- 1Department of Cardiac, Vascular and Endovascular Surgery and Transplantology, Medical University of Silesia in Katowice, Silesian Center for Heart Diseases, Zabrze, Poland
- 2Student Scientific Association of Adult Cardiac Surgery, Department of Cardiac, Vascular and Endovascular Surgery and Transplantology, Medical University of Silesia in Katowice, Katowice, Poland
Introduction: Pericardial mesothelioma is an exceedingly rare pericardial neoplasm. It has atypical clinical symptoms and imaging characteristics that often lead to an inconclusive diagnosis. The diagnosis of a rare tumor such as pericardial mesothelioma, which can present with a variety of manifestations, requires a multidisciplinary approach.
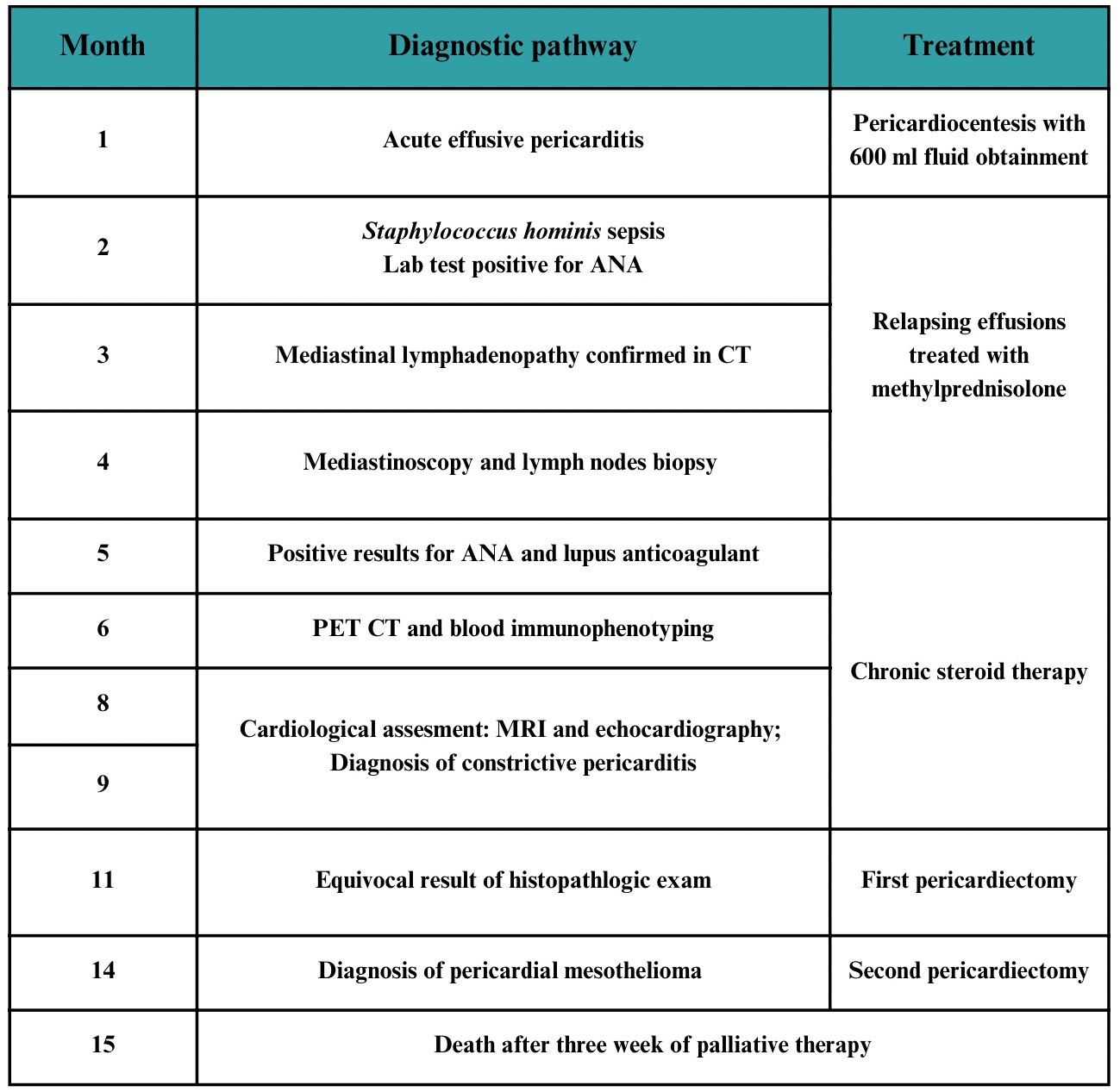
Case presentation: A 36-year-old Caucasian female patient without significant past medical history was admitted to the hospital with chest pain and a high fever and was diagnosed with acute pericarditis. The following month, the patient was treated for sepsis; during this hospitalization, lab tests for antinuclear antibodies (ANA) turned out to be positive. Concurrently, mediastinal lymphadenopathy was observed. Given the suspicion of mediastinal lymphoma, a mediastinoscopy with lymph node biopsy was performed. Following a negative biopsy result, positron emission tomography combined with computed tomography (PET/CT) and blood immunophenotyping were performed. Both tests ruled out a diagnosis of lymphoma. Concurrently, the patient was hospitalized in the rheumatology department due to positive ANA results. There, in addition to the ANA titer at a level of 1:320, lupus anticoagulant was detected. The patient was diagnosed with systemic lupus erythematosus (SLE) and initiated on chronic steroid therapy. As heart failure progressed, the patient was admitted to the cardiology department. Tissue Doppler echocardiography and cardiac magnetic resonance imaging (MRI) revealed features indicative of constrictive pericarditis. The patient underwent a pericardiectomy with satisfactory results. However, the pathology result of the pericardium remained equivocal. The patient was readmitted 3 months later with severe circulatory failure, and a salvage procedure of pericardiectomy was performed. Histopathological examination of the sections confirmed the diagnosis of pericardial epithelioid mesothelioma. The patient died after 3 weeks of palliative care.
