- 1Department of Pediatric Gastroenterology, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Department of Pediatric Hematology and Oncology, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Background: Asparaginase-associated pancreatitis (AAP) is a major challenge for continuing asparaginase therapy. We aimed to investigate the acute and long-term complications and survival rates related to first and second AAP episodes in Chinese children with haematological malignancies.
Methods: We retrospectively analysed clinical data of children with pancreatitis who received asparaginase chemotherapy for acute lymphoblastic leukaemia (ALL), acute mixed cell leukaemia, and non-Hodgkin’s lymphoma at Shanghai Children’s Medical Center from November 2013 to November 2023.
Results: Of the 76 children included in the study, 12 had local complications (15.79%), with no deaths recorded. Systemic complications manifested in 28 patients (36.84%), resulting in 3 deaths (3.95%). Four patients (5.26%) developed long-term complications (chronic pancreatitis or insulin-dependent diabetes mellitus). No significant differences in local or long-term complications were recorded between children in the asparaginase re-exposed (n=39) and non-re-exposed (n=45) groups. Among the re-exposed patients, eight (25.81%) experienced a second attack without fatalities or complications. Survival analysis of intermediate- to high-risk patients revealed a significantly higher event-free survival (EFS) rate for the re-exposed group than for the non-re-exposed group. The second AAP episode’s occurrence and severity had no relation to the first AAP episode’s severity, and the second AAP episode was significantly less severe than the first (p<0.001).
Conclusions: The second AAP episode’s occurrence is unrelated to the first AAP episode’s severity, and the second AAP episode’s severity is significantly lower than that of the first. Further, asparaginase therapy could improve EFS in children with intermediate and high-risk ALL.
1 Introduction
Asparaginase is a basic chemotherapy component in paediatric acute lymphoblastic leukaemia (ALL) and lymphoma. It inhibits protein synthesis in tumour cells by breaking down asparagine in plasma into aspartic acid and ammonia, leading to cell death (1). Extensive clinical data and studies have demonstrated that asparaginase chemotherapy in children with leukaemia and lymphoma can improve remission and overall survival rates. However, asparaginase is associated with various toxicities, including hypersensitivity, pancreatitis, hyperlipidaemia, hepatotoxicity, thrombosis, and osteonecrosis, which may lead to the discontinuation of asparaginase therapy (2). This could potentially be associated with a decreased event-free survival rate in children, with asparaginase-associated pancreatitis (AAP) being the primary cause of discontinuation (3–6).
Previous studies have indicated that severe pancreatitis is the main reason for asparaginase interruption. Children with mild AAP are usually allowed to complete asparaginase treatment as scheduled. However, the incidence of severe pancreatitis in patients with AAP is 39.5%, which means that more than one-third of children with AAP may discontinue asparaginase. Some have argued that because the truncation of asparaginase is a key factor in the relapse of haematological malignancies in children, it is necessary to re-evaluate the risks of AAP and the criteria for re-exposure to asparaginase (7). The Ponte Di Legno Toxicity Working Group (PTWG) gathered comprehensive data on AAP in children with ALL from 18 countries (8). This large cohort study investigated the severity, associated complications, and risk factors of AAP for first episodes and second episodes after re-exposure to asparaginase. The risk of a second AAP episode after re-exposure to asparaginase was not related to the first AAP episode’s severity. More importantly, compared to the first AAP episode, the overall risk of complications and mortality from a second AAP event was not higher. Accordingly, asparaginase re-exposure should be determined mainly based on the anticipated requirement for asparaginase to achieve effective anti-leukaemia results.
We retrospectively analysed the acute and long-term complications and survival rates related to the first and second AAP episodes in Chinese children with haematological malignancies.
2 Materials and methods
2.1 Patient information
The Institutional Review Board of Shanghai Children’s Medical Center, which is affiliated with Shanghai Jiao Tong University School of Medicine, Shanghai, approved this study (SCMCIRB-YKW2021021). All the participants’ legal guardians provided written informed consent. Using the World Health Organization’s classification of myeloid neoplasms and acute leukaemia (9), a retrospective analysis was conducted on children treated with asparaginase for ALL, acute mixed-cell leukaemia, and non-Hodgkin lymphoma (NHL) at Shanghai Children’s Medical Center diagnosed with AAP between November 2013 and November 2023. The collected data included demographics, medication details, clinical manifestations related to pancreatitis, laboratory results, imaging findings, treatment, acute and long-term complications, re-exposure status, and prognosis. The median follow-up duration was 907.5 days. The inclusion criterion was children under 18 years of age diagnosed with acute pancreatitis following asparaginase administration. The exclusion criteria included pancreatitis from other causes, pre-existing abnormal pancreatic enzymes or imaging findings prior to asparaginase therapy, and the absence of relevant clinical data on AAP.
2.2 Diagnostic criteria and definitions
The standard diagnosis of AAP (according to the revised Atlanta criteria by the PTWG) requires that acute pancreatitis be diagnosed within 50 days after asparaginase therapy, with at least two of three specified criteria: (1) abdominal symptoms indicative of pancreatitis (acute, persistent, and severe upper abdominal pain); (2) serum amylase or lipase levels, or both, three times or more above the upper limit of normal (ULN); and (3) imaging findings characteristic of acute pancreatitis (8, 10).
The AAP severity was graded according to the PTWG guidelines: Grade 1, mild pancreatitis, with clinical manifestations and pancreatic enzymes at least three times the ULN for a maximum of 72 hours; Grade 2, severe pancreatitis, with clinical manifestations and/or pancreatic enzymes at least three times the ULN for more than 72 hours, or haemorrhagic pancreatitis, pancreatic abscess, or pseudocysts; and Grade 3, death from AAP (10).
