- 1Department of Neurosurgery, Tübingen University Hospital, Tübingen, Germany
- 2Department of Pathology, Hospital Álvaro Cunqueiro, Vigo, Spain
Introduction: Intracranial schwannomas represent a rare group of intracranial tumors, with purely motor nerve schwannomas being the rarest of them. The anatomical proximity of these tumors to the brainstem may present a radiological challenge in differentiating them from intra–axial brainstem tumors, which can influence further decision–making and treatment options.
Methods: We report on a 47–year–old male patient who was diagnosed with a large cystic intracranial tumor with radiological features suggestive of an intrinsic brainstem glioma.
Results: After discussing treatment options and risks based on a presumed radiological diagnosis, microsurgical treatment via lateral–suboccipital craniotomy in semi–sitting position, under continuous intraoperative neuromonitoring was performed. Intraoperative findings proved that the tumor was an extra–axial schwannoma originating from the left trochlear nerve. Gross total removal of the lesion was achieved.
Conclusion: Due to their rarity, non–specific symptoms and the possibility to mimicking intra–axial brainstem tumors on imaging, these tumors may present a diagnostic challenge and should be taken into account during treatment decision-making.
Introduction
Intracranial schwannomas represent approximately 8% of central nervous system (CNS) tumors, representing a relatively uncommon group (1). Non-vestibular schwannomas of purely motor nerves, such as the trochlear nerve, are considered to be the rarest of all cranial nerve schwannomas (1, 27). Due to their proximity to the brainstem, radiological differentiation between trochlear nerve schwannomas and intrinsic brainstem gliomas can sometimes be challenging, potentially influencing decision-making concerning treatment. We present a case of a rare cystic trochlear schwannoma with imaging features suggestive of a brainstem glioma.
Case presentation
A 47-year-old male patient, who himself works as a medical colleague in pathology, presented at our center in November 2012. He had been complaining of dizziness when turning his head for eight months, which lasted for a few seconds at a time. Climbing stairs with turns or lying down quickly in bed were trigger factors. Additionally, he reported paresthesia of the left cheek corresponding to the maxillary branch of the trigeminal nerve and some minor difficulties in swallowing. Patient’s past medical history was unremarkable except for mild asthma. Family medical history was positive for lattice (reticular) corneal dystrophy that required corneal transplantation and lung cancer from mother’s side, as well as arterial hypertension and diabetes type II from father’s side.
A neurologic examination revealed hypoesthesia in the area of the left V2 and unsystematic dizziness. The status of other cranial nerves was intact. No motor or sensory long-tract signs were observed. Muscle reflexes were symmetric, Babinski sign was negative and the finger–to nose–test was without evidence of dysmetria. The patient´s gait was unimpaired (Figure 1).

Figure 1. Timeline showing clinical course (purple boxes) since the beginning of the symptoms 8 months before surgery, diagnostic work-up (red boxes), treatment (blue box) and follow-up (green boxes).
A head MRI revealed a cystic, inhomogeneously enhancing tumor that appeared to originate within the brainstem with supra- and infratentorial extension and strong perifocal vasogenic edema. The diagnosis of pilocytic astrocytoma was presumed, and a differential diagnosis of cystic cranial nerve schwannoma was considered (Figure 2). Treatment options and risks based on the suspected diagnosis were discussed with the patient. The decision was made to proceed with a microsurgical removal of the lesion under continuous intraoperative neuromonitoring.
