- 1Cancer Center, Department of Pathology, Zhejiang Provincial People’s Hospital(Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, China
- 2Department of Pathology, Tongxiang First People’s Hospital, Tongxiang, China
- 3Cancer Center, Department of Radiation Oncology, Zhejiang Provincial People’s Hospital(Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, China
- 4Cancer Center, Department of Pulmonary and Critical Care Medicine, Zhejiang Provincial People’s Hospital(Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, China
- 5Cancer Center, Department of Radiology, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, China
Thoracic SMARCA4-deficient undifferentiated tumors (SMARCA4-UTs), recently recognized as a rare malignancy described in the 5th edition of the World Health Organization Classification of Tumors, are characterized by an inactivating mutation in SMARCA4, most commonly found in the mediastinum of male smokers. Despite the aggressive nature and poor prognosis associated with these tumors, which have a median survival time of approximately 4-7 months, no standardized treatment guidelines are currently established. There are currently no reported cases of extended progression-free survival (PFS) in SMARCA4-UT patients treated with surgery and immunotherapy. Here, we report the clinical features and genomic information of a SMARCA4-UT case in which the patient responded significantly to a combination therapy involving surgery, immunotherapy, and amlotinib. A 56-year-old male non-smoker presented with a mass in the superior lobe of left lung and left hilar adenopathy. A left upper lobectomy and lymphadenectomy were performed, and postoperative pathology confirmed that the tumor was Thoracic SMARCA4-UT. The patient subsequently received chemotherapy with pemetrexed and carboplatin. Five months post-operation, the disease progressed with left adrenal metastasis and mediastinal adenopathy. An adrenalectomy was performed, followed by whole exon sequencing (WES). SMARCA4, SMARCA2 and SMARCA1 gene mutations were detected in this case. Given a tumor proportion score (TPS) of 60% for programmed death-ligand 1(22C3)immunoexpression and high TMB(361.32 muts/Mb), a combination of Pembrolizumab plus anlotinib was initiated as a second-line approach. After 46 cycles, the patient demonstrated no disease progression with a PR lasting 31 months and long progression-free survival(PFS) of 43 months. The lung tumor was initially detected in September 2020, and the patient remained alive at the latest follow-up in November 2024. This case offers a long-term follow-up of the effectiveness and safety of combining pembrolizumab and anlotinib in advanced SMARCA4-UT, and substantiates the role of long-term immunotherapy in preventing radiographic/clinical recurrence following surgery. This case illustrates new potential efficacy of immunotherapy in combination with surgery as a treatment approach of SMARCA4-UT.
Introduction
Thoracic SMARCA4-deficient undifferentiated tumor (SMARCA4-UT) was first recognized as a distinct entity in the 5th edition of the 2021 World Health Organization Classification of Tumors (1). It is a high-grade malignancy that commonly occur in the mediastinum, lung, pulmonary hilum, and pleura. SMARCA4-UT often affects young to middle-aged adults with heavy smoking history and a striking male predominance (2–5). Effective treatments have not yet been developed, and the prognosis remains poor, with a median survival of 4-7 (range, 1-13)months (2). Due to the high probability of early recurrence, upfront surgery is not an effective treatment (6). Although radiotherapy and chemotherapy have limited efficacy, immune checkpoint inhibitors (ICIs) have shown promising results in some patients (7–12). However, given the limited number of patients and short survival duration during the follow-up, rare long-term follow-up cases have been reported in advanced SMARCA4-UT patients. Herein, we report a case of Thoracic SMARCA4-UT with adrenal metastasis, who underwent surgical resections and was successfully treated with Pembrolizumab plus anlotinib, achieving a PR lasting 31 months and long progression-free survival (PFS) of 43 months.
Case description
A 56-year-old male, non-smoker, presented with recurrent low fever in September 2020 with no prior history of tumors. Chest computed tomography (CT) revealed a solid mass in the superior lobe of left lung adjacent the pleura, measuring 5.5x5.0x4.0 cm, accompanied by left hilar adenopathy (Figure 1A). PET-CT showed no bone and distant metastasis. Comprehensive laboratory tests were within normal limits, and blood tests showed no abnormal squamous cell carcinoma antigen (SCC), carcinoembryonic antigen (CEA), or neuron-specific enolase (NSE) levels. On October 10, 2020, the patient underwent a left upper lobectomy and lymphadenectomy. Postoperative pathology revealed a solid tumor structure with large epithelioid cells, obvious atypia, and prominent nucleoli (Figures 2A, B). Immunohistochemistry was positive for SMARCB1/INI1, focally positive for cytokeratin and negative for TTF-1 (Figure 2C), NapsinA, P40 (Figure 2D), SMARCA4 (Figure 2E), CK5/6, Nut, Vimentin, EBER. Ki-67 was as high as 70% (Figure 2F). These findings led to a diagnosis of Thoracic SMARCA4-UT, classified as stage IIIA (pT3N1M0).