Persistent complications owing to AAP were characterised by a long-term requirement for insulin therapy and recurring abdominal pain lasting for at least one year following diagnosis of asparaginase-associated complications, including insulin-dependent diabetes mellitus, chronic pancreatitis, and recurrent abdominal pain (8, 11). Local complications owing to AAP included pancreatic pseudocysts, peripancreatic fluid collections, acute necrotic collections, and walled-off necrosis (12).
Systemic complications owing to AAP included systemic inflammatory response syndrome (SIRS), sepsis, acute respiratory distress syndrome (ARDS), multiple organ dysfunction syndrome (MODS), intra-abdominal hypertension, and abdominal compartment syndrome (12–15).
2.3 Outcomes
The primary outcome was a comparison of acute and long-term complications of AAP in the first or second episode following re-exposure to asparaginase. Secondary outcomes included event-free survival (EFS) in all children and children in the intermediate-high risk group with AAP.
2.4 Statistical methods
Analyses were performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 9.0.0 (GraphPad Software, Boston, MA, USA) software. Based on the results of the Shapiro–Wilk test, continuous variables are expressed as means and standard deviations (x ± s) or medians and interquartile ranges (M [p25, p75]), and the comparison of normally and non-normally distributed variables of groups were performed using the t-test and Mann–Whitney U test, respectively. Categorical variables are presented as numbers and percentages and were analysed using the chi-squared or Fisher’s exact tests (when the expected cell number was less than 5). We utilised Kaplan–Meier curves to demonstrate survival probability and performed the log-rank test to compare survival differences between patient groups. To analyse the potential risk factors associated with EFS in children with AAP, we employed multivariate Cox regression models, presenting hazard ratios (HRs) and 95% confidence intervals (CIs). EFS was defined as the interval between AAP diagnosis and the date of the first or last follow-up event, with relapse, death, or a second malignancy considered competing events. Statistical significance was set at p<0.05.
3 Results
3.1 Patient characteristics
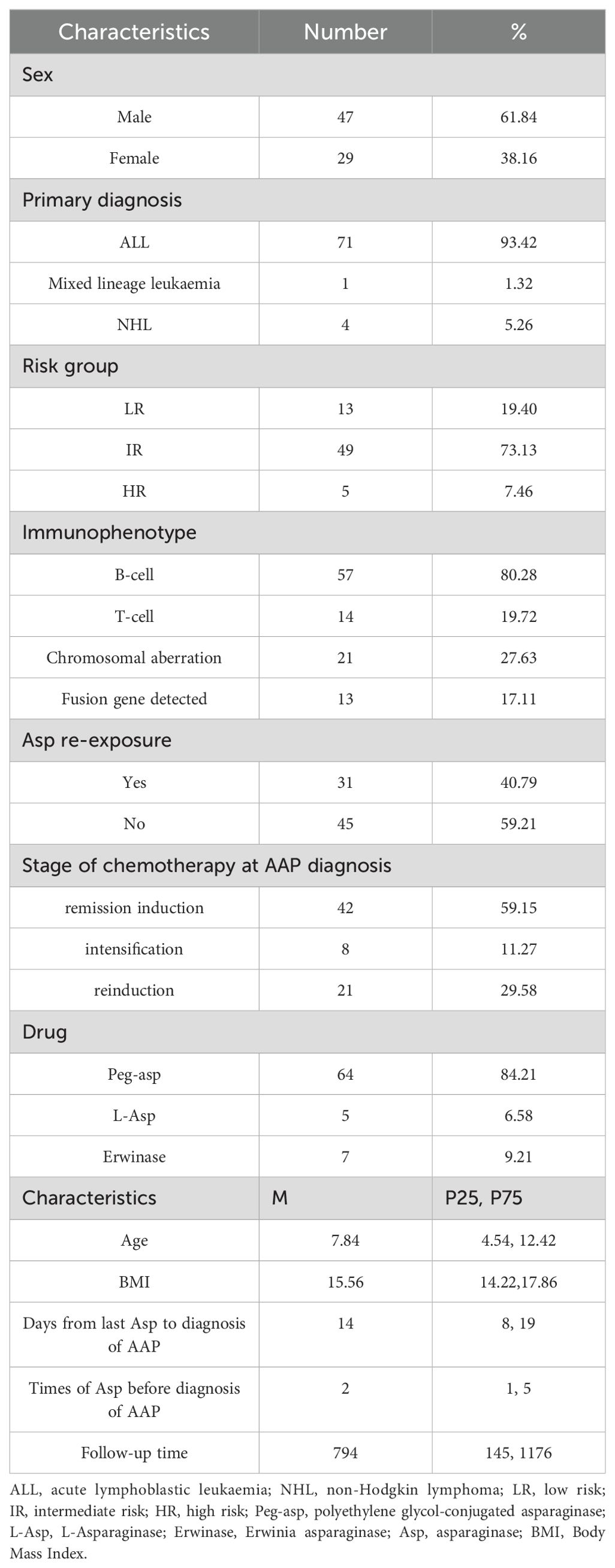
A total of 76 patients diagnosed with acute AAP (47 male and 29 female) were enrolled in the study. The median age at the time of AAP diagnosis was 7.84 (4.54, 12.42) years. Seventy-one patients were diagnosed with ALL after excluding 4 children who were diagnosed with AAP after recurrence of ALL, 49 in the intermediate-risk group, 13 in the low-risk group, and 5 in the high-risk group. Fifty-seven cases were of the B-cell type, and 14 were of the T-cell type. Thirteen cases had abnormal chromosome numbers, and 21 cases were positive for fusion genes, including TEL-AML1 (n=8), TCF3-PBX1 (n=2), MLL-r (n=2), and Ph+/BCR-ABL (n=9). One patient presented with acute mixed-cell leukaemia, and four had NHL. In the study population, 31 patients continued asparaginase therapy, and 45 discontinued it.
