
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 15 November 2024
Sec. Head and Neck Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1473889
 Melanie Townsend1
Melanie Townsend1 Alexandra E. Kejner2
Alexandra E. Kejner2 Farzad Nourollah-Zadeh3
Farzad Nourollah-Zadeh3 Fabio A.P. Rizzante4
Fabio A.P. Rizzante4 Tina R. Woods3
Tina R. Woods3 Sonali Rathore3
Sonali Rathore3 Douglas Alterman4
Douglas Alterman4 Sorin Teich4
Sorin Teich4 William G. Albergotti2
William G. Albergotti2 Jason G. Newman2
Jason G. Newman2 Angela J. Yoon2,3*
Angela J. Yoon2,3*Proliferative verrucous leukoplakia (PVL) is an aggressive and distinct type of oral precancerous lesion characterized by warty surfaced white plaque diffusely involving oral mucosa. Surgical excision is the treatment of choice. However, PVL has persistent and recurrent growth patterns, requiring multiple surgical procedures. Surgical intervention is especially challenging if PVL extends between teeth limiting access. These interproximally located lesions have a high propensity to undergo malignant transformation. We report a case of a 53-year-old man with recurrent PVL diffusely covering the maxillary and mandibular gingiva. Despite complete surgical excisions, PVL recurred, and a focal area in the interproximal mandibular gingiva progressed to invasive squamous cell carcinoma requiring marginal resection. The remaining PVL areas were treated with topical imiquimod (toll-like receptor 7 agonist) for six months, resulting in complete clinical and histological resolution. Topical agents can cover a larger surface area and penetrate in between interproximal areas. Importantly, it allows for maximal local exposure with minimal systemic toxicity, essential for long-term treatment and prophylactic use of the agent to prevent relapse.
Proliferative verrucous leukoplakia (PVL) is a long-evolving, aggressive, and irreversible precancerous condition with a malignant transformation rate of 63.9% and a recurrence rate of 67.2% (1–3). Etiology is unknown, with a weaker association with tobacco and alcohol consumption (3). Although the PVL pathogenesis seems to involve the activation of immunological pathways that can be associated with human papillomavirus (HPV) infection, there is no firm evidence of HPV involvement (4). The most common site is gingiva, in which lesions initially appear as white patches that proliferate with time to become multifocal, later confluent, and diffuse verrucous plaques (1). Current treatment modalities include complete removal with scalpel excision or laser ablation (1–3). Despite research efforts, no approved therapeutic strategies to halt PVL recurrence exist (1). Toll-like receptor (TLR) 7 agonists, imiquimod, FDA-approved medication for cutaneous neoplastic lesions, have shown promise in off-label use for other oral precancerous and cancerous lesions (5, 6). There are limited reports of successful treatment of PVL with imiquimod. Here, we report a case of the off-label use of imiquimod to treat recurrent PVL and prevent relapse.
A 53-year-old man presented to the Otolaryngology in 2022 with histology-confirmed carcinoma-in-situ of the right maxillary gingiva presenting as flat, ~3 cm erythroleukoplakia and a smaller lesion of mandibular gingiva. He had no other illnesses or history of smoking or alcohol abuse. Additional biopsies were obtained to assess for invasive squamous cell carcinoma (SCC), which showed high-grade epithelial dysplasia but no carcinoma. The in-situ hybridization for high-risk human papillomavirus types 16 and 18 was negative. The patient had KTP laser ablation of all the maxillary and mandibular lesions. At a 1-month follow-up, the lesion recurred in both maxillary and mandibular gingiva, and re-biopsy showed moderate epithelial dysplasia. A second round of laser ablation was performed. At the next 1-month follow-up, recurrent PVL diffusely involving maxillary and mandibular gingiva was noted. Repeated biopsies showed moderate epithelial dysplasia in the maxillary gingiva but invasive SCC of the right posterior mandibular gingiva. PET/CT showed no evidence of nodal involvement or distant metastasis (T1N0M0). Marginal resection of the posterior mandible along with the extraction of three teeth and the sentinel lymph node biopsy of the neck were performed. The patient also underwent oral cavity brachytherapy 3000 cGy in 10 fractions in 1 week (2 fractions per day). The timeline is summarized in Figure 1A.
