- 1Department of Hematology, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 2Lymphoma and Cell Therapy Research Center, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 3Department of Hospital Pathology, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 4Division of Nuclear Medicine, Department of Radiology, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 5Department of Hematology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
Introduction: Peripheral T-cell lymphomas (PTCLs) have poor outcomes in the relapsed/refractory (R/R) setting. In this study, we evaluated the efficacy of dexamethasone, L-asparaginase, ifosfamide, carboplatin, and etoposide (DL-ICE) chemotherapy followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT) in patients with R/R PTCLs.
Methods: We retrospectively analyzed 80 adult patients with R/R PTCLs treated with DL-ICE chemotherapy between September 2009 and March 2023. Patients achieving complete or partial remission were eligible for consolidative allo-HSCT. Overall survival (OS) and progression-free survival (PFS) were evaluated.
Results: The overall response rate to DL-ICE was 37.5%, with 30% achieving complete remission (CR). With a median follow-up of 96.4 months, the median OS and PFS were 8.9 and 3.8 months, respectively. Seventeen patients (21%) underwent allo-HSCT, including 11 with non-CR status. The 5-year OS was significantly higher in the allo-HSCT group compared to that in the group with chemotherapy alone (64.7% vs 18.3%, p <0.001). Multivariate analysis identified advanced stage, EBV viremia, and non-CR status as poor prognostic factors.
Discussion: DL-ICE chemotherapy demonstrated modest activity in R/R PTCLs. Consolidation with allo-HSCT, even in patients who do not achieve CR, resulted in long-term survival in a subset of patients. Early consideration of allo-HSCT may improve outcomes for patients with R/R PTCLs.
1 Introduction
Peripheral T-cell lymphomas (PTCLs) are a heterogeneous group of aggressive non-Hodgkin lymphomas with poor prognosis, particularly in the relapsed/refractory (R/R) setting (1). Despite advances in front-line therapy, 30–40% of patients experience relapse or refractory disease (2). The prognosis for R/R PTCLs remains poor, with a median overall survival (OS) of <1 year when treated with conventional salvage chemotherapy alone (3). High-dose chemotherapy followed by autologous stem cell transplantation (auto-SCT) has been the standard approach for chemosensitive relapse. However, outcomes remain poor, with 3-year progression-free survival (PFS) rates of only 20–30% (4). This has led to increased interest in allogeneic hematopoietic stem cell transplantation (allo-HSCT) as a potentially curative option for R/R PTCLs (5). L-asparaginase has shown promising activity in extranodal NK/T-cell lymphoma (6). Interestingly, some reports have highlighted its effectiveness against PTCL NOS (7), leading to its incorporation into salvage regimens for other T-cell lymphomas. The combination of dexamethasone, L-asparaginase, ifosfamide, carboplatin, and etoposide (DL-ICE) represents a novel salvage approach for R/R PTCLs, building upon established ICE regimens (8). Recent studies have highlighted the potential benefit of allo-HSCT in R/R PTCLs, even in patients who did not achieve complete remission (CR) (9). However, the optimal timing and patient selection for allo-HSCT remain controversial. Additionally, the role of Epstein–Barr virus (EBV) in prognosis and treatment response has recently gained attention3. This study evaluated the efficacy and safety of DL-ICE chemotherapy followed by allo-HSCT in patients with R/R PTCLs. We hypothesized that this approach would improve outcomes compared with historical data with conventional salvage therapy alone and sought to identify prognostic factors that could guide treatment decisions.
2 Methods
2.1 Patient Selection
This retrospective study included adult patients (≥18 years) with histologically confirmed R/R PTCLs treated at Seoul St. Mary’s Hospital and Yeouido St. Mary’s Hospital between September 2009 and March 2023. Eligible PTCL subtypes included PTCL not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell lymphoma, extranodal NK/T-cell lymphoma (ENKTL), and other rare subtypes. Patients must have received at least one prior line of therapy and to have measurable disease at the time of relapse. Candidates whose skin allergic test were positivity for L-asparaginase were excluded from the study. The study was approved by the Institutional Review Board of the Catholic Medical Center (XC23RADI0045). Patient consent was not needed because of the retrospective nature of the study.
2.2 Treatment Protocol
All patients received DL-ICE chemotherapy consisting of:
● Dexamethasone 20 mg/day on days 1–4
● L-asparaginase 4,000 IU/m2 on days 1–4
● Ifosfamide 5 g/m2 over 24 h on day 1
● Carboplatin AUC 5 on day 1
● Etoposide 100 mg/m2 on days 1–3
Cycles were repeated every 21–28 days for a maximum of four cycles. Granulocyte colony-stimulating factor support was administered according to institutional guidelines. All patients were hospitalized until chemotherapy was completed to monitor for side effects.