During the remission induction period, 42 children with ALL experienced AAP, whereas 8 developed AAP during the intensification period and 21 during the re-induction period. One child with MPAL developed AAP in induced remission after recurrence, 2 of the children with NHL developed AAP in induced remission and 2 in consolidation. Patients developed AAP after a median of 14 (8, 9) days from the last asparaginase dose, with a median of 2 (1, 5) administrations. Different asparaginase formulations were used in the patients: 64 patients received polyethylene glycol-conjugated asparaginase (PEG asparaginase), five received L-asparaginase, and seven were treated with Erwinase (Porton Biopharma, Salisbury, England) before the AAP event. Baseline characteristics of the study cohort are shown in Table 1.
3.2 Impact of the first AAP episode
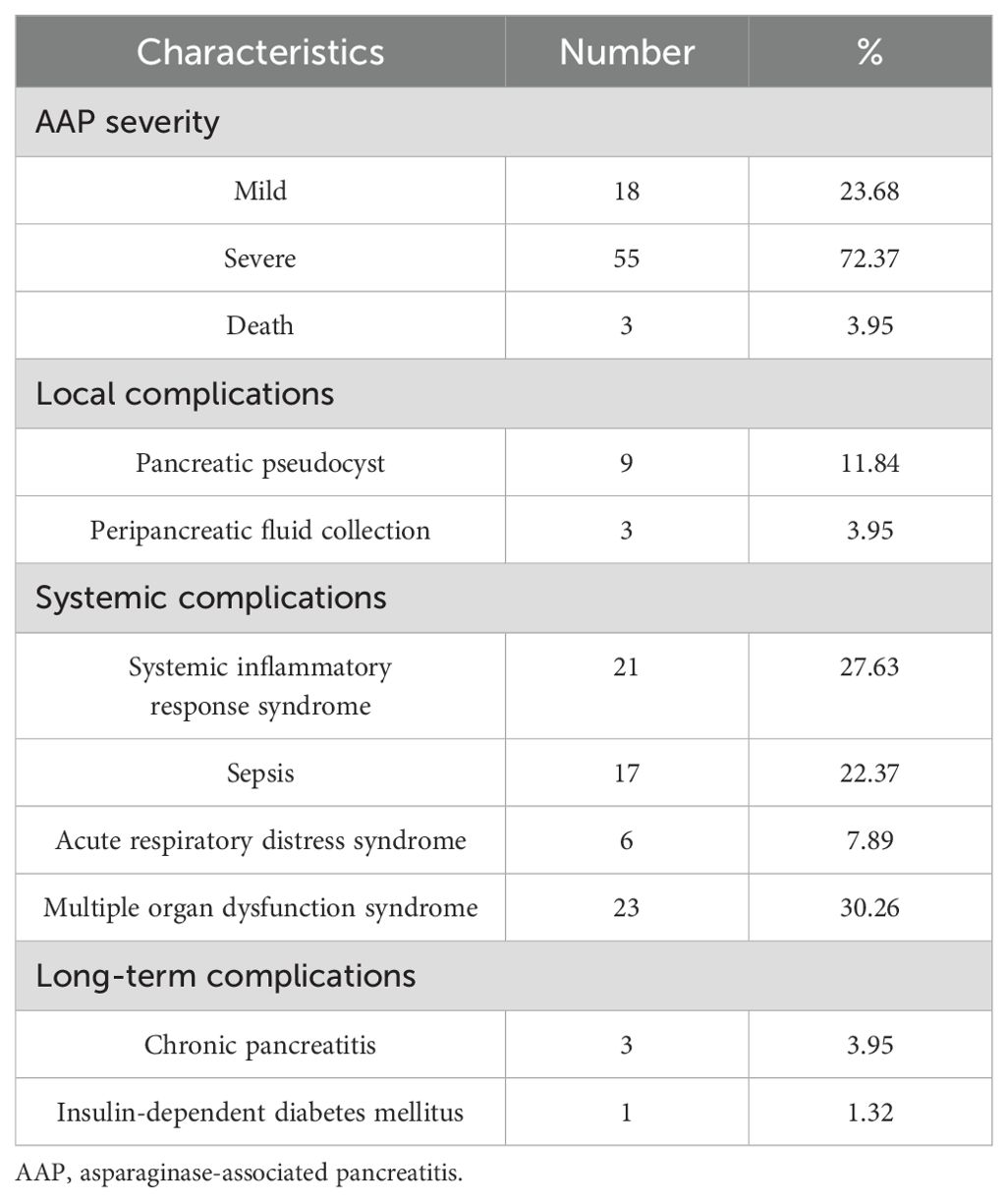
According to the PTWG pancreatitis grading, 18 cases (23.68%) were Grade 1, 55 (72.37%) were Grade 2, and 3 (3.95%) died from AAP (classified as Grade 3). Twelve patients (15.79%) experienced local complications, including nine cases of pancreatic pseudocysts and three cases of peripancreatic fluid collections. Pseudocysts and peripancreatic fluid collections resolved after a median of 131 and 7 days, respectively, with one case of a pseudocyst progressing to chronic pancreatitis. Systemic complications occurred in 28 patients (36.84%), including SIRS in 21, sepsis in 17, ARDS in 6, and MODS in 23, resulting in 3 deaths. Long-term complications were observed in four patients (5.26%), including three with chronic pancreatitis (3.95%) and one with insulin-dependent diabetes mellitus (1.32%). After endoscopic retrograde cholangiopancreatography (ERCP), the three cases of chronic pancreatitis did not recur, and the child with diabetes mellitus discontinued insulin therapy after 392 days of follow-up (Table 2).
