- 1Department of Oral and Maxillofacial Pathology, School of Dentistry, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Khuzestan, Iran
- 2Social Determinants of Health Research Center, Department of Public Health, School of Health, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Khuzestan, Iran
Background: Oral squamous cell carcinoma (OSCC) is the most frequent oral cancer worldwide. Despite advances in OSCC treatment, the mortality rate has not decreased in recent years. Therefore, the aim of this investigation was to assess the survival rate as a factor reflecting the quality aspects of care and background parameters that influence survival in patients with OSCC.
Methods: This is a retrospective analysis of 165 patients with OSCC who were registered in the Khuzestan cancer registry system in 2014 to 2018. The data were collected in two parts: demographic information and survival information. Demographic and background variables include age, gender, marital status, ethnicity, employment status, insurance status, and educational status. Survival information was also collected through phone calls to patients or their families. The survival rate of the patients was analyzed using the log-rank test and the influencing factors were analyzed with the Cox regression test.
Results: In this study, 165 patients, 43 women (26.1%) and 122 men (73.9%), with OSCC were included. The follow-up period of the patients was 5 years (2014–2019), during which 74 patients died. One, three, and five-year survival rates were 93.34%, 71.51%, and 44.84%, respectively. The results showed that age (χ2 = 4.410, p < 0.05) and employee status (χ2 = 10.205, p < 0.05) were associated with survival rate in OSCC patients based on the log-rank test results, while Cox regression analysis, after including all variables in the model and adjusting them, showed that all variables were not associated with survival rate (p > 0.05).
Conclusion: Since all background factors were not associated with survival rate, efforts should continue to identify effective factors and investigate the relationship between survival and pathological factors such as recurrence status, distant metastasis, type of treatment, and involved organs.
Introduction
Oral cancer comprises a group of neoplasms that involve any area of the oral cavity, oropharynx, and salivary glands (1). However, this term is often used instead of oral squamous cell carcinoma (OSCC), which represents the most common neoplasms of the mouth. It is estimated that over 90% of all oral neoplasms are of the OSCC type (2). In the United States, more than 30,000 patients are diagnosed annually with various types of oral cancers, with over 8,000 individuals dying due to cancer (3).
The data from the Global Cancer Observatory indicate that the annual incidence of OSCC in 2020 was 377,713 cases worldwide, with the highest number in Asia (248,360), followed by Europe (65,279) and North America (27,469). The 5-year prevalence of OSCC is estimated to be close to 1 million (959,248) and follows the same pattern of incidence (4). The available data on the trend of OSCC in Iran is extremely limited and inadequate. Furthermore, there has been a lack of updates on the epidemiological data of OSCC in recent years.
However, according to the scientific report published by the Islamic Republic of Iran Ministry of Health in 2003, oral cancer ranks among the top 10 prevalent cancers in three provinces for women and in five provinces for men, out of all the cancers that have affected both genders (5).
Multiple clinical, pathological, and molecular markers play a role in predicting the prognosis of oral cancer. Various studies have been conducted to determine prognostic factors in patients with OSCC and showed that factors such as location, stage, time from diagnosis to treatment, local recurrence, tumor differentiation, degree of keratinization, and pattern of lymph-vascular invasion have a significant correlation with 5-year survival (6, 7). In a retrospective study, Narges Gholizadeh et al. examined predictive factors for survival rate in OSCC in Iran. Data collection was from medical records of patients with oral cancer from 2009 to 2012 in the cancer department of the Islamic Republic of Iran Ministry of Health. The finding showed that the overall 5-year survival rate was 40.24% (SE = 5.5). Moreover, age and regular follow-up had a statistically significant relationship with survival rate (8).
OSCC is usually diagnosed in advanced stages; thus, despite advances in diagnostic and treatment methods, the mortality rate remains high (9). Public health professionals have long been focusing on the survival rate as an indicator of quality of screening, diagnostic, and treatment programs. Hence, according to the limited number of survival studies in southern Iran, the current study was conducted using cancer registry data in Khuzestan province from 2014 to 2018 to estimate the overall 5-year survival rate of OSCC in the Khuzestan province and identify associated demographic and background factors to understand the current situation, enable national and international comparisons, and plan strategies to control this disease in the future.
Material and methods
Patient and data collection
The current study was a cross-sectional study conducted using a descriptive–analytical method. The study population consisted of all patients with OSCC who were registered in the Khuzestan cancer registry system from 2014 to 2018. The sampling method was census. The data collection was divided into two parts. The first part included background parameters of the patients, such as age (under 55, greater than or equal to 55), gender (male, female), marital status (single, married), ethnicity, education (illiterate, below diploma, diploma and above), employment status (housewife, retired, employee, others) and insurance type (public health, supplemental health), which were collected by referring to the Khuzestan cancer registry database. The second part—information about the overall 5-year survival rate—was collected through phone call after obtaining the consent of the patients or their relatives and ensuring confidentiality. The inclusion criteria for the study included individuals’ willingness to participate in the study. Individuals who did not complete the questionnaire and those who had a history of systemic chronic diseases at the time of cancer diagnosis were excluded from the study. This study was approved by the Ethics Committee in Ahvaz Jundishapur University of Medical Sciences (ethics code: IR.AJUMS.REC.1402.201).
Statistical analysis
The data were analyzed using the statistical software SPSS 26 (IBM SPSS Statistics, Armonk, NY, USA). Data were presented as frequency (percentage) for categorical variables. The follow-up period began after the initial treatment for each patient. It ended when the patient expired, experienced tumor recurrence, or was lost to follow-up. The significance of the curves was assessed using the log-rank test. Potential risk factors and pathological characteristics were further analyzed using a multivariate Cox regression model to obtain independent predictors of survival. The final model was constructed in stages, and differences in clinical characteristics were examined using the χ2 test. In all analyses, p < 0.05 was considered significant.
