- 1Integrated Biology of Rare Tumors Unit, Department of Research, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
- 2Head and Neck Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
- 3Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy
- 4Evaluative Epidemiology Unit, Department of Epidemiology and Data Science, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
- 5Retired, Milan, Italy
Oral cavity squamous cell carcinoma (OCSCC) predominantly affects the tongue and the floor of the mouth, primarily in patients over 50 years of age. Incidence and mortality rates vary significantly worldwide, influenced by geographic areas and demographic characteristics. Epidemiological studies revealed an increase in incidence of OCSCC among young adults (YA) <44 years old. This narrative review, provides updated information on the incidence, risk factors, and prognosis of YA-OCSCC using data published from 2018 to 2023 from different geographic locations. The studies indicate that the incidence of YA-OCSCC in Asia is approximately twice that in the US and that the incidence is strongly linked to risk factors such as betel quid chewing, tobacco use, and high alcohol consumption. The prognosis for YA-OCSCC, compared to that in older patients, shows similar or better overall survival, even in cases with relapses, but worse 5-year disease-free survival, despite receiving similar treatments. Consequently, a concerted effort is crucial to raise awareness about the cessation of tobacco and areca nut use, alcohol control, and the promotion of healthy lifestyle behaviors. Recent molecular data on YA-OCSCC suggests a potential profile characterized by epidermal growth factor receptor overexpression, low tumor mutation burden and an attenuated immune response. Upon confirmation in larger cohorts of YA-OCSCC patients from different geographical areas, the validated markers could aid in selecting tailored treatments.
1 Introduction
Oral cavity squamous cell carcinoma (OCSCC) is the predominant histotype among oral cavity malignant tumors, comprising over 90% of cases. GLOBOCAN estimates (2020) (1) indicate that there are approximately 380,000 new OCSCC cases worldwide, with an age-standardized mortality rate of 2.8 per 100,000 in men and 1.0 per 100,000 in women. The Global Cancer Observatory (GCO) (2), an interactive web-based platform maintained by the International Agency for Research on Cancer (IARC), reports that, among the six continents, Asia has the highest incidence of oral cancer (including lip and tongue) (65.8% of all cases), followed by Europe (17.3%) and North America (7.3%) (Figure 1A). Furthermore, GCO (2), offers projections for new cases between 2022 and 2045 through the “Cancer tomorrow” link (https://gco.iarc.fr/tomorrow). These projections suggest that the greatest increase in new cases will occur in Africa, with the smallest increase in Europe (Figure 1B). Notably, incidence and mortality rates vary significantly worldwide, influenced by geographic areas and demographic characteristics. Additionally, over 70% of OCSCC deaths occur in countries with a low or medium human development index (3).
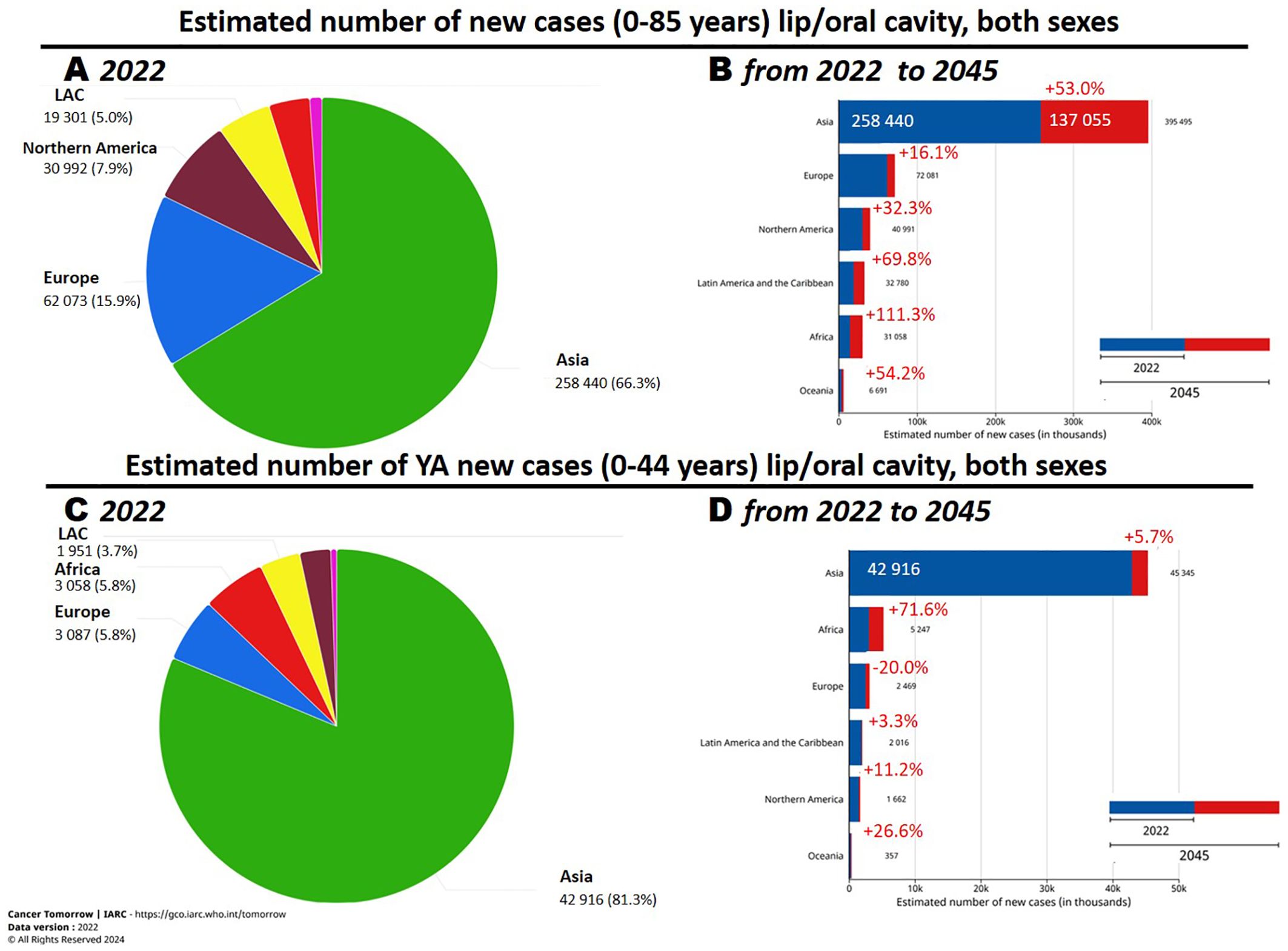
Figure 1. (A, C) Number of new oral cavity cancer cases in 2022 and (B, D) estimated changes in 2045. Reprinted with permission from the online data visualization tools for exploring the global cancer burden provided by the IARC (International Agency for Research on Cancer) Global Cancer Observatory. Figures “Cancer Today. Absolute numbers, Incidence, Both sexes, in 2022. Lip, oral cavity. Continents”, “Cancer Today. Absolute numbers, Incidence, Both sexes, age [0-44], in 2022. Lip, oral cavity. Continents”, “Cancer Today. Age-Standardized Rate (World) per 100 000, Incidence, Both sexes, in 2022. Lip, oral cavity. Continents”, “Cancer Today. Age-Standardized Rate (World) per 100 000, Incidence, Both sexes, age [0-44], in 2022. Lip, oral cavity. Continents” Respective URLs (accessed on 17th June 2024): https://gco.iarc.fr/today/en/dataviz/pie?mode=population&group_populations=0&cancers=1 https://gco.iarc.fr/today/en/dataviz/pie?mode=population&group_populations=0&cancers=1 &age_end=8&populations=903_904_905_908_909_935 https://gco.iarc.fr/today/en/dataviz/bars?mode=population&cancers=1 https://gco.iarc.fr/today/en/dataviz/bars?mode=population&cancers=1&age_end=8 Copyright 2024. Permission from IARC obtained on 17th June 2024.
