- 1Genomic Oncology Research Group, Department of Physiology and Medical Physics, RCSI University of Medicine and Health Sciences, Dublin, Ireland
- 2Department of Medical Oncology, Beaumont Hospital, Dublin, Ireland
- 3Cancer Clinical Trials and Research Unit, Beaumont Hospital, Dublin, Ireland
- 4Medical Oncology Group, Department of Medicine, RCSI University of Medicine and Health Sciences, Dublin, Ireland
Metastatic meningioma is rare, occurring in less than 1% of patients, and very few case studies have been reported, in particular for those that have spread to the lungs. Here we describe a rare case of metastatic meningioma to the lungs. Following a discussion at a medical oncology multi-disciplinary team meeting, whole genome sequencing was requested in November 2021 and discussed at a neurosurgical molecular tumor board in June 2022. Sequencing was performed on matched longitudinal collected samples of the primary tumor resection, the re-excised recurrent tumor after adjuvant radiation therapy, the lung metastases before treatment with sunitinib, and one paired blood sample for tumor-normal analysis. Whole genome characterization and clonal evolution analysis confirmed neurofibromatosis 2 (NF2) gene loss as the main driver of this cancer. In the same cancer clone as NF2, we identified a BRCA2 (p.E51K) mutation was present in all tumors, which may represent a potential driver event, though evidence supporting this is currently limited. Although this mutation is predicted to potentially influence homologous recombination, its clinical relevance as a biomarker for PARP inhibition remains speculative and requires further investigation. We also noted a SETD2 (p.S1885N) mutation that was present only in the recurrent tumor which was identified as a predicted biomarker of response to WEE1 inhibition. There was a stepwise increase in tumor mutational burden (TMB) from the primary meningioma to lung metastases, suggesting this patient may have been a candidate for immunotherapy.
1 Introduction
Meningioma is the most common type of primary brain tumor (1) with most being slow-growing benign low-grade tumors treatable using surgery and adjuvant radiotherapy. Tumors are classified according to the World Health Organization (WHO) grading system: Grade 1, 2, or 3 (2). Compared to WHO Grade 1 tumors, high-grade tumors are less common, with WHO Grade 2 (atypical) malignant meningiomas occurring at an age-adjusted incidence rate of ~9.12% per year (1). These tumors are more likely to be invasive and recur locally following initial treatment in 30%-50% of all patients (1). Metastatic meningioma is rare, occurring in less than 1% of patients (3), with lung the most common site of metastasis (4). Due to their rarity, very few case studies have been reported. Genomic characterization of meningioma has overall improved our understanding of the underlying tumor biology and helped refine tumor classification and identify potential alterations for targeted therapy (5–12). Sequencing efforts have primarily focused on low-grade meningiomas, while characterization of high-grade and/or metastatic tumors remains relatively infrequent (7); however, integrated genomic analyses have highlighted key pathways, such as the co-mutation of SMARCB1 in atypical meningiomas (13). The loss of chromosome 22 is one of the most common genomic alterations that affect meningiomas (14). On this chromosome, inactivation of the tumor suppressor gene neurofibromatosis 2 (NF2), which encodes for the protein Merlin, is the main driver of 50% of meningiomas (9, 14). Compared to low-grade Merlin-intact tumors, NF2-altered tumors are more likely to be high-grade tumors with less favorable clinical outcomes (9). Here, we present a case report of metastatic meningioma to the lungs where longitudinal collected tumor samples underwent whole genome sequencing. The aim was to better understand the molecular drivers of this metastatic meningioma and identify any potentially actionable alterations in the lung metastases. At present, there are no standard treatments for metastatic meningioma (8) and, as such, comprehensive genomic profiling of such cases not only furthers our knowledge of this rare entity in cancer but also may guide personalized medicine decision-making.
2 Case description
Presentation and history
A 52-year-old man with an Eastern Cooperative Oncology Group (ECOG) performance status of 1 initially presented with an isolated episode of collapse in October 2013. Prior to this presentation, he and his family had noticed behavioral changes, worsening short-term memory, and headaches not relieved by simple analgesia. A brain MRI scan depicted a large bifrontal mass lesion (Figure 1A) which straddled the midline on either side of the interhemispheric fissure in the anterior cranial fossa, measuring 7cm in width and almost 6cm in the anteroposterior dimension, consistent with a meningioma. Due to his escalating symptoms, he underwent a resection. The resection was performed via a bi-coronal flap. The elevation of one flap intraoperatively was noted to be difficult due to adherence to the meningioma. The meningioma was enucleated and a frozen sample at the time of surgery confirmed a meningioma. The base of the tumor arose from the underside of the sagittal sinus. The anterior aspect adhered to the falx, and the ipsilateral layer of the falx and surrounding tissues underwent diathermy. A Simpson 2 resection was achieved. This resected specimen confirmed a WHO grade 2 meningioma with focal rhabdoid changes. The specimen was moderately cellular, with large, ovoid nuclei and centrally located nucleoli with frequent mitosis and copious amounts of eosinophilic cytoplasm. Some cells showed intracytoplasmic ill-defined hyaline structures consistent with rhabdoid change. The proliferation index as assessed by MIB-1 was elevated at approximately 10%-15%. Adjuvant radiation of 60Gy/30 fractions was completed following recovery from surgical resection and a discussion at a neurosurgical multidisciplinary team meeting. Following this, the patient began a surveillance program with MRI scans performed every 3 months initially, which demonstrated postoperative encephalomalacia and gliosis within both the anterior and parasagittal frontal lobes. The interval increased to 6 months until the end of 2016 when a brain MRI scan demonstrated a local recurrence (Figure 1A). This MRI brain scan with contrast that showed the recurrence of his disease depicted two areas of lobulated enhancing tissue within the resection cavity. However, it was also noted that there was surrounding T2/FLAIR hyperintensity within the frontal lobes, consistent with post-radiation change. During this period, the patient remained asymptomatic with an ECOG performance status of 1 and he continued to work. However, due to further progression over two subsequent MRI scans, which showed increasing areas of enhancement extending from the prior resection cavity to the right frontal horn resulting in a midline shift, and discussion with his neurosurgical team, he opted to proceed with re-resection in September 2018. A histological examination demonstrated a recurrence of the WHO grade 2 meningioma. The patient completed a further course of radiation therapy (54Gy/30fractions), and recovered well, returning to work following recovery from radiation. Microsatellite instability (MSI) testing of the specimen resected in 2018 confirmed a microsatellite stable (MSS) tumor. Further surveillance MRI was performed at 3-month intervals until May of 2020 when the patient presented with an episode of collapse associated with a 2-month history of grade 1 dyspnoea and dry cough. Diagnostic imaging with a CT scan of his thorax, abdomen, and pelvis with contrast performed during this admission demonstrated multiple bilateral pulmonary masses (Figure 1A). Bronchoscopy and biopsy of the pulmonary lesions confirmed metastatic meningioma.