3.3 Re-exposure to asparaginase after AAP
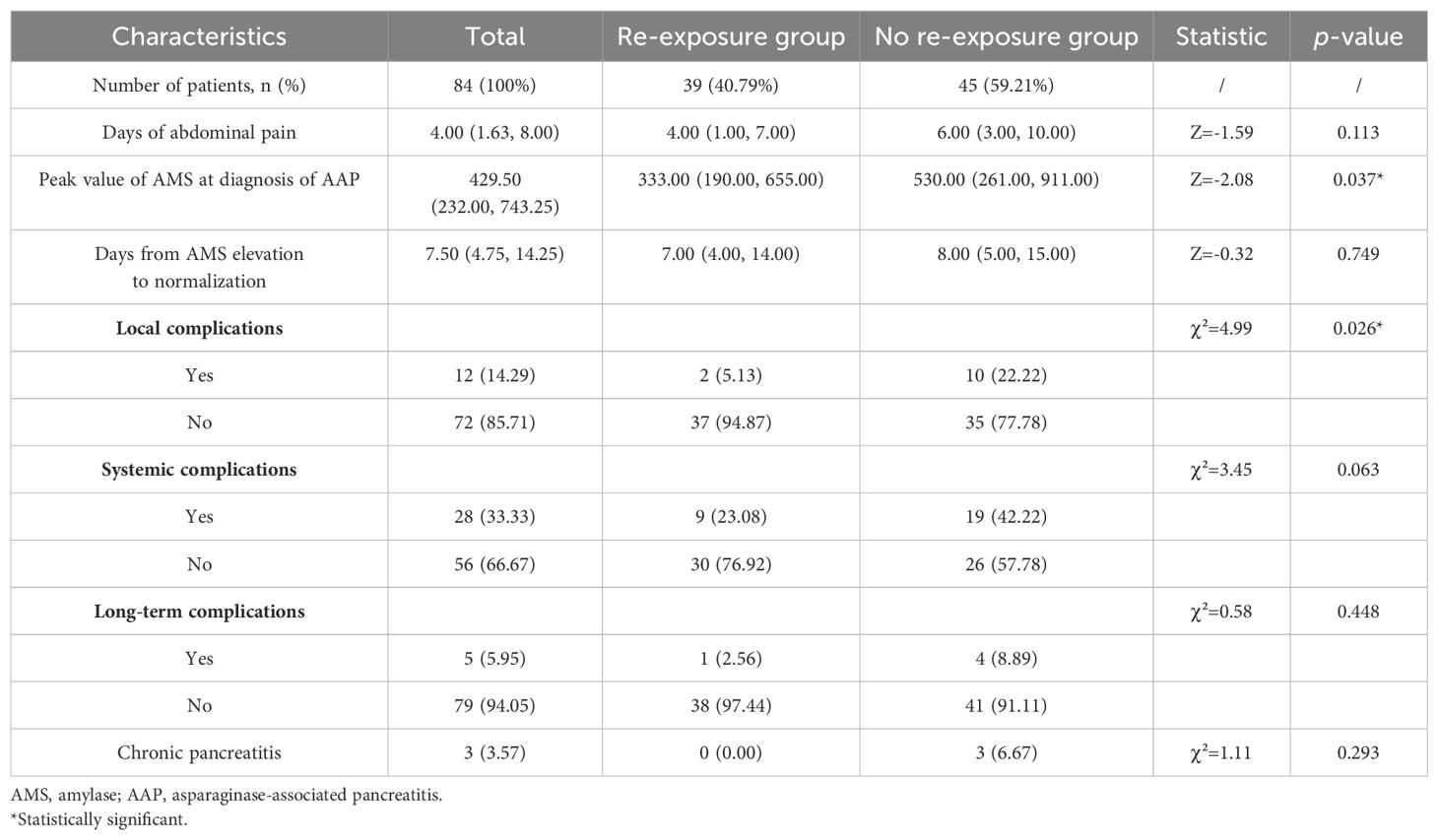
Based on re-exposure to asparaginase after the first episode of AAP, the 84 patients with AAP were divided into re-exposure group (n=39) and non-re-exposure group (n=45). There were no significant differences between the two groups in terms of the number of days of abdominal pain (p=0.113), the interval between the onset and normalisation of serum amylase levels (p=0.749), systemic complications (p=0.063), and long-term complications (p=0.448). However, the highest serum amylase levels (p=0.037) and the incidence of local complications (p=0.026) in the re-exposure group were significantly lower than those in the non-re-exposure group (Table 3).
Among the 31 children who were re-exposed to asparaginase after AAP, eight (25.81%) experienced a second attack, including seven cases of Grade 1 and one case of Grade 2 AAP, with no deaths reported. There were no local, systemic or long-term complications in the re-exposed children.
