
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 02 December 2024
Sec. Genitourinary Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1461165
Primary renal epithelioid angiosarcoma (EAS) is extremely rare and carries a poor prognosis. Herein, we present a case of renal EAS in an 81-year-old male patient who complained of hematuria for 1 year. A computerized tomography (CT) scan revealed an occupying lesion at the upper pole of the left kidney, with scattered calcifications, along with retroperitoneal lymph node metastasis and possible lung metastasis. A laparoscopic palliative nephrectomy was performed, and postoperative pathology confirmed a malignant tumor with necrosis in the left kidney. Immunohistochemistry (IHC) revealed positive expression for CD31, CD10, and vimentin, consistent with the diagnosis of EAS. Although EAS is a rare, aggressive, and often misdiagnosed condition, IHC can help confirm its diagnosis, and in our case, the scattered calcifications observed on CT imaging might be helpful in its differential diagnosis.
Angiosarcoma is a rare soft tissue sarcoma originating from endothelial cells, accounting for 1%–2% of all sarcomas (1–3). Epithelioid angiosarcoma (EAS), a morphological subtype, is highly aggressive and has a poor prognosis (3, 4). While EAS is most commonly found in the skin and deep soft tissues, it can also occur in bones, adrenal glands, breasts, and liver (1, 4). Renal EAS is exceptionally rare, with only a few reported cases to date (5–8). The case of primary renal EAS presented here differs in certain aspects from those previously described and could significantly contribute to improving the early diagnosis of this condition.
An 81-year-old male patient with a 5-year history of hypertension presented to our hospital with gross hematuria, accompanied by frequent urination and urgency, persisting for 1 year. A computerized tomography (CT) scan revealed a quasi-circular soft tissue mass originating from the upper pole of the left kidney, measuring approximately 72 × 54 mm in diameter. The mass contained scattered calcifications and showed mild enhancement after contrast, along with retroperitoneal lymphadenopathy (Figures 1A–C). The CT findings suggested renal cancer, with possible retroperitoneal lymph node and lung metastases. Following a comprehensive evaluation, a laparoscopic palliative nephrectomy was performed. Gross examination revealed a tumor near the upper pole of the left kidney, measuring 60 × 50 × 50 mm. The tumor appeared solid, grayish-brown, rough, and brittle, with unclear corticomedullary junction in the surrounding kidney (Figure 1D). Microscopically, at low magnification, the tumor cells were abundant and diffusely arranged in nests and cords, with some areas displaying branched cavities and red blood cell accumulation (Figures 1E, F). At higher magnification, irregular vascular cavities are rich in red blood cells, and individual tumor cells contain red blood cells in their cytoplasm (Figures 1G, H). The tumor cells varied in size and displayed epithelioid features, with polygonal or spindle shapes, indistinct cell boundaries, abundant eosinophilic cytoplasm, nuclear size variation, prominent nucleoli, and visible mitotic figures (Figures 1I, J). Immunohistochemistry (IHC) showed positive staining for CD31, vimentin, and CD10 (Figure 2), and negative staining for cytokeratin (CK), CK7, CK19, P504S, PAX-8, CAIX, HMB-45, MELAN-A, S100, SMA, D2-40, and calretinin, leading to the diagnosis of renal EAS. The patient was transferred to the intensive care unit (ICU) postoperatively, but due to complications, including pulmonary infection and his advanced age, he was unable to be weaned from the ventilator. Ultimately, the family decided to discontinue further treatment.
