- Department of Neurosurgery and Neurorestoration, Klinikum Klagenfurt am Wörthersee, Klagenfurt, Austria
Background: Skull base tumors represent a small subset of intracranial neoplasm. Due to their proximity to critical neurovascular structures, their resection often leads to morbidity. As a result, surgical interventions can exacerbate symptoms or cause new deficits, thereby impacting the patients’ perceived quality of life (QoL). The factors influencing QoL in patients with skull base tumors remain underexplored. This systematic review aims to synthesize current research on QoL outcomes and identify potential factors influencing QoL in these patients.
Methods: A systematic literature review was conducted in PubMed using the keywords “Skull Base” AND “Quality of Life.” A total of 815 studies published up to January 31, 2024, were screened. After abstract review, 656 studies were excluded, and 159 studies underwent full-text review. The wide variability in study methodologies and utilized QoL instruments made only a descriptive comparison possible.
Results: In total, 113 studies were systematically reviewed. Publications focusing on the same tumor type or localization were compared. The majority of studies addressed tumors of the anterior skull base, with pituitary adenomas, meningiomas and vestibular schwannomas being the most commonly represented. The impact of surgery on QoL is often underestimated by caregivers and has a more profound effect on patients than expected by surgeons. A transient decline in QoL after surgery was observed across almost all studies regardless of localization and entity. Factors influencing QoL included age, gender, tumor localization, surgical approach, tumor type, extent of resection, preoperative clinical status and neurological deficits. Radiotherapy and recurrent surgeries were predictors of poorer QoL. Early psychological intervention in complex tumors appears to enhance QoL. Some successful sealing techniques, such as nasoseptal flaps and lumbar drains, affected QoL. However, variability in study methodologies reduced the validity of the findings.
Conclusion: This review highlights the significant impact of skull base tumor surgery on patients’ QoL. Given the major oncological and surgical challenges presented by skull base tumors, their treatment significantly affects QoL, and gross total resection (GTR) should not always be the primary goal. Additionally, recognizing and addressing the modifiable and non-modifiable factors influencing QoL is crucial for improving patient outcomes and providing personalized care.
Introduction
Tumors at the skull base, while representing only a small subset of intracranial neoplasms, present considerable challenges in neurosurgery due to their proximity to critical neurovascular structures. This anatomical complexity necessitates highly specialized surgical approaches, often carrying a significant risk of morbidity (1).
Skull base tumors are a diverse group of adult and pediatric neoplasms and exhibit considerable heterogeneity in their originating tissue and dignity, encompassing a wide range of different histological tumor entities (2). These tumors typically arise outside the brain parenchyma and can develop in distinct anatomical compartments of the skull base such as the meninges (e.g. meningiomas), sellar region (e.g. pituitary adenomas or craniopharyngiomas), cranial nerves (e.g. schwannomas) or bone and cartilage tissue (e.g. chordomas or chondrosarcomas) (3). The estimated incidence of these tumors varies significantly depending on the tumor type, with pituitary adenomas being the most common, occurring at an incidence of approximately 2.7 per 100,000 individuals in the United States (4).
Most skull base tumors show limited responsiveness to chemotherapy. As a result, surgical resection and radiotherapy remain the primary therapeutic modalities (2). However, the proximity of these tumors to critical neurovascular structures, such as the cranial nerves, the brainstem and major blood vessels, poses a significant risk during surgical intervention, often making complete resection difficult or impossible (1). Consequently, surgery is typically the initial step in treatment, aimed at reducing tumor burden, followed by adjuvant radiotherapy to control residual tumor tissue.
Despite the benefits of surgery and radiotherapy, certain tumor types, such as sarcomas and chordomas, demonstrate resistance to conventional radiation therapy. In these cases, more advanced therapeutic techniques, such as particle beam therapy, have emerged as promising additional tools, offering enhanced precision and efficacy in targeting radioresistant tumors while sparing surrounding healthy tissue (5).
Historically, research on skull base tumors has concentrated on clinical endpoints such as mortality rates, surgical complications, the extent of tumor resection, responses to radiation therapy and overall survival rates (6–8). These factors are crucial for evaluating the efficacy of treatment modalities and for predicting long-term outcomes. However, they do not fully capture the comprehensive impact of the disease and its treatment on patients’ daily lives.
Quality of life (QoL) has emerged as an equally important outcome measure. It is a multidimensional construct that encompasses physical, psychological and social aspects of health from the patient’s perspective (9). These dimensions help understand the broader impacts of medical interventions, extending beyond immediate clinical outcomes. The diagnosis of a skull base tumor itself can carry a significant psychological burden, potentially leading to anxiety and depression (10, 11). Surgical interventions, while often necessary for managing or curing the disease, can exacerbate these issues, especially if they result in noticeable physical or functional deficits.
The recovery period for these patients can be demanding, involving rehabilitation, adjustment to new limitations, undergoing adjuvant therapy and coping with the fear of recurrence, all of which can further influence the patient’s quality of life (12, 13).
In the last decades, there were no validated instruments available specifically designed to measure such complex outcomes. As a result, tools like custom questionnaires and the Karnofsky Performance Status Scale (KPS) were employed to indirectly assess QoL. Originally developed to evaluate the ability of cancer patients to perform ordinary tasks, the KPS primarily quantifies a patient’s functional status and predicts their capacity to endure therapies. This scale is used predominantly by physicians to measure physical independence, rather than capturing the subjective well-being of the patient (14).
Over time, more advanced QoL assessment tools have been developed that directly measure the patient’s experience, such as the 36-Item Short Form Survey (SF-36). The SF-36 is a reliable and validated instrument which consists of 36 questions split into eight categories that explore both the physical and psychological dimensions of health, including physical functioning, role limitations due to physical or emotional problems, vitality, emotional well-being, social functioning, pain and general health perception (15). This multifaceted approach to assess various health dimensions makes the SF-36 a widely used questionnaire across various fields of medicine, not just skull base oncology.
While general QoL instruments like the SF-36 cover a broad array of health aspects, certain anatomical locations require more specialized instruments. The Anterior Skull Base Questionnaire (ASBQ), for instance, is specifically designed to assess QoL facets relevant to anterior skull base pathologies. It provides a validated and comprehensive evaluation through 35 questions divided into six subdomains: performance, physical function, energy and vitality, pain, specific symptoms and emotional impact (16).
Other QoL instruments frequently utilized in skull base surgery, such as the Anterior Skull Base Nasal Inventory (ASK-12) and Sinonasal Outcome Test (SNOT-22), focus on sinonasal quality of life. These tools primarily assess nasal symptoms, neurological symptoms, emotional burden and quality of sleep, thus addressing only specific components of the overall QoL (17, 18).
While a wide variety of validated QoL instruments are available today, the ones mentioned above are the most frequently used to assess QoL in the studies we have reviewed.
This systematic review aims to investigate and mine current research focusing on QoL outcomes following the resection of skull base tumors. We will examine how these outcomes are assessed, the tools used to measure QoL, and the effect of various surgical approaches on patient-reported quality of life. By highlighting patient-centered measures, we aim to promote a more comprehensive understanding of treatment impacts, guiding both clinical decision-making and patient care strategies in skull base oncology.
Methods
To ensure a robust and transparent approach to our literature search and analysis, this systematic review is designed to comply with the PRISMA guidelines (19), as illustrated by the PRISMA flowchart (Figure 1).
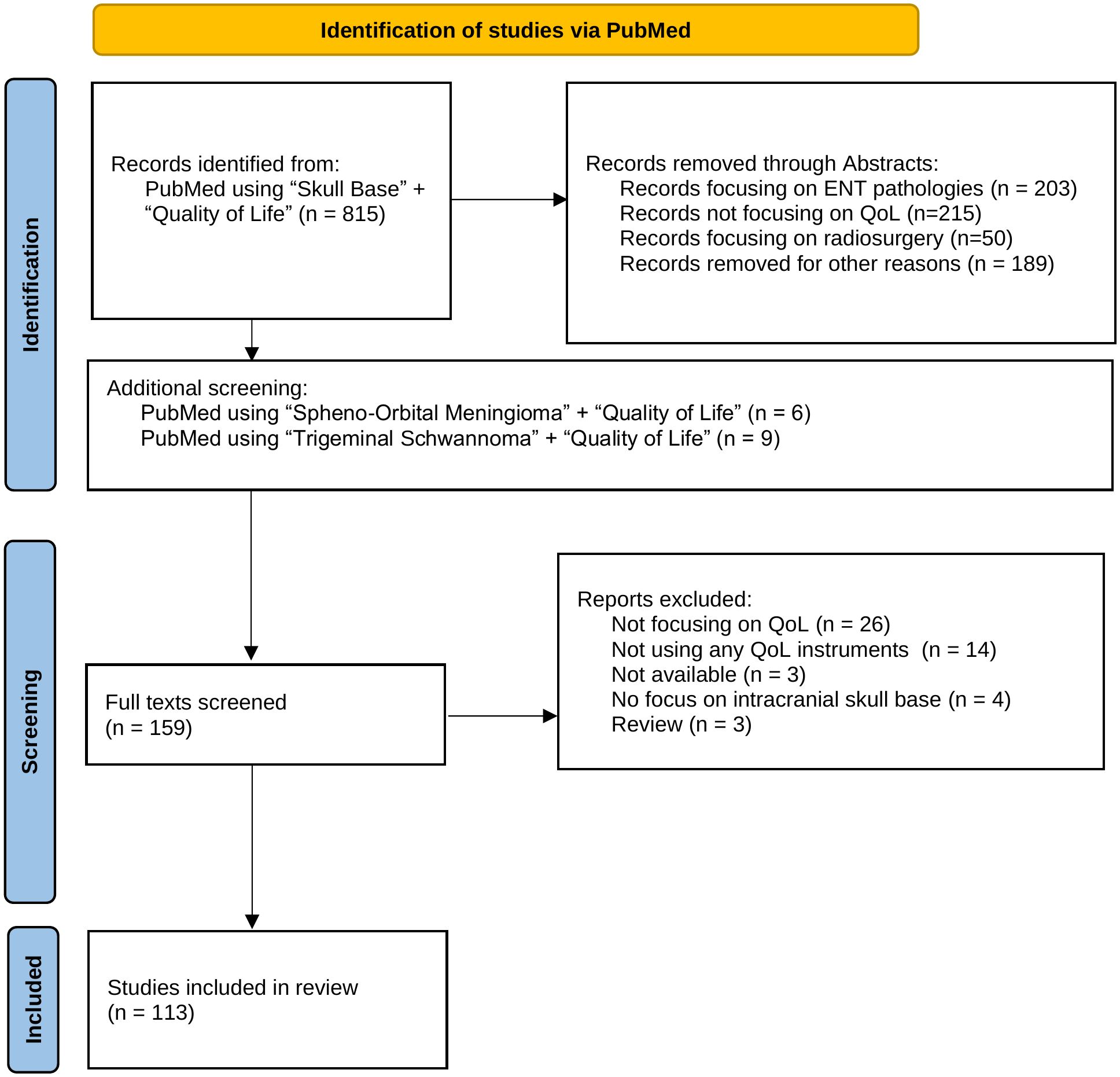
Figure 1. This flow chart outlines the systematic process of selecting studies for inclusion in the review, detailing the number of records identified, screened, and assessed for eligibility, as well as the reasons for exclusions at each stage.
We conducted the systematic literature review by searching PubMed using the keywords “Skull Base” AND “Quality of Life.” This search included all studies published up to January 31, 2024. Our initial search yielded 815 publications. Following a screening of abstracts, 159 studies were selected for detailed evaluation. We excluded 656 studies based on the following criteria: lack of focus on quality of life, primary involvement with ENT pathologies, studies evaluating radiosurgery techniques, or those not centrally addressing skull base pathologies.
The selected 159 articles underwent full-text review by the first two authors. Further exclusions were applied for studies that did not employ a validated quality of life assessment tool.
In cases where certain tumor types were underrepresented, we performed additional, targeted literature searches and cross-referenced existing findings. This methodological step was crucial to ensure that no significant studies were overlooked, resulting in the inclusion of one more study.
The final collection comprised 113 studies and we systematically compared the outcomes across these studies to identify factors that significantly impact the quality of life following skull base tumor resection. Publications focusing on more than one tumor identity were discussed for every single tumor identity. In the corresponding tables, these studies have been marked with an asterisk (*). Additionally, our analysis assessed the variety and frequency of quality of life assessment tools used, and examined the distribution of studies by tumor type and location to identify any patterns or gaps in the research landscape. To determine the country of origin for each study, we recorded the country of the first author’s affiliated institution.
Figures presented in this study were created using Microsoft PowerPoint for initial layouts and basic graphics and refined in Affinity Designer 2.5. The ggplot2 library in R was used for the visualization of bar charts.
Results
113 articles were included in this review, with the majority being published after 2010 (Figure 2A). The five most commonly utilized quality of life assessment tools included the SNOT-22 (n=44), the ASBQ (n=26), the SF-36 (n=24), the KPS (n=13) and the ASK-12 (n=6) (Figure 2B). The majority of the studies originated from the USA (n=34), United Kingdom (n=13), Australia (n=12), China (n=12), and Germany (n=11) (Figure 2C). Each study included in this review specifically targeted distinct tumor types or particular regions of the skull base (Figure 3).
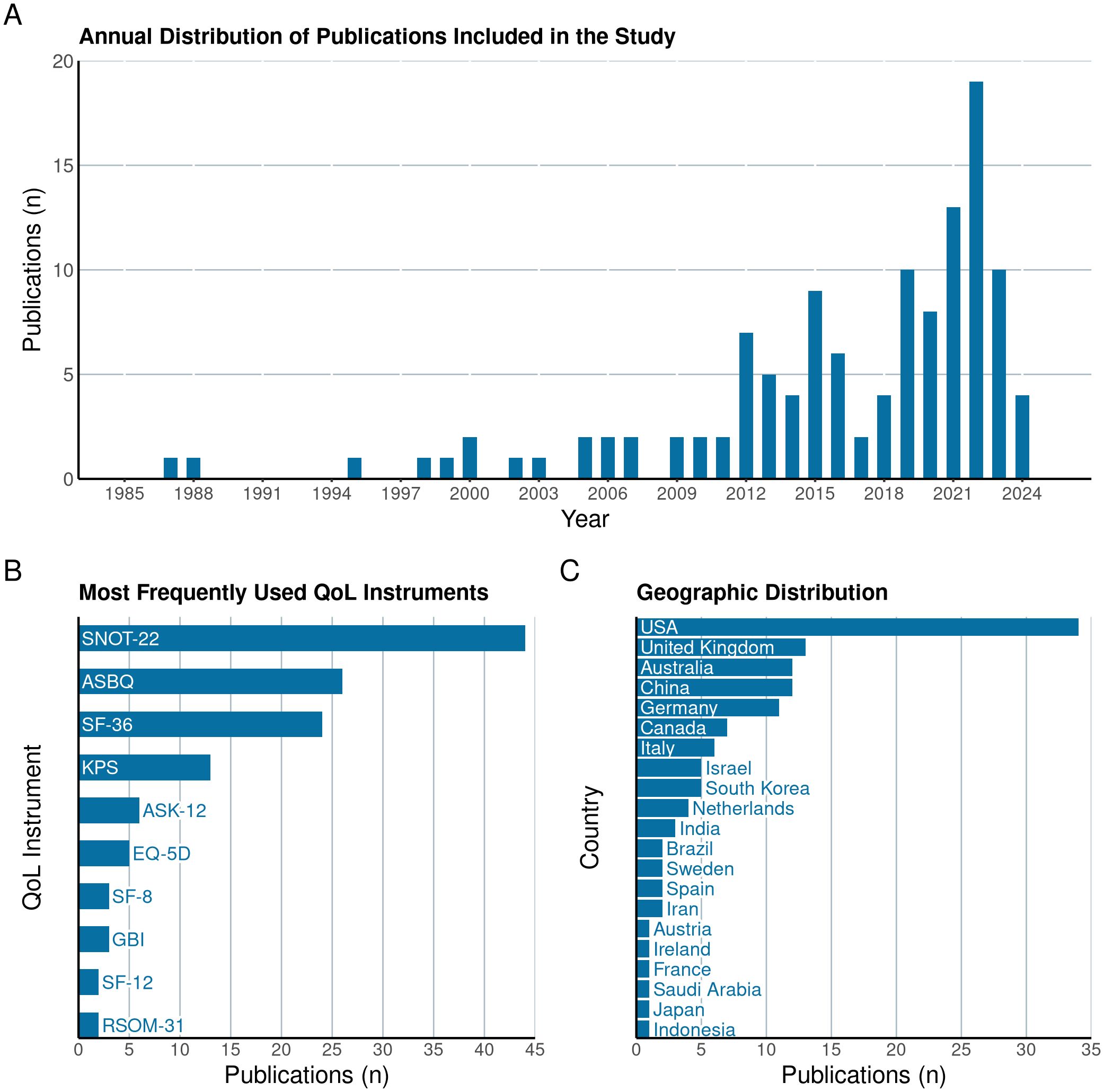
Figure 2. Illustration of the annual distribution of publications (A) included in the systematic review, highlighting trends in research volume over time. The figure also details the most frequently utilized Quality of Life (QoL) instruments in these studies (B) and the countries of origin of the included research (C).