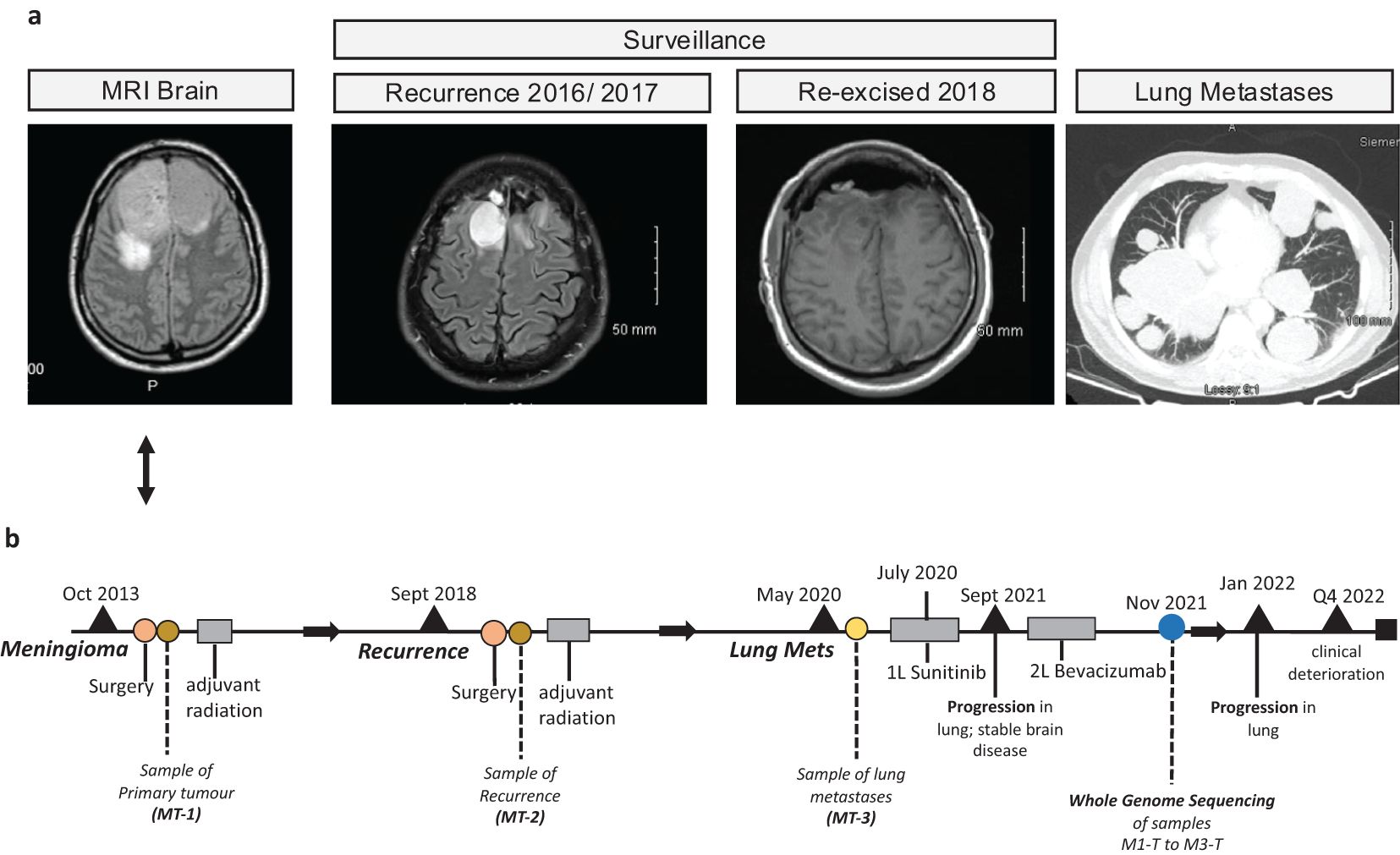
Figure 1. Case clinical history. (A) Images from magnetic resonance imaging (MRI) brain scans from the meningioma lung metastases case are presented here. (B) Graphical timeline plot summarizing the sample collection, treatment, and clinical event history for this case.
The standard of care adjuvant therapy in high-grade or atypical meningiomas is radiation, however, in this case, referral for consideration of systemic treatment was made due to the recurrence after radiation of new and progressive systemic disease. In July 2020, the patient was referred to medical oncology where he was commenced on sunitinib based on data from other case reports (15). These case reports showed that, in a small exploratory cohort of 13 patients, sunitinib is active in patients with recurrent/atypical meningioma with a progression-free survival of 5.2 months (15). He remained on sunitinib for 14 months and tolerated it well, with regular disease monitoring including both CT and MRI. A repeat CT scan of the thorax, abdomen, and pelvis and a brain MRI scan performed after 14 months of treatment, identified progression of the disease in the thorax, but stable disease in the brain. At this point, the patient was switched to bevacizumab based on a systemic review carried out by Franke et al. (16) but his disease progressed further after 4 months of this treatment, with progression again isolated to the disease in the lungs.
Following a discussion at a medical oncology multi-disciplinary team meeting, whole genome sequencing (WGS) was requested in November 2021 and discussed at a neurosurgical molecular tumor board (MTB) in June 2022. Detailed descriptions of the WGS, mutation, and copy number variant analyses are supplied in the Supplementary Material. Unfortunately, due to significant clinical deterioration and presentation with multiple episodes of seizure over the months preceding the MTB, the patient was not administered any further lines of systemic anti-cancer treatment.