Conclusions: In the differential diagnosis of relapsing and resultant constrictive pericarditis, neoplastic processes that may mimic systemic rheumatic diseases should also be considered. Pericardial mesothelioma is a very rare diagnosis and may result in increased ANA titers, particularly anti-dense fine speckled 70 (DFS70) antibodies.
Introduction
Constrictive pericarditis is a serious consequence of chronic pericarditis. The pericardium becomes overgrown, with fibrous thickening and also calcifications, which impairs the diastolic function of the heart. Rarely, the cause of constrictive pericarditis may be neoplastic processes. Primary cardiac neoplasms are rare entities with a prevalence of 0.001%–0.056% and account for 0.3% to 0.7% of all cardiac cancers (1). Pericardial mesothelioma has atypical clinical symptoms and imaging characteristics that often lead to inconclusive diagnosis. The lack of effective chemotherapy treatment is associated with a poor prognosis (2). This case study presents a detailed diagnostic pathway of a patient with pericardial mesothelioma, which manifested as lymphoma and rheumatic disease.
Case presentation
A 36-year-old female patient with a history of bilateral conjunctivitis (8 months earlier) was admitted to the hospital with chest pain and high fever. She was diagnosed with acute pericarditis. Pericardiocentesis was performed, yielding 600 mL of fibrinous exudate, with negative microbiological results (Figure 1).
One month later, the patient was readmitted with symptoms of a very severe pulsatile headache, which did not respond to standard pain treatment. Neuroinfection was ruled out by the cerebrospinal fluid examination and imaging studies. Based on elevated inflammatory markers and one positive blood culture for Staphylococcus hominis, antibiotic therapy with cloxacillin was initiated. The patient was treated for 14 days for bacteremia of unknown origin.
Additional investigations were performed at this time. In echocardiographic assessment, no vegetations were seen on cardiac valves, and the pericardium was slightly thickened with trace amounts of fluid (2 mm). Laboratory tests revealed antinuclear antibodies (ANA) with a nuclear dense fine speckled type. A computed tomography (CT) scan showed mediastinal lymphadenopathy with lymph nodes up to 2 cm.
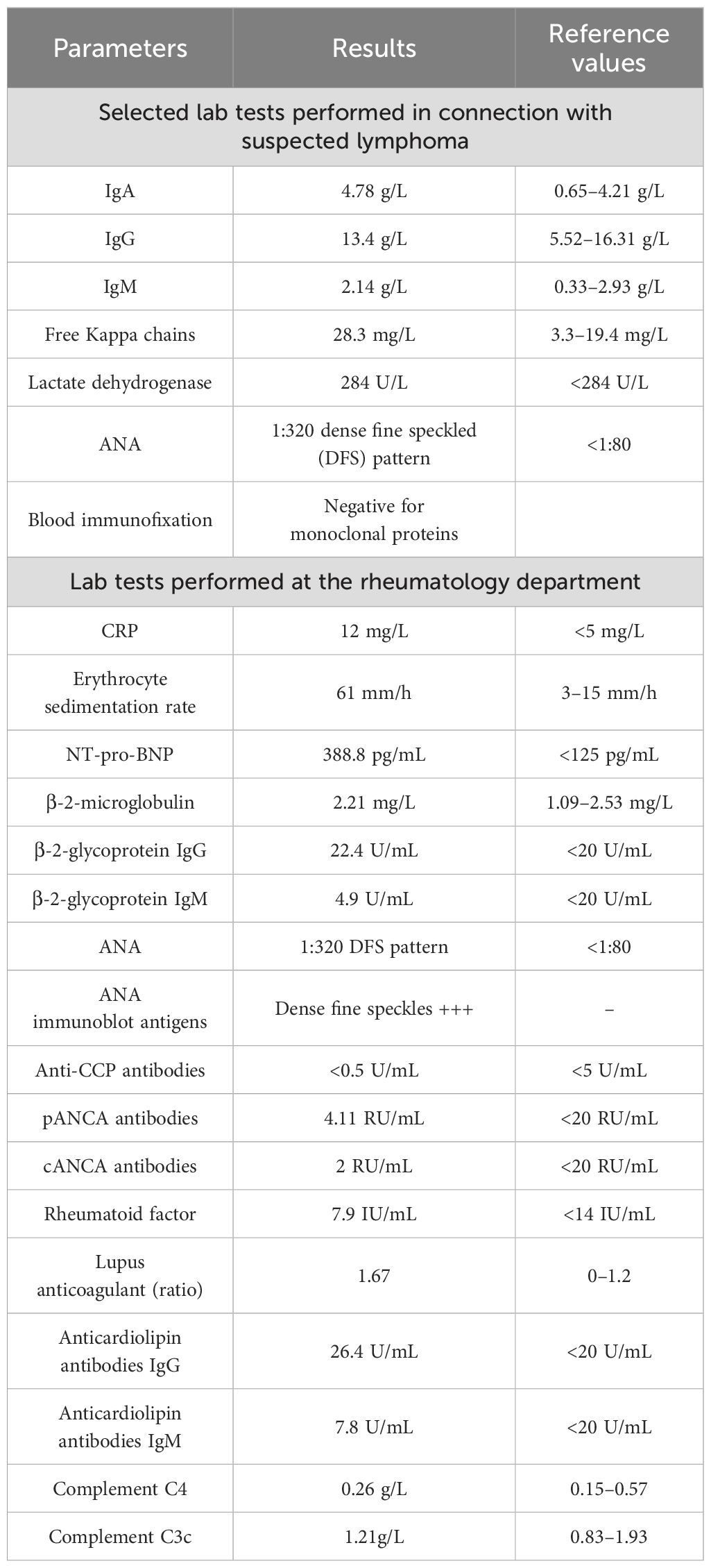
During a subsequent hospitalization due to severe dyspnea, in the following month, CT scans confirmed mediastinal lymphadenopathy, characterized by multiple lymph nodes measuring 1–2 cm in size, as well as a new 85 × 56 mm tumor not seen on a previous CT scan, located anterior to the ascending aorta. Complex lab tests were performed (Table 1). Because of the suspicion of mediastinal lymphoma, mediastinoscopy with lymph node sampling was performed. Histopathological analysis showed only reactive lesions. Immunohistochemical examination revealed expression of the following molecules: CD20 (+), CD30 (−), CD15 (−), CD3 (−), BCl-2 (+), and Ki 67 (+).
