- 1Department of Oncology, Changzhou Tumor Hospital Affiliated to Soochow University, Changzhou, China
- 2Department of Pathology, Changzhou Tumor Hospital Affiliated to Soochow University, Changzhou, China
Advanced metastatic cardia cancer is an intractable malignance with poor prognosis. It is often accompanied by upper digestive tract obstruction, which seriously affects the quality of patients. Therefore, effective relief of eating obstruction is an important goal in the treatment of cardia cancer. Immune checkpoint inhibitors (ICIs) have shown significant efficacy in cardia cancer, but only a small percentage of patients will benefit from them due to immune resistance. Oncolytic viruses have been shown to enhance the efficacy of ICIs by altering the immune microenvironment. This indicates that oncolytic virus has the potential value of overcoming the immune resistance of cardia cancer. Here, we present a case with local recurrent and multiple metastatic cardia cancer accompanied by eating obstruction. After 4 cycles of chemotherapy plus ICI therapy, the patient´s metastases were significant shrink, but the recurrent carida lesion were almost unchanged. Then we implemented exploratory local injection of recombinant human adenovirus type 5(H101) into recurrent cardia lesion by painless gastroscopy. Surprisingly, the cardia lesion shrank significantly, and the eating obstruction was greatly relieved. We also observed a significant increase of infiltrated CD4+T cells in biopsy tissues after H101 treatment. Our study not only conformed the value of oncolytic viruses to reverse ICI resistance in patients with gastric cancer, but also revealed its underlying impact on immune microenvironment.
Introduction
Advanced cardia cancer is a highly aggressive and heterogeneity malignance with a poor five-year survival rate (1). Patients are often suffer from eating obstruction, which can seriously affect the patient’s quality of life, which can seriously affect the patient’s quality of life, resulting in poor nutrition and even shorter survival. Therefore, it is a difficult and urgent problem to be solved in clinic practice. In recent years, immune checkpoint inhibitors (ICIs) have shown significant efficacy in cardia cancer, but only a small subgroup of patients will benefit from them due to immune resistance (2–5). Therefore, overcoming immune resistance is an imperative task.
Oncolytic viruses have been shown to enhance the efficacy of ICIs by directly lysing tumor cells and altering the immune microenvironment (6, 7). However, there are few reports about the effect of oncolytic viruses on reversing immune resistance of cardia cancer. We have a patient diagnosed with metastatic cardia cancer who underwent 4 cycles of immune checkpoint inhibitor combined with chemotherapy without no shrink in cardia lesions. What’s worse, the patient was suffered from the symptom of eating obstruction. However, the patient refused radiotherapy for fear of its toxic side effects. Considering the unique anti-tumor mechanism of oncolytic virus and its mild toxic side effects, we innovatively injected H101 into the cardia lesions by electronic gastroscope. Surprisingly, the cardia lesions were significantly reduced, and the parent symptom of eating obstruction were significantly relieved. The details are reported below.
Case presentation
In April 2021, a 72-year-old man was diagnosed with cardia cancer and subsequently underwent radical gastrectomy. Pathological diagnosis reported a poorly differentiated ulcerative adenocarcinoma (stage ШB,T3N3M0). He received oral S-1 (40mg twice daily for 14 days, discontinued for 7 days, and repeated every 21 days) as adjuvant chemotherapy regimen for about 4 months, but discontinued due to significant gastrointestinal reaction.
In February 2023, he was admitted to our department with an worsen eating obstruction. Chest and abdominal computed tomography (CT) scan showed cardia recurrence and multiple metastases in liver, peritoneum, abdominal cavity and retroperitoneal lymph nodes. Sintilimab (200mg, intravenously every three weeks) combined with nanoparticle albumin-bound paclitaxel(300mg, intravenously every three weeks) was admitted as first-line treatment after recurrence. After 4 cycles of chemotherapy plus ICI therapy, the patient´s metastases were significant shrink (Figure 1), but the cardia lesions were almost unchanged. As the patient strongly requested further relief of eating obstruction, we implemented exploratory local injection of H101 into recurrent cardia lesion by painless gastroscopy with the patient’s full knowledge and consent. Since June 9, 2023, H101 (5.0×1011 virus particles/0.5ml every time) was multipoint injected by painless gastroscopy every six weeks (8), 1 day before Sintilimab in each cycle. We observed on CT and gastroscopy images that the cardiac lesion were significantly reduced after two H101 treatment (Figure 2). Meanwhile, the patient´s eating obstruction symptom was subsequently relieved. Before and after two H101 treatment, we biopsied the cardia lesions and performed immunohistochemical staining of CD4+T cells and CD8+T cells respectively, results revealed a significant promotion of CD4+ T cell infiltration after H101 treatment (Figure 3). Unfortunately, no significant infiltration of CD8+T cells was found after H101 treatment (Figure 4). Then Sintilimab combined with S-1(60mg orally twice daily for 14 days) was admitted as maintenance antitumor therapy to date. (The timeline of treatments is shown in Table 1).