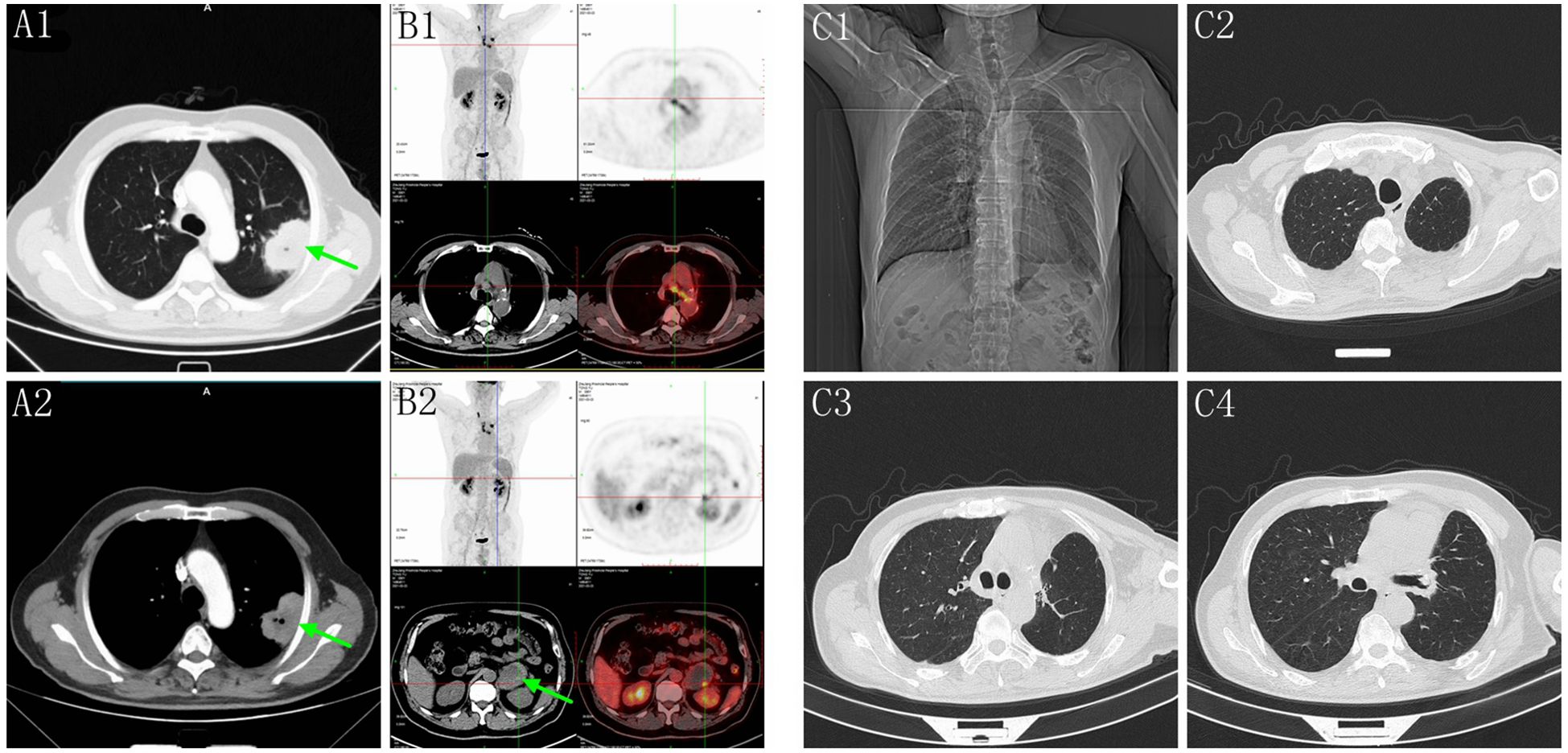
Figure 1. (A1, A2) Chest CT showed a solid mass in the superior lobe of left lung (green arrow). (B1, B2) Five months post-surgery, PET-CT revealed mediastinal adenopathy and a circular mass in the left adrenal gland measuring 8.5x5.6x4.3 cm (green arrow). (C1–C4) Chest CT showed no recurrence and the mediastinal lymph nodes were significantly reduced on September 26, 2024.