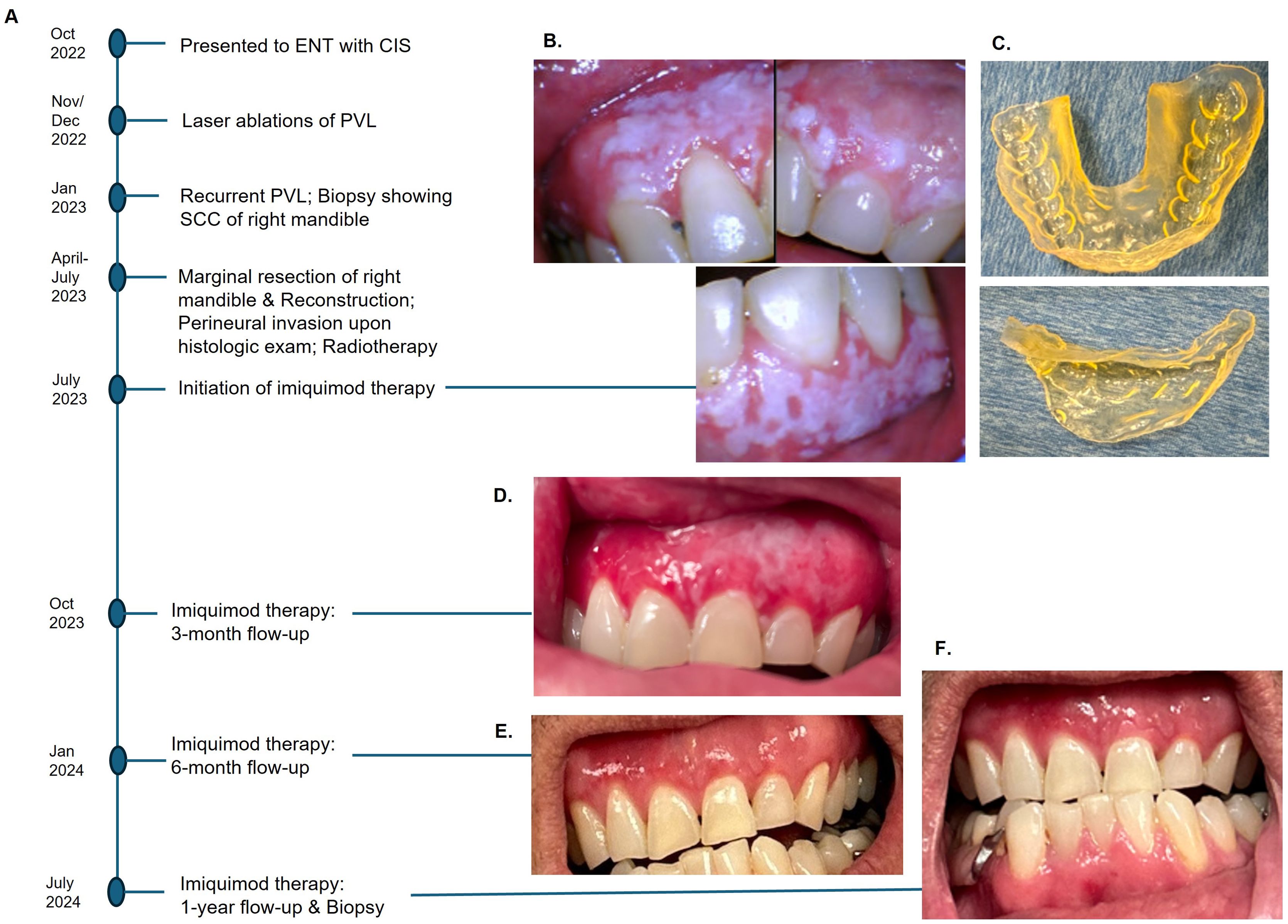
Figure 1. Events in chronological order (A). Diffuse PVL of maxillary gingiva and left mandibular gingiva after marginal resection of the right posterior mandible (B), 3-D printed customized tray to hold imiquimod cream (C). A significant reduction of PVL was observed at 3-month follow-up (D), and no clinically visible lesion at 6-month post-therapy (E). No clinical or histologic evidence of recurrence after a year with continued use of imiquimod (F).