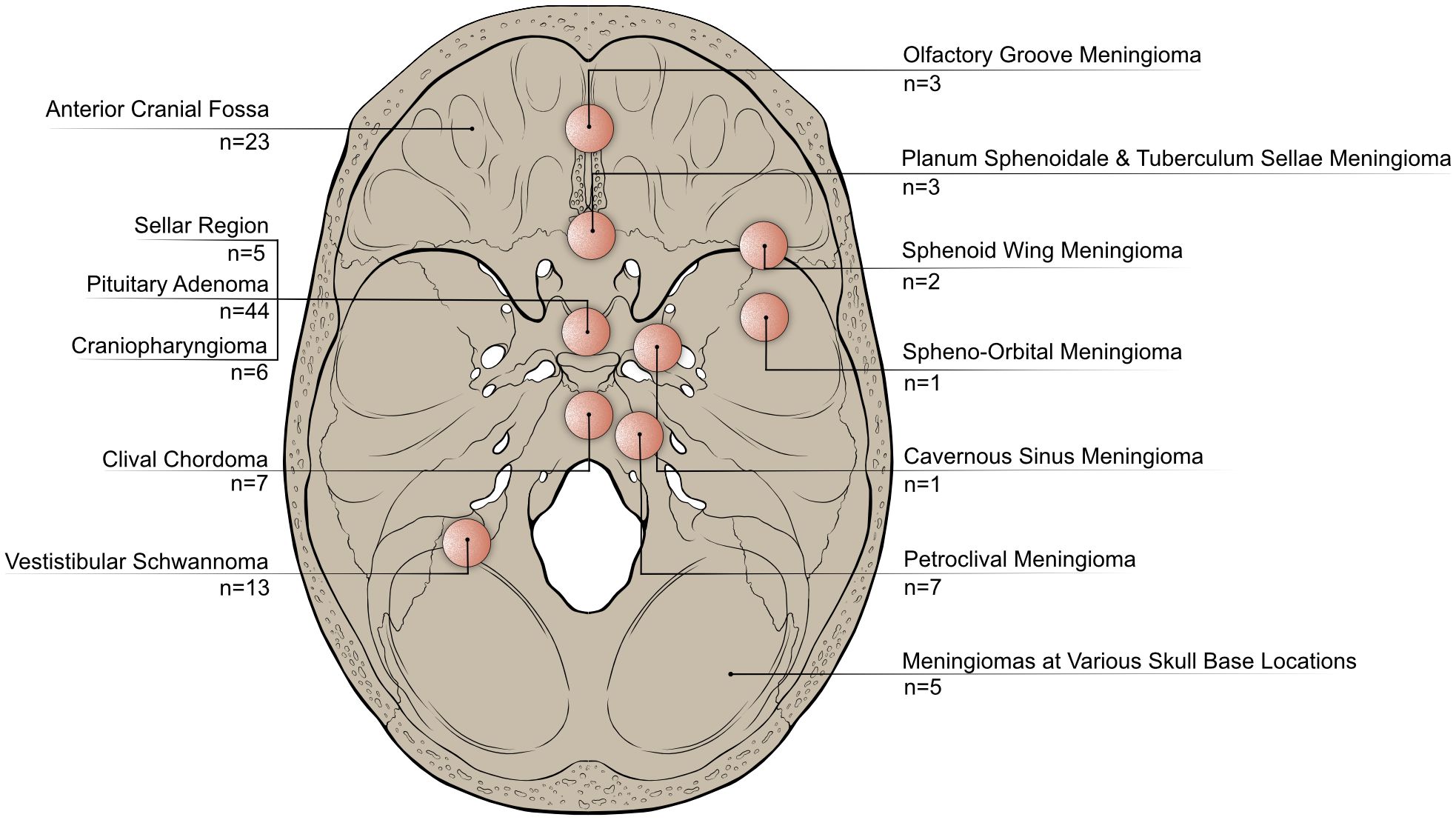
Figure 3. Categorization of publications included in this review based on the anatomical locations or tumor entities they focus on. This highlights variations in research focus across different anatomical regions or types of skull base tumors.
Most publications focused on pituitary adenomas (n=44), different tumor identities located in the anterior skull base (n=23) and meningiomas (n=22).
Tumors of the anterior skull base
Tumors of the anterior skull base constitute a significant portion of skull base tumors, spanning a wide spectrum of different benign and malignant lesions. Historically, open surgical approaches were standard in the treatment of these lesions, including those that are highly invasive and often require extensive surgical intervention. Studies identified that focus on these tumors have been summarized in Table 1.
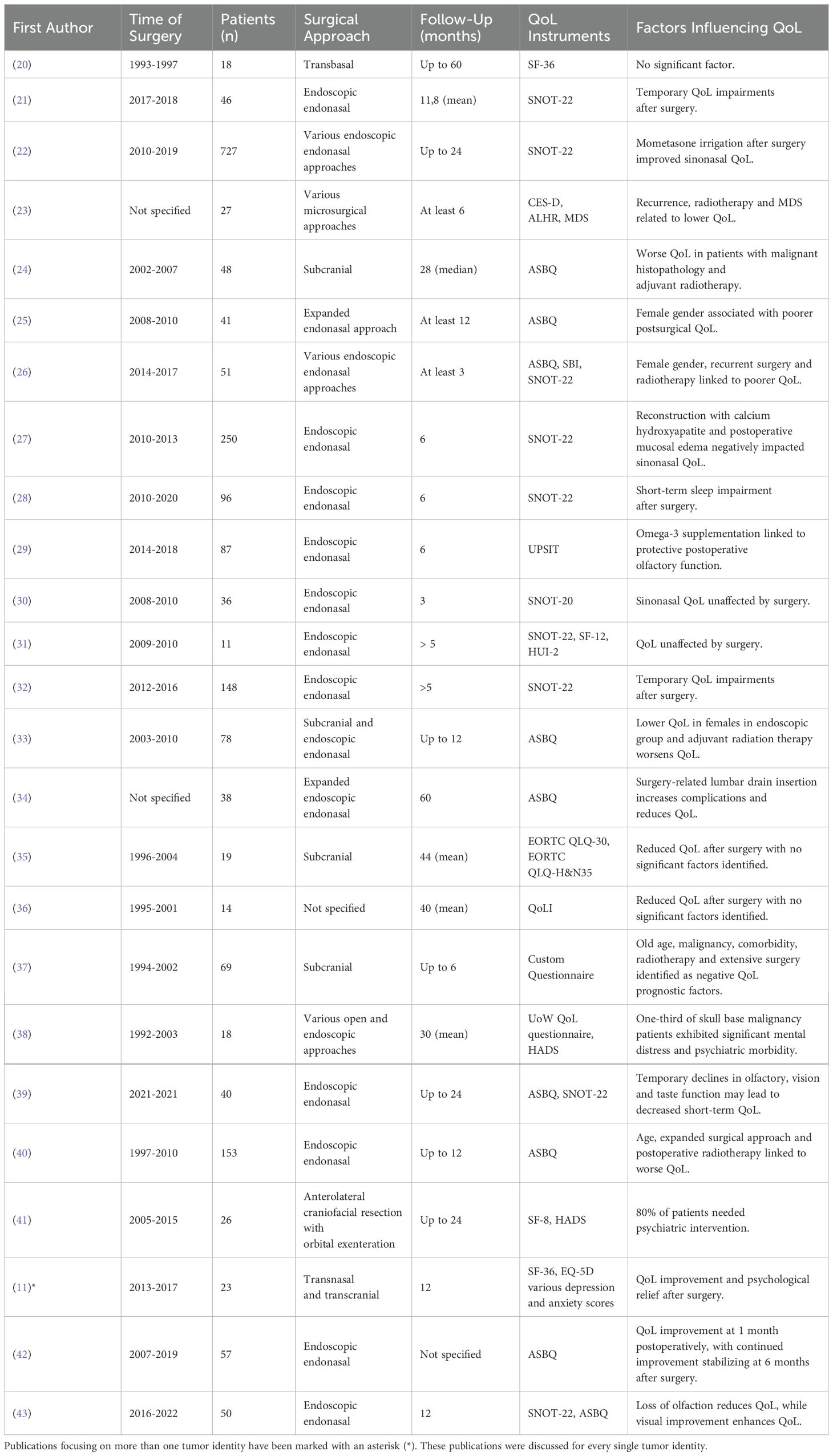
Table 1. Studies investigating QoL in patients after resection of various tumors located in the anterior skull base.
Recent advancements have increasingly supported the use of endoscopic endonasal approaches for treating anterior skull base lesions, where appropriate. While these techniques are not suitable for all tumors, they have been shown to improve QoL outcomes when compared to traditional open approaches like the subcranial approach, particularly as measured by the ASBQ (24). Furthermore, long-term QoL studies affirm the benefits of endoscopic methods for eligible lesions at the anterior skull base (26).
Earlier studies highlight the challenges associated with open surgery. High morbidity rates and significant disruptions in returning to work were noted among patients undergoing complex tumor resections (20). These issues are reflected in the diminished role function scores, indicating a negative impact on the patients perceived capacity to work (35, 36).
Studies suggests that QoL typically declines immediately following anterior skull base tumor resection, but generally returns to baseline within 6 to 12 months after surgery (24, 28, 37). Emotional and financial difficulties, as well as sleep disturbances, are common after surgery (35). Additionally, sinonasal QoL issues, such as nasal crusting or olfactory impairments, affect approximately two-thirds of patients (23). These conditions, as measured by the SNOT-22, often show improvement as early as 3 to 6 months following surgery (21, 27, 32, 39).
Some studies focusing specifically on meningiomas in the anterior skull base demonstrated significant improvement in QoL as early as one month after resection, with further improvements observed up to the six-month follow-up (42). However, more aggressive resections (Simpson Grade I) tend to result in higher rates of cranial nerve deficits (44). While visual improvement after surgery significantly impacts QoL, the loss of olfaction or taste is considered less critical (45). These neurological deficits were found to significantly decrease QoL (23, 39).
Significant disparities in QoL outcomes have been observed among patients with malignant and benign skull base pathologies (24). Patients with malignant pathologies experienced significantly lower QoL scores six months after surgery. However, there was a notable improvement in their QoL twelve months after surgery, as measured by the SNOT-22, HUI-2, and SF-36 (24, 31). In contrast, QoL scores for patients with benign tumors remained stable throughout the postoperative period (24).
Patients with malignant tumors of the anterior skull base often experience significant mental distress and psychiatric morbidity, necessitating the use of psychotropic medication in up to 80% of cases (35, 38, 41). Those undergoing extensive open cranial surgery may benefit from early psychiatric and psychological interventions, which can help them return to normal psychological health approximately two years post-surgery (41). In contrast, patients with benign lesions often experience significant psychological relief following tumor resection, whether through open or endoscopic approaches (46).
Adjuvant radiotherapy significantly worsened physical functioning, role performance and vitality. Along with recurrent surgery, it was strongly linked to poorer quality of life outcomes, measured using the ASBQ, SBI, and SNOT-22 test (24, 26, 36, 47).
Several studies identified female gender as a predictor of poorer QoL outcomes following surgery, with significant reductions in all domains of the ASBQ. Female patients reported decreases in general performance, physical function, vitality, pain and emotional impact by 18 to 32%, whereas male patients noted improvements of up to 18% in these areas (24–26).
Other factors linked to poorer postoperative QoL include older age, comorbidities and more extensive surgeries (37). The use of a preventive lumbar drain for cerebrospinal fluid (CSF) leaks in transsphenoidal endoscopic tumor resection was associated with increased complications, longer hospital stays and overall decreased QoL (34).
Conversely, certain postoperative regimes, such as omega-3 supplementation after endoscopic transnasal surgery, might improve QoL due to its potential protective effects on olfactory function (29). Postoperative irrigation with mometasone twice a day significantly reduced postoperative SNOT-22 scores compared to budesonide and saline (22).
Tumors of the sellar region
The sellar region is the site of origin for various tumors arising from different tissue types, with adenomas and meningiomas being the most common. In recent years, the endoscopic transnasal approach has become a widely adopted surgical approach when suitable, leading to numerous studies that evaluate QoL using sinonasal QoL instruments such as the ASK-12 and SNOT-22 test Table 2.
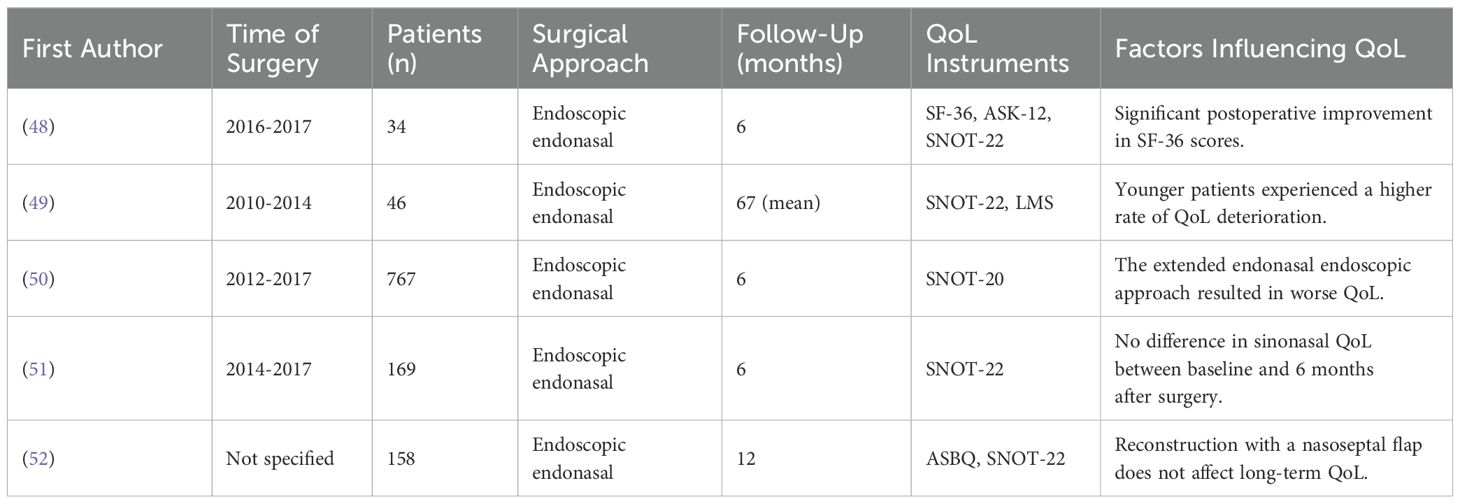
Table 2. Studies investigating QoL in patients after surgery of various different tumors in the sellar region.