The following month, positron emission tomography with computed tomography (PET/CT) scan was performed to exclude lymphoma, which ruled out this diagnosis. The tumor located anteriorly to the aorta described in a previous CT scan turned out to be a normodense fluid reservoir with reduced dimensions of 44 × 35 mm with reduced fluorodeoxyglucose uptake. However, the maximal standardized uptake value (SUVmax) in the pericardial sac was 8.7, which was interpreted as an inflammatory lesion. The final exclusion of lymphoma was made based on the performed blood immunophenotyping.
During a 3-month period of hematological diagnostic evaluation, the patient was concurrently hospitalized in the rheumatology department. The patient still experienced fever episodes and relapsing pericardial effusions treated pro tempore with methylprednisolone. There, in addition to the ANA, lupus anticoagulant was detected and a diagnosis of systemic lupus erythematous (SLE) was made based on the EULAR criteria (3). In view of the patient’s abnormal Schirmer test, Sjögren’s syndrome was also suspected. Since that time, the patient had been on chronic treatment with prednisone. Interestingly, the patient exhibited laboratory features of antiphospholipid syndrome, with positive results for β-2-microglobulin, β-2-glycoprotein, and lupus anticoagulant; however, these features did not persist for more than 12 weeks. Additionally, the patient showed no clinical features of the syndrome. Throughout the aforementioned period of hematology and rheumatology evaluation, the patient had persistently elevated C-reactive protein (CRP) levels, and the ANA titer increased to the 1:3,200 level.
At 6 months, as heart failure progressed, the patient was admitted to the cardiology department for further treatment. Strain echocardiography, tissue Doppler echocardiography, and cardiac magnetic resonance imaging (MRI) revealed features indicative of constrictive pericarditis. MRI showed a hyper-intense space around the entire heart with separation up to 9 mm in front of the right atrium (RA), up to 9 mm on the diaphragmatic wall of the right ventricle (RV), and up to 8 mm behind the posterolateral wall of the left ventricle (LV) (Figure 2). Numerous hypo-intense bands were noted, suggesting dense, organized fluid with numerous fibrous bands. The visceral pericardium was thickened up to 5 mm around the entire heart. After intravenous administration of a contrast agent, there was marked enhancement of the pericardial lamina around the whole heart. The patient was accepted for pericardiectomy.
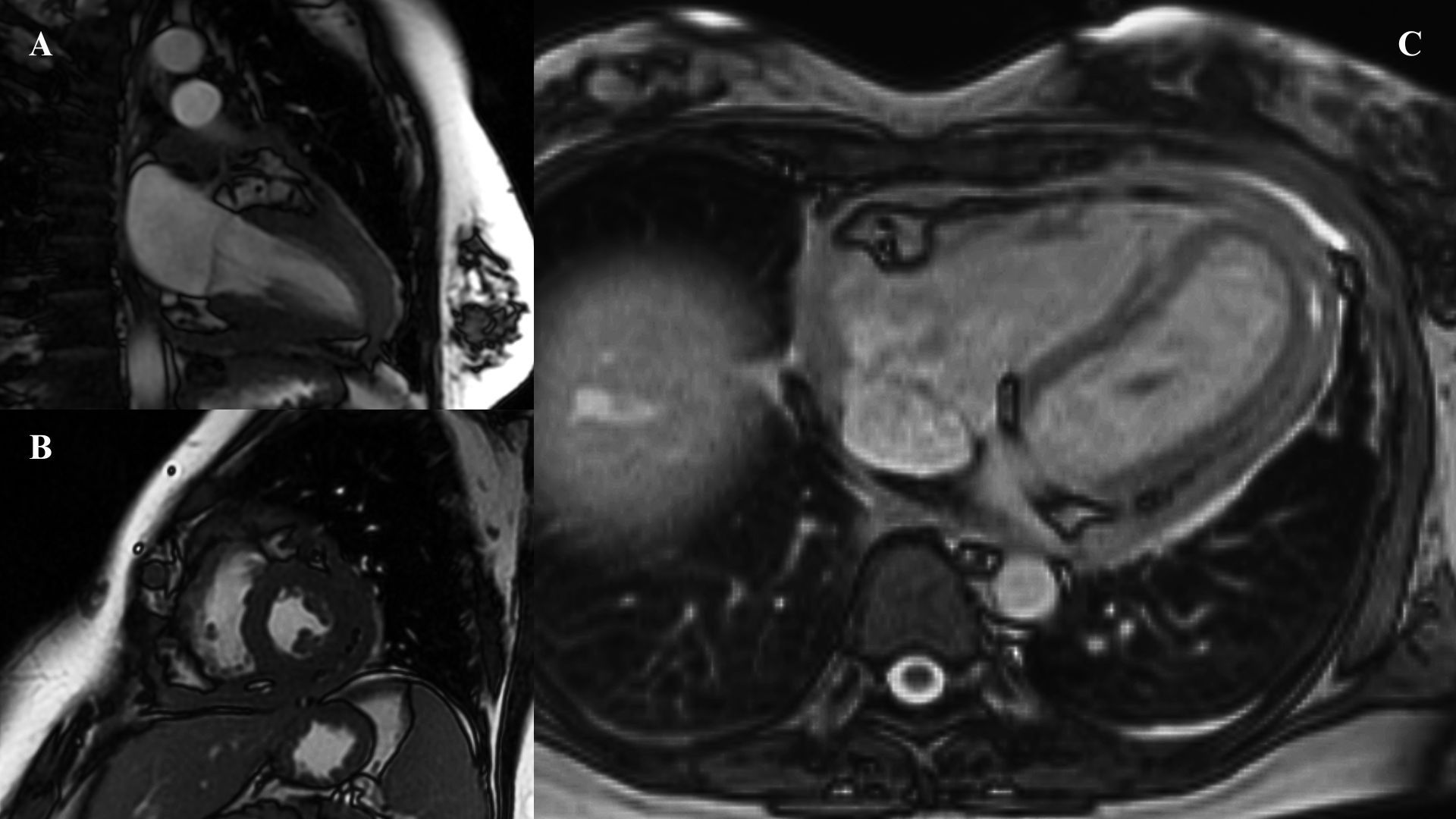
Figure 2. (A, B) First performed MRI scan—short and long axis. (C) MRI performed before pericardiectomy.