Results
Patient characteristics
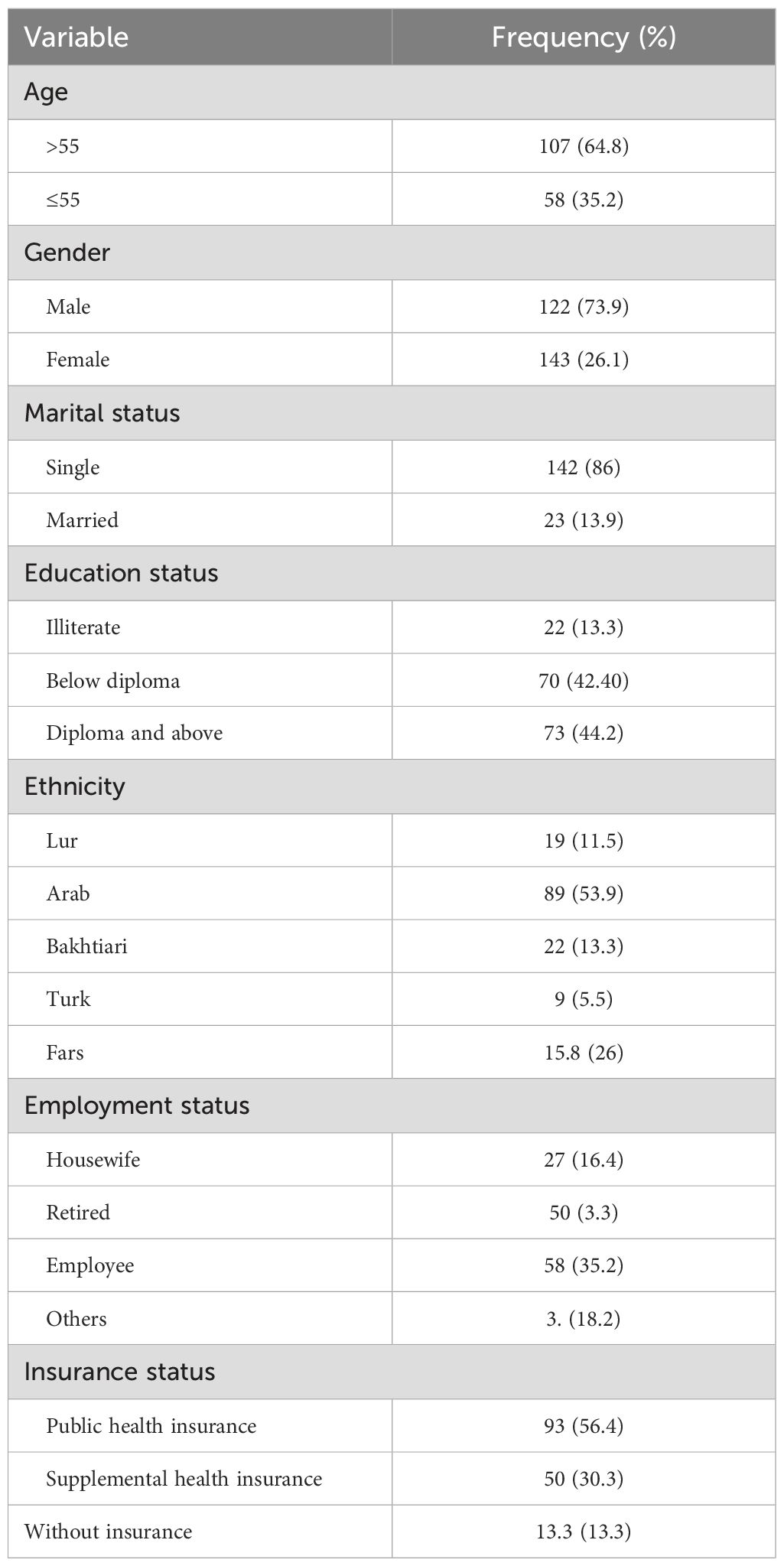
In this study, 165 patients, 43 women (26.1%) and 122 men (73.9%), with OSCC who were undergoing chemotherapy treatment were included. Patients were divided into five ethnic groups: Lur (11.5%), Fars (15.8%), Arab (53.9%), Turk (5.5%), and Bakhtiari (13.3%). Patients were categorized into two groups: over 55 years old (35.2%) and under 55 years old (64.8%). Additionally, individuals were divided into three educational categories: illiterate (13.3%), below diploma (42.4%), and diploma and above (44.2%) (Table 1).
Survival analysis
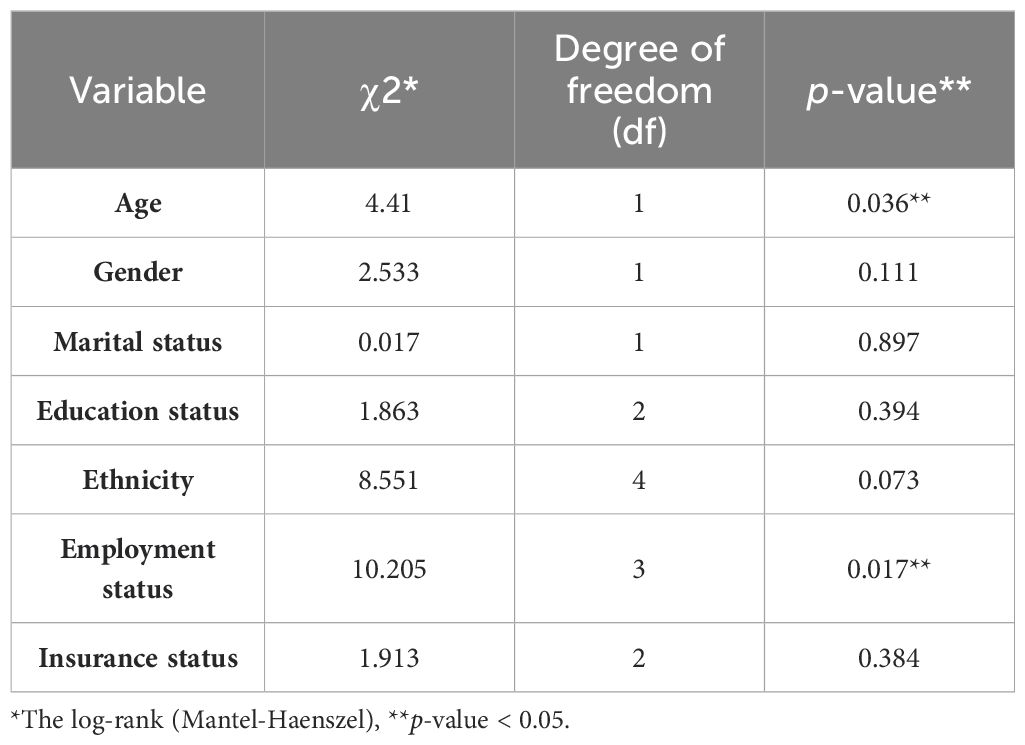
The 1-, 2-, 3-, 4-, and 5-year survival for OSCC was 93.34%, 81.21%, 71.51%, 56.96%, and 44.84%, respectively. Based on the log-rank test results in Table 2, age (df = 2, χ2 = 4.410, p = 0.036) and employment status (df = 2, χ2 = 10.205, p = 0.017) were found to be associated with the overall 5-year survival rate, while other variables such as gender, marital status, ethnicity, insurance status, and education status were not associated with the overall 5-year survival rate (Figures 1–4).