While many studies report no significant change in the long-term ASK-12 and SNOT-22 scores before and after tumor resection in the sellar region, the SNOT-22 scores can deteriorate following surgery in the sellar region, typically worsening for a period of 3 to 12 weeks before returning to baseline levels within 3 to 6 months (49). In one study, tumors requiring an extended endoscopic endonasal approach were associated with worsened sinonasal QoL compared to those treated with a standard transsellar approach, measured by the SNOT-22 (50). However, other studies using the same measure reported no decline in sinonasal QoL in patients undergoing the extended approach (51). In contrast, QoL assessments using the SF-36 questionnaire generally show a significant improvement after surgery (48, 51). To address CSF leaks, a common complication of transnasal surgery, nasoseptal flaps are frequently used for reconstruction. However, these flaps seem to have little effect on the long-term quality of life outcomes (52).
Age significantly influences postoperative quality of life outcomes, with younger patients exhibiting a greater deterioration in quality of life following the resection of tumors in the sellar region compared to older individuals (49).
Pituitary adenomas
Table 3 provides a summary of the studies identified that predominantly focus on the quality of life in patients undergoing pituitary adenoma surgery. Studies encompassing multiple tumor types, including those involving patients with pituitary adenomas, are specifically annotated in the table.
Preoperative QoL, as measured by the ASBQ, was notably lower in female patients, those with diabetes, visual deficits, endocrinopathy, functioning adenomas, or headaches compared to patients with incidental adenomas (54, 88, 92). Additionally, QoL measured by the SF-36 questionnaire indicated decreased QoL in six of its eight domains preoperatively in patients with pituitary adenomas (82).
After surgery, QoL typically declined transiently in the first 2-4 weeks, particularly in sinonasal health and physical functioning, before improving to above baseline levels by 6-12 weeks and continuing to improve throughout the first postoperative year (43, 53, 74, 77, 82, 84, 92). Long-term improvements in QoL were observed following endoscopic surgery (65), exceeding preoperative levels (65), even among frail patients who experienced comparable visual and endocrine outcomes to their non-frail counterparts (63).
Postoperative nasal symptoms such as nasal discharge, pain and nasal whistling as well as issues with smell and taste significantly affected physical QoL (69, 87). These symptoms, peaking in the initial days after surgery (54), led to QoL impairments in domains such as sleep, mood, appetite, sexual desire, nutrition, health, hobbies and social interactions (59). However, these impairments typically resolved or significantly improved within three months after surgery, particularly in the domains of physical well-being, vitality and pain (11, 54, 57, 67, 73, 87). Several studies reported that olfactory and taste-specific QoL impairments, initially present after surgery, were no longer measurable 1 to 12 months later (53, 60, 70, 83, 89). Improvements in vision or visual field deficits were particularly associated with favorable QoL outcomes, which were measurable as early as three months after surgery (57, 67, 70).
In contrast to physical and social QoL, psychological QoL tended to improve directly postoperatively and three months after surgery, psychological QoL returned to baseline (54), with some studies reporting normalization of mental functions only after one year (57). Significant improvements in overall postoperative QoL were driven by improved emotional states of the patients (11, 73).
Previous sinonasal surgery, smoking, and the use of a nasoseptal flap were linked to worse rhinologic symptoms and QoL (62, 66, 71). Although the nasoseptal flap could cause worse sinonasal morbidity and headache in the immediate postoperative period, it did not have a long-term negative impact on QoL, with patients typically returning to baseline by 3-6 months after surgery (66, 80, 84, 91). In contrast, other studies found no impairment in sinonasal QoL and olfactory function after surgery (93, 94), even when using a nasoseptal flap (85).
Several studies demonstrated that gross total resection (GTR) resulted in better postoperative QoL compared to subtotal resection, as measured by ASBQ and SNOT-22 (65, 84, 86). However, other studies showed no significant difference in QoL based on the extent of resection (73, 74). Female sex and older age were associated with worse postoperative QoL (43, 77), although age was not a consistent factor across all studies (92).
Functioning pituitary adenomas were associated with worse QoL, as measured by RSOM-31 and EES-Q QoL instruments (54, 91), although this was not universally observed across all studies (71, 73) and some authors report a preoperative endocrinopathy as a factor associated with better postoperative QoL measured by the ASBQ-35 (92). Patients with Cushing’s disease reported significant QoL benefits from surgery, particularly in physical health domains. Prolactinoma and non-functioning pituitary adenoma patients also experienced significant QoL improvements three months after surgery (43). In contrast, ACTH-secreting adenomas were associated with worse sinonasal QoL after surgery. Tumor size did not significantly affect postoperative QoL (92).
Comparative studies of surgical approaches found that endoscopic techniques yielded better QoL outcomes measured by SF-36 and SNOT-22 compared to microscopic approaches (75). Conversely, other studies showed opposite results using the ASK, SF-8, and EQ-5D questionnaires (76, 78). Various endoscopic approaches have been explored in the literature, revealing only minor differences in QoL due to headache or olfactory function that were negligible in long-term follow-ups (47, 58, 60, 61, 79, 81, 90). Cerebrospinal fluid leaks during surgery did not significantly reduce QoL after surgery (73), although some studies noted slight negative associations (88).
Craniopharyngioma
Table 4 summarizes studies related to craniopharyngiomas, which frequently present surgical challenges due to their location and expansive growth. Studies involving multiple tumor types, including craniopharyngiomas, have been specifically annotated in the table.
A longitudinal study spanning over 20 years demonstrated that the overall QoL for patients, after resection of a craniopharyngioma, was relatively high, as measured by the SF-36 and KPS indices (95). Gross total resection is associated with a higher QoL (84, 96), while tumor recurrence or the need for additional resections tends to worsen QoL. Patients who experience visual improvement after surgery tend to report higher QoL scores, whereas persistent visual deficits lasting over a year, as well as hypopituitarism, have been shown to significantly worsen QoL (96).
Gender differences also appear to influence QoL outcomes, with female patients exhibiting lower QoL (96).
The studies we investigated found no significant differences in QoL outcomes among the various surgical techniques used for the resection of craniopharyngiomas. The primary methods fall into two main categories: endoscopic endonasal approaches and transcranial approaches (81, 96, 97). Typically, the endoscopic endonasal approach may lead to short-term, self-limited impairments in sinonasal related QoL. Moreover, techniques such as the use of a nasoseptal flap or gasket seal reconstruction in an endoscopic approach do not result in a long-term decrease in sinonasal QoL (86).
Meningiomas
Meningiomas are among the most common types of skull base tumors and can develop in any part of the skull base, affecting various neurovascular structures and causing a wide range of symptoms. The choice of surgical approach for removing these tumors depends on their size and location, factors that can significantly influence patient QoL Table 5.
When the specific location of the meningioma at the skull base is not considered, resection commonly results in a temporary decline in QoL postoperatively. Typically, QoL returns to baseline levels about 12 months after surgery (99). Most studies report no significant long-term impairments in QoL following meningioma surgery (13, 99, 100). However, one study noted a decrease in QoL among patients over the age of 55 (98).
Surgical complications, including CSF leaks, wound infections and accidental cranial nerve injuries, can impact patients QoL following surgery (100). Conversely, other data indicates that surgical complications do not affect QoL (13). Severe complications such as postoperative hemorrhage and associated prolonged ICU stays can lead to functional deterioration after meningioma resection (101). Additionally, while one study observed improvements in neuropsychological functions after surgery (99), another reported no changes (13). However, neither study found these neuropsychological outcomes to influence the overall perceived QoL.
The anatomical location of meningiomas within the skull base plays a significant factor in postoperative QoL. Meningiomas situated in the posterior fossa are associated with poorer QoL outcomes compared to those located in the anterior or middle cranial fossa (13). This disparity may be attributed to the fact that the posterior fossa contains surgically highly demanding meningiomas, such as petroclival meningiomas, which present more complex challenges during resection.
Petroclival meningiomas
Petroclival meningiomas, despite their typically benign pathology, present significant surgical challenges due to their proximity to critical anatomical structures. The complex anatomy and difficult access of this region have driven the development of surgical techniques aimed at minimizing morbidity while achieving complete resection and maintaining the QoL for patients. However, the impact of surgery on QoL is often underestimated by caregivers and has a more profound effect on patients than expected by surgeons (102). The results of our findings are summarized in Table 6.
Postoperatively, patients typically experience a decline in QoL, which generally improves to preoperative levels within a year after surgery. Long-term follow-ups have shown that QoL even surpass preoperative levels, as measured by the KPS. However, it is important to note that severely disabled patients with a preoperative KPS score below 70 tend to have poorer outcomes one year after surgery (104).
Achieving a surgical cure often necessitates a gross total resection. However, studies have indicated that gross total resection of petroclival meningiomas can result in worse postoperative QoL compared to subtotal resection (105, 107). While aiming for gross total resection, careful attention must be paid to protecting anatomical structures, as lower cranial nerve palsies can prevent patients from returning to a normal life and significantly diminishing postoperative QoL (103). This is particularly crucial given the high risk of new postsurgical neurological deficits associated with petroclival meningioma surgery (108, 109).
Additionally, patients with preoperative brainstem compression due to the tumor have been shown to experience significantly better QoL after surgery (102, 107). The impact of other anatomical factors, such as cavernous sinus infiltration, remains controversial, with some studies indicating no effect on QoL (102) and others suggesting an influence (107).
Sphenoid wing meningiomas
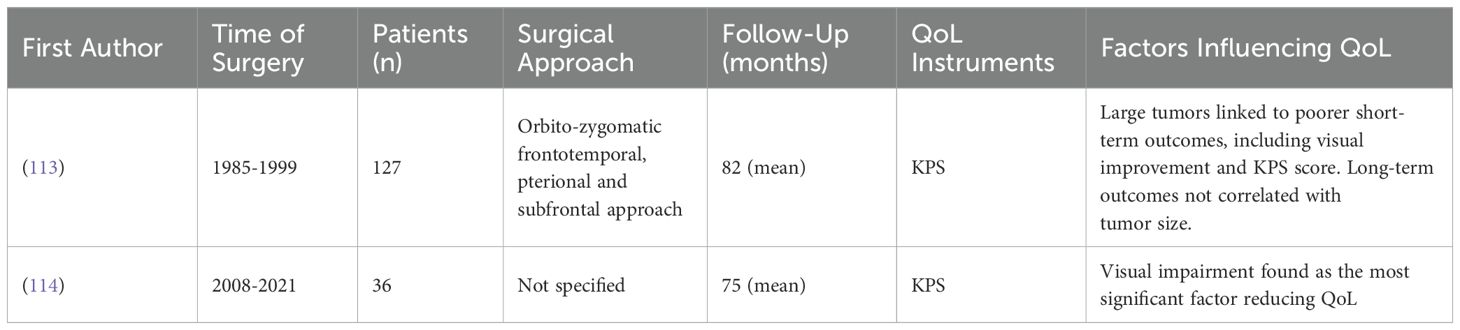
Sphenoid wing meningiomas can present a significant challenge for neurosurgeons aiming for complete and safe removal, particularly medial sphenoid wing meningiomas, which are associated with the poorest neurological functional outcomes, second only to petroclival meningiomas. These tumors negatively impact postoperative quality of life and have the highest recurrence rates among meningiomas (110–112). Two studies have investigated the quality of life in patients with sphenoid wing meningiomas, both specifically focusing on medial sphenoid wing meningiomas (Table 7).
Visual impairment has been identified as a significant factor contributing to both preoperative and postoperative reduced QoL in patients with medial sphenoid wing meningiomas that infiltrate the cavernous sinus (114).
Tumor recurrence and progression pose the major long-term risks following resection and the initial surgery is of crucial importance. It was observed that larger medial sphenoid wing meningiomas are associated with poorer immediate clinical outcomes, including less visual improvement and lower KPS scores and present greater challenges for complete removal. However, in the long-term, tumor size did not correlate with overall outcomes measured by KPS (113).
Spheno-orbital meningiomas
Spheno-orbital meningiomas are rare and our search identified only one study (Table 8) examining the QoL following their resection. This study reported a significant improvement in QoL, as measured by the European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire (EORTC QLQ). However, the analysis was limited to comparing preoperative QoL with assessments made three months after surgery and they identified no factors that significantly influenced the QoL outcomes (115).
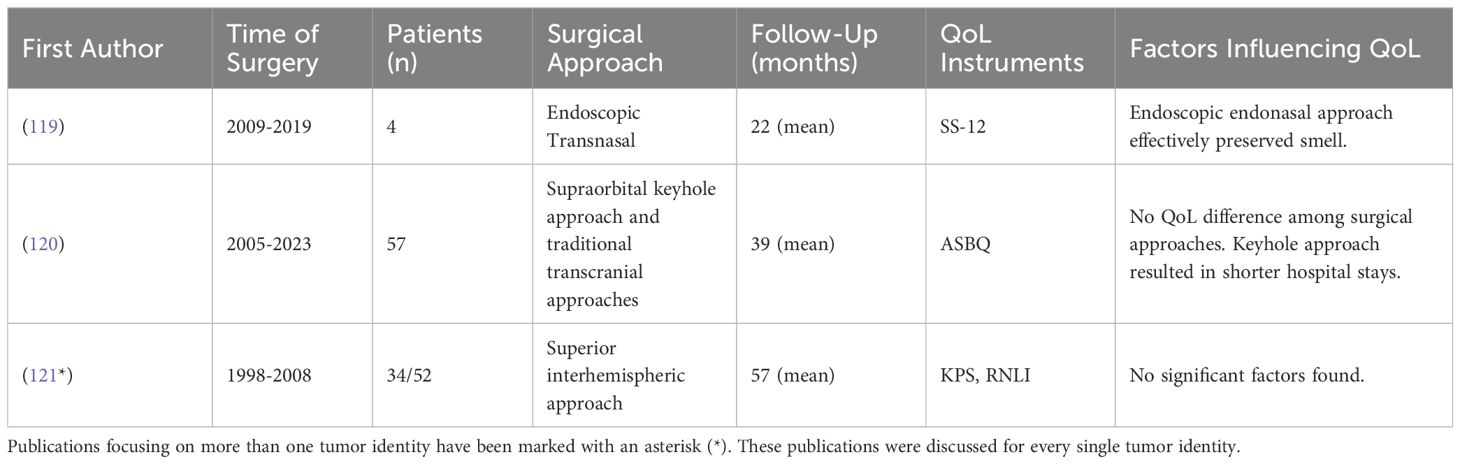
Table 8. Studies investigating QoL in patients after spheno-orbital meningioma surgery and cavernous sinus meningioma.