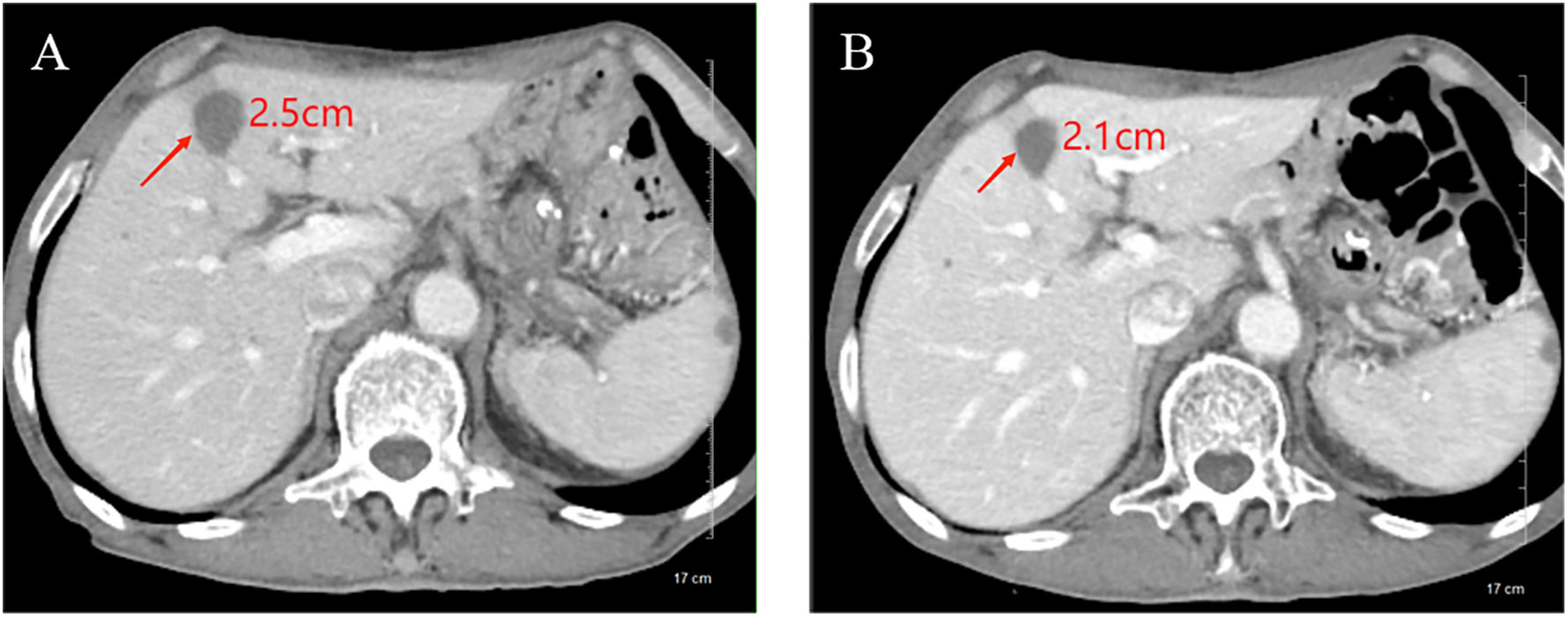
Figure 1. Comparison of longest diameter of liver metastases on CT images before and after chemotherapy plus ICI therapy. (A) Before treatment, the longest diameter of liver metastases was about 2.5cm; (B) After treatment, the longest diameter of liver metastases was reduced to 2.1cm.