It was decided to re-evaluate the diagnosis of SLE. The patient denied skin rashes, psoriatic lesions, Raynaud’s sign, muscle weakness, sacral pain, or morning stiffness. At that time, the patient also no longer met the criteria for the active disease process of SLE in the SLE Risk Probability Index. The patient was evaluated for 4.5 points: 1.5 points for serositis and 3 points for ANA. A score >7 points is indicative of a diagnosis of SLE. Sjögren’s syndrome was also excluded as anti-Ro and anti-La antibodies remained negative.
The pericardial sac was excised from the anterior wall, and the inferior and superior vena cava outlets were dissected, including the pulmonary artery. Postoperative period was uneventful and an improvement in diastolic cardiac function was achieved. Histopathological analysis of the excised material remained inconclusive. Inflammatory–necrotic lesions were observed, along with resorptive granuloma featuring a purulent inflammatory infiltrate. Cell atypia of a very minor degree, with a reactionary character, was noted with low mitotic index (one mitotic figure per 10 high-power fields). Results of immunohistochemistry showed positive Calretinin, WT1, and BAP1 with negative BerEP4, classifying the immunoprofile as equivocal. No membrane staining was observed in epithelial cells during the PD-L1 22C3 qualitative immunohistochemical assay.
However, the patient was readmitted 3 months later with severe circulatory failure. After obtaining the patient’s consent, a repeat pericardiectomy was performed as an immediate salvage procedure. Perioperative risk in EuroSCORE II (European System for Cardiac Operative Risk Evaluation) reached 28%. The procedure revealed massive adhesions from the previous surgery and thick calcified pericardial laminae fused together with the myocardium. During the reoperation, sections were taken for histopathological examination, the result of which revealed the diagnosis of pericardial epithelioid mesothelioma. It is worth mentioning that the patient did not report a history of asbestos exposure.
Pathomorphological exam revealed neoplastic cells with marked atypia (Figure 3). Immunohistochemistry reaction showed positive results for the following: BAP1, Calretinin, CK 5/6, Podop (D-20), WT1 (+), and Ki-67 at the level of 20%.
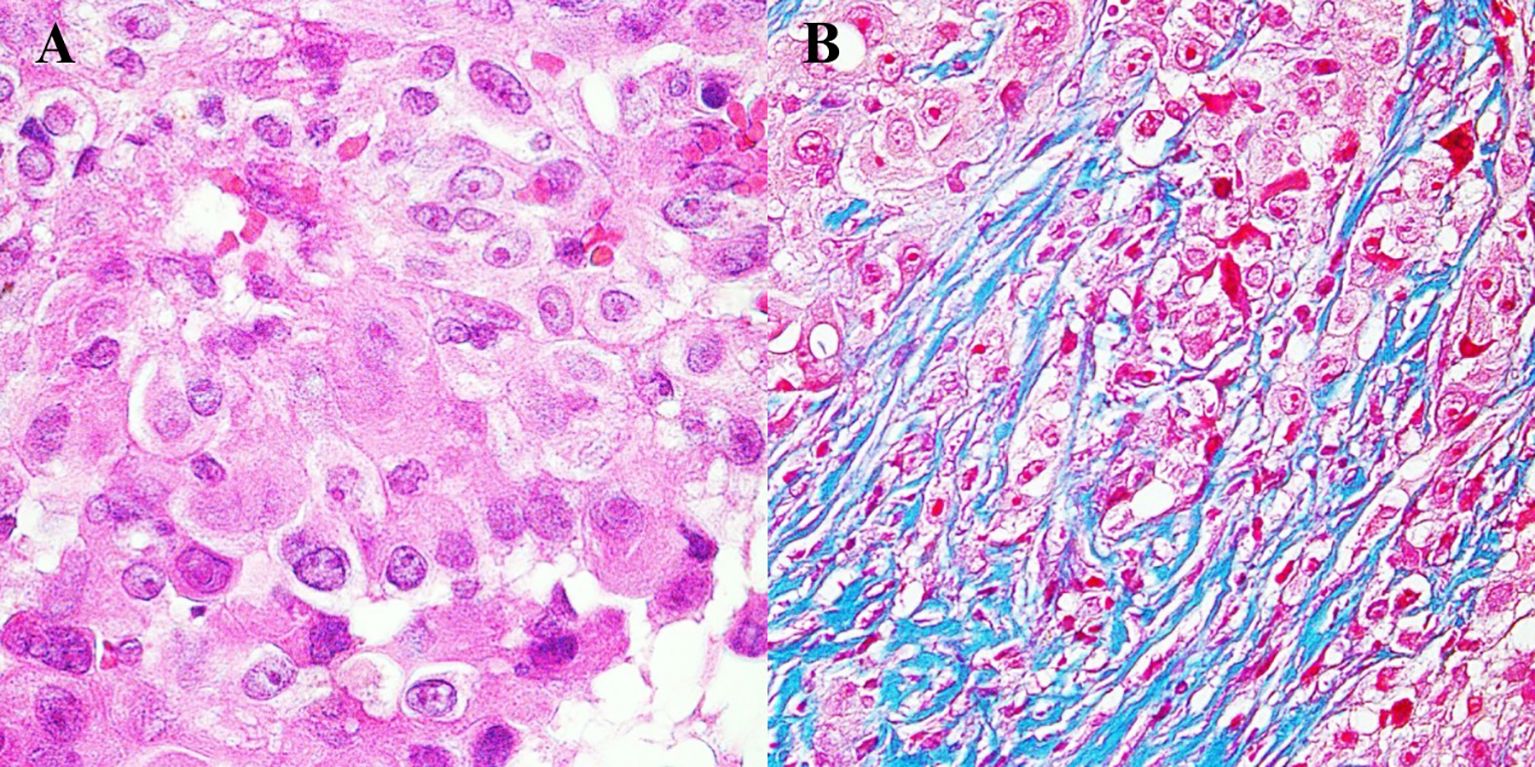
Figure 3. Histopathological image of mesothelioma. (A) Tumor cells with visible nuclei. Hematoxylin and eosin staining, magnification 500×. (B) Tumor cells infiltrating connective tissue fibers. Masson staining, magnification 500×.