Cavernous sinus meningiomas
Cavernous sinus meningiomas are the most prevalent primary tumors of the cavernous sinus, yet they comprise only about 1% of all intracranial meningiomas (117). A single study investigating the QoL of patients with cavernous sinus meningiomas was found (Table 8). This study indicated a tendency for improved KPS scores in patients who underwent adjuvant stereotactic radiosurgery compared to those who had only microsurgical resection, potentially due to better tumor control; however, the changes were not statistically significant (116).
Olfactory groove meningiomas
Olfactory groove meningiomas, which develop above the cribriform plate, can grow to substantial sizes before detection (118). The resection of these tumors can be achieved through various surgical approaches, depending on the surgeon’s preference and the tumor size. We identified three studies examining the QoL in patients with olfactory groove meningioma (Table 9).
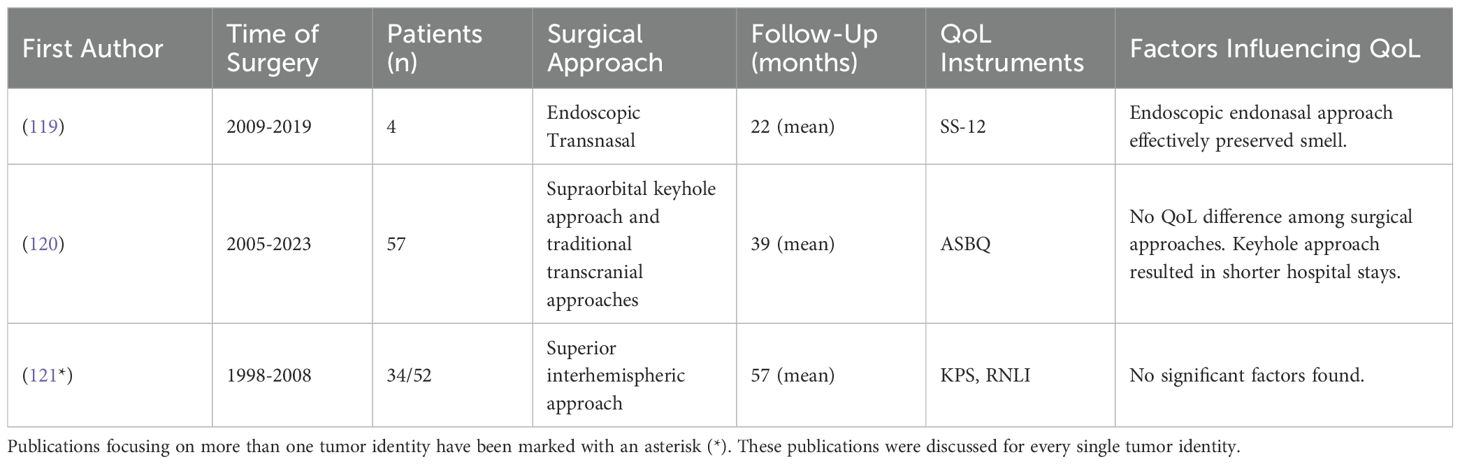
In selected cases, the endoscopic transnasal approach has demonstrated a good rate of smell preservation (119), while the supraorbital keyhole approach is associated with reduced postoperative edema and shorter hospital stays compared to traditional open approaches (120). However, the choice of surgical approach did not affect the overall QoL for these patients (120). One study using the Reintegration to Normal Living Index (RNLI) found that patients undergoing resection via the superior interhemispheric approach experienced a moderately reduced QoL, without identifying any specific factors influencing this outcome (121).
Tuberculum sellae and planum sphenoidale meningiomas
Tuberculum sellae and planum sphenoidale meningiomas originate in close proximity. Given that most studies we have reviewed involve cohorts with both types of meningiomas, we have combined them into a single section (Table 10). These studies primarily focus on evaluating the effectiveness of various surgical techniques and also assess quality of life outcomes.
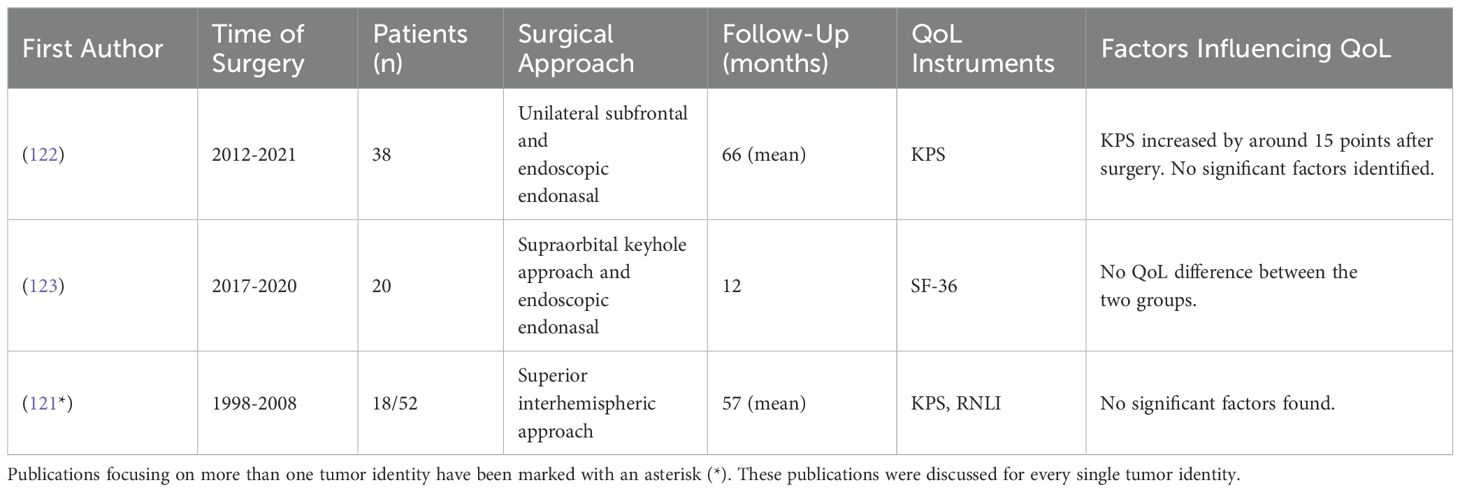
Table 10. Studies investigating QoL in patients after tuberculum sellae & planum sphenoidale meningioma surgery.
QoL, as indirectly measured by the KPS, generally shows improvement after surgery, indicating an enhancement in patients’ functional status (121, 122). Comparing different surgical approaches such as the supraorbital keyhole approach, the endoscopic endonasal approach and the unilateral subfrontal approach revealed no significant differences in QoL outcomes. Furthermore, the choice of surgical approach does not significantly impact the rates of gross total resection or postoperative vision outcomes, suggesting no indirect influence on QoL through these factors (122, 123).
Vestibular schwannomas
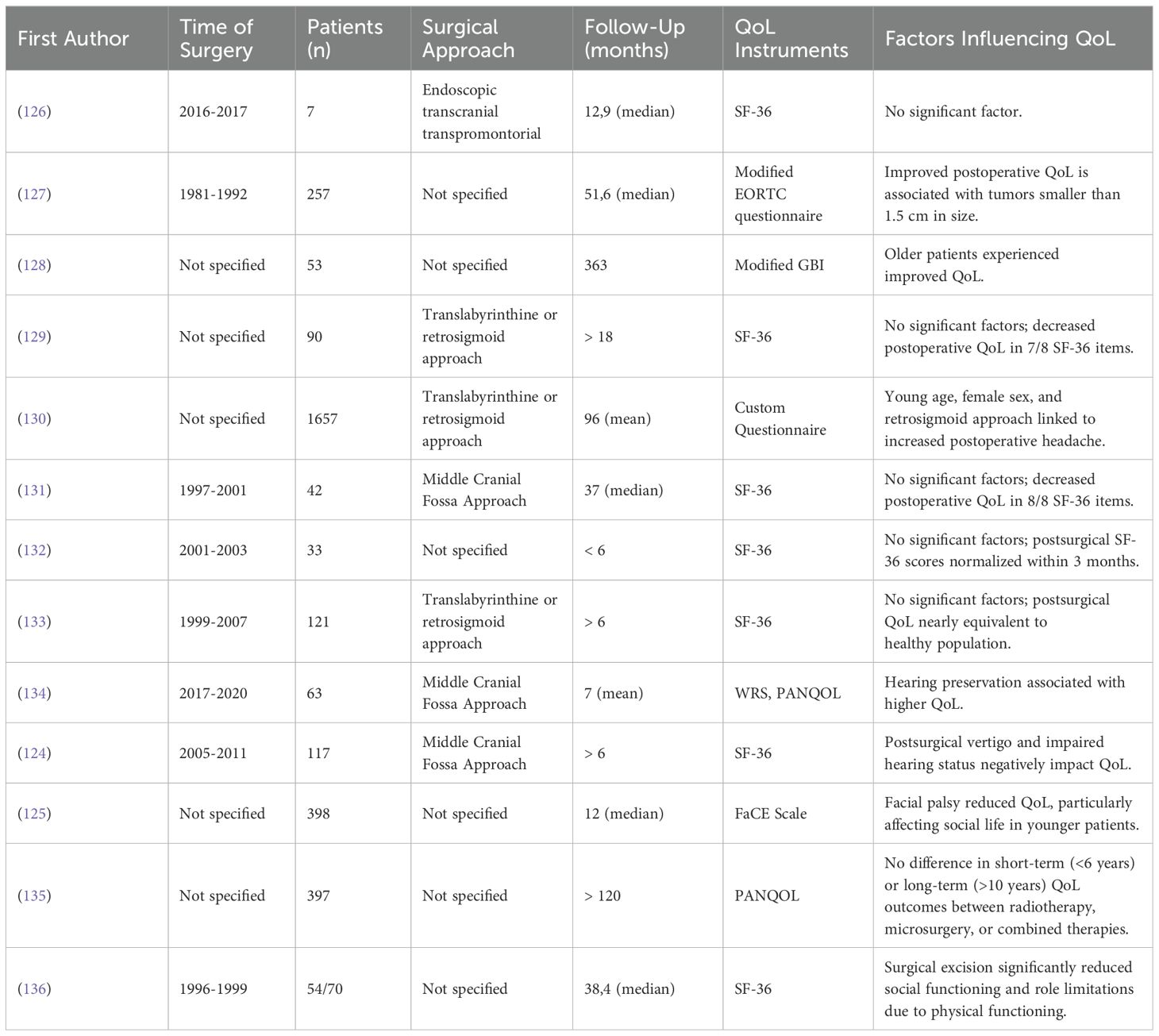
Given the close proximity of vestibular schwannomas to critical structures such as the facial and vestibulocochlear nerves, surgical resection of these tumors can result in significant neurological deficits such as facial palsy, hearing loss or vertigo (124, 125). The results of our findings are summarized in Table 11.
Additionally, psychological factors such as depression, anxiety and sleep disorders further compound the challenges, negatively impacting the postoperative QoL in these patients (131).
Contrasting perspectives emerge regarding the overall post-surgical QoL in these patients. Some research suggests that quality of life remains stable postoperatively (126, 133). However, other studies (128, 131, 132, 136) indicate a post-surgical decline in QoL, which appears to normalize within six months post-surgery (132).
Smaller vestibular schwannomas with less than 1.5 cm in diameter have been associated with a more favorable postoperative quality of life (127). This finding is in contrast to other studies (128, 129) who report no significant impact of tumor size on postoperative QoL.
A particularly challenging complication is postoperative facial palsy, which significantly lowers QoL in social domains, notably among younger women under 40 years (125). Hearing preservation has been found critical for postoperative QoL with better preoperative hearing levels correlating with improved postoperative outcomes and QoL (124, 134).
Another aspect is the choice of surgical approach. Postoperative headaches have been linked to the retrosigmoid approach, showing a noticeable decrease in QoL, particularly among younger women, compared to the translabyrinthine or middle cranial fossa approaches (130). Otherwise, it was found that the surgical approach or even the treatment modality (Microsurgery, radiotherapy or combined therapy) generally does not affect postoperative QoL (129).
The economic impact on younger patients is also significant, with some studies noting a decrease in QoL due to financial stress, a factor less impactful on older patients who may possess greater financial reserves or be at a different career stage (128). However, such findings were not consistently reported across all studies (129).
Clival chordomas
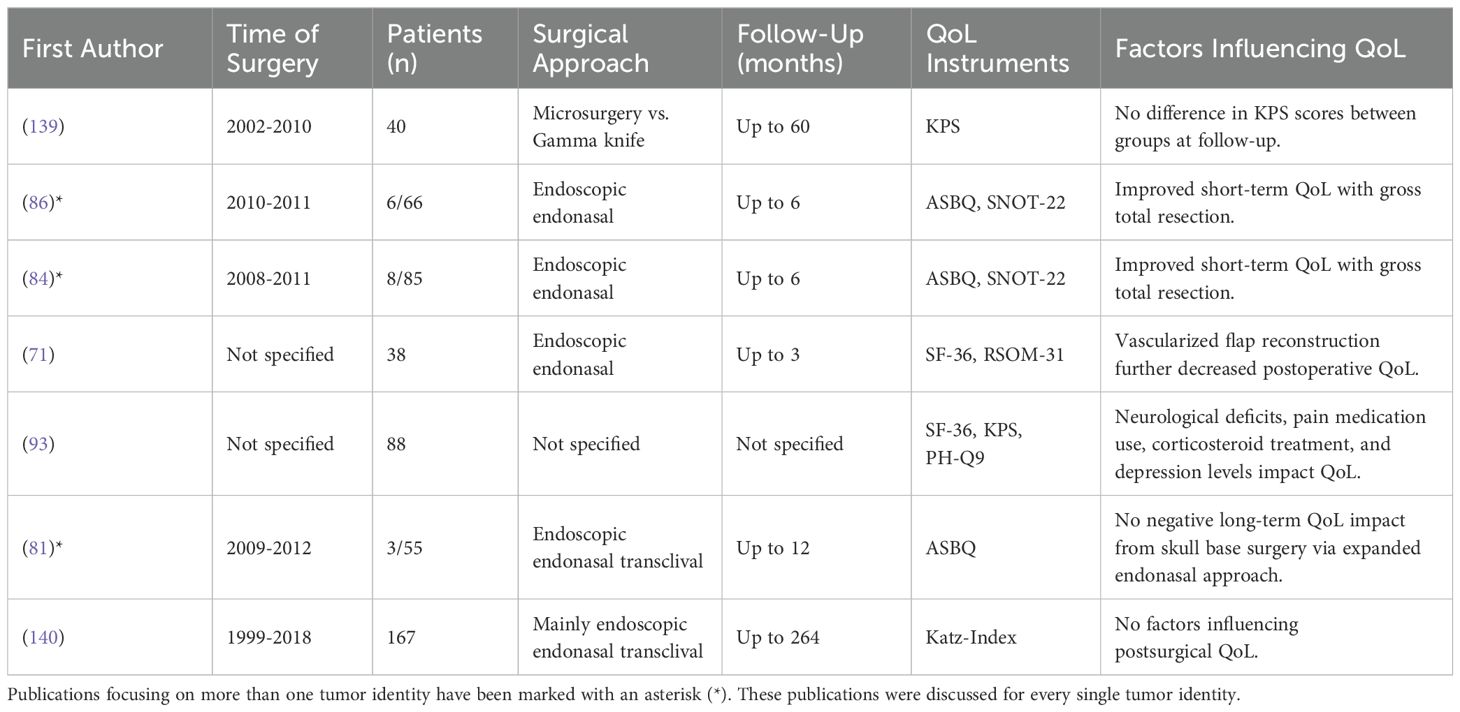
Clival chordomas, although histologically classified as low-grade tumors, demonstrate clinically malignant behaviors due to their diffusely infiltrative growth patterns and high rates of recurrence and tumor-related mortality (137, 138). Given the aggressive nature of the disease and the necessity for comprehensive removal, the challenge of achieving a surgical outcome that effectively manages the disease while also preserving the patient’s quality of life is crucial. The results of our findings are summarized in Table 12. The endoscopic endonasal approach has become a popular approach for resecting clival chordomas as it offers reduced morbidity compared to more extensive transcranial and transfacial approaches (141, 142).