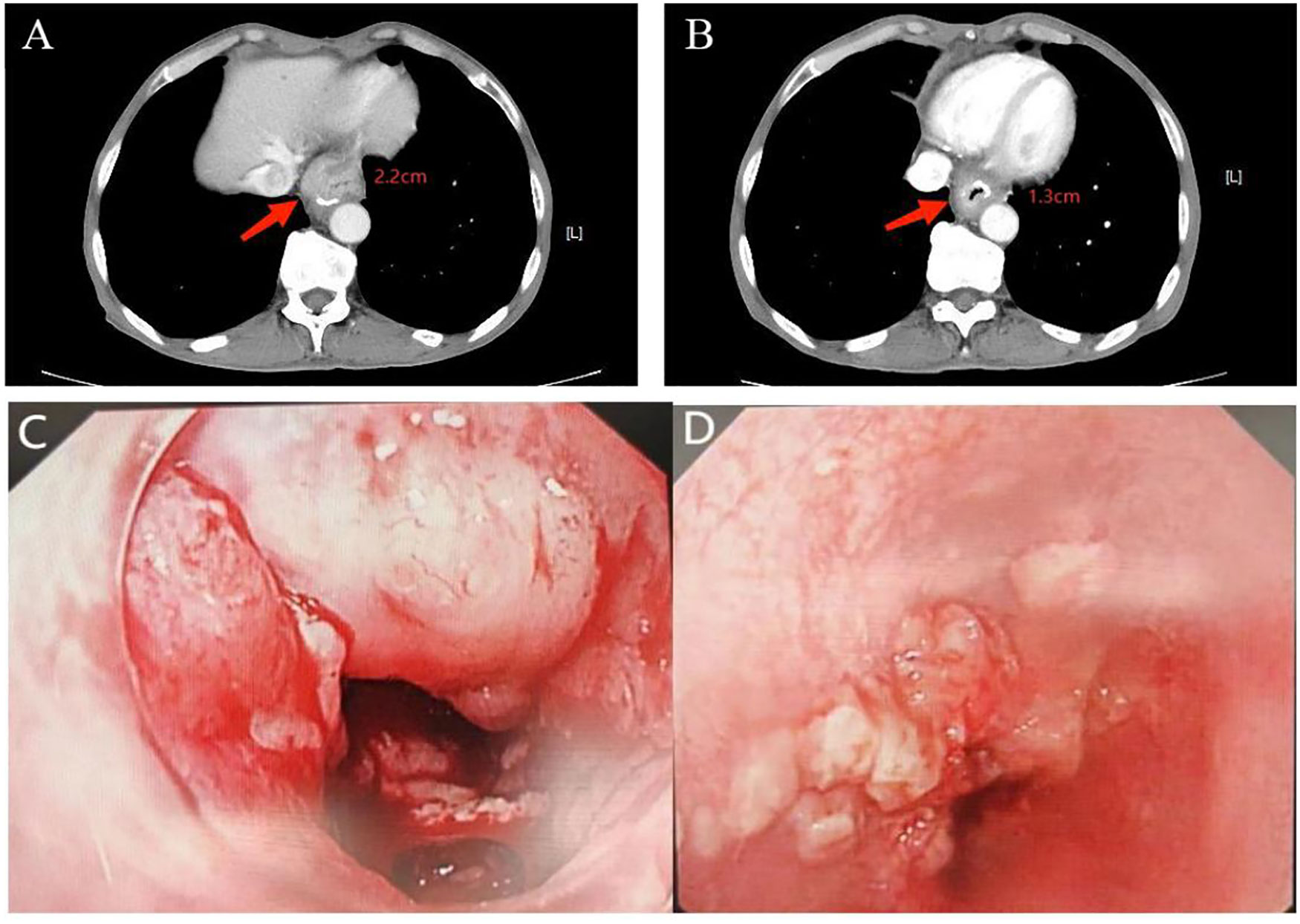
Figure 2. Comparison of cardia lesion thickness on CT and gastroscopy images before and after H101 treatment. (A) Before H101 treatment, the thickest part of the cardia lesion was about 2.2cm; (B) After H101 treatment, the thickest part was significantly reduced to 1.3cm; (C) Before H101 treatment, gastroscopy showed that the cardia lesions were large and protruding into the cavity; (D) After H101 treatment, gastroscopy showed that the cardia lesions were significantly reduced and the local stenosis was significantly improved.