Patients achieving CR or partial remission (PR) following DL-ICE were considered for consolidative allo-HSCT. Lack of available donors or patients’ disagreement with allo-HSCT were reasons for allocation to autologous HSCT (auto-HSCT). For auto-HSCT, patients received G-CSF (filgrastim, 10 µg/kg) for mobilization 48 h after the last cycle of DL-ICE infusion. Apheresis was performed when leukocyte and peripheral CD34 counts were elevated. In ASCT, patients were treated with reduced-intensity conditioning using the BuMelTT protocol, which consisted of busulfan (2.4 mg/kg) on days -8 to -6, melphalan (40 mg/m²) on days -5 to -4, and thiotepa on days -3 to -2, as previously described (10).
In the allo-HSCT group, patients received a fludarabine, melphalan, and total body irradiation (FMT) regimen comprising 30 mg/m² fludarabine on days -9 to -4, 70 mg/m² melphalan on day -3, and total body irradiation of 400 cGy on days -2 to -1. Anti-thymocyte globulin (rabbit ATG, 2.5–5.0 mg/kg; Genzyme Transplant, Cambridge, MA, USA) was administered for graft-versus-host disease (GVHD) prophylaxis in mismatched grafts on days -2 to -1. After transplantation, cyclosporine was used for full matched sibling donors, while tacrolimus was administered for other types of donors to control GVHD. Methotrexate was infused on days 1, 3, 6, and 11 (5 mg/m² for fully matched sibling donors and 10 mg/m² for other donors) (11).
2.3 Response Assessment and Statistical Analysis
Treatment response was assessed using computed tomography (CT) and positron emission tomography after two cycles and at the end of treatment. In patients from the auto- and allo-HSCT groups, assessment was performed before transplantation, utilizing the Lugano classification (12). CR is defined as the disappearance of target lymphoma lesions