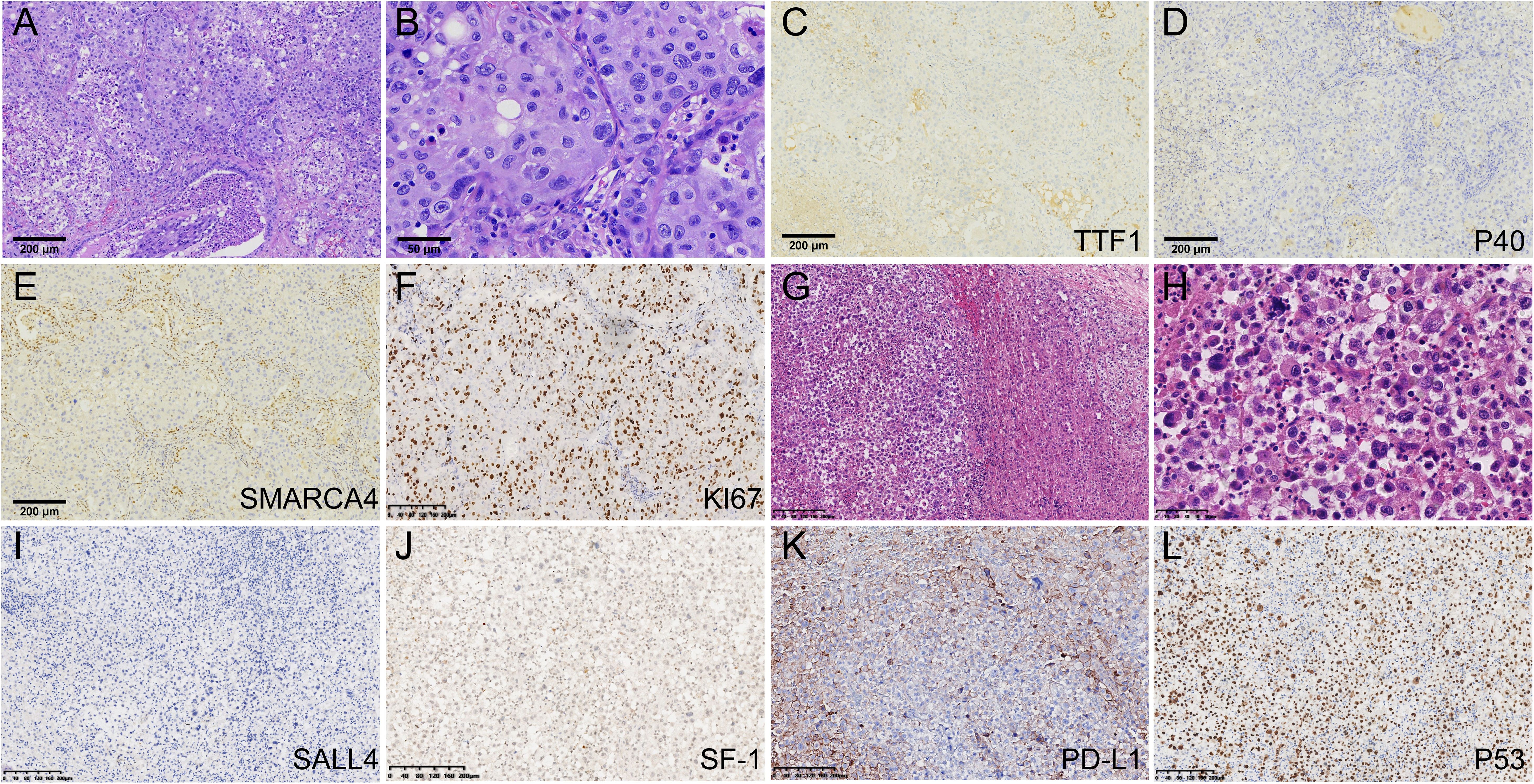
Figure 2. Pathological findings and immunohistochemical results. (A) The lung tumor cells arranged in sheets and were surrounded by an infiltration of inflammatory cells(HE100). (B) Large tumor cells showed obvious atypia, and prominent nucleoli(HE400). (C) TTF-1 staining was negative(x100). (D) P40 staining was negative(100). (E) SMARCA4 staining was negative in the tumor cells(100). (F) ki67 was as high as 70%. (G, H) The tumor cells of left adrenal gland were poorly differentiated, displaying discohesion, marked atypia and prominent nucleoli. (I) SALL4 staining was negative. (J) SF-1 was negative in the tumor cells. (K) PD-L1 TPS score was 60%. (L) P53 is overexpressed.
Subsequently, the patient received chemotherapy (pemetrexed approximately 960 mg day 1 and carboplatin approximately 670 mg day 1 every 4 weeks) with Neulasta support for sustained leukocyte enhancement from December 5, 2020 to March 6, 2021. Blood tests during chemotherapy showed white blood cells at 4.4x10^9/L, hemoglobin at 97 g/L, neutrophil count at 1.9x10^9/L, and platelet count at 245x10^9/L, albumin at 36.7 g/L, ALT at 38 U/L, AST at 28 U/L, creatinine at 87 μmol/L, and urea at 7.48 mmol/L. The patient developed mild anemia and minor adverse reactions throughout the course of chemotherapy.