Genomic characterization of meningioma lung metastases
WGS was performed on samples taken from the primary tumor resection (M1-T), the re-excised recurrent tumor (M2-T) after adjuvant radiation therapy, the lung metastases (M3-T) before treatment with sunitinib, and one paired blood sample for tumor-normal analysis (Figure 1B; Supplementary Figure S1). WGS data was used for genomic characterization of somatic single nucleotide variants (SNVs), insertions and deletions (InDels), and copy number alterations (SCNAs) across all tumor samples. We used the Bi et al.’s genomic sequencing of high-grade meningioma tumor samples (7) and Nassiri et al.’s molecular subgroups (11) as a reference for molecular classification and characterization of the meningioma tumors profiled here (Supplementary Figure S2).
The genome-wide somatic copy number profiles were remarkably similar across all tumor sample timepoints (Figure 2A). Chromosome 22q loss encompassing the NF2 gene was present in all tumors along with SCNAs previously reported in Grade 2 and Grade 3 meningioma primary tumors including chr1p, chr6q, chr10, chr14q, chr18p, and q copy number loss. In the NF2 gene, a C>T nonsense (stop gained) mutation was detected in the primary tumor (M1-T) and lung metastases (M3-T) at variant allele frequency (VAF) estimates of 32% and 100% (Figures 2B, C). This suggests that in combination with chromosome 22q loss, biallelic NF2 inactivation occurred in these tumors. Structural variant calling identified a large number of intrachromosomal inversions in both the primary tumor (n=114) and lung metastases (n=398) (Figures 2D, E) with few interchromosomal translocations (TRAs) identified.
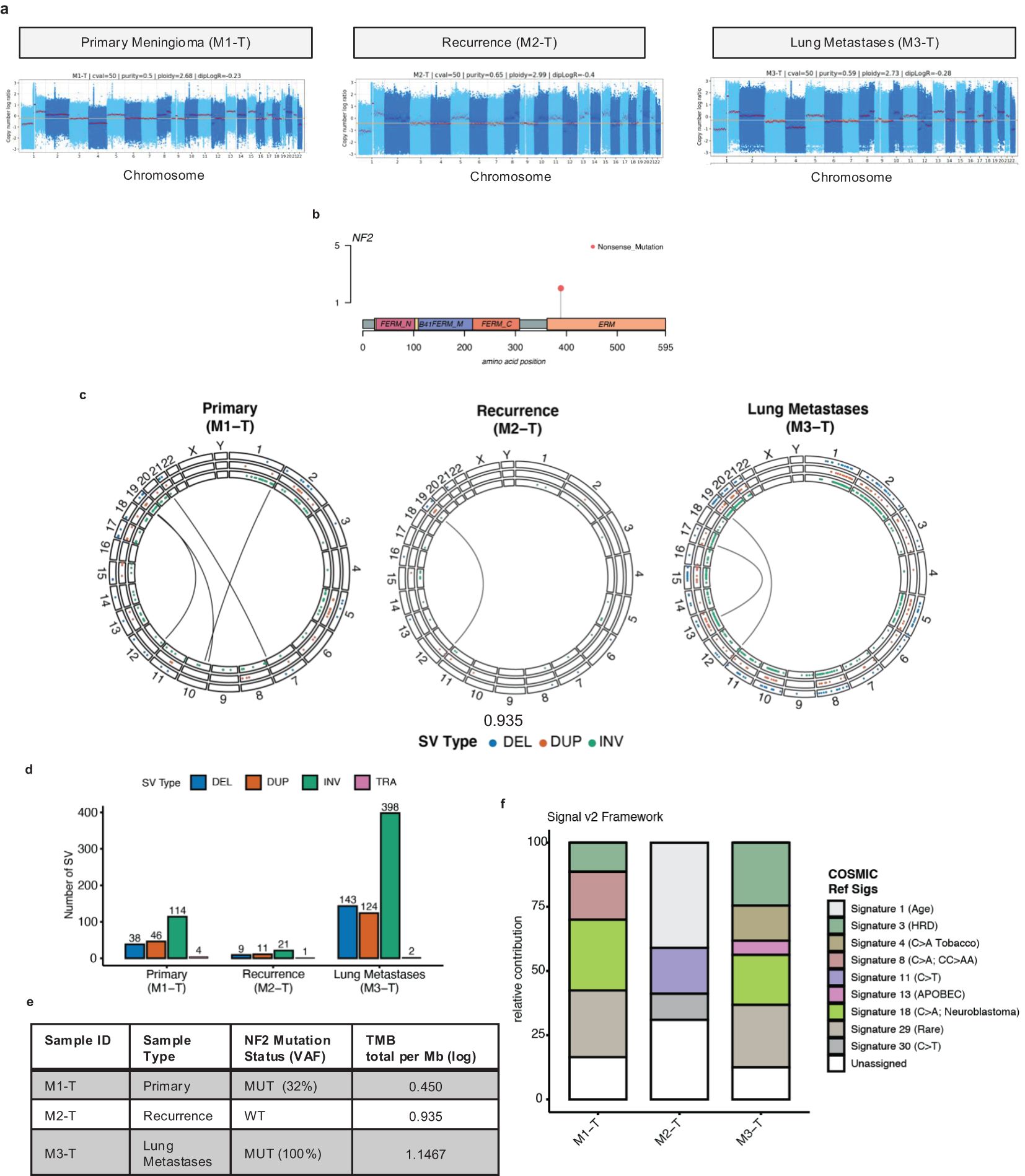
Figure 2. Genome-wide characterization of meningioma lung metastases tumor samples. (A) Somatic copy number log ratio (tumor over normal) coverage plots for the primary tumor (M1-T), recurrence (M2-T), and lung metastases (M3-T). The red horizontal line indicates a diploid log ratio reference. (B) Lolliplot of a recurrent missense mutation Q389* (orange dot) identified in the Merlin protein encoded by the NF2 gene from the WGS data (C) Circos plots of genome-wide chromosomal structural variation [DEL: deletions (blue dots); DUP: duplications (orange dots); INV: inversions (green dots); inter chromosomal translocations (TRA) indicated by black center line links] across all tumor samples. (D) Bar chart of the number of somatic SVs detected in each tumor sample with the colors indicating SV type [DEL (blue), DUP (orange), INV (green), TRA (pink)]. (E) Summary of the nonsynonymous TMB estimates [log total per megabase (Mb)] for tumor samples (M1-T, M2-T, M3-T). (F) Stacked bar chart of the relative contribution (0-100) of the COSMIC reference mutational signatures (Signature 1-30) detected in each tumor sample (left-right) using the Signal (v2) mutational signature profiling framework.