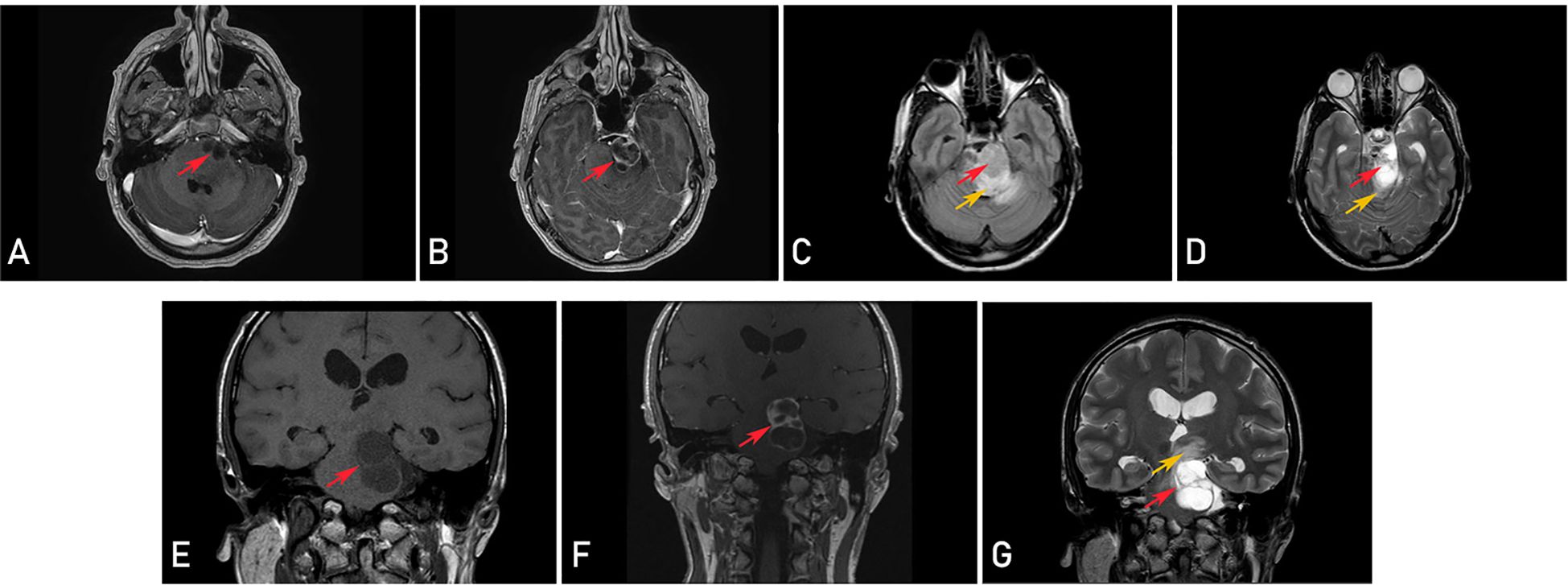
Figure 2. Preoperative magnetic resonance imaging (MRI) revealing a cystic, inhomogeneously enhancing tumor (red arrows) and perifocal vasogenic edema (orange arrows) in axial (T1-weighted contrast-enhanced (A, B), T2-fluid attenuated (FLAIR) (C) and T2-weighted (D) sequences) and coronal plane (T1-weighted (E), T1-weighted contrast-enhanced (F) and T2-weighted (G) sequences).
The operation was conducted in a semi-sitting position. Following the retrosigmoid craniotomy, the dura mater was incised under the microscope, along the transverse and sigmoid sinus. The cerebellomedullary cistern was opened and cerebrospinal fluid (CSF) was released, which lowered the pressure in the posterior cranial fossa. After microsurgical preparation of the cerebellopontine angle cistern, the nerve complex VII and VIII were identified, as well as a large cystic structure above them. Xanthochrome fluid was drained from a cyst, and the trigeminal nerve was visualized. It was tightly attached to the yellowish hard tumor capsule. Microsurgical dissection of the tumor capsule from the trigeminal nerve and petrosal vein was then performed. Then the solid part of the tumor was debulked with an ultrasonic aspirator while several tumor pieces were sent for histopathological frozen section examination. The frozen specimen results indicated that the tumor was most likely a schwannoma, thereby excluding the diagnosis of glioma. Following further reduction of the tumor, the trochlear nerve was identified. Neurolysis of the nerve was initiated, but it was observed that the nerve completely dissolved into the tumor. Using a meticulous microsurgical technique, the tumor capsule and the solid part of the tumor were dissected from the oculomotor nerve and the brainstem surface. This procedure resulted in a gross total resection of the tumor with the preservation of all neurovascular structures, except for the trochlear nerve, which was thought to be the source of the tumor. Based on these findings, the intraoperative diagnosis of the cystic trochlear nerve schwannoma was established.
Postoperatively, the patient reported diplopia, which improved during the course of the hospital stay. A surveillance MRI confirmed the gross total resection of the tumor and signs of a resolving edema in the brainstem. The final histopathologic examination revealed tumor tissue interspersed with nerve fibers exhibiting the typical features of a WHO grade I schwannoma (Supplementary Image).
Three months following surgery the patient reported the persistence of hypoesthesia in the V2 segment of the trigeminal nerve, though with a decreasing tendency. The examination confirmed the persistence of trochlear nerve palsy, which was satisfactorily compensated by prismatic lenses.