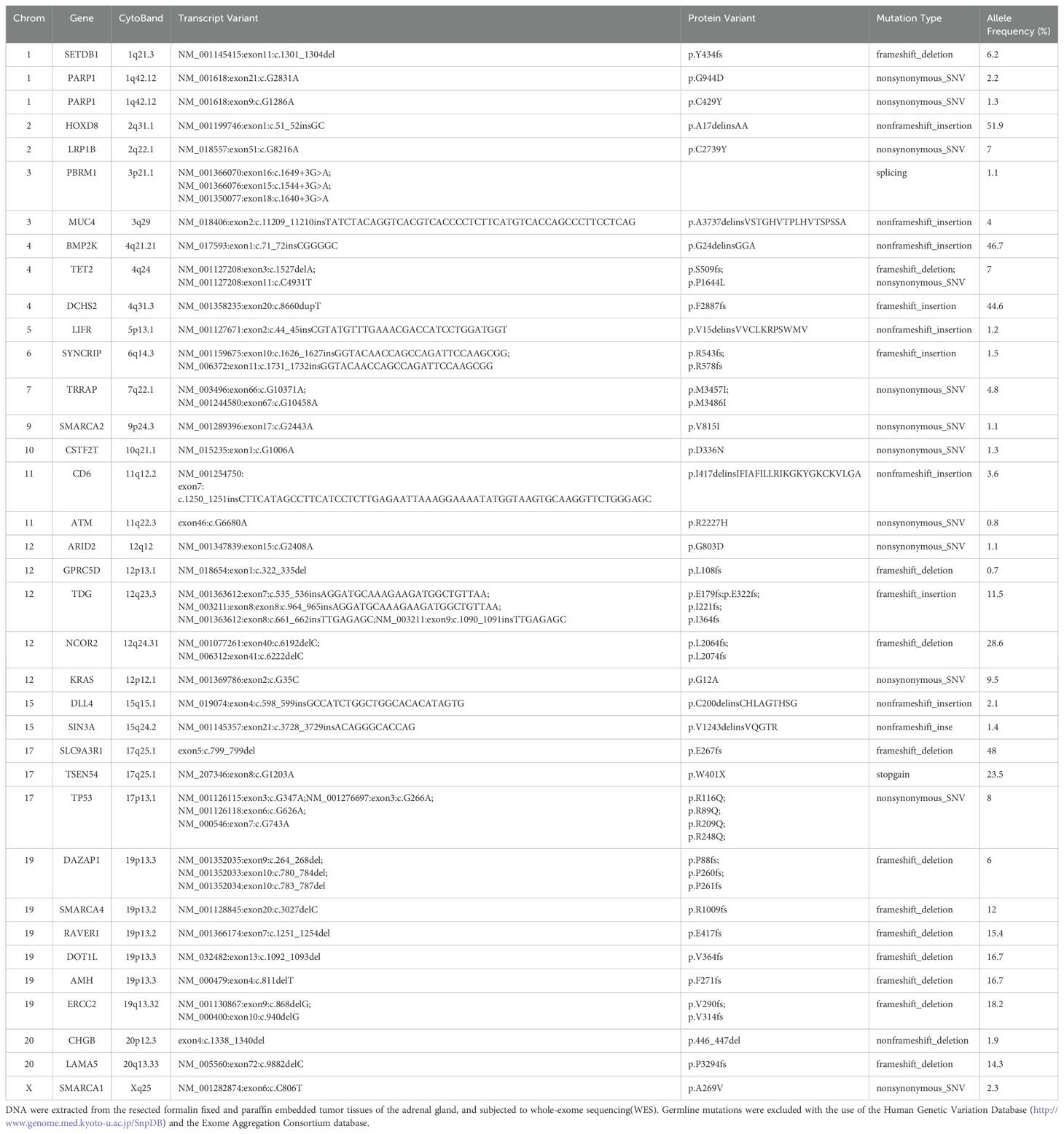
Five months post-surgery, PET-CT revealed mediastinal adenopathy suggesting recurrence, and a circular mass in the left adrenal gland measuring 8.5x5.6x4.3 cm on March 23, 2021 (Figure 1B). No anomalies were detected in the contralateral adrenal gland. Left adrenalectomy was performed on March 26, 2021. Histologically, the tumor cells were poorly differentiated, displaying discohesion, marked atypia and prominent nucleoli (Figures 2G, H). Immunohistochemistry was positive for INI1, and negative for SMARCA4, TTF-1, NapsinA, NUT, SALL4 (Figure 2I), and SF-1 (Figure 2J). PD-L1 (clone 22C3) tumor proportion score (TPS) was 60% (Figure 2K), and P53 is overexpressed (Figure 2L). DNA were extracted from paraffin embedded tumor tissues of the left adrenal gland, and underwent whole-exome sequencing (WES) (AZENTA Life Science [Suzhou] Co. Ltd, Suzhou China). The sequencing results revealed a frameshift deletion in SMARCA4 gene p.R1009fs (c.3027delC), a nonsynonymous SNP in SMARCA2 gene p.V815I (c.G2443A) and a nonsynonymous SNV in SMARCA1 gene p.A269V (c.C806T). This was along with mutations in KRAS (p.G12A), LRP1B (p.C2739Y), ARID2 (p.G803D) and TP53. WES data analysis showed that the tumor mutation burden (TMB) was 361.32 muts/Mb. The genomic profile is demonstrated in Table 1. Consequently, the final diagnosis was SMARCA4-UT with adrenal metastasis, classified as Stage IV rT0N1M1b.
Due to high PD-L1 expression and TMB, ICIs plus antiangiogenic agents were initiated as second-line treatment (pembrolizumab 200 mg D1, anlotinib 10 mg qd D1-D14 every 3 weeks) from April 2021 to October 2024. The patient was regularly monitored during treatment, and no evidence of disease progression was observed on chest and abdominal CT, brain MR scans. After 14 cycles of combined therapy, the patient had a partial response (PR), and chest CT showed a significant reduction of mediastinal lymph nodes on April 14, 2022. No severe toxicity or delayed toxicity occurred during treatment. Subsequently, the patient successfully completed 32 cycles of combined therapy, consisting of pembrolizumab 200 mg Day 1 and anlotinib 10 mg qd Day 1 to Day 14, repeated every 3 weeks as a sequential treatment. A recent chest CT showed no recurrence on September 26, 2024, with a significant reduction in mediastinal lymph nodes (Figure 1C).