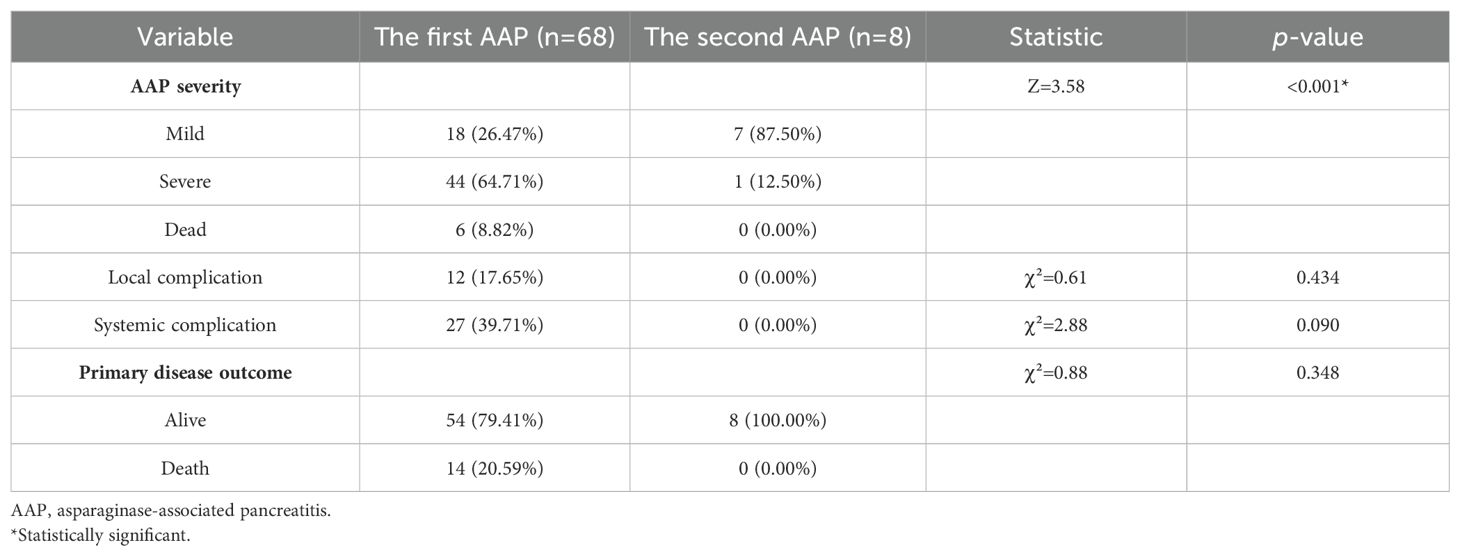
There was a significant difference in the severity of the first AAP episode compared to that of the second (p<0.001), with the first being more severe. There was no significant correlation between the first AAP episode’s severity and the occurrence of a second episode (p=1.000), and there were no significant differences in the occurrence of local (p=0.434) or systemic complications (p=0.090) or survival outcomes (p=0.348) between the first and second AAP episodes (Table 4). In the same patient, the severity of the second AAP episode was not significantly related to the severity of the first (p=0.063), and there was no significant correlation in terms of systemic complications (p=0.125).
3.4 Survival analysis
After excluding five children with non-ALL and four children diagnosed with AAP after relapse of ALL, the EFS of all enrolled children who were re-exposed to asparaginase was not significantly higher than that in children who were not re-exposed (76.4% vs. 54.4%, respectively; log-rank p=0.084) (Figure 1). Multivariate Cox regression analysis, after adjusting for age, sex, immunotyping and presence of chromosomal abnormalities and fusion genes, showed that asparaginase re-exposure had no significant effect on the EFS of children (p=0.092; HR: 2.510; 95% CI: 0.861-7.315) (Figure 2). However, survival analysis of intermediate-risk and high-risk patients revealed that the EFS of children in the asparaginase re-exposure group was increased compared to that of children in the non-re-exposure group (82.2% vs 49.3%, respectively; log-rank p=0.025) (Figure 1). In the multivariate Cox regression analysis, after adjusting for age, sex immunotyping, and the presence of chromosomal abnormalities and fusion genes, the presence of asparaginase re-exposure was an independent risk factor for EFS in children with intermediate-high risk ALL (p=0.010; HR: 5.957; 95% CI: 1.519–23.361) (Figure 2).
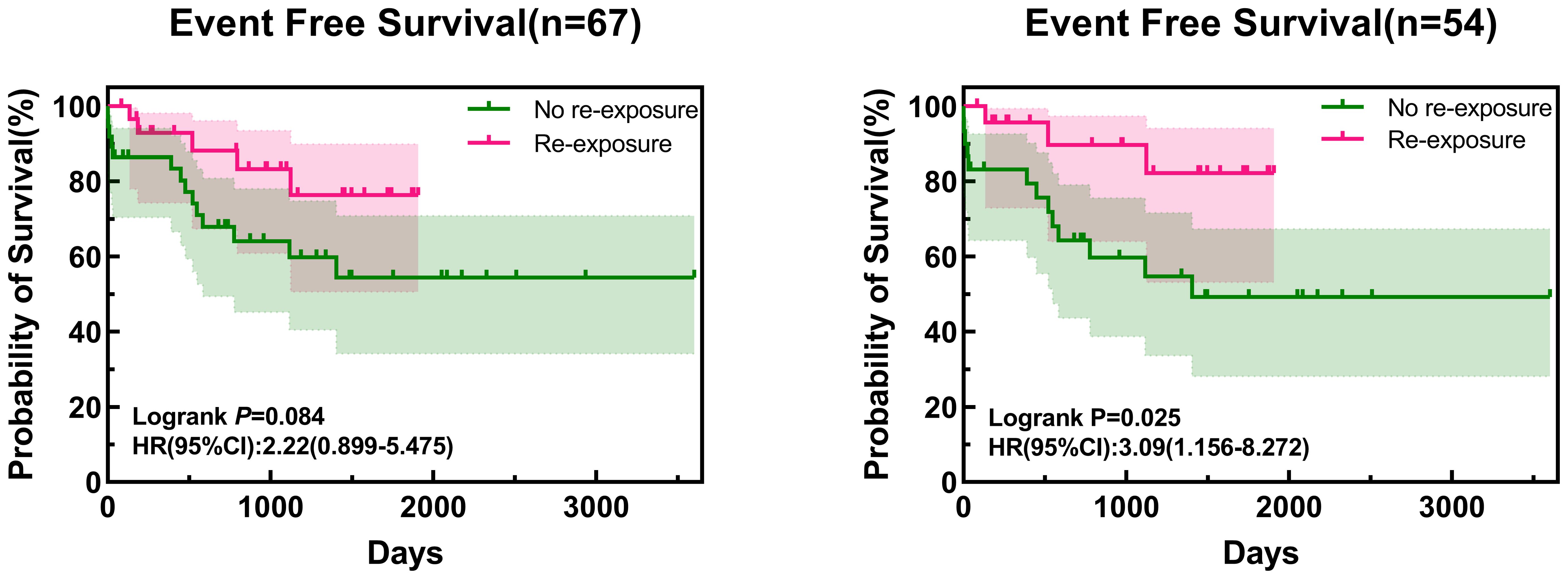
Figure 1. Survival curve of all patients and intermediate- to high-risk patients. HR, hazard ratio; CI, confidence interval.