OCSCC involves the tongue and the mouth floor, predominantly in patients >50 years of age, with a male-to-female ratio of about 2:1. However, epidemiological studies have observed a notable incidence of cases, especially tongue cancer, in young adults (YA) <44 years old. Although the estimates found in GCO are for tumors of the oral cavity including lips, in the age group 0-44 years the incidence of tumors of the lips is much lower than that of the oral cavities, less than 10% of the total, so it can be assumed that for this specific age group the estimates provided by GCO are a good approximation for OCSCC tumors (http://rarecarenet.istitutotumori.mi.it/analysis.php).
In 2020 GCO identified that approximately 17% of OCSCC were diagnosed in young–adult patients (Figure 1C) and “Cancer tomorrow” link estimates that the number of new OCSCC cases from 2020 and 2040 will have the higher increase in Africa and a relevant decrease in Europe (Figure 1D).Before 2018, we found only one paper analyzing big population data, all the others were hospital-based and the analyzed small cohort. The paper analyzed a comprehensive screening of the Taiwanese general population aged >18 years between 2004 and 2009 (> 18 million individuals). From this cohort > 4 million persons were identified as high-risk population and included in the screening. 4,110 OCSCC were identified and 42% of them were in the YA age range (4). A recent epidemiological study, based on a population cancer registry in Taiwan, depicting long-term trends of OCSCC from 1980 to 2019, identified Taiwan as having the highest incidence worldwide (5). Indeed, between 1980–1984 and 2015–2019, the age-standardized incidence rates increased from 4.19 to 27.19 per 100,000 in men and from 1.16 to 2.8 per 100,000 in women.
At the end of 2018, a seminal review aimed at analyzing early-onset OCSCC within the USA summarized the literature on risk factors, outcomes, and molecular analyses (6). Although this review referenced numerous studies, the vast majority were published before 2015, focused on a single geographic area, and involved limited patient cohorts. Furthermore no data about molecular genetic aspects were reported. Over the past seven years, an increasing body of evidence has emerged from various geographic areas. Therefore, the objectives of this “narrative review” are: i)present and discuss data published from 2018 to 2023; ii) summarize the new data about genetic predisposition, viral infections, and the preliminary identification of molecular data as potential risk factors or biomarkers.
2 Search strategy and selection criteria
A literature search was conducted using the PubMed database to identify studies pertinent to the epidemiology, risk factors, prognosis, and molecular data in YA-OCSCC patients. The search spanned from January 2018 to November 2023 and letters, editorials, study protocols, case reports, short communications, and non-English articles were excluded. Independent researchers, three in molecular biology and two in the clinical management of patients with head and neck squamous cell carcinoma (HNSCC), evaluated the articles for quality and thematic relevance. Additionally, the references of all included papers were reviewed to identify further studies of interest. The final selection was revised by two senior researchers.
3 Incidence of YA-OCSCC and associated risk factors in different countries
OCSCC constitutes a major global health challenge (1), with its incidence notably higher in certain geographic regions. India is regarded as the global epicenter of OCSCC (7), however, a limitation in analyzing the Indian context is that cancer registries encompass merely 10% of the population, predominantly in urban areas, while 72% of the population resides in rural settings. Consequently, the incidence rates reported may be an underestimation of the true figures (7).
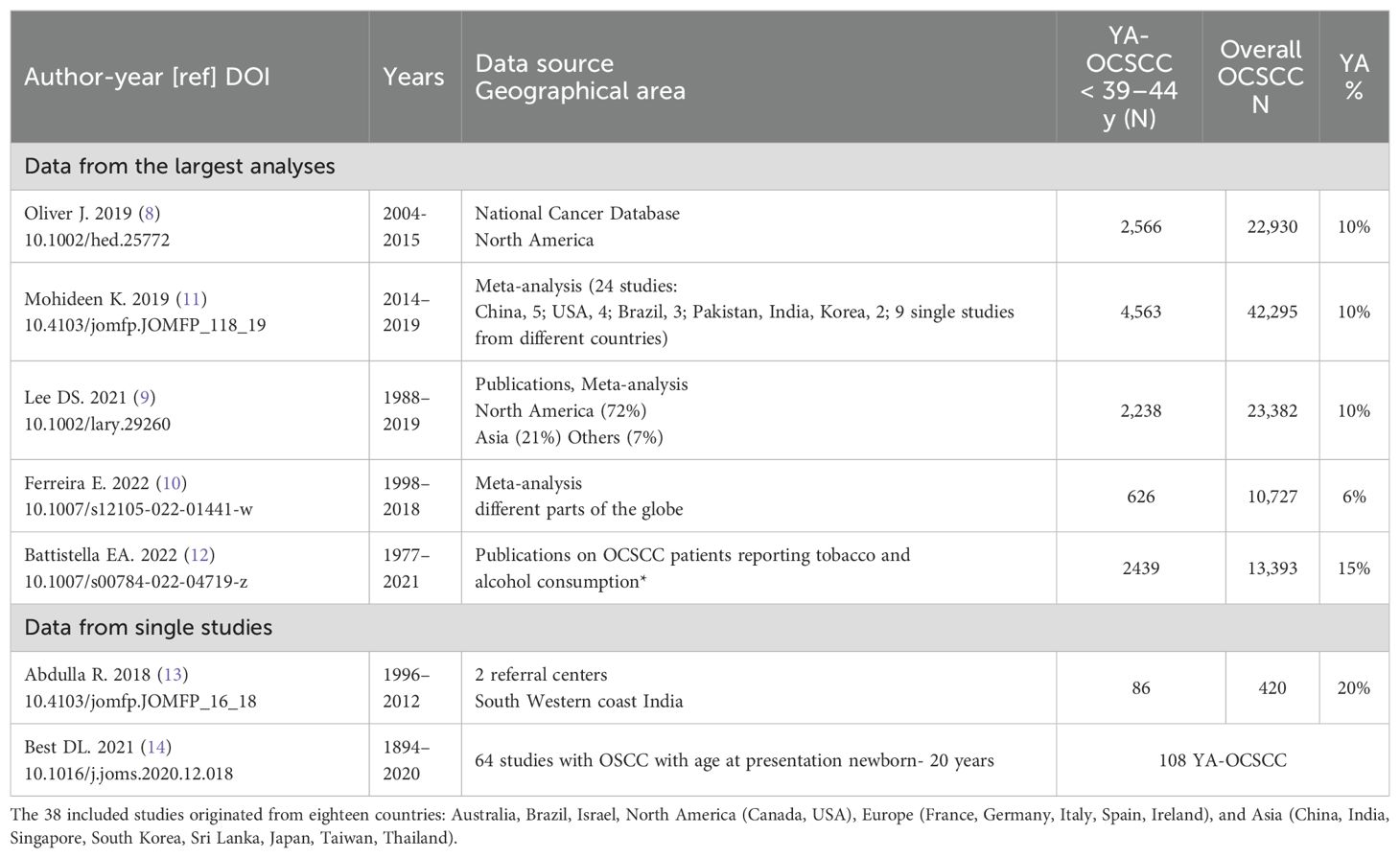
Five recent studies on YA-OCSCC incidence, with a high number of included cases (8–12) are reported in Table 1. Two studies (8, 9) reported that the incidence of YA-OCSCC in North America was around 10%. According to “Tracking changes in the age distribution of head and neck cancer in the United States from 1975 to 2016” (data from the SEER-NCI program), the mean age at HNSCC diagnosis has increased over the last 40 years, with YA-OCSCC being the only HNSCC subset to demonstrate a significant increase in proportion, likely due to a rise in diagnoses among young women (15). We also included in Table 1 two individual studies: the first from two referral centers in India (13) and the second discussing risk factors, prognosis, and treatment strategies for YA-OCSCC in patients aged newborn to 20 years (14).
