- Department of Gynaecology, III, Women’s and Children’s Hospital of Ningbo University, Ningbo, Zhejiang, China
Objective: To assess the effectiveness and tolerability of both PD-1 and PD-L1 inhibitors in advanced cervical cancer (CC), focusing on varying PD-L1 levels.
Methods: A comprehensive exploration was carried out on EMBASE, PubMed, Cochrane Library databases as well as Web of Science up to May 25, 2024, for studies involving advanced CC patients receiving PD-1/PD-L1 inhibitors. Inclusion criteria were studies reporting objective response rate (ORR), disease control rate (DCR), median progression-free survival (PFS), as well as median overall survival (OS). Data extraction and quality assessment were performed by two reviewers using the JBI Case Series Critical Appraisal Checklist, followed by a meta-analysis via STATA/MP 16.0.
Results: Five eligible studies comprising 223 patients were chosen. ORR and DCR were 42% (95% CI: 17%-66%, P = 0.00) and 70% (95% CI: 22%-117%, P = 0.00), respectively, in the PD-L1 positive patients and were 36% (95% CI: 17%-54%, P = 0.00) and 47% (95% CI: 30%-63%, P = 0.00), respectively, in patients with PD-L1 negativity. For patients exhibiting PD-L1 positivity, median PFS and median OS were 3.98 months (95% CI: 0.80–7.16, P = 0.01) and 11.26 months (95% CI: 3.01–12.58, P = 0.00), respectively.
Conclusion: With PD-1/PD-L1 inhibitors, PD-L1 positive CC patients demonstrate superior ORR, DCR, median PFS, and median OS, underscoring PD-L1 as one biomarker for immunotherapy response.
Introduction
Cervical cancer (CC) is still a significant contributor to cancer-related mortality in women worldwide, particularly in middle- and low-income countries (1). According to 2020 data, there were approximately 604,127 new cases of cervical cancer worldwide, and 341,831 deaths, with age-standardised incidence and mortality rates of 13.3 and 7.2 per 100,000 women, respectively (2). Despite great progress in both screening and vaccination, a majority of patients still experience serious disease or recurrence and have limited therapy options and unfavourable prognoses (3, 4). Traditional therapies, including chemotherapy, radiation as well as surgery, have presented limited efficacy in these stages of the disease, entailing the exploration of innovative therapy (5).
With the advent of immunotherapy, cancer treatment has been revolutionized bringing hope for patients suffering from advanced tumours. Programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors have presented encouraging results in cancers as one class of immune checkpoint inhibitors, including melanoma, bladder cancer as well as non-small cell lung cancer (6, 7). These inhibitors lift the immune system’s capability of recognizing and eliminating cancer cells by disrupting the binding between PD-1 on T cells and PD-L1 on tumour cells (7). The PD-L1 quantification on tumour cells is commonly assessed using the Combined Positive Score (CPS). It has emerged as one potential biomarker for forecasting the reaction to PD-1/PD-L1 inhibitors (8). CPS is determined by assessing the proportion of PD-L1-positive tumour cells and immune ones relative to the total viable tumour ones (9). Preliminary clinical studies indicate a possibility of exhibiting better reactions to PD-1/PD-L1 inhibitors in patients having higher CPS, which implies a potential stratified therapy (10, 11).
The meta-analysis is to assess the effectiveness and tolerability of both PD-1 and PD-L1 inhibitors in treating advanced CC systematically, with a particular focus on different PD-L1 expressions. Data were integrated from various high-quality studies to comprehensively understand the potential of these immune therapies in improving the outcomes of advanced CC patients.
Methods
Based on implementation under the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions, this study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (12). The current study was formally registered on the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) (ID: INPLASY202460062).
Search strategy
We performed an extensive search across various databases like Web of Science, PubMed, EMBASE, as well as the Cochrane Library, encompassing articles published before May 25, 2024. The search was restricted to studies published exclusively in the English language with the following terms for search: “Uterine Cervical Neoplasms” OR “CC” AND “Immune Checkpoint Inhibitors” OR “PD-1 Inhibitor” OR “PD-L1 Inhibitor”. We performed a manual review to the reference lists of the encompassed articles for identifying additional related research. The particular search process is detailed in Supplementary File 1.
Inclusion and exclusion criteria
Studies were encompassed if they met the criteria below:
1. Patients were confirmed with advanced or recurrent CC, regardless of subtype.
2. Patients received treatment by PD-1 or PD-L1 inhibitors alone or in conjunction with other therapies.
3. Retrospective analyses or stage II clinical trials.
4. Included studies assessed relevant clinical outcomes, such as PFS, ORR, OS, DCR, as well as AEs, using RECIST 1.1 criteria (13).
5. Tumour PD-L1 was assessed and quantified as one CPS, which was calculated as the percentage of PD-L1-stained cells divided by the sum of viable tumour cells multiplied by 100. The definition of positivity was established as having a CPS of 1 or higher.
The exclusion criteria were:
1. Animal research, meta-analyses, reviews, duplicate reports, letters or case reports.
2. Studies with fewer than 10 patients.
Two reviewers conducted a thorough screening of articles independently, assessing their eligibility according to pre-established criteria Disagreements/discrepan were resolved through consensus between the two reviewers or with the assessment of one-third reviewers if necessary.
Data extraction and quality evaluation
Through one predefined extraction form, two reviewers extracted data. The extracted data encompassed baseline patient characteristics, study characteristics, and predefined outcomes (ORR, DCR, PFS, OS). The quality of clinical studies was evaluated via the JBI Case Series Critical Appraisal Checklist (14).
Statistical analyses
Analyses were conducted via STATA/MP 16.0. Inter-study heterogeneity was judged via the chi-square test as well as the I² statistic. Random-effects models (REM) were adopted when I²≥50% (indicating high heterogeneity), and fixed-effects models (FEM) were adopted when I²<50% (implying low heterogeneity) (15). The robustness of the pooled results was judged via sensitivity analyses. Egger’s test was conduc to evaluate the possible publication bias.