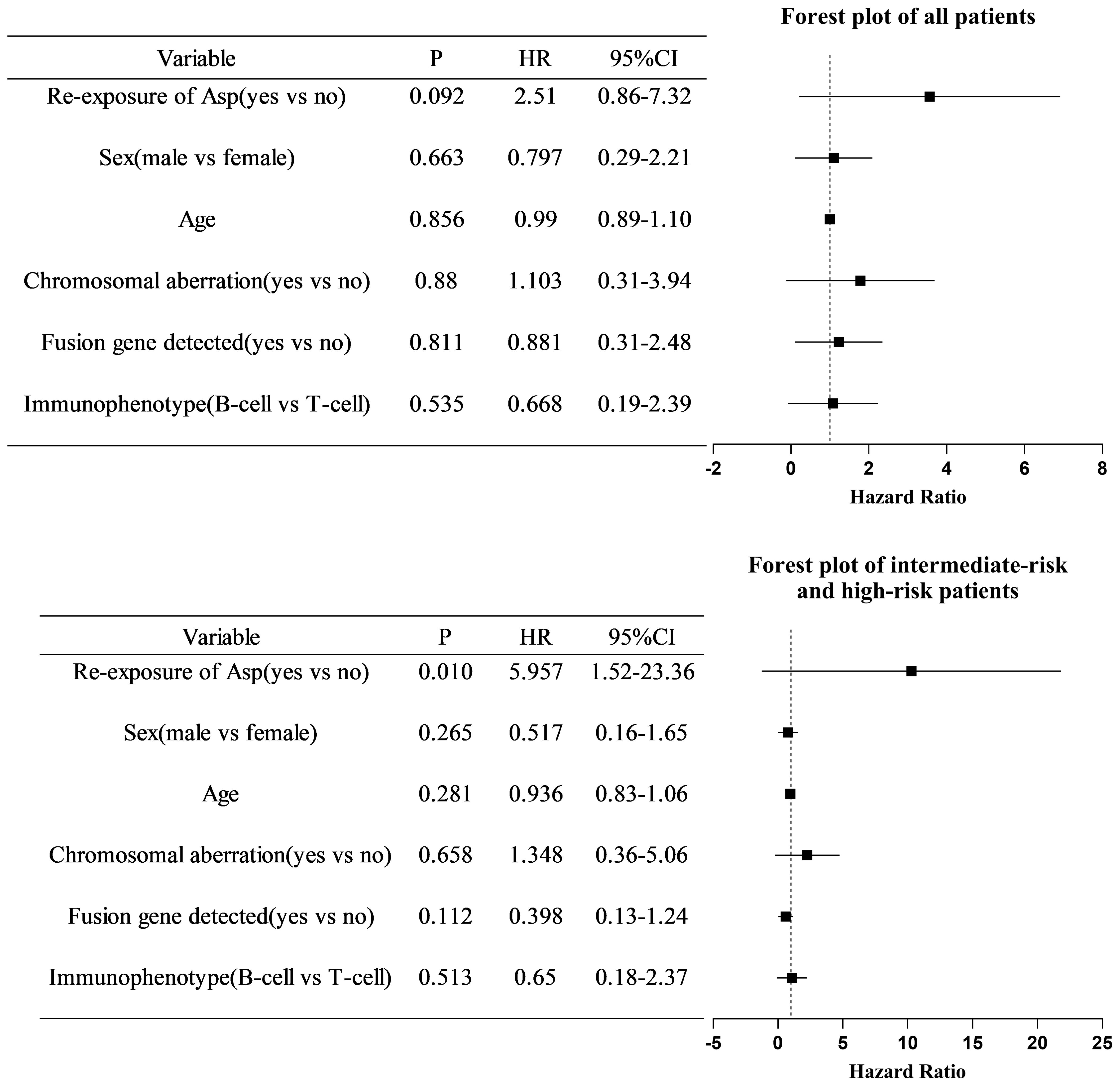
Figure 2. Multivariate COX regression analysis of risk factors for event-free survival in all patients and intermediate- to high-risk patients. COX, cyclooxygenase; HR, hazards ratio; CI, confidence interval; Asp, asparaginase.
4 Discussion
Acute and long-term complications in children with AAP are a primary concern for clinicians, serving as a key indicator for identifying patients who should have restricted exposure to asparaginase. This study assessed acute and long-term complications of first and second AAP episodes in children with haematological tumours, and it is the largest single-centre study on AAP in children in China.
Few studies have examined the complications of AAP in children. Kearney et al. (16) reported that of 403 patients with ALL, 28 (7%) experienced AAP, with five (18%) developing pseudocysts. None of the patients progressed to chronic pancreatitis, long-term exocrine pancreatic insufficiency, or required long-term insulin therapy. Among the 28 patients with AAP, 16 (57%) were re-exposed to asparaginase, and 10 experienced a second episode of AAP, one of which resulted in a pseudocyst, whereas the others recovered spontaneously, with no deaths from AAP and no decline in EFS. Mauney et al. (17) documented 34 patients with AAP, with 7 (20.6%) requiring admission to the intensive care unit, 17 (50%) having organ failure or pancreatic necrosis, 23 (67.6%) developing ascites, 10 (29.4%) having peripancreatic fluid, and 18 (52.9%) having pleural effusions. Despite severe complications following AAP, there were no deaths directly attributed to AAP. Our study found that the incidence of local complications was 15.79%, including nine instances of pancreatic pseudocysts and three of peripancreatic fluid, which subsequently resolved. Except for one child with a pseudocyst that progressed to chronic pancreatitis, none of the other children developed long-term complications or died owing to local issues. The systemic complication incidence rate was 36.84%, including 21 cases of SIRS, 17 of sepsis, 6 of ARDS, and 23 of MODS, resulting in three deaths. Long-term complications were observed in 5.26% of the patients, including three with chronic pancreatitis and one with insulin-dependent diabetes mellitus. Treatment with ERCP resulted in no recurrence in the three cases with chronic pancreatitis. The proportion of severe AAP cases in this study was higher than that in other studies, which may be related to the difference in the definition of AAP grade and chemotherapy regimen. Acute complications and long-term complications had little impact on the children’s quality of life. Deaths from AAP were primarily owing to systemic complications but did not exceed those caused by other forms of pancreatitis (2.0%–11.1%) (18).