The patients exhibited favorable tolerance towards the combination therapy. During the first month, the patient had mild pruritus and rash. After 8 months, the patient developed hypertension, managed with irbesartan. After 9 months, the patient felt chest tightness, and was diagnosed with coronary atherosclerotic heart disease and unstable angina pectoris. Coronary artery stenting was performed. After 16 months, the patient had subclinical hypothyroidism and was treated with levothyroxine. After 41 months, the patient developed adrenocortical hypofunction and was given hydrocortisone acetate tablets. Other clinical assessments, including body weight, blood and urine routine, hepatic and renal function, blood glucose and electrocardiograms, remained within normal limits. These side effects were classified as grade II according to CTCAE 5.0, and the patient did not have any serious immune-related side effects. The patient successfully completed 46 cycles of pembrolizumab combined with anlotinib and achieved a PR duration of 31 months and long PFS of 43 months at time of this report. The patient remained alive at the latest follow-up in November 2024. A summary of the patient's clinicial course is provided in Figure 3.
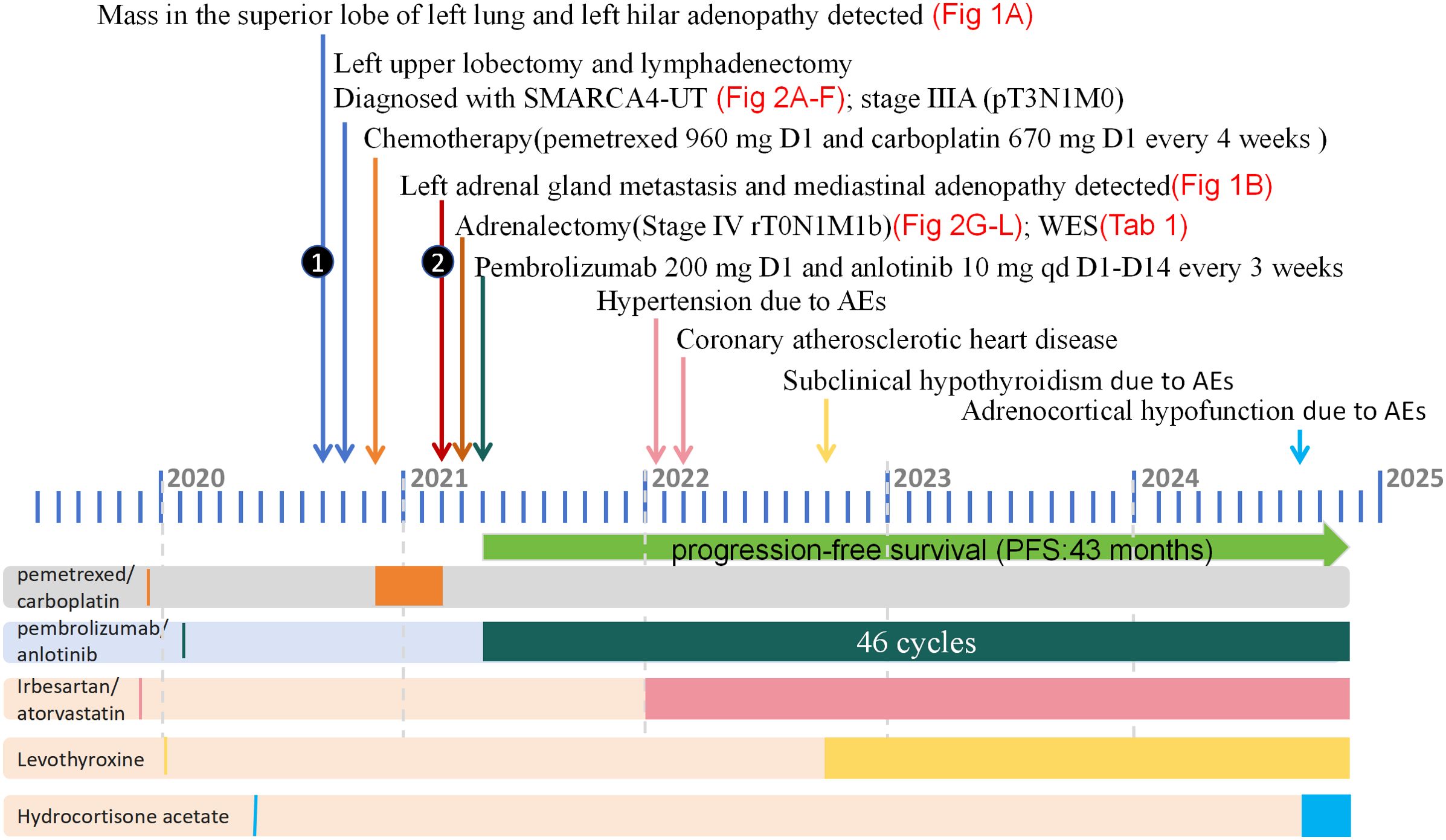
Figure 3. The diagnosis and treatment process of this case from September 2020 to November 2024. Surgery, chemotherapy, and immunotherapy are all represented in the upper part of the figure, with different colors representing different treatment modalities displayed in the lower part of the panel. SMARCA4-UT, SMARCA4-deficient undifferentiated tumor; WES, whole-exome sequencing; AE, adverse event.
