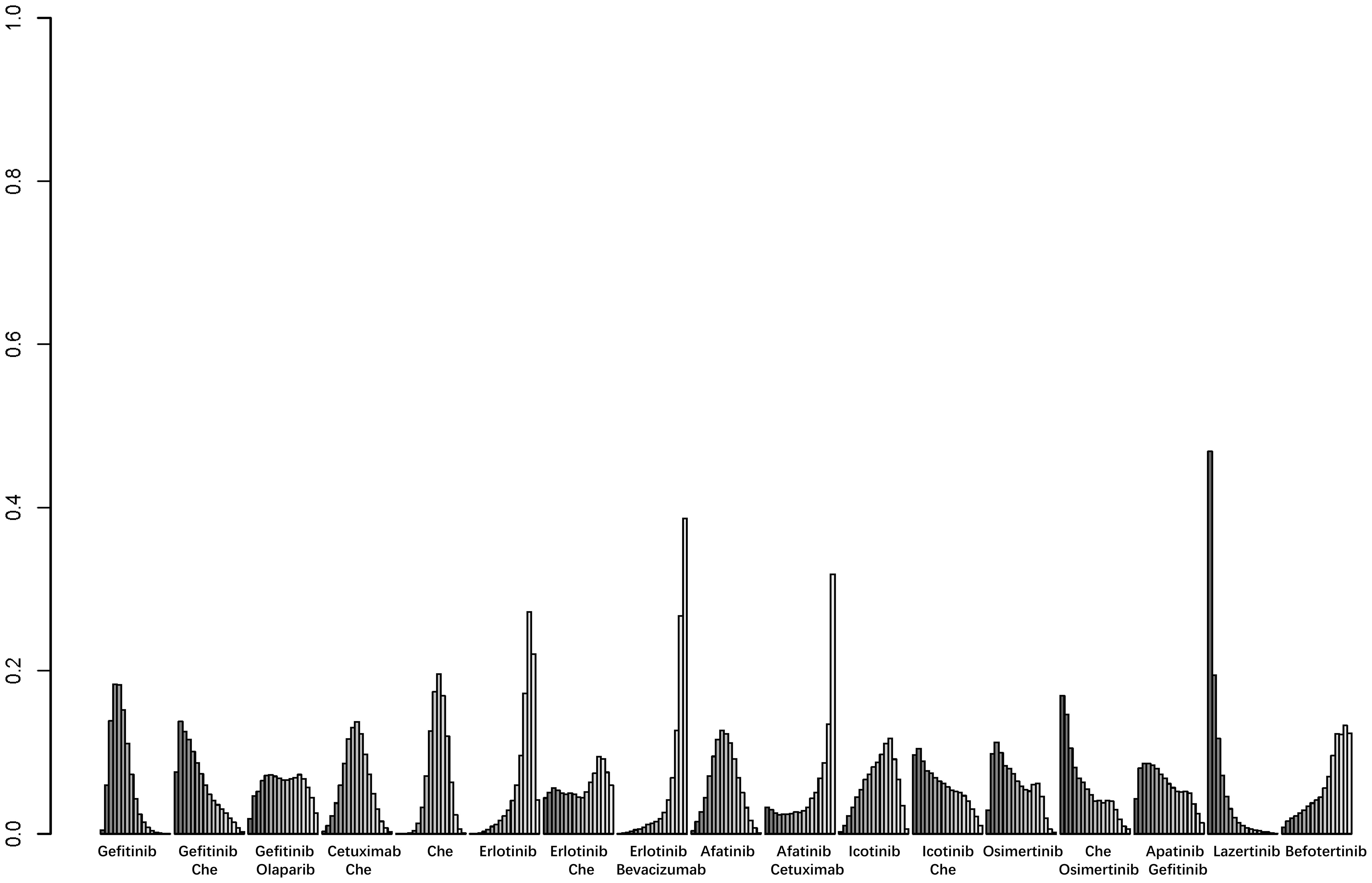
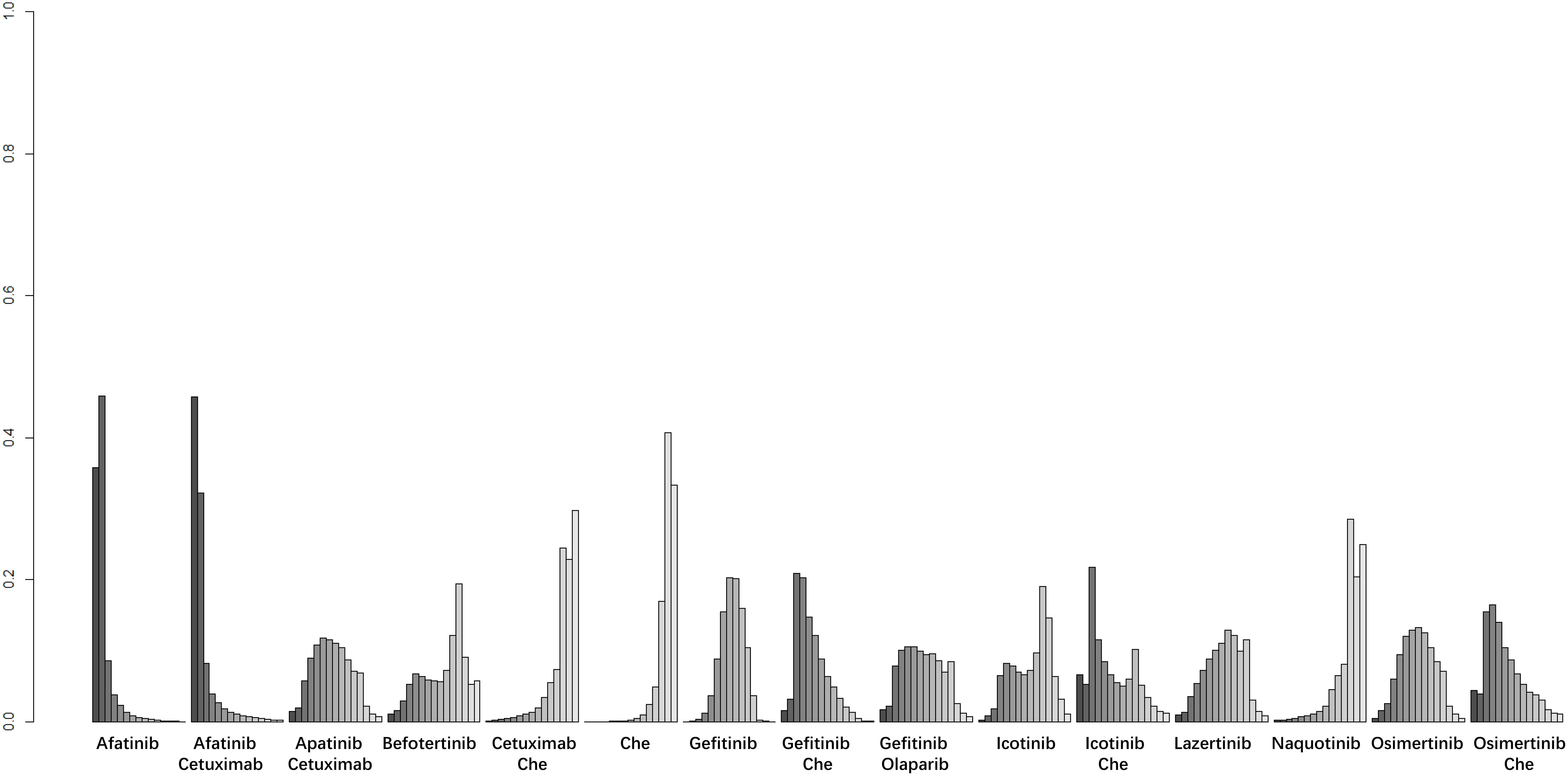
A total of 25 studies reported PFS outcomes for 19 interventions: Chemotherapy; Afatinib; Afatinib + Cetuximab; Apatinib + Gefitinib; Befotertinib; Cetuximab + Chemotherapy; Erlotinib; Erlotinib + Bevacizumab; Erlotinib + Chemotherapy; Gefitinib; Gefitinib + Chemotherapy; Gefitinib + Olaparib; Icotinib; Icotinib + Chemotherapy; Lazertinib; Naquotinib; Osimertinib; Osimertinib + Bevacizumab; Osimertinib + Chemotherapy (8, 10, 28–31, 33, 35–49, 51). The network structure for different interventions was shown in Figure 3A. The network meta-analysis results indicated that Afatinib (HR=0.28, 95% CI: 0.1, 0.79), Apatinib + Gefitinib (HR=0.3, 95% CI: 0.09, 0.91), Erlotinib (HR=0.25, 95% CI: 0.12, 0.54), Erlotinib + Bevacizumab (HR=0.18, 95% CI: 0.06, 0.53), Erlotinib + Chemotherapy (HR=0.21, 95% CI: 0.06, 0.64), Gefitinib (HR=0.43, 95% CI: 0.23, 0.71), Gefitinib + Chemotherapy (HR=0.27, 95% CI: 0.13, 0.58), Gefitinib + Olaparib (HR=0.31, 95% CI: 0.09, 0.95), Lazertinib (HR=0.19, 95% CI: 0.06, 0.58), Osimertinib + Chemotherapy (HR=0.07, 95% CI: 0.01, 0.37), Osimertinib (HR=0.12, 95% CI: 0.03, 0.41), and Osimertinib + Bevacizumab (HR=0.14, 95% CI: 0.03, 0.74) were