A follow-up examination conducted 11 years after the removal of the tumor revealed the persistence of a palsy of the left trochlear nerve. However, the use of prismatic lenses effectively mitigated the issue of double vision. The patient has been able to resume his occupational and daily activities without difficulty. There was no evidence of tumor recurrence on the surveillance cranial MRI (Figure 3).
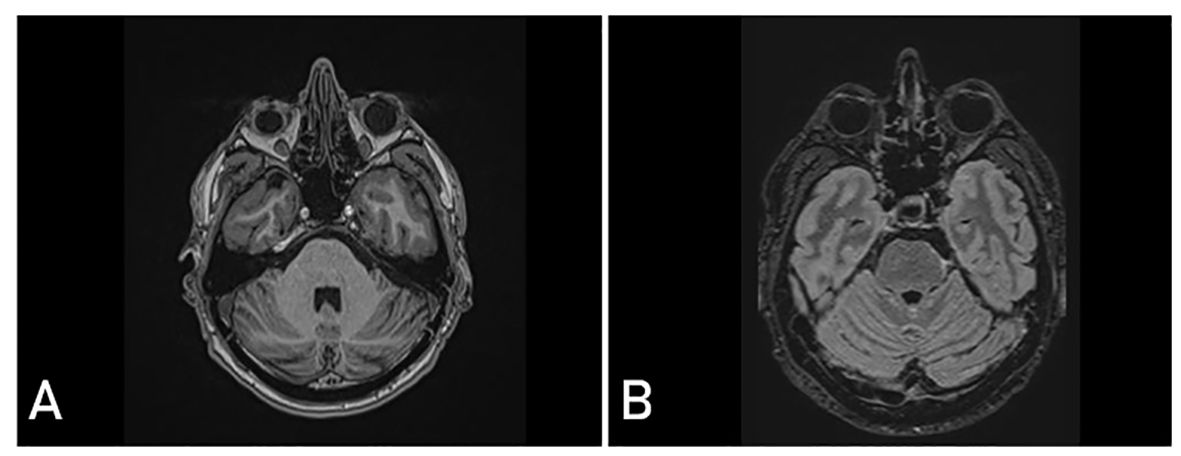
Figure 3. Postoperative magnetic resonance imaging (MRI) 11 years after surgery showing complete realignment of the brainstem and no signs of tumor recurrence in T1-weighted contrast - enhanced (A) and T2 fluid attenuated (FLAIR) (B) sequences.
Discussion
Intracranial schwannomas are benign tumors that arise from Schwann cells in the nerve sheath of cranial nerves and represent approximately 8% of all intracranial tumors (1). Most commonly they arise from sensory nerves or mixed motor–sensory nerves (1). In the majority of the cases, there is an association between intracranial schwannomas and neurofibromatosis type 2 (2). Purely motor nerve schwannomas, especially sporadic, are rare, and schwannomas originating from the trochlear nerve are considered to be the rarest (3).
A literature search of the PubMed and Web of Science databases was conducted using the following terms: “trochlear nerve”, “tumor”, “schwannoma”, “neurinoma” and “surgery”. The search was limited to English-language sources published between 1976 and 2024 (Supplementary Table 1). This yielded a total of 45 documented cases of surgically treated trochlear nerve schwannomas with histological confirmation. The median age was 44 years, with age at time of treatment ranging from 12 to 72 years. The majority of patients were in the fifth (23.9%), sixth (21.7%) or seventh (21.7%) decade of life. There was no difference in gender distribution (male-to-female ratio 1.09:1).
Celli et al. categorized trochlear nerve schwannomas into three groups: cisternal, cisternocavernous and cavernous (2). The majority of trochlear schwannomas are of the cysternal type and are located in the ambient cistern. However, in four cases, the tumor was located in the pineal region (3, 4, 8, 10, 11). Large cisternal type trochlear schwannomas typically extend supra- and infratentorial, exerting compression on the brainstem and adjacent cranial nerves, which contributes to the variety of unspecific symptoms they cause (12). In the literature, the most common symptoms are diplopia, headache and ataxia. Other reported symptoms included focal neurological deficits, including long tract signs or other cranial nerves palsies (mostly cranial nerves V and VII), tinnitus, vertigo and hearing loss (8). Cases of unusual presentation such as pathological laughter, atypical facial pain or persistent hiccups were also described (13–15). In cases complicated by intratumoral hemorrhage, signs of increased intracranial pressure, such as nausea and vomiting, were observed. Additionally, sudden onset or acute exacerbation of preexisting symptoms was noted (9, 12, 15–17).
