- Department of Clinical Studies and Nutritional Epidemiology, Nutrition Biomed Research Institute, Melbourne, VIC, Australia
Introduction: Distant metastases following standard treatment for locally advanced rectal cancer (LARC) are typically associated with poor disease-free survival. We report on a 52-year-old Australian male of Dutch ancestry with no family history of colorectal cancer or significant medical history who experienced bleeding per rectum for several months prior to a colonoscopy in July 2010. He was subsequently diagnosed with Stage IIb LARC.
Case presentation: Despite treatment with curative intent, a distant recurrence to his left lung was detected in May 2012, upstaging him to Stage IV rectal cancer. He had repeated distant metastatic recurrences over the next 8 years, and treatment included multiple surgeries, chemotherapies, radiation treatments, a “watch and wait” period of 20 months, and personalised dietary management. Genetic and nutrigenomic testing identified that the case had KRAS and MTHFR mutations. As part of his dietary management, the case also had his levels of folate, vitamin B12, and vitamin D regularly monitored because of his genetic predisposition and history of deficiency for these key nutrients. Apart from changes in his CEA levels, sudden increases in the patient’s folate levels, inconsistent with dietary exposures preceded detection of each new distant recurrence, with significant decreases in the levels at the next follow-up measurement.
Conclusion: A multimodal approach to this patient’s management appeared to contribute to his long-term survival of nearly 10 years from the initial diagnosis. Multidisciplinary management, including the use of additional biomarkers, may enhance survival rates in other similar cases with advanced disease resistant to differing therapies, and with potentially poor prognosis.
Introduction
Over the past few decades, there has been an increasing trend in the number of younger cases diagnosed with advanced colorectal cancer (CRC) (1). Rectal cancer comprises around one-third of all CRC cases and is a distinct and heterogeneous disease presenting with different metabolic and genetic profiles, disease patterns, and responses to available treatments (2). While standard treatment for LARC, such as neoadjuvant combined chemotherapy (usually fluoropyridines) and radiotherapy (nCRT), along with surgery, has improved local disease control, distant metastatic recurrence reportedly occurs in approximately 30% of patients (3, 4). Distant metastasis is the leading cause of cancer-related death among LARC patients with a median survival between 24 to 36 months (5). The 5-year relative survival rate for Stage IV rectal cancer has been estimated at around 14% (6).
One of the biomarkers that have been associated with aggressive disease, poor treatment responses, and low overall survival rates is the Kirsten Rat Sarcoma viral oncogene homolog (KRAS) gene mutation (3, 7, 8). Epigenetic factors, such as DNA methylation patterns, are also reported to be associated with an increase in the number of younger patients with non-hereditary disease (9). Methylation is a dynamic process involved in regulation of gene expression by the addition of a methyl group to the 5-carbon position of the cytosine ring of DNA (10). DNA methylation can be modified by environmental factors, such as diet, with abnormal methylation patterns associated with cancer development and progression (10). Methylenetetrahydrofolate reductase (encoded by the MTHFR gene) is an enzyme involved in methylation and can influence DNA synthesis and repair (11). Genetic variations in the MTHFR gene have been associated with differing responses to treatments of rectal cancer as well as influencing folate metabolism and bioavailability (3, 11).