on CT, with normalization of 18F-fluorodeoxyglucose PET uptake in all involved sites (Deauville score of 1–3). PR was defined as a ≥50% regression in the target mass (Deauville score of 4–5). Progressive disease (PD) was characterized by a >25% increase in the target mass or the presence of new lesions (13).
OS was calculated from the start of DL-ICE until death from any cause or the last follow-up. PFS was calculated from the start of DL-ICE until disease progression, death, or the last follow-up. The presence of EBV in situ hybridization was detected using EBV-encoded small RNA oligonucleotides added to formalin-fixed paraffin-embedded samples, following the Inform EBER Probe Assay Protocol (Ventana Medical Systems Inc., Oro Valley, AZ, USA). EBV DNA load in peripheral blood was measured using real-time quantitative polymerase chain reaction at baseline and during follow-up. EBV viremia was defined >500 copies/mL. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate Cox proportional hazards models were employed to identify prognostic factors. Statistical analyses were performed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, 2022).
3 Results
3.1 Patient Characteristics
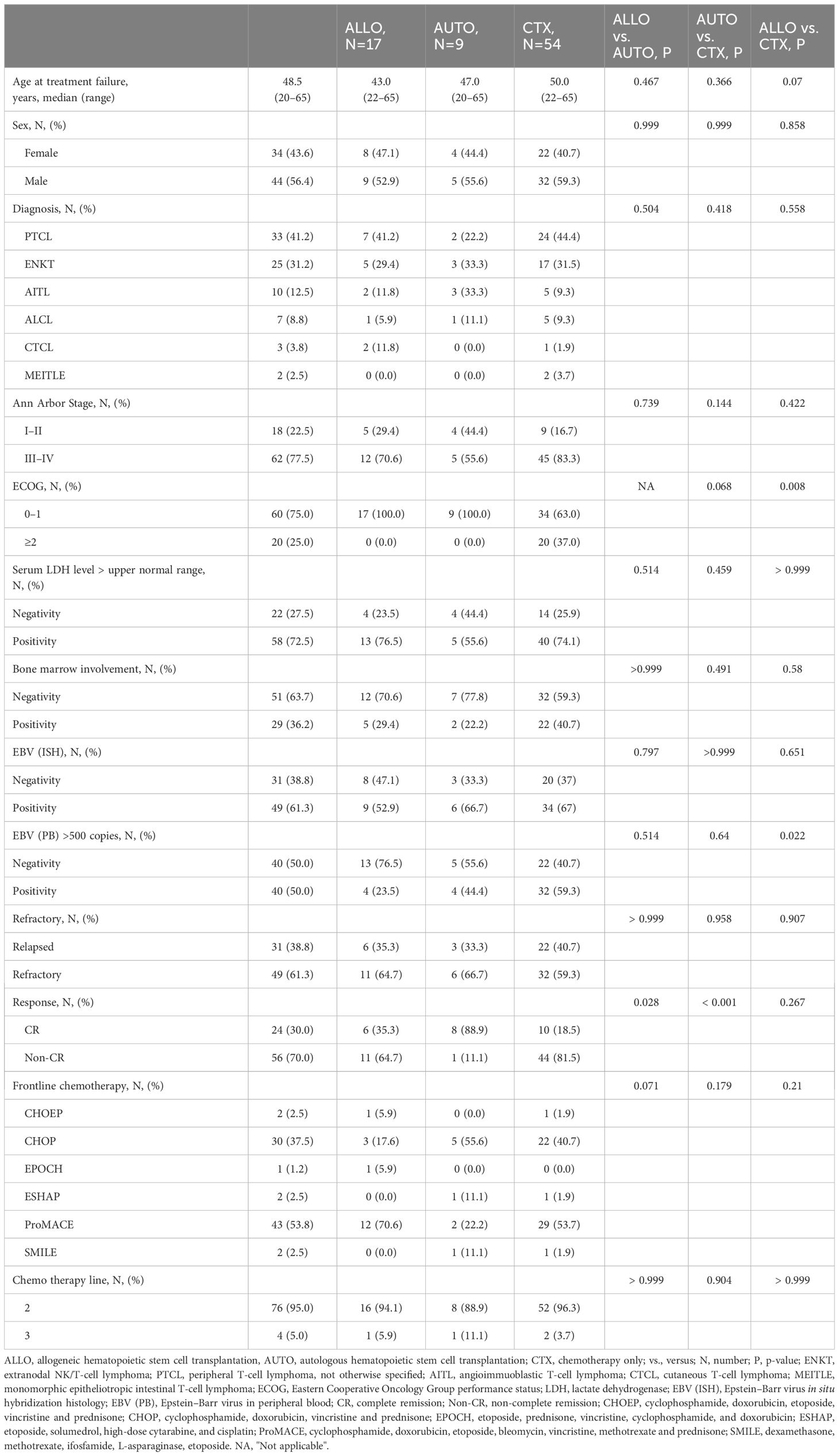
In total, 80 patients with R/R PTCLs were included in the analysis. The median age was 48.5 years (range: 20–65), and 56% was male. PTCL subtypes included PTCL-NOS (41%), ENKTL (31%), AITL (13%), and others (15%). Most patients had advanced-stage disease (78%) and elevated lactate dehydrogenase (LDH) (73%) at relapse. Approximately 39% of the patients had primary refractory disease, while 61% had relapsed after the initial response. Moreover, 50% had detectable EBV viremia at baseline (Table 1).
3.2 Response to DL-ICE and Survival Outcomes