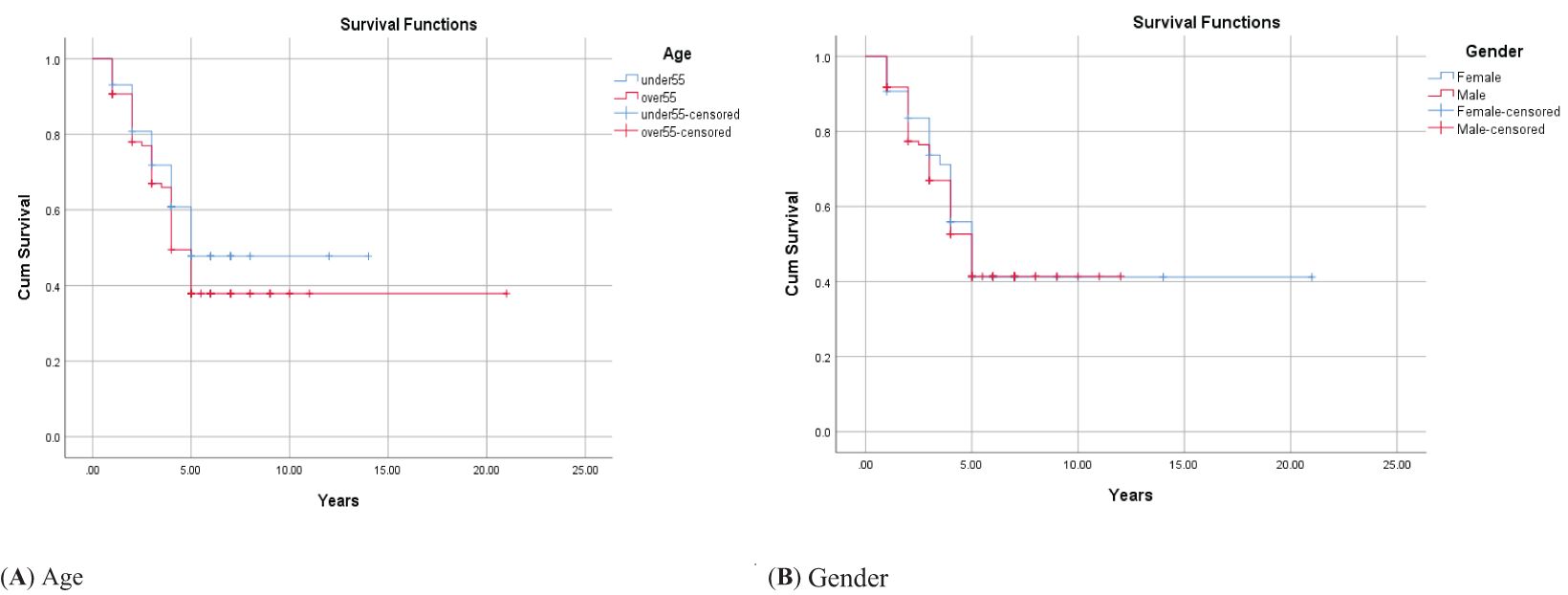
Figure 1. Adjusted parametric survival curves illustrating OSCC-specific survival by age (A) and gender (B), p < 0.001 by the log-rank test.