Overall, the SCNA profiles here are characteristic of higher-grade meningioma tumors and of the MG4 proliferative and hypermitotic tumor subtypes reported by Nassiri et al. and Choundary et al. respectively (11, 12). Next, the tumor mutational burden (TMB) was calculated as the total number of nonsynonymous somatic mutations divided by the exome region length in megabases (Mb) (Figure 2F). This analysis identified a twofold increase in TMB from 0.450 (log TMB/Mb) in the primary meningioma tumor to 0.935 (log TMB/Mb) in the recurrence and 1.1467 (log TMB/Mb) in the lung metastases. The primary and recurrence TMBs were in the same range as previously reported nonsynonymous TMB estimates for higher-grade, NF2-altered recurrent meningioma tumors (Supplementary Figure S2). Next, in order to investigate which mutagenic process may have led to a stepwise increase in TMB and chromosomal instability with disease progression, we performed a mutational signature analysis of somatic single nucleotide variants (Figure 2F; Supplementary Figure S3). Mutational signatures extracted from tumor samples were fitted to Catalogue Of Somatic Mutations In Cancer (COSMIC) reference signatures (Signatures 1-30). In both the primary meningioma and lung metastases we detected Signature 3 [associated with homologous repair deficiency (HRD)], Signature 18 (C>A; etiology unknown; found in neuroblastoma) and Signature 29 (Rare; C>A mutation in tobacco chewing). Signature 4, a C>A mutation pattern associated with exposure to tobacco smoking [this case is an ex-smoker (~25 pack years)] and APOBEC-associated Signature 13 were detected only in the lung metastases, with Signature 8 (C>A; found in medulloblastoma) found only in the primary meningioma. None of these signatures were detected in the recurrence, which was overall dominated by a C>T mutation context pattern associated with three mutational signatures: Signature 1 (ageing), Signature 11 (alkylating agents in glioblastoma and melanoma), and Signature 30 (BER deficiency). In the recurrence, we did detect a predicted loss of function driver mutation in SETD2, a reported DNA MMR regulator (17), however, the MSS classification given by MSI testing, performed by the molecular pathology team in 2018, was microsatellite stable.
Next, in order to better understand which, if any, driver mutations besides NF2 may be associated with cancer initiation, progression, and metastases, we used Cancer Genome Interpreter (CGI) to assign known or predicted novel driver status to nonsynonymous mutations. Following this, for clonal evolution analysis, PyCloneVI and ClonEvol were used to infer clonal population structure and clonal ordering from copy number and purity-adjusted VAFs from somatic mutations across all tumor sample timepoints (Figure 3A; Supplementary Figure S4). Five tumor clones (labeled Cluster/Clone #1 to #5) were detected across the primary meningioma, recurrence, and lung metastases sample timepoints (Figure 3C). The primary tumor was composed of two clones: Clone #3 and Clone #5, with Clone #3 present at a clonal prevalence of 0.915. This clone was stably maintained in both the recurrence and lung metastases at clonal prevalence values of 0.676 and 0.968 respectively. Nonsynonymous predicted driver mutations in Clone #3 included NF2 (p.Q389*), as previously described above, BRCA2 (p.E51K), and SEC23B (p.G285V) (Figure 3B). This suggests Clone #3 led to the initiation and development of the primary meningioma tumor with NF2 the main driver event. Our analysis also suggests that the presence of BRCA2 may represent a secondary or potentially modifying genetic event; however, this interpretation warrants cautious consideration, particularly given the established role of NF2 mutations in meningioma. Clone #5 was detected only in the primary tumor and contained mutations in genes previously reported to be frequently mutated (> 2 tumors) in high-grade meningioma including LRP1B (7). In addition to Clone #3, the recurrence was composed of two other clones, Clone #1 and Clone #4. Clone #4 contained the driver mutation, previously detailed above, in SETD2 (p.S1885N) and it appears to have not seeded the lung metastases, however, Clone #1 did. The lung metastases were composed of Clone #3, Clone #1, and Clone #2 (Figures 3D, E). Although SMARCB1 mutations have previously been shown to be co-mutated in atypical meningiomas (13), we did not identify any SMARCB1 mutations in our study.