Results
Literature search
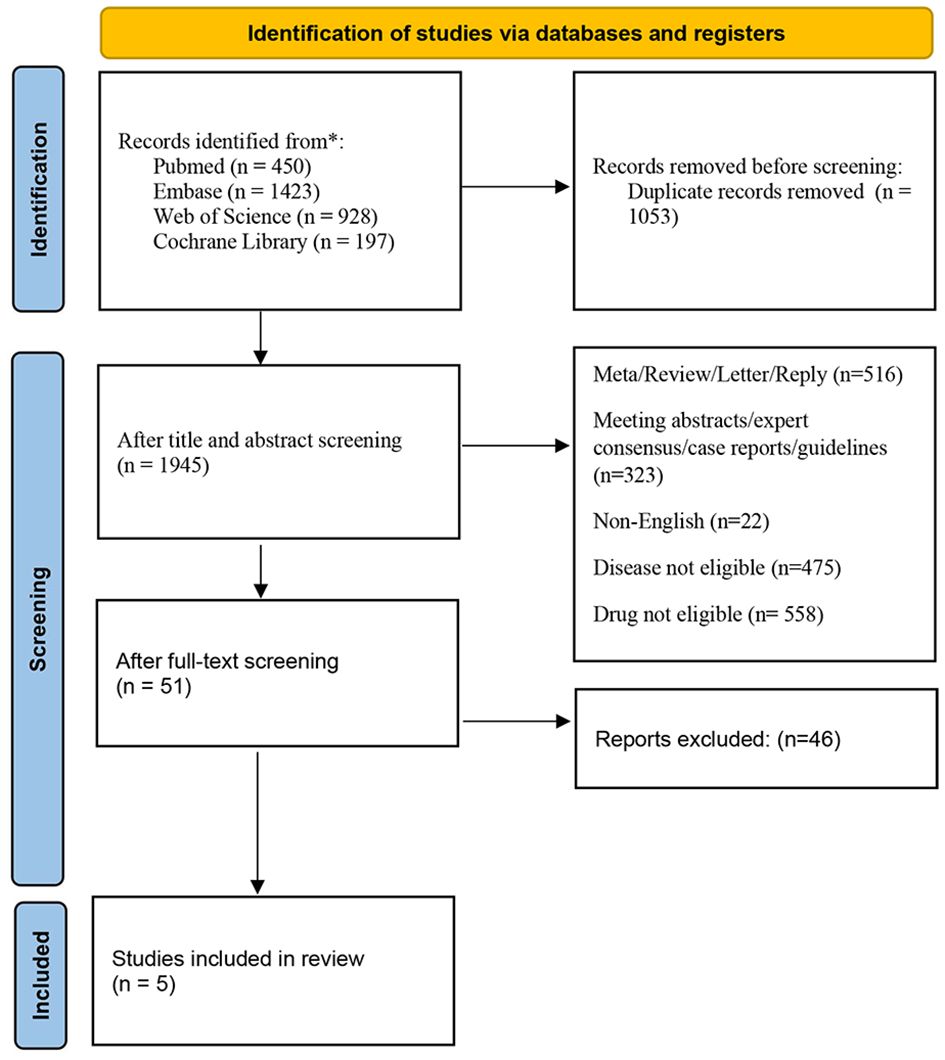
The initial search strategy yielded 2,998 relevant articles. After removing 1,053 duplicate studies, we screened titles and abstracts, causing the exclusion of 1,894 studies not fulfilling the inclusion criteria. Subsequently, we performed a detailed review of the whole texts of the left 51 potentially eligible papers, and ultimately selected 5 trials for the final analysis (16–19). The process of selecting studies is depicted in Figure 1. All eligible research data were obtained from published manuscripts.