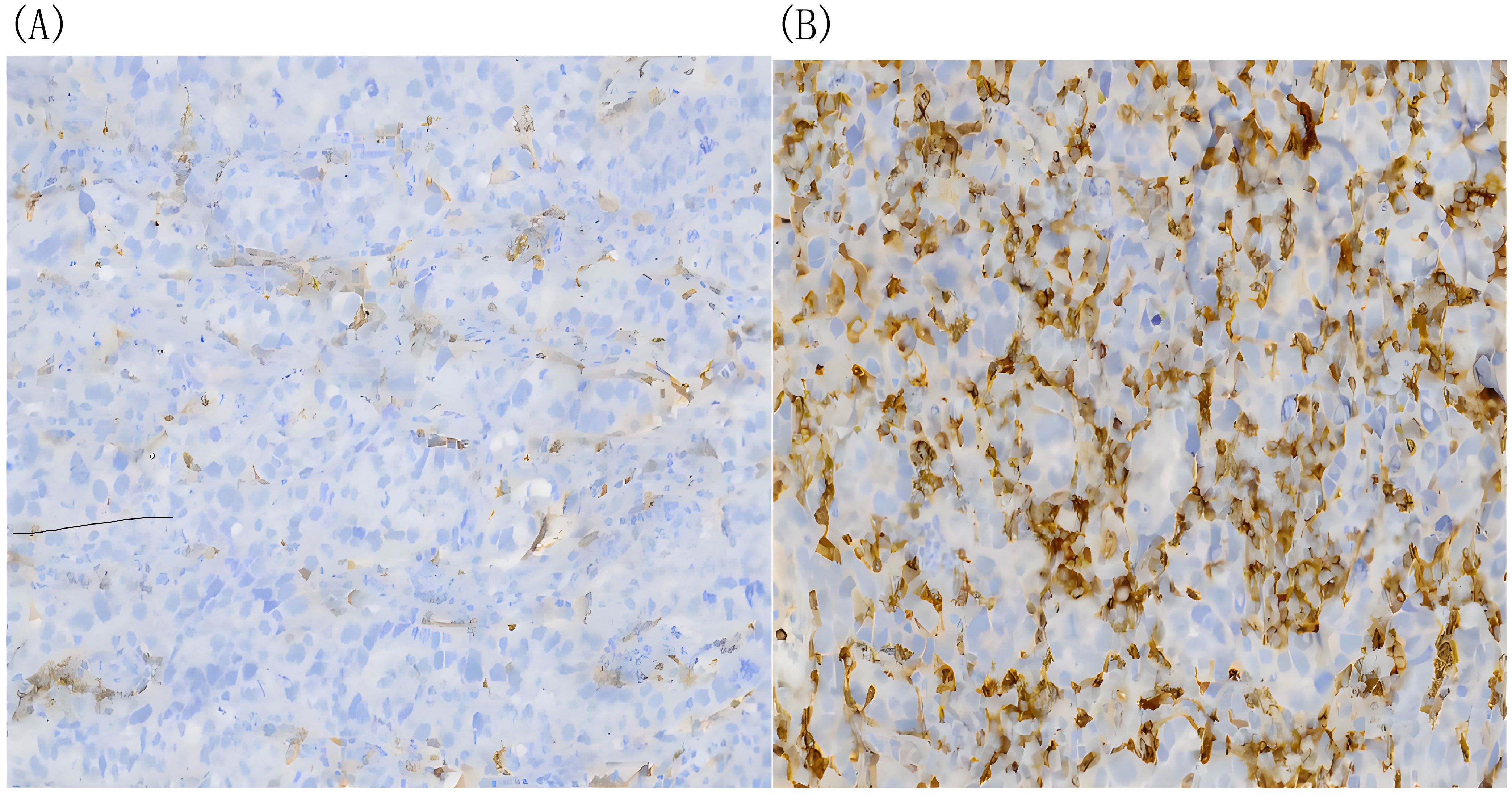
Figure 3. The density of infiltrating CD4+T cells tested by immunohistochemical DAB staining. (A) Before H101 treatment, the density of infiltrating CD4+ T cells in cardia lesion is low(-);(B) After H101 treatment, the density of infiltrating CD4+ T cells increased significantly(+++). Reagent information: Rabbit monoclonal to CD4, Product code: ab133616, Abcam (Shanghai) Trading Co. LTD. Quantitative method: The percentage of artificially counted positive cells in all cells of 10 times microscope field: Count the proportion of positive cells accurately located by IHC markers in the total cells and score (using nucleus as the cell localization standard). The standard is as follows: 25% of the total cell number is (-), 25%-50% is (+), 50-75% is (++), and more than 75% is (+++).