The effect of folate on colorectal carcinogenesis is complex and appears to have a “dual modulatory” role, with both high and low levels having been implicated in cancer risk, dependent on timing, dosing, and form of the nutrient (12, 13). This was evident from the varying responses to different folate levels reported in animal models (12). One animal study on rats found that folate deficiency led to a reduced risk of CRC (14), while another study on rats reported that low folate status increased the risk of CRC and that moderate increases in dietary folate were shown to be protective against developing the disease (13). It was also reported that no appreciable differences were observed at slightly higher folate doses (greater than 4 times the dose), while exceptionally high levels (1,000 times the dose) led to the development of neoplasms (13). Human intervention studies have investigated the effect of supplementation with folic acid (FA), the synthetic form of the nutrient for prevention of recurrent colorectal adenomas (CRAs) (12). One randomised clinical trial (RCT) allocated participants to either 1 mg/day of FA or a placebo for 3 years and found that this did not reduce the risk of CRAs (15). After an additional intervention and follow-up period, participants in the FA group had a higher incidence of advanced and multiple CRAs suggesting that FA supplementation may increase the risk of neoplasia (15). Two other RCTs using FA supplementation at 1 mg/day (16) and 0.5 mg/day (17) reported no effect on the risk of CRA recurrence or increased risk of advanced or multiple CRAs. The results from these RCTs highlight potential metabolic differences with FA, which may affect folate metabolism and biological pathways (12). Additionally, when administered at higher doses, FA, in some circumstances, may increase the risk of recurrence and disease progression, e.g., in participants with undetected microscopic CRAs (12).
While not all (18–20), several early epidemiological studies have reported inverse associations between folate levels and colorectal cancer (21–25). A large Danish cohort study reported that dietary folate had a protective effect against developing both colon and rectal cancer among those who consumed more than 10 g of alcohol per day (26). Variations in the potential effect of folate on CRC risk according to body site, gender, and genetic polymorphisms were also noted in a large Dutch study (27). In this study, dietary intakes of folate (pre folic acid fortification era) were associated with a decreased risk of rectal cancer but not colon cancer. Reduced risk was also observed for men but not women, and this association was most pronounced in those with KRAS mutated tumours (27).
We present a 52-year-old male of Dutch ancestry who was diagnosed with LARC and had nCRT, surgery, and adjuvant chemotherapy (aCT). He also had KRAS (G13D) and MTHFR (C667T rs1801133 and A198C rs1801131) mutations and had his first distant recurrence in his left lung within 2 years of initial diagnosis and treatment. Following this first distant recurrence, a nutritional assessment of the case identified potential nutritional deficiencies and metabolic issues based on nutrigenomics testing and a review of his medical history (vitamin D deficiency) and dietary intake, e.g., high alcohol and low folate intakes. From this point onward, he had personalised dietary management focussing on his folate, vitamin B12, and vitamin D levels, all of which were monitored and managed along with his standard medical care. The patient survived nearly 10 years, with eight of these years having multiple distant recurrences to both lungs, and later his liver.
Case presentation
The patient was a 52-year-old white Australian businessman of Dutch ancestry. He had no family or personal history of cancer and had an unremarkable prior medical history consisting of irregular respiratory and viral infections, and, more recently, hypercholesterolaemia and vitamin D deficiency, which were managed by a statin (rosuvastatin 5 mg/day) and a supplement (intermittent use of OsteoVit D 1,000 IU/day), respectively. His daily alcohol consumption exceeded four standard drinks, and he was a past smoker of 10+ pack-years which he had ceased 30 years ago. At diagnosis (T3N0M0), the patient weighed 117 kg (BMI: 32 kg/m²) and was advised to lose 20 kg by his colorectal surgeon in preparation for the resection of his primary tumour to achieve good pathologic response and preservation of his anal sphincter/anus. Surgery was scheduled to follow neoadjuvant chemoradiotherapy (nCRT), using infusional 5-fluorouracil (5-FU), and radiotherapy (50.4 Grays). Table 1 presents a summary of the multidisciplinary management of the patient over the 10-year history of his disease.