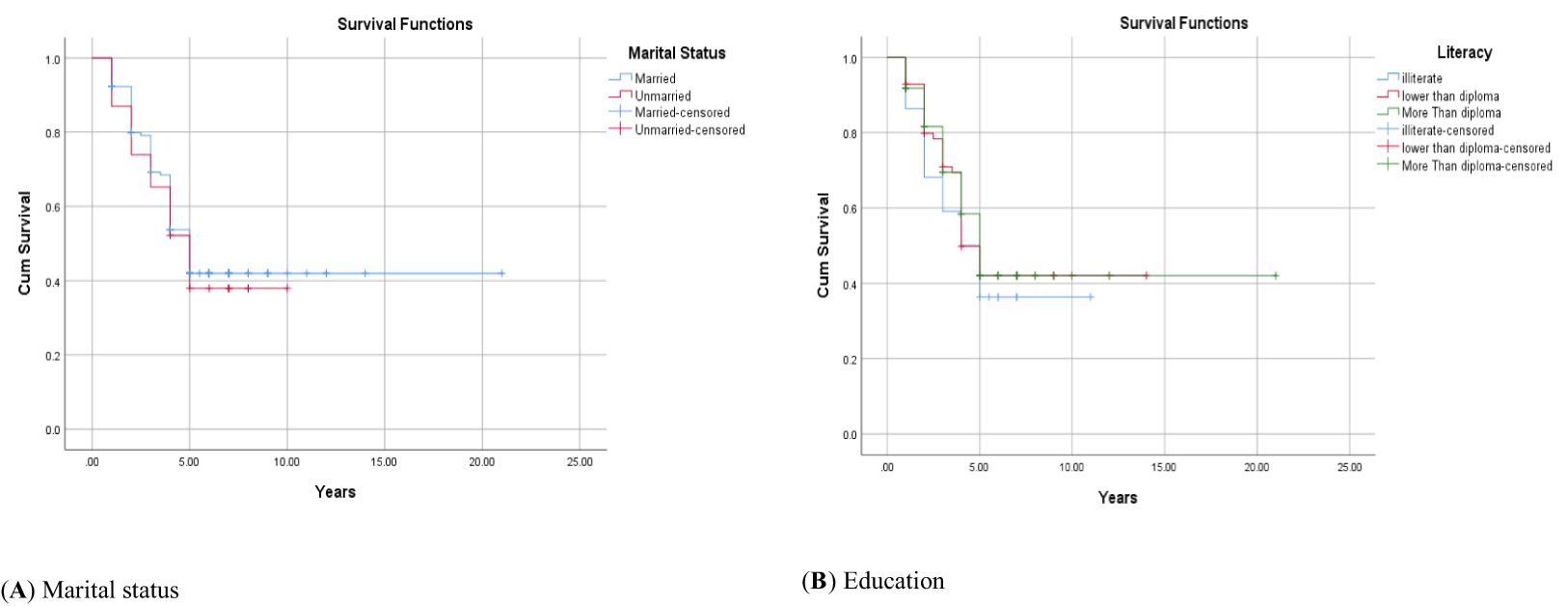
Figure 2. Adjusted parametric survival curves illustrating OSCC-specific survival by marital status (A) and education (B), p < 0.001 by log-rank test.
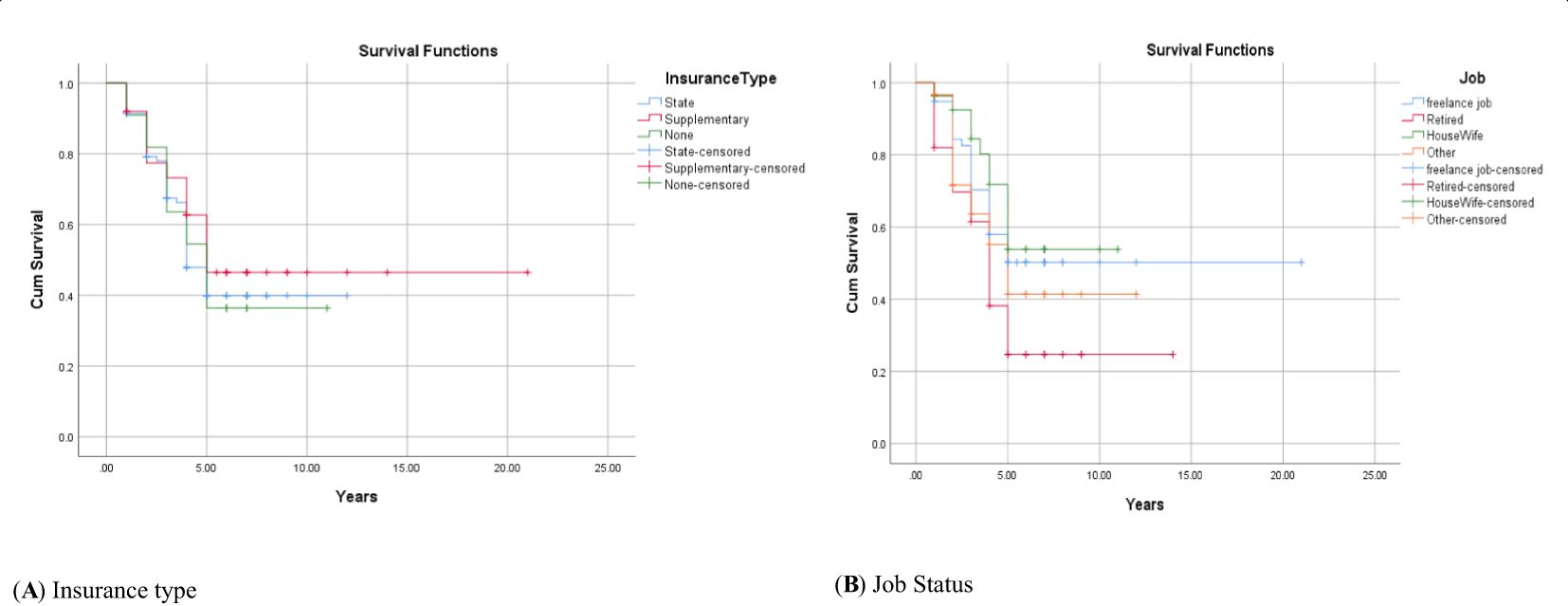
Figure 3. Adjusted parametric survival curves illustrating OSCC-specific survival by insurance type (A) and job status (B), p < 0.001 by log-rank test.