The patient was disqualified from oncological treatment due to the lack of effective chemotherapy and the fact that pericardiectomy is the treatment of choice. The patient died after 3 weeks of palliative care.
The timeline of the diagnostic and therapeutic pathway is presented in Figure 1.
Discussion
Primary pericardial mesothelioma (PPM) originates from the mesothelial cells of the pericardium. It constitutes less than 1% of all malignant mesotheliomas but accounts for nearly 50% of all primary pericardial tumors (4). Risk factors for pericardial mesothelioma are debated, although the most commonly reported are asbestos exposure, radiotherapy (including breast cancer treatment), chemotherapy, smoking, and a history of cardiovascular diseases (5), but the patient described had no such history.
During the diagnostic process of the patient, at the time of relapsed pericarditis, presumed diagnoses of mediastinal lymphoma and SLE were made before the eventual diagnosis of pericardial mesothelioma was established. Negative PET/CT was interpreted for exclusion of lymphoma, and due to positive ANA, SLE remains as an alternative diagnosis. At that time, engaging a multidisciplinary team discussion could have provided valuable insights, potentially suggesting the necessity of multimodal imaging or facilitating a more thorough evaluation of the likelihood of an alternative diagnosis of SLE.
Lymphoma as the initial diagnosis
Initially, the clinical presentation raised strong suspicion of mediastinal lymphoma, with features such as mediastinal lymphadenopathy with pericardial involvement. Mediastinal lymphadenopathy may indicate a malignant process, such as mediastinal lymphoma. Notably, secondary cardiac lymphoma occurs in approximately 9%–24% of patients with lymphoma (6). The pericardium is the most commonly involved cardiac structure, often leading to pericardial effusion (7) and, in severe cases, cardiac tamponade (8) (9). Interestingly, pericardial involvement has also been reported as the initial presentation of lymphoma in some cases, underscoring its diagnostic significance (10). Given that secondary pericardial involvement is far more common than primary pericardial malignancies, a hematologic diagnostic pathway remains a rational and essential approach for the described patient. Further investigation focused on exclusion of the more common diagnosis through biopsy, PET/CT, and blood immunophenotyping.
Suspicion of SLE
The case described above highlights that other cancers can also arise in the presence of positive ANA. Several cases have reported systemic lupus erythematosus (SLE)-like features associated with pericardial mesothelioma. McGuigan described a case of PPM presenting with SLE-like symptoms but with low titers of ANA (11). Similarly, Mensi reported a case of PPM characterized by non-erosive polyarthritis, photosensitive rash, sicca syndrome, and recurrent episodes of pericarditis with pericardial effusion. This patient also exhibited high ANA titers, similar to the case described earlier (12). Additionally, Rakhra documented a case of pleural mesothelioma presenting with SLE seropositivity, where serological findings included positive ANA, low-titer anti-double-stranded DNA antibodies (15 IU/mL), and rheumatoid factor (RF) (16 IU/mL) (13). In contrast, the patient in our case had positive ANA, negative RF, and positive lupus anticoagulant. The diagnostic challenge posed by these overlapping features, along with the risk of misdiagnosis, often leads to the late identification of the cancer, contributing to a high mortality rate.
ANA may be associated with a variety of cancers and may have potential anti-tumor activity on an antibody-dependent cell-mediated cytotoxicity (14). The development of autoantibodies results from the breakdown of immunological tolerance, stemming from B- and T-cell dysregulation (15, 16).
The study by Solans-Laqué showed ANA seropositivity with a prevalence of 43.7% in gynecological cancers and 26.6% in lung cancer (17). The presence of ANA is particularly common in patients with lymphomas, with reports indicating a prevalence as high as 31.5% (18). Barreno-Rocha reported the presence of both lupus anticoagulant and ANA in 23.3% of the analyzed patients (19). In the patient with coexisting mediastinal lymphadenopathy, this condition initially led clinicians to suspect lymphoma. Since lymphoma can metastasize from the mediastinum to the pericardium, it should be excluded through biopsy, immunophenotyping, and PET.
Determining the specific type of ANA against nuclear antigens is also crucial in the further rheumatologic diagnostic process. The patient described above had a homogeneous and speckled luminescence pattern of ANA. Notably, in Gauderon’s analysis, the presence of this particular pattern was significantly associated with the absence of cancer, a finding that was not confirmed by the patient’s clinical course (20). Conversely, Cheng’s meta-analysis identified anti-dense fine speckled 70 (DFS70) antibodies as having high specificity for excluding systemic autoimmune rheumatic diseases (21). However, it is important to note that positive DFS70 antibodies can occur in 3.2% of SLE cases and in 10% of Sjögren’s syndrome cases (22). Therefore, their presence cannot unequivocally rule out the possibility of diagnosing these diseases.