significantly better than chemotherapy in improving PFS. Furthermore, Erlotinib, Erlotinib + Bevacizumab, Erlotinib + Chemotherapy, Gefitinib + Chemotherapy, Lazertinib, Osimertinib, Osimertinib + Bevacizumab, and Osimertinib + Chemotherapy were significantly better than Cetuximab + Chemotherapy (HR=0.26, 95% CI: 0.07, 0.89; HR=0.18, 95% CI: 0.04, 0.79; HR=0.21, 95% CI: 0.04, 0.94; HR=0.28, 95% CI: 0.08, 0.96; HR=0.19, 95% CI: 0.04, 0.85; HR=0.12, 95% CI: 0.02, 0.59; HR=0.14, 95% CI: 0.02, 0.98; HR=0.07, 95% CI: 0.01, 0.5, respectively). No significant differences were found among the other pairwise comparisons (Table 2). Based on cumulative probability results, Osimertinib + Chemotherapy (SUCRAs: 93.4%), Osimertinib (SUCRAs: 84.61%), and Osimertinib + Bevacizumab (SUCRAs: 76.31%) might be the top three measures for improving PFS (Figure 4).
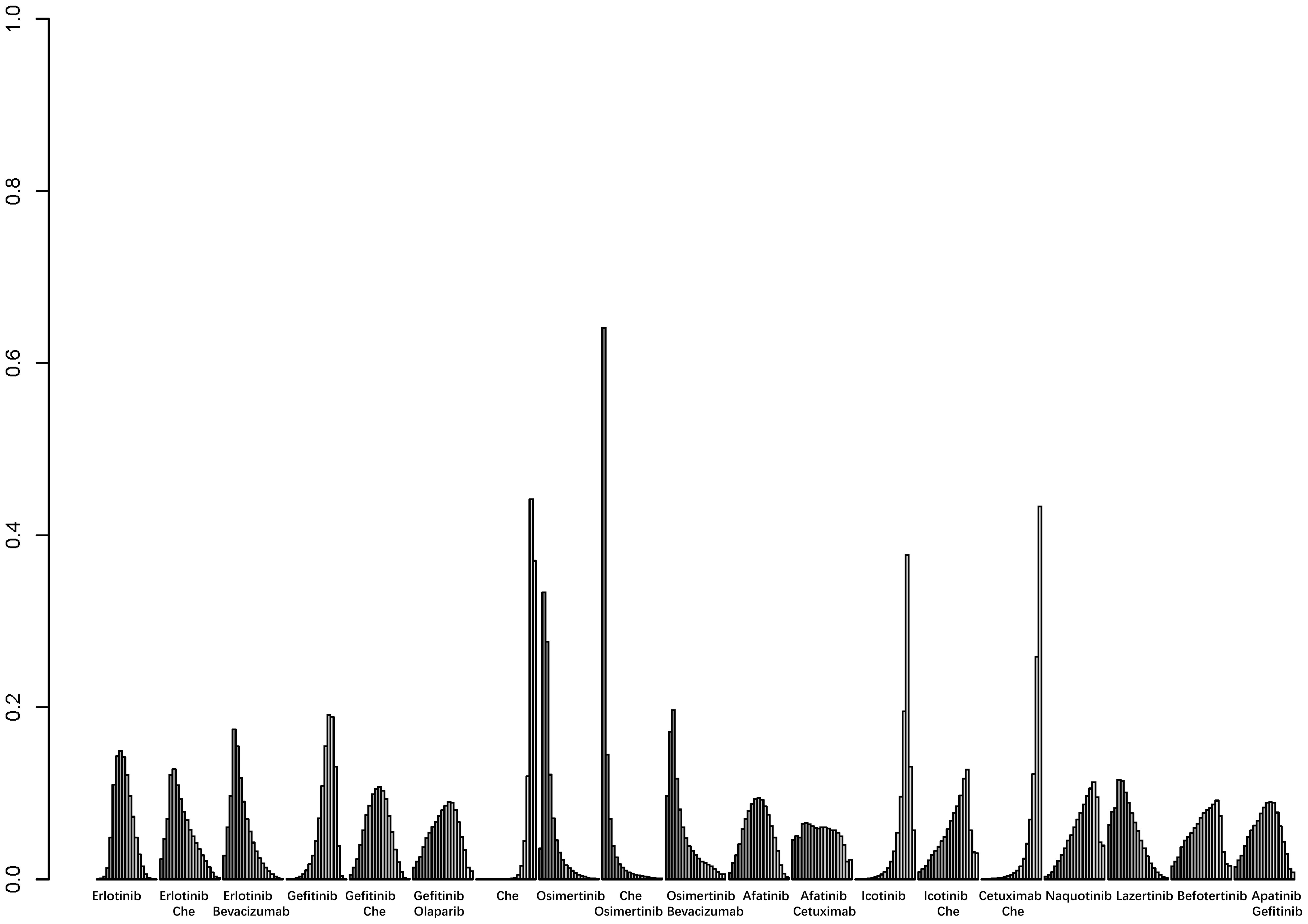
Figure 4. Result of probability ranking for optimal PFS among different intervention measures. The horizontal axis represents the various intervention measures, and the vertical axis indicates the probability of ranking for each intervention measure. Each bar graph shows the probability of different intervention measures being ranked from first position to last position.