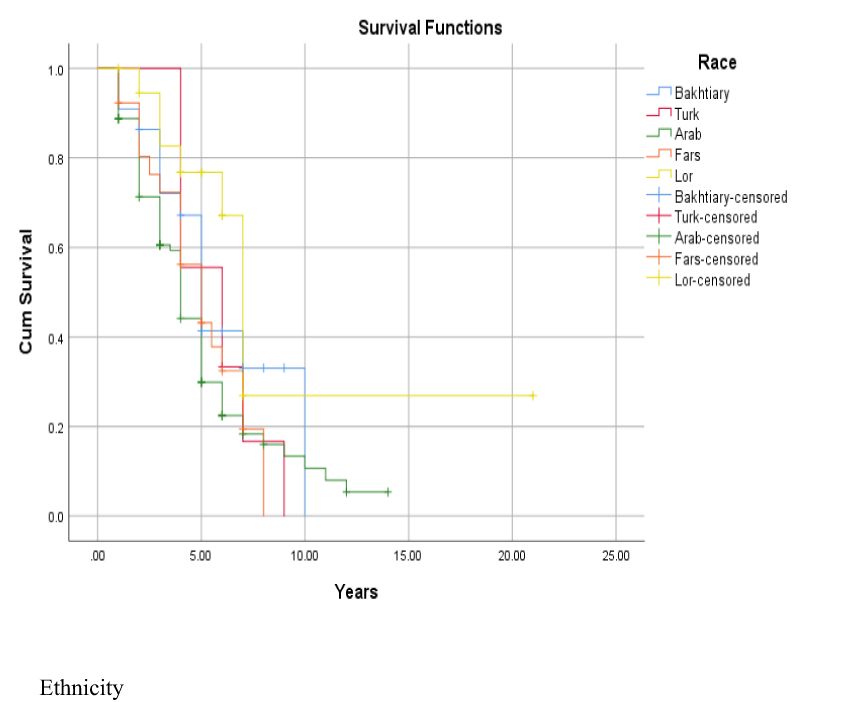
Figure 4. Adjusted parametric survival curves illustrating OSCC-specific survival by ethnicity, p < 0.001 by log-rank test.
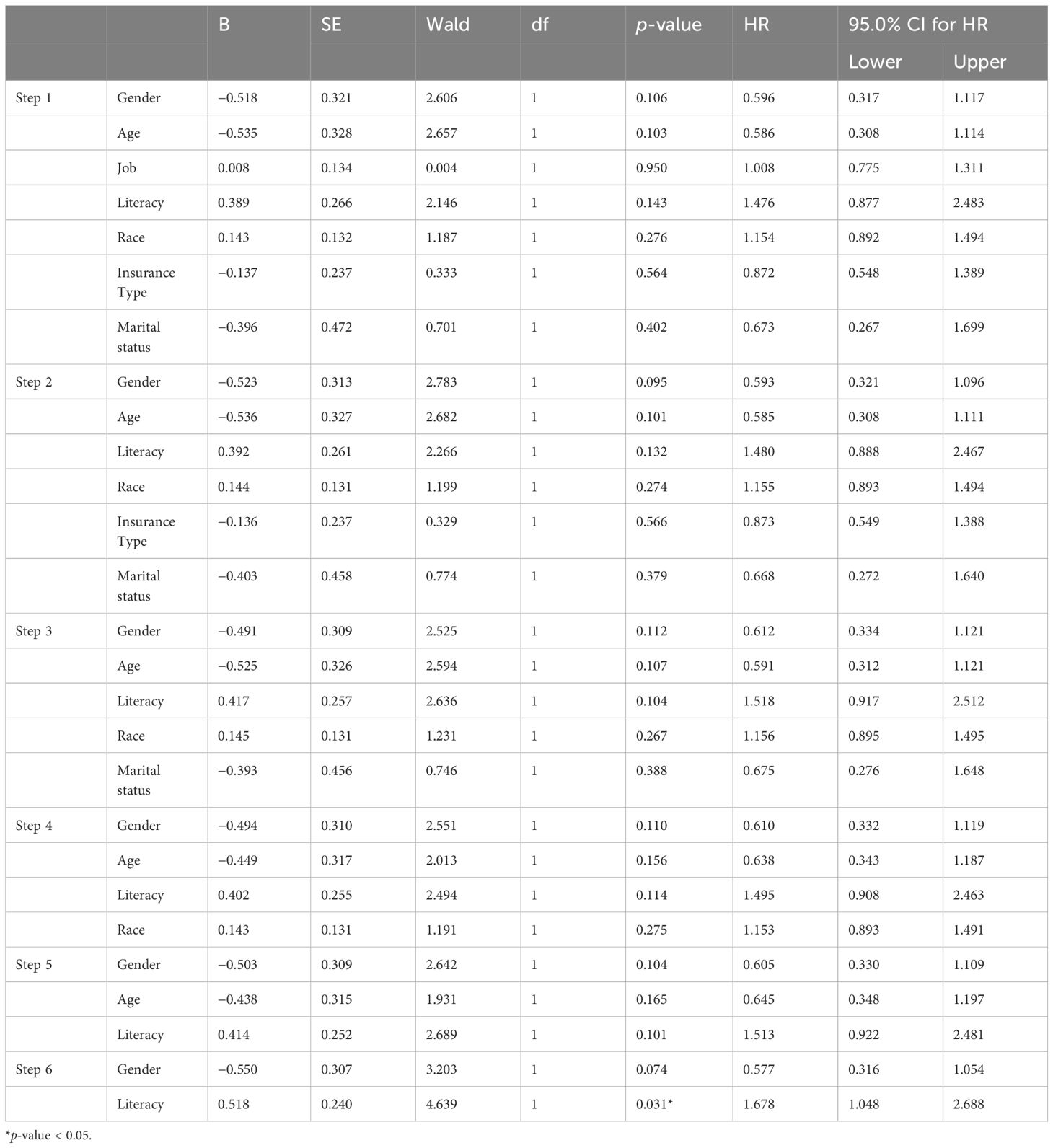
The results of Cox regression analysis, after including all variables in the model and adjusting them, indicate that none of them were statistically significant (p-value > 0.05). Therefore, we sequentially removed each variable from our model, starting with the ones with higher p-values, and obtained the results shown in Table 3. However, all variables were not to be significant. This analysis was using the “backward method”, which consists of six steps, as displayed in Table 3. Based on the findings presented in Table 3, it is evident that the hazard ratio (HR) for various variables was close to 1, especially during the initial stages of Cox regression.