The most recent meta-analysis on YA-OCSCC (10), including different continents, did not identify an increase in YA-OCSCC incidence within the analyzed timeframe (1998–2018). However, the YA-OCSCC trend in incidence in the population under study (South America, China, Europe, India, and South Africa) was 6%, while in India, it was more than double at 13%. The single study (13) reporting a 20% prevalence of YA-OCSCC appears to confirm its higher prevalence in Asia compared to that of older patients. The established risk factors for YA-OCSCC include tobacco use, high alcohol consumption, and betel quid chewing (16). In North America and Europe, alcohol consumption and tobacco smoking are widespread and may persist throughout life. In South-Central Asia, tobacco smoking and betel quid chewing are more common practices.
Figure 2 summarizes the factors involved in the development of YA-OCSCC, categorized according to their likelihood of causing the tumor. Approximately 600 million people chew betel quid in India and Southeast Asia (17). Betel quid typically contains areca nut, betel leaf, catechu, slaked lime, and often tobacco (18). The areca nut produces carcinogenic nitrosamines in the saliva of chewers, leading to oral preneoplastic disorders with a high likelihood of progressing to cancer (18). Consequently, IARC has classified it as a Group 1 carcinogen (19). Areca nut, the fruit of the Areca catechu palm, is widely cultivated in Asia and the Pacific Islands, where it is more popular (19). Betel nut chewing begins at a very young age in Micronesia, resulting in a high number of school children developing oral mucosal lesions (20). Efforts by the World Health Organization to control betel nut chewing face challenges due to its deep cultural roots, including religious significance in some parts of Southeast Asia and India (17).
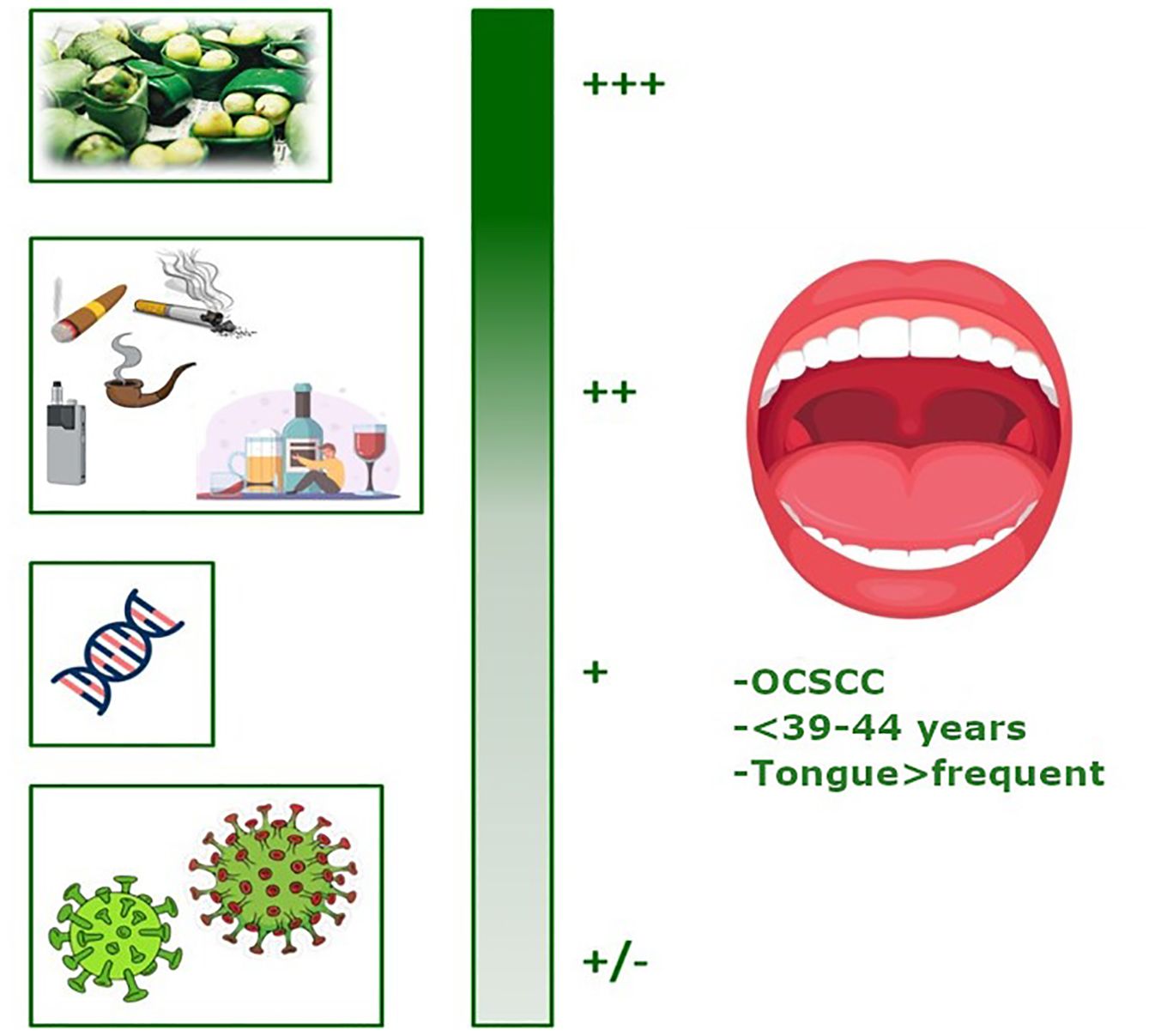
Figure 2. Graphical representation of the factors involved in the development of YA-OCSCC (ranked by probability). Created with BioRender.com.
The new evidences confirm that the Betel quid chewing represents the factor with the highest probability of causing cancer (Figure 2: +++).