Written informed consent was obtained from the patient for the publication of all clinical data and images.
Discussion
SMARCA4, located on chromosome 19p13, encodes for BRG1, one of the two ATPase subunits of the SWI/SNF (BRG1/BRM associated factor or BAF) complex, vital for chromatin remodeling and genomic regulation (5, 13). Inactivation of SMARCA4 (BRG1) has been suggested to be involved in the pathogenesis of some undifferentiated carcinomas of the lung, ovary, gastrointestinal tract, uterus, and other organs (14). Histologically, thoracic SMARCA4-UT typically have solid growth patterns with sheets of variably discohesive high-grade undifferentiated cells with eosinophilic cytoplasm, numerous mitoses, and prominent nucleoli (1–3). Most cases also exhibit a variable degree of rhabdoid morphology and necrosis (3). Immunohistochemically, SMARCA4-UT have a simultaneous deficiency of SMARCA4 (BRG1) and SMARCA2 (BRM) (5). Next-generation sequencing (NGS) are beneficial for the diagnosis. A review of recent literature indicates that the genomic mechanisms leading to SMARCA4 inactivation are variable, including mainly nonsense and frameshift mutations, with missense mutations, splice-site mutations, or deletions being less common (2, 3). Differential diagnoses for SMARCA4-UT include NUT carcinoma, lymphoma, mediastinal germ-cell tumor, round cell sarcomas, and neuroendocrine carcinoma, as well as various types of sarcomas (15).
Common metastatic sites for SMARCA4-UT include lymph nodes, bone, adrenal glands, and liver. A recent literature review of 102 documented cases with variable details revealed that adrenal involvement was present in 14 (14%) of the cases, while 20 (20%) exhibited other forms of intra-abdominal disease, most frequently affecting the liver and peritoneum (3). Coexisting bulky abdominal disease may make determination of primary site difficult (5). In clinical practice, the presence of a large adrenal mass may prompt the clinical consideration of an adrenal primary tumor, especially when the rhabdoid morphology may closely mimic an oncocytic adrenocortical carcinoma. Steroidogenic factor-1 (SF-1) is considered one of the most useful markers for distinguishing adrenal cortical lineage from other tumors (16). While one might consider the possibility of primary adrenal SMARCA4-deficient undifferentiated tumors, comprehensive genomic characterization and immunohistochemical feature of adrenocortical carcinomas have not revealed alterations in the SMARCA4 gene (17). Given the presence of a lung mass with left hilar adenopathy and considering the overall pathological and genetic characteristics of this case, negative expression of SF-1, it was concluded that the patient had a primary thoracic SMARCA4-UT with metastasis to the adrenal gland. This determination underscores the importance of a thorough evaluation of the tumor’s characteristics and metastatic patterns to accurately identify the primary site and guide treatment strategies.
SMARCA4-UT is a rare, highly aggressive malignancy, with limited response to chemotherapy and surgery. All patients essentially have disease progression or recurrence, and the cause of death was local complications due to disease burden (18). Recent studies have reported that ICIs show promising results in the treatment of SMARCA4-UT (7–12). Consensus predictors of immunotherapy efficacy include PD-L1 expression levels, TMB and MSI status. However, based on a review of previously published cases in SMARCA4-UT that had some response to ICIs, PD-L1 ranges from 0 to 100% (7–12). Thus, immunotherapy efficacy seems to be independent of PD-L1 expression. In the present case, PD-L1 TPS score was 60%, and WES TMB is 361.32 muts/Mb, greater than 199 muts/Mb, belonging to high TMB (19). In many cancer types, higher TMB was associated with poorer survival, in contrast to ICI-treated patients in whom higher TMB was associated with longer survival (20). Therefore, high TMB may be a valid indicator of immunotherapy in SMARCA4-UT. The biological mechanisms driving antitumor activity of ICI in SMARCA4-UT remains unclear, and further studies are required to identify predictive biomarkers and patients who will benefit from immunotherapy.
KEAP1, STK11, ARID1A, KRAS, and NF1 mutations are the second most common mutations in SMARCA4-UT (2). KEAP1 and STK11 mutations have been identified as associated with immunotherapy resistance in lung cancer (21). In this case, KRAS (p.G12A) mutation was found, but no KEAP1 or STK11 mutation were found. Studies have shown that KRAS mutation is associated with worse overall survival in PD-L1 negative NSCLC, and this association is largely driven by comutations with STK11 and KEAP1, which are enriched in PD-L1 negative tumors (22). Moreover, LRP1B and DNA damage repair genes (PARP1/2, ERCC1-4, MSH2-6, ATM, BRCA1/2, etc.) mutations have been shown to be associated with favorable outcomes to ICIs in lung cancer (23, 24). We also found LRP1B (p.C2739Y), ERCC2 (p.V290fs, pV314FS), PARP1 (p.Y434fs, p.G944D) and ATM (p.R2227H) mutations in this case. Therefore, these genetic characteristics may account for the good response to immunotherapy. We detected a nonsynonymous SNP in SMARCA2 gene p.V815I (c.G2443A) and a nonsynonymous SNV in SMARCA1 gene p.A269V (c.C806T), which are not registered in the Catalogue Of Somatic Mutations In Cancer database (25), and their oncogenicity is unknown.