The anti-DFS70 antibody, also known as DFS70, Lens epithelium-derived growth factor (LEDGF), or DNA-binding transcription co-activator p75, is an autoantibody closely associated with the dense fine speckled (DFS) pattern (23). DFS70 is overexpressed in various cancers and has oncogenic functions as an oncoprotein, participating in the transcriptional activation of cancer-associated genes and mRNA splicing (24). It promotes cancer cell proliferation and enhances the tumorigenic and metastatic properties of neoplasms (25).
Diagnosis and difficulty in diagnosis of pericardial mesothelioma
Constrictive pericarditis is a typical manifestation of pericardial mesothelioma (2). It is a complication of chronic or recurrent pericarditis; thus, it is likely to be a symptom that appears at a late stage of cancer development. Symptoms of tamponade may also be present, sometimes being the first manifestation of the disease (26). Analyzing the case retrospectively, early signs of mesothelioma included a large pericardial effusion, noticeable pericardial thickening, and mediastinal lymphadenopathy, observed alongside the exclusion of more common causes, such as metastasis from lymphoma.
Unfortunately, the usefulness of both biopsy and pericardial fluid cytology is limited. The diagnostic yield of pericardial fluid cytology is often low, with only 24% of cases showing malignant cells, according to Nilsson’s analysis (27). The false-negative rates of pericardial biopsy in detecting malignant pericardial effusions have been reported to be 40%–44.7% (28). In cases of constrictive pericarditis, the pericardium typically exhibits thickening due to collagen fibrosis with areas of hyalinization, thick-walled blood vessels, and minimal chronic inflammation (29). As highlighted in the case report, such histological findings can introduce significant challenges in the evaluation of sections taken during pericardiectomy. This can delay the diagnosis, which ideally should be made before pericardial constriction occurs, as it complicates the ability to perform radical resection of neoplastic tissue. Tissue samples may be taken from fibrotic areas without obvious atypia, underscoring the need for very precise and extensive sampling during the pericardiectomy procedure.
Other considerations should be given to the immunohistochemistry of mesothelioma lineage. What makes the case described interesting is the equivocal immunoprofile of the pericardial tissue taken during the first pericardiectomy. Such results caused a delay in the diagnosis and treatment of the patient. The negligible atypia suggested inflammatory changes in the mesothelium. Benign reactive mesothelial proliferation, even with atypia, may be caused by infection, collagen vascular disease, surgery, or trauma (30). The tissue showed low mitotic activity—one mitotic figure per 10 high-power fields, compared to other pericardial mesothelioma cases reported by Karadžić, which had five mitotic figures per 10 high-power fields (31). Elliot asserts that mitotic activity may be increased in mesothelial hyperplasia; however, atypical mitoses should not be present (32). In such ambiguous situations, an immunohistochemical reaction is indicated. A positive reaction for WT1 and Calretinin confirms mesothelial proliferation, while the absence of BerEP4 indicates no epithelial proliferation (33). The loss of BAP1 is known as a highly specific marker for distinguishing malignant mesothelioma from reactive proliferation (34). At that time, considering the rarity of mesothelioma, the patient’s tissue was classified as inflammatory mesothelial proliferation.
In patients with recurrent pericardial effusions of unclear etiology, multimodal imaging plays a pivotal role in establishing a definitive diagnosis. Among these modalities, cardiac MRI is particularly valuable in the assessment of pericardial tumors, offering superior anatomic delineation and the ability to characterize tissue composition. It also aids in identifying tumor infiltration, as well as necrotic and fibrotic lesions (35, 36).
Pericardial mesothelioma, a rare and aggressive tumor, typically appears homogeneously isointense on T1-weighted images and heterogeneous on T2-weighted images, with gadolinium enhancement that can sometimes be irregular due to necrotic tissue components (36–38). These imaging features are critical in distinguishing mesothelioma from other pericardial pathologies.
Liu et al. emphasized the importance of multimodal imaging in such cases, recommending the combined use of echocardiography, contrast-enhanced echocardiography, cardiac MRI, and PET/CT to achieve a comprehensive diagnostic evaluation (36). This approach not only enhances diagnostic accuracy but also helps to determine the extent of local invasion and the presence of distant metastases, which are key to planning appropriate management strategies.
The role of PET/CT in diagnosing mesothelioma warrants attention. In the current case, the patient’s thickened pericardium, measuring 18 mm and exhibiting an SUVmax of 8.7, was interpreted as indicative of chronic pericarditis. However, reported cases utilizing PET/CT for mesothelioma diagnosis have typically shown an SUVmax greater than 10. Notably, some studies have documented increased diffuse uptake in the pericardium, with SUVmax values reaching 19.5 in both the right and LVs (39), as well as a localized pericardial mass with an SUVmax of 12.9 (40). In contrast, Hyeon’s analysis revealed that among 11 patients with malignancy, the median SUVmax was only 3.4, suggesting that the interpretation of the current patient’s results may not be accurate (41).
The primary treatment is excision of the tumor through pericardiectomy; however, this approach is beneficial only for localized tumors and before the potential process of constriction begins (42). There are also reported cases of response and prolonged survival in patients treated with pemetrexed and platinum-based chemotherapy (43, 44).