Data about tobacco smoking and alcohol consumption (Figure 2:++) indicated that are still the most prominent causes of cancer worldwide and are considered significant for OCSCC occurrence, and, as evidenced by studies published in the recent years, the comparison of the proportion of individuals reporting tobacco and alcohol consumption showed that these habits were more prevalent in the older group (48.4% and 45.8%, respectively) than in the younger group (39.5% and 30.9%, respectively). Schantz et al. (21) reported that the proportion of YA patients with a history of tobacco use (cigarette smoking or tobacco chewing) decreased from 64% to 35% between 1944 and 1984. A similar trend was observed in patients who were drinkers.
During the same period, the popularity of smokeless tobacco products, such as electronic cigarettes (ECs) or vaping, has increased worldwide, driven by the belief that they are safer alternatives to traditional smoking. These studies found that although EC users have a lower cancer incidence than do conventional cigarette smokers, their cancer burden remains twice that of nonsmokers (22). Campbell et al. (23) conducted an extensive study in US referral centers evaluating the risk factors associated with YA-OCSCC. This was the first study to report an association between YA-OCSCC and EC use in the US. Despite the absence of a control group, the proportion of EC use among YA-OCSCC patients (12.4%) exceeded the national rates for similar age groups (5–6%), suggesting a potential role for EC in tumor development. Furthermore, a recent systematic review found that EC users tend to be YA males with higher education levels. This provides valuable insights for refining intervention strategies aimed at EC users to enhance the effectiveness of anti-smoking efforts (24).
The medium-low risk factors included inherited genetic alterations (Figure 2: +), such as those associated with Fanconi anemia (FA). FA, a rare hereditary disease typically autosomal recessive, is caused by mutations in one of 22 known FA-related genes (25). It is characterized by a deficiency in DNA damage repair, particularly inter-strand DNA crosslink repair, leading to bone marrow failure (26). FA also increases genomic instability in the epithelial cells of the head and neck region. Patients with FA have a significantly elevated risk of malignancy by age 40, with OCSCC being the most common solid tumor observed (the estimated incidence of OCSCC is higher in patients with FA than in the general population). Moreover, as the life expectancy of patients with FA improves, the incidence of OCSCC (27) may rise over time.
Some YA-OCSCC patients without FA present with a monoallelic germline alteration in an FA gene and a second risk factor, such as APOBEC alteration (26). Additionally, other genetic predispositions are implicated in YA-OCSCC. For example, the rs6942067 GG genotype, a single nucleotide polymorphism upstream of the DCBLD1 gene, was significantly more prevalent in the Cancer Genome Atlas HNSCC cohort among young, HPV-negative non-smokers with HNSCC (28). Furthermore, polymorphisms in the major histocompatibility complex class I-related chain A (MICA), which are critical for eliminating malignant tumors, have been associated with carcinogenesis in adolescents and YA with OCSCC, suggesting their potential as cancer markers in this age group (29). Studies on the impact of age at diagnosis indicate that YA-OCSCC risk increases with a positive family history of cancer (16). The International Head and Neck Cancer Epidemiology Consortium’s observation of this association (30) led Mahmood et al. (31) to conclude that genetic factors are likely to play key roles. Additionally, Best reported that OCSCC in individuals under 21 years of age, who lack a predisposing condition, likely represents a rare entity with an etiology that is not well understood (Table 1) (14).
Certain virus infections (Figure 2: +/-) may be potential risk factors. Over the past few decades, HPV infection has emerged as a risk factor for HNSCC, particularly in the oropharynx, most often originating from the tonsillar crypt epithelium. HPV oncoproteins inactivate specific proteins, disrupting cell cycle regulation, causing genetic instability, and increasing the proliferation of cancer cells, thereby promoting tumor development (16). Studies have indicated a higher incidence of HPV-related OCSCC in younger patients, typically 5–10 years younger than those who are HPV-negative and usually without established risk factors such as tobacco and alcohol consumption (32). Patients with HPV-related OCSCC exhibit distinct clinical and epidemiological features, including younger age at diagnosis, high-risk sexual behaviors, and improved survival rates, differentiating these cancers from HPV-negative ones (33). Hepatitis C virus (HCV) is another viral risk factor for OCSCC. Su et al. (34) studied the population in Taiwan, where both viral hepatitis and OCSCC are endemic, from 2000 to 2005. Comparing 21,199 adults with chronic HCV to 84,796 sex/age-matched subjects without viral hepatitis, they discovered that HCV-positive patients developed OCSCC at an earlier age (<50 years) than those in the viral hepatitis-free control group.
A recent systematic review and meta-analysis examining the epidemiology of OCSCC in non-smokers indicate a distinct profile compared to smokers with OCSCC, with a majority of cases being women (54–82%) and patients typically younger (<50 years) or older (>70 years) than their male counterparts at the time of diagnosis (35). The authors propose a multifactorial etiology while acknowledging that the current literature on the topic of non-smokers with OCSCC is limited, emphasizing the need for further study.
3.1 Outcome of YA-OCSCC
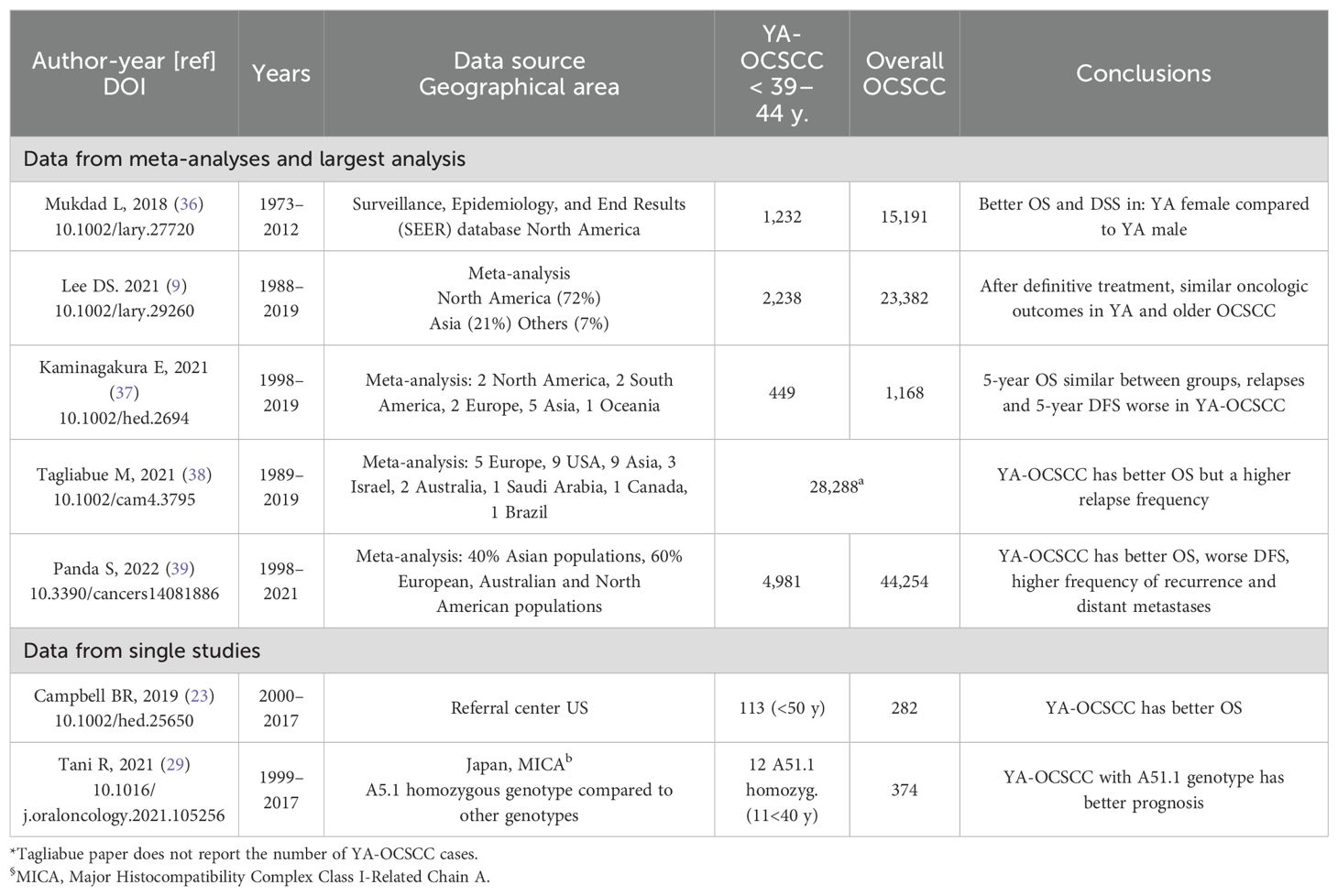
Whether age is an independent prognostic factor in OCSCC is debatable (Table 2). Recent retrospective studies have indicated that YA-OCSCC patients exhibit better outcomes than their older counterparts (23, 29, 48). Registry data, from Surveillance Epidemiology and End Result (SEER) (36) and one meta-analysis (39), consistently demonstrated superior overall survival (OS) in YA-OCSCC compared to older patients with OCSCC. However, two separate meta-analyses (9, 37), reported similar outcomes for both YA and older individuals. Moreover, a worse disease-free survival (DFS) (37, 39), and a higher risk of distant metastasis (39) have been observed in YA-OCSCC patients.