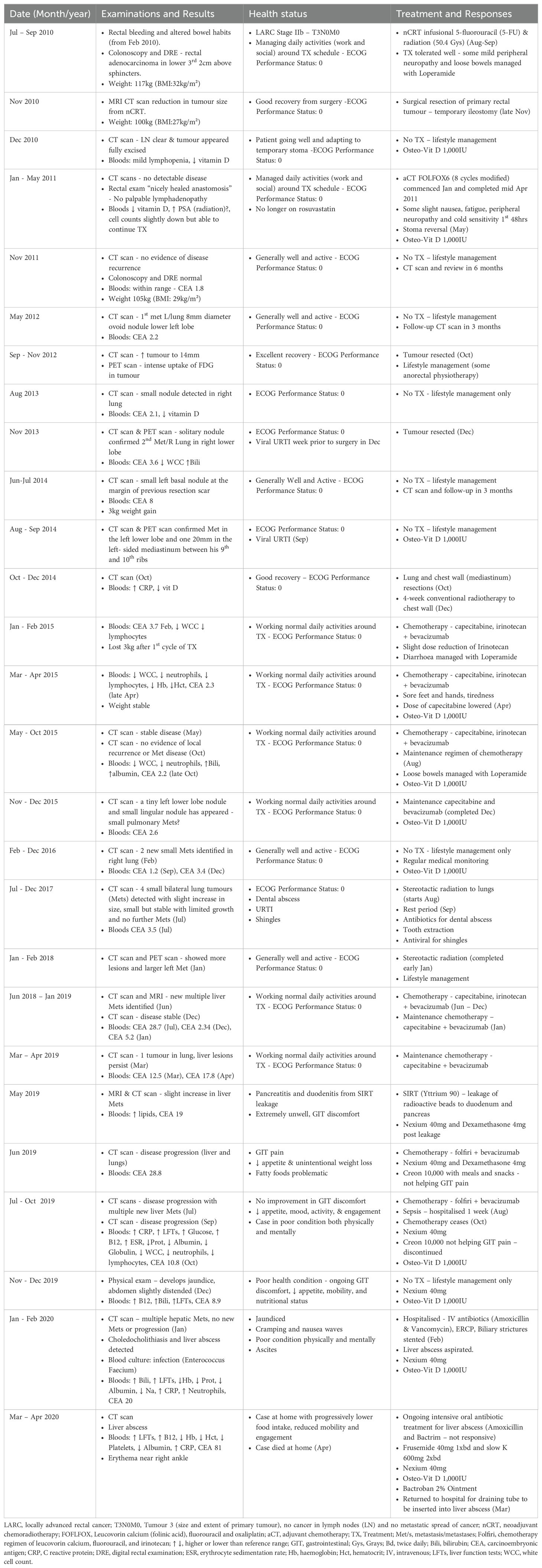
Table 1. Physical examinations, treatments, results, and responses over 10-year history of recurrent metastatic rectal cancer.
Due to the potential adverse side effects from nCRT (e.g., anaemia and neutropaenia) and the need to maintain his nutritional status for the upcoming surgery, dietary modification focussed on modest calorie restriction, mainly via a significant reduction in alcohol consumption and energy-dense “discretionary” foods. The patient modified his dietary intake from diagnosis and intentionally lost a total of 17 kg, weighing 100 kg (BMI: 27 m²/kg) by the time of his surgery approximately 4 months after his initial diagnosis.
The nCRT was effective in downsizing the rectal adenocarcinoma and downstaging his disease for surgery, which involved an open ultra-low anterior resection of his tumour with hand-sewn coloanal anastomosis with diverting loop ileostomy in late November 2010. Surgical resection of his tumour was successful, and the patient recovered well with no indication to require a long-term ileostomy. He was considered disease free at this stage.
Given the option, the patient chose to have adjuvant chemotherapy (aCT) with eight cycles of modified FOLFOX6 in early January 2011 and underwent an ileostomy reversal approximately 6 months later in May 2011. The patient returned to having regular/normal bowel function following some physiotherapy and dietary adjustment, e.g., an increase in soluble fibre and reduced lactose intake.
The patient kept well and was able to perform all daily activities (ECOG Performance status 0) and had three monthly medical follow-ups without any indication of disease recurrence. However, almost 2 years post initial diagnosis in May 2012, the first recurrence of distant disease was detected in the case’s left lung. No other lesions were identified anywhere at the time of his video-assisted thoracoscopic surgical (VATS) wedge resection to his left lower lung lesion in October 2012.