Study characteristics
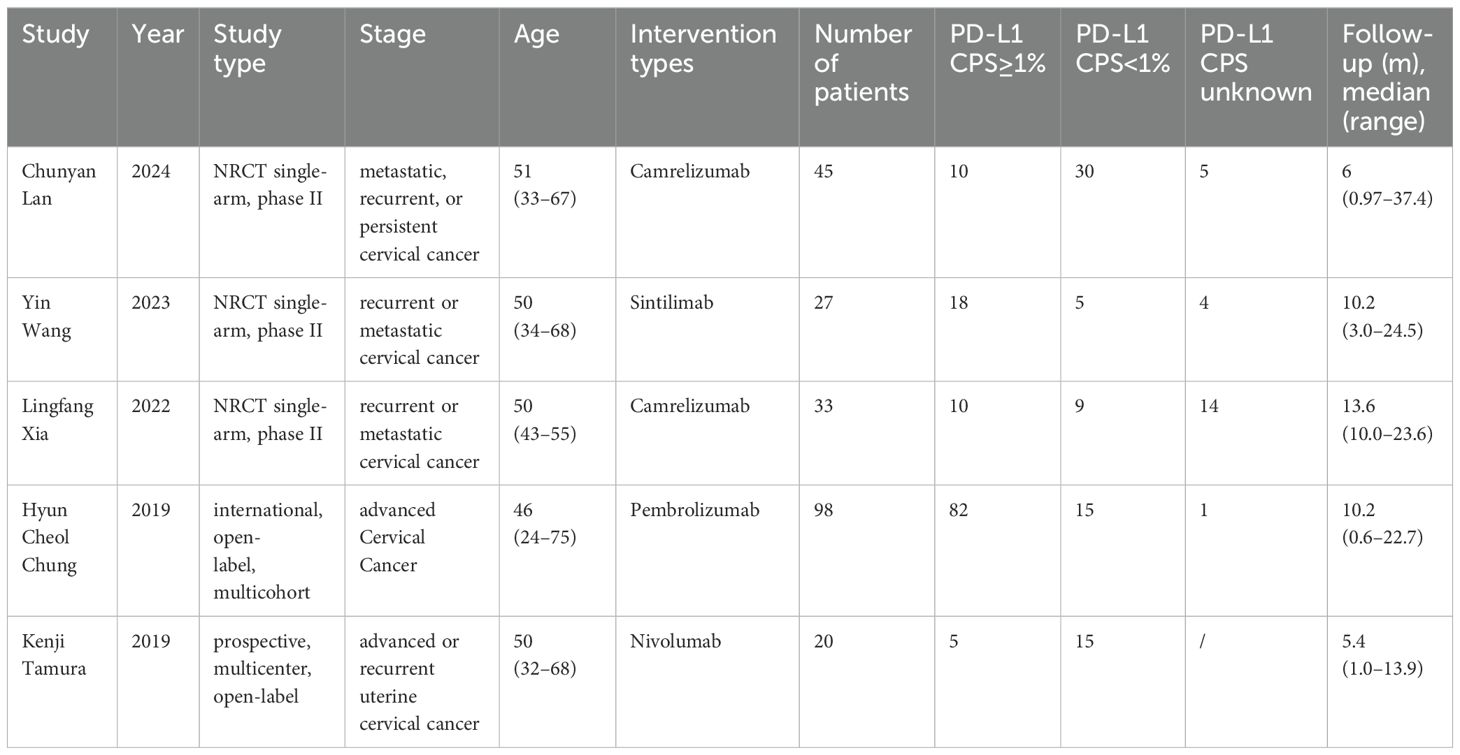
Totally, 5 studies were included in the final analysis Table 1 presents their detailed characteristics.
Quality assessment
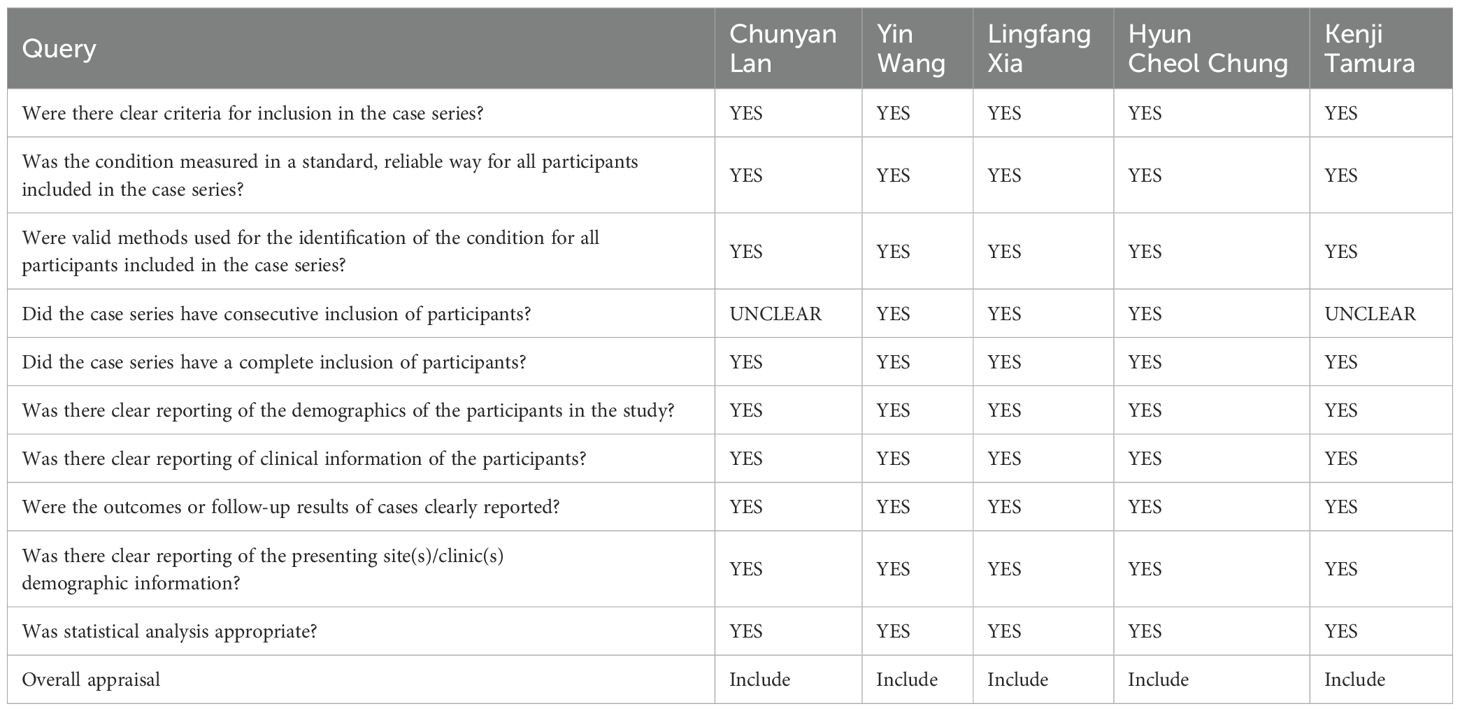
On the basis of the JBI Critical Appraisal Checklist for Case Series, five clinical studies were evaluated, comprising ten items that examine the quality of case reports including case selection, evaluation of the disease or health problem, and case data presentation. The assessment results are provided in Table 2.