The overall response rate to DL-ICE chemotherapy was 37.5%, including 30% CR and 7.5% PR. These results are comparable with other salvage regimens, such as DHAP (dexamethasone, high-dose cytarabine, and cisplatin) and GDP (gemcitabine, dexamethasone, and cisplatin), which have reported overall response rates of 30–50% in R/R PTCLs (14, 15). With a median follow-up of 96.4 months, the median OS and PFS for the entire cohort were 8.9 (95% CI: 7.9–22.6) months and 3.8 (95% CI: 3.0–7.0) months, respectively, similar to those reported in the SCHOLAR-1 study, which found a median OS of 6.3 months in refractory aggressive non-Hodgkin lymphomas (14) (Figures 1A, B). Seventeen patients (21%) proceeded to allo-HSCT, including six in CR and 11 with less than CR. Median PFS for in the allo-HSCT group were 8.7 months. The 5-year OS rate was significantly higher in the allo-HSCT group than in those receiving chemotherapy alone (64.7% vs. 18.3%, p <0.001) Nine patients were allocated to auto-HSCT, including eight patients who achieved CR and one patient with less than CR. The median PFS was 16.7 months, and the 5-year OS rate was 33.3% (Figures 1C, D). Notably, patients undergoing allo-HSCT with less than CR achieved a 5-year OS of 63.6%, is consistent with recent studies suggesting that allo-HSCT can overcome chemoresistance in PTCLs through a graft-versus-lymphoma effect (Figures 2A, B) (16). Detailed treatment courses in patients underwent HSCT described in Figure 2C.
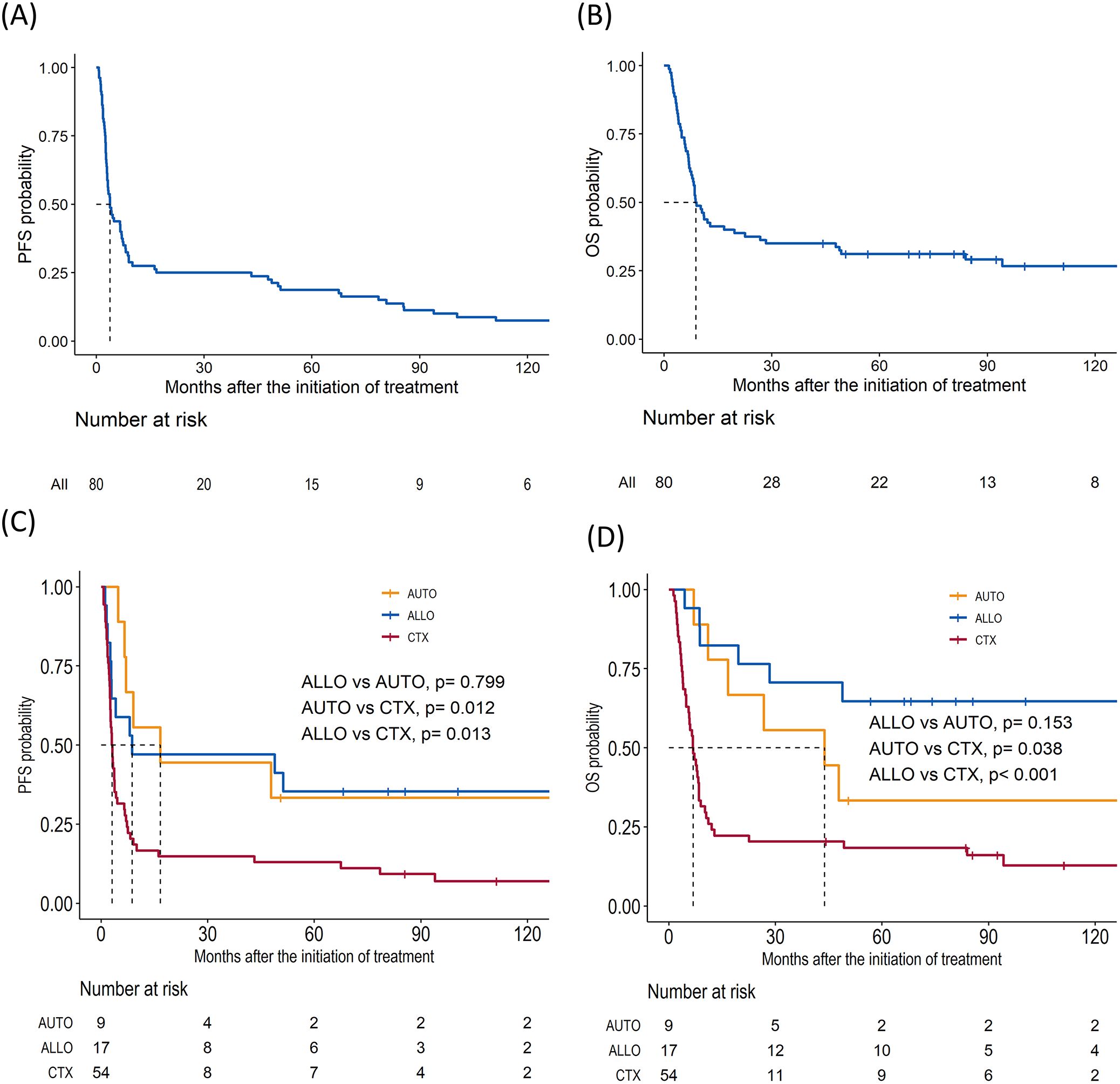
Figure 1. Survival outcomes for patients with relapsed/refractory peripheral T-cell lymphomas who underwent salvage chemotherapy. (A) Progression-free survival (PFS) in total cohort. (B) Overall survival (OS). (C) PFS of allogeneic hematopoietic stem cell transplantation (ALLO), autologous hematopoietic stem cell transplantation (AUTO), and chemotherapy only group (CTX). (D) OS of ALLO, AUTO, and CTX.