Intracranial schwannomas exhibit distinctive characteristics on MRI. On T2–weighted images, they appear as a heterogeneously hyperintense lesions. They appear as a low or intermediate signal lesions on T1–weighted images, with avid enhancement after intravenous contrast administration (18) (Supplementary Table 2).
Although not so common like in skull base meningioma, presence of peritumoral brain edema (PTBE) in patients with vestibular schwanommas (VS) is also reported in the literature (5 – 10%). Since the mechanism of formation is still unclear, it is the topic of ongoing research. Direct compression of the brainstem leading to a decrease of cerebral blood flow and a slowed metabolism of nearby cells can cause cytotoxic edema. On the other hand, some research stated that PTBE was actually vasogenic brain edema. Hong-Hai You et al. reported about strong relationship among Vascular Endothelial Growth Factor (VEGF) expression, tumor angiogenesis, and PTBE formation in patients with VS (29).
In our review, the presence of a cyst was reported in 22 cases. Due to their proximity to the brainstem, cisternal schwannomas may appear on imaging as intrinsic brainstem lesion (5, 6). This may lead to the diagnostic uncertainty and influence decision-making concerning the appropriate treatment. In our case, according to the preoperative radiographic findings, intrinsic brainstem glioma was suspected. The presumed differential diagnosis was cystic cranial nerve schwannoma. Considering the eloquent location of brainstem gliomas, the therapy of choice in many cases is biopsy for histological confirmation followed by radiation in case of progression (24). In our case the decision was made to remove the tumor, which proved to be a correct decision given that the tumor was extra-axial and benign and a gross total resection was achieved. Conversely, cases of intrinsic brainstem glioma with imaging characteristics of a cranial nerve schwannoma have also been reported (7, 24).
In the literature, the most commonly utilized approach for the resection of the trochlear nerve schwannoma is the subtemporal transtentorial (32.6%) approach, followed by a lateral - suboccipital approach (26.1%) (8). In addition, pterional and anterior or posterior transpetrosal approaches were described, as well as transventricular transvelar and paraoccipital posterior interhemispheric transtentorial approaches to the lesions in the pineal region (3, 11, 15, 19–21). To the best of our knowledge, this is the first reported case of a cystic trochlear nerve schwannoma that has been operated via the lateral-suboccipital approach in the semi-sitting position, an approach that has been demonstrated to be an effective technique for the removal of large tumors, resulting in improved tumor resection and nerve preservation (22).
The trochlear nerve is the sole cranial nerve to emerge from the brainstem on the dorsal side and crosses the midline. Additionally, it is the longest and the thinnest of all cranial nerves (12). Despite its inherent vulnerability to injury, trochlear nerve palsy was observed preoperatively in only 21 patients (45.6%) in our review. In the majority of cases (82.6%) gross total resection of the tumor was achieved. Trochlear nerve palsy was reported in 33 patients (71.7%) in the postoperative course. Treatment modalities for diplopia include prism lenses and strabismus surgery (25). Fujiwara et al. reported that even some patients whose trochlear nerve was cut intraoperatively did not develop diplopia after surgery. This may be the result of the fusion of the visual fields occurring prior to surgery during tumor progression (23).
Regarding the management of trochlear nerve schwannomas, Torun et al. in their 2018 literature review and the group of authors in their 2022 consensus statement on behalf of the European Association of Neurosurgical Societies (EANS) skull base section propose a multidisciplinary and tailored approach (25, 28). Observation with clinical and radiological follow-up may be sufficient for smaller tumors in patients without major symptoms. Lock et al. reported on a case of a trochlear schwannoma that was not surgically treated. During a 22–year follow-up period, no tumor progression was observed (26). In patients with larger tumors and symptoms caused by mass effect, surgery is the therapy of choice (28). Ozoner et al. reported in their literature review that there was no difference in outcome with respect to subtotal or total tumor resection (8). In our 11–year follow-up, cranial MRI showed no evidence of tumor recurrence with complete realignment of the brainstem and complete resolution of the brainstem vasogenic edema. Farrokhi et al. observed no tumor recurrence in their 12–year postoperative follow-up (3). For small tumors with a tendency to grow or residual tumor, stereotactic radiosurgery may be the therapy of choice, although the potential vulnerability of the trochlear nerve should be considered (27, 28).