Therefore, attention should be paid to systemic complications of AAP. Similarly, the incidences of chronic pancreatitis and insulin-dependent diabetes mellitus were relatively low, with minimal effect on the children’s quality of life. In our study, 31 children with AAP were exposed to asparaginase again, resulting in eight patients experiencing a second attack. The incidence of local and systemic complications in the second episode of AAP was not higher than in the first episode, and the recurrence of AAP was unrelated to the severity of the first episode of AAP. Even in the same child, the incidence of complications with recurrent AAP did not increase compared to the first episode, which aligns with the findings from Wolthers et al. (8).
Previous research has suggested that re-exposure to asparaginase should be considered only in children with mild AAP, which is defined as pancreatic enzymes exceeding three times the UNL and clinical symptoms lasting less than 72 hours, and absence of imaging evidence of pancreatic pseudocysts or necrosis (10, 19). However, Raja et al. (20) reported 45 AAP cases, 12 of which occurred after re-exposure to asparaginase, including three severe AAP cases at the first exposure. They found that further asparaginase therapy did not trigger pancreatitis recurrence, suggesting a broader consideration of the criteria for re-exposure to asparaginase after the first AAP episode. Similarly, in our study, 22 patients with severe AAP were re-exposed to asparaginase, and no complications or deaths occurred. We also believe that withdrawal should be done with caution so as not to affect the survival of patients with ALL.
Multiple studies have provided evidence that the truncation of asparaginase raises the haematological cancer relapse likelihood. In an international study by Rank et al. (21), which included 2,448 patients with ALL aged 1.0–45.9 years (including 168 patients with AAP), there was no significant increase in the 5-year cumulative recurrence rate among non-re-exposed patients with AAP compared to re-exposed patients with AAP, and there was no difference in the recurrence risk between patients with and those without AAP. Neither AAP nor asparaginase truncation owing to AAP was considered associated with an increased recurrence risk. Gupta et al. (22) enrolled 5,195 patients treated under the AALL0331 protocol and 3,001 patients treated under the AALL0232 protocol (aged 1–30.99 years) and found that in high-risk patients with ALL, asparaginase discontinuation was associated with worse disease-free survival (DFS), whereas it did not affect DFS in low-risk patients. Our study compared the EFS of children with AAP who were re-exposed to asparaginase with those who were not. The results showed that, regardless of risk stratification, asparaginase truncation did not increase tumour relapse or death risk in children with ALL. However, for children with ALL in the intermediate-high risk group, the EFS of children with AAP with interrupted asparaginase therapy was significantly lower than that of children who were re-exposed, with a 5.96-fold increased risk of tumour relapse or death. This may be owed to the demand for the intensity of asparaginase treatment in children with ALL in the intermediate and high-risk groups such that the use of a full course of asparaginase has an important impact on the prognosis of these patients, and insufficient exposure to asparaginase will lead to an increased risk of relapse. Therefore, from the perspective of the impact of asparaginase withdrawal on haematological tumour relapse, for children with intermediate and high-risk ALL, the potential dangers of being re-exposed to asparaginase must be carefully weighed against the need for asparaginase to ensure survival.
The study has certain limitations. The retrospective nature and lack of systematic AAP screening present, potential for selection biases in favour of patients with more noticeable symptoms. AAP can easily be confused with sepsis if pancreatic enzyme levels are not measured. The study involved a limited number of participants and had insufficient follow-up time for certain children. Survival analysis was not possible owing to the limited number of patients with ALL in the low-risk group who had confirmed AAP. The conclusions drawn require further validation through multicentre studies with larger sample sizes.
In conclusion, this study demonstrated that (1) in ALL treatment, identifying AAP early is essential to preventing progression to systemic complications and reducing mortality; (2) active treatment and multidisciplinary cooperation can effectively mitigate the harm caused by severe AAP; (3) the occurrence of a second AAP episode is not related to the severity of the first, and the severity of a second AAP episode is significantly lower than that of the first; and (4) further asparaginase therapy could improve the EFS of children with intermediate and high-risk ALL. Given the significance of asparaginase in treating paediatric haematological malignancies, the decision to reintroduce asparaginase should be mainly based on its effectiveness in combating leukaemia, even in children with severe AAP.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
HT: Writing – original draft. CC: Writing – review & editing. WH: Writing – original draft. SS: Writing – review & editing. JZ: Writing – original draft. SD: Writing – original draft. ZD: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from the Science and Technology Commission of Shanghai Municipality of China (Grant Number: 22Y11921800).