The patient was referred to an oral and maxillofacial pathologist for non-surgical management of residual PVL. At baseline, an intraoral camera was used to capture all lesions in detail (Figure 1B), and cone-beam CT was performed to ensure no osseous involvement. A 3-D printed custom tray was fabricated to allow retention of the imiquimod 5% cream in place (Figure 1C). The patient was instructed to apply two single packets of imiquimod cream on the affected mucosal areas, one for maxillary gingiva and one for mandibular gingiva, once every other day before bedtime. The patient was directed to massage the cream into mucosa using gloved fingers, place the custom tray over it, and squeeze the appliance to cause the cream to diffuse between the teeth to reach interproximal areas. After 20 minutes of application, the cream was rinsed thoroughly with water.
A significant resolution of PVL was noted at the three-month post-therapy visit, with thinner leukoplakia foci remaining in the left maxillary gingiva (Figure 1D). No significant adverse effects were reported. The entire gingiva had an erythematous appearance, most likely due to application site reaction. By six months post-therapy, the lesion was no longer visible (Figure 1E). The patient opted to continue with two packets every other day to prevent recurrence. At 12 months follow-up, there was no evidence of PVL recurrence (Figure 1F). Multiple biopsies were taken from the maxillary and mandibular gingiva, which all showed normal mucosa without evidence of epithelial dysplasia. Photomicrographs comparing the initial biopsy of the right maxillary gingiva showing carcinoma-in-situ and the post-treatment biopsy of the same location demonstrating normal mucosa are illustrated in Figure 2. The patient will continue with the twice-a-week imiquimod regimen for a year. If no recurrence is observed, the dose schedule will be changed to once a week to prevent recurrent PVL.
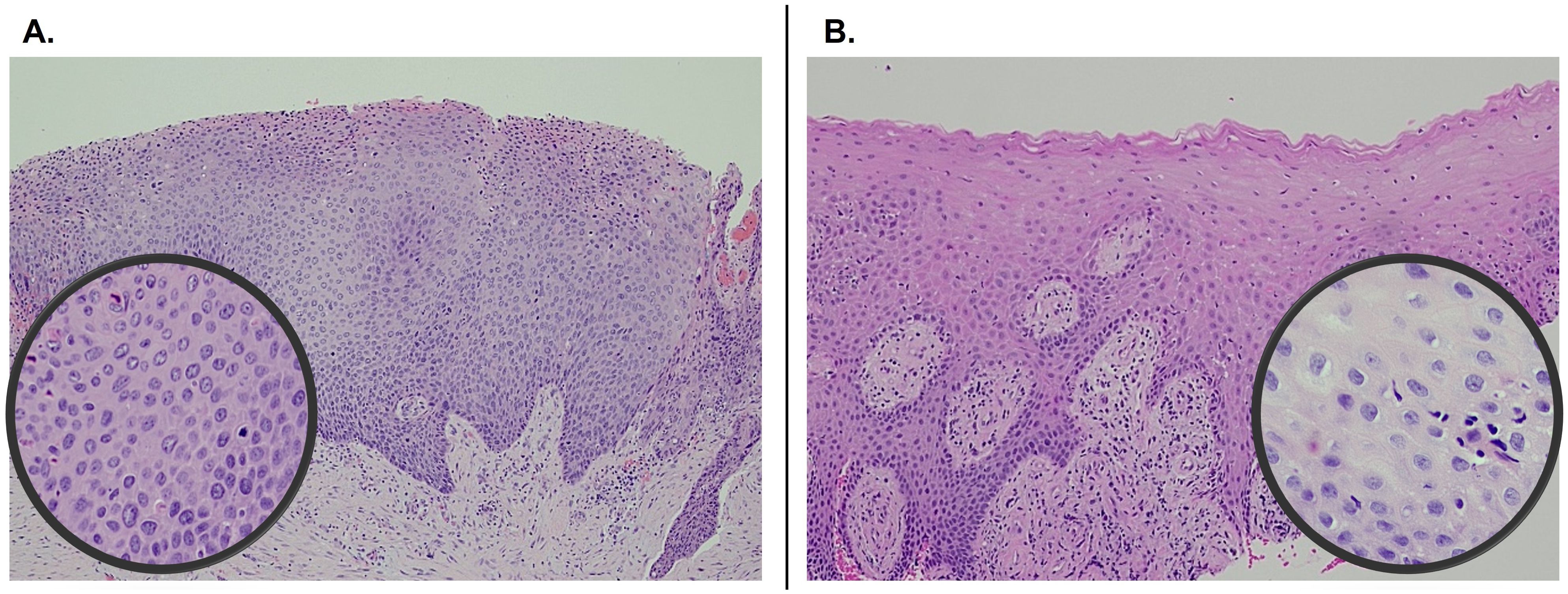
Figure 2. Pre- and post-treatment comparison of histopathology of right maxillary gingiva showing carcinoma-in-situ at initial presentation (A) and normal mucosa 1-year following imiquimod treatment (B). Both photomicrographs were captured at 100x and the insets at 400x.