3.4.3 Overall survival
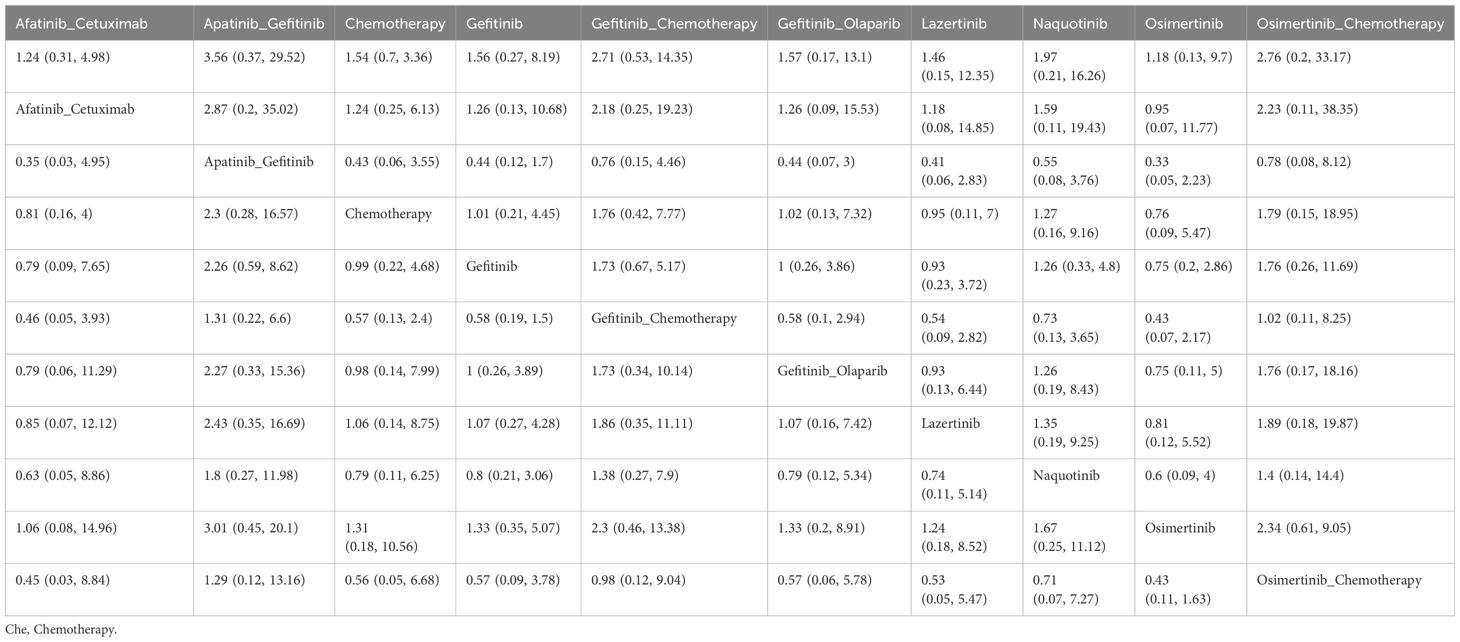
A total of 22 studies reported OS outcomes for 17 interventions: Chemotherapy; Afatinib; Afatinib + Cetuximab; Apatinib + Gefitinib; Befotertinib; Cetuximab + Chemotherapy; Erlotinib; Erlotinib + Bevacizumab; Erlotinib + Chemotherapy; Gefitinib; Gefitinib + Chemotherapy; Gefitinib + Olaparib; Icotinib; Icotinib + Chemotherapy; Lazertinib; Osimertinib; Osimertinib + Chemotherapy (8, 12, 27, 28, 32, 33, 35–38, 40–45, 47, 48, 50–52). The network structure for different interventions is shown in Figure 3B. The network meta-analysis results indicated that Lazertinib (HR=0.56, 95% CI: 0.32, 0.99) was significantly better than chemotherapy in improving OS. Lazertinib (HR=0.44, 95% CI: 0.21, 0.9) and Osimertinib (HR=0.63, 95% CI: 0.4, 0.98) were significantly better than Erlotinib. Additionally, Lazertinib (HR=0.37, 95% CI: 0.15, 0.85) and Osimertinib (HR=0.53, 95% CI: 0.28, 0.98) were significantly better than Erlotinib + Bevacizumab. No significant differences were found among the other pairwise comparisons (Supplementary Table 1). Based on cumulative probability results, Lazertinib (SUCRAs: 89.72%), Gefitinib (SUCRAs: 72.07%), and Osimertinib + Chemotherapy (SUCRAs: 70.74%) might be the top three measures for improving OS (Figure 5).
3.4.4 Objective response rate
A total of 18 studies were included for analysis (8, 12, 30–33, 35–39, 41–44, 46–48), involving the following 15 interventions: Afatinib, Afatinib + Cetuximab, Apatinib + Gefitinib, Befotertinib, Cetuximab + Chemotherapy, Chemotherapy, Gefitinib, Gefitinib + Chemotherapy, Gefitinib + Olaparib, Icotinib, Icotinib + Chemotherapy, Lazertinib, Naquotinib, Osimertinib, Osimertinib + Chemotherapy. The network structure for different interventions was shown in Figure 3C. The network meta-analysis results showed that Afatinib (HR=2.73, 95% CI: 1.88, 3.98), Afatinib + Cetuximab (HR=2.77, 95% CI: 1.52, 5.02), Apatinib + Gefitinib (HR=1.65, 95% CI: 1.02, 2.85), Gefitinib (HR=1.58, 95% CI: 1.24, 2.12), and Gefitinib + Chemotherapy (HR=1.86, 95% CI: 1.35, 2.7) had higher ORR compared to chemotherapy. Afatinib and Afatinib + Cetuximab also showed higher ORR compared to Cetuximab + Chemotherapy (HR=2.6, 95% CI: 1.41, 4.83; HR=2.63, 95% CI: 1.22, 5.69; respectively). Afatinib and Afatinib + Cetuximab showed higher ORR compared to Naquotinib (HR=2.53, 95% CI: 1.28, 4.72; HR=2.56, 95% CI: 1.1, 5.53; respectively). and Afatinib showed significantly higher ORR compared to Cetuximab + Chemotherapy (HR=2.6, 95% CI: 1.41, 4.83). Other pairwise intervention differences were not statistically significant (Supplementary Table 2). Based on cumulative probability results, Afatinib (SUCRAs: 92.27%), Afatinib + Cetuximab (SUCRAs: 90.95%), and Gefitinib + Chemotherapy (SUCRAs: 69.05%) might be the top three measures for ORR (Figure 6).