Studies indicate that even extended endoscopic endonasal approaches do not negatively influence long-term QoL and only lead to temporary short-term impairments in general and sinonasal QoL (84, 86, 139). Comparisons with other treatment modalities, such as gamma knife surgery, also show no difference in QoL (139).
Gross total resection significantly improves the recovery of postoperative sinonasal QoL (84, 86). The use of a vascularized flap in endoscopic endonasal surgery is associated with more pronounced sinonasal symptoms compared to approaches that do not utilize the flap. Specifically, studies have indicated that such approaches can negatively affect physical and mental QoL at least up to three months post-surgery (71), highlighting the need for careful consideration of surgical techniques to minimize these effects. Additionally, the use of corticosteroids and pain medication correlates with reduced QoL after surgery (93).
Most studies utilize sinonasal QoL instruments. However, it should be noted that the resection of clival chordomas can lead to a variety of complications, such as neurological deficits or CSF leaks, which can increase the burden of the disease for the patient. Neurological deficits such as sensory deficits and bowel and bladder dysfunction can significantly impact the QoL in these patients and diplopia has been linked to anxiety and depression and was often already present prior to surgery (93). While gross total resection should be attempted, avoiding neurological deficits is paramount to preserving the patient’s QoL.
Discussion
This systematic review represents the first comprehensive evaluation of factors that influence QoL following the resection of skull base tumors across various anatomical locations. Whereas previous reviews have primarily focused on specific areas, such as the anterior skull base (143), or on particular approaches like the endoscopic endonasal approach (144), our extensive review covers a broad range of skull base locations and surgical techniques. This approach provides a more holistic perspective on postoperative QoL in patients with skull base tumors.
However, this literature review also demonstrates that most publications dealing with quality of life focus on the anterior skull base and the endoscopic endonasal approach. Hence, the most common used tools in this review were the SNOT -22 and the ASBQ, mainly evaluating the sinonasal outcome and quality of life. This leads to a potential bias, as other aspects of quality of life or other surgical approaches are less frequently discussed.
Our examination of the literature has revealed several key factors that may impact QoL following surgery.
Sociodemographic factors
We identified age and gender as two key sociodemographic factors that influence QoL after surgery.
Research has consistently shown that female gender is associated with poorer QoL outcomes in various skull base tumors (24–26, 37, 43, 96, 98, 100). The mechanism for this disparity is not clear and may stem from a combination of biological, psychological and social factors. Biologically, hormonal differences could influence symptom severity and recovery trajectories (145). Psychologically, women may experience higher levels of distress or depression related to diagnosis and treatment, which can adversely affect QoL (146, 147). Socially, women often face greater challenges in balancing treatment with familial and caregiving responsibilities (148). This complex interplay highlights the need for gender-specific considerations in the management and support structures for tumor patients to optimize their QoL after surgery.
Age also appears to be a significant determinant of QoL. Numerous studies have demonstrated that older patients often experience a reduced QoL following the resection of skull base tumors (45, 98, 100). Conversely, research indicates that younger patients may suffer a more rapid deterioration in QoL compared to older individuals. This may be attributed to the greater economic impact experienced by younger patients, who often face substantial challenges in balancing recovery with employment and financial responsibilities (49, 128).
Tumor localization
Patients undergoing surgery for meningiomas in the anterior or middle cranial fossa generally report a higher postoperative QoL compared to those with tumors located in the posterior fossa (13). The proximity of posterior fossa tumors to critical brainstem and neurovascular structures means that more aggressive resections in this area tend to lead to neurological deficits, which are strongly correlated with reduced quality of life QoL for patients (103). However, in cases of petroclival meningiomas where the brainstem was compressed preoperatively, patients generally experience a significantly improved QoL after surgery (107).
Regardless of the tumor entity, QoL in patients with anterior skull base tumors typically declines immediately following resection. However, it generally returns to baseline levels within 6 to 12 months postoperatively (24, 28, 37). Endonasal approaches may initially disrupt nasal and sinus function, resulting in temporary discomfort and a reduced QoL, particularly in the sinonasal domain.
Tumor entity
Individuals with malignant pathologies, particularly in the anterior skull base, exhibited significantly lower QoL scores six months after surgery compared to patients with benign lesions. However, these patients demonstrated considerable improvements in QoL twelve months after surgery. In contrast, patients with benign tumors tended to experience a more stable QoL throughout their postoperative recovery period (24, 31).
The majority of studies examining meningioma resections at various skull base locations have shown a significant improvement in QoL after surgery (98, 100, 121, 122). Conversely, a smaller number of studies report no change in QoL following the surgical intervention (13, 99). Upon closer examination of meningioma location, petroclival meningiomas and medial sphenoid wing meningiomas are notably associated with a negative impact on QoL. This correlation might be attributed to poor neurological functional outcomes and the highest recurrence rates among meningiomas (110–112).
Patients undergoing resection of pituitary adenomas typically experience an improvement in QoL after surgery, following a transient decline primarily due to sinonasal symptoms related to the endonasal approach (43, 74, 82). These patients usually exhibit a good preoperative QoL, and the psychological relief experienced after surgery plays a crucial role in their overall QoL improvement (46). In contrast to tumor size (92), endocrinopathy negatively impacts the QoL for patients with pituitary adenomas (54, 91) and relief from these endocrine disorders has been linked to improved QoL outcomes (43). Patients with prolactinomas may experience improvements in QoL as early as three months after surgery (43), whereas those with acromegaly or Cushing disease generally require significantly more time to recover their QoL (43, 149). This difference may be attributed to the residual effects on appearance, mood and metabolism that persist even after hormonal levels have normalized (150–152) However, it is important to note that examining QoL specifically related to endocrinopathy falls beyond the scope of this review and has been extensively discussed in previous reviews (153, 154).
Surgical approach
For most skull base tumors, a variety of surgical approaches are utilized for tumor resection. The choice of approach generally depends on the surgeon’s experience and preference.
However, particularly for tumors located in the pituitary region and the anterior skull base, endoscopic approaches have been widely adopted due to their minimally invasive nature and the panoramic view they provide the surgeon. While endoscopic endonasal approaches are associated with a higher incidence of CSF leaks (24, 26, 73, 75, 121, 123), our findings indicate no significant impact on the QoL for patients from these leaks. However, the prophylactic insertion of a lumbar drain has been associated with poorer QoL after surgery, persisting as long as 12 months after the procedure. Patients who received lumbar drains experienced higher morbidity, longer hospital stays and a reduction in QoL potentially stemming from associated side effects such as discomfort, headaches or infections (34). In contrast, the use of nasoseptal flaps for reconstruction and prevention of CSF leaks is correlated with worsened rhinologic symptoms and headaches in the immediate postoperative period. However, these effects do not appear to impact long-term QoL (62, 66, 71, 80, 86, 91).
Few studies have compared different surgical approaches and their impact on QoL. Such comparisons were primarily limited to variations of the endonasal approach, which revealed only minor differences in long-term sinonasal QoL, particularly with expanded endoscopic approaches used for more complex tumors (50, 51, 61, 78, 79). However, most studies we have included lack comparisons of different open transcranial approaches or the comparison between open and endonasal approaches in terms of perceived QoL outcomes for patients.
Gross total resection and neurological deficits
Gross total resection (GTR) is the objective in most tumor surgeries, whenever feasible. This is particularly crucial in malignant tumors, where achieving complete resection is associated with longer survival and reduced recurrence rates. However, achieving GTR in skull base tumors often presents numerous challenges due to the proximity to critical neurovascular structures.
The studies included in this review indicate that the quality of life following GTR of skull base lesions generally improves or remains unchanged, irrespective of the surgical approach employed. The positive effect is particularly evident in cases of craniopharyngioma, where GTR is often linked to a significantly enhanced QoL. The correlation is likely due to the reduced likelihood of tumor recurrence, the decreased need for subsequent surgical interventions and the reduced necessity for adjuvant radiotherapy (96). Although pursuing GTR in cases of craniopharyngiomas may result in endocrinopathy, the overall benefits of GTR seem to outweigh the decrease in QoL caused by new endocrine disorders (96, 155).
In contrast, patients with petroclival meningiomas often experience a deterioration in QoL after gross total resection (105, 107). This decline may be attributed to the vastly different spectrum of complications associated with resecting petroclival meningiomas compared to craniopharyngiomas. The proximity of petroclival meningiomas to the lower cranial nerves and the brainstem significantly increases the likelihood of neurological deficits, which are associated with poor postoperative QoL (107). Therefore, it is necessary for the surgeon to balance the pursuit of gross total resection with the patient’s QoL after surgery and tailor the surgical plan for each individual patient (109).
In meningioma patients, a more aggressive resection tend to lead to a greater incidence of cranial nerve deficits, which can significantly hinder a patient’s ability to return to normal life and substantially diminish their QoL (44, 103). However, not all cranial nerve deficits uniformly impact QoL in the same way.
The severity and type of deficit play critical roles in determining the extent of impact. For example, cranial nerve deficits affecting motor function and thus enabling actions such as swallowing, may be more debilitating and disruptive compared to sensory deficits. Particularly, changes in vision significantly influence QoL both before and after surgery, with postoperative improvements in vision strongly correlating with enhanced QoL for the patient (23, 39, 57, 100, 156). While some publications consider the loss of olfaction or taste to be less impactful (45), the patient’s occupation and leisure activities can significantly influence how anosmia affects their quality of life (157).
Furthermore, the individual’s ability to adapt to these changes also varies, with some patients managing to find effective coping strategies that mitigate the impact on their daily lives. This complexity underscores the need for a personalized approach in postoperative care, aimed at addressing specific deficits and supporting overall well-being.
Vestibular schwannomas present significant challenges that can impact postoperative quality of life, with outcomes varying widely across different studies and neurosurgical centers. Due to the proximity to the facial and vestibulocochlear cranial nerves, complications typically result in neurological deficits related to their functions. Notably, younger women may experience drastic impairments in QoL due to postoperative facial palsy (125), whereas hearing loss affects QoL independently of gender (124, 134). Although the size of the tumor significantly influences the complexity of the surgery, its impact on QoL is less clear. Only one study has found a correlation between larger tumor size (> 1,5cm) and worse postoperative QoL (127), whereas two other studies reported no impact on QoL (128, 129).
Implications for clinical practice
The presented literature offers several key insights for clinicians. The evidence consistently shows a transient decline in QoL after surgery across almost all studies, regardless of the tumor’s anatomical location or entity. Interestingly, this decline tends to recover to baseline levels postoperatively and in some cases, particularly with tumors treated at the anterior skull base, patient’s quality of life surpasses preoperative levels. This could be attributed to the predominance of less invasive endoscopic surgeries in this region, which are associated with faster recoveries and less impactful long-term sinonasal outcomes compared to traditional open surgeries (158). However, we found no clear evidence demonstrating that endonasal approaches are superior to open approaches with regard to quality of life.
It is important to highlight that changes in QoL are significantly influenced by the patient’s preoperative clinical status. Patients who were asymptomatic prior to surgery often experience a deterioration in QoL postoperatively (37). This observation brings to light the complexity of measuring QoL of patients who undergo surgery not because of current symptoms but to prevent future complications, a common scenario in skull base tumors. This preventative aspect of surgical intervention is often not captured in QoL assessments, emphasizing the need for developing more nuanced survey instruments that can capture the preventative necessity of skull base surgery.
However, our review of the current literature highlights the significant impact of non-modifiable factors such as age and sex on QoL outcomes, alongside modifiable factors like psychological support. Early psychological interventions, especially for patients undergoing treatment for complex tumors, appear to enhance QoL, suggesting the importance of integrated care models that address both physical and mental health after surgery (41).
Moreover, the severity of the tumor (malignant versus benign), the necessity of radiotherapy and recurrent surgeries are predictors of poorer QoL outcomes (31, 37, 96). This underscores the need for a tailored follow-up strategy that allocates more resources to high-risk patients to mitigate these effects.
Gross total resection, while often the primary goal in skull base surgery, should not always be considered, if followed by cranial nerve or other neurological deficits, diminishing the quality of life of patients. Surgical planning should include the patient’s individual perception which neurological deficits they could endure. This often depends on the patient’s occupation or leisure activities, making this decision highly individual.
The demographic characteristics of the skull base tumor population present additional challenges. Many patients are elderly with multiple comorbidities and depending on the tumor and treatment type, may have a shortened life expectancy. These factors complicate data collection and longitudinal study follow-ups, making large-scale, statistically significant conclusions difficult. Moreover, the histological variability of these tumors adds another layer of complexity in interpreting the impact on QoL.
It is crucial to recognize the multifaceted nature of QoL and the potential discrepancy between patient-reported outcomes and clinical assessments by healthcare providers (102). Regular collection of self-reported QoL data is vital, particularly given the improving survival rates for patients with skull base tumors. Such data not only provide insights into the patient’s recovery trajectory but also help in adjusting care plans to enhance overall well-being of the patients.
Limitations
Our study has several limitations. This literature review was conducted using PubMed, other databases were not explored. Consequently, some studies addressing quality of life following skull base tumor resection may have been omitted. However, additional targeted literature searches were performed to address underrepresented tumor types. To our knowledge, this is the first review encompassing many different tumor types and anatomical localizations.
The variability in scores, tumor types, localizations and treatment modalities across the studies presented prevented direct comparisons. Therefore, this review cannot provide definitive conclusions regarding quality of life. Nevertheless, it offers insights into potential influential factors.
Most studies included in this review focus on anterior skull base tumors and the endoscopic endonasal approach. Consequently, the most frequently used assessment tools were the SNOT-22 and the ASBQ, which predominantly evaluate sinonasal quality of life. This focus may introduce bias, as other aspects of quality of life and different surgical approaches are less frequently discussed.
Additionally, this review only considered publications related to surgical treatment of skull base tumors and did not explicitly evaluate the impact of radiotherapy, conservative treatments, or other treatment modalities.
Conclusion
The transient decrease in QoL following skull base tumor resection is a commonly observed outcome across various anatomical locations and tumor entities. The recovery timelines and outcomes are influenced by a wide variety of factors such as tumor entity, anatomical localization, surgical techniques, patient demographics, and psychosocial considerations. Recognizing and addressing the factors influencing QoL is important for improving patient outcomes and emphasizing individualized care.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material Further inquiries can be directed to the corresponding author/s.