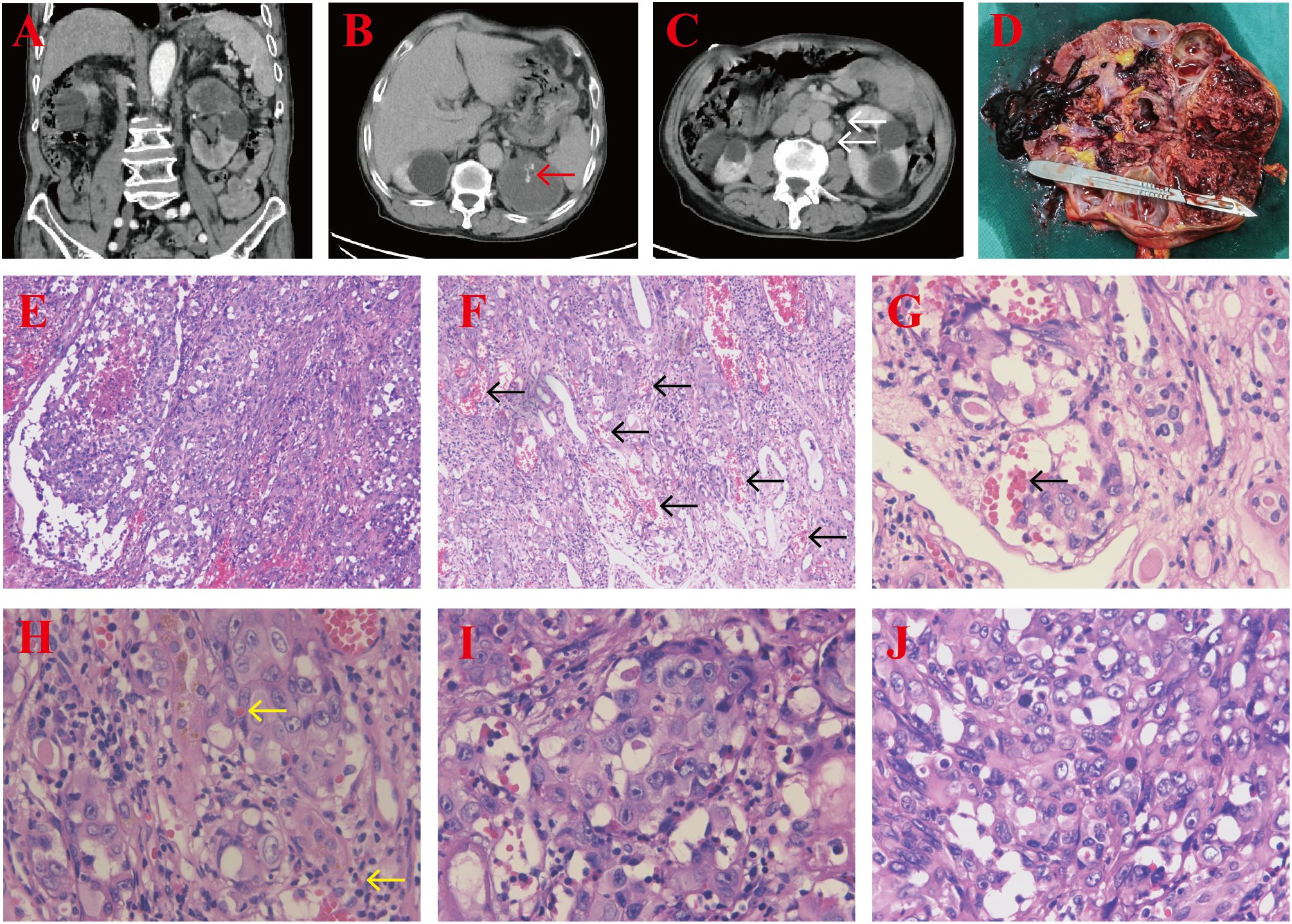
Figure 1. (A–C) CT scan showing a quasi-circular soft tissue mass in the upper pole of the left kidney with scattered calcifications (red arrow) and retroperitoneal lymphadenopathy (white arrows). (D) The cut section of the tumor revealed a solid, grayish-brown, rough, and brittle lesion, with an unclear corticomedullary junction in the surrounding kidney tissues. (E, F) H&E-stained section demonstrating abundant tumor cells arranged in nests and cords, along with irregular vascular spaces (black arrows) (magnification, ×100). (G, H) Irregular vascular cavities are rich in red blood cells (black arrow), and individual tumor cells contain red blood cells in their cytoplasm (yellow arrows) (magnification, ×400), H&E staining. (I, J) The tumor cells exhibit epithelioid features, with varying nuclear sizes, prominent nucleoli, and visible mitotic figures (magnification, ×400), H&E staining.