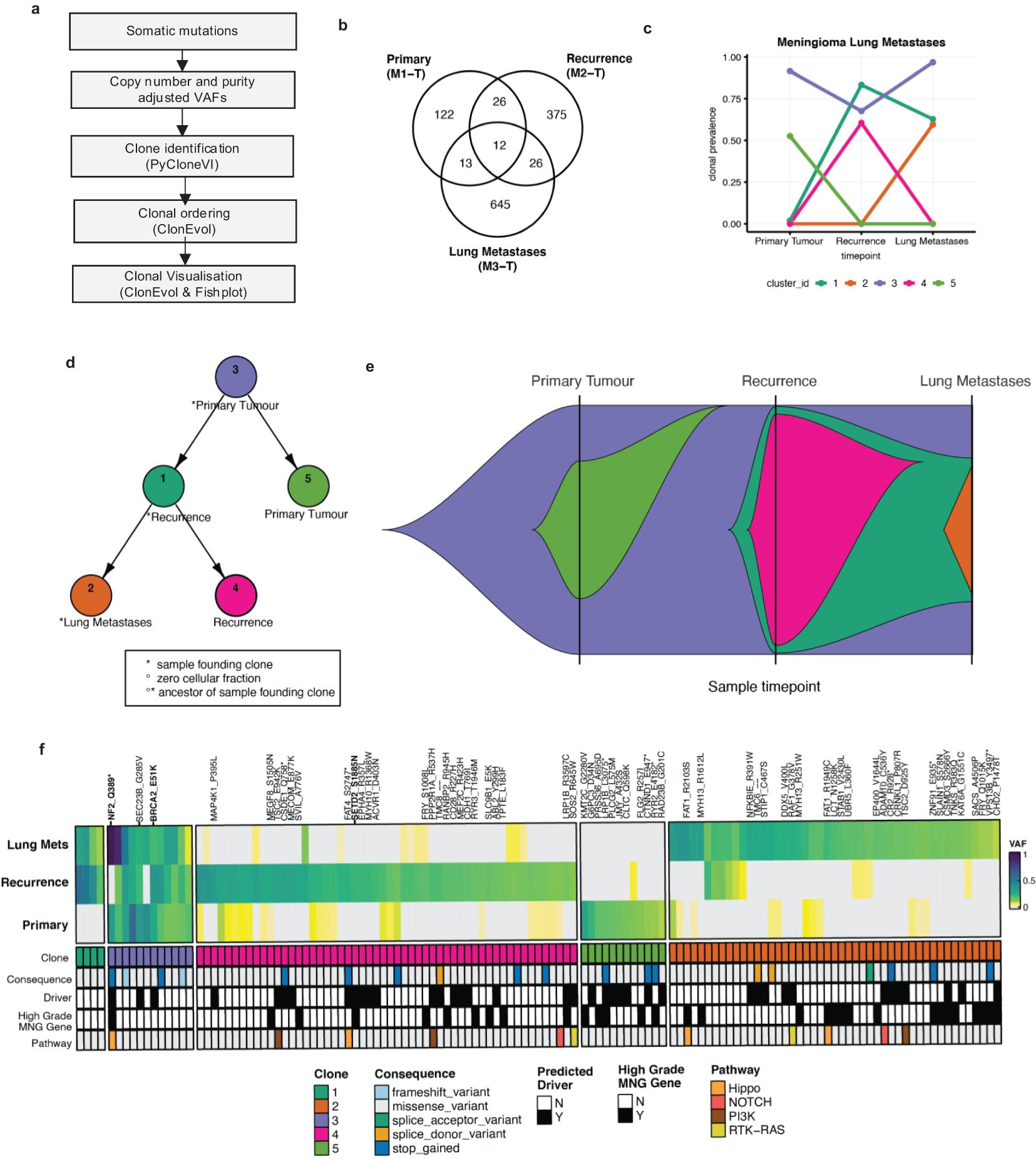
Figure 3. Clonal evolution in the meningioma lung metastases. (A) High-level graphical overview of clonal evolution analysis workflow. (B) Venn diagram of the nonsynonymous somatic mutation counts from all tumor samples. (C) Line plot of clonal prevalence (0-1.0) changes across all tumor sample timepoints (left to right). Five tumor clones [Clone #1 (dark green), #2 (orange), #3 (purple), #4 (pink), #5 (purple)] were identified using PyCloneVI. (D) Node tree plot shows the clonal ordering of the tumor clones from ClonEvol. (E) Fish plot shows a graphical representation of the clonal evolution across all tumor sample timepoints for the primary tumor (left), recurrence (middle), and lung metastases (right). (F) Heatmap of VAF (0-1.0) values for a subset of the total nonsynonymous somatic mutations for the primary meningioma (bottom), recurrence (middle), and lung metastases (top). The mutations were selected for heatmap visualization if they were classified as either a predicted driver mutation by the Cancer Genome Interpreter (CGI), annotated as a frequently mutated gene in high-grade meningioma (MNG) tumors, or if the mutation was present in 2 or more tumor samples. The mutations shown in the heatmap are sorted by PyCloneVI clone assignment. The mutations are additionally annotated by consequence type and canonical oncogenic pathway membership.
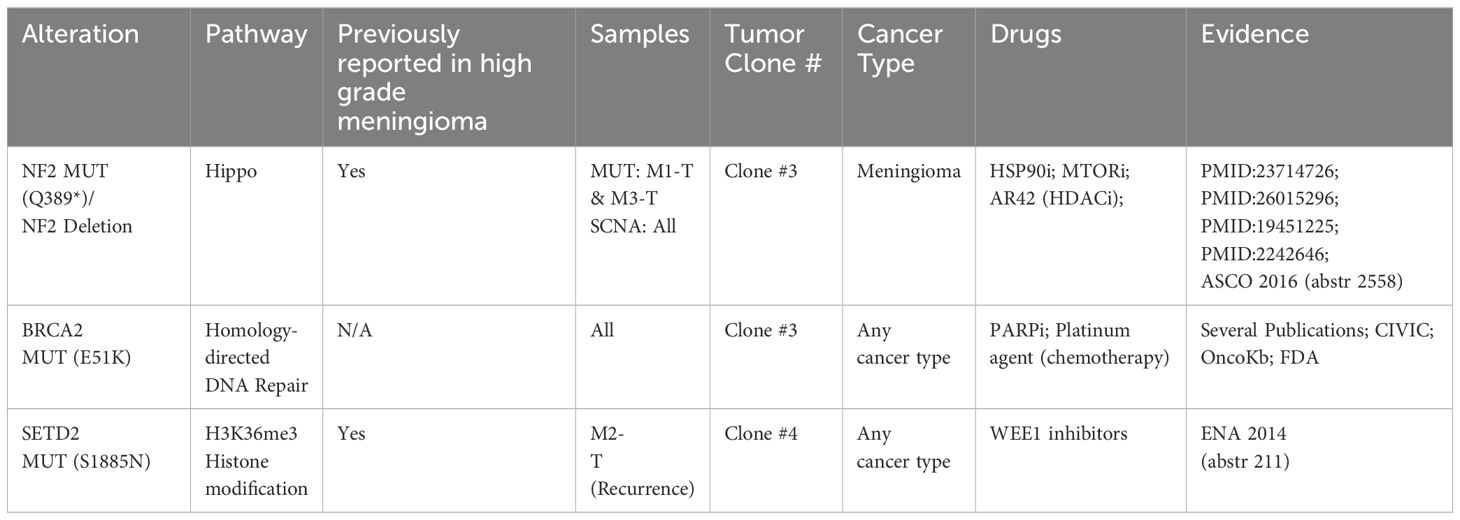
Next, having characterized the clonal composition, evolution, and dynamics of the meningioma lung metastases, we used this information along with CGI annotation to identify which mutations were drug response biomarkers (Table 1). The only predicted drug-responsive biomarkers detected were NF2 (p.Q389* mutation/copy number loss or deletion) and BRCA2 (p.E51K mutation) in Clone #3, present in all three tumors and SETD2 (p.S1885N mutation) present only in Clone #4 in the recurrence (Figure 3F).