Author contributions
VS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. TR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TK: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ASBQ, Anterior Skull Base Questionnaire; ALHR, Atkinson Life Happiness Rating; CES-D, Centre for Epidemiologic Studies Depression Scale; FACT-H&N, Functional Assessment of Cancer Therapy – Head and Neck; GTR, Gross Total Resection; KPS, Karnofsky Performance Scale; MDS, Midface Dysfunction Scale; STR, Subtotal Resection; SBI, Skull Base Inventory; QoL, Quality of Life; UPSIT, University of Pennsylvania Smell Identification Test; HUI, Health Utilities Index; SNOT, Sino Nasal Outcome Test; SF, Short-Form Health Survey; QoLI, Quality of Life Index; UoW QoL, University of Washington Quality of Life Questionnaire; HADS, Hospital Anxiety and Depression Scale; LMS, Lund-Mackay Score; ASK-12, Anterior Skull Base Nasal Inventory-12; EES-Q, Endoscopic Endonasal Sinus and Skull Base Surgery Questionnaire; ENS6Q, Empty Nose Syndrome 6 Item Questionnaires; CSS, Chronic Sinusitis Survey; GBI, Glasgow Benefit Inventory; RSOM-31, Rhinosinusitis Outcome Measure-31; BAST-24, Barcelona Smell Test 24; CSF, Cerebrospinal Fluid; EORTC QLQ C-30, EORTC of Cancer Quality of Life Core Questionnaire; SS-12, Sniffin’ Sticks 12-item smell identification test; RNLI, Reintegration to Normal Living Index; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
References
1. Burton BN, Hu JQ, Jafari A, Urman RD, Dunn IF, Bi WL, et al. An updated assessment of morbidity and mortality following skull base surgical approaches. Clin Neurol Neurosurg. (2018) 171:109–15. doi: 10.1016/j.clineuro.2018.06.015
2. Raskin S, Bornhorst M. Biological treatments of neurofibromatosis type 2 and other skull base disorders. Otolaryngol Clin North Am. (2021) 54:789–801. doi: 10.1016/j.otc.2021.05.004
3. Bi WL, Santagata S. Skull base tumors: neuropathology and clinical implications. Neurosurgery. (2022) 90:243–61. doi: 10.1093/neuros/nyab209
4. McDowell BD, Wallace RB, Carnahan RM, Chrischilles EA, Lynch CF, Schlechte JA. Demographic differences in incidence for pituitary adenoma. Pituitary. (2011) 14:23–30. doi: 10.1007/s11102-010-0253-4
5. Iannalfi A, Riva G, Ciccone L, Orlandi E. The role of particle radiotherapy in the treatment of skull base tumors. Front Oncol. (2023) 13:1161752. doi: 10.3389/fonc.2023.1161752
6. Irish JC, Gullane PJ, Gentili F, Freeman J, Boyd JB, Brown D, et al. Tumors of the skull base: Outcome and survival analysis of 77 cases. Head Neck. (1994) 16:3–10. doi: 10.1002/hed.2880160103
7. Janecka IP, Sen C, Sekhar LN, Ramasastry S, Curtin HD, Barnes EL, et al. Cranial base surgery. Results in 183 patients. J Neurooncol. (1994) 20:281–9. doi: 10.1007/BF01053044
8. O’Malley BW, Janecka IP. Evolution of outcomes in cranial base surgery. Semin Surg Oncol. (1995) 11:221–7. doi: 10.1002/ssu.2980110307
9. Witgert M, Veramonti T, Hanna E. Instruments for estimation of health-related quality of life in patients with skull base neoplasms. Skull Base. (2010) 20:005–10. doi: 10.1055/s-0029-1242978
10. De Almeida J, Vescan A, Witterick I, Gullane P, Gentili F, Ringash J, et al. Changes experienced in quality of life for skull base surgical patients: A qualitative case study. J Neurol Surg Part B Skull Base. (2014) 76:129–44. doi: 10.1055/s-0034-1371520
11. Wagner A, Shiban Y, Kammermeier V, Joerger A-K, Lange N, Ringel F, et al. Quality of life and emotional burden after transnasal and transcranial anterior skull base surgery. Acta Neurochir (Wien). (2019a) 161:2527–37. doi: 10.1007/s00701-019-04062-5
12. Chow VJ, Tsetsos N, Poutoglidis A, Georgalas C. Quality of life in sinonasal tumors: an up-to-date review. Curr Opin Otolaryngol Head Neck Surg. (2022) 30:46–57. doi: 10.1097/MOO.0000000000000774
13. Fisher FL, Zamanipoor Najafabadi AH, van der Meer PB, Boele FW, Peerdeman SM, Peul WC, et al. Long-term health-related quality of life and neurocognitive functioning after treatment in skull base meningioma patients. J Neurosurg. (2022) 136:1077–89. doi: 10.3171/2021.4.JNS203891
14. Timmermann C. [amp]]lsquo;Just give me the best quality of life questionnaire’: the Karnofsky scale and the history of quality of life measurements in cancer trials. Chronic Illn. (2013) 9:179–90. doi: 10.1177/1742395312466903
15. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. (1992) 30:473–83. doi: 10.1097/00005650-199206000-00002
16. Gil Z, Abergel A, Spektor S, Shabtai E, Khafif A, Fliss DM. Development of a cancer-specific anterior skull base quality-of-life questionnaire. J Neurosurg. (2004) 100:813–9. doi: 10.3171/jns.2004.100.5.0813
17. Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item sinonasal outcome test. Clin Otolaryngol. (2009) 34:447–54. doi: 10.1111/j.1749-4486.2009.01995.x
18. Little AS, Jahnke H, Nakaji P, Milligan J, Chapple K, White WL. The anterior skull base nasal inventory (ASK nasal inventory): a clinical tool for evaluating rhinological outcomes after endonasal surgery for pituitary and cranial base lesions. Pituitary. (2012) 15:513–7. doi: 10.1007/s11102-011-0358-4
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
20. Lang DA, Honeybul S, Neil-Dwyer G, Evans BT, Weller RO, Gill J. The extended transbasal approach: clinical applications and complications. Acta Neurochir (Wien). (1999) 141:579–85. doi: 10.1007/s007010050346
21. Gohil J, Stanley A, Jamaluddin M, Singh A, Shah S, George T, et al. Prospective study of sinonasal outcome following endoscopic skull base surgery. Neurol India. (2022) 70:1137. doi: 10.4103/0028-3886.349666
22. Salmon MK, Kshirsagar RS, Eide JG, Sweis AM, Davin K, Prasad A, et al. Postoperative mometasone irrigations improve quality of life in skull base tumor patients. World J Otorhinolaryngol - Head Neck Surg. (2023) 9:314–9. doi: 10.1002/wjo2.99
23. Palme CE, Irish JC, Gullane PJ, Katz MR, Devins GM, Bachar G. Quality of life analysis in patients with anterior skull base neoplasms. Head Neck. (2009) 31:1326–34. doi: 10.1002/hed.21102
24. Abergel A, Fliss D, Margalit N, Gil Z. A prospective evaluation of short-term health-related quality of life in patients undergoing anterior skull base surgery. Skull Base. (2010) 20:027–33. doi: 10.1055/s-0029-1242982
25. Cavel O, Abergel A, Margalit N, Fliss D, Gil Z. Quality of life following endoscopic resection of skull base tumors. J Neurol Surg Part B Skull Base. (2012) 73:112–6. doi: 10.1055/s-0032-1301392
26. Molteni G, Sacchetto A, Saccardo T, Gulino A, Marchioni D. Quality of life evaluation after trans-nasal endoscopic surgery for skull base tumors. Am J Rhinol Allergy. (2021) 35:507–15. doi: 10.1177/1945892420972045
27. Ahn J-C, Cho S-W, Kim D-K, Han DH, Kim D-Y, Rhee C-S, et al. Recovery period of sinonasal quality of life and its associated factors after endoscopic endonasal approach for anterior skull base tumors. Acta Otolaryngol (Stockh.). (2019) 139:461–6. doi: 10.1080/00016489.2019.1574982
28. Alshammari DM, Almomen A, Taha M, Albahrna H, Alshammari S. Quality of life and morbidity after endoscopic endonasal skull base surgeries using the sinonasal outcomes test (SNOT): A tertiary hospital experience. Int J Otolaryngol. (2021) 2021:1–6. doi: 10.1155/2021/6659221
29. Yan CH, Rathor A, Krook K, Ma Y, Rotella MR, Dodd RL, et al. Effect of omega-3 supplementation in patients with smell dysfunction following endoscopic sellar and parasellar tumor resection: A multicenter prospective randomized controlled trial. Neurosurgery. (2020) 87:E91–8. doi: 10.1093/neuros/nyz559
30. Hanson M, Patel PM, Betz C, Olson S, Panizza B, Wallwork B, et al. Sinonasal outcomes following endoscopic anterior skull base surgery with nasoseptal flap reconstruction: a prospective study. J Laryngol Otol. (2015) 29:S41–6. doi: 10.1017/S002221511500047X
31. Ransom ER, Doghramji L, Palmer JN, Chiu AG. Global and disease-specific health-related quality of life after complete endoscopic resection of anterior skull base neoplasms. Am J Rhinol Allergy. (2012) 26:76–9. doi: 10.2500/ajra.2012.26.3713
32. Wu V, Cusimano MD, Lee JM. Extent of surgery in endoscopic transsphenoidal skull base approaches and the effects on sinonasal morbidity. Am J Rhinol Allergy. (2018) 32:52–6. doi: 10.2500/ajra.2018.32.4499
33. Abergel A, Cavel O, Margalit N, Fliss DM, Gil Z. Comparison of quality of life after transnasal endoscopic vs open skull base tumor resection. Arch Otolaryngol Head Neck Surg. (2012) 138:142–7. doi: 10.1001/archoto.2011.1146
34. Huo CW, King J, Goldschlager T, Dixon B, Chen Zhao Y, Uren B, et al. The effects of cerebrospinal fluid (CSF) diversion on post-operative CSF leak following extended endoscopic anterior skull base surgery. J Clin Neurosci. (2022) 98:194–202. doi: 10.1016/j.jocn.2022.02.006
35. Riechelmann H, Meling D, Messer P, Richter H, Rettinger G, Antoniadis G. Subkranialer Zugang bei Malignomen mit Beteiligung der Frontobasis. Laryngo-Rhino-Otol. (2006) 85:426–34. doi: 10.1055/s-2006-925021
36. Woertgen C, Rothoerl RD, Hosemann W, Strutz J. Quality of life following surgery for Malignancies of the anterior skull base. Skull Base. (2007) 17:119–23. doi: 10.1055/s-2006-953513
37. Gil Z, Abergel A, Spektor S, Cohen JT, Khafif A, Shabtai E, et al. Quality of life following surgery for anterior skull base tumors. Arch Otolaryngol Neck Surg. (2003) 129:1303. doi: 10.1001/archotol.129.12.1303
38. Martinez-Devesa P, Barnes ML, Alcock CJ, Kerr RSC, Milford CA. Evaluation of quality of life and psychiatric morbidity in patients with Malignant tumours of the skull base. J Laryngol Otol. (2006) 120:1049–54. doi: 10.1017/S0022215106002477
39. Xu H, Li W, Zhang H, Wang H, Hu L, Sun X, et al. The impact of endoscopic endonasal surgery on quality of life in patients with Malignant tumors of the anterior skull base: A prospective study. Cancer Manage Res Volume. (2023) 15:523–35. doi: 10.2147/CMAR.S409091
40. Castelnuovo P, Lepera D, Turri-Zanoni M, Battaglia P, Bolzoni Villaret A, Bignami M, et al. Quality of life following endoscopic endonasal resection of anterior skull base cancers. J Neurosurg. (2013) 119:1401–9. doi: 10.3171/2013.8.JNS13296
41. Mukoyama N, Nishio N, Kimura H, Kishi S, Tokura T, Kimura H, et al. Prospective evaluation of health-related quality of life in patients undergoing anterolateral craniofacial resection with orbital exenteration. J Neurol Surg Part B Skull Base. (2020) 81:585–93. doi: 10.1055/s-0039-1694010
42. Liu JQ, Wang ZL, Zhang Y, Qi P, Yan W, Wei XT, et al. Impact of endoscopic endonasal approach on quality of life in patients with anterior skull base intra-extracranial extension meningioma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2022) 57:923–30. doi: 10.3760/cma.j.cn115330-20210924-00628
43. Castle-Kirszbaum M, Wang YY, King J, Goldschlager T. Quality of life after endoscopic surgical management of pituitary adenomas. Neurosurgery. (2022) 90:81–91. doi: 10.1227/NEU.0000000000001740
44. Schneider M, Schuss P, Güresir Á, Wach J, Hamed M, Vatter H, et al. Cranial nerve outcomes after surgery for frontal skull base meningiomas: the eternal quest of the maximum-safe resection with the lowest morbidity. World Neurosurg. (2019) 125:e790–6. doi: 10.1016/j.wneu.2019.01.171
45. Castle-Kirszbaum M, Kam J, Dixon B, Goldschlager T, King J, Wang YY. Surgical outcomes and longitudinal quality of life after endoscopic endonasal surgery for anterior skull base meningioma. J Neurosurg. (2022) 137:953–60. doi: 10.3171/2021.11.JNS212090
46. Wagner A, Shiban Y, Lange N, Joerger A-K, Hoffmann U, Meyer B, et al. The relevant psychological burden of having a benign brain tumor: a prospective study of patients undergoing surgical treatment of cranial meningiomas. J Neurosurg. (2019b) 131:1840–7. doi: 10.3171/2018.8.JNS181343
47. Conrad J, Blaese M, Becker S, Huppertz T, Ayyad A, Ringel F. Sinonasal outcome after endoscopic transnasal surgery—A prospective rhinological study. Oper Neurosurg. (2023) 24:223–31. doi: 10.1227/ons.0000000000000532
48. Schreiber A, Bertazzoni G, Ferrari M, Rampinelli V, Verri P, Mattavelli D, et al. Nasal morbidity and quality of life after endoscopic transsphenoidal surgery: A single-center prospective study. World Neurosurg. (2019) 123:e557–65. doi: 10.1016/j.wneu.2018.11.212
49. Riley CA, Tabaee A, Conley L, Amine M, Soneru CP, Anand VK, et al. Long-term sinonasal outcomes after endoscopic skull base surgery with nasoseptal flap reconstruction. Laryngoscope. (2019) 129:1035–40. doi: 10.1002/lary.27637
50. Seo MY, Nam D, Kong D, Lee JJ, Ryu G, Kim HY, et al. Quality of life after extended versus transsellar endoscopic skull base surgery from 767 patients. Laryngoscope. (2019) 129:1318–24. doi: 10.1002/lary.27630
51. Choi KJ, Ackall FY, Truong T, Cheng TZ, Kuchibhatla M, Zomorodi AR, et al. Sinonasal quality of life outcomes after extended endonasal approaches to the skull base. J Neurol Surg Part B Skull Base. (2019) 80:416–23. doi: 10.1055/s-0038-1675592
52. Castle-Kirszbaum M, Wang YY, King J, Uren B, Dixon B, Zhao YC, et al. Patient wellbeing and quality of life after nasoseptal flap closure for endoscopic skull base reconstruction. J Clin Neurosci. (2020) 74:87–92. doi: 10.1016/j.jocn.2020.01.072
53. Cong Z, Zhu J, Sun H, Tang C, Yang J, Ma C. Endoscopic 1½-transseptal approach for pituitary surgery. Front Oncol. (2023) 12:1116408. doi: 10.3389/fonc.2022.1116408