Proliferative verrucous leukoplakia (PVL) is difficult to manage due to the widespread nature of the lesion requiring extensive surgical excision (1–3). The challenge is further compounded by the lesions affecting hard-to-reach interproximal dentate areas, a high recurrence rate, and malignant transformation potential; this, in turn, necessitates multiple surgeries and frequent biopsies (1–3). The most commonly used treatment modalities for PVL are scalpel excision, laser ablation, and electrocautery, among other invasive procedures (1). In attempts to prevent recurrence, various therapies, including retinoids, vitamin A, antioxidants, and cyclooxygenase inhibitors, have been tried without clear efficacy (3).
Imiquimod is a toll-like receptor (TLR) 7 agonist exhibiting anti-tumoral effects mediated by initiating apoptosis of neoplastic cells and activating innate and adaptive immunity (7, 8). Imiquimod increases tumor cell apoptosis by shifting the pro- and anti-apoptotic Bcl factors toward the pro-apoptotic Bax protein and by stimulating the release of mitochondrial cytochrome c into the cytosol, activating caspase-9 and caspase-3 (9, 10). Upon binding to TLRs, imiquimod activates nuclear factor-kB, which stimulates the production of pro-inflammatory cytokines (interleukin-6/8) and other components of innate immunity (8). The end result is T cell-mediated anti-tumoral response (8–11). The time for absorption of drugs into the oral mucosa is short, and any residual medication is washed with saliva after 5 to 10 minutes of application (12, 13). Residual imiquimod mixed in saliva can irritate the oropharynx, resulting in pharyngitis. With the trays allowing retention of the imiquimod in place, the medication was applied for 20 minutes and then thoroughly rinsed.
There are a limited number of studies assessing the efficacy of imiquimod in managing PVL (Table 1). Martinez-Lopez et al. (14) reported successful treatment of a 66-year-old woman with PVL of the lower lip and gingiva, applied three nights a week for six weeks, and no recurrence was observed at the six-month follow-up. Alhadlaq et al. (15) reported the study of oral leukoplakia treatment with imiquimod, in which 70.6% of the lesions were multi-focal/proliferative. Imiquimod was applied five days a week for six weeks. At six months follow-up, 75% of the patients were recurrence-free. There are few other reports of utilizing imiquimod to treat multifocal, diffuse, or recurrent oral neoplasm, including oral precancer and cancer and florid oral papillomatosis (16–20).
The adverse events associated with imiquimod include application site mucositis and discomfort, nausea, and influenza-like symptoms (11). The serum level of active drugs is very low or undetectable during the imiquimod therapy (21). While the exact cause of systemic adverse events is unclear, studies show that imiquimod-related flu-like symptom is encountered during the first treatment cycle in patients treated on the head and neck compared to the body and are independent of age and circulating levels of cytokines during treatment (21). A long-term application of imiquimod for a mean duration of 6.3 years demonstrated safety without serious adverse events (22). Similar to our case, other studies reported that imiquimod is effective and safe for patients with oral precancers. Importantly, the studies that had patients placed on a long-term maintenance dose of imiquimod report no recurrence (17, 18, 20).
Metronomic chemotherapy (MCT) and drug repurposing (i.e., off-label use of imiquimod) have emerged as promising therapeutic approaches for the treatment of various neoplasms (23). Similar to imiquimod, MCT allows oral administration of chemotherapeutic regimens at lower doses without long drug-free intervals and exerts an immunomodulatory effect in the tumor microenvironment (23). However, MCT is associated with more severe systemic toxicity compared to imiquimod, which includes nausea, minor-grade fatigue, mild to severe leucopenia, lymphopenia, neutropenia, and anemia (11, 23).