Figure 2. Impact on survival outcomes of hematopoietic stem cell transplantation (HSCT) by subgroups. (A) Kaplan–Meier curve of overall survival (OS) in patients with less than complete remission (CR) before HSCT (B) OS in CR. (C) Swimmers plot of patients who underwent HSCT.
3.3 Prognostic Factors
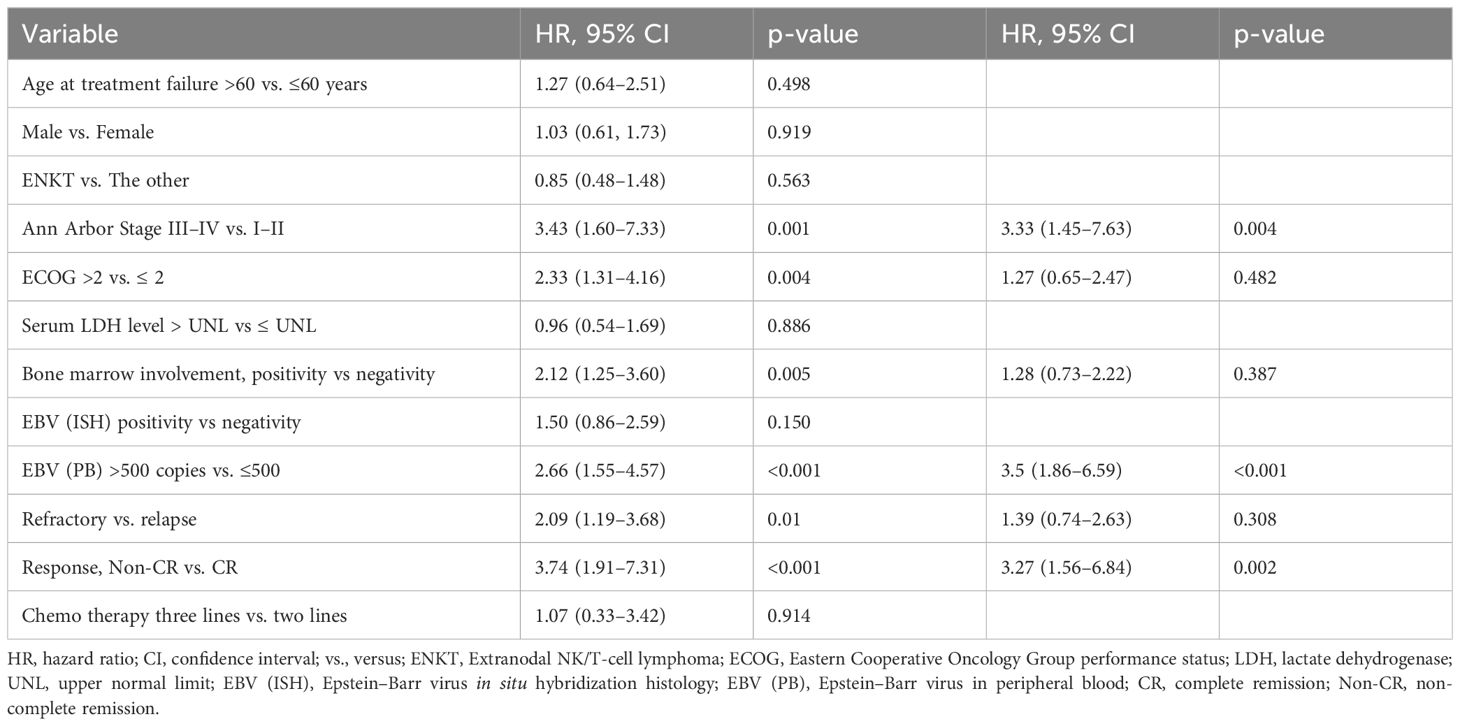
In multivariate analysis, advanced Ann Arbor stage (hazard ratio [HR]: 3.33, 95% confidence interval [CI]: 1.45–7.63, p=0.004), EBV viremia >500 copies/mL (HR: 3.50, 95% CI: 1.86–6.59, p <0.001), and failure to achieve CR with DL-ICE (HR: 3.27, 95% CI: 1.56–6.84, p=0.002) were independently associated with inferior OS (Table 2).
3.4 Toxicity in the Salvage Chemotherapy and Post-Allo-HSCT Complications
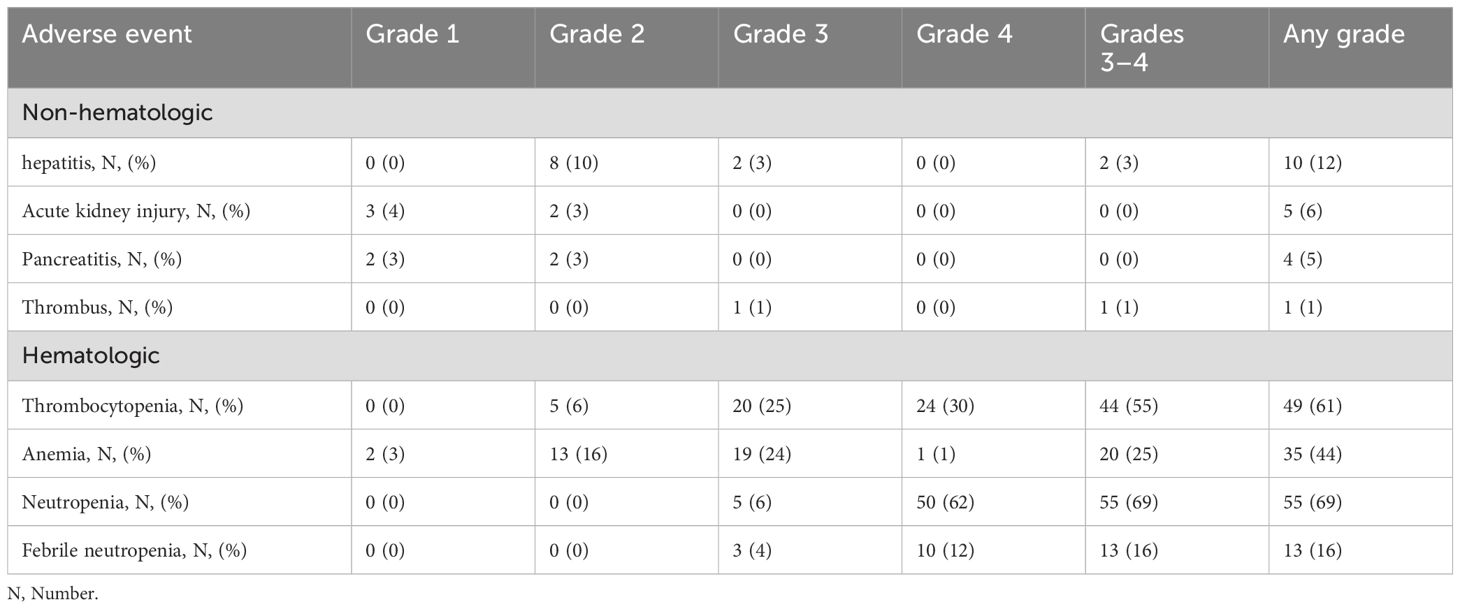
All patients were admitted until the completion of chemotherapy to monitor for L-asparaginase-related toxicity. The most common grade 3–4 toxicities with DL-ICE were hematologic, including neutropenia (69%), thrombocytopenia (55%), and anemia (25%). Febrile neutropenia occurred in 16% of patients. These rates are comparable with those reported with other intensive salvage regimens (17). Non-hematologic toxicities were generally mild, with grade 3–4 events, including hepatitis (3%) and thrombosis (1%) (Table 3). The incorporation of L-asparaginase did not appear to significantly increase toxicity compared to standard ICE chemotherapy (8). Among allo-HSCT recipients, acute GVHD grades II–IV occurred in 41% of patients, while chronic GVHD developed in 35%. There were three cases of transplant-related mortality. These complication rates are consistent with those reported in other studies of allo-HSCT for PTCLs (16).