Meta-analysis results
Comparison of ORR by PD-L1 CPS
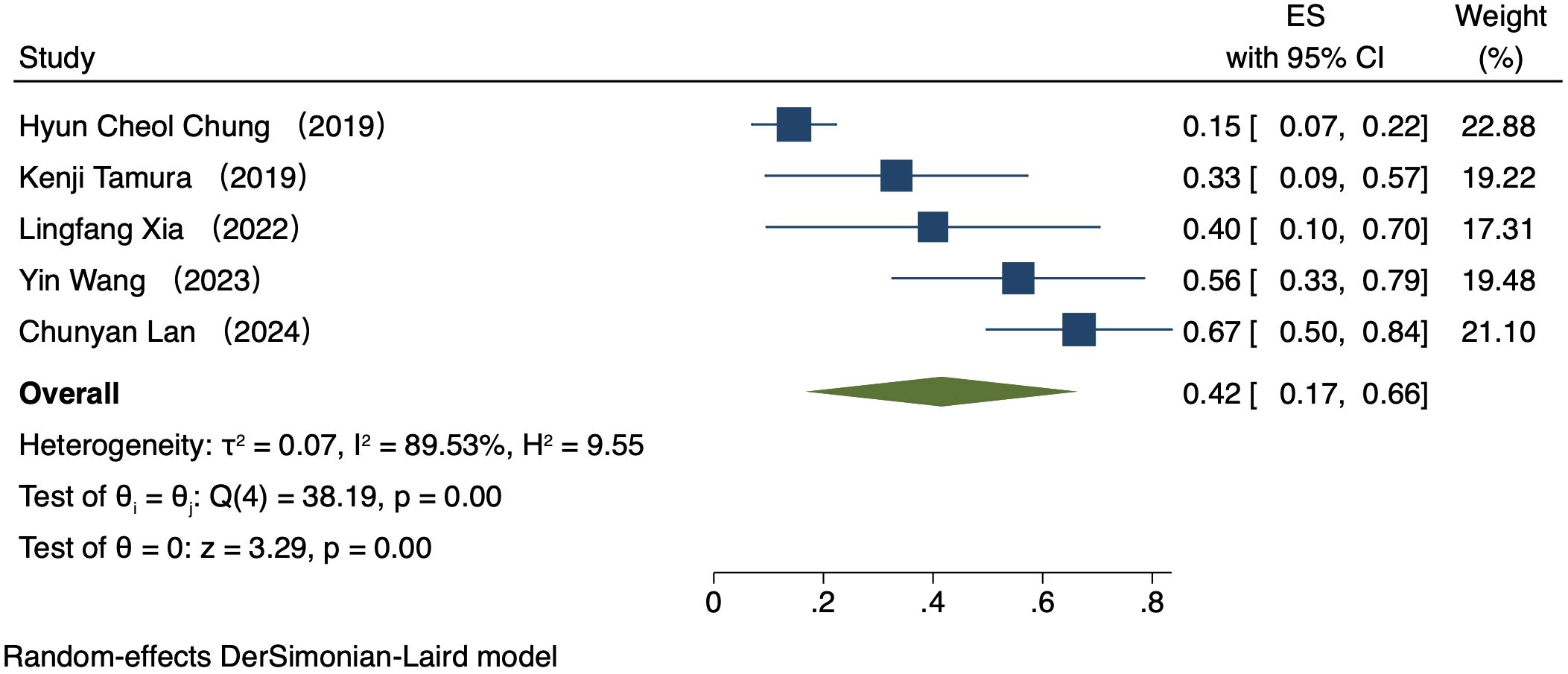
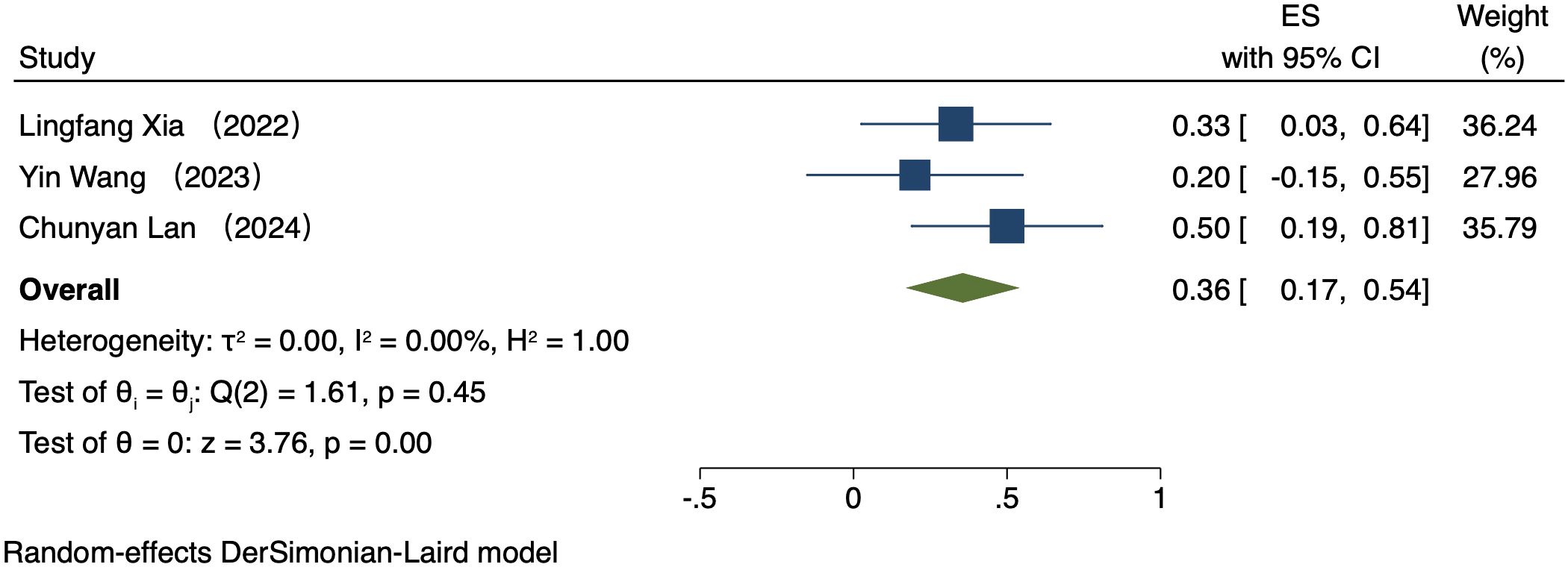
Five studies (223 patients) analyzed ORR by PD-L1 CPS (16–20). In patients exhibiting PD-L1 positivity, a REM was used because of notable heterogeneity (I² = 89.53%, P = 0.00). The ORR was 42% (95% CI: 17%-66%, P = 0.00, Figure 2). In patients exhibiting PD-L1 negativity, a FEM was used because of low heterogeneity (I² = 0.00%, P = 0.45). The ORR was 36% (95% CI: 17%-54%, P = 0.00, Figure 3).