3.4.5 Grade ≥3 adverse events
A total of 11 studies were included for analysis (12, 30, 32, 33, 35, 39, 42, 43, 46, 48), involving the following 11 interventions: Afatinib, Afatinib + Cetuximab, Apatinib + Gefitinib, Chemotherapy, Gefitinib, Gefitinib + Chemotherapy, Gefitinib + Olaparib, Lazertinib, Naquotinib, Osimertinib, Osimertinib + Chemotherapy. The network structure for different interventions was shown in Figure 3D. The network meta-analysis results indicated no statistically significant differences in the incidence of grade ≥3 adverse events between any treatment and chemotherapy or other treatments (Table 3). Based on cumulative probability results, Afatinib (SUCRAs: 74.93%), Osimertinib (SUCRAs: 69.42%), and Afatinib + Cetuximab (SUCRAs: 61.87%) might be the top three measures in terms of safety (Figure 7).
3.4.6 Local inconsistency analysis results
Node-splitting analysis was used to confirm the consistency of any two intervention schemes in any closed loop. This study analyzed the closed loops in four outcome indicators. For the PFS indicator, the indirect effect of Gefitinib alone compared to chemotherapy alone was more significant in improving PFS than the direct effect (P=0.038). Additionally, there were inconsistent estimates in the comparisons of Gefitinib + Chemotherapy with chemotherapy alone (P=0.036) and Gefitinib alone (P=0.034). For the OS indicator, the indirect effect of Gefitinib alone compared to chemotherapy alone significantly improved PFS (P=0.049). The indirect estimate of Gefitinib + Chemotherapy compared to Gefitinib alone was slightly higher than the direct comparison (P=0.464). In other closed loops and remaining outcome indicators, the P-values for the consistency between direct and indirect estimates were all greater than 0.05.
3.4.7 Subgroup analysis
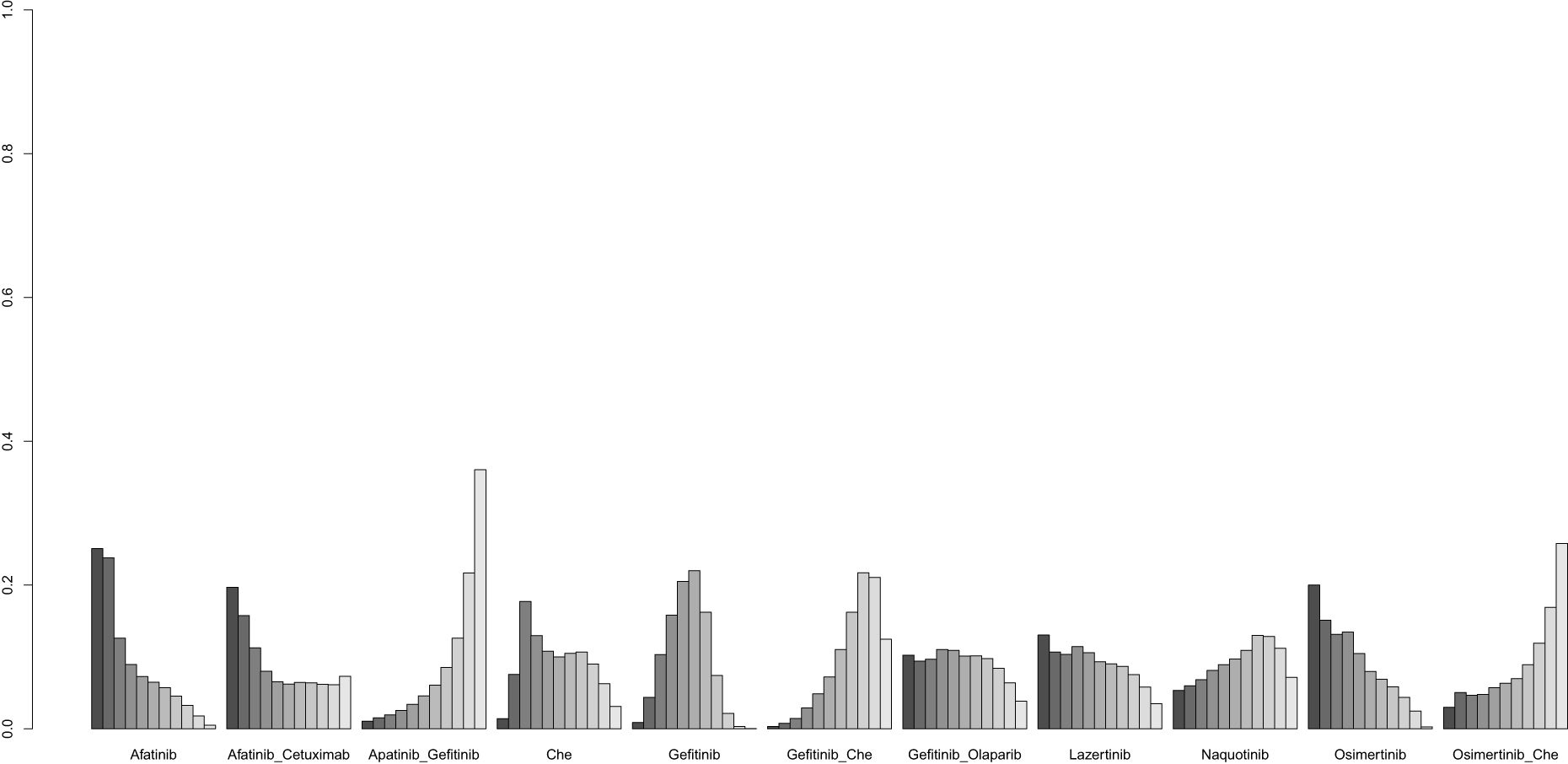
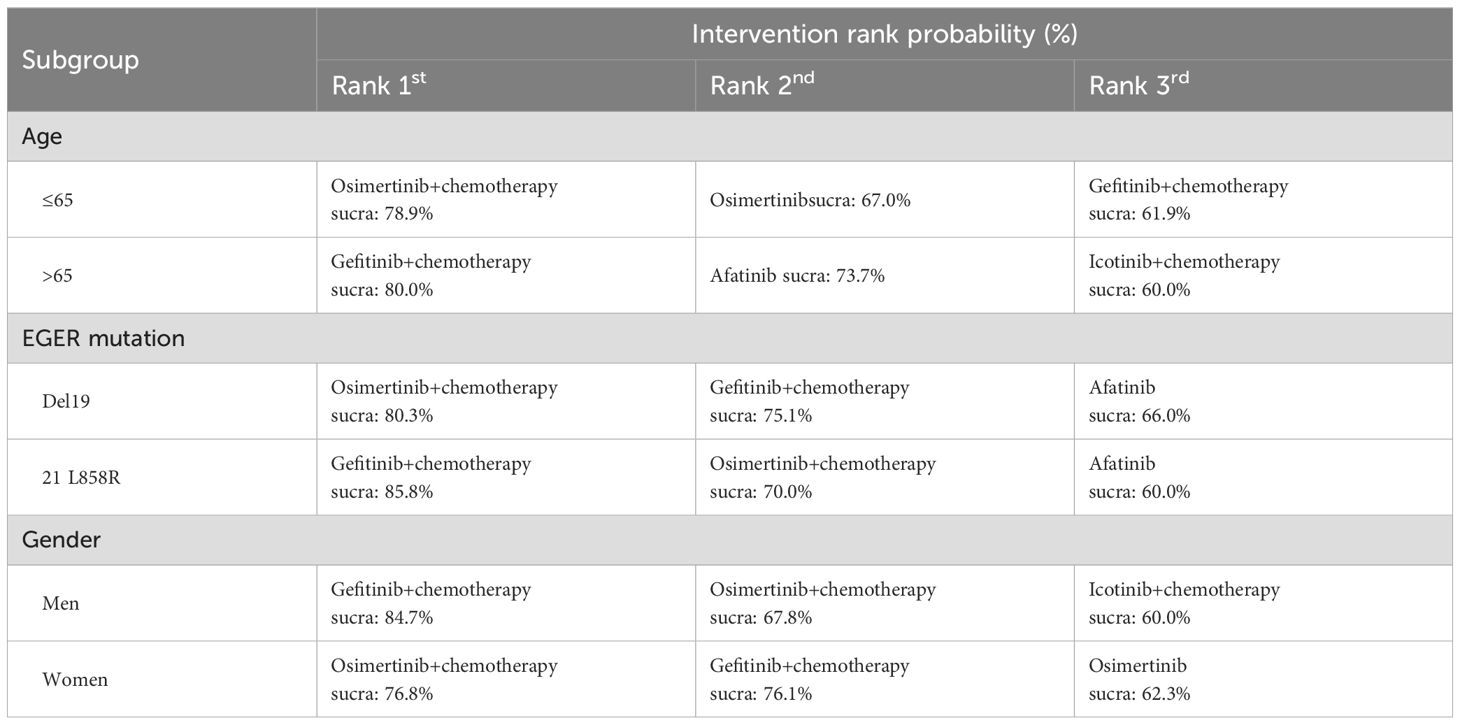
Table 4 presents the results of subgroup network meta-analyses stratified by age (>65/≤65 years), EGFR mutation type (exon 19 deletion/21 L858R), and gender (male/female). The findings indicate that Osimertinib combined with chemotherapy demonstrates the most significant improvement in PFS among patients aged ≤65 years, those with exon 19 deletions, and females. In contrast, Gefitinib combined with chemotherapy emerges as a more effective treatment option for patients aged >65 years, those with the 21 L858R mutation, and males. The subgroup analysis network plots and ranking diagrams are provided in Supplementary Figures 1 and 2.
4 Discussion
To our knowledge, this is the first NMA comparing the efficacy and safety of various first-line treatment options for advanced or metastatic EGFR mutation-positive NSCLC. The NMA analyzed the latest data from 30 eligible randomized controlled trials. Our findings indicate that Osimertinib + Chemotherapy is the most effective measure for improving PFS, Lazertinib is the best measure for improving OS, and Afatinib is the most prominent for enhancing ORR. Despite Afatinib's superior safety profile, the incidence of grade 3 or higher adverse events was not significantly different among all treatment regimens.