These observations may be influenced by various confounding factors. Older individuals are often frailer than young adults; thus, it is possible that YA-OCSCC patients received more intensive treatment. Moreover, older individuals typically have more comorbidities, which suggests that competing causes of death could have affected the mortality rates reported in the studies. Additionally, while some research indicates that YA-OCSCC patients may experience poorer DFS, it is conceivable that their potentially better tolerance of salvage/palliative treatments may have favorably impacted their post-recurrence survival.
Based on the available literature and quality of the studies, no final conclusion can be drawn on the independent prognostic role of age for OCSCC. However, while conducting translational research studies on prognostic signatures for OCSCC patients, age should be regarded as a relevant clinical variable to be included in multivariable models.
3.2 Molecular data
The heterogeneity of HNSCC and OCSCC, along with the challenges in distinguishing hereditary factors from environmental ones, complicates the identification of a single set of genes involved in the diseases’ pathogenesis (16, 31). Furthermore, Mishra et al. (49) conducted an extensive literature survey and analysis to generate chromosome maps that specifically highlight OSCC-related mutations.
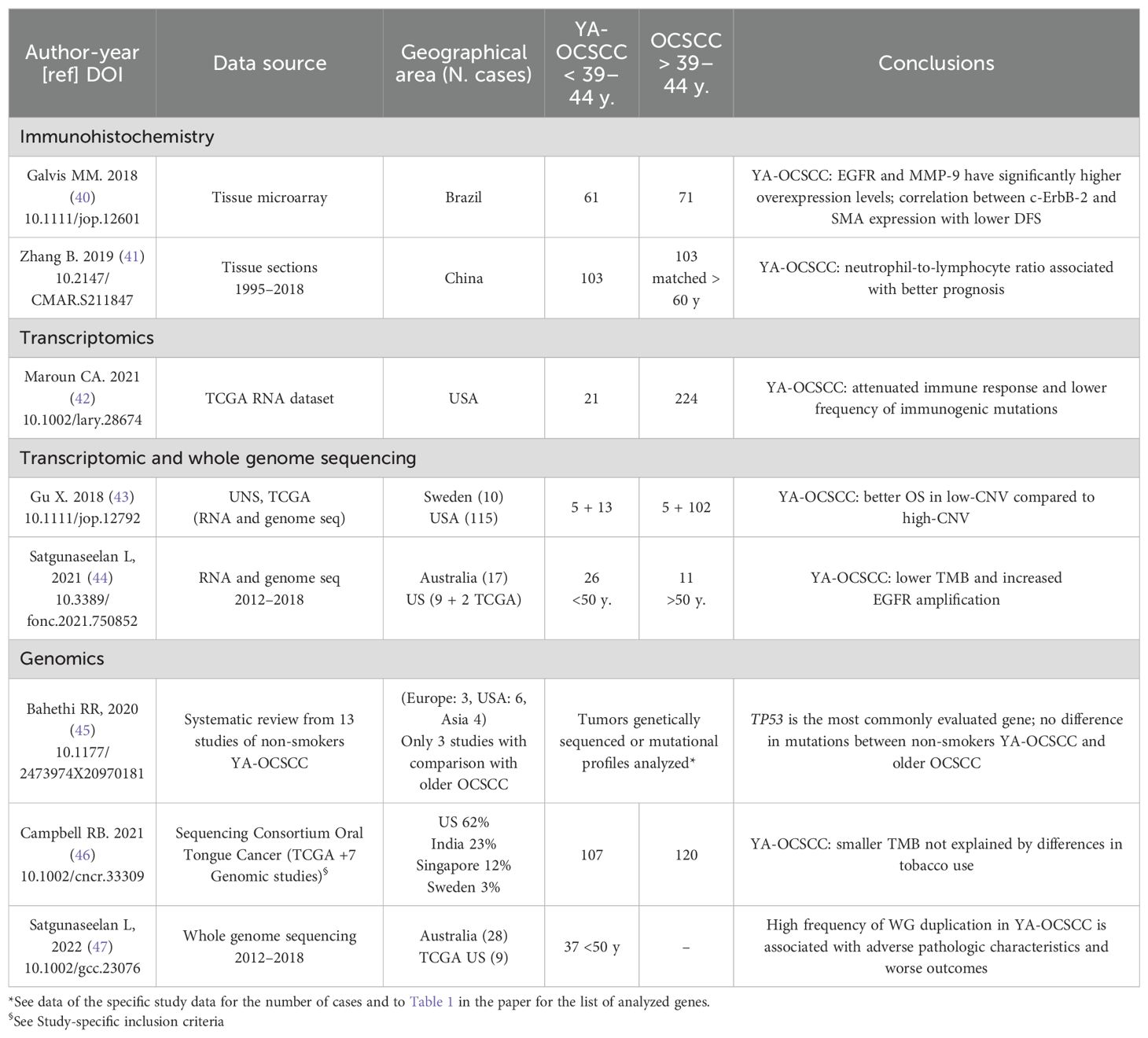
Recently, numerous experimental approaches have concentrated on the link between high-risk genes and YA-OCSCC. Kolegova et al. (16) recently summarized the specific genetic, epigenetic, transcriptomic, and proteomic alterations associated with YA-OCSCC. They emphasized the distinct tumor microenvironment and immune landscape in these patients. On these bases, we chose several recent studies that identified novel potential risk factors or biomarkers for early onset and/or prognosis (Table).