Comparison of DCR by PD-L1 CPS
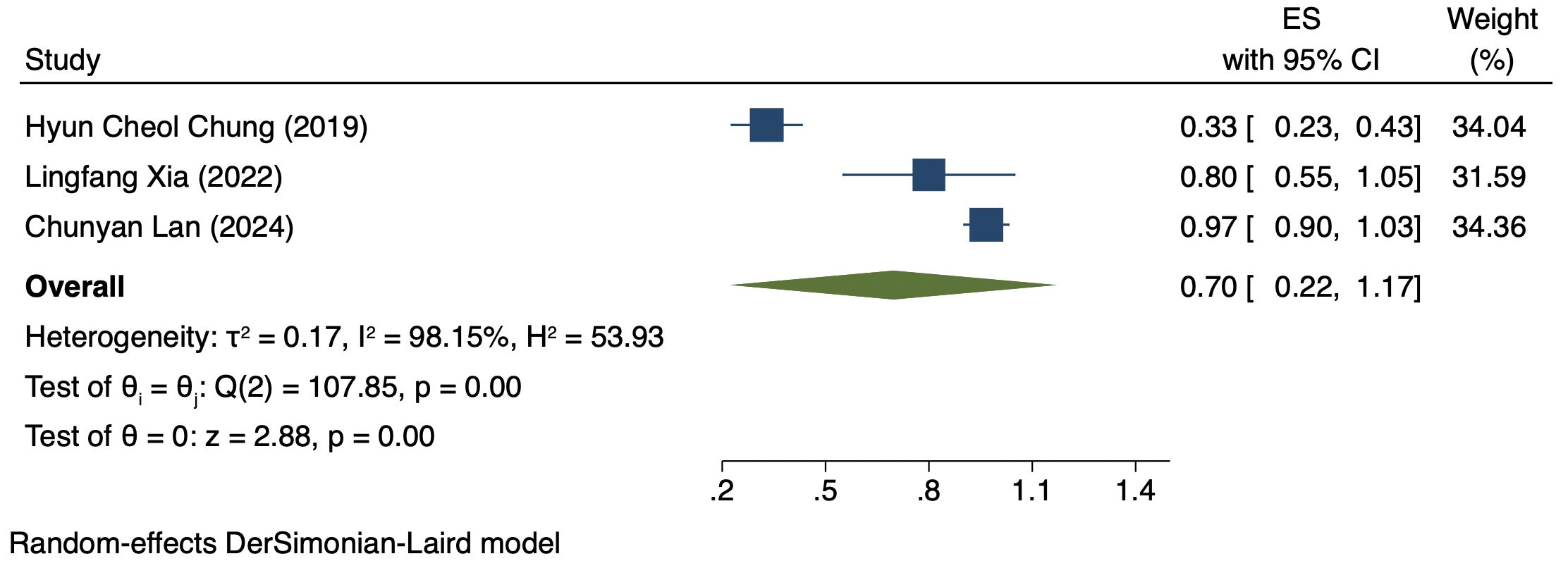
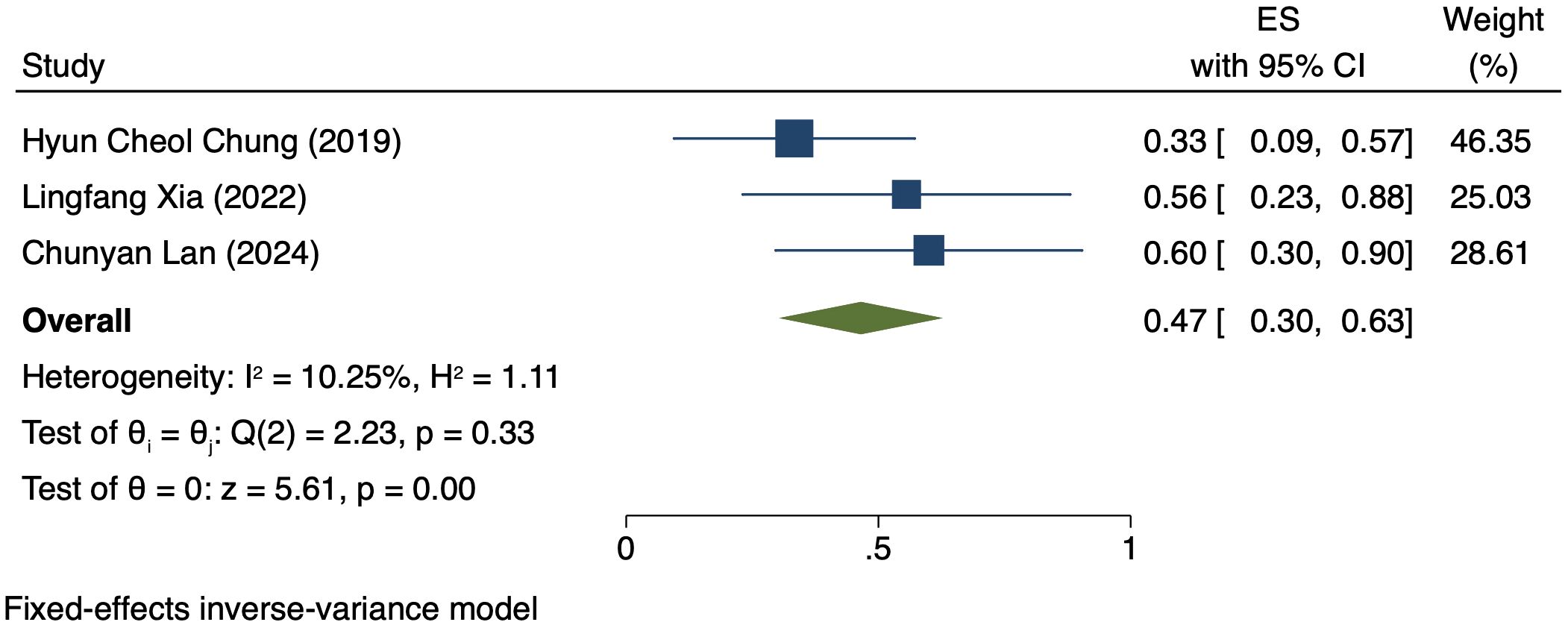
Three studies (176 patients) analyzed DCR by PD-L1 CPS (17, 19, 21). In PD-L1 positive patients, a REM was used because of notable heterogeneity (I² = 98.15%, P = 0.00). The DCR was 70% (95% CI: 22%-117%, P = 0.00), as shown in Figure 4. In PD-L1 negative patients, a FEM was used because of low heterogeneity (I² = 