Discussion
Based on our knowledge, the current study is the first scientific report to examine the 5-year survival rate of patients with OSCC in southern Iran. The results of the current study indicate that age and occupation were associated with the overall 5-year survival rate in OSCC patients based on the log-rank test, while Cox regression analysis, after including all variables in the model and adjusting them, showed that all variables including age, gender, marital status, ethnicity, employment status, insurance status, and educational status were not associated with the overall 5-year survival rate. Further investigation provides potential explanations for these findings and discusses how these findings can be applied in clinical practice.
In this study, the 5-year survival rate in patients with OSCC was reported to be 44.84%. Gholizadeh et al. (8) reported a survival rate of 40.24%, Jafari et al. (10) reported a survival rate of 49.40%, and Tajmiririahi et al. (11) reported a survival rate 41.7% in OSCC patients. These studies have been conducted on the Iranian population in the last 5 years. The possible reason for the higher overall 5-year survival rate in the current study may be the potential advancements in diagnostic and therapeutic methods for OSCC in recent years. Additionally, the overall 5-year survival rate in the present study was lower compared to reports from some developed countries, e.g., the United States, but higher compared to survival rates in studies from developing countries, e.g., Uganda. For example, in the study by Zanoni et al. (12), the 5-year survival rate in 2,082 patients with OSCC was 64.3% in the United States, while in the study by Asio et al. (13) in Uganda, it was only 20.7%. The difference in the overall 5-year survival rate in this research compared to other studies in developed countries may be due to the better healthcare infrastructure and better access of patients to medical facilities in Iran.
In the current study, it has been shown that older patients with OSCC have a better overall 5-year survival rate compared to younger patients, but when other risk factors were adjusted, this association disappeared. Consistent with the current study, Oh et al. (14) and Chang et al. (15), who specifically investigated the impact of age on survival in patients with oral cancers, after examining data of 5,518 individuals found that age is not an independent predictor of overall survival in OSCC. Furthermore, in another study, which classified age relatively similar to the present study, the results also showed that age does not have a significant difference in the survival of patients with OSCC (16). However, there are contradictory findings, which report older patients’ worse survival due to comorbidities and adverse drug effects, while other studies have linked younger individuals to worse survival due to the CCND1 gene polymorphism (which is associated with the early onset of head and neck cancer) (17, 18). Overall, the impact of age on OSCC prognosis is still debatable (17). Although some studies have reached contradictory findings, they are not able to precisely explain the cause and pathological mechanism; therefore, further research with larger sample sizes is necessary.
The current study has shown that gender is not associated with 5-year survival rates in patients with OSCC. Similar to the current study, the studies by Siriwardena et al. (19), Lo et al. (20), Lee et al. (21), and Honorato et al. (22) did not find a significant relationship between survival rates and gender in patients with oral cancer. However, contradictory to this study, the study by Choi et al. (23) showed gender as an important factor in the prognosis and survival of OSCC cancer. Oh et al. (24) also introduced being female as the most important predictor of the overall 5-year survival rate in young and middle-aged non-smoking women. It seems that this disease is often diagnosed late, and at that time, background variables cannot play a significant role. However, it is suggested that for further investigations, cohort studies should be conducted to examine the role of gender through exploring behavioral, hormonal, genetic factors, and differences in immunological mechanisms between men and women.
The results of the current study indicated that employment type was associated with the overall 5-year survival rate in patients with OSCC in such a way that housewives had a higher survival rate compared to other occupational groups. However, in the multivariable analyses, this association was eliminated, suggesting the potential mediating factors in this relationship. Some studies have shown a positive impact of returning to work on the survival of head and neck cancer patients, which may be due to higher income, increased physical activity, and better emotional status, potentially having a positive effect on the survival of patients with oral cancer (15).
No association was found between education level and 5-year survival rate in patients with OSCC in the present study. The results of Asio’s study in Uganda (13) and in the Fengqiong Liu study in China on patients with oral cancer also showed no association between education level and survival rate (25). However, contrary to the current study, some scientists believe that oral cancer is strongly influenced by social factors (26, 27). For example, in Brazil and Taiwan, patients with a lower income and education level had a higher mortality rate due to oral cancer (26, 28). Furthermore, data from a study in India showed that patients with a lower educational background have lower survival rates for breast cancer and oral cavity cancers, which is attributed to having a more advanced stage of the disease at the time of diagnosis. On the other hand, education level may be considered as a substitute for socio-economic status, which can be related to survival (25). However, the current study did not find any association between education and the overall 5-year survival rate.
Marital status was not identified as an independent predictor of the overall 5-year survival rate in OSCC patients in the Cox regression analysis. Contrary to the current study, several studies have introduced marriage as a protective factor for the survival of patients (26, 29–32). These studies explained that married patients may receive support in adopting a healthy lifestyle and a positive perspective (30). Additionally, another study listed smoking as a factor for lower survival in unmarried individuals (29). Despite the above studies, the research team did not find a study consistent with the findings of the current study and suggests conducting further studies to examine the role of marital status in the overall 5-year survival rate of OSCC patients.
The current study has shown that insurance type is not related to survival rate. In contrast to the current study, other studies have shown the superiority of private insurance over Medicare and Medicare disability in the survival of patients with head and neck cancers (33). Another study has also shown that insurance status and household income level are important independent predictors of overall survival in throat squamous cell carcinoma (34). In other words, individuals with private insurance started early treatment and had much better outcomes compared to uninsured patients or recipients of Medicaid or Medicare. Perhaps the possible reason for not finding a correlation between the type of insurance and the overall 5-year survival rate in the present study is that this disease is often diagnosed late, and at that time, background variables cannot play a significant role as influential factors.