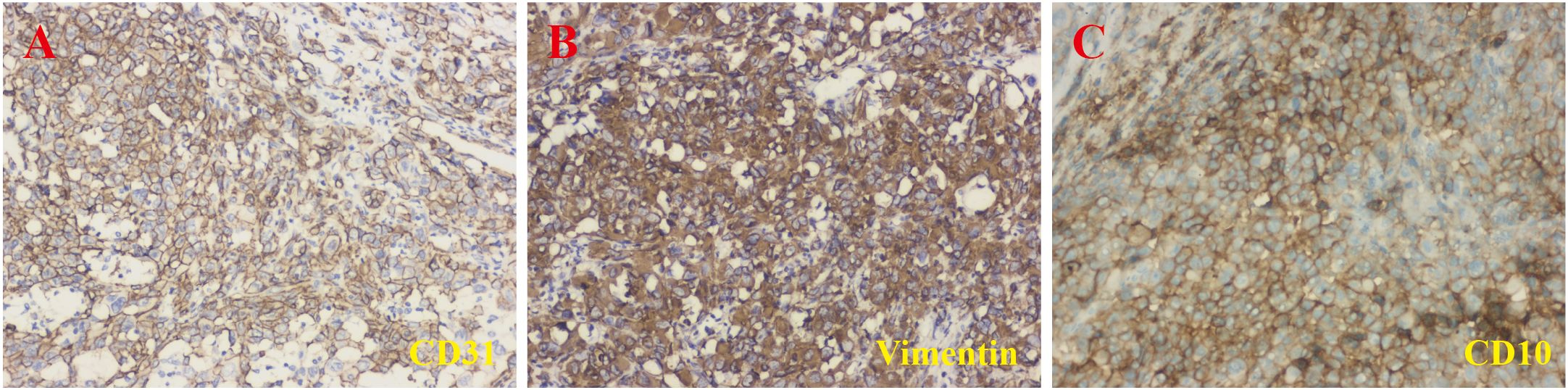
Figure 2. Immunohistochemical staining results. The lesion showed positive staining for CD31 (A), vimentin (B), and CD10 (C) (magnification, ×200).
There are seven cases (including this case) of renal EAS reported to date, and the clinical presentation, imaging features, histopathology, and immunohistochemical characteristics are quite similar (5–10). The clinical presentation of EAS often lacks specificity, making preoperative diagnosis challenging. EAS typically occurs in the elderly, and the patient in this case was 81 years old, consistent with previous reported cases (5, 7, 8). Moreover, the clinical symptoms associated with renal EAS may include flank pain, hematuria, and abdominal discomfort, which are common to various renal diseases (11). Herein, the patient presented with gross hematuria, similar to the presentation reported by Liu et al. (7). Other patients with renal EAS may seek medical attention due to pain in the renal area (5). These symptoms are common in other types of renal tumors, making renal EAS diagnosis challenging. As a result, it is difficult for clinicians to make a preliminary differential diagnosis based solely on clinical presentation.
Similarly, the imaging characteristics of EAS often lack specificity, which can lead to challenges in diagnosis. The imaging findings may overlap with those of other renal masses, including renal cell carcinoma and renal epithelioid angiomyolipoma (EAML). For instance, renal EAML can present similarly on imaging studies, often showing a heterogeneous mass with areas of necrosis and fat, which can complicate the differential diagnosis (12). In the present case, the tumor showed mild enhancement after contrast, which could help differentiate it from the significant enhancement typically seen in renal tumors. In a previously reported case of renal EAS, the CT scan revealed a large multilocular lesion in the lower part of the left kidney, characterized by low density and a predominantly fluid-filled content (5). In our case, the CT scan showed a cystic-solid lesion at the upper pole of the left kidney with clear boundaries, low density, and progressive enhancement. These findings suggest that the CT appearance of renal EAS may not exhibit distinctive features. Moreover, in our patient, scattered calcifications were observed within the tumor tissue. Calcifications are often associated with renal cancer, particularly in cases involving osseous metaplasia (13, 14). In a case of bladder angiosarcoma, calcifications within the tumor were identified via ultrasonography, CT, and cystoscopy (15). Similarly, calcifications were also documented in another angiosarcoma case (16). Calcifications in tumors are typically dystrophic, occurring within necrotic regions (15). In one renal EAS case, the CT scan also showed a rounded density shadow, with a ring-like calcification of the wall (10). In our case, necrosis was present in the tumor, and the CT scan showed speckled calcifications in the center of the mass, which may be a specific feature of EAS.