Initial genetic testing of the patient indicated that he had a KRAS (G13D) mutation, NRAS/BRAF wild type, and PD-L1 (IHC = 0%). Additional nutrigenomics testing was also conducted at diagnosis of the first distant recurrence to assist in optimising the patient’s nutritional and health status. This identified variations in several genes active in the one carbon and methylation pathway, including methylenetetrahydrofolate reductase (MTHFR—C667T and A1298C), 5-methyltetrahydrofolate-homocysteine methyltransferase reductase (MTRR), and Transcobalamin 2 (TCN2) genetic variants, which are also involved with folate and vitamin B12 metabolism and adequacy (10). Based on these results, the patient was genetically predisposed to reduced folate metabolism, lower vitamin B12 status, and higher homocysteine levels. From this point in his multidisciplinary management, the patient’s folate and vitamin B12 measures were monitored regularly along with other regular biomarkers such as his CEA, haematology, and biochemistry. The patient also had his vitamin D levels regularly checked because he had a history of vitamin D deficiency for several years prior to his initial diagnosis. This was the only supplement that he took across the course of his disease, and dosage was adjusted based on his blood levels and seasonal changes.
Given his excellent recovery (ECOG Performance Status 0), regular medical monitoring continued, but no further medical intervention followed this initial lung surgery. However, in August 2013, a second distant recurrence was detected in the patient’s right lung, and he had an excision of the right lower lobe tumour in December 2013. Regular medical follow-ups ensued, and in June 2014, another small nodule was detected in his left lung, which was confirmed as a metastatic tumour on a follow-up scan in September 2014, along with an additional moderately avid tumour undetected by CT scan but identified by PET scan in the case’s left-sided mediastinum between his 9th and 10th ribs. The tumour in the left side of his chest wall was consistent with the location of his drainage tube from the earlier resection in 2012 suggesting that some seeding had occurred. The patient underwent surgical resection of these new lesions in October 2014. Around this time, the patient was discovered to have a small liver cyst of 6 mm also detected on a CT scan.
Following the late 2014 resections, the patient underwent post-surgery radiation to his left-sided chest wall (50 Gy in 20 fractions) and chemotherapy for the first time in 4 years since his aCT in early 2011. The new chemotherapy regimen consisted of capecitabine, irinotecan, and bevacizumab from January 2015 until July 2015 with a lowered maintenance dose of capecitabine and bevacizumab only, until December 2015. A follow-up CT scan in early February 2016 identified two new small tumours in the patient’s right lung. He continued to have regular medical monitoring but was not prescribed any active medical treatment from January 2016 until August 2017.
During this “watch and wait” period, the patient’s health was exclusively managed via lifestyle interventions, predominantly by a personalised dietary plan. A pattern had emerged, as indicated in Table 2, whereby just prior to the detection of a new distant recurrence, the patient’s folate levels would rapidly increase, which was inconsistent with his dietary exposures that included, at the time, high alcohol and low folate intake, typically associated with low folate levels. This was directly followed by a significant decrease in folate levels. As the patient was not on any nutritional supplement that contained folate/folic acid, it was suggestive that the disease may be involved in these changes. This was evident at detection of the first recurrence, when the patient’s red cell folate levels were 1,888 nmol/L (CEA was 2.2 µg/L) and subsequently dropped to 1,100 nmol/L, which was also inconsistent with dietary exposures and in the absence of any medical treatment. Similarly, red cell folate was 1,826 nmol/L (CEA was 2.1 µg/L) at detection of the second lung metastasis and dropped to 665 nmol/L, with a corresponding increase in CEA to 3.6 µg/L. Red cell folate was 2,053 nmol/L (CEA was 8 µg/L) at detection of the third recurrence in the left lung and chest wall, with folate levels dropping further to 525 nmol/L and CEA increasing to 12.9 µg/L at follow-up measurements (see Figure 1).