Acknowledgments
We would like to thank Editage (www.editage.cn) for the English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bender C, Maese L, Carter-Febres M, Verma A. Clinical utility of pegaspargase in children, adolescents and young adult patients with acute lymphoblastic leukemia: A review. Blood Lymphat Cancer. (2021) 11:25–40. doi: 10.2147/BLCTT.S245210
2. Schmidt MP, Ivanov AV, Coriu D, Miron IC. L-asparaginase toxicity in the treatment of children and adolescents with acute lymphoblastic leukemia. J Clin Med. (2021) 10:4419. doi: 10.3390/jcm10194419
3. Gottschalk Højfeldt S, Grell K, Abrahamsson J, Lund B, Vettenranta K, Jónsson ÓG, et al. Relapse risk following truncation of pegylated asparaginase in childhood acute lymphoblastic leukemia. Blood. (2021) 137:2373–82. doi: 10.1182/blood.2020006583
4. Pession A, Valsecchi MG, Masera G, Kamps WA, Magyarosy E, Rizzari C, et al. Long-term results of a randomized trial on extended use of high dose l-asparaginase for standard risk childhood acute lymphoblastic leukemia. J Clin Oncol. (2005) 23:7161–7. doi: 10.1200/JCO.2005.11.411
5. Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: Results of dana-farber consortium protocol 91-01. Blood. (2001) 97:1211–8. doi: 10.1182/blood.V97.5.1211
6. Raja RA, Schmiegelow K, Frandsen TL. Asparaginase-associated pancreatitis in children. Br J Haematol. (2012) 159:18–27. doi: 10.1111/bjh.2012.159.issue-1
7. Pemmaraju N, Rytting ME. Questions on asparaginase-associated pancreatitis. Lancet Oncol. (2017) 18:1148–9. doi: 10.1016/S1470-2045(17)30610-1
8. Wolthers BO, Frandsen TL, Baruchel A, Attarbaschi A, Barzilai S, Colombini A, et al. Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: An observational ponte di legno toxicity working group study. Lancet Oncol. (2017) 18:1238–48. doi: 10.1016/S1470-2045(17)30424-2
9. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood. (2016) 127:2391–405. doi: 10.1182/blood-2016-03-643544
10. Schmiegelow K, Attarbaschi A, Barzilai S, Escherich G, Frandsen TL, Halsey C, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: A delphi consensus. Lancet Oncol. (2016) 17:e231–e9. doi: 10.1016/S1470-2045(16)30035-3
11. Skipper MT, Albertsen BK, Schmiegelow K, Andrés-Jensen L. Long-term effects of asparaginase-associated pancreatitis. Pediatr Blood Cancer. (2023) 70:e30528. doi: 10.1002/pbc.30528
12. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: Revision of the atlanta classification and definitions by international consensus. Gut. (2013) 62:102–11. doi: 10.1136/gutjnl-2012-302779
13. Nasa P, Chanchalani G, Juneja D, Malbrain ML. Surgical decompression for the management of abdominal compartment syndrome with severe acute pancreatitis: A narrative review. World J Gastrointest Surg. (2023) 15:1879–91. doi: 10.4240/wjgs.v15.i9.1879
14. Matthay MA, Arabi Y, Arroliga AC, Bernard G, Bersten AD, Brochard LJ, et al. A new global definition of acute respiratory distress syndrome. Am J Respir Crit Care Med. (2024) 209:37–47. doi: 10.1164/rccm.202303-0558WS
15. Srzić I, Nesek Adam V, Tunjić Pejak D. Sepsis definition: What's new in the treatment guidelines. Acta Clin Croat. (2022) 61:67–72. doi: 10.20471/acc.2022.61.s1.11
16. Kearney SL, Dahlberg SE, Levy DE, Voss SD, Sallan SE, Silverman LB. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr Blood Cancer. (2009) 53:162–7. doi: 10.1002/pbc.22076
17. Mauney EE, Power-Hays A, Flamand Y, Vrooman L, Silverman LB, Grover AS. Clinical characteristics and short-term outcomes of children with asparaginase-associated pancreatitis. J Pediatr Gastroenterol Nutr. (2022) 74:402–7. doi: 10.1097/MPG.0000000000003334
18. Restrepo R, Hagerott HE, Kulkarni S, Yasrebi M, Lee EY. Acute pancreatitis in pediatric patients: Demographics, etiology, and diagnostic imaging. AJR Am J Roentgenol. (2016) 206:632–44. doi: 10.2214/AJR.14.14223
19. Liu C, Yang W, Devidas M, Cheng C, Pei D, Smith C, et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. (2016) 34:2133–40. doi: 10.1200/JCO.2015.64.5812
20. Raja RA, Schmiegelow K, Albertsen BK, Prunsild K, Zeller B, Vaitkeviciene G, et al. Asparaginase-associated pancreatitis in children with acute lymphoblastic leukaemia in the nopho all2008 protocol. Br J Haematol. (2014) 165:126–33. doi: 10.1111/bjh.2014.165.issue-1
21. Rank CU, Wolthers BO, Grell K, Albertsen BK, Frandsen TL, Overgaard UM, et al. Asparaginase-associated pancreatitis in acute lymphoblastic leukemia: Results from the nopho all2008 treatment of patients 1-45 years of age. J Clin Oncol. (2020) 38:145–54. doi: 10.1200/JCO.19.02208
Keywords: asparaginase, leukaemia, pancreatitis, event-free survival, complications
Citation: Tang H-j, Chen C-c, Hu W-t, Shen S-h, Zeng J-q, Ding S and Deng Z-h (2024) Effects of asparaginase-associated pancreatitis in children with haematological tumours. Front. Oncol. 14:1472049. doi: 10.3389/fonc.2024.1472049
Received: 28 July 2024; Accepted: 23 September 2024;
Published: 08 October 2024.
Edited by:
Luca Lo Nigro, Azienda Ospedaliero Universitaria Policlinico - San Marco, ItalyReviewed by:
Sandeep Batra, Riley Hospital for Children, United StatesGiulia Angela Restivo, Civico Hospital, Italy
Copyright © 2024 Tang, Chen, Hu, Shen, Zeng, Ding and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhao-hui Deng, ZHpocmpAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Hui-jiao Tang
Hui-jiao Tang Chang-cheng Chen2†
Chang-cheng Chen2† Wen-ting Hu
Wen-ting Hu Shu-hong Shen
Shu-hong Shen Zhao-hui Deng
Zhao-hui Deng