Notably, the geographical areas vary between studies on protein expression (Brazil and China) and those on genomics/transcriptomics alterations (North America, Australia, and Europe), allowing for only limited comparisons. However, where comparisons were feasible, Epidermal growth factor receptor (EGFR) protein over-expression (40) was corroborated by gene amplification (44). The tumor mutation burden (TMB) was observed to be lower in YA-OCSCC compared to older OCSCC patients in two distinct studies, one encompassing North America and Australia (44), and the other Asia and North America (46). Moreover, Parzefall et al., revealed that protein kinase C alpha (PRKCA), highly expressed in oral tongue squamous cell carcinoma (OTSCC) young patients without alcohol and smoking history, is associated with a poor prognosis and it can be used as a biomarker to predict high-risk patients (50).
In addition to the data reported in Table 3 two recent studies have highlighted the role of the tumor immune environment in YA-OCSCC (22, 50). The first study (51), despite being limited to a small sample size (11 patients <44 years and five patients >44 years), provides detailed immunologic data that suggest older patients have significantly higher percentages of FOXP3 + T-cells and elevated expression levels of signatures related to anti-tumor immune activity. The second study (22), which reviewed molecular data from the literature, found that whole transcriptomic analysis of smokers, vapers, and non-users of tobacco products revealed dysregulation of immune-related and mitochondrial genes in both vapers and smokers. Similar to traditional smoking, vaping increases resistance to anti-cancer therapies, causes oral microbiome dysbiosis, and promotes suppression of the host immune system.
4 Conclusions
The update of the YA-OCSCC literature and the evaluation of IARC data about the YA-OCSCC incidence enabled to better define the burden of this disease in different geographic regions and to suggest its association with the major risk factors (betel quid chewing, tobacco use, and high alcohol consumption).
Based on the “Cancer tomorrow” link [https://gco.iarc.fr/tomorrow], the estimated number of new YA-OCSCC cases between 2020 and 2040 is expected to increase in Africa and decrease in Europe. Moreover, concerted efforts by healthcare professionals, policymakers, and the community are expected to enhance awareness among YAs through education on tobacco, including also e-cigarette and areca nut use cessation, alcohol control, and the promotion of a healthy lifestyle (3, 12, 37). For cases not associated with lifestyle factors, new genetic alterations/predispositions in YA-OCSCC have been identified as potential risk factors. However, due to the limited number of cases, further studies involving larger cohorts and high-throughput genomic approaches are necessary.
Recent YA-OCSCC prognostic studies, which compared outcomes with those of older patients with OCSCC, indicated similar or better OS, even in cases with relapse and a worse 5-year DFS. These findings suggest that young age alone may not warrant age-specific treatment selection beyond the standard of care. Thus, as per international guidelines (52), radical surgery should always be pursued, when feasible, with postoperative radiotherapy for loco-regionally advanced disease (i.e., stage III-Iva/b). The use of adjuvant concomitant chemoradiation is limited to cases with non-radical resections and/or lymph node involvement with extracapsular spread (53). Due to the relevant toxicities related to chemo-radiotherapy, concurrent adjuvant treatments are usually reserved to fit patients without major comorbidities. Given that older subjects are more frequently frail than younger individuals counterparts, we cannot exclude that elderly patients may have received less intensive treatments compared to YA-OCSCC. This potential confounder may have impacted on patient survival and, ultimately, on prognostic studies in general.
Molecular data studies associated with YA-OCSCC remain exploratory, having been performed in a limited number of cases. However, across different geographic areas the following profile has been proposed for YA-OCSCC: i) overexpression of EGFR; ii) low TMB; and iii) an attenuated immune response promoted by smoking, snuffing, vaping, and oral microbiome dysbiosis. When tested in a large cohort of YA-OCSCC patients, the identified markers could contribute to the selection of tailored treatments.
By analyzing epidemiologic cohorts and clinical studies, relevant etiological and molecular characteristics unique to YA-OCSCC have been identified. However, further research is needed to explore additional factors, such as the oral microbiome, sex, ethnicity, and various underlying genetic elements, that may influence the development and outcomes in YA-OCSCC patients.
Author contributions
DL: Writing – original draft, Writing – review & editing. EM: Writing – review & editing. SC: Writing – original draft, Writing – review & editing. CB: Writing – review & editing. ET: Writing – review & editing. LB: Writing – review & editing. SC: Writing – original draft, Writing – review & editing. AT: Writing – review & editing. LL: Writing – review & editing. LD: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research and the manuscript preparation were supported by a generous donation of private funds raised in memory of Paolo Sardella. This research was partially funded by Italian Ministry of Health “Ricerca Corrente” funds. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
LL declares the following conflicts of interest: research funds donated directly to the institute for clinical trials from AstraZeneca, BMS, Boehringer Ingelheim, Celgene International, Eisai, Exelixis, Debiopharm International SA, Hoffmann-La Roche Ltd, IRX Therapeutics, Medpace, Merck-Serono, MSD, Novartis, Pfizer, Roche, and Buran; occasional fees for participation as a speaker at conferences/congresses or as a scientific consultant for advisory boards from AstraZeneca, Bayer, MSD, Merck-Serono, AccMed, Neutron Therapeutics, Inc., and Alentis. SC declares occasional fees for participation as a speaker at conferences/congresses from AccMed; support for attending meetings and/or travel from AccMed, MultiMed Engineers srl, Care Insight sas.
The remaining authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. IARC. Available online at: https://gco.iarc.fr (accessed June 17, 2024).