When considering PFS improvement, the top three treatments were Osimertinib + Chemotherapy, Osimertinib, and Osimertinib + Bevacizumab. Overall, Osimertinib, a third-generation EGFR-TKI, demonstrated outstanding performance. A previous network meta-analysis that included 17 trials indicated that Osimertinib-containing regimens were most likely to rank first in terms of PFS, regardless of the presence of brain metastases, which was consistent with our results (53). Additionally, a previous clinical trial found that the PFS of Osimertinib combined with Bevacizumab was shorter than that of Osimertinib alone in NSCLC patients with the EGFR T790M mutation (54), and we found the same result in an untreated population. Holleman et al. conducted a network meta-analysis that included data from 3539 EGFR mutation-positive NSCLC patients, comparing five EGFR-TKIs, including Afatinib, Dacomitinib, Erlotinib, Gefitinib, and Osimertinib in first-line treatment (55). The cumulative probability ranking indicated that Osimertinib had potentially better efficacy in terms of PFS compared to all other TKIs. Furthermore, the PFS improvement effect of Osimertinib combined with chemotherapy was the best, consistent with the effects observed by Hosomi et al. and Noronha et al. in their comparisons of first-generation EGFR-TKIs combined with or without chemotherapy in patients with advanced EGFR mutation-positive NSCLC (56, 57). Although the exact mechanism of the benefit of Osimertinib combined with chemotherapy remains unclear, as pointed out by the FLAURA-2 researchers, chemotherapy may complement Osimertinib's targeted action through its non-selective anti-tumor actions and overcome tumor heterogeneity (48).
Subgroup analyses of PFS under different population characteristics were conducted using network meta-analysis. Overall results indicate that Osimertinib combined with chemotherapy and Gefitinib combined with chemotherapy demonstrate optimal efficacy across subgroups stratified by age, EGFR mutation type, and gender. Among patients aged ≤65 years, those with exon 19 deletion mutations, or females, Osimertinib plus chemotherapy exhibited the most significant improvement in PFS. Previous real-world studies have identified factors associated with the efficacy of osimertinib, such as EGFR exon 19 deletions and female gender, which significantly enhance PFS outcomes with osimertinib treatment (58, 59). Additionally, a global Phase III trial on osimertinib in treatment-naïve EGFR-mutant NSCLC patients reported PFS of 21.4 months and 14.4 months for exon 19 deletion and L858R mutation patients, respectively, indicating a trend toward superior PFS in the exon 19 deletion group compared to the L858R group (12). In elderly patients, Gefitinib combined with chemotherapy was found to be the most effective treatment option, followed by Osimertinib combined with chemotherapy. In contrast, in younger patients, third-generation EGFR-TKIs (e.g., Osimertinib) combined with chemotherapy showed superior PFS improvement. Previous studies have revealed that elderly patients are more prone to lower BMI, which may compromise the efficacy of osimertinib due to potential cachexia—a condition characterized by significant weight loss primarily due to skeletal muscle and fat mass depletion (60, 61) (Furthermore, physiological decline in elderly patients may lead to reduced tolerance to potent drugs, making the lower-toxicity Gefitinib plus chemotherapy regimen a more suitable choice.