10.25%, P = 0.33). The DCR was 47% (95% CI: 30%-63%, P = 0.00), as shown in Figure 5.
Median PFS in patients exhibiting PD-L1 positivity
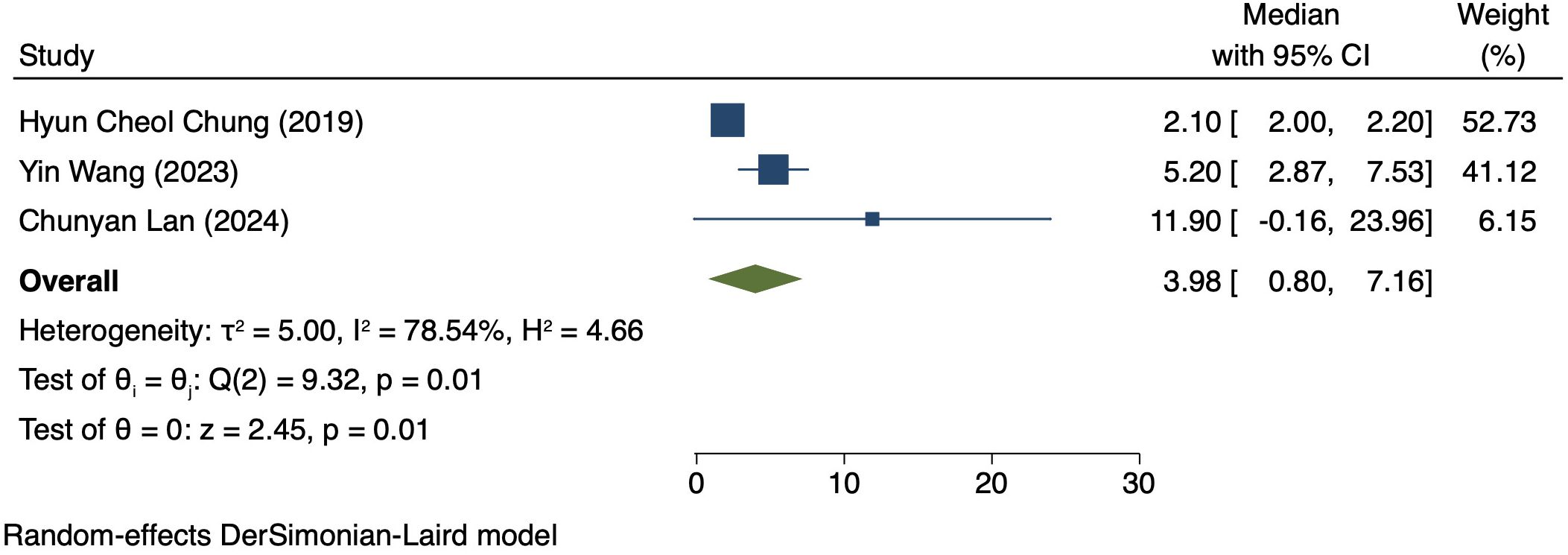
Three studies (170 patients) analyzed PFS in Patients exhibiting PD-L1 positivity (16, 17, 20). A REM was used because of notable heterogeneity (I² = 78.54%, P = 0.01). The PFS was 3.98 months (95% CI: 0.80–7.16, P = 0.01), as shown in Figure 6.