3 Discussion
Metastatic meningioma is rare with limited treatment options. Here, molecular-based classification of longitudinal tumors using WGS data, from a case of metastatic meningioma, identified features reported to be characteristic of MG4 proliferative (11, 12) and hypermitotic (12) tumor subtypes. Common characteristics of these tumor molecular subtypes are a higher TMB relative to all other subtypes, risk of recurrence, unfavorable outcomes, and high levels of aneuploidy including copy number loss in chr22q, 1p, and, specific to the MG4 subtype, chr10 loss amongst others (7, 11, 12, 18). Molecular profiling from patients with primary atypical meningiomas, albeit in a small number of patients, has been described previously (19, 20). Within a group of 22 patients profiled by Barresi et al., TMB ranged from 2.19mut/Mb to 12.68mut/Mb at a single timepoint (19). In this case, TMB was assessed in the primary meningioma and the recurrence, showing that the TMB increased over time. Chromosome 1p and 10p loss in particular has been shown to be a strong predictor of decreased recurrence-free survival for patients with WHO grade 2 meningioma following gross total resection (18). In Choudhury et al.’s study, the hypermitotic subtype was shown to have decreased immune infiltration compared to an immune-rich subtype (12), suggesting an immunosuppressive tumor microenvironment that may respond to immune checkpoint inhibition. Others have reported that higher-grade meningiomas are composed of several mutations which are predicted to be neoantigens (7). Interestingly, here we identified a stepwise increase in tumor mutational burden from the primary meningioma and recurrence to the lung metastases, suggesting this patient may have been a candidate for immunotherapy (8). Recently, there have been a few phase II clinical trials evaluating the efficacy of anti-programmed death ligand 1 (PD-1) inhibitors in recurrent/progressive grade 2/3 meningioma (21, 22). Immunotherapy response in these trials has been variable, with one trial reporting treatment with nivolumab did not significantly increase the 6-month progression-free survival (PFS-6) (22), while in another, treatment with pembrolizumab did increase PFS-6 (21). However, notably in both trials, there were reports of a long-term durable response to immune checkpoint inhibition in a subset of meningiomas with elevated TMBs, including one patient with distal metastatic disease, treated with pembrolizumab who had a PFS lasting ~20 months. Mismatch repair deficiency (MMRd) is rare in meningioma but was found to be the probable cause of elevated TMB in at least one of the exceptional responder cases (17). Here, MSI testing and mutational signature analysis showed little evidence of MMRd in these tumors. Interestingly, in Bi et al.’s study (7), they also observed one case with an elevated level of TMB where there was no evidence for mismatch repair defects, suggesting that the rate of mutagenesis is due to some other mutagenic process. Consistent with this study, we identified a prevalence of C>T transitions, in particular in the recurrence, reported previously in meningioma to be associated with exposure to adjuvant radiation. In the lung metastases, given the smoking status of this patient, the prevalence of C>A mutations are likely due to tobacco-associated mutagenesis.
Clonal evolution analysis confirmed NF2 gene loss as the main driver of this cancer. However, interestingly, in the same cancer clone as NF2, we identified a potential BRCA2 driver mutation (p.E51K) present in all tumors along with a SETD2 (p.S1885N) mutation present only in the recurrence. BRCA-associated protein 1 (BAP1), a tumor suppressor gene whose mutation is often accompanied by NF2 disruption, has previously been described in both in vitro and in vivo models and is associated not just with meningioma, but also has a significant association with malignant mesothelioma in mouse models (23–26). However, in our study, in both the primary meningioma and lung metastases, the mutational signature analysis indicated some evidence for a mutational signature associated with HRD. Typically, HRD is present in BRCA1/2-mutated or BRCA-like tumors and so it could be possible the increase in TMB is due to this type of DNA repair pathway defect, not MMRd. Although not well described in meningioma, BRCA2 mutations are predicted to be a biomarker of drug response to PARP inhibition (27). While our analysis identified a BRCA2 (p.E51K) mutation present alongside NF2 gene loss in the same cancer clone across all tumor samples, it is important to acknowledge that the evidence supporting BRCA2 (p.E51K) as a driver mutation in meningioma is not well-established. Current databases, including ClinVar and OncoKB, classify this variant as being of uncertain biological significance. Furthermore, the absence of this mutation from key somatic cancer mutation references such as COSMIC further emphasizes this ambiguity. The interpretation of BRCA2 (p.E51K) as a driver mutation in this context warrants careful consideration, especially given that NF2 is a well-recognized primary driver in meningiomas. However, the presence of BRCA2 raises the possibility of a secondary role, either as a non-driver mutation or a modifier effect that could impact tumor behavior. While this mutation may represent a potential biomarker of interest, particularly for its predicted responsiveness to PARP inhibitors, definitive conclusions regarding its role and therapeutic implications require further validation.
A SETD2 mutation, which was identified as a predicted biomarker of response to WEE1 inhibition was noted in the recurrence sample (28). Although studies on WEE1 inhibition in meningiomas are limited, preclinical evidence from other brain tumors suggest that WEE1 inhibitors can enhance the effects of radiation and induce tumor cell death (29).
In this patient, for the first- and second-line treatment of their lung metastases, there was a response to sunitinib and bevacizumab for 14 and 4 months respectively. The use of tyrosine kinase inhibition such as sunitinib and monoclonal antibodies targeting anti-angiogenic pathways such as vascular endothelial growth factor (VEGF) signaling has shown antitumoral activity in phase II trials for recurrent meningioma (15, 30, 31). Other tyrosine kinase inhibitors that have also been explored in NF2-associated schwannomas and meningiomas include crizotinib, brigatinib, and dasatinib (32–34). Furthermore, a recent case report demonstrated that it may be possible to use concurrent anti-PD-1 and anti-VEGF in cases of recurrent high-grade metastatic meningioma (35).