Regarding the improvement in OS, the top three treatment options were Lazertinib, Gefitinib, and Osimertinib + Chemotherapy. Lazertinib is a potent, orally available, blood-brain barrier-penetrable, irreversible third-generation EGFR-TKI. In our results, only Lazertinib showed a statistically significant improvement in OS compared to chemotherapy. Jeon et al. evaluated Lazertinib's efficacy against Osimertinib using external controls, finding that Lazertinib did not reach the median OS, whereas the Osimertinib group had a median OS of 29.8 months, suggesting Lazertinib's potential for superior survival benefits (62). Furthermore, Lee et al. reviewed and compared the efficacy outcomes of Lazertinib versus platinum-based chemotherapy in patients with EGFR mutation-positive NSCLC (63). Their results indicated that Lazertinib had excellent OS (32.23 months vs. 18.73 months; adjusted HR: 0.45, 95% CI: 0.29-0.69). In summary, Lazertinib may shows promise for OS improvement as a first-line treatment.
For ORR, Afatinib appears to be the best treatment option. Afatinib is a second-generation targeted drug that can covalently bind with the epidermal growth factor and other receptors, and it is irreversible. It effectively reduces medication time and prolongs the replacement probability of reversible non-covalent binding sites, thus inhibiting tyrosine kinase activity and the proliferation and metastasis of tumors. A review evaluating clinical outcomes in NSCLC patients with uncommon EGFR mutations found an ORR of 60.6% for the Afatinib group, slightly higher than 50.3% for the Osimertinib group, although the difference was not statistically significant (64). Another systematic review focusing on uncommon EGFR mutations included 38 studies with a total of 1836 patients, supporting the use of Afatinib for G719X, S768I, E709X, and L747X mutations, as well as compound rare mutations (65). Additionally, in a network meta-analysis conducted by Holleman et al., Afatinib and Osimertinib performed the best in ORR compared to other drugs (Dacomitinib, Erlotinib, Gefitinib), with a probability of 46% for both drugs (55). However, previous clinical trials have shown that approximately 60% of patients with acquired resistance to EGFR-TKIs (Erlotinib, Gefitinib, and Afatinib) develop a new mutation (T790M) in the drug target, which has been shown to alter drug binding and enzymatic activity of the mutant EGF receptor (66).
Regarding safety outcomes, all evaluated treatment options showed no statistically significant differences in the incidence of grade 3 or higher adverse events compared to chemotherapy or other regimens. Notably, Afatinib was identified as the most optimal treatment option in the cumulative probability ranking for safety, followed by Osimertinib. Previous real-world studies indicate that Afatinib is as effective and well-tolerated in routine clinical practice as in clinical trials, performing well in patients with certain uncommon EGFR mutations, brain metastases, and elderly patients (67, 68). Concerning Osimertinib, a clinical trial demonstrated that the incidence of grade 3 or higher adverse events in the Osimertinib group was similar to that in the control EGFR-TKI group (Gefitinib or Erlotinib), despite a longer exposure time in the Osimertinib group (13). Zhao et al. conducted a network meta-analysis that showed Icotinib had the highest probability (80%) of causing grade 3 or higher adverse events, followed by Osimertinib, although there was no statistically significant difference between Icotinib and Osimertinib (RR=2.92; 95% CI: 0.21-40.49). A meta-analysis of Osimertinib for advanced NSCLC with EGFR mutations revealed that the most common adverse events were diarrhea and rash, with combined incidences of 44% and 42%, respectively, followed by dry skin (29%). Moreover, most patients tolerated Osimertinib well (69).
4.1 Limitations
However, our study has several limitations. First, some trials in this study did not report or reach OS, potentially leading to a risk of bias in the results. Second, our NMA did not differentiate between specific forms of chemotherapy, and there may be efficacy differences between different chemotherapy regimens. Third, due to the limited number of current studies and the lack of OS subgroup analysis results from some studies, the data volume for subgroup analysis would be significantly reduced if considered, so we did not further explore subgroups to avoid impacting the power of the tests. Finally, since most trials were open-label, the lack of blinding might affect the credibility of the results.
5 Conclusion
In summary, our study indicates that Osimertinib is the preferred treatment option for untreated advanced or metastatic non-squamous NSCLC with EGFR mutations, considering both survival and safety. In particular, the combination of Osimertinib and chemotherapy demonstrates excellent survival benefits, providing crucial reference points for clinical decision-making for these patients. Nevertheless, our conclusions require further validation through more high-quality clinical studies with double-blind, multicenter, large-sample, and longer follow-up periods.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
MZ: Conceptualization, Resources, Writing – original draft, Writing – review & editing. LS: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1498518/full#supplementary-material
Supplementary Figure 1 | Subgroup analysis Network map.
Supplementary Figure 2 | Result of probability ranking for optimal PFS among different intervention measures in the subgroups. A, age; B, EGER mutation; C, gender.
Supplementary Text 1 | Search strategy.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun (Lond). (2022) 42:937–70. doi: 10.1002/cac2.v42.10
3. Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. (2022) 72:409–36. doi: 10.3322/caac.21731
4. Chouaid C, Bensimon L, Clay E, Millier A, Levy-Bachelot L, Huang M, et al. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer. (2019) 127:44–52.
5. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. (2015) 5:2892–911.
6. Tan DS, Mok TS, Rebbeck TR. Cancer genomics: diversity and disparity across ethnicity and geography. J Clin Oncol. (2016) 34:91–101.
7. Passaro A, Jänne PA, Mok T, Peters S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer. (2021) 2:377–91. doi: 10.1038/s43018-021-00195-8
8. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. (2009) 361:947–57. doi: 10.1056/NEJMoa0810699
9. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. (2017) 18:1454–66. doi: 10.1016/S1470-2045(17)30608-3
10. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. (2012) 13:239–46. doi: 10.1016/S1470-2045(11)70393-X
11. Cooper AJ, Sequist LV, Lin JJ. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat Rev Clin Oncol. (2022) 19:499–514. doi: 10.1038/s41571-022-00639-9
12. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
13. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. (2020) 382:41–50. doi: 10.1056/NEJMoa1913662
14. Lamb YN. Osimertinib: A review in previously untreated, EGFR mutation-positive, advanced NSCLC. Target Oncol. (2021) 16:687–95. doi: 10.1007/s11523-021-00839-w
15. Chen Y, Wen S, Wu Y, Shi L, Xu X, Shen B. Efficacy and safety of first-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) combined with chemotherapy or antiangiogenic therapy as first-line treatment in patients with EGFR-mutant non-small cell lung cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. (2021) 163:103393. doi: 10.1016/j.critrevonc.2021.103393
16. Greenhalgh J, Boland A, Bates V, Vecchio F, Dundar Y, Chaplin M, et al. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev. (2021) 3:Cd010383.
17. Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, Dundar Y, et al. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev. (2016) 5):Cd010383. doi: 10.1002/14651858.CD010383.pub2
18. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
19. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. (2019) 366:l4898. doi: 10.1136/bmj.l4898
20. Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages. PloS One. (2014) 9:e115065. doi: 10.1371/journal.pone.0115065
21. Gelman A, Rubin DB. Markov chain Monte Carlo methods in biostatistics. Stat Methods Med Res. (1996) 5:339–55. doi: 10.1177/096228029600500402
22. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. (2004) 23:3105–24. doi: 10.1002/sim.v23:20
23. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29:932–44. doi: 10.1002/sim.v29:7/8
24. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. (2013) 33:641–56. doi: 10.1177/0272989X12455847
25. Trinquart L, Chatellier G, Ravaud P. Adjustment for reporting bias in network meta-analysis of antidepressant trials. BMC Med Res Methodol. (2012) 12:150. doi: 10.1186/1471-2288-12-150
26. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
27. Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. (2009) 373:1525–31. doi: 10.1016/S0140-6736(09)60569-9
28. Jänne PA, Wang X, Socinski MA, Crawford J, Stinchcombe TE, Gu L, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol. (2012) 30:2063–9. doi: 10.1200/JCO.2011.40.1315
29. Kenmotsu H, Wakuda K, Mori K, Kato T, Sugawara S, Kirita K, et al. Randomized phase 2 study of osimertinib plus bevacizumab versus osimertinib for untreated patients with nonsquamous NSCLC harboring EGFR mutations: WJOG9717L study. J Thorac Oncol. (2022) 17:1098–108. doi: 10.1016/j.jtho.2022.05.006
30. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. (2014) 15:213–22. doi: 10.1016/S1470-2045(13)70604-1
31. Yu H, Zhang J, Wu X, Luo Z, Wang H, Sun S, et al. A phase II randomized trial evaluating gefitinib intercalated with pemetrexed/platinum chemotherapy or pemetrexed/platinum chemotherapy alone in unselected patients with advanced non-squamous non-small cell lung cancer. Cancer Biol Ther. (2014) 15:832–9. doi: 10.4161/cbt.28874
32. Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. (2015) 16:141–51. doi: 10.1016/S1470-2045(14)71173-8
33. An C, Zhang J, Chu H, Gu C, Xiao F, Zhu F, et al. Study of gefitinib and pemetrexed as first-line treatment in patients with advanced non-small cell lung cancer harboring EGFR mutation. Pathol Oncol Res. (2016) 22:763–8. doi: 10.1007/s12253-016-0067-4
34. Yang JC, Srimuninnimit V, Ahn MJ, Lin CC, Kim SW, Tsai CM, et al. First-Line Pemetrexed plus Cisplatin followed by Gefitinib Maintenance Therapy versus Gefitinib Monotherapy in East Asian Never-Smoker Patients with Locally Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer: Final Overall Survival Results from a Randomized Phase 3 Study. J Thorac Oncol. (2016) 11:370–9. doi: 10.1016/j.jtho.2015.11.008
35. Han B, Jin B, Chu T, Niu Y, Dong Y, Xu J, et al. Combination of chemotherapy and gefitinib as first-line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: A randomized controlled trial. Int J Cancer. (2017) 141:1249–56. doi: 10.1002/ijc.v141.6
36. Patil VM, Noronha V, Joshi A, Choughule AB, Bhattacharjee A, Kumar R, et al. Phase III study of gefitinib or pemetrexed with carboplatin in EGFR-mutated advanced lung adenocarcinoma. ESMO Open. (2017) 2:e000168. doi: 10.1136/esmoopen-2017-000168
37. Shi YK, Wang L, Han BH, Li W, Yu P, Liu YP, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. (2017) 28:2443–50. doi: 10.1093/annonc/mdx359
38. Herbst RS, Redman MW, Kim ES, Semrad TJ, Bazhenova L, Masters G, et al. Cetuximab plus carboplatin and paclitaxel with or without bevacizumab versus carboplatin and paclitaxel with or without bevacizumab in advanced NSCLC (SWOG S0819): a randomised, phase 3 study. Lancet Oncol. (2018) 19:101–14. doi: 10.1016/S1470-2045(17)30694-0
39. Kelly RJ, Shepherd FA, Krivoshik A, Jie F, Horn L. A phase III, randomized, open-label study of ASP8273 versus erlotinib or gefitinib in patients with advanced stage IIIB/IV non-small-cell lung cancer. Ann Oncol. (2019) 30:1127–33. doi: 10.1093/annonc/mdz128
40. Stinchcombe TE, Jänne PA, Wang X, Bertino EM, Weiss J, Bazhenova L, et al. Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced EGFR-mutant non-small cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. (2019) 5:1448–55. doi: 10.1001/jamaoncol.2019.1847
41. Xu L, Qi Q, Zhang Y, Cui J, Liu R, Li Y. Combination of icotinib and chemotherapy as first-line treatment for advanced lung adenocarcinoma in patients with sensitive EGFR mutations: A randomized controlled study. Lung Cancer. (2019) 133:23–31. doi: 10.1016/j.lungcan.2019.05.008
42. Garcia-Campelo R, Arrieta O, Massuti B, Rodriguez-Abreu D, Granados ALO, Majem M, et al. Combination of gefitinib and olaparib versus gefitinib alone in EGFR mutant non-small-cell lung cancer (NSCLC): A multicenter, randomized phase II study (GOAL). Lung Cancer. (2020) 150:62–9. doi: 10.1016/j.lungcan.2020.09.018
43. Cortot AB, Madroszyk A, Giroux-Leprieur E, Molinier O, Quoix E, Bérard H, et al. First-line afatinib plus cetuximab for EGFR-mutant non-small cell lung cancer: results from the randomized phase II IFCT-1503 ACE-lung study. Clin Cancer Res. (2021) 27:4168–76. doi: 10.1158/1078-0432.CCR-20-4604
44. Zhao H, Yao W, Min X, Gu K, Yu G, Zhang Z, et al. Apatinib plus gefitinib as first-line treatment in advanced EGFR-mutant NSCLC: the phase III ACTIVE study (CTONG1706). J Thorac Oncol. (2021) 16:1533–46. doi: 10.1016/j.jtho.2021.05.006
45. Gijtenbeek RGP, van der Noort V, Aerts J, Staal-van den Brekel JA, Smit EF, Krouwels FH, et al. Randomised controlled trial of first-line tyrosine-kinase inhibitor (TKI) versus intercalated TKI with chemotherapy for EGFR-mutated nonsmall cell lung cancer. ERJ Open Res. (2022) 8. doi: 10.1183/23120541.00239-2022