3. Barsouk A, Aluru JS, Rawla P, Saginala K, Barsouk A. Epidemiology, risk factors, and prevention of head and neck squamous cell carcinoma. Med Sci. (2023) 11:42. doi: 10.3390/medsci11020042
4. Chuang SL, Su WWY, Chen SLS, Yen AMF, Wang CP, Fann JCY, et al. Population-based screening program for reducing oral cancer mortality in 2,334,299 Taiwanese cigarette smokers and/or betel quid chewers. Cancer. (2017) 123:1597–609. doi: 10.1002/cncr.30517
5. Chou CW, Lin CR, Chung YT, Tang CS. Epidemiology of oral cancer in Taiwan: A population-based cancer registry study. Cancers (Basel). (2023) 15:2175. doi: 10.3390/cancers15072175
6. Campbell BR, Netterville JL, Sinard RJ, Mannion K, Rohde SL, Langerman A, et al. Early onset oral tongue cancer in the United States: A literature review. Physiol Behav. (2018) 176:139–48. doi: 10.1016/j.oraloncology.2018.10.009
7. Mohan P, Richardson A, Potter JD, Coope P, Paterson M. Opportunistic screening of oral potentially Malignant disorders: A public health need for India. JCO Glob Oncol. (2020) 6:688–96. doi: 10.1200/jgo.19.00350
8. Oliver JR, Wu SP, Chang CM, Roden DF, Wang B, Hu KS, et al. Survival of oral tongue squamous cell carcinoma in young adults. Head Neck. (2019) 41:2960–8. doi: 10.1002/hed.25772
9. Lee DS, Ramirez RJ, Lee JJ, Valenzuela CV, Zevallos JP, Mazul AL, et al. Survival of young versus old patients with oral cavity squamous cell carcinoma: A meta-analysis. Physiol Behav. (2019) 131:678–87. doi: 10.1002/lary.29260.Survival
10. Ferreira e Costa R, Leão MLB, Sant’Ana MSP, Mesquita RA, Gomez RS, Santos-Silva AR, et al. Oral squamous cell carcinoma frequency in young patients from referral centers around the world. Head Neck Pathol. (2022) 16:755–62. doi: 10.1007/s12105-022-01441-w
11. Mohideen K, Krithika C, Jeddy N, Bharathi R, Thayumanavan B, Sankari SL. Meta-analysis on risk factors of squamous cell carcinoma of the tongue in young adults. J Oral Maxillofac Pathol. (2019) 23:450–7. doi: 10.4103/jomfp.JOMFP_118_19
12. Batistella EÂ, Gondak R, Rivero ERC, Warnakulasuriya S, Guerra E, Porporatti AL, et al. Comparison of tobacco and alcohol consumption in young and older patients with oral squamous cell carcinoma: a systematic review and meta-analysis. Clin Oral Investig. (2022) 26:6855–69. doi: 10.1007/s00784-022-04719-z
13. Abdulla R, Adyanthaya S, Kini P, Mohanty V, D'Souza N, Subbannayya Y. Clinicopathological analysis of oral squamous cell carcinoma among the younger age group in coastal Karnataka, India: A retrospective study. J Oral Maxillofac Pathol. (2018) 22:180–7. doi: 10.4103/jomfp.JOMFP_16_18
14. Best DL, Spresser W, Shivers P, Edwards SP, Ward BB. Squamous cell carcinoma of the tongue in young patients: A case series and literature review. J Oral Maxillofac Surg. (2021) 79:1270–86. doi: 10.1016/j.joms.2020.12.018
15. Bajpai S, Zhang N, Lott DG. Tracking changes in age distribution of head and neck cancer in the United States from 1975 to2016. Clin Otolaryngol. (2021) 46:1205–12. doi: 10.1111/coa.13817
16. Kolegova ES, Patysheva MR, Larionova IV, Fedorova IK, Kulbakin DE, Choinzonov EL, et al. Early-onset oral cancer as a clinical entity: aetiology and pathogenesis. Int J Oral Maxillofac Surg. (2022) 51:1497–509. doi: 10.1016/j.ijom.2022.04.005
17. Regulation P. RISKS OF BETEL QUID & TOBACCO USE. (2017) 129(12):1215–20. Available online at: https://publichealthlawcenter.org/sites/default/files/resources/Health-Risks-Betel-Quid-and-Tobacco-2017.pdf (Accessed on September 13, 2024).
18. Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, et al. A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. (2009) 10:1033–4. doi: 10.1016/S1470-2045(09)70326-2
19. Narayanan AM, Yogesh A, Chang MP, Finegersh A, Orosco RK, Moss WJ. A survey of areca (Betel) nut use and oral cancer in the commonwealth of the Northern Mariana Islands. Hawaii J Health Soc Welf. (2020) 79:112–6.
20. Narayanan AM, Finegersh AF, Chang MP, Orosco RK, Moss WJ. Oral cavity cancer outcomes in remote, betel nut-endemic pacific islands. Ann Otol Rhinol Laryngol. (2020) 129(12):1215–20. doi: 10.1177/0003489420934846
21. Schantz SP, Byers RM, Goepfert H. Tobacco and cancer of the tongue in young adults. JAMA. (1988) 259:1943–4. doi: 10.1001/jama.1988.03720130021012
22. Maan M, Abuzayeda M, Kaklamanos EG, Jamal M, Dutta M, Moharamzadeh K. Molecular insights into the role of electronic cigarettes in oral carcinogenesis. Crit Rev Toxicol. (2023) 53:1–14. doi: 10.1080/10408444.2023.2190764
23. Campbell BR, Sanders CB, Netterville JL, Sinard RJ, Rohde SL, Langerman A, et al. Early onset oral tongue squamous cell carcinoma: Associated factors and patient outcomes. Head Neck. (2019) 41:1952–60. doi: 10.1002/hed.25650
24. Herzog C, Jones A, Evans I, Raut JR, Zikan M, Cibula D, et al. Cigarette smoking and E-cigarette use induce shared DNA methylation changes linked to carcinogenesis. Cancer Res. (2024) 84:1898–914. doi: 10.1158/0008-5472.CAN-23-2957
25. Nalepa G, Clapp DW. Fanconi anaemia and cancer: An intricate relationship. Nat Rev Cancer. (2018) 18:168–85. doi: 10.1038/nrc.2017.116
26. Beddok A, Krieger S, Castera L, Stoppa-Lyonnet D, Thariat J. Management of Fanconi Anemia patients with head and neck carcinoma: Diagnosis and treatment adaptation. Oral Oncol. (2020) 108:104816. doi: 10.1016/j.oraloncology.2020.104816
27. Amenábar JM, Torres-Pereira CC, Tang KD, Punyadeera C. Two enemies, one fight: An update of oral cancer in patients with Fanconi anemia. Cancer. (2019) 125:3936–46. doi: 10.1002/cncr.32435
28. Cardin GB, Bernard M, Bahig H, Nguyen-Tan PF, Ballivy O, Filion E, et al. Single nucleotide polymorphism rs6942067 is a risk factor in young and in non-smoking patients with hpv negative head and neck squamous cell carcinoma. Cancers (Basel). (2020) 12:55. doi: 10.3390/cancers12010055
29. Tani R, Ito N, Matsui K, Yamasaki S, Hamada A, Tokumaru K, et al. MICA A5.1 homozygous genotype is associated with a risk for early-onset oral cancer. Oral Oncol. (2021) 116:105256. doi: 10.1016/j.oraloncology.2021.105256
30. Winn DM, Lee YC, Hashibe M, Boffetta P, Agudo A, Ahrens W, et al. The INHANCE consortium: Toward a better understanding of the causes and mechanisms of head and neck cancer. Oral Dis. (2015) 21:685–93. doi: 10.1111/odi.12342
31. Mahmood N, Hanif M, Ahmed A, Jamal Q, Saqib, Khan A. Impact of age at diagnosis on clinicopathological outcomes of oral squamous cell carcinoma patients. Pakistan J Med Sci. (2018) 34:595–9. doi: 10.12669/pjms.343.14086