4 Discussion
This study evaluated the efficacy of the DL-ICE chemotherapy regimen followed by allo-HSCT in patients with R/R PTCLs. The overall response rate of 37.5% to DL-ICE is modest but comparable to that of other salvage regimens in this setting (14, 15). Notably, the incorporation of L-asparaginase did not significantly increase toxicity compared with standard ICE chemotherapy, suggesting that this regimen is a viable option for patients who have failed previous platinum-based salvage therapy. However, L-asparaginase-related toxicities, such as pancreatitis, thromboembolism, and hepatitis, necessitate caution. Additionally, we excluded patients who tested positive for an allergic reaction to L-asparaginase, thereby reducing the risk of anaphylactic shock.
In comparison to previous reports, the PFS for patients receiving ICE therapy did not show a significant improvement, with our cohort demonstrating a PFS of 3.8 months compared to the reported range of 3.4–4.4 months. However, patients who underwent allo-HSCT exhibited a superior OS rate compared to the 1-year OS rates of 51.0–59.6% previously reported (18). The most striking finding of this study was the superior long-term survival observed in patients who proceeded to allo-HSCT, even among those with less than CR. The 5-year OS of 64.7% for transplanted patients compares favorably with historical data for conventional salvage approaches (3, 4). This supports the potential benefit of the graft-versus-lymphoma effect in T-cell malignancies, as previously suggested (16). Notably, 11 of the 17 transplanted patients had less than CR at the time of allo-HSCT, yet achieved a 5-year OS of 63.6%. This finding challenges the traditional paradigm that requires CR prior to transplantation and suggests that allo-HSCT should be considered earlier in the disease course for eligible patients with R/R PTCLs, rather than pursuing multiple lines of salvage therapy. This approach is further supported by recent studies indicating that an increased number of treatment lines prior to transplant is associated with worse outcomes (16).
The role of L-asparaginase in the DL-ICE regimen deserves special attention. While L-asparaginase has shown remarkable efficacy in extranodal NK/T-cell lymphoma, its utility in other PTCL subtypes remains limited. Our study suggests that incorporating L-asparaginase into the ICE backbone provides additional benefits without significantly increasing toxicity. This is particularly important given the limited treatment options for patients with R/R PTCLs. Future studies should consider a randomized comparison of ICE versus DL-ICE to definitively assess the contribution of L-asparaginase in this setting. The timing of allo-HSCT in the treatment course of R/R PTCLs remains to be addressed. Our data suggest that earlier referral for transplantation, even in patients who have not achieved CR, may be beneficial (19). However, the decision to proceed with transplant must be balanced against the risks of transplant-related mortality and GVHD.
In our cohort, the rates of acute and chronic GVHD were considerable but manageable and in line with previous reports (20). Strategies to mitigate GVHD while preserving the graft-versus-lymphoma effect, such as post-transplant cyclophosphamide, which has shown tolerable outcomes in patients with PTCLs, warrant further investigation in this patient population (21). We recommend considering allo-HSCT over auto-HSCT due to the low CR rates and the short PFS observed in our cohort. Waiting for CR appears less effective in the R/R setting compared to the chemotherapy-naive context. However, in situations where donor availability is limited, a careful application of auto-HSCT may still offer survival benefits in patients who achieve CR.
The identification of advanced stage and chemoresistance as poor prognostic factors is consistent with previous findings in PTCLs (22). Furthermore, the strong prognostic impact of EBV viremia, which is a potential biomarker for PTCLs and other lymphomas (23–25), underscores the possible role of EBV-directed therapies, such as rituximab or EBV-specific cytotoxic T lymphocytes, in the management of EBV-positive PTCLs (26).
While rituximab has been utilized in the treatment of EBV-positive B-cell lymphomas, its role in T-cell lymphomas remains inadequately defined. Emerging therapies, such as EBV-specific cytotoxic T lymphocytes or small molecule inhibitors targeting EBV-driven pathways, may provide new avenues for treatment (27). Additionally, employing EBV DNA load as a biomarker for assessing treatment response and early relapse detection could potentially inform treatment decisions and enhance patient outcomes.
Notably, although our results with allo-HSCT are promising, not all patients are candidates for this intensive intervention. For those ineligible for transplant, novel targeted therapies and immunotherapies are urgently required. Recent advances with agents, such as brentuximab vedotin for CD30-positive PTCLs and checkpoint inhibitors for specific PTCL subtypes, have shown considerable potential (28). Integrating these novel agents into salvage regimens or as maintenance therapy post-transplant may further improve patient outcomes.