Conclusions
Following recurrent effusive or constrictive pericarditis with no convincing etiology diagnosis, unexplained laboratory or imaging abnormalities should prompt a review by a multidisciplinary team to guide further diagnostic pathways. Multimodality imaging, including PET/CT, MRI, and echocardiography, is essential to consider rare diagnoses such as pericardial mesothelioma.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving humans because the case description is a retrospective analysis, which is not considered by the bioethics committee. No experimental treatment or diagnostic tests were undertaken in the patient. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
GH: Supervision, Writing – review & editing. MKa: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. MKr: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. TH: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Medical University of Silesia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. McGehee E, Gerber DE, Reisch J, Dowell JE. Treatment and outcomes of primary pericardial mesothelioma: a contemporary review of 103 published cases. Clin Lung Cancer. (2019) 20:e152–7. doi: 10.1016/j.cllc.2018.11.008
3. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2019) 71:1400–12. doi: 10.1002/art.40930
4. Ekmektzoglou KA, Samelis GF, Xanthos T. Heart and tumors: location, metastasis, clinical manifestations, diagnostic approaches and therapeutic considerations. J Cardiovasc Med. (2008) 9:769–77. doi: 10.2459/JCM.0b013e3282f88e49
5. Marinaccio A, Consonni D, Mensi C, Mirabelli D, Migliore E, Magnani C, et al. Association between asbestos exposure and pericardial and tunica vaginalis testis Malignant mesothelioma: a case-control study and epidemiological remarks. Scandinavian J Work Environ Health. (2020) 46:609–17. doi: 10.5271/sjweh.3895
6. Zhao Y, Huang S, Ma C, Zhu H, Bo J. Clinical features of cardiac lymphoma: an analysis of 37 cases. J Int Med Res. (2021) 49:300060521999558. doi: 10.1177/0300060521999558
7. Paraskevaidis IA, Michalakeas CA, Papadopoulos CH, Anastasiou-Nana M. Cardiac tumors. ISRN Oncol. (2011) 2011:208929. doi: 10.5402/2011/208929
8. Rognoni A, Cavallino C, Lupi A, Secco GG, Rossi L, Bongo AS. Cardiac tamponade caused by a large lymphoma involving the pericardium and the right heart chambers. Emergency Med. (2012) 2:105. doi: 10.4172/2165-7548.1000105
9. Lim WJ, Kaisbain N, Bakar RA, Hadi HA, Mohamed Yusof AK. Secondary cardiac lymphoma presenting with cardiac tamponade and cardiac mass: a case report. Cardio-oncology. (2024) 10:31. doi: 10.1186/s40959-024-00202-8
10. Muthusamy P, Ebrom S, Cohle SD, Khan N. Pericardial involvement as an initial presentation of anaplastic large cell lymphoma. Can Family phys Med famille canadien. (2014) 60:638–41.
11. McGuigan L, Fleming A. Pericardial mesothelioma presenting as systemic lupus erythematosus. Ann Rheum Dis. (1984) 43:515–7. doi: 10.1136/ard.43.3.515
12. Mensi C, Romano A, Berti A, Dore R, Riboldi L. A second case of pericardial mesothelioma mimicking systemic lupus erythematosus in the literature in over 30 years: a case report. J Med Case Rep. (2017) 11:85. doi: 10.1186/s13256-017-1237-z
13. Rakhra A, Munir A, Chilukuri RS, Nahas J. A rare case of Malignant mesothelioma presenting with systemic lupus erythematosus seropositivity: A case report and review of literature. Cureus. (2019) 11:e4092. doi: 10.7759/cureus.4092
14. Vlagea A, Falagan S, Gutiérrez-Gutiérrez G, Moreno-Rubio J, Merino M, Zambrana F, et al. Antinuclear antibodies and cancer: a literature review. Crit Rev Oncology/Hematol. (2018) 127:42–9. doi: 10.1016/j.critrevonc.2018.05.002
15. Meng X, Layhadi JA, Keane ST, Cartwright NJK, Durham SR, Shamji MH. Immunological mechanisms of tolerance: central, peripheral and the role of T and B cells. Asia Pacific Allergy. (2023) 13:175–86. doi: 10.5415/apallergy.0000000000000128
16. Wang R, Zhao H, Liu Y, Kang B, Cai J. Antinuclear antibodies with a nucleolar pattern are associated with a significant reduction in the overall survival of patients with leukemia: a retrospective cohort study. Front Oncol. (2021) 11:631038. doi: 10.3389/fonc.2021.631038
17. Solans-Laqué R, Pérez-Bocanegra C, Salud-Salvia A, Fonollosa-Plá V, Rodrigo MJ, Armadans L, et al. Clinical significance of antinuclear antibodies in Malignant diseases: association with rheumatic and connective tissue paraneoplastic syndromes. Lupus. (2004) 13:159–64. doi: 10.1191/0961203304lu521oa
18. Zou HY, Gu X, Yu WZ, Wang Z, Jiao M. Detection of serum antinuclear antibodies in lymphoma patients. Genet Mol Res (GMR). (2015) 14:16546–52. doi: 10.4238/2015.December.11.1
19. Barreno-Rocha SG, Guzmán-Silahua S, Cardona-Muñoz EG, Zavala-Cerna MG, Muñoz Gaytan DE, Riebeling-Navarro C, et al. Frequency of autoantibodies on non-Hodgkin lymphoma. Healthc (Basel Switzerland). (2023) 11:2210. doi: 10.3390/healthcare11152210
20. Gauderon A, Roux-Lombard P, Spoerl D. Antinuclear antibodies with a homogeneous and speckled immunofluorescence pattern are associated with lack of cancer while those with a nucleolar pattern with the presence of cancer. Front Med. (2020) 7:165. doi: 10.3389/fmed.2020.00165
21. Cheng C-F, Shih M-C, Lan T-Y, Li K-J. Anti-DFS70 antibodies for differentiating systemic autoimmune rheumatic disease in patients with positive ANA tests: a systematic review and meta-analysis. Diagnostics. (2021) 11:1592. doi: 10.3390/diagnostics11091592
22. Conrad K, Röber N, Rudolph S, Mahler M. DFS70 antibodies – biomarkers for the exclusion of ANA-associated autoimmune rheumatic diseases. LaboratoriumsMedizin. (2015) 38:299–307. doi: 10.1515/labmed-2015-0040
23. Meng J, Wang R, Luo Y, Li S, Bai Y, Song N, et al. Prevalence and clinical characteristics of the dense fine speckled pattern: indirect immunofluorescence-antinuclear antibody screening in the Chinese population. Scandinavian J Immunol. (2023) 97:e13233. doi: 10.1111/sji.13233
24. Singh DK, Gholamalamdari O, Jadaliha M, Ling L, Lin YC, Zhang Y, et al. PSIP1/p75 promotes tumorigenicity in breast cancer cells by promoting the transcription of cell cycle genes. Carcinogenesis. (2017) 38:966–75. doi: 10.1093/carcin/bgx085
25. Singh PK, Plumb MR, Ferris AL, Iben JR, Wu X, Fadel HJ, et al. LEDGF/p75 interacts with mRNA splicing factors and targets HIV-1 integration to highly spliced genes. Genes Dev. (2015) 29:2287–97. doi: 10.1101/gad.269396.115
26. Matsuyama S, Imazuru T, Uchiyama M, Ota H, Iida M, Shimokawa T. Primary Malignant pericardial mesothelioma presenting with cardiac tamponade. Int J Surg Case Rep. (2020) 73:253–6. doi: 10.1016/j.ijscr.2020.07.054
27. Nilsson A, Rasmuson T. Primary pericardial mesothelioma: Report of a patient and literature review. Case Rep Oncol. (2009) 2:125–32. doi: 10.1159/000228894
28. Saab J, Hoda RS, Narula N, Hoda SA, Geraghty BE, Nasar A, et al. Diagnostic yield of cytopathology in evaluating pericardial effusions: Clinicopathologic analysis of 419 specimens. Cancer Cytopathol. (2017) 125:128–37. doi: 10.1002/cncy.21790
29. Fallek Boldes O, Dahan S, Segal Y, Ben-Ami Shor D, Huber RK, Barshack I, et al. Characteristics of pericardial biopsy: 100 cases in a single center. Israel Med Assoc J. (2019) 21:183–8.