Conclusion
Due to their rarity and non-specific symptoms, trochlear nerve schwannomas may present a diagnostic challenge and should be considered in the differential diagnosis of the unclear expansive lesions of the brainstem and cerebellopontine angle. For large tumors requiring surgical treatment, a lateral-suboccipital approach in a semi-sitting position may offer the possibility of a safe gross total tumor resection and a good outcome.
Data availability statement
The original contributions presented in the study are included in the article/ (Supplementary Material). Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MF: Writing – original draft. PH: Writing – review & editing. JR: Writing – review & editing. MT: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1474372/full#supplementary-material
References
1. Luo W, Ren X, Chen S, Liu H, Sui D, Lin S. Intracranial intraparenchymal and intraventricular schwannomas: report of 18 cases. Clin Neurol Neurosurg. (2013) 115:1052–7. doi: 10.1016/j.clineuro.2012.10.029
2. Celli P, Ferrante L, Acqui M, Mastronardi L, Fortuna A, Palma L. Neurinoma of the third, fourth, and sixth cranial nerves: A survey and report of a new fourth nerve case. Surg Neurol. (1992) 38:216–24. doi: 10.1016/0090-3019(92)90172-J
3. Farrokhi MR, Ghaffarpasand F, Taghipour M, Derakhshan N. Transventricular transvelar approach to trochlear nerve schwannoma: novel technique to lesions of inferior pineal region. World Neurosurg. (2018) 114:274–80. doi: 10.1016/j.wneu.2018.03.132
4. Lan YH, Li YC, Chang CN, Zhang B, Lu YJ. Trochlear schwannoma arising from transition zone of nerve sheath in the pineal region: case report and review of the literature. World Neurosurg. (2020) 137:218–25. doi: 10.1016/j.wneu.2020.02.019
5. Garen PD, Harper CG, Teo C, Johnston IH. Cystic schwannoma of the trochlear nerve mimicking a brain-stem tumor. Case Rep J Neurosurg. (1987) 67:928–30. doi: 10.3171/jns.1987.67.6.0928
6. Shenoy SN, Raja A. Cystic trochlear nerve neurinoma mimicking intrinsic brainstem tumour. Br J Neurosurg. (2004) 18:183–6. doi: 10.1080/02688690410001681073
7. Xie HM, Richard SA, Lan Z. A petroclival glioma mimicking trigeminal schwannoma: A case report. Med (Baltimore). (2021) 100:e27792. doi: 10.1097/MD.0000000000027792
8. Ozoner B, Gungor A, Ture H, Ture U. Surgical treatment of trochlear nerve schwannomas: case series and systematic review. World Neurosurg. (2022) 162:e288–300. doi: 10.1016/j.wneu.2022.03.006
9. Ohba S, Miwa T, Kawase T. Trochlear nerve schwannoma with intratumoral hemorrhage: case report. Neurosurgery. (2006) 58:E791. doi: 10.1227/01.NEU.0000204307.99246.17
10. da Cunha MLV, Miranda MHF, Cecconello GL. Trochlear nerve schwannoma: case report and literature review. Braz Neurosurg. (2017) 36:178–84. doi: 10.1055/s-0037-1603919
11. Chaudhry NS, Ahmad FU, Morcos JJ. Pineal region schwannoma arising from the trochlear nerve. J Clin Neurosci. (2016) 32:159–61. doi: 10.1016/j.jocn.2016.05.004
12. Lei J, Li Y, Wan X, Wang J, You C, Zhao K, et al. Hemorrhagic schwannoma of the trochlear nerve: case report and a review of the literature. Front Oncol. (2023) 12:1097155. doi: 10.3389/fonc.2022.1097155
13. Nadkarni TD, Goel A. Trochlear nerve neurinoma presenting as pathological laughter. Br J Neurosurg. (1999) 13:212–3. doi: 10.1080/02688699944023
14. Veshchev I, Spektor S. Trochlear nerve neuroma manifested with intractable atypical facial pain: case report. Neurosurgery. (2002) 50:889–91. doi: 10.1097/00006123-200204000-00043
15. Hatae R, Miyazono M, Kohri R, Maeda K, Naito S. Trochlear nerve schwannoma with intratumoral hemorrhage presenting with persistent hiccups: A case report. J Neurol Surg Rep. (2014) 75:e183–8. doi: 10.1055/s-0034-1378156