32. Martinez RCP, Sathasivam HP, Cosway B, Paleri V, Fellows S, Adams J, et al. Clinicopathological features of squamous cell carcinoma of the oral cavity and oropharynx in young patients. Br J Oral Maxillofac Surg. (2018) 56:332–7. doi: 10.1016/j.bjoms.2018.03.011
33. Menezes FDS, Latorre MDRDO, Conceição GMS, Curado MP, Antunes JLF, Toporcov TN. The emerging risk of oropharyngeal and oral cavity cancer in HPV-related subsites in young people in Brazil. PloS One. (2020) 15:e0232871. doi: 10.1371/journal.pone.0232871
34. Su FH, Chang SN, Chen PC, Sung FC, Huang SF, Chiou HY, et al. Positive association between hepatitis C infection and oral cavity cancer: a nationwide population-based cohort study in Taiwan. PloS One. (2012) 7:e48109. doi: 10.1371/journal.pone.0048109
35. Heller MA, Nyirjesy SC, Balsiger R, Talbot N, VanKoevering KK, Haring CT, et al. Modifiable risk factors for oral cavity cancer in non-smokers: A systematic review and meta-analysis. Oral Oncol. (2023) 137:106300. doi: 10.1016/j.oraloncology.2022.106300
36. Mukdad L, Heineman TE, Alonso J, Badran KW, Kuan EC, St. John MA. Oral tongue squamous cell carcinoma survival as stratified by age and sex: A surveillance, epidemiology, and end results analysis. Laryngoscope. (2019) 129:2076–81. doi: 10.1002/lary.27720
37. Kaminagakura E, Tango RN, Cruz-Perez D, Bonan R, Yamamoto de Almeida L, de Almeida Lança ML, et al. Oral squamous cell carcinoma outcome in adolescent/young adult: Systematic review and meta-analysis. Head Neck. (2022) 44:548–61. doi: 10.1002/hed.26940
38. Tagliabue M, Belloni P, De Berardinis R, Gandini S, Chu F, Zorzi S, et al. A systematic review and meta-analysis of the prognostic role of age in oral tongue cancer. Cancer Med. (2021) 10:2566–78. doi: 10.1002/cam4.3795
39. Panda S, Mohanty N, Panda S, Mishra L, Gopinath D, Sahoo A, et al. Are survival outcomes different for young and old patients with oral and oropharyngeal squamous cell carcinoma? A systematic review and meta-analysis. Cancers (Basel). (2022) 14:1886. doi: 10.3390/cancers14081886
40. Miranda Galvis M, Santos-Silva AR, Freitas Jardim J, Paiva Fonseca F, Lopes MA, de Almeida OP, et al. Different patterns of expression of cell cycle control and local invasion-related proteins in oral squamous cell carcinoma affecting young patients. J Oral Pathol Med. (2018) 47:32–9. doi: 10.1111/jop.12601
41. Zhang B, Du W, Gan K, Fang Q, Zhang X. Significance of the neutrophil-to-lymphocyte ratio in young patients with oral squamous cell carcinoma. Cancer Manag Res. (2019) 11:7597–603. doi: 10.2147/CMAR.S211847
42. Maroun CA, Zhu G, Fakhry C, Gourin CG, Seiwert TY, Vosler PS, et al. An immunogenomic investigation of oral cavity squamous cell carcinoma in patients aged 45 Years and younger. Laryngoscope. (2021) 131:304–11. doi: 10.1002/lary.28674
43. Gu X, Coates PJ, Boldrup L, Wang L, Krejci A, Hupp T, et al. Copy number variation: A prognostic marker for young patients with squamous cell carcinoma of the oral tongue. J Oral Pathol Med. (2019) 48:24–30. doi: 10.1111/jop.12792
44. Satgunaseelan L, Porazinski S, Strbenac D, Istadi A, Willet C, Chew T, et al. Oral squamous cell carcinoma in young patients show higher rates of EGFR amplification: implications for novel personalized therapy. Front Oncol. (2021) 11:750852. doi: 10.3389/fonc.2021.750852
45. Bahethi RR, Stepan KO, Pinotti R, Li R, Agrawal N, Puram SV, et al. Genetic mutations in young nonsmoking patients with oral cavity cancer: A systematic review. OTO Open. (2020) 4:2473974X20970181. doi: 10.1177/2473974X20970181
46. Campbell BR, Chen Z, Faden DL, Agrawal N, Li RJ, Hanna GJ, et al. The mutational landscape of early- and typical-onset oral tongue squamous cell carcinoma. Cancer. (2021) 127:544–53. doi: 10.1002/cncr.33309
47. Satgunaseelan L, Strbenac D, Willet C, Chew T, Sadsad R, Wykes J, et al. Whole genome duplication in oral squamous cell carcinoma in patients younger than 50 years: implications for prognosis and adverse clinicopathological factors. Genes Chromosomes Cancer. (2022) 61:561–71. doi: 10.1002/gcc.23076
48. van Monsjou HS WV, Lopez-Yurda MI, Hauptmann M, van den Brekel MW, Balm AJ. Oral and oropharyngeal squamous cell carcinoma in young patients: the Netherlands Cancer Institute experience. Head Neck. (2014) 36:1391. doi: 10.1002/HED
49. Mishra MK, Gupta S, Shivangi, Sharma M, Sehgal S. The repertoire of mutational signatures in tobacco- and non-tobacco-induced oral cancer. Clin Transl Oncol. (2023) 25:3332–44. doi: 10.1007/s12094-023-03192-8
50. Parzefall T, Schnoell J, Monschein L, Foki E, Liu DT, Frohne A, et al. PRKCA overexpression is frequent in young oral tongue squamous cell carcinoma patients and is associated with poor prognosis. Cancers (Basel). (2021) 13:2082. doi: 10.3390/cancers13092082
51. Chatzopoulos K, Sotiriou S, Collins AR, Kartsidis P, Schmitt AC, Chen X, et al. Transcriptomic and immunophenotypic characterization of tumor immune microenvironment in squamous cell carcinoma of the oral tongue. Head Neck Pathol. (2021) 15:509–22. doi: 10.1007/s12105-020-01229-w
52. Machiels JP, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2020) 31:1462–75. doi: 10.1016/j.annonc.2020.07.011
53. Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. (2005) 27:843–50. doi: 10.1002/hed.20279
Keywords: oral cavity squamous cell carcinoma, young adult, incidence, risk factors, outcome, molecular data
Citation: Lenoci D, Moresco E, Cavalieri S, Bergamini C, Torchia E, Botta L, Canevari S, Trama A, Licitra L and De Cecco L (2024) Oral cancer in young adults: incidence, risk factors, prognosis, and molecular biomarkers. Front. Oncol. 14:1452909. doi: 10.3389/fonc.2024.1452909
Received: 21 June 2024; Accepted: 05 September 2024;
Published: 20 September 2024.
Edited by:
Markus Brunner, Medical University of Vienna, AustriaReviewed by:
Rong-Hui Xia, Shanghai Jiao Tong University, ChinaLara Maria Alencar Ramos Innocentini, University of São Paulo, Brazil
Franciane Schroeder, Federal University of Rio Grande do Sul, Brazil
Copyright © 2024 Lenoci, Moresco, Cavalieri, Bergamini, Torchia, Botta, Canevari, Trama, Licitra and De Cecco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Cavalieri, U3RlZmFuby5jYXZhbGllcmlAaXN0aXR1dG90dW1vcmkubWkuaXQ=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Deborah Lenoci
Deborah Lenoci Elisa Moresco2†
Elisa Moresco2† Stefano Cavalieri
Stefano Cavalieri Cristiana Bergamini
Cristiana Bergamini Laura Botta
Laura Botta Silvana Canevari
Silvana Canevari Annalisa Trama
Annalisa Trama Lisa Licitra
Lisa Licitra Loris De Cecco
Loris De Cecco