Furthermore, the heterogeneity of PTCLs presents significant challenges in developing standardized treatment protocols. Our study encompassed various PTCL subtypes, each exhibiting distinct biological characteristics and treatment sensitivities. Future research should aim to establish subtype-specific treatment strategies, potentially guided by molecular profiling and biomarker analysis. This personalized approach could optimize treatment selection and enhance outcomes across the spectrum of PTCLs.
This study has several limitations, including its retrospective design and relatively small sample size. We defined transplant eligibility based on an upper age limit of 65 years. Additional studies are required to address the salvage chemotherapy outcomes for those ineligible for transplant. The heterogeneity of PTCL subtypes and the absence of a control group also restrict definitive conclusions. Notably, only two patients were treated with an asparaginase-containing regimen, while the ENKT subtype represented 25 patients in our cohort. Our centers utilize ProMACE as the primary first-line chemotherapy for the ENKT subtype. Despite this, the conclusions remain relevant, as PTCL-NOS was the most prevalent subtype in our cohort, and no significant survival differences were observed with the ENKTCL subtype. Prospective, randomized studies are necessary to establish the optimal sequencing of novel agents, chemotherapy, and allo-HSCT in R/R PTCLs.
In conclusion, DL-ICE chemotherapy followed by allo-HSCT represents a feasible and potentially effective strategy for patients with R/R PTCLs. The achievement of long-term survival in a subset of patients underscores the importance of timely transplantation. Delaying transplantation in hopes of achieving a better chemotherapy response may result in patients becoming ineligible for transplant and consequently lead to poorer outcomes. Early consideration of allo-HSCT, even for patients with less than CR, may provide the best chance for cure in this challenging patient population. Future studies should focus on optimizing patient selection for allo-HSCT, exploring novel strategies to reduce post-transplant relapse and GVHD, and developing personalized treatment strategies based on PTCL subtype and molecular characteristics.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Institutional Review Board of the Catholic Medical Center (XC23RADI0045). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Patient consent was not needed because of the retrospective nature of the study.
Author contributions
TK: Formal analysis, Investigation, Writing – original draft. TK: Data curation, Writing – review & editing. EH: Resources, Writing – review & editing. GM: Resources, Writing – review & editing. SC: Validation, Writing – review & editing. YJ: Conceptualization, Formal analysis, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. (2008) 26:4124–30. doi: 10.1200/JCO.2008.16.4558
2. Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. (2013) 31:1970–6. doi: 10.1200/JCO.2012.44.7524
3. Bellei M, Foss FM, Shustov AR, Horwitz SM, Marcheselli L, Kim WS, et al. The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, International T-Cell Project. Haematologica. (2018) 103:1191–7. doi: 10.3324/haematol.2017.186577
4. d’Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. (2012) 30:3093–9. doi: 10.1200/JCO.2011.40.2719
5. Schmitz N, Truemper L, Bouabdallah K, Ziepert M, Leclerc M, Cartron G, et al. A randomized phase 3 trial of autologous vs allogeneic transplantation as part of first-line therapy in poor-risk peripheral T-NHL. Blood. (2021) 137:2646–56. doi: 10.1182/blood.2020008825
6. Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. (2011) 29:4410–6. doi: 10.1200/JCO.2011.35.6287
7. Zhang Y, Xu W, Liu H, Li J. Therapeutic options in peripheral T cell lymphoma. J Hematol Oncol. (2016) 9:37. doi: 10.1186/s13045-016-0267-0
8. Moskowitz CH, Bertino JR, Glassman JR, Hedrick EE, Hunte S, Coady-Lyons N, et al. Ifosfamide, carboplatin, and etoposide: A highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin's lymphoma. J Clin Oncol. (1999) 17:3776–85. doi: 10.1200/JCO.1999.17.12.3776
9. Mamez AC, Dupont A, Blaise D, Chevallier P, Forcade E, Ceballos P, et al. Allogeneic stem cell transplantation for peripheral T cell lymphomas: a retrospective study in 285 patients from the Societe Francophone de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC). J Hematol Oncol. (2020) 13:56. doi: 10.1186/s13045-020-00892-4
10. Yoon JH, Min GJ, Park SS, Jeon YW, Lee SE, Cho BS, et al. Autologous hematopoietic cell transplantation using dose-reduced intravenous busulfan, melphalan, and thiotepa for high-risk or relapsed lymphomas. Bone Marrow Transpl. (2019) 54:330–3. doi: 10.1038/s41409-018-0289-z