There are certain limitations associated with using in silico tools, such as CGI, to determine variant significance. While CGI integrates data from multiple databases and algorithms to predict variant impact, not all variants of uncertain significance can be conclusively classified as drivers without further biological validation. Specifically, the classification of the BRCA2 (p.E51K) mutation as a driver remains inconclusive, necessitating additional studies to explore its functional impact in meningiomas. We also noted a high number of structural variants (SVs) in both the primary tumor and lung metastases, however relevant evidence connecting specific SVs to known cancer pathways remains limited.
Despite these limitations, in this case report, molecular characterization and clonal evolution analysis of longitudinal tumors using WGS data identified potentially actionable alterations in meningioma metastases to the lungs. Unfortunately, due to significant clinical deterioration over the months preceding the molecular tumor board, the patient was not administered further lines of systemic anti-cancer treatment. However, this report highlights how clonal evolution analysis and comprehensive genomic alteration profiling can help further our knowledge of this rare entity in cancer but may also guide personalized medicine decision-making.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical approval was not required for the studies involving humans because the study was conducted in accordance with the local legislation and institutional requirements. Informed consent to conduct the study was obtained from the patient and was recorded in the patient’s notes by the patient’s clinician. This patient provided informed consent, in the presence of his next of kin, for whole genome sequencing and for the use of the case including radiological findings and clinical course. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
NC: Conceptualization, Formal analysis, Writing – original draft. OF: Conceptualization, Writing – original draft, Data curation, Investigation. LG: Conceptualization, Investigation, Writing – review & editing. BH: Conceptualization, Investigation, Supervision, Writing – review & editing. SF: Conceptualization, Supervision, Writing – review & editing. ST: Conceptualization, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the North-East Cancer Research and Education Trust (NECRET) (Grant number:1696) and by a Health Research Charities (HRCI), Health Research Board (HRB), and Breakthrough Cancer Research joint funding scheme (HRCI-HRB-2020-023).
Acknowledgments
We would like to acknowledge the Irish Centre for High End Computing (https://www.ichec.ie/) for the use of HPC infrastructure for sequencing data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1483126/full#supplementary-material
References
1. Low JT, Ostrom QT, Cioffi G, Neff C, Waite KA, Kruchko C, et al. Primary brain and other central nervous system tumors in the United States (2014-2018): A summary of the CBTRUS statistical report for clinicians. Neurooncol Pract. (2022) 9:165–82. doi: 10.1093/nop/npac015
2. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
3. Dalle Ore CL, Magill ST, Yen AJ, Shahin MN, Lee DS, Lucas CG, et al. Meningioma metastases: incidence and proposed screening paradigm. J Neurosurg. (2020) 132:1447–55. doi: 10.3171/2019.1.JNS181771
4. Bailey DD, Montgomery EY, Garzon-Muvdi T. Metastatic high-grade meningioma: A case report and review of risk factors for metastasis. Neurooncol Adv. (2023) 5:vdad014. doi: 10.1093/noajnl/vdad014
5. Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. (2013) 45:285–9. doi: 10.1038/ng.2526
6. Yew A, Trang A, Nagasawa DT, Spasic M, Choy W, Garcia HM, et al. Chromosomal alterations, prognostic factors, and targeted molecular therapies for Malignant meningiomas. J Clin Neurosci. (2013) 20:17–22. doi: 10.1016/j.jocn.2012.02.007
7. Bi WL, Greenwald NF, Abedalthagafi M, Wala J, Gibson WJ, Agarwalla PK, et al. Genomic landscape of high-grade meningiomas. NPJ Genom Med. (2017) 2. doi: 10.1038/s41525-017-0023-6
8. Preusser M, Brastianos PK, Mawrin C. Advances in meningioma genetics: novel therapeutic opportunities. Nat Rev Neurol. (2018) 14:106–15. doi: 10.1038/nrneurol.2017.168
9. Brastianos PK, Galanis E, Butowski N, Chan JW, Dunn IF, Goldbrunner R, et al. Advances in multidisciplinary therapy for meningiomas. Neuro Oncol. (2019) 21:i18–31. doi: 10.1093/neuonc/noy136
10. Lee YS, Lee YS. Molecular characteristics of meningiomas. J Pathol Transl Med. (2020) 54:45–63. doi: 10.4132/jptm.2019.11.05
11. Nassiri F, Liu J, Patil V, Mamatjan Y, Wang JZ, Hugh-White R, et al. A clinically applicable integrative molecular classification of meningiomas. Nature. (2021) 597:119–25. doi: 10.1038/s41586-021-03850-3
12. Choudhury A, Magill ST, Eaton CD, Prager BC, Chen WC, Cady MA, et al. Meningioma DNA methylation groups identify biological drivers and therapeutic vulnerabilities. Nat Genet. (2022) 54:649–59. doi: 10.1038/s41588-022-01061-8
13. Harmanci AS, Youngblood MW, Clark VE, Coskun S, Henegariu O, Duran D, et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun. (2017) 8:14433. doi: 10.1038/ncomms14433
14. Ruttledge MH, Sarrazin J, Rangaratnam S, Phelan CM, Twist E, Merel P, et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet. (1994) 6:180–4. doi: 10.1038/ng0294-180
15. Kaley TJ, Wen P, Schiff D, Ligon K, Haidar S, Karimi S, et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. (2015) 17:116–21. doi: 10.1093/neuonc/nou148