30. Zeren EH, Demirag F. Benign and Malignant mesothelial proliferation. Surg Pathol Clinics. (2010) 3:83–107. doi: 10.1016/j.path.2010.03.010
31. Karadzic R, Kostic-Banovic L, Antovic A, Celar M, Katic V, Ilic G, et al. Primary pericardial mesothelioma presenting as constrictive pericarditis. Arch Oncol. (2005) 13:150–2. doi: 10.2298/AOO0504150K
32. Fels Elliott DR, Jones KD. Diagnosis of mesothelioma. Surg Pathol Clinics. (2020) 13:73–89. doi: 10.1016/j.path.2019.10.001
33. Chapel DB, Schulte JJ, Husain AN, Krausz T. Application of immunohistochemistry in diagnosis and management of Malignant mesothelioma. Trans Lung Cancer Res. (2020) 9:S3–S27. doi: 10.21037/tlcr.2019.11.29
34. Cigognetti M, Lonardi S, Fisogni S, Balzarini P, Pellegrini V, Tironi A, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Modern Pathol. (2015) 28:1043–57. doi: 10.1038/modpathol.2015.65
35. Behairy NH, Moharram AN. Role of computed tomography and magnetic resonance imaging in diagnosing pericardial lesions. Egypt J Radiol Nucl Med. (2012) 43:389–95. doi: 10.1016/j.ejrnm.2012.06.004
36. Liu J, Wang Z, Yang Y, Xiong Y, Wang W, Qiu J, et al. Multimodal diagnostic workup of primary pericardial mesothelioma: A case report. Front Cardiovasc Med. (2021) 8:758988. doi: 10.3389/fcvm.2021.758988
37. Kaminaga T, Takeshita T, Kimura I. Role of magnetic resonance imaging for evaluation of tumors in the cardiac region. Eur Radiol. (2003) 13:L1–L10. doi: 10.1007/s00330-002-1789-0
38. Tjeerdsma G, Brouwer J, Van Veldhuisen DJ. Images in cardiology. Rapid progression of pericardial Malignant mesothelioma. Heart (British Cardiac Society). (1998) 79:616–8. doi: 10.1136/hrt.79.6.618
39. Edel JP, Balink H. 18F-FDG PET/CT revealing constrictive pericarditis as the only manifestation of Malignant mesothelioma. Clin Nucl Med. (2019) 44:55–6. doi: 10.1097/RLU.0000000000002349
40. Aga F, Yamamoto Y, Norikane T, Nishiyama Y. A case of primary pericardial mesothelioma detected by 18F-FDG PET/CT. Clin Nucl Med. (2012) 37:522–3. doi: 10.1097/RLU.0b013e3182478c13
41. Hyeon CW, Yi HK, Kim EK, Park SJ, Lee SC, Park SW, et al. The role of 18F-fluorodeoxyglucose-positron emission tomography/computed tomography in the differential diagnosis of pericardial disease. Sci Rep. (2020) 10:21524. doi: 10.1038/s41598-020-78581-y
42. Godar M, Liu J, Zhang P, Xia Y, Yuan Q. Primary pericardial mesothelioma: a rare entity. Case Rep Oncol Med. (2013) 2013:283601. doi: 10.1155/2013/283601
43. Doval DC, Pande SB, Sharma JB, Rao SA, Prakash N, Vaid AK. Report of a case of pericardial mesothelioma with liver metastases responding well to pemetrexed and platinum-based chemotherapy. J Thorac Oncol. (2007) 2:780–1. doi: 10.1097/JTO.0b013e31811f3acd
Keywords: pericardial mesothelioma, constrictive pericarditis, anti-nuclear antibodies, systemic rheumatic disease, mediastinal lymphoma
Citation: Hirnle G, Kapałka M, Krawiec M and Hrapkowicz T (2025) Pericardial mesothelioma mimicking mediastinal lymphoma and systemic rheumatic disease: a case report. Front. Oncol. 14:1481373. doi: 10.3389/fonc.2024.1481373
Received: 15 August 2024; Accepted: 16 December 2024;
Published: 17 January 2025.
Edited by:
Wouter Kok, Amsterdam University Medical Center, NetherlandsReviewed by:
Yashwant Kumar, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaXiaomeng Shi, Emory University, United States
Copyright © 2025 Hirnle, Kapałka, Krawiec and Hrapkowicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michał Kapałka, bWljaGFsLmthcGFsa2EwMEBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Grzegorz Hirnle1†
Grzegorz Hirnle1† Michał Kapałka
Michał Kapałka