54. Joustra GE, Ten Dam E, Vermeulen KM, Korsten-Meijer AGW, Appelman APA, Feijen RA. Prospective evaluation of multidimensional health-related quality of life after endoscopic endonasal surgery for pituitary adenomas using the endoscopic endonasal sinus and skull base surgery questionnaire. Laryngoscope Investig Otolaryngol. (2023) 8:7–15. doi: 10.1002/lio2.1004
55. Zhong Y, Deng Z, Chen H, Qiu Q. Evaluation of sinonasal-related quality of life of 49 patients undergoing endoscopic skull base surgery. Braz. J. Otorhinolaryngol.. (2024) 90:101337. doi: 10.1016/j.bjorl.2023.101337
56. Weiland T, Gellner V, Pondorfer P, Hortobagyi D, Maitz E, Kiss P, et al. Endoscopic trans-sphenoidal pituitary surgery does not impact postoperative nasal quality of life. Eur Arch Otorhinolaryngol. (2024) 281:245–56. doi: 10.1007/s00405-023-08203-6
57. Sunil A, Thakar S, Aryan S, Hegde A. Changes in sinonasal and overall quality of life following endoscopic endonasal surgery for non-functioning pituitary adenomas: results of A prospective observational study. Neurol India. (2022) 70:2357. doi: 10.4103/0028-3886.364068
58. Zhu J, Wen G, Tang C, Cong Z, Cai X, Yang J, et al. One-and-a-half nostril versus binostril endoscopic transsphenoidal approach to the pituitary adenomas: A prospective randomized controlled trial. Front Surg. (2022) 9:1007883. doi: 10.3389/fsurg.2022.1007883
59. Zhang X, Li Y, Zhang D, Huang F, Zhong Y, Xu X. Olfaction disorders in patients with pituitary adenoma after endoscopic transsphenoidal surgery: a qualitative study. Ann Palliat Med. (2022) 11:2235–46. doi: 10.21037/apm-21-2920
60. Zhong J, Gu Y, Zheng J, Yang B, Qi Z, Li T, et al. A modified microscopic-endoscopic bilateral transseptal approach for pituitary adenomas: comparisons of nasal outcome and quality of life using the microscopic transnasal approach. Front Oncol. (2022) 12:778704. doi: 10.3389/fonc.2022.778704
61. Ferreli F, Lasagna C, Canali L, Baram A, Bono BC, Tropeano MP, et al. A randomized prospective comparative study on sinonasal morbidity and quality of life of transsphenoidal endoscopic surgery for pituitary adenomas: endonasal versus trans-septal approach. Eur Arch Otorhinolaryngol. (2024) 281:257–66. doi: 10.1007/s00405-023-08216-1
62. Hallén T, Olsson DS, Farahmand D, Esposito D, Olofsson A-C, Jakobsson S, et al. Sinonasal Symptoms and Self-Reported Health before and after Endoscopic Pituitary Surgery—A Prospective Study. J Neurol Surg Part B Skull Base. (2022) 83:e160–8. doi: 10.1055/s-0041-1722929
63. Castle-Kirszbaum M, Wang YY, King J, Goldschlager T. Frailty does not preclude surgical success after endoscopic transsphenoidal surgery for pituitary adenomas. Pituitary. (2021) 24:922–9. doi: 10.1007/s11102-021-01166-z
64. Dolci RLL, de Moraes LT, de Carvalho ACM, Rickli JCK, de Souza JL, Encinas WE, et al. Quality-of-life evaluation for patients submitted to nasal endoscopic surgery for resection of pituitary tumours. Eur Arch Otorhinolaryngol. (2021) 278:1411–8. doi: 10.1007/s00405-020-06381-1
65. McCoul ED, Bedrosian JC, Akselrod O, Anand VK, Schwartz TH. Preservation of multidimensional quality of life after endoscopic pituitary adenoma resection. J Neurosurg. (2015) 123:813–20. doi: 10.3171/2014.11.JNS14559
66. Shay A, Sturgis M, Ritz EM, Beer-Furlan A, Muñoz L, Byrne R, et al. Prior smoking and nasoseptal flap usage adversely impact quality of life and healing after endoscopic pituitary surgery. Neurosurg Focus. (2020) 48:E17. doi: 10.3171/2020.3.FOCUS2050
67. Green FR, Sanders MI, Davies P, Mirza S, Sinha S. Quality of life outcomes after transnasal endoscopic pituitary surgery using the Glasgow Benefit Inventory. Br J Neurosurg. (2022) 36:720–7. doi: 10.1080/02688697.2022.2106352
68. Little AS, Kshettry VR, Rosen MR, Rehl RM, Haegen TW, Rabinowitz MR, et al. Postoperative postoperative oral antibiotics and sinonasal outcomes following endoscopic transsphenoidal surgery for pituitary tumors study: a multicenter, prospective, randomized, double-blinded, placebo-controlled study. Neurosurg. (2021) 89:769–76. doi: 10.1093/neuros/nyab301
69. Van Der Meulen M, Verstegen MJT, Lobatto DJ, Kleijwegt MC, Pereira AM, Biermasz NR, et al. Impact of patient-reported nasal symptoms on quality of life after endoscopic pituitary surgery: a prospective cohort study. Pituitary. (2022) 25:308–20. doi: 10.1007/s11102-021-01199-4
70. Bedrosian JC, McCoul ED, Raithatha R, Akselrod OA, Anand VK, Schwartz TH. A prospective study of postoperative symptoms in sinonasal quality-of-life following endoscopic skull-base surgery: dissociations based on specific symptoms. Int Forum Allergy Rhinol. (2013) 3:664–9. doi: 10.1002/alr.21161
71. Alobid I, Enseñat J, Mariño-Sánchez F, Rioja E, De Notaris M, Mullol J, et al. Expanded Endonasal Approach using Vascularized Septal Flap Reconstruction for Skull Base Tumors has a Negative Impact on Sinonasal Symptoms and Quality of Life. Am J Rhinol Allergy. (2013) 27:426–31. doi: 10.2500/ajra.2013.27.3932
72. Zimmer LA, Shah O, Theodosopoulos PV. Short-term quality-of-life changes after endoscopic pituitary surgery rated with SNOT-22. J Neurol Surg Part B Skull Base. (2014) 75:288–92. doi: 10.1055/s-0034-1372464
73. Carmel Neiderman NN, Wengier A, Dominsky O, Ringel B, Warshavsky A, Horowitz G, et al. A prospective evaluation of quality of life in patients undergoing extended endoscopic endonasal surgery for benign pituitary gland lesion. J Neurol Surg Part B Skull Base. (2022) 83:e386–94. doi: 10.1055/s-0041-1730322
74. Wu TJ, Chen A, Wells C, Heaney AP, Bergsneider M, Wang MB. Sinonasal quality of life outcomes after endoscopic endonasal transsphenoidal surgery with posterior septum free mucosal graft reconstruction. J Neurol Surg Part B Skull Base. (2021) 82:528–33. doi: 10.1055/s-0040-1716678
75. Cho J, Grayson JW, Christensen J, Winder MJ, Sheehy J, Steel T, et al. Long-term sinonasal function following transnasal pituitary surgery: A comparison of surgical approach. Am J Rhinol Allergy. (2020) 34:361–8. doi: 10.1177/1945892419896788
76. Hong SD, Nam D-H, Seol HJ, Choi NY, Kim HY, Chung S-K, et al. Endoscopic binostril versus transnasal transseptal microscopic pituitary surgery: sinonasal quality of life and olfactory function. Am J Rhinol Allergy. (2015) 29:221–5. doi: 10.2500/ajra.2015.29.4165
77. Little AS, Kelly D, Milligan J, Griffiths C, Prevedello DM, Carrau RL, et al. Predictors of sinonasal quality of life and nasal morbidity after fully endoscopic transsphenoidal surgery. J Neurosurg. (2019a) 122:1458–65. doi: 10.3171/2014.10.JNS141624
78. Little AS, Kelly DF, Milligan J, Griffiths C, Prevedello DM, Carrau RL, et al. Comparison of sinonasal quality of life and health status in patients undergoing microscopic and endoscopic transsphenoidal surgery for pituitary lesions: a prospective cohort study. J Neurosurg. (2019b) 123:799–807. doi: 10.3171/2014.10.JNS14921
79. Hong SD, Nam D-H, Kong D-S, Kim HY, Chung S-K, Dhong H-J. Endoscopic modified transseptal transsphenoidal approach for maximal preservation of sinonasal quality of life and olfaction. World Neurosurg. (2016) 87:162–9. doi: 10.1016/j.wneu.2015.12.050
80. Jalessi M, Jahanbakhshi A, Amini E, Kamrava SK, Farhadi M. Impact of nasoseptal flap elevation on sinonasal quality of life in endoscopic endonasal approach to pituitary adenomas. Eur Arch Otorhinolaryngol. (2016) 273:1199–205. doi: 10.1007/s00405-015-3729-z
81. Rioja E, Bernal-Sprekelsen M, Enriquez K, Enseñat J, Valero R, De Notaris M, et al. Long-term outcomes of endoscopic endonasal approach for skull base surgery: a prospective study. Eur Arch Otorhinolaryngol. (2016) 273:1809–17. doi: 10.1007/s00405-015-3853-9
82. Kuan E, Yoo F, Chyu J, Oh A, Bergsneider M, Wang M. Quality of Life before and after Endoscopic Pituitary Surgery as Measured by the Short-Form-36. J Neurol Surg Part B Skull Base. (2018) 79:314–8. doi: 10.1055/s-0037-1608648
83. Hura N, Orlov CP, Khalafallah AM, Mukherjee D, Rowan NR. Impact of routine endoscopic skull base surgery on subjective olfaction and gustation outcomes. Oper Neurosurg. (2021) 21:137–42. doi: 10.1093/ons/opab137
84. McCoul ED, Anand VK, Bedrosian JC, Schwartz TH. Endoscopic skull base surgery and its impact on sinonasal-related quality of life. Int Forum Allergy Rhinol. (2012b) 2:174–81. doi: 10.1002/alr.21008
85. Chou CT, Valappil B, Mattos JL, Snyderman CH, Gardner PA, Fernandez-Miranda JC, et al. The effect of nasoseptal flap elevation on post-operative olfaction and sinonasal quality of life: A prospective double-blinded randomized controlled trial. Am J Rhinol Allergy. (2021) 35:353–60. doi: 10.1177/1945892420957505
86. McCoul ED, Anand VK, Schwartz TH. Improvements in site-specific quality of life 6 months after endoscopic anterior skull base surgery: a prospective study: Clinical article. J Neurosurg. (2012a) 117:498–506. doi: 10.3171/2012.6.JNS111066
87. Hegde R, Prodan V, Futera K, Hathorn I, Gohil R, Hughes MA. Exploring the influence of nasal morbidity on quality of life following endoscopic endonasal skull base surgery: a retrospective cohort study of 95 patients. Neurosurg Rev. (2023) 47:13. doi: 10.1007/s10143-023-02240-9
88. Buchlak QD, Esmaili N, Bennett C, Wang YY, King J, Goldschlager T. Predictors of improvement in quality of life at 12-month follow-up in patients undergoing anterior endoscopic skull base surgery. PloS One. (2022) 17:e0272147. doi: 10.1371/journal.pone.0272147
89. Raikundalia MD, Huang RJ, Chan L, Truong T, Kuchibhatla M, Merchant J, et al. Olfactory-specific quality of life outcomes after endoscopic endonasal surgery of the sella. Allergy Rhinol. (2021) 12:215265672110450. doi: 10.1177/21526567211045041
90. Sowerby LJ, Gross M, Broad R, Wright ED. Olfactory and sinonasal outcomes in endoscopic transsphenoidal skull-base surgery. Int Forum Allergy Rhinol. (2013) 3:217–20. doi: 10.1002/alr.21103
91. Georgalas C, Badloe R, Van Furth W, Reinartz S, Fokkens WJ. Quality of life in extended endonasal approaches for skull base tumours. Rhinol J. (2012) 50:255–61. doi: 10.4193/Rhino12.050
92. Castle-Kirszbaum M, Wang YY, King J, Kam J, Goldschlager T. Quality of life and surgical outcomes in incidental pituitary adenomas undergoing endoscopic endonasal resection. J Neurosurg. (2023) 138:567–73. doi: 10.3171/2022.5.JNS2286
93. Diaz RJ, Maggacis N, Zhang S, Cusimano MD. Determinants of quality of life in patients with skull base chordoma: Clinical article. J Neurosurg. (2014) 120:528–37. doi: 10.3171/2013.9.JNS13671
94. Chaaban MR, Chaudhry AL, Riley KO, Woodworth BA. Objective assessment of olfaction after transsphenoidal pituitary surgery. Am J Rhinol Allergy. (2015) 29:365–8. doi: 10.2500/ajra.2015.29.4206
95. Shi X, Wang L, Wu B, Zhang Y, Zhou Z. Long-term outcomes after a transcranial microsurgical approach to craniopharyngiomas: a 20-year clinical follow-up study. Neurosurg Rev. (2023) 46:34. doi: 10.1007/s10143-022-01942-w
96. Patel KS, Raza SM, McCoul ED, Patrona A, Greenfield JP, Souweidane MM, et al. Long-term quality of life after endonasal endoscopic resection of adult craniopharyngiomas. J Neurosurg. (2015) 123:571–80. doi: 10.3171/2014.12.JNS141591
97. Marx S, Tsavdaridou I, Paul S, Steveling A, Schirmer C, Eördögh M, et al. Quality of life and olfactory function after suprasellar craniopharyngioma surgery—a single-center experience comparing transcranial and endoscopic endonasal approaches. Neurosurg Rev. (2021) 44:1569–82. doi: 10.1007/s10143-020-01343-x
98. Jones SH, Iannone AF, Patel KS, Anchouche K, Raza SM, Anand VK, et al. The impact of age on long-term quality of life after endonasal endoscopic resection of skull base meningiomas. Neurosurgery. (2016) 79:736–45. doi: 10.1227/NEU.0000000000001360
99. Zweckberger K, Hallek E, Vogt L, Giese H, Schick U, Unterberg AW. Prospective analysis of neuropsychological deficits following resection of benign skull base meningiomas. J Neurosurg. (2017) 127:1242–8. doi: 10.3171/2016.10.JNS161936
100. Karsy M, Jensen MR, Guan J, Ravindra VM, Bisson EF, Couldwell WT. EQ-5D quality-of-life analysis and cost-effectiveness after skull base meningioma resection. Neurosurgery. (2019) 85:E543–52. doi: 10.1093/neuros/nyz040
101. Tariciotti L, Fiore G, Carapella S, Remore LG, Schisano L, Borsa S, et al. A frailty-adjusted stratification score to predict surgical risk, post-operative, long-term functional outcome, and quality of life after surgery in intracranial meningiomas. Cancers. (2022) 14:3065. doi: 10.3390/cancers14133065
102. Lang DA, Neil-Dwyer G, Garfield J. Outcome after complex neurosurgery: the caregiver’s burden is forgotten. J Neurosurg. (1999) 91:359–63. doi: 10.3171/jns.1999.91.3.0359
103. Neil-Dwyer G, Lang DA, Davis A. Outcome from complex neurosurgery: an evidence based approach. Acta Neurochir (Wien). (2000) 142:367–71. doi: 10.1007/s007010050444
104. Natarajan SK, Sekhar LN, Schessel D, Morita A. Petroclival meningiomas: multimodality treatment and outcomes at long-term follow-up. Neurosurgery. (2007) 60:965–81. doi: 10.1227/01.NEU.0000255472.52882.D6
105. Batish A, Gupta S, Mohanty M, Tripathi M, Salunke P, Aggarwal A. Surgical outcome analysis of large and giant petroclival meningiomas with special reference to quality of life issues. Neurol India. (2022) 70:897. doi: 10.4103/0028-3886.349614
106. Almefty R, Dunn IF, Pravdenkova S, Abolfotoh M, Al-Mefty O. True petroclival meningiomas: results of surgical management. J Neurosurg. (2014) 120(1):10–51. doi: 10.3171/2013.8.JNS13535
107. Zhao Z, Yuan X, Zou H, Jiang W, Liao Y, Luo D, et al. Microsurgical removal and prognostic analysis of petroclival meningiomas. Zhonghua Wai Ke Za Zhi. (2014) 52:508–13.