16. Franke AJ, Skelton WI, Woody LE, Bregy A, Shah AH, Vakharia K, et al. Role of bevacizumab for treatment-refractory meningiomas: A systematic analysis and literature review. Surg Neurol Int. (2018) 9:133. doi: 10.4103/sni.sni_264_17
17. Dunn IF, Du Z, Touat M, Sisti MB, Wen PY, Umeton R, et al. Mismatch repair deficiency in high-grade meningioma: a rare but recurrent event associated with dramatic immune activation and clinical response to PD-1 blockade. JCO Precis Oncol. (2018). doi: 10.1200/PO.18.00190
18. Vaubel RA, Kumar R, Weiskittel TM, Jenkins S, Dasari S, Uhm JH, et al. Genomic markers of recurrence risk in atypical meningioma following gross total resection. Neurooncol Adv. (2023) 5:vdad004. doi: 10.1093/noajnl/vdad004
19. Barresi V, Simbolo M, Fioravanzo A, Piredda ML, Caffo M, Ghimenton C, et al. Molecular profiling of 22 primary atypical meningiomas shows the prognostic significance of 18q heterozygous loss and CDKN2A/B homozygous deletion on recurrence-free survival. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13040903
20. Du Y, Lu T, Huang S, Ren F, Cui G, Chen J. Somatic mutation landscape of a meningioma and its pulmonary metastasis. Cancer Commun (Lond). (2018) 38:16. doi: 10.1186/s40880-018-0291-2
21. Brastianos PK, Kim AE, Giobbie-Hurder A, Lee EQ, Wang N, Eichler AF, et al. Phase 2 study of pembrolizumab in patients with recurrent and residual high-grade meningiomas. Nat Commun. (2022) 13:1325. doi: 10.1038/s41467-022-29052-7
22. Bi WL, Nayak L, Meredith DM, Driver J, Du Z, Hoffman S, et al. Activity of PD-1 blockade with nivolumab among patients with recurrent atypical/anaplastic meningioma: phase II trial results. Neuro Oncol. (2022) 24:101–13. doi: 10.1093/neuonc/noab118
23. Xu D, Yin S, Shu Y. NF2: An underestimated player in cancer metabolic reprogramming and tumor immunity. NPJ Precis Oncol. (2024) 8:133. doi: 10.1038/s41698-024-00627-5
24. Yap TA, Aerts JG, Popat S, Fennell DA. Novel insights into mesothelioma biology and implications for therapy. Nat Rev Cancer. (2017) 17:475–88. doi: 10.1038/nrc.2017.42
25. Badhai J, Pandey GK, Song JY, Krijgsman O, Bhaskaran R, Chandrasekaran G, et al. Combined deletion of Bap1, Nf2, and Cdkn2ab causes rapid onset of Malignant mesothelioma in mice. J Exp Med. (2020) 217. doi: 10.1084/jem.20191257
26. Kukuyan AM, Sementino E, Kadariya Y, Menges CW, Cheung M, Tan Y, et al. Inactivation of bap1 cooperates with losses of nf2 and cdkn2a to drive the development of pleural Malignant mesothelioma in conditional mouse models. Cancer Res. (2019) 79:4113–23. doi: 10.1158/0008-5472.CAN-18-4093
27. Lee JM, Ledermann JA, Kohn EC. PARP Inhibitors for BRCA1/2 mutation-associated and BRCA-like Malignancies. Ann Oncol. (2014) 25:32–40. doi: 10.1093/annonc/mdt384
28. Pfister SX, Markkanen E, Jiang Y, Sarkar S, Woodcock M, Orlando G, et al. Inhibiting WEE1 selectively kills histone H3K36me3-deficient cancers by dNTP starvation. Cancer Cell. (2015) 28:557–68. doi: 10.1016/j.ccell.2015.09.015
29. Mir SE, De Witt Hamer PC, Krawczyk PM, Balaj L, Claes A, Niers JM, et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell. (2010) 18:244–57. doi: 10.1016/j.ccr.2010.08.011
30. Mair MJ, Berghoff AS, Brastianos PK, Preusser M. Emerging systemic treatment options in meningioma. J Neurooncol. (2023) 161:245–58. doi: 10.1007/s11060-022-04148-8
31. Schroeder RD, Angelo LS, Kurzrock R. NF2/merlin in hereditary neurofibromatosis 2 versus cancer: biologic mechanisms and clinical associations. Oncotarget. (2014) 5:67–77. doi: 10.18632/oncotarget.1557
32. Troutman S, Moleirinho S, Kota S, Nettles K, Fallahi M, Johnson GL, et al. Crizotinib inhibits NF2-associated schwannoma through inhibition of focal adhesion kinase 1. Oncotarget. (2016) 7:54515–25. doi: 10.18632/oncotarget.10248
33. Chang LS, Oblinger JL, Smith AE, Ferrer M, Angus SP, Hawley E, et al. Brigatinib causes tumor shrinkage in both NF2-deficient meningioma and schwannoma through inhibition of multiple tyrosine kinases but not ALK. PloS One. (2021) 16:e0252048. doi: 10.1371/journal.pone.0252048
34. Sagers JE, Beauchamp RL, Zhang Y, Vasilijic S, Wu L, DeSouza P, et al. Combination therapy with mTOR kinase inhibitor and dasatinib as a novel therapeutic strategy for vestibular schwannoma. Sci Rep. (2020) 10:4211. doi: 10.1038/s41598-020-60156-6
Keywords: meningioma, lung metastases, whole genome sequencing, targeted therapies, driver mutations
Citation: Cosgrove N, Fitzpatrick OM, Grogan L, Hennessy BT, Furney SJ and Toomey S (2025) Case report: Clonal evolution analysis of a rare case of meningioma lung metastases identifies actionable alterations in matched longitudinal tumour samples. Front. Oncol. 14:1483126. doi: 10.3389/fonc.2024.1483126
Received: 19 August 2024; Accepted: 22 November 2024;
Published: 28 January 2025.
Edited by:
Kristin Huntoon, University of Arizona, United StatesReviewed by:
Andrea Carai, Bambino Gesù Children’s Hospital (IRCCS), ItalyDanielle F Miyagishima, Yale University, United States
Noor Ul Huda Maria, Mount Sinai Hospital, United States
Copyright © 2025 Cosgrove, Fitzpatrick, Grogan, Hennessy, Furney and Toomey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simon J. Furney, c2ltb25mdXJuZXlAcmNzaS5pZQ==; Sinead Toomey, c2luZWFkdG9vbWV5QHJjc2kuaWU=
†These authors have contributed equally to this work
 Nicola Cosgrove
Nicola Cosgrove Orla M. Fitzpatrick2,3†
Orla M. Fitzpatrick2,3† Simon J. Furney
Simon J. Furney Sinead Toomey
Sinead Toomey