16. Liu P, Bao Y, Zhang W. Trochlear nerve schwannoma with repeated intratumoral hemorrhage. J Craniofac Surg. (2016) 27:e528–9. doi: 10.1097/SCS.0000000000002816
17. Yamamoto M, Jimbo M, Ide M, Kubo O. Trochlear neurinoma. Surg Neurol. (1987) 28:287–90. doi: 10.1016/0090-3019(87)90308-9
18. Skolnik AD, Loevner LA, Sampathu DM, Newman JG, Lee JY, Bagley LJ, et al. Cranial nerve schwannomas: diagnostic imaging approach. Radiographics. (2016) 36:1463–77. doi: 10.1148/rg.2016150199
19. Younes WM, Hermann EJ, Krauss JK. Cisternal trochlear nerve schwannoma: improvement of diplopia after subtotal tumour excision. Br J Neurosurg. (2012) 26:107–9. doi: 10.3109/02688697.2011.592053
20. Boucher AB, Michael LM 2nd. The middle fossa approach for the removal of a trochlear schwannoma. Case Rep Neurol Med. (2014) 2014:672314. doi: 10.1155/2014/672314
21. Kohama M, Murakami K, Endo T, Watanabe M, Tominaga T. Surgical and histological observations of trochlear neurinoma: case report. Neurol Med Chir (Tokyo). (2009) 49:217–20. doi: 10.2176/nmc.49.217
22. Wang SS, Tatagiba M. The semisitting retrosigmoid technique for removal of large vestibular schwannoma: 2-dimensional operative video. Oper Neurosurg (Hagerstown). (2023) 25:e216–7. doi: 10.1227/ons.0000000000000745
23. Fujiwara E, Adachi K, Tateyama S, Hasegawa M, Hirose Y. Frequency of diplopia after intraoperative nerve disturbance in trochlear nerve schwannoma: A case report and systematic review. Neurol Med Chir (Tokyo). (2021) 61:591–7. doi: 10.2176/nmc.oa.2021-0079
24. Roessler K, Heynold E, Coras R, Lücking H, Buchfelder M. Successful surgery of exophytic brainstem glioma mimicking cerebellar-pontine angle tumor: case report and review of literature. World Neurosurg. (2019) 128:202–5. doi: 10.1016/j.wneu.2019.05.053
25. Torun N, Laviv Y, Jazi KK, Mahadevan A, Bhadelia RA, Matthew A, et al. Schwannoma of the trochlear nerve-an illustrated case series and a systematic review of management. Neurosurg Rev. (2018) 41:699–711. doi: 10.1007/s10143-016-0783-y
26. Lock JH, Biousse V, Newman NJ. Trochlear nerve schwannoma: A 22 year follow-up. Clin Exp Ophthalmol. (2020) 48:257–8. doi: 10.1111/ceo.13664
27. Inoue T, Shima A, Hirai H, Suzuki F, Matsuda M. Trochlear nerve schwannoma treated with gamma knife after excision: A case report and review of the literature. J Neurol Surg Rep. (2015) 76:e248–52. doi: 10.1055/s-0035-1564059
28. Bal J, Bruneau M, Berhouma M, Cornelius JF, Cavallo LM, Daniel RT, et al. Management of non-vestibular schwannomas in adult patients: A systematic review and consensus statement on behalf of the EANS skull base section. Part I: oculomotor and other rare non-vestibular schwannomas (I, II, III, IV, VI). Acta Neurochir (Wien). (2022) 164:285–97. doi: 10.1007/s00701-021-05048-y
Keywords: trochlear nerve, schwannoma, neurinoma, surgery, case report
Citation: Fimic M, Haas P, Ortiz Rey JA and Tatagiba M (2024) Surgical management of a large cystic trochlear nerve schwannoma mimicking a brainstem glioma: a case report. Front. Oncol. 14:1474372. doi: 10.3389/fonc.2024.1474372
Received: 01 August 2024; Accepted: 14 October 2024;
Published: 11 November 2024.
Edited by:
Abdulqadir J. Nashwan, Hamad Medical Corporation, QatarReviewed by:
Shilpa Rao, National Institute of Mental Health and Neurosciences (NIMHANS), IndiaNeil Miller, Johns Hopkins Medicine, United States
Joe M. Das, Imperial College London, United Kingdom
Copyright © 2024 Fimic, Haas, Ortiz Rey and Tatagiba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miroslav Fimic, bWlyb3NsYXYuZmltaWNAZ21haWwuY29t
 Miroslav Fimic
Miroslav Fimic Patrick Haas
Patrick Haas Jose Antonio Ortiz Rey
Jose Antonio Ortiz Rey Marcos Tatagiba
Marcos Tatagiba