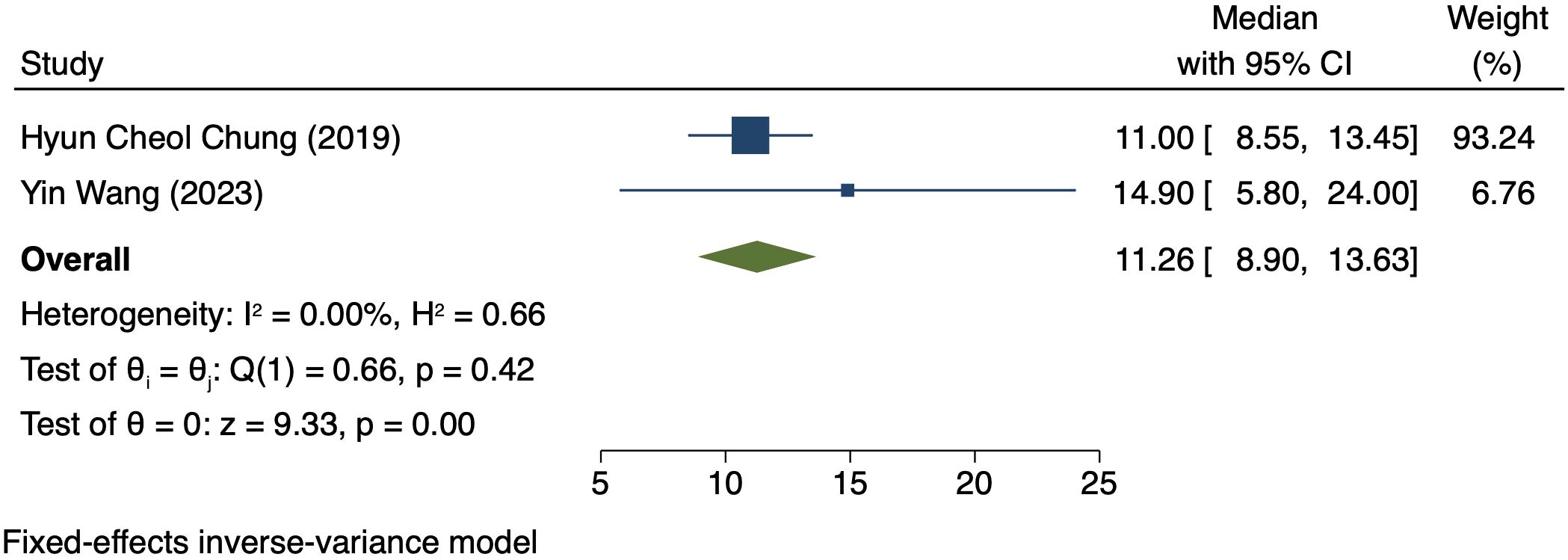
Median OS in patients exhibiting PD-L1 positivity
Two studies (125 patients) analyzed OS in patients exhibiting PD-L1 positivity (16, 20). A FEM was used due to low heterogeneity (I² = 0.00%, P = 0.42). The OS was 11.26 months (95% CI: 3.01–12.58, P = 0.00, Figure 7).
Sensitivity analysis
By sequentially excluding each study, a sensitivity analysis was performed for assessing its impact on the summary results. According to the analysis results, no individual study significantly impacts the overall 95% CI of the summary results, indicating a relatively robust of the meta-analysis results. The results are presented in Supplementary File 2.
Publication bias
To ensure the validity of the meta-analysis, publication bias was judged via Egger’s test. The p-value of 0.79 (> 0.05), indicates no notable publication bias.
Discussion
This study comparatively analyzed ORR and DCR among patients who had different PD-L1 CPS, focusing on assessing the efficacy disparity between groups exhibiting PD-L1 positivity and PD-L1 negativity. The results revealed an ORR of 42% (95% CI: 17%-66%) and 36% (95% CI: 17%-54%) in the group exhibiting PD-L1 positivity and group exhibiting PD-L1 negativity, respectively. This difference suggests a possibly larger response rate of PD-L1-positive patients to immunotherapy. The underlying mechanism for it can be explained by the interaction between PD-L1 with the immune system. PD-L1, a cell surface protein frequently found on tumour cells, binds to the PD-1 receptor on T cells, suppressing the activity of T cells and helping tumour cells evade immune system attacks (22, 23). In tumours expressing PD-L1, tumour cells can more effectively utilize this mechanism to evade immune surveillance. Thus, these patients possibly have a better response to immune checkpoint inhibitors like PD-1/PD-L1 inhibitors, as these drugs are able to disrupt the binding of PD-1/PD-L1 with restore T cell-mediated tumour attack (24, 25). DCR was also compared among patients who had different PD-L1 CPS. The group exhibiting PD-L1 positivity and group exhibiting PD-L1 negativity had a DCR of 70% (95% CI: 22%-117%) and 47% (95% CI: 30%-63%), respectively. These findings imply the high value of PD-L1 expression in immune therapy response further (26). for more deeply probing into the survival outcomes of patients exhibiting PD-L1 CPS positivity, we analyzed the PFS and OS and found a PFS and OS of 3.98 months (95% CI: 0.80–7.16) and 7.80 months (95% CI: 3.01–12.58), respectively, in patients exhibiting PD-L1 CPS positivity. The findings imply the possibility of experiencing improved long-term survival rates among PD-L1 CPS-positive patients receiving immune therapy (27–29).
These results underscore the high value of PD-L1 in immune therapy. Patients exhibiting PD-L1 positivity demonstrated better efficacy in multiple key outcome measures in contrast to patients exhibiting PD-L1 negativity, indicating PD-L1 as an effective biomarker for identifying patients with a larger likelihood of favorable response to immune therapy in patients (30, 31).
Whereas, the current research also has certain limitations. First, a noticeable heterogeneity in the analysis could affect the stability of the results. Second, the included studies with relatively small sample sizes mostly consisted of non-controlled trials, limiting the generalizability and persuasiveness of the findings. Additionally, because of lack of enough pathological data, we could not further investigate the treatment response based on different types of CC tissue. studies included in this analysis predominantly involved Asian patients, raising uncertainty about the generalizability of these findings to other populations. Therefore, further validation of these findings is warranted through the implementation of large-scale randomized controlled trials (RCTs) in the future (32, 33).
In conclusion, PD-L1 expression is crucial in immune therapy, with PD-L1 CPS-positive patients demonstrating better efficacy in terms of ORR, DCR, median PFS, and median OS in contrast to patients exhibiting PD-L1 negativity. While the initial findings are encouraging, additional research is required to ascertain the wide applicability as well as long-term implications of these findings (34).