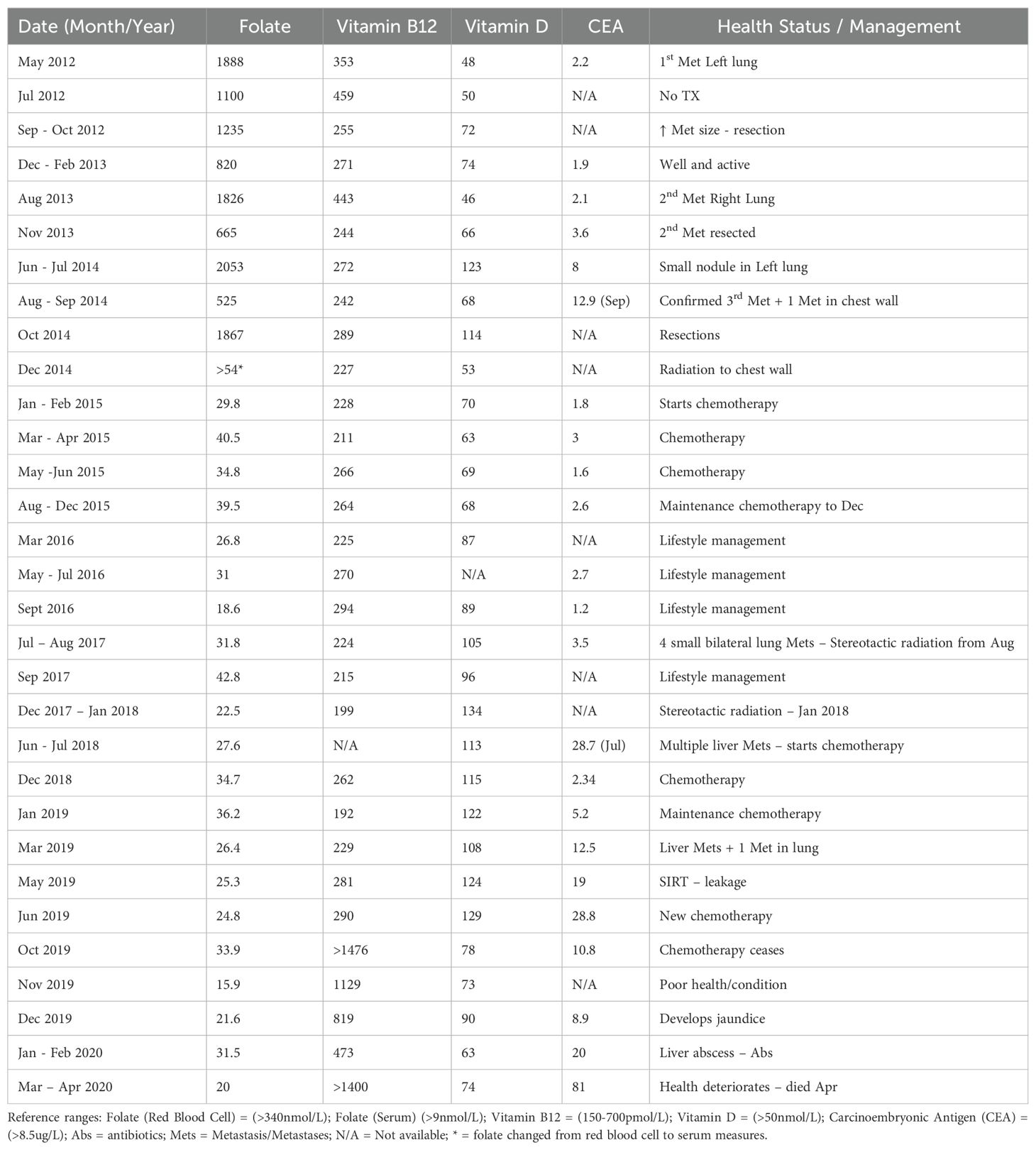
Table 2. Folate, Vitamin B12, Vitamin D, and CEA levels from first distance recurrence of rectal cancer.