46. Cho BC, Ahn MJ, Kang JH, Soo RA, Reungwetwattana T, Yang JC, et al. Lazertinib versus gefitinib as first-line treatment in patients with EGFR-mutated advanced non-small-cell lung cancer: results from LASER301. J Clin Oncol. (2023) 41:4208–17. doi: 10.1200/JCO.23.00515
47. Lu S, Zhou J, Jian H, Wu L, Cheng Y, Fan Y, et al. Befotertinib (D-0316) versus icotinib as first-line therapy for patients with EGFR-mutated locally advanced or metastatic non-small-cell lung cancer: a multicentre, open-label, randomised phase 3 study. Lancet Respir Med. (2023) 11:905–15. doi: 10.1016/S2213-2600(23)00183-2
48. Planchard D, Jänne PA, Cheng Y, Yang JC, Yanagitani N, Kim SW, et al. Osimertinib with or without chemotherapy in EGFR-mutated advanced NSCLC. N Engl J Med. (2023) 389:1935–48. doi: 10.1056/NEJMoa2306434
49. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. (2011) 12:735–42. doi: 10.1016/S1470-2045(11)70184-X
50. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol. (2015) 26:1877–83. doi: 10.1093/annonc/mdv276
51. Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. (2014) 15:1236–44. doi: 10.1016/S1470-2045(14)70381-X
52. Yamamoto N, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, et al. Erlotinib plus bevacizumab vs erlotinib monotherapy as first-line treatment for advanced EGFR mutation-positive non-squamous non-small-cell lung cancer: Survival follow-up results of the randomized JO25567 study. Lung Cancer. (2021) 151:20–4. doi: 10.1016/j.lungcan.2020.11.020
53. Zhu Y, Liu C, Xu Z, Zou Z, Xie T, Xing P, et al. Front-line therapy for brain metastases and non-brain metastases in advanced epidermal growth factor receptor-mutated non-small cell lung cancer: a network meta-analysis. Chin Med J (Engl). (2023) 136:2551–61. doi: 10.1097/CM9.0000000000002468
54. Cho BC, Lee KH, Cho EK, Kim D-W, Lee J-S, Han J-Y, et al. 1258O Amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in combination with lazertinib, a 3rd-generation tyrosine kinase inhibitor (TKI), in advanced EGFR NSCLC. Ann Oncol. (2020) 31. doi: 10.1016/j.annonc.2020.08.1572
55. Holleman MS, van Tinteren H, Groen HJ, Al MJ, Uyl-de Groot CA. First-line tyrosine kinase inhibitors in EGFR mutation-positive non-small-cell lung cancer: a network meta-analysis. Onco Targets Ther. (2019) 12:1413–21. doi: 10.2147/OTT.S189438
56. Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. (2020) 38:115–23. doi: 10.1200/JCO.19.01488
57. Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. (2020) 38:124–36. doi: 10.1200/JCO.19.01154
58. van Veelen A, Veerman GDM, Verschueren MV, Gulikers JL, Steendam CMJ, Brouns AJWM, et al. Exploring the impact of patient-specific clinical features on osimertinib effectiveness in a real-world cohort of patients with EGFR mutated non-small cell lung cancer. Int J Cancer. (2024) 154:332–42. doi: 10.1002/ijc.v154.2
59. Igawa S, Ono T, Kasajima M, Ishihara M, Hiyoshi Y, Kusuhara S, et al. Impact of EGFR genotype on the efficacy of osimertinib in EGFR tyrosine kinase inhibitor-resistant patients with non-small cell lung cancer: a prospective observational study. Cancer Manag Res. (2019) 11:4883–92. doi: 10.2147/CMAR.S207170
60. Nishikawa H, Goto M, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Cancer cachexia: its mechanism and clinical significance. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22168491
61. Shepshelovich D, Xu W, Lu L, Fares A, Yang P, Christiani D. Body mass index (BMI), BMI change, and overall survival in patients with SCLC and NSCLC: a pooled analysis of the International Lung Cancer Consortium. J Thorac Oncol. (2019) 14:2024. Erratum.
62. Jeon HL, Kwak M, Kim S, Yu HY, Shin JY, Jung HA. Comparative effectiveness of lazertinib in patients with EGFR T790M-positive non-small-cell lung cancer using a real-world external control. Sci Rep. (2024) 14:14659. doi: 10.1038/s41598-024-65220-z
63. Lee J, Lee H, Yoon D, Choi EY, Woo J, Jo B, et al. Lazertinib versus Platinum-Based Chemotherapy with Epidermal Growth Factor Receptor (EGFR)-Positive Non-Small-Cell Lung Cancer after Failing EGFR-Tyrosine Kinase Inhibitor: A Real-World External Comparator Study. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16122169
64. Wang C, Zhao K, Hu S, Dong W, Gong Y, Xie C. Clinical outcomes of afatinib versus osimertinib in patients with non-small cell lung cancer with uncommon EGFR mutations: A pooled analysis. Oncologist. (2023) 28:e397–405. doi: 10.1093/oncolo/oyad111
65. Borgeaud M, Parikh K, Banna GL, Kim F, Olivier T, Le X, et al. Unveiling the landscape of uncommon EGFR mutations in NSCLC-A systematic review. J Thorac Oncol. (2024) 19:973–83. doi: 10.1016/j.jtho.2024.03.016
66. Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. (2018) 29:i10–i9. doi: 10.1093/annonc/mdx703
67. Wang S, Li J. Second-generation EGFR and ErbB tyrosine kinase inhibitors as first-line treatments for non-small cell lung cancer. Onco Targets Ther. (2019) 12:6535–48. doi: 10.2147/OTT.S198945
68. Hsu PC, Lee SH, Chiu LC, Lee CS, Wu CE, Kuo SC, et al. Afatinib in untreated stage IIIB/IV lung adenocarcinoma with major uncommon epidermal growth factor receptor (EGFR) mutations (G719X/L861Q/S768I): A multicenter observational study in Taiwan. Target Oncol. (2023) 18:195–207. doi: 10.1007/s11523-023-00946-w
Keywords: EGFR mutation-positive, non-small cell lung cancer, non-squamous, first-line treatment, network meta-analysis
Citation: Zhang M and Sun L (2025) First-line treatment for advanced or metastatic EGFR mutation-positive non-squamous non-small cell lung cancer: a network meta-analysis. Front. Oncol. 14:1498518. doi: 10.3389/fonc.2024.1498518
Received: 19 September 2024; Accepted: 27 November 2024;
Published: 15 January 2025.
Edited by:
Matiullah Khan, AIMST University, MalaysiaReviewed by:
Kang Qin, University of Texas MD Anderson Cancer Center, United StatesLiyun Miao, Nanjing Drum Tower Hospital, China
Copyright © 2025 Zhang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Sun, c3VubGFuNjIwM0AxNjMuY29t
 Mengyao Zhang
Mengyao Zhang Lan Sun
Lan Sun