Finally, the current study showed that ethnicity is not a significant factor in relation to the overall 5-year survival rate of patients with OSCC. These findings were consistent with the findings of a study by Mahboubi et al. (35) on Asian and non-Asian cancer patients, which showed that Asian patients generally had worse outcomes compared to non-Asian patients. Additionally, another study showed that the survival rate of OSCC in black people was lower than that in white people. A comparison among Iranian ethnic groups also showed that Kurds had the lowest survival rate and Turks had the highest survival rate in colorectal cancer (36). The participants in the current study live in a province and have numerous interactions with each other, which may lead to similar lifestyles. Further studies are necessary to gain a comprehensive understanding of the complex interactions between ethnicity, environmental exposure, and genetic predisposition in OSCC with the overall 5-year survival rate. Studying diseases in different ethnic groups can help identify reasons.
This research was derived from the Khuzestan cancer registry system and published for the first time. On the other hand, this study had some limitations. To determine the vital condition, we called the phone numbers of the patients registered in the cancer registration system. Some phone numbers have been changed or incorrectly recorded. In addition, it was not possible to find all the patients due to immigration. The authors did not collect information regarding the treatments of the included patients and deleterious habits (such as smoking and alcohol use), tumor location, or TNM classification. Finally, the data of the cancer registration system do not include the clinical characteristics of patients such as tumor stage and pathological grade.
Conclusion
Based on the findings of the present study, since none of the demographic variables were associated with the overall survival rate, efforts should continue to identify effective factors and investigate the relationship between pathological factors such as recurrence status, distant metastasis, type of treatment, and involved organs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1402.201). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because we used data from cancer registry whose were registered in time of diagnosis.
Author contributions
BK: Conceptualization, Supervision, Writing – review & editing, Validation. AG: Data curation, Investigation, Project administration, Writing – original draft. MC: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors are grateful to the participants, field workers, and co-researchers involved in this study. This work was a part of a dissertation of Dr. Arian Ghoreyshvandi (U-02119) at the School of Dentistry.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Markopoulos AK. Current aspects on oral squamous cell carcinoma. Open dentistry J. (2012) 6:126. doi: 10.2174/1874210601206010126
2. Coletta RD, Yeudall WA, Salo T. Grand challenges in oral cancers. Front Oral Health. (2020). 1:3. doi: 10.3389/froh.2020.00003
3. Cote SE, Singh H. Chapter 45 - Dental diseases and disorders. in Immigrant Medicine, ed. P. F. Walker, E. D. Barnett (W.B. Saunders) (2007), 597–610. doi: 10.1016/B978-0-323-03454-8.50049-8
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
5. Goya M. Iranian Annual Cancer Registration Report 2003-2009. Ministry of Health and Medical Education, Health Deputy Center for Disease Control and Prevention (2010).
6. Sklenicka S, Gardiner S, Dierks EJ, Potter BE, Bell RB. Survival analysis and risk factors for recurrence in oral squamous cell carcinoma: does surgical salvage affect outcome? J Oral Maxillofac Surg. (2010) 68:1270–5. doi: 10.1016/j.joms.2009.11.016
7. Subramaniam N, Balasubramanian D, Low T-HH, Murthy S, Clark JR, Thankappan K, et al. Factors affecting survival in surgically salvaged locoregional recurrences of squamous cell carcinoma of the tongue. J Oral Maxillofac Surge. (2018) 76:1133. e1–e6. doi: 10.1016/j.joms.2017.12.029
8. Gholizadeh N, Razavi HSE, Haftkhani GJ, Sheykhbahaei N. Predictive factors of survival rate in oral squamous cell carcinoma: a retrospective study in Iran. J Contemp Med Sci. (2019) 5:101–5. doi: 10.22317/jcms.v5i2.572
9. Silva LC, Faustino ISP, Ramos JC, Colafemina ACE, Di Pauli-Paglioni M, Leite AA, et al. The importance of early treatment of oral squamous cell carcinoma: Case report. Oral Oncol. (2023) 144:106442. doi: 10.1016/j.oraloncology.2023.106442
10. Jafari A, Esmaeili N, Najafi S, Emami Razavi H. Survival rate in patients with oral squamous cell carcinoma. J Dermatol Cosmetic. (2018) 8:195–203.
11. Tajmirriahi N, Razavi SM, Shirani S, Homayooni S, Gasemzadeh G. Evaluation of metastasis and 5-year survival in oral squamous cell carcinoma patients in Isfahan (2001–2015). Dental Res J. (2019) 16:117–21.
12. Zanoni DK, Montero PH, Migliacci JC, Shah JP, Wong RJ, Ganly I, et al. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. (2019) 90:115–21. doi: 10.1016/j.oraloncology.2019.02.001
13. Asio J, Kamulegeya A, Banura C. Survival and associated factors among patients with oral squamous cell carcinoma (OSCC) in Mulago hospital, Kampala, Uganda. Cancers Head Neck. (2018) 3:1–10. doi: 10.1186/s41199-018-0036-6
14. Oh LJ, Satgunaseelan L, Asher R, Veness M, Smee R, Goldstein D, et al. Young age is not a predictor of disease specific survival in oral cancer: A multi-institutional study. Oral Oncol. (2021) 115:105162. doi: 10.1016/j.oraloncology.2020.105162