The diagnosis of EAS often relies on pathology and immunohistochemical staining, as clinical symptoms and imaging findings alone cannot distinguish EAS from other renal malignancies. The histopathology differential diagnosis of renal EAS is complex due to its overlapping features with other renal tumors, including clear cell renal cell carcinoma, malignant melanoma, EAML, and malignant mesothelioma (7), and the differential diagnostic basis is shown in Table 1. Primary renal EAS could easily mimic epithelial tumor morphologically and immunohistochemically, and may lead to misdiagnosis, but the combined use of endothelial cell markers such as FVIIIRA and CD31 can help confirm its diagnosis (9). Common vascular and epithelial markers, such as CD31, CD34, ERG, and factor VIII, are frequently used to support a definitive diagnosis. However, the positive expression of all these markers is not necessary to confirm the diagnosis (17). Herein, the tumor showed positive expression for CD31, vimentin, and CD10, which led to the final diagnosis of EAS. CD34 positivity has been documented to vary from 40% to 100%, usually staining in regions with abundant vessel formation (18). In the current case, the tumor cells were positive for CD31 but negative for CD34, along with the reported cases (7, 10). As shown in the results in Table 2, the positivity rate for CD34 in five renal EAS (two cases were not applicable) cases was 60%. In other reported cases, markers such as CK may also show positive expression (7). However, in our patient, CK was negative. Studies have shown that CK is not a necessary marker for the diagnosis of EAS (19). Moreover, Iacovelli et al. found that in the epithelial-like subtype, the expression of CK and CD31 is more prominent, while the spindle cell subtype is primarily characterized by higher vimentin expression and negative CK expression (20). This pattern appears to align with the immunohistochemical features observed in our patient. Furthermore, CD10 is a specific immunomarker for renal cell carcinoma, but its expression in EAS is rarely reported. In a case of renal EAS described by Singh et al., tumor cells exhibited positive expression of CD10 (8), in line with the positive expression for CD10 in our patient. When CD10 is expressed in EAS, it may complicate the differentiation from renal cell carcinoma with sarcomatoid differentiation. The renal EAS is easily misdiagnosed as epithelioid hemangioendothelioma (EHE), as it could show similar characteristics in immunohistochemical staining, such as being positive for CD31, CD34, and ERG, but the results of Ki-67, WWTR1-CAMTA1, and YAP1-TFE3 might be helpful in differential diagnosis (21, 22). Therefore, when diagnosing EAS based on immunohistochemistry, it is essential to carefully evaluate the relevance of specific markers to arrive at a more accurate diagnosis.
EAS is a rare disease with a poor prognosis, and its occurrence in the kidney is even more uncommon. Renal EAS lacks characteristic clinical presentations, making the diagnosis largely reliant on pathology and immunohistochemical results. In this case, the presence of calcifications within the tumor tissue identified by CT scan may aid in diagnosing EAS. Nevertheless, further cases are needed for validation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. BC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing. JS: Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. KC: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. YZ: Data curation, Methodology, Project administration, Resources, Software, Writing – review & editing. TL: Data curation, Methodology, Project administration, Resources, Software, Writing – review & editing. DT: Funding acquisition, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. KT: Conceptualization, Funding acquisition, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the High-level Talent Project of the First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine (GYZYYFY-BS-2023(14)), Guizhou Traditional Chinese Medicine Tumor Inheritance and Science and Technology Innovation Talent Base (No. Deaf leader [2018] No. 3), and Guizhou Provincial University of traditional Chinese medicine and ethnic medicine cancer prevention and Treatment Medical Transformation Engineering Research Center (Grant Nos. [2023] No. 037).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1461165/full#supplementary-material
1. Navarro Sanchez JM, Oommen T, Lum C, Halford Z, Vierkoetter K. Mediastinal epithelioid angiosarcoma, new insights into an uncommon diagnosis: A case report and literature review. Hawaii J Health Soc Welf. (2023) 82:208–12.
2. Sevim S, Gedik E, Ozakinci H, Kaygusuz G, Enneli D. Primary epithelioid angiosarcoma of endometrium. Pol J Pathol. (2023) 74:219–22. doi: 10.5114/pjp.2023.132302
3. Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. (2010) 11:983–91. doi: 10.1016/S1470-2045(10)70023-1
4. Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. (2011) 135:268–72. doi: 10.5858/135.2.268
5. Waqas M, Rahim W, Shohab D, Khawaja MA, Ali Z, Mamoon N. Primary renal epithelioid angiosarcoma. J Coll Physicians Surg Pak. (2018) 28:S66–8. doi: 10.29271/jcpsp.2018.03.S66
6. Iannaci G, Crispino M, Cifarelli P, Montella M, Panarese I, Ronchi A, et al. Epithelioid angiosarcoma arising in schwannoma of the kidney: report of the first case and review of the literature. World J Surg Oncol. (2016) 14:29. doi: 10.1186/s12957-016-0789-5
7. Liu H, Huang X, Chen H, Wang X, Chen L. Epithelioid angiosarcoma of the kidney: A case report and literature review. Oncol Lett. (2014) 8:1155–8. doi: 10.3892/ol.2014.2292
8. Singh C, Xie L, Schmechel SC, Manivel JC, Pambuccian SE. Epithelioid angiosarcoma of the kidney: a diagnostic dilemma in fine-needle aspiration cytology. Diagn Cytopathol. (2012) 40 Suppl 2:E131–139. doi: 10.1002/dc.v40.2s
9. Li N, Li W, Li Z. Primary renal epithelioid angiosarcoma with transitional cell carcinoma in renal pelvis. Zhonghua Wai Ke Za Zhi. (1997) 35:294–5.