Our follow-up period is limited to six months after the patient had a complete resolution of PVL. With a mean follow-up of 79 months, the recurrence rate was 67.2% [95% CI: 48.3-81.8] (2). The average time to malignant transformation is four years (24). Hence, a long-term follow-up is needed to accurately assess the efficacy of imiquimod in preventing recurrence and malignant transformation.
We report the efficacy and safety of imiquimod therapy for an aggressive precancerous oral lesion with high malignant transformation potential. PVL is also associated with a high recurrence rate. As such, long-term maintenance therapy after the initial resolution of the lesion may be necessary to prevent relapse.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Medical University of South Carolina. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MT: Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. AK: Conceptualization, Investigation, Supervision, Writing – review & editing. FN-Z: Data curation, Investigation, Writing – review & editing. FR: Data curation, Investigation, Methodology, Writing – review & editing. TW: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. SR: Formal analysis, Investigation, Visualization, Writing – review & editing. DA: Investigation, Resources, Supervision, Writing – review & editing. ST: Investigation, Resources, Supervision, Writing – review & editing. WA: Data curation, Investigation, Writing – review & editing. JN: Conceptualization, Investigation, Supervision, Writing – review & editing. AY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the NIH/NCI R21CA252441 (AY) and Hollings Cancer Center Support Grant (P30 CA138313).
We would like to thank Peyton Galatolie for coordinating patient care.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
PET/CT, Positron emission tomography/computed tomography scan; PVL, Proliferative verrucous leukoplakia; SCC, squamous cell carcinoma; TLR, Toll-like receptor.
1. Abadie WM, Partington EJ, Fowler CB, Schmalbach CE. Optimal management of proliferative verrucous leukoplakia: A systematic review of the literature. Otolaryngol Head Neck Surg. (2015) 153:504–11. doi: 10.1177/0194599815586779
2. Prano-Haro A, Bagan L, Baga JV. Recurrences following treatment of proliferative verrucous leukoplakia: A systematic review and meta-analysis. J Oral Path Med. (2021) 50:820–8. doi: 10.1111/jop.13178
3. Staines K, Rogers H. Proliferative verrucous leukoplakia: a general dental practitioner-focused clinical review. Br Dent J. (2024) 236:297–301. doi: 10.1038/s41415-024-7066-8
4. de Mello Palma V, Frank LA, Balinha DM, Rados PV, Pohlmann AR, Guterres SS, et al. Is imiquimod a promising drug to treat oral mucosa diseases? A scoping review and new perspectives. Br J Clin Pharmacol. (2024) 90:427–39. doi: 10.1111/bcp.15923
5. Amaral AL, Lwaleed BA, Bouquot JE, Andrade SA. How safe is off-label use of imiquimod in oral lesion? Br J Clin Pharmacol. (2024) 90:427–39. doi: 10.1038/s41432-024-01026-2
6. Satish T, Khan S, Levin M, Carvajal R, Yoon AJ. Treatment of recurrent mucosal melanoma of the oral cavity with topical imiquimod and pembrolizumab achieves complete histopathologic remission. J Immunother Cancer. (2021) 9:e001219. doi: 10.1136/jitc-2020-001219
7. Burns RP, Ferbel B, Tomai M, Miller R, Gaspari AA. The imidazoquinolines, imiquimod and R-848, induce functional, but not phenotypic, maturation of human epidermal langerhans' Cells. Clin Immunol. (2000) 94:13–23. doi: 10.1006/clim.1999.4804
8. Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol. (2002) 27:571–7. doi: 10.1046/j.1365-2230.2002.01151.x
9. Navi D, Huntley A. Imiquimod 5 percent cream and the treatment of cutaneous Malignancy. Dermatol Online J. (2004) 10:4. doi: 10.5070/D34VW339W4