108. Gillard DM, Jiam NT, Morshed RA, Bhutada AS, Crawford ED, Braunstein SW, et al. Differences in hearing, balance, and quality-of-life outcomes in petroclival versus nonpetroclival posterior fossa meningiomas. Otol Neurotol. (2023) 44:e333–7. doi: 10.1097/MAO.0000000000003864
109. Giammattei L, Di Russo P, Starnoni D, Passeri T, Bruneau M, Meling TR, et al. Petroclival meningiomas: update of current treatment and consensus by the EANS skull base section. Acta Neurochir (Wien). (2021) 163:1639–63. doi: 10.1007/s00701-021-04798-z
110. Bonnal J, Thibaut A, Brotchi J, Born J. Invading meningiomas of the sphenoid ridge. J Neurosurg. (1980) 53:587–99. doi: 10.3171/jns.1980.53.5.0587
111. Al-Mefty O. Clinoidal meningiomas. J Neurosurg. (1990) 73:840–9. doi: 10.3171/jns.1990.73.6.0840
112. Mathiesen T, Lindquist C, Kihlström L, Karlsson B. Recurrence of cranial base meningiomas. Neurosurgery. (1996) 39:2–9. doi: 10.1097/00006123-199607000-00002
113. Liu D, Yuan X, Liu Q, Jiang X, Jiang W, Peng Z, et al. Large medial sphenoid wing meningiomas: long-term outcome and correlation with tumor size after microsurgical treatment in 127 consecutive cases. Turk Neurosurg. (2012) 22(6):547–57. doi: 10.5137/1019-5149.JTN.5142-11.1
114. Da Silva CE, Zanatta C, Thibes AC, Vidaletti T. Sphenoid wing meningiomas with secondary cavernous sinus invasion: surgical results and algorithm for treatment at a single Brazilian center. World Neurosurg. (2022) 163:e635–46. doi: 10.1016/j.wneu.2022.04.050
115. Aman RA, Wisyesa K, Nugroho AW, Ichwan S, Tandian D, Ashari S, et al. Pre-and post-surgical health-related quality of life evaluation of spheno-orbital meningioma patients based on EORTC QLQ-C30 questionnaire at dr. Cipto mangunkusumo general hospital. Acta Neurol Taiwanica. (2020) 29:99–102.
116. Nanda A, Thakur JD, Sonig A, Missios S. Microsurgical resectability, outcomes, and tumor control in meningiomas occupying the cavernous sinus. J Neurosurg. (2016) 125:378–92. doi: 10.3171/2015.3.JNS142494
117. Meling TR, Da Broi M, Scheie D, Helseth E. Meningiomas: skull base versus non-skull base. Neurosurg Rev. (2019) 42:163–73. doi: 10.1007/s10143-018-0976-7
118. Hentschel SJ, DeMonte F. Olfactory groove meningiomas. Neurosurg Focus. (2003) 14:1–5. doi: 10.3171/foc.2003.14.6.4
119. Orgain CA, Kuan EC, Alvarado R, Adappa ND, Jonker BP, Lee JYK, et al. Smell preservation following unilateral endoscopic transnasal approach to resection of olfactory groove meningioma: A multi-institutional experience. J Neurol Surg Part B Skull Base. (2020) 81:263–7. doi: 10.1055/s-0039-1688794
120. Bander ED, Pandey A, Yan J, Giantini-Larsen AM, Schwartz A, Estin J, et al. Olfactory groove meningiomas: supraorbital keyhole versus orbitofrontal, frontotemporal, or bifrontal approaches. J Neurosurg. (2023) 140(6):1–8. doi: 10.3171/2023.10.JNS231432
121. Lévêque S, Derrey S, Martinaud O, Gérardin E, Langlois O, Fréger P, et al. Superior interhemispheric approach for midline meningioma from the anterior cranial base. Neurochirurgie. (2011) 57:105–13. doi: 10.1016/j.neuchi.2011.08.001
122. Li Y, Zhang C, Su J, Qin C, Wang X, Li Y, et al. Individualized surgical treatment of giant tuberculum sellae meningioma: Unilateral subfrontal approach vs. endoscopic transsphenoidal approach. Front Surg. (2022) 9:990646. doi: 10.3389/fsurg.2022.990646
123. Torales J, Di Somma A, Alobid I, Lopez M, Hoyos J, Ferres A, et al. Endonasal versus supraorbital approach for anterior skull base meningiomas: Results and quality of life assessment from a single-surgeon cohort. Neurocir Engl Ed. (2024) 35:S2529849623000485. doi: 10.1016/j.neucie.2023.12.001
124. Scheich M, Ginzkey C, Reuter E, Harnisch W, Ehrmann D, Hagen R. Quality of life after microsurgery for vestibular schwannoma via the middle cranial fossa approach. Eur Arch Otorhinolaryngol. (2014) 271:1909–16. doi: 10.1007/s00405-013-2671-1
125. Leong SC, Lesser TH. A national survey of facial paralysis on the quality of life of patients with acoustic neuroma. Otol Neurotol. (2015) 36:503–9. doi: 10.1097/MAO.0000000000000428
126. Moon I, Cha D, Nam S-I, Lee H-J, Choi J. The feasibility of a modified exclusive endoscopic transcanal transpromontorial approach for vestibular schwannomas. J Neurol Surg Part B Skull Base. (2019) 80:082–7. doi: 10.1055/s-0038-1667061
127. Irving RM, Beynon GJ, Viani L, Hardy DG, Baguley DM, Moffat DA. The patient’s perspective after vestibular schwannoma removal: quality of life and implications for management. Am J Otol. (1995) 16:331–7.
128. Nikolopoulos TP, Johnson I, O’Donoghue GM. Quality of life after acoustic neuroma surgery. Laryngoscope. (1998) 108:1382–5. doi: 10.1097/00005537-199809000-00024
129. Da Cruz MJ, Moffat DA, Hardy DG. Postoperative quality of life in vestibular schwannoma patients measured by the SF36 health questionnaire. Laryngoscope. (2000) 110:151–5. doi: 10.1097/00005537-200001000-00027
130. Ryzenman JM, Pensak ML, Tew JM. Headache: A quality of life analysis in a cohort of 1,657 patients undergoing acoustic neuroma surgery, results from the acoustic neuroma association. Laryngoscope. (2005) 115:703–11. doi: 10.1097/01.mlg.0000161331.83224.c5
131. Baumann I, Polligkeit J, Blumenstock G, Mauz P-S, Zalaman IM, Maassen MM. Quality of life after unilateral acoustic neuroma surgery via middle cranial fossa approach. Acta Otolaryngol (Stockh.). (2005) 125:585–91. doi: 10.1080/00016480510026935
132. Sandooram D, Hornigold R, Grunfeld B, Thomas N, Kitchen N, Gleeson M. The effect of observation versus microsurgical excision on quality of life in unilateral vestibular schwannoma: A prospective study. Skull Base. (2010) 20:047–54. doi: 10.1055/s-0029-1242985
133. Cheng S, Naidoo Y, Da Cruz M, Dexter M. Quality of life in postoperative vestibular schwannoma patients. Laryngoscope. (2009) 119:2252–7. doi: 10.1002/lary.20217
134. La Monte OA, Tawfik KO, Khan U, Schwartz M, Friedman R. Analysis of hearing preservation in middle cranial fossa resection of vestibular schwannoma. Otol Neurotol. (2022) 43:395–9. doi: 10.1097/MAO.0000000000003445
135. Lodder WL, van der Laan BFAM, Lesser TH, Leong SC. The impact of acoustic neuroma on long-term quality-of-life outcomes in the United Kingdom. Eur Arch Otorhinolaryngol. (2018) 275(3):709–17. doi: 10.1007/s00405-018-4864-0
136. Kelleher MO, Fernandes MF, Sim DW, O’Sullivan MG. Health-related quality of life in patients with skull base tumours. Br J Neurosurg. (2002) 16:16–20. doi: 10.1080/02688690120114183
137. Al-Mefty O, Borba LAB. Skull base chordomas: a management challenge. J Neurosurg. (1997) 86:182–9. doi: 10.3171/jns.1997.86.2.0182
138. George B, Bresson D, Herman P, Froelich S. Chordomas. Neurosurg Clin N Am. (2015) 26:437–52. doi: 10.1016/j.nec.2015.03.012
139. Shu Z, Hou Y, Wang Y, Tang X. Comparison of prognosis of skull base chordoma treated by surgical resection and gamma knife surgery. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2011) 36:359–62. doi: 10.3969/j.issn.1672-7347.2011.04.015
140. Cavallo LM, Mazzatenta D, d’Avella E, Catapano D, Fontanella MM, Locatelli D, et al. The management of clival chordomas: an Italian multicentric study. J Neurosurg. (2020) 135:93–102. doi: 10.3171/2020.5.JNS20925
141. Komotar RJ, Starke RM, Raper DMS, Anand VK, Schwartz TH. The endoscope-assisted ventral approach compared with open microscope-assisted surgery for clival chordomas. World Neurosurg. (2011) 76:318–27. doi: 10.1016/j.wneu.2011.02.026
142. Fernandez-Miranda JC, Gardner PA, Snyderman CH, Devaney KO, Mendenhall WM, Suárez C, et al. Clival chordomas: A pathological, surgical, and radiotherapeutic review: Clival chordomas. Head Neck. (2014) 36:892–906. doi: 10.1002/hed.23415
143. Kirkman M, Borg A, Al-Mousa A, Haliasos N, Choi D. Quality-of-life after anterior skull base surgery: A systematic review. J Neurol Surg Part B Skull Base. (2013) 75:073–89. doi: 10.1055/s-0033-1359303
144. Gui CH, Tham AC. Quality of life after endoscopic skull base surgery with a nasoseptal flap: a systematic review. J Laryngol Otol. (2022) 136:1164–9. doi: 10.1017/S0022215121004667
145. Buchanan FF, Myles PS, Cicuttini F. Effect of patient sex on general anaesthesia and recovery. Br J Anaesth. (2011) 106:832–9. doi: 10.1093/bja/aer094
146. Peleg-Oren N, Sherer M, Soskolne V. Effect of gender on the social and psychological adjustment of cancer patients. Soc Work Health Care. (2003) 37:17–34. doi: 10.1300/J010v37n03_02
147. Fehrenbach MK, Brock H, Mehnert-Theuerkauf A, Meixensberger J. Psychological distress in intracranial neoplasia: A comparison of patients with benign and Malignant brain tumours. Front Psychol. (2021) 12:664235. doi: 10.3389/fpsyg.2021.664235
148. Clarke NE, McCarthy MC, Downie P, Ashley DM, Anderson VA. Gender differences in the psychosocial experience of parents of children with cancer: a review of the literature. Psychooncology. (2009) 18:907–15. doi: 10.1002/pon.1515
149. Biermasz NR, Van Thiel SW, Pereira AM, Hoftijzer HC, Van Hemert AM, Smit JWA, et al. Decreased quality of life in patients with acromegaly despite long-term cure of growth hormone excess. J Clin Endocrinol Metab. (2004) 89:5369–76. doi: 10.1210/jc.2004-0669
150. Kauppinen-Mäkelin R, Sane T, Sintonen H, Markkanen H, Välimäki MJ, Löyttyniemi E, et al. Quality of life in treated patients with acromegaly. J Clin Endocrinol Metab. (2006) 91:3891–6. doi: 10.1210/jc.2006-0676
151. Sievers C, Dimopoulou C, Pfister H, Lieb R, Steffin B, Roemmler J, et al. Prevalence of mental disorders in acromegaly: a cross-sectional study in 81 acromegalic patients. Clin Endocrinol (Oxf.). (2009) 71:691–701. doi: 10.1111/j.1365-2265.2009.03555.x
152. Imran SA, Tiemensma J, Kaiser SM, Vallis M, Doucette S, Abidi E, et al. Morphometric changes correlate with poor psychological outcomes in patients with acromegaly. Eur J Endocrinol. (2016) 174:41–50. doi: 10.1530/EJE-15-0888
153. Andela CD, Scharloo M, Pereira AM, Kaptein AA, Biermasz NR. Quality of life (QoL) impairments in patients with a pituitary adenoma: a systematic review of QoL studies. Pituitary. (2015) 18:752–76. doi: 10.1007/s11102-015-0636-7
154. Castle-Kirszbaum M, Biermasz N, Kam J, Goldschlager T. Quality of life in Prolactinoma: A systematic review. Pituitary. (2024) 27:239–47. doi: 10.1007/s11102-024-01392-1
155. De Vile CJ, Grant DB, Hayward RD, Kendall BE, Neville BG, Stanhope R. Obesity in childhood craniopharyngioma: relation to post-operative hypothalamic damage shown by magnetic resonance imaging. J Clin Endocrinol Metab. (1996) 81:2734–7. doi: 10.1210/jcem.81.7.8675604
156. Unteroberdörster M, Müller O, Özkan N, Pierscianek D, Hadamitzky M, Kleist B, et al. Impact of optic canal decompression on visual outcome in subtotal resected skull base meningiomas. J Neurosurg Sci. (2020) 64:440–5. doi: 10.23736/S0390-5616.17.04020-6
157. Ved R, Mo M, Hayhurst C. Olfactory Outcomes after Resection of Tuberculum Sella and Planum Sphenoidale Meningiomas via a Transcranial Approach. J Neurol Surg Part B Skull Base. (2022) 83:296–304. doi: 10.1055/s-0040-1722671
Keywords: quality of life, skull base surgery, neurooncology, systematic review, patient-reported outcome measures
Citation: Sperl V, Rhomberg T and Kretschmer T (2024) Determinants of quality of life following resection of skull base tumors: a systematic review. Front. Oncol. 14:1473261. doi: 10.3389/fonc.2024.1473261
Received: 30 July 2024; Accepted: 08 October 2024;
Published: 20 December 2024.
Edited by:
Maria Teresa Pedro, Universitaetsklinikum Ulm, GermanyCopyright © 2024 Sperl, Rhomberg and Kretschmer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Rhomberg, dGhvbWFzLnJob21iZXJnLjFAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Veronika Sperl
Veronika Sperl Thomas Rhomberg*†
Thomas Rhomberg*† Thomas Kretschmer
Thomas Kretschmer