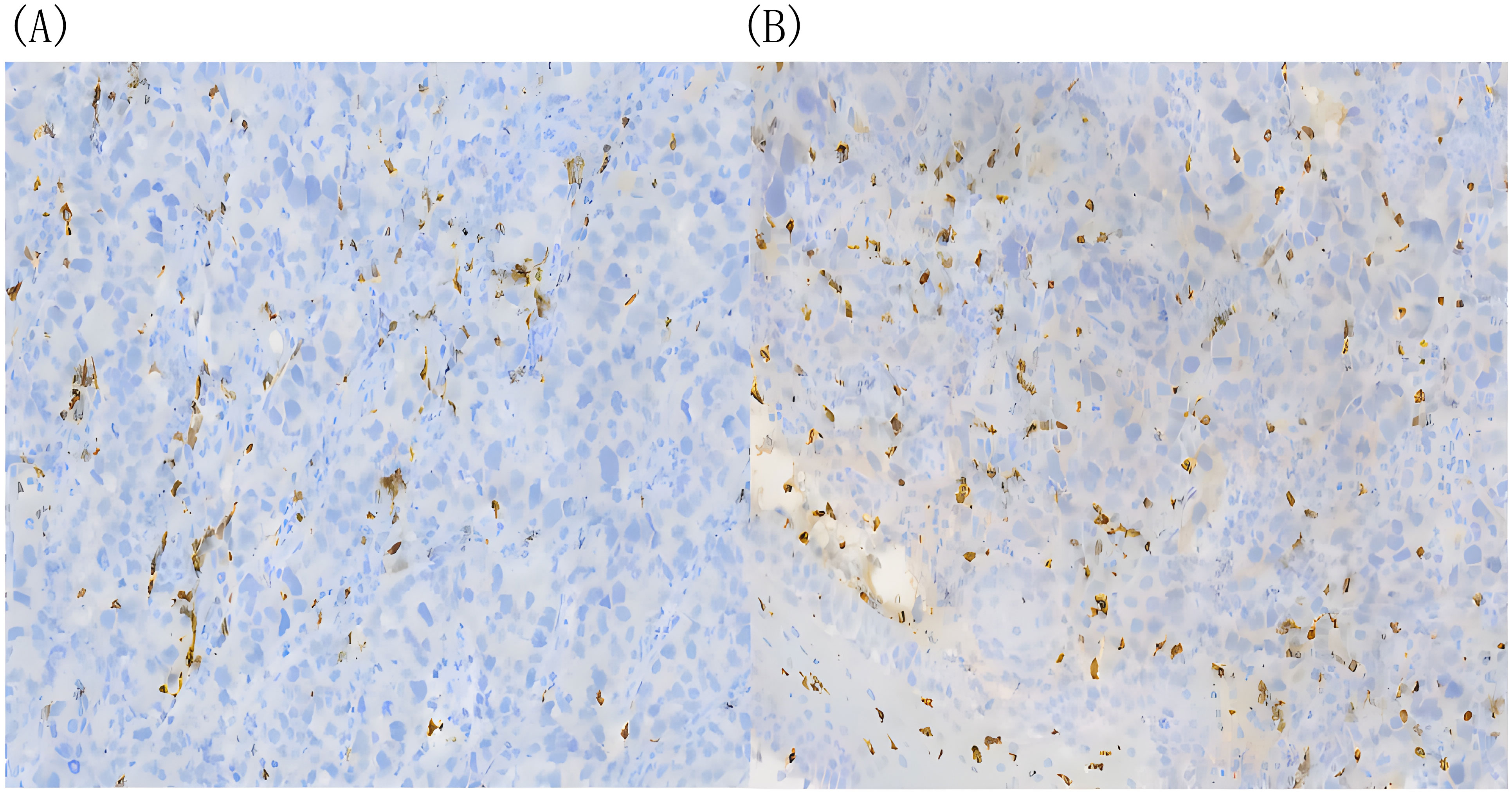
Figure 4. There was no significant difference in infiltrating CD8+ T cells before and after H101 treatment. (A) Before H101 treatment, the density of infiltrating CD8+ T cells in cardia lesion is low(+);(B) After H101 treatment, the density of infiltrating CD8+ T cells increased slightly (+). Reagent information: Rabbit monoclonal to CD8, Product code: ab217344, Abcam (Shanghai) Trading Co. LTD.
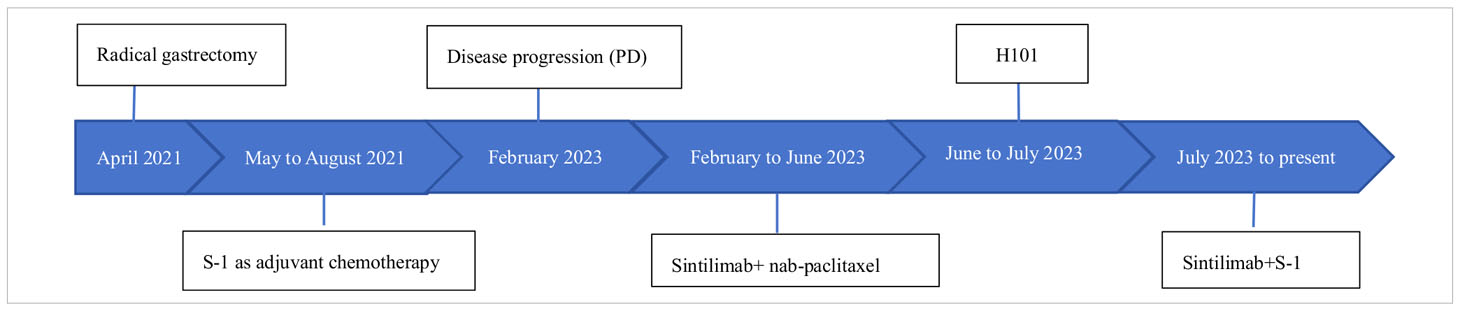
Table 1. This table provides detailed information about the patient’s medical history, including the timeline of treatments.
Discussion
Here, We present a case involving an individual diagnosed with advanced cardia cancer who exhibited favorable responses to a combination therapy involving oncolytic virus, chemotherapy, and immunotherapy. We innovatively treated cardia cancer by local injection of oncolytic virus through gastroscopy and effectively reversed immune resistance. Following H101 treatment, the patient experienced notable reduction in cardia lesions, improvement in eating obstruction, and modest activation of the local tumor immune microenvironment, fostering infiltration of CD4+ T cells. Research in cancer immunotherapy has mainly focused on CD8+ T cells in the tumor microenvironment (TME), However, it has been reported that antitumor immunity cannot be induced unless the tumor cells have the MHC class II binding neoantigens, which are recognized by CD4+ T cells. CD4+ T cells are likely to be the driving force of the cancer immunity cycle, allowing for a continuous supply of cytotoxic lymphocytes(CTLs) to the TME. Additionally, studies also have demonstrated that after oncolytic viruses infect tumor cells, they induce cell lysis and the release of tumor antigens and other immunostimulatory molecules. While this process can trigger an immune response, these antigens and stimulatory factors may predominantly recruit and activate helper T cells (CD4+ T cells) rather than cytotoxic T cells (CD8+ T cells). CD4+ T cells play a crucial role in coordinating subsequent immune responses by secreting cytokines (9–11). This conclusion is consistent with our test results. No significant infiltration of CD8+T cells was observed after H101 treatment, possibly due to the deviation of the biopsy specimen location from the injection site, and lack of enough tissue samples to fully observe. The deeper reason may be due to the special characteristics of oncolytic virus to change the TME. Moreover, subsequent to the localized administration of H101, we observed effective control over distant liver metastases, thereby further substantiating the synergistic potential of oncolytic viruses in conjunction with immunotherapy.
Oncolytic viruses (OVs) represent a widely employed strategy for localized tumor treatment. Engineered to selectively target and destroy tumor cells while sparing normal tissue, they hold promise in cancer therapy. However, their clinical application remains constrained. After the approval of the herpes virus-based drug T-VEC by the US Food and Drug Administration (FDA) in 2015, other OVs such as adenovirus, coxsackie virus, and measles virus have since entered clinical investigation (12, 13). In a recent phase II clinical study, H101 also found to trigger a proliferative burst of CXCR6+ and GZMK+CD8+ T cells in malignant ascites (14). Nonetheless, OVs alone often fail to elicit robust therapeutic responses against malignant tumors, encountering obstacles like limited tumor penetration, constraints associated with local drug delivery, and pre-existing immune responses against the virus (15). These challenges underscore the need for innovative approaches to enhance the efficacy of OVs in cancer treatment.
To further enhance therapeutic efficacy, numerous genetically engineered oncolytic viruses have undergone development and clinical trials. A phase 1/2 clinical investigation demonstrated the feasibility and safety of LOAd703, an oncolytic adenovirus carrying transgenes encoding TMZ-CD40L and 4-1BBL, in combination with nab-paclitaxel and gemcitabine for treating patients with advanced pancreatic ductal adenocarcinoma (16). Additionally, efforts to diminish the extracellular matrix and facilitate oncolytic virus diffusion within tumors have led to modifications incorporating hyaluronidase or relaxin into oncolytic viruses, showing promising efficacy in preclinical studies (14, 17). In addition, to bolster dendritic cell maturation, GM-CSF fragments are integrated into viral genes to further stimulate immune activation (18).
Presently, immune checkpoint inhibitors demonstrate substantial effectiveness against advanced solid tumors (5, 19). Nonetheless, a majority of patients do not derive benefit, likely attributed to the heterogeneous nature of the tumor immune microenvironment. An expanding body of research indicates that modifying the tumor immune microenvironment holds promise for augmenting the effectiveness of immune checkpoint inhibitors and overcoming immune resistance (20, 21). Oncolytic viruses offer a compelling approach by efficiently lysing tumor cells to release antigens, inducing immunogenic cell death, fostering immune cell infiltration, and bolstering anti-tumor immunity (22). A preclinical study showed that oncolytic parapoxvirus ovis can induce GasderminE-mediated pyroptosis and activate anti-tumor immunity, adding new evidence for oncolytic viruses to stimulate anti-tumor immunity (23). A phase II trial found that H101 can trigger a proliferation burst of CXCR6+ and GZMK+CD8+T cells in malignant ascites, enhancing tumor-specific T cell cytotoxicity (14). Furthermore, biopsy results from another phase II trial, following treatment with a triple-mutated, third-generation oncolytic herpes simplex virus type 1, revealed an increase in tumor-infiltrating CD4+/CD8+ lymphocytes, while the count of Foxp3+ cells remained low (24). These findings suggest the effective activation of anti-tumor immunity by oncolytic viruses.
However, investigations into combining oncolytic viruses with immunotherapy for solid tumors predominantly reside in Phase 1/2 clinical trials, necessitating additional trials to conclusively establish the efficacy of this combination. This study underscores that oncolytic viruses, functioning as localized treatments, can manage primary lesion reduction and immune activation. Immunotherapy, serving as a systemic approach, can potentiate the cytotoxicity of immune cells and efficiently control lesions.
Conclusion
We present a case study of a patient diagnosed with cardia cancer who underwent local administration of oncolytic virus H101 following ineffective treatment with paclitaxel combined with PD-1 inhibitors. Remarkably, this approach exhibited promising efficacy and immune-stimulating effects, offering insights into the selection of subsequent-line immunotherapy for cardia cancer. This finding unveils further therapeutic avenues for exploration.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Changzhou Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft. MX: Data curation, Investigation, Supervision, Writing – review & editing. JM: Data curation, Formal analysis, Investigation, Software, Writing – review & editing. YB: Investigation, Supervision, Validation, Writing – review & editing. CF: Software, Methodology, Writing – review & editing. QG: Formal analysis, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zeng H, Ran X, An L, Zheng R, Zhang S, Ji JS, et al. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health. (2021) 6:e877–e87. doi: 10.1016/S2468-2667(21)00157-2
2. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London England). (2017) 390:2461–71. doi: 10.1016/S0140-6736(17)31827-5
3. Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu M-H, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet (London England). (2018) 392:123–33. doi: 10.1016/S0140-6736(18)31257-1
4. Shitara K, van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee K-W, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:1571–80. doi: 10.1001/jamaoncol.2020.3370
5. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (London England). (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
6. Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. (2017) 170:1109–19.e10. doi: 10.1016/j.cell.2017.08.027
7. Wang L, Chard Dunmall LS, Cheng Z, Wang Y. Remodeling the tumor microenvironment by oncolytic viruses: beyond oncolysis of tumor cells for cancer treatment. J immunotherapy Cancer. (2022) 10:e004167. doi: 10.1136/jitc-2021-004167
8. Zhang R, Cui Y, Guan X, Jiang XJ. A recombinant human adenovirus type 5 (H101) combined with chemotherapy for advanced gastric carcinoma: A retrospective cohort study. Front Oncol. (2021) 9:752504. doi: 10.3389/fonc.2021.752504
9. Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. (2019) 574:696–701. doi: 10.1038/s41586-019-1671-8
10. Ferris ST, Durai V, Wu R, Theisen DJ, Ward JP, Bern MD, et al. cDC1 prime and are licensed by CD4(+) T cells to induce anti-tumour immunity. Nature. (2020) 584:624–9. doi: 10.1038/s41586-020-2611-3
11. Borst J, Ahrends T, Babala N, Melief CJM, Kastenmuller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. (2018) 18:635–47. doi: 10.1038/s41577-018-0044-0
12. Greig SL. Talimogene laherparepvec: first global approval. Drugs. (2016) 76:147–54. doi: 10.1007/s40265-015-0522-7
13. Zhang B, Huang J, Tang J, Hu S, Luo S, Luo Z, et al. Intratumoral OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: a multicenter, phase I/II clinical trial. J immunotherapy Cancer. (2021) 9:e002224. doi: 10.1136/jitc-2020-002224
14. Zhang Y, Qian L, Chen K, Gu S, Meng Z, Wang J, et al. Oncolytic adenovirus in treating Malignant ascites: a phase II trial and longitudinal single-cell study. Mol Ther. (2024) 32:2000–20. doi: 10.1016/j.ymthe.2024.04.029
15. Kiyokawa J, Kawamura Y, Ghouse SM, Acar S, Barçın E, Martínez-Quintanilla J, et al. Modification of extracellular matrix enhances oncolytic adenovirus immunotherapy in glioblastoma. Clin Cancer Res. (2021) 27:889–902. doi: 10.1158/1078-0432.CCR-20-2400
16. Musher BL, Rowinsky EK, Smaglo BG, Abidi W, Othman M, Patel K, et al. LOAd703, an oncolytic virus-based immunostimulatory gene therapy, combined with chemotherapy for unresectable or metastatic pancreatic cancer (LOKON001): results from arm 1 of a non-randomised, single-centre, phase 1/2 study. Lancet Oncol. (2024) 25:488–500. doi: 10.1016/S1470-2045(24)00079-2
17. Jung KH, Choi IK, Lee HS, Yan HH, Son MK, Ahn HM, et al. Oncolytic adenovirus expressing relaxin (YDC002) enhances therapeutic efficacy of gemcitabine against pancreatic cancer. Cancer Lett. (2017) 396:155–66. doi: 10.1016/j.canlet.2017.03.009
18. Zhu L, Huang J, Zhang S, Cai Q, Guo X, Liu B, et al. oHSV2-mGM repolarizes TAMs and cooperates with αPD1 to reprogram the immune microenvironment of residual cancer after radiofrequency ablation. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2024) 178:117060. doi: 10.1016/j.biopha.2024.117060
19. Qin S, Kudo M, Meyer T, Bai Y, Guo Y, Meng Z, et al. Tislelizumab vs sorafenib as first-line treatment for unresectable hepatocellular carcinoma: A phase 3 randomized clinical trial. JAMA Oncol. (2023) 9:1651–9. doi: 10.1001/jamaoncol.2023.4003
20. Meng Y, Ye F, Nie P, Zhao Q, An L, Wang W, et al. Immunosuppressive CD10(+)ALPL(+) neutrophils promote resistance to anti-PD-1 therapy in HCC by mediating irreversible exhaustion of T cells. J Hepatol. (2023) 79:1435–49. doi: 10.1016/j.jhep.2023.08.024
21. Xie D, Tian Y, Hu D, Yang Y, Hu Z, Mangold KA, et al. Oncolytic adenoviruses expressing checkpoint inhibitors for cancer therapy. Signal transduction targeted Ther. (2023) 8:436. doi: 10.1038/s41392-023-01683-2
22. Svensson-Arvelund J, Cuadrado-Castano S, Pantsulaia G, Kim K, Aleynick M, Hammerich L, et al. Expanding cross-presenting dendritic cells enhances oncolytic virotherapy and is critical for long-term anti-tumor immunity. Nat Commun. (2022) 13:7149. doi: 10.1038/s41467-022-34791-8
23. Lin J, Sun S, Zhao K, Gao F, Wang R, Li Q, et al. Oncolytic Parapoxvirus induces Gasdermin E-mediated pyroptosis and activates antitumor immunity. Nat Commun. (2023) 14:224. doi: 10.1038/s41467-023-35917-2
Keywords: cardia cancer, immune checkpoint inhibitors, recombinant human adenovirus type 5, CD4+ T cell, CD8+ T cell, immune microenvironment
Citation: Zhao Q, Xiao M, Ma J, Fu C, Gao Q and Bi Y (2024) Reverse resistance to immune checkpoint inhibitor in a patient with recurrent cardia cancer by intratumoral injection of recombinant human adenovirus type 5: a case report and literature review. Front. Oncol. 14:1465664. doi: 10.3389/fonc.2024.1465664
Received: 16 July 2024; Accepted: 10 September 2024;
Published: 11 November 2024.
Edited by:
Guohao Wang, National Institutes of Health (NIH), United StatesReviewed by:
Chao Quan, National Institute on Alcohol Abuse and Alcoholism (NIH), United StatesShufeng Sun, National Institutes of Health (NIH), United States
Huixian Zhou, Albert Einstein College of Medicine, United States
Copyright © 2024 Zhao, Xiao, Ma, Fu, Gao and Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanzhi Bi, Yml5YW56aGkyMjJAMTYzLmNvbQ==
 Qiu Zhao
Qiu Zhao Min Xiao1
Min Xiao1