Conclusions
Herein, we report a case of 4-year long-term survival in a patient with Thoracic SMARCA4-UT and adrenal metastases who underwent surgeries and a combination therapy of pembrolizumab and anlotinib, achieving a PR duration of 31 months and PFS of 43 months. The lung tumor was initially detected in September 2020, and the patient remained alive at the latest follow-up in November 2024. Our case provides a long-term follow-up of the efficacy and safety of immunotherapy in advanced SMARCA4-UT, and highlights the potential of long-term immunotherapy in preventing radiographic/clinical recurrence after surgery. This illustrates new potential of immunotherapy in combination with surgery as an effective therapy in the treatment of SMARCA4-UT, and imaging changes can evaluate efficacy and long-term prognosis in these patients. Clinical trials exploring the role of ICI monotherapy or the combination of ICIs with other pathway targeting in SMARCA4-UT are needed, as are studies focused on predictive biomarkers and patient selection.
Data availability statement
The data presented in the study are deposited in the SRA database in NCBI, accession number PRJNA119820. The direct link is https://www.ncbi.nlm.nih.gov/sra.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Zhejiang Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TD: Data curation, Funding acquisition, Investigation, Writing – original draft, Conceptualization, Methodology. MX: Formal Analysis, Investigation, Methodology, Writing – original draft, Visualization. HZ: Conceptualization, Data curation, Formal Analysis, Software, Writing – original draft. SW: Methodology, Project administration, Software, Validation, Writing – original draft. HW: Data curation, Supervision, Validation, Visualization, Writing – original draft. ZG: Funding acquisition, Resources, Supervision, Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Zhejiang medicine health science and technology plan general project (grant number 2024KY728); the National Natural Science Foundation of China(Grant number No.82072577); and Natural Science Foundation of Zhejiang Province of China(Grant number No. LY21H160002).
Acknowledgments
All authors would like to thank the patient and his family for allowing this case study to be published.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1453895/full#supplementary-material
References
1. Yoshida A, Boland JM, Jain D, Le Loarer F, Rekhtman N. Thoracic SMARCA4-deficient undifferentiated tumour. In: World Health Organization classification of Tumours, WHO classification of thoracic Tumours, 5th ed. International Agency for Research on Cancer, Lyon, France (2021). p. 111–4.
2. Le Loarer F, Watson S, Pierron G, de Montpreville VT, Ballet S, Firmin N, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic Malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet. (2015) 47:1200–5. doi: 10.1038/ng.3399
3. Rekhtman N, Montecalvo J, Chang JC, Alex D, Ptashkin RN, Ai N, et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol. (2020) 15:231–47. doi: 10.1016/j.jtho.2019.10.023
4. Sauter JL, Graham RP, Larsen BT, Jenkins SM, Roden AC, Boland JM. SMARCA4-deficient thoracic sarcoma: a distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Mod Pathol. (2017) 30:1422–32. doi: 10.1038/modpathol.2017.61
5. Yoshida A, Kobayashi E, Kubo T, Kodaira M, Motoi T, Motoi N, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol. (2017) 30:797–809. doi: 10.1038/modpathol.2017.11
6. Luo J, Ding B, Campisi A, Chen T, Teng H, Ji C. Molecular, clinicopathological characteristics and surgical results of resectable SMARCA4-deficient thoracic tumors. J Cancer Res Clin Oncol. (2023) 149:4455–63. doi: 10.1007/s00432-022-04359-6
7. Takada K, Sugita S, Murase K, Kikuchi T, Oomori G, Ito R, et al. Exceptionally rapid response to pembrolizumab in a SMARCA4-deficient thoracic sarcoma overexpressing PD-L1: A case report. Thorac cancer. (2019) 10:2312–5. doi: 10.1111/1759-7714.13215
8. Tanaka S, Hayashi S, Isobe Y, et al. Positive outcome of first-line therapy for a SMARCA4-deficient thoracic sarcomatoid tumor. Int Cancer Conf J. (2021) 10:112–5. doi: 10.1007/s13691-021-00472-4
9. Iijima Y, Sakakibara R, Ishizuka M, et al. Notable response to nivolumab during the treatment of SMARCA4-deficient thoracic sarcoma: a case report. Immunotherapy. (2020) 12:563–9. doi: 10.2217/imt-2019-0142