Conclusion
The meta-analysis verifies that CC patients exhibiting PD-L1 positivity have superior efficacy regarding ORR, DCR, median PFS, as well as median OS when receiving PD-1/PD-L1 inhibitor therapy in contrast to patients exhibiting PD-L1 negativity. These findings support the utilization of PD-L1 as one biomarker for forecasting the advanced CC patients’ reaction to immunotherapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JY: Conceptualization, Data curation, Investigation, Writing – original draft. HY: Investigation, Methodology, Writing – original draft. YZ: Data curation, Formal analysis, Writing – original draft. MlZ: Formal analysis, Investigation, Writing – original draft. MyZ: Formal analysis, Investigation, Project administration, Writing – original draft. QW: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1454372/full#supplementary-material
Supplementary File 1 | Search strategy.
Supplementary File 2 | Sensitivity Analysis.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health. (2023) 11:e197–206. doi: 10.1016/S2214-109X(22)00501-0
3. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
4. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. (2020) 8:e191–203. doi: 10.1016/S2214-109X(19)30482-6
5. Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv72–83. doi: 10.1093/annonc/mdx220
6. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. (2018) 359:1350–5. doi: 10.1126/science.aar4060
7. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. (2015) 348:56–61. doi: 10.1126/science.aaa8172
8. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. (2015) 14:847–56. doi: 10.1158/1535-7163.MCT-14-0983
9. Ilie M, Long-Mira E, Bence C, Butori C, Lassalle S, Bouhlel L, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. (2016) 27:147–53. doi: 10.1093/annonc/mdv489
10. Chung H, Delord J-P, Perets R, Italiano A, Shapira-Frommer R, Manzuk L, et al. Pembrolizumab treatment of advanced cervical cancer: updated results from the phase II KEYNOTE-158 study. Gynecologic Oncol. (2021) 162:S27. doi: 10.1016/S0090-8258(21)00696-X
11. Rischin D, Gil-Martin M, González-Martin A, Braña I, Hou JY, Cho D, et al. PD-1 blockade in recurrent or metastatic cervical cancer: Data from cemiplimab phase I expansion cohorts and characterization of PD-L1 expression in cervical cancer. Gynecol Oncol. (2020) 159:322–8. doi: 10.1016/j.ygyno.2020.08.026
12. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med. (2009) 6:e1000100.
13. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
14. Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. (2020) 18:2127–33.
15. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
16. Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. (2019) 37:1470–8. doi: 10.1200/JCO.18.01265
17. Lan C, Lu H, Zhou L, Liao K, Liu J, Xie Z, et al. Long-term survival outcomes and immune checkpoint inhibitor retreatment in patients with advanced cervical cancer treated with camrelizumab plus apatinib in the phase II CLAP study. Cancer Commun (Lond). (2024) 44:654–69. doi: 10.1002/cac2.12547
18. Tamura K, Hasegawa K, Katsumata N, Matsumoto K, Mukai H, Takahashi S, et al. Efficacy and safety of nivolumab in Japanese patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma: Multicenter, open-label phase 2 trial. Cancer Sci. (2019) 110:2894–904. doi: 10.1111/cas.14148
19. Xia L, Zhou Q, Gao Y, Hu W, Lou G, Sun H, et al. A multicenter phase 2 trial of camrelizumab plus famitinib for women with recurrent or metastatic cervical squamous cell carcinoma. Nat Commun. (2022) 13:7581. doi: 10.1038/s41467-022-35133-4
20. Wang Y, Zhao J, Liang H, Liu J, Huang S, Zou G, et al. Efficacy and safety of sintilimab plus albumin-bound-paclitaxel in recurrent or metastatic cervical cancer: a multicenter, open-label, single-arm, phase II trial. EClinicalMedicine. (2023) 65:102274. doi: 10.1016/j.eclinm.2023.102274
21. Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. (2018) 67:1481–9. doi: 10.1007/s00262-018-2226-9
22. Friedman JM. “Neurofibromatosis 1” In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, et al, editors. GeneReviews(®). Seattle, WA, United States: University of Washington, Seattle (1993).
23. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
24. Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. (2014) 515:558–62. doi: 10.1038/nature13904
25. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. (2015) 372:2018–28. doi: 10.1056/NEJMoa1501824
26. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
27. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
28. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. (2015) 373:1803–13. doi: 10.1056/NEJMoa1510665
29. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. (2015) 372:320–30. doi: 10.1056/NEJMoa1412082
30. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. (2014) 515:568–71. doi: 10.1038/nature13954
31. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. (2014) 515:563–7. doi: 10.1038/nature14011
32. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. (2017) 376:1015–26. doi: 10.1056/NEJMoa1613683
33. Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
Keywords: PD-1inhibitors, PD-L1 expression, cervical cancer, PD-L1 inhibitors, meta-analysis
Citation: Yang J, Yu H, Zhang Y, Zhu M, Zhang M and Wang Q (2024) Efficacy of PD-1 or PD-L1 inhibitors for the therapy of cervical cancer with varying PD-L1 expression levels: a single-arm meta-analysis. Front. Oncol. 14:1454372. doi: 10.3389/fonc.2024.1454372
Received: 25 June 2024; Accepted: 26 July 2024;
Published: 20 August 2024.
Edited by:
Wenyi Jin, City University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Gang Tian, Sichuan Province Engineering Technology Research Center of Molecular Diagnosis of Clinical Diseases, ChinaYing Yang, Jinhua Municipal Central Hospital, China
Copyright © 2024 Yang, Yu, Zhang, Zhu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiming Wang, bTEzOTU3ODg1MTYyXzFAMTYzLmNvbQ==
 Jie Yang
Jie Yang Haizan Yu
Haizan Yu Qiming Wang
Qiming Wang