15. Chang T-S, Chang C-M, Ho H-C, Su Y-C, Chen L-F, Chou P, et al. Impact of young age on the prognosis for oral cancer: a population-based study in Taiwan. PloS One. (2013) 8:e75855. doi: 10.1371/journal.pone.0075855
16. Sim YC, Hwang J-H, Ahn K-M. Overall and disease-specific survival outcomes following primary surgery for oral squamous cell carcinoma: analysis of consecutive 67 patients. J Korean Assoc Oral Maxillofac Surgeons. (2019) 45:83. doi: 10.5125/jkaoms.2019.45.2.83
17. Yang J, Guo K, Zhang A, Zhu Y, Li W, Yu J, et al. Survival analysis of age-related oral squamous cell carcinoma: a population study based on SEER. Eur J Med Res. (2023) 28:413. doi: 10.1186/s40001-023-01345-7
18. Zheng Y, Shen H, Sturgis EM, Wang L-E, Eicher SA, Strom SS, et al. Cyclin D1 polymorphism and risk for squamous cell carcinoma of the head and neck: a case–control study. Carcinogenesis. (2001) 22:1195–9. doi: 10.1093/carcin/22.8.1195
19. Siriwardena B, Tilakaratne A, Amaratunga E, Tilakaratne W. Demographic, aetiological and survival differences of oral squamous cell carcinoma in the young and the old in Sri Lanka. Oral Oncol. (2006) 42:831–6. doi: 10.1016/j.oraloncology.2005.12.001
20. Lo W-L, Kao S-Y, Chi L-Y, Wong Y-K, Chang RC-S. Outcomes of oral squamous cell carcinoma in Taiwan after surgical therapy: factors affecting survival. J Oral Maxillofac surge. (2003) 61:751–8. doi: 10.1016/S0278-2391(03)00149-6
21. Lee Y-C, Young C-K, Chien H-T, Chin S-C, Iandelli A, Liao C-T, et al. Characteristics and outcome differences in male and female oral cavity cancer patients in Taiwan. Medicine. (2021) 100:e27674. doi: 10.1097/MD.0000000000027674
22. Honorato J, Rebelo M, Dias F, Camisasca D, Faria P, e Silva GA, et al. Gender differences in prognostic factors for oral cancer. Int J Oral Maxillofac surge. (2015) 44:1205–11. doi: 10.1016/j.ijom.2015.04.015
23. Choi K-K, Kim M-J, Yun P-Y, Lee J-H, Moon H-S, Lee T-R, et al. Independent prognostic factors of 861 cases of oral squamous cell carcinoma in Korean adults. Oral Oncol. (2006) 42:208–17. doi: 10.1016/j.oraloncology.2005.07.005
24. Oh LJ, Asher R, Veness M, Smee R, Goldstein D, Iyer NG, et al. Effect of age and gender in non-smokers with oral squamous cell carcinoma: Multi-institutional study. Oral Oncol. (2021) 116:105210. doi: 10.1016/j.oraloncology.2021.105210
25. Mathew A, George PS, Kunnambath R, Mathew BS, Kumar A, Syampramod R, et al. Educational status, cancer stage, and survival in South India: a Population-based study. JCO Global Oncol. (2020) 6:1704–11. doi: 10.1200/GO.20.00259
26. Wong Y-K, Tsai W-C, Lin J-C, Poon C-K, Chao S-Y, Hsiao Y-L, et al. Socio-demographic factors in the prognosis of oral cancer patients. Oral Oncol. (2006) 42:893–906. doi: 10.1016/j.oraloncology.2005.12.007
27. Iype E, Pandey M, Mathew A, Thomas G, Sebastian P, Nair M. Oral cancer among patients under the age of 35 years. J postgraduate Med. (2001) 47:171–6.
28. Dantas TS, de Barros Silva P, Sousa EF, da Cunha M, de Aguiar ASW, Costa FWG, et al. Influence of educational level, stage, and histological type on survival of oral cancer in a Brazilian population: a retrospective study of 10 years observation. Medicine. (2016) 95:e2314. doi: 10.1097/MD.0000000000002314
29. Osazuwa-Peters N, Simpson MC, Boakye EA, Mohammed KA, Zhao L, Challapalli SD, et al. Differences in the sociodemographic correlates of HPV-associated cancer survival in the United States. Cancer Res. (2018) 78:4255. doi: 10.1158/1538-7445.AM2018-4255
30. Li Y, Ma X, Guan C, Yang X. Analysis of the influence of marital status on prognosis of prostate cancer patients based on big data. Am J Clin Exp Urol. (2022) 10:320.
31. Ming W, Zuo J, Han J, Wang Y, Chen J. Propensity-score matching analysis for marital status and survival of oral and oropharyngeal squamous cell carcinoma based on SEER database. (2021) 41(15_suppl):e20000. doi: 10.21203/rs.3.rs-1071238/v1
32. Shi X, Zhang T-t, Hu W-p, Ji Q-h. Marital status and survival of patients with oral cavity squamous cell carcinoma: a population-based study. Oncotarget. (2017) 8:28526. doi: 10.18632/oncotarget.16095
33. Kwok J, Langevin SM, Argiris A, Grandis JR, Gooding WE, Taioli E. The impact of health insurance status on the survival of patients with head and neck cancer. Cancer: Interdiscip Int J Am Cancer Soc. (2010) 116:476–85. doi: 10.1002/cncr.v116:2
34. Shin JY, Yoon JK, Shin AK, Blumenfeld P, Mai M, Diaz AZ. Association of insurance and community-level socioeconomic status with treatment and outcome of squamous cell carcinoma of the pharynx. JAMA Otolaryngology–Head Neck Surge. (2017) 143:899–907. doi: 10.1001/jamaoto.2017.0837
35. Mahboubi K, Nakoneshny S, Sauro K, Hart R, Matthews TW, Dort J, et al. The association of ethnicity and oncologic outcomes for oral cavity squamous cell carcinoma (OSCC). Cancers (Basel). (2024) 16:2117. doi: 10.3390/cancers16112117
Keywords: oral, squamous cell carcinoma, OSCC, survival, cancer, Iran
Citation: Karimi B, Ghoreyshvandi A and Cheraghi M (2024) Survival outcomes and contributing factors in oral squamous cell carcinoma patients in Khuzestan province, southwest of Iran. Front. Oncol. 14:1472190. doi: 10.3389/fonc.2024.1472190
Received: 29 July 2024; Accepted: 22 October 2024;
Published: 16 December 2024.
Edited by:
Sven Eric Niklander, Universidad Andres Bello, ChileReviewed by:
Felipe Silveira, University of the Republic, UruguayRené Andrés Martinez-Flores, Universidad Andrés Bello, Chile
Copyright © 2024 Karimi, Ghoreyshvandi and Cheraghi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Cheraghi, bWFyaWFjaGVyYWdoaUBnbWFpbC5jb20=
 Babak Karimi1
Babak Karimi1 Maria Cheraghi
Maria Cheraghi