10. Schön MP, Schön M. Imiquimod: mode of action. Br J Dermatol. (2007) 157:8–13. doi: 10.1111/j.1365-2133.2007.08265.x
11. Zhang X, Xie Y, Wang L. Rare cutaneous side effects of imiquimod: A review on its mechanisms, diagnosis, and management. Dermatol Ther. (2023) 13:1909–34. doi: 10.1007/s13555-023-00978-0
12. Remiro PFR, Nagahara MHT, Ghezzi M, Filippini A, Demurtas A, Pescina S, et al. An alternative device for the topical treatment of oral cancer: Development and ex-vivo evaluation of Imiquimod-loaded polysaccharides formulations. Pharmaceutics. (2022) 14:2573. doi: 10.3390/pharmaceutics14122573
13. Bartlett JA, Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. (2012) 13:1110–5. doi: 10.1208/s12249-012-9839-7
14. Martinez-Lopez A, Armadas AF. Successful treatment of proliferative verrucous leukoplakia with 5% topical imiquimod. Dermatol Ther. (2017) 30:e12413. doi: 10.1111/dth.12413
15. Alhadlaq MA, Villa A, Sroussi H, Ikeda K, Veluppillai P, Treister N, et al. Novel management approach to oral leukoplakia: topical imiquimod treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. (2023) 136:e160. doi: 10.1016/j.oooo.2023.07.022
16. Mane S, Patilsoman B, Bhate P, Das D, Malusare P, Tomar N. To evaluate the efficacy and tolerability of topical 5% Imiquimod in cases of oral leukoplakia: A pilot study. Indian Acad Oral Med Radiol. (2021) 33:27–31. doi: 10.4103/jiaomr.jiaomr_147_20
17. Mullins R, Ansell M, Laverick S. Treatment of oral dysplasia with 5% imiquimod cream: Short communication. Br J Oral Maxillofac Surg. (2016) 54:1028–9. doi: 10.1016/j.bjoms.2016.01.030
18. Wester A, Eyler J, Swan J. Topical Imiquimod for the palliative treatment of recurrent oral squamous cell carcinoma. JAAD Case Rep. (2017) 3:329–31. doi: 10.1016/j.jdcr.2017.04.008
19. Wenzel K, Saka B, Zimmermann R, Gundlach KKH, Barten M, Gross G. Malignant conversion of florid oral and labial papillomatosis during topical immunotherapy with imiquimod. Med Microbiol Immunol. (2003) 192:161–4. doi: 10.1007/s00430-002-0172-8
20. Ruiz-Huertas P, Borrego-Luque A, Toledano-Valero P, Manzotti C, Rollon-Mayordomo A. Oral florid papillomatosis: Topical treatment with 5% imiquimod in orabase. Clin Exp Dent Res. (2022) 8:858–62. doi: 10.1002/cre2.557
21. Bhatia N. Local skin reactions and the onset of influenza-like signs and symptoms induced by imiquimod. JAAD Int. (2022) 7:113–8. doi: 10.1016/j.jdin.2022.01.010
22. Grussendorf-Conen EI, Jacobs S, Rübben A, Dethlefsen U. Topical 5% imiquimod long-term treatment of cutaneous warts resistant to standard therapy modalities. Dermatology. (2002) 205:139–45. doi: 10.1159/000063909
23. Jan N, Sofi S, Qayoom H, Shabir A, Haq BU, Macha MA, et al. Metronomic chemotherapy and drug repurposing: A paradigm shift in oncology. Heliyon. (2024) 10:e24670. doi: 10.1016/j.heliyon.2024.e24670
Keywords: Proliferative verrucous leukoplakia, topical imiquimod, toll-like receptor 7 agonist, oral neoplasm, treatment
Citation: Townsend M, Kejner AE, Nourollah-Zadeh F, Rizzante FAP, Woods TR, Rathore S, Alterman D, Teich S, Albergotti WG, Newman JG and Yoon AJ (2024) Eradication of proliferative verrucous leukoplakia with toll-like receptor 7 agonist (topical imiquimod): a case report. Front. Oncol. 14:1473889. doi: 10.3389/fonc.2024.1473889
Received: 06 August 2024; Accepted: 29 October 2024;
Published: 15 November 2024.
Edited by:
Andreas Dietz, Leipzig University, GermanyReviewed by:
Julia Chang, National Taiwan University, TaiwanCopyright © 2024 Townsend, Kejner, Nourollah-Zadeh, Rizzante, Woods, Rathore, Alterman, Teich, Albergotti, Newman and Yoon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela J. Yoon, eW9vbmFAbXVzYy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.