10. Xie JJ, Liu F, Li B, Zhang H, Ren FM, Zhang YH, et al. Epithelioid angiosarcoma of the kidney: report of a case. Zhonghua Bing Li Xue Za Zhi. (2020) 49:1328–30. doi: 10.3760/cma.j.cn112151-20200402-00283
11. Wang P, He J, Wan X, Ren P, Tang K. Clinical challenges and management of primary renal epithelioid angiomyolipoma of duplex kidney with paraneoplastic syndrome. J Int Med Res. (2021) 49:3000605211032493. doi: 10.1177/03000605211032493
12. Cao QH, Liu F, Xiao P, Tian XY, Li B, Li Z. Coexistence of renal epithelioid angiomyolipoma and clear cell carcinoma in patients without tuberous sclerosis. Int J Surg Pathol. (2012) 20:196–200. doi: 10.1177/1066896911413576
13. Cakici MC, Kir G, Akalin MK, Yildirim A. Clear cell renal cell carcinoma with osseous metaplasia: Two extremely rare cases and review of the literature. Arch Esp Urol. (2020) 73:651–4.
14. Lai BM, Ka SY, Kan WK, Lam MW, Lee TF, Lui YH, et al. Case 237: renal cell carcinoma with osseous metaplasia. Radiology. (2017) 282:293–8. doi: 10.1148/radiol.2016140817
15. Rallabandi HB, Swain M, Gowrishankar S, Sinha S. Postradiation angiosarcoma of bladder with extensive osseous metaplasia. Indian J Pathol Microbiol. (2016) 59:78–80. doi: 10.4103/0377-4929.178234
16. Balamurali G, du Plessis DG, Wengoy M, Bryan N, Herwadkar A, Richardson PL. Thorotrast-induced primary cerebral angiosarcoma: case report. Neurosurgery. (2009) 65:E210–211; discussion E211. doi: 10.1227/01.NEU.0000348294.05571.D9
17. Matoso A, Epstein JI. Epithelioid angiosarcoma of the bladder: A series of 9 cases. Am J Surg Pathol. (2015) 39:1377–82. doi: 10.1097/PAS.0000000000000444
18. Jennings TA, Peterson L, Axiotis CA, Friedlaender GE, Cooke RA, Rosai J. Angiosarcoma associated with foreign body material. A Rep three cases Cancer. (1988) 62:2436–44. doi: 10.1002/1097-0142(19881201)62:11<2436::aid-cncr2820621132>3.0.co;2-j
19. Brown JG, Folpe AL, Rao P, Lazar AJ, Paner GP, Gupta R, et al. Primary vascular tumors and tumor-like lesions of the kidney: a clinicopathologic analysis of 25 cases. Am J Surg Pathol. (2010) 34:942–9. doi: 10.1097/PAS.0b013e3181e4f32a
20. Iacovelli R, Orlando V, Palazzo A, Cortesi E. Clinical and pathological features of primary renal angiosarcoma. Can Urol Assoc J. (2014) 8:E223–226. doi: 10.5489/cuaj.1585
21. Rosenbaum E, Jadeja B, Xu B, Zhang L, Agaram NP, Travis W, et al. Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets. Mod Pathol. (2020) 33:591–602. doi: 10.1038/s41379-019-0368-8
Keywords: scattered calcifications, pathology, renal, epithelioid angiosarcoma, case report
Citation: Zhai J, Che B, Shen J, Cen K, Zhang Y, Li T, Tang D and Tang K (2024) Case report: A rare case of renal epithelioid angiosarcoma. Front. Oncol. 14:1461165. doi: 10.3389/fonc.2024.1461165
Received: 08 July 2024; Accepted: 11 November 2024;
Published: 02 December 2024.
Edited by:
Pranab Dey, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Parikshaa Gupta, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaCopyright © 2024 Zhai, Che, Shen, Cen, Zhang, Li, Tang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaifa Tang, ZG9jLnRhbmdrZkBob3RtYWlsLmNvbQ==; Dongxin Tang, dGFuZ2Rvbmd4aW5Ac2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.