11. Jeon YW, Yoon S, Min GJ, Park SS, Park S, Yoon JH, et al. Clinical outcomes of fludarabine and melphalan with an 800 cGy total body irradiation conditioning regimen in patients with refractory or relapsed aggressive non-Hodgkin lymphoma undergoing allogeneic hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk. (2019) 19:345–55.e7. doi: 10.1016/j.clml.2019.03.023
12. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. (2014) 32:3059–68. doi: 10.1200/JCO.2013.54.8800
13. Cheson BD, Ansell S, Schwartz L, Li G, Advani R, Jacene HA, et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. (2016) 128:2489–96. doi: 10.1182/blood-2016-05-718528
14. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. (2017) 130:1800–8. doi: 10.1182/blood-2017-03-769620
15. O’Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. (2011) 29:1182–9. doi: 10.1200/JCO.2010.29.9024
16. Hamadani M, Ngoya M, Sureda A, Bashir Q, Litovich CA, Finel H, et al. Outcome of allogeneic transplantation for mature T-cell lymphomas: impact of donor source and disease characteristics. Blood Adv. (2022) 6:920–30. doi: 10.1182/bloodadvances.2021005899
17. Biagi JJ, Herbert KE, Smith C, Abdi E, Leahy M, Falkson C, et al. A phase II study of dexamethasone, ifosfamide, cisplatin and etoposide (DICE) as salvage chemotherapy for patients with relapsed and refractory lymphoma. Leuk Lymphoma. (2005) 46:197–206. doi: 10.1080/10428190400014884
18. Tay T, Somasundaram N, Lim C, Khoo LP, Goh AZK, Lee YS, et al. Treatment outcomes of T and natural-killer/T-cell lymphoma with ifosfamide, carboplatin and etoposide chemotherapy. Cancer Rep. (2022) 5:e1552. doi: 10.1002/cnr2.1552
19. Du J, Yu D, Han X, Zhu L, Huang Z. Comparison of allogeneic stem cell transplant and autologous stem cell transplant in refractory or relapsed peripheral T-cell lymphoma: A systematic review and meta-analysis. JAMA Netw Open. (2021) 4:e219807. doi: 10.1001/jamanetworkopen.2021.9807
20. Qian L, Wu Z, Shen J. Advances in the treatment of acute graft-versus-host disease. J Cell Mol Med. (2013) 17:966–75. doi: 10.1111/jcmm.12093
21. Sterling CH, Hughes MS, Tsai H-L, Yarkony K, Fuchs EJ, Swinnen LJ, et al. Allogeneic blood or marrow transplantation with post-transplantationcyclophosphamide for peripheral t cell lymphoma: The importance of graft source. Transplantation and Cellular therapy (2023) 29:267. doi: 10.1016/j.jtct.2022.12.009
22. Sibon D. Peripheral T-cell lymphomas: therapeutic approaches. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14092332
23. Kim SJ, Yoon DH, Jaccard A, Chng WJ, Lim ST, Hong H, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. (2016) 17:389–400. doi: 10.1016/S1470-2045(15)00533-1
24. Kim T, Min G, Jeon Y, Park S, Park S, Shin S, et al. Impact of Epstein-Barr virus on peripheral t-cell lymphoma not otherwise specified and angioimmunoblastic t-cell lymphoma. Front Oncol. (2022) 11:797028. doi: 10.3389/fonc.2021.797028
25. Kanakry JA, Li H, Gellert LL, Lemas MV, Hsieh W, Hong F, et al. Plasma Epstein-Barr virus DNA predicts outcome in advanced Hodgkin lymphoma: correlative analysis from a large North American cooperative group trial. Blood. (2013) 121:3547–53. doi: 10.1182/blood-2012-09-454694
26. Nie M, Bi XW, Zhang WW, Sun P, Xia Y, Liu PP, et al. Consolidative treatment after salvage chemotherapy improves prognosis in patients with relapsed extranodal natural killer/T-cell lymphoma. Sci Rep. (2016) 6:23996. doi: 10.1038/srep23996
27. Kanakry JA, Ambinder RF. EBV-related lymphomas: new approaches to treatment. Curr Treat Options Oncol. (2013) 14:224–36. doi: 10.1007/s11864-013-0231-y
Keywords: L-asparaginase, non-Hodgkin lymphomas, relapsed/refractory, stem cell transplantation, T-cell lymphomas
Citation: Kim T-Y, Kim T-J, Han EJ, Min GJ, Cho S-G and Jeon Y (2024) DL-ICE as a bridge to allogeneic transplantation in relapsed/refractory PTCL: survival outcomes and prognostic factors. Front. Oncol. 14:1461268. doi: 10.3389/fonc.2024.1461268
Received: 08 July 2024; Accepted: 21 November 2024;
Published: 09 December 2024.
Edited by:
Luca Castagna, Azienda Ospedaliera Ospedali Riuniti Villa Sofia Cervello, ItalyReviewed by:
Cinzia Pellegrini, Sant’Orsola-Malpighi Polyclinic, ItalyCole Sterling, Johns Hopkins University, United States
Copyright © 2024 Kim, Kim, Han, Min, Cho and Jeon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youngwoo Jeon, bmF0aXZlNDdAY2F0aG9saWMuYWMua3I=
 Tong-Yoon Kim
Tong-Yoon Kim Tae-Jung Kim
Tae-Jung Kim Eun Ji Han4
Eun Ji Han4 Gi June Min
Gi June Min Seok-Goo Cho
Seok-Goo Cho Youngwoo Jeon
Youngwoo Jeon