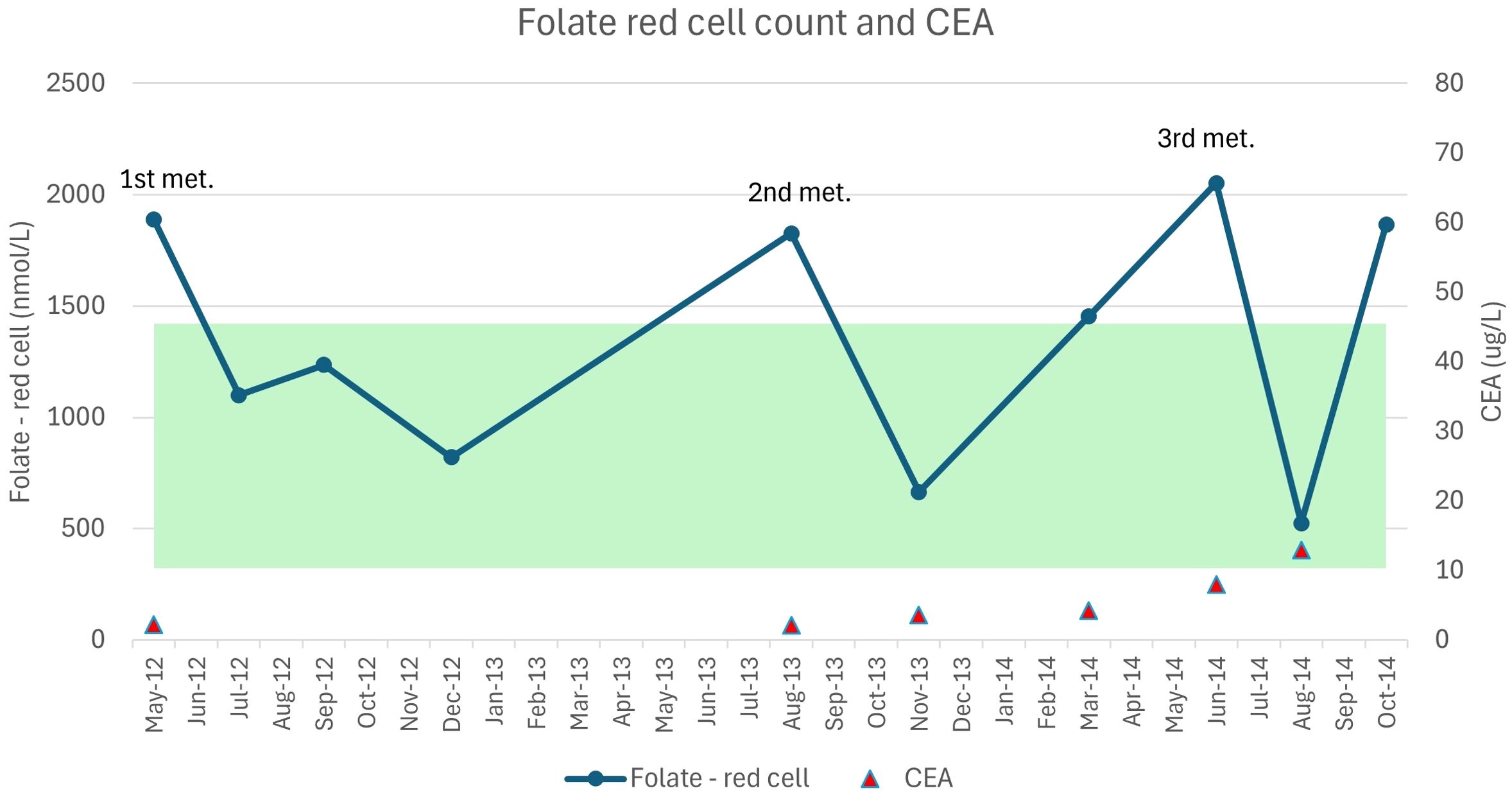
Figure 1. Red cell folate measures and the