10. Kawachi H, Kunimasa K, Kukita Y, et al. Atezolizumab with bevacizumab, paclitaxel and carboplatin was effective for patients with SMARCA4-deficient thoracic sarcoma. Immunotherapy. (2021) 13:799–806. doi: 10.2217/imt-2020-0311
11. Henon C, Blay JY, Massard C, et al. Long lasting major response to pembrolizumab in a thoracic Malignant rhabdoid-like SMARCA4-deficient tumor. Ann Oncol. (2019) 30:1401–3. doi: 10.1093/annonc/mdz160
12. Pokhrel A, Yadav R, Manvar KK, et al. Chemotherapy and immune checkpoint inhibitors in a case of SMARCA4-dUT: A case report and review of literature. J Investig Med High Impact Case Rep. (2023) 11:23247096231176220. doi: 10.1177/23247096231176220
13. Chatzopoulos K, Boland JM. Update on genetically defined lung neoplasms: NUT carcinoma and thoracic SMARCA4-deficient undifferentiated tumors. Virchows Arch. (2021) 478:21–30. doi: 10.1007/s00428-020-03011-3
14. Tian Y, Xu L, Li X, Li H, Zhao M. SMARCA4: Current status and future perspectives in non-small-cell lung cancer. Cancer letters. (2023) 554:216022. doi: 10.1016/j.canlet.2022.216022
15. Perret R, Chalabreysse L, Watson S, Serre I, Garcia S, Forest F, et al. SMARCA4-deficient thoracic sarcomas: clinicopathologic study of 30 cases with an emphasis on their nosology and differential diagnoses. Am J Surg Pathol. (2019) 43:455–65. doi: 10.1097/PAS.0000000000001188
16. Mete O, Asa SL, Giordano TJ, Papotti M, Sasano H, Volante M. Immunohistochemical biomarkers of adrenal cortical neoplasms. Endocr Pathol. (2018) 29:137–49. doi: 10.1007/s12022-018-9525-8
17. Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell. (2016) 30:363. doi: 10.1016/j.ccell.2016.07.013
18. Crombé A, Alberti N, Villard N, Pilleul F, Buy X, Le Loarer F, et al. Imaging features of SMARCA4-deficient thoracic sarcomas: a multi-centric study of 21 patients. Eur Radiol. (2019) 29:4730–41. doi: 10.1007/S00330-019-06017-X
19. Budczies J, Allgäuer M, Litchfield K, Rempel E, Christopoulos P, Kazdal D, et al. Optimizing panel-based tumor mutational burden (TMB) measurement. Ann Oncol. (2019) 30:1496–506. doi: 10.1093/annonc/mdz205
20. Valero C, Lee M, Hoen D, Wang J, Nadeem Z, Patel N, et al. The association between tumor mutational burden and prognosis is dependent on treatment context. Nat Genet. (2021) 53:11–5. doi: 10.1038/s41588-020-00752-4
21. Cordeiro de Lima VC, Corassa M, Saldanha E, Freitas H, Arrieta O, Raez L, et al. STK11 and KEAP1 mutations in non-small cell lung cancer patients: Descriptive analysis and prognostic value among Hispanics (STRIKE registry-CLICaP). Lung Cancer. (2022) 170:114–21. doi: 10.1016/j.lungcan.2022.06.010
22. Sun L, Handorf EA, Zhou Y, Borghaei H, Aggarwal C, Bauman J. Outcomes in patients treated with frontline immune checkpoint inhibition (ICI) for advanced NSCLC with KRAS mutations and STK11/KEAP1 comutations across PD-L1 levels. Lung Cancer. (2024) 190:107510. doi: 10.1016/j.lungcan.2024.107510
23. Brown LC, Tucker MD, Sedhom R, Schwartz EB, Zhu J, Kao C, et al. LRP1B mutations are associated with favorable outcomes to immune checkpoint inhibitors across multiple cancer types. J Immunother Cancer. (2021) 9:e001792. doi: 10.1136/jitc-2020-001792
24. Averbuch I, Tschernichovsky R, Icht O, Goldstein DA, Mutai R, Dudnik E, et al. Correlations between pathogenic variants in DNA repair genes and anticancer treatment efficacy in stage IV non-small cell lung cancer: A large real-world cohort and review of the literature. Thorac Cancer. (2023) 14:1589–96. doi: 10.1111/1759-7714.14902
Keywords: lung cancer, SMARCA4-UT, immune checkpoint inhibitors, anti-angiogenic agent, prognosis, genen mutation
Citation: Duan T, Xu M, Zhang H, Wu S, Wang H and Guo Z (2024) Long-term follow-up of combination therapy with pembrolizumab and anlotinib in thoracic SMARCA4-deficient undifferentiated tumor: a case report and molecular features. Front. Oncol. 14:1453895. doi: 10.3389/fonc.2024.1453895
Received: 24 June 2024; Accepted: 18 November 2024;
Published: 11 December 2024.
Edited by:
Eswar Shankar, The Ohio State University, United StatesReviewed by:
Shiv Verma, Case Western Reserve University, United StatesBalaji Chandrasekaran, Texas A and M University, United States
Copyright © 2024 Duan, Xu, Zhang, Wu, Wang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenying Guo, aXZ5Z3VvY25AMTI2LmNvbQ==
 Ting Duan
Ting Duan Mingxin Xu2
Mingxin Xu2 Haibo Zhang
Haibo Zhang