
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 16 September 2024
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1465395
This article is part of the Research Topic Community Series in Reducing Adverse Effects of Cancer Immunotherapy: Volume II View all 18 articles
Background: Immune checkpoint inhibitors (ICIs) have become a prevalent tool in anti-tumor therapy in recent years. They may cause immune-related adverse events (irAEs) including potentially life-threatening cardiovascular toxicities such as myocarditis.
Case presentation: In this report, we describe a 69-year-old man with recurrent esophageal cancer who developed myocarditis after receiving three cycles of sintilimab combined with nab-paclitaxel. Despite a rising cardiac troponin I (cTnI), he initially reported no discomfort. He was later suspected of having with sintilimab-induced myocarditis. Although treatment with methylprednisolone reduced his cTnI levels, he still experienced significant discomfort. Moreover, he developed pneumonia and septic shock.
Conclusion: In our literature search to identify all reported cases of sintilimab-associated adverse events involving myocarditis, we found 14 patients, including those with esophageal cancer, thymoma, lung cancer, gastric cancer, hepatobiliary carcinoma, and chordoma. The primary treatment for ICI-induced cardiotoxicity is methylprednisolone. However, the long-term or high-dose use of steroids can also induce side effects, which have not been the focus of these case reports. This is the first reported case of asymptomatic immune-mediated myocarditis occurring during the treatment of esophageal cancer with sintilimab. It is also the first to address the side effects of methylprednisolone used in the treatment of sintilimab-related myocarditis. To facilitate an early diagnosis, regular monitoring is required during sintilimab treatment. We should also focus on the prevention and management of adverse effects related to steroid use.
Immune checkpoint inhibitors (ICIs) have emerged as promising anti-tumor therapies in recent years. However, they can cause distinct immune-related adverse events (irAEs), owing to the inhibition of immunological inhibitory mechanisms. Cardiovascular irAEs are more common than were initially reported in early ICI clinical trials (1). ICIs induced cardiovascular toxicities, including myocarditis, pericarditis, arrhythmias, cardiomyopathy, and acute coronary syndrome. Of these, myocarditis has been the most extensively studied due to its incidence and severity. The mortality attributed to ICI-myocarditis ranges from 36% to 67% (2–4). Sintilimab, a programmed cell death-1 (PD-1) inhibitor, has been approved for various cancers in China, including esophageal cancer. We searched for reports on sintilimab-related adverse events involving myocarditis (5–18), identifying 14 patients. The primary strategy for managing ICI-induced cardiotoxicity is immunosuppressive therapy (6). However, long-term or high doses of steroid use can also induce side effects, which were outside the scope of these case reports. Herein, we reported a 69-year-old man with recurrent esophageal squamous cell carcinoma, who received sintilimab combined with nab-paclitaxel for 3 cycles. His cardiac troponin I (cTnI) continued to increase significantly; however he had not complained of any discomfort. After treatment with methylprednisolone, his cTnI levels decreased, but he experienced multiple discomforts, such as leg cramps, weakness while walking, sore throat, elevated blood glucose, and even he developed a septic shock. This is the first reported case of asymptomatic immune-mediated myocarditis occurring during the treatment of esophageal cancer with sintilimab. It is also the first report to address the side effects caused by methylprednisolone in the treatment of sintilimab-related myocarditis. During the sintilimab treatment phase, regular monitoring is essential for early diagnosis. Additionally, managing and preventing the side effects associated with steroid should use should also be our primary focuses.
A 69-year-old man with hypertension and diabetes presented at our hospital in June 2021, reporting dysphagia that had started two months earlier. External pathological examination revealed esophageal squamous cell carcinoma (ESCC). He underwent surgery in mid-June 2021, which confirmed the diagnosis of ESCC, staged at T3N1M0. He received four cycles chemotherapy with nab-paclitaxel (CSPC, OUYI, Pharmaceutical Co, Ltd) and nidaptin (Nanjing Xiansheng Dongyuan Pharmaceutical Co., Ltd) from August to October 2021. In February 2023, during a follow-up visit to the hospital, a positron emission tomography/computed tomography (PET/CT) scan showed a soft tissue mass adjacent to the left side of the esophageal hiatus and above the retroperitoneal abdominal trunk, exhibiting increased fluorodeoxyglucose (FDG) metabolism. This indicated a potential tumor recurrence or metastasis, possibly affecting the abdominal trunk (Figure 1A). From March to April 2023, he received three cycles of nab-paclitaxel (300 mg at Day 1), and sintilimab (Innovent Biologics Inc.) (200mg at Day 1).
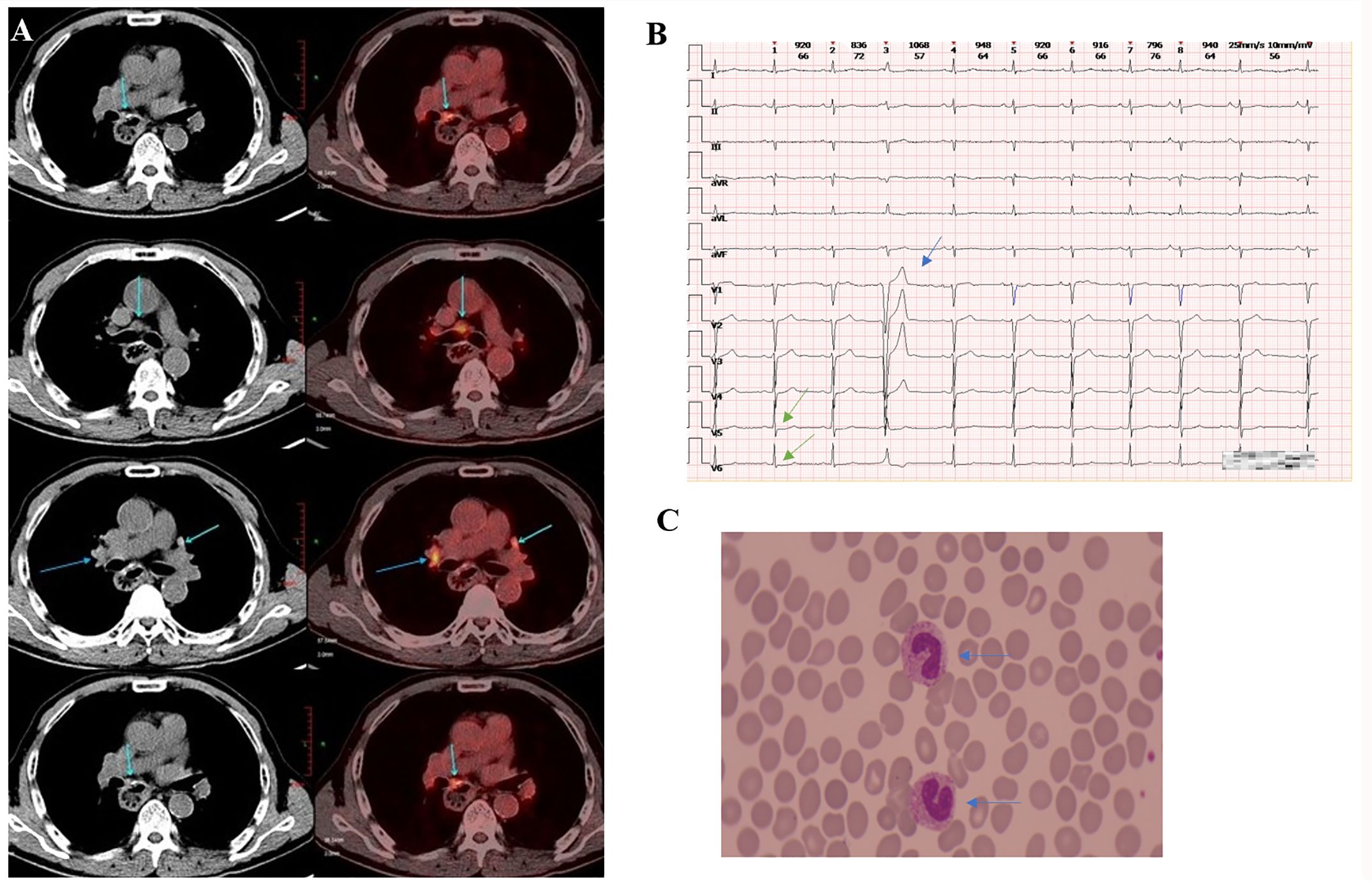
Figure 1. Clinical data of patients. (A) The PET/CT scan (taken in late February 2023) showed a soft tissue mass adjacent to the left side of the esophageal hiatus and above the retroperitoneal abdominal trunk, exhibiting increased FDG metabolism. (B) The ECG (taken in early June 2023) revealed mild ST segment depression in leads V5 and V6 (marked by the green arrow) and T-wave changes (indicated by the blue arrow), premature atrial contractions, short strands of atrial tachycardia, and premature ventricular contractions. (C) The blood smear examination (taken in late June, 2023) indicates a left shift in neutrophil nuclei.
When the patient was scheduled for his fourth cycle of treatment in late May 2023, the baseline cTnI monitoring revealed an abnormal elevation (cTnI 1.85μg/L, normal ≤0.050μg/L). Other cardiac markers, including creatine kinase (CK), B-type natriuretic peptide (BNP), lactate dehydrogenase (LDH), and D-dimer were normal. The electrocardiogram (ECG) indicated a sinus rhythm with a left deviation of electrical axis -35° and low QRS complex voltage in limb leads. An echocardiography showed mild aortic regurgitation, moderate mitral and tricuspid regurgitation, with no abnormalities in left ventricular diastolic and systolic function. The following day his cTnI significantly increased to 4.145μg/L. The patient had not complained of any discomfort. He was administered glucocorticoids (methylprednisolone 40 mg/day intravenous) for 3 days. However, his cTnI level further increased to 6.384μg/L. He declined the cardiac magnetic resonance (CMR) as he did not experience any heart-related symptoms. The dose of methylprednisolone was increased to 120 mg/day and administered for 3 days. Subsequently, the cTnl level decreased to 3.013μg/L but later increased to 4.904μg/L. A repeat ECG revealed mild ST-segment depression and T wave changes, premature atrial contractions, short strands of atrial tachycardia, and premature ventricular contractions (Figure 1B). An echocardiography indicated an ejection fraction (EF) of 60% and left atrial enlargement. Concurrently, the patient began experiencing chest tightness.
After ruling out immune pneumonia and acute coronary events, and considering the correlation between the sintilimab use and the examination results, a cardiology consultation strongly suspected sintilimab-induced myocarditis. High-dose steroids, specifically methylprednisolone pulse dosing 1000 mg/day, was administered intravenously for 3 days. The dose was then tapered to 120 mg/day for 4 days, 80mg/day for 2 days, and finally 40mg/day. To prevent infection, compound sulfamethoxazole tablets (SMZ) 0.96g were given orally every 12 hours (q12h). His cTnI decreased to 0.169 μg/ L (Figure 2A). During this period, he experienced cramps in his lower limbs, unsteady walking, sore throat and hyperglycemia.

Figure 2. Changes in biochemical markers. (A) Levels of Troponin I before and after methylprednisolone treatment. When the MPS dose was adjusted to 120mg/d, cTnI showed a brief decrease, but then increased again. When the MPS dose was 1000mg/d, it was found that cTnI significantly decreased, and as the MPS dose decreased, cTnI did not rebound and gradually returned to normal levels. (B) Levels of PCT and CRP before and after antibiotic therapy. With the adjustment of antibiotics, the inflammatory markers CRP and PCT of the patient gradually decreased. However, discontinuation of medication during discharge led to a further increase in inflammatory markers, which were reduced when antibiotics were reused. cTnI, cardiac troponin I; MPS, methylprednisolone; d, day. PCT, procalcitonin; CRP, C-reactive protein; po, per os; q8h, every 8 hours; q12h, every 12 hours; SMZ, compound sulfamethoxazole tablets; ETP, ertapenem; TIENAM, imipenem and cilastatin sodium; MEPM, meropenem; SCF, sulbactam and cefoperazone; TZP, piperacillin sodium and tazobactam sodium; FCZ, fluconazole.
On Day 16 of methylprednisolone treatment (the 22nd day of hospitalization), the patient developed a fever of 39.9°C with chills, and was subsequently treated with ertapenem 1g. The next day, he had persistent fever (38.1°C) and tachypnea. Labs showed leukocytosis (white blood cells of 25.01x10^9/L), neutrophilia (24.73x10^9/L), elevated C-reactive protein (CRP) (140mg/L), increased procalcitonin (PCT) (15.57ng/ml), reduced PaO2/FiO2 (163.5 mmHg), and high lactic acid (2.8 mmol/L). CT scan indicated scattered lung inflammation. We initiated treatment with imipenem and cilastatin sodium (TIENAM) at 1g every 8 hours (q8h). Subsequently, the patient experienced fatigue and his blood pressure dropped to 80/40mmhg. Considering these symptoms indicative of septic shock, we immediately started pressor support and fluid resuscitation. He was then transferred to the infection department for continued treatment. The blood smear indicated infection, evidenced by left shift of nucleus (Figure 1C). The patient was treated with meropenem 1 g q12h for 7 days, and with sulbactam and cefoperazone (SCF) 2 g q12h for 5 days. His PCT level decreased to 0.12ng/ml, and his CRP decreased to 17.6mg/L (Figure 2B). The patient’s symptoms improved, and vital signs were relatively stable. He was discharged after 32 days of hospitalization. However, the patient was readmitted to the hospital 12 days after his discharge due to oral pain and fever, where he was diagnosed with pneumonia and oral candidiasis. Fluconazole was administered for gargling. He received piperacillin sodium and tazobactam sodium (TZP) 4.5g q8h for 3 days, followed by TIENAM 1g q8h for 5 days. He was discharged after 8 days of admission. After more than one month of follow-up, the patient reported no significant discomfort. Figure 3 provides a summary the treatment for this patient.

Figure 3. The treatment chart of the patient. nab-PTX, nab-paclitaxel; NDP, Nedaplatin; c, cycle; PET/CT, positron emission tomography/computed tomography; ICI, immune checkpoint inhibitors; MPS, methylprednisolone; d, day; po, per os; q8h,every 8 hours;q12h,every 12 hours;SMZ, compound sulfamethoxazole tablets; ETP, ertapenem; TIENAM, imipenem and cilastatin sodium; MEPM, meropenem; SCF, sulbactam and cefoperazone;TZP, piperacillin sodium and tazobactam sodium; FCZ, fluconazole.
From the patient’s perspective, the adverse events (AEs) associated with ICI did not cause significant discomfort, unlike the side effects of methylprednisolone, which notably distressed him. Prompt anti-infection measures, along with attentive care from the medical staff, helped establish a rapport with him, contributing significantly to alleviating his anxiety.
In recent years, ICIs for cancer have emerged as a potentially powerful therapeutic approach. Sintilimab is a recombinant human immunoglobulin G4 monoclonal antibody that targets PD-1. According to the ORIENT-15 trial (19), sintilimab when used in combination with chemotherapy, demonstrated significant advantages in both overall survival and progression-free survival for patients with ESCC. ICIs, however, carry the risk of disrupting immunological balance, leading to the development of autoreactive T cells and other irAEs. In the report (19), the most frequent adverse events related to treatment and classified as grade 3 or worse included a decrease in neutrophil count, decrease in white blood cell count, anaemia, and hypokalemia. Notably, immune myocarditis occurred in 1 case (0.3%) of the placebo-chemotherapy group, not in sintilimab-chemotherapy group (19). Although cardiovascular toxicity is a rare irAE, it has been reported frequently with the widespread use of ICIs. ICIs induce cardiovascular toxicities, including myocarditis, pericarditis, arrhythmias, cardiomyopathy, and acute coronary syndrome. Because of its onset and severity, myocarditis has been the most extensively investigated. The mortality rate attributed to ICIs-myocarditis is high, ranging from 36% to 67% (2–4).
The pathogenesis of ICI-associated myocarditis is currently unknown. Won et al. generated a clinically relevant mouse model for ICI-associated myocarditis by administering an αPD-1 monoclonal antibody (mAb). They found that the number and activity of cardiac-myosin-specific T cells were increased in these mice, indicating a contribution of heart autoantigen-reactive T cells to ICI-associated myocarditis. PD-1-expressing cardiac-myosin-specific T cells are present in the heart during naive conditions, and these T cells are under control by the PD-1/PD-L1 pathway in naive conditions, leading to the development of myocarditis (20). The study conducted by Matsumori revealed that the disruption of the PD-1 receptor, an inhibitory receptor found on T cells, resulted in fatal myocarditis in mice (21).
We conducted a comprehensive search of PubMed to identify all reported cases of sintilimab-associated myocarditis, as detailed in references (5–18). After reviewing all cases and excluding duplicate reports, the clinical features, treatment, and outcomes of these patients were compiled in Table 1. Among the 14 patients, 3 had thymoma. 6 had non-small cell lung cancer, 1 had gastric cancer.1 had extrahepatic cholangiocarcinoma, 1 had hepatocellular carcinoma, 1 had chordoma and 1 had esophageal cancer. Despite the report by Hong et al. (18), a patient with esophageal cancer treated with sintilimab developed inflammatory myopathy, which also exhibited signs of myocarditis. The immune inflammation reported in their case involves multiple areas. Our patient, however, had asymptomatic myocarditis, and although the course of the disease was potentially dangerous, its onset was insidious. Among the reported cases, some primarily presented with extensive myositis (8, 10, 17, 18). Additionally, several cases were associated with myasthenia gravis (6, 8, 10, 15). Furthermore, one case was complicated by autoimmune hepatitis (11), while another had rhabdomyolysis (17).Immune myocarditis can occur at any time during sintilimab therapy, with the longest interval (5) being 4 months after 5 courses, and the shortest (10) being 1 week after 1 course. However, most cases developed myocarditis after the first course of sintilimab administered (6–8, 10–15). In our case, immune myocarditis occurred 3 weeks after 3 cycles.
The clinical manifestations of ICI-induced myocarditis may include fatigue, muscle weakness, myalgias, chest pain, diplopia, ptosis, shortness of breath, orthopnoea, lower extremity oedema, palpitations, lightheadedness/dizziness, syncope, and cardiogenic shock (1). However, the patient in this case report did not exhibit these typical signs, similar to some case reports (9, 13). cTnI is a specific and sensitive biomarker for myocardial injury. A positive troponin test, with troponin I or T levels exceeding the 99th percentile of the upper reference limit, may indicate a cardiovascular irAE (1). In our case, the patient’s cTnI levels were significantly elevated. Given the patient’s lack of previous heart issues but a history of ICI therapy, we strongly suspected ICI-induced myocarditis after ruling out acute myocardial infarction, pulmonary embolism and aortic dissection. Although nab-paclitaxel was used in combination with sintilimab, myocarditis caused by nab-paclitaxel is rare (22, 23).
Myocarditis can present with variable and complex clinical symptoms, making it a diagnostic challenge (24). Patients who have started ICI therapy within the past 8 weeks, have experienced one or more non-cardiac irAEs, and exhibit troponin elevations with atypical trends should be suspected of having myocarditis (24). The diagnosis is suspected based on new cardiac symptoms, new ECG changes, and/or a new increase in troponin levels. Cardiovascular imaging should be performed to confirm cardiac dysfunction and exclude other possible causes (24, 25). Despite being the gold standard for diagnosing ICI-associated myocarditis, endomyocardial biopsy is rarely performed due to the intrusive nature of the procedure and the heightened risk of heart perforation. CMR is considered the premier non-invasive imaging method for myocarditis diagnosis. However, in cases with ICI myocarditis, its sensitivity might be diminished (1). Some scholars also believe that novel biomarkers, including inflammatory and myocardial injury biomarkers, need to be considered in the early diagnosis of myocarditis (21). Regrettably, our patient opted against undergoing the CMR as he didn’t experience any discomfort.
According to the ASCO Guideline (26), our patient falls into the G2 category of Cardiovascular Toxicities. The guideline specifies that in cases of G2 or higher toxicity, immunotherapy with ICIs should be held, and treatment should be discontinued (26). Therefore, the patient discontinued sintilimab treatment. For patients presenting with grade≥ 2 cardiovascular toxicities, the early initiation of high-dose corticosteroids (1-2 mg/kg/d of prednisone) is advisable, as it is likely to be beneficial without AEs (26). Initially, we administered methylprednisolone 40 mg/day for 2 days to the patient, but there was still increase an cTnI levels. Subsequently, the dosage of methylprednisolone was adjusted to 120 mg/day for 4 days. This led to a short-term decline in cTnI levels, which subsequently rose again, accompanied by changes in the patient’s electrocardiogram. According to guidelines, for myocarditis, an intravenous dose of 1000 mg/day of methylprednisolone should be prescribed (1). For patients unresponsive to hormone treatment, the recommendation of other immunomodulatory drugs is advised (1). Consequently, we introduced high-dose steroids with methylprednisolone pulse dosing 1000 mg/day intravenous for 3 days, after which the dose was gradually decreased. The patient’s cTnI levels gradually decreased. However, this was accompanied by the development of several new conditions, including side effects caused by methylprednisolone such as walking weakness, hyperglycemia, oral candidiasis, pneumonia, and even septic shock. In the review of these case reports (5–18), there was rare mention of the side effects associated with methylprednisolone or other immunomodulatory drugs.
For patients needing long-term steroids, especially the elderly, diabetes or immunocompromised, it’s vital to prevent opportunistic infections and implement proactive strategies to manage toxicity (26). Prophylaxis against Pneumocystis jirovecii pneumonia (PJP) should be considered for patients undergoing treatment with a prednisone equivalent of ≥ 20 mg/d for four or more weeks or ≥ 30 mg for three weeks or more. We administered SMZ to the patient on the first day of initiating 120 mg/d methylprednisolone to prevent PJP. However, the patient still experienced pulmonary infections and septic shock. Septic shock, a potentially life-threatening condition, occurs when sepsis leads to cellular metabolism abnormalities and abnormalities low blood pressure. The patient exhibited symptoms including fever, elevated heart rate, tachypnea, leukocytosis, elevated plasma CRP and PCT, arterial hypotension, arterial hypoxemia, and hyperlactatemia, leading to a diagnosis of septic shock (27). The mortality rate of severe sepsis and septic shock is currently approximated at 20 to 30%. Inappropriate or delayed antibiotic treatment correlates with increased mortality (27). Fortunately, timely intervention, including antibiotics, led to patient improvement. However, post-discharge, the patient experienced recurrent fever, pneumonia, and oral candidiasis. Managing steroid-related complications requires a long-term, comprehensive approach. Decision-making should involve a multidisciplinary strategy and consider institutional guidelines.
We present a case of a male patient with recurrent esophageal cancer, who, after receiving sintilimab combination with chemotherapy, subsequently developed immune-related myocarditis. While methylprednisolone treatment ameliorated the myocarditis, it concurrently induced a series of infections. To our knowledge, this report is notable for being the first to document asymptomatic immune myocarditis during the sintilimab treatment of esophageal cancer. It is also the first report to address the side effects of methylprednisolone during the treatment of sintilimab-related myocarditis. However, our report is not without limitations. Primarily, we did not employ CMR to detect myocardial abnormalities. Secondly, initially, the dosage of methylprednisolone administered was that prescribed for cardiovascular toxicities rather than specifically for myocarditis, resulting in a suboptimal initial dose.
In summary, myocarditis caused by sintilimab is not completely controllable. We should be cautious about ICI-induced myocarditis. For an early diagnosis, routine monitoring is required during treatment. If confirmed myocarditis occurs, intravenous methylprednisolone should be prescribed aggressively and sufficiently. We should also focus on the prevention and management of adverse effects related to steroid use. Meanwhile, more clinical instances must be encountered to gain additional experience.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the committee of Zhejiang Provincial People’s Hospital (QT2024017). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
QZ: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. ZQ: Conceptualization, Data curation, Writing – review & editing. GW: Investigation, Supervision, Writing – review & editing. PY: Supervision, Writing – review & editing. QW: Formal analysis, Writing – review & editing. JQ: Formal analysis, Writing – review & editing. JJ: Funding acquisition, Writing – review & editing. DY: Conceptualization, Data curation, Methodology, Writing – review & editing, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Zhejiang Province Public Welfare Technology Application Research Project of China No. LGF22H160080. Basic Scientific Research Funds of Department of Education of Zhejiang Province (No. KYQN202128).
We thank the patient for providing the necessary medical history required for this case report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Thuny F, Naidoo J, Neilan TG. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur Heart J. (2022) 43:4458–68. doi: 10.1093/eurheartj/ehac456
2. Cozma A, Sporis ND, Lazar AL, Buruiana A, Ganea AM, Malinescu TV, et al. Cardiac toxicity associated with immune checkpoint inhibitors: A systematic review. Int J Mol Sci. (2022) 23:10948. doi: 10.3390/ijms231810948
3. Brumbaugh AD, Narurkar R, Parikh K, Fanucchi M, Frishman WH. Cardiac immune-related adverse events in immune checkpoint inhibition therapy. Cardiol Rev. (2019) 27:97–107. doi: 10.1097/crd.0000000000000217
4. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. (2018) 391:933. doi: 10.1016/s0140-6736(18)30533-6
5. Zheng S, Zhang H, Hu B, Zhou J, Wen L, Li M. A case of acute myocarditis induced by pd-1 inhibitor (Sintilimab) in the treatment of large cell neuroendocrine carcinoma. Heliyon. (2023) 9:e16874. doi: 10.1016/j.heliyon.2023.e16874
6. Wang C, Zhong B, He J, Liao X. Immune checkpoint inhibitor sintilimab-induced lethal myocarditis overlapping with myasthenia gravis in thymoma patient: A case report. Medicine. (2023) 102:e33550. doi: 10.1097/md.0000000000033550
7. Liu X, Zeng Z, Cao J, Li X, Muhetaer M, Jin Z, et al. Sintilimab-induced myocarditis in a patient with gastric cancer: A case report and literature review. J Cardiovasc Dev Dis. (2023) 10:422. doi: 10.3390/jcdd10100422
8. Lin X, Guan W, Li B, Deng H, Chen Y, Yang Y, et al. A case report and literature review on respiratory failure with immune checkpoint inhibitors: A life-threatening adverse event. Immunopharmacol immunotoxicology. (2023) 45:780–7. doi: 10.1080/08923973.2023.2228480
9. Hu Y, Liu C, Jin S, Yi Z, Wang C, Pan X, et al. A case of subclinical immune checkpoint inhibitor-associated myocarditis in non-small cell lung cancer. BMC pulmonary Med. (2023) 23:119. doi: 10.1186/s12890-023-02417-4
10. Yin B, Xiao J, Wang X, Li X, Guan Y, Chen J, et al. Myocarditis and myositis/myasthenia gravis overlap syndrome induced by immune checkpoint inhibitor followed by esophageal hiatal hernia: A case report and review of the literature. Front Med. (2022) 9:950801. doi: 10.3389/fmed.2022.950801
11. Liu S, Ma G, Wang H, Yu G, Chen J, Song W. Severe cardiotoxicity in 2 patients with thymoma receiving immune checkpoint inhibitor therapy: A case report. Medicine. (2022) 101:e31873. doi: 10.1097/md.0000000000031873
12. Lin Y, Yuan X, Chen L. Immune myocarditis related to sintilimab treatment in a patient with advanced lung adenocarcinoma: A case report. Front Cardiovasc Med. (2022) 9:955527. doi: 10.3389/fcvm.2022.955527
13. Ji H, Wen Z, Liu B, Chen H, Lin Q, Chen Z. Sintilimab induced iciam in the treatment of advanced hcc: A case report and analysis of research progress. Front Immunol. (2022) 13:995121. doi: 10.3389/fimmu.2022.995121
14. Yang ZX, Chen X, Tang SQ, Zhang Q. Sintilimab-induced myocarditis overlapping myositis in a patient with metastatic thymoma: A case report. Front Cardiovasc Med. (2021) 8:797009. doi: 10.3389/fcvm.2021.797009
15. Liang S, Yang J, Lin Y, Li T, Zhao W, Zhao J, et al. Immune myocarditis overlapping with myasthenia gravis due to anti-pd-1 treatment for a chordoma patient: A case report and literature review. Front Immunol. (2021) 12:682262. doi: 10.3389/fimmu.2021.682262
16. Bi H, Ren D, Wang Q, Ding X, Wang H. Immune checkpoint inhibitor-induced myocarditis in lung cancer patients: A case report of sintilimab-induced myocarditis and a review of the literature. Ann palliative Med. (2021) 10:793–802. doi: 10.21037/apm-20-2449
17. Xing Q, Zhang ZW, Lin QH, Shen LH, Wang PM, Zhang S, et al. Myositis-myasthenia gravis overlap syndrome complicated with myasthenia crisis and myocarditis associated with anti-programmed cell death-1 (Sintilimab) therapy for lung adenocarcinoma. Ann Transl Med. (2020) 8:250. doi: 10.21037/atm.2020.01.79
18. Hong G, Zhao H, Yin Y, Shen H, Zeng Z, Yang J, et al. Sintilimab-induced inflammatory myopathy in a patient with esophageal cancer: A case report. Front Immunol. (2023) 14:1253463. doi: 10.3389/fimmu.2023.1253463
19. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (Orient-15): multicentre, randomised, double blind, phase 3 trial. Bmj. (2022) 377:e068714. doi: 10.1136/bmj-2021-068714
20. Won T, Kalinoski HM, Wood MK, Hughes DM, Jaime CM, Delgado P, et al. Cardiac myosin-specific autoimmune T cells contribute to immune-checkpoint-inhibitor-associated myocarditis. Cell Rep. (2022) 41:111611. doi: 10.1016/j.celrep.2022.111611
21. Matsumori A. Myocarditis and autoimmunity. Expert Rev Cardiovasc Ther. (2023) 21:437–51. doi: 10.1080/14779072.2023.2219895
22. Hao W, Zhang J, Wang Y, Fang B, Jin S, Yuan J, et al. Immune-related adverse events associated with nab-paclitaxel/paclitaxel combined with immune checkpoint inhibitors: A systematic review and network meta-analysis. Front Immunol. (2023) 14:1175809. doi: 10.3389/fimmu.2023.1175809
23. Kogure Y, Iwasawa S, Saka H, Hamamoto Y, Kada A, Hashimoto H, et al. Efficacy and safety of carboplatin with nab-paclitaxel versus docetaxel in older patients with squamous non-small-cell lung cancer (Capital): A randomised, multicentre, open-label, phase 3 trial. Lancet Healthy Longev. (2021) 2:e791–800. doi: 10.1016/s2666-7568(21)00255-5
24. Suarez-Almazor ME, Pundole X, Abdel-Wahab N, Johnson DB, Gupta D, Glezerman I, et al. Multinational association of supportive care in cancer (Mascc) 2020 clinical practice recommendations for the management of immune-mediated cardiovascular, rheumatic, and renal toxicities from checkpoint inhibitors. Support Care Cancer. (2020) 28:6159–73. doi: 10.1007/s00520-020-05710-8
25. Raschi E, Rossi S, De Giglio A, Fusaroli M, Burgazzi F, Rinaldi R, et al. Cardiovascular toxicity of immune checkpoint inhibitors: A guide for clinicians. Drug Saf. (2023) 46:819–33. doi: 10.1007/s40264-023-01320-5
26. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: asco guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/jco.21.01440
Keywords: case report, sintilimab, myocarditis, immune-related adverse events, methylprednisolone, septic shock
Citation: Zhou Q, Qin Z, Wu G, Yan P, Wang Q, Qu J, Jiang J and Ye D (2024) Sintilimab-induced myocarditis suspected in a patient with esophageal cancer and followed septic shock: case report and literature review. Front. Oncol. 14:1465395. doi: 10.3389/fonc.2024.1465395
Received: 16 July 2024; Accepted: 29 August 2024;
Published: 16 September 2024.
Edited by:
Cleber Machado-Souza, Pelé Pequeno Príncipe Research Institute, BrazilReviewed by:
Massimo Baudo, Lankenau Institute for Medical Research, United StatesCopyright © 2024 Zhou, Qin, Wu, Yan, Wang, Qu, Jiang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Da Ye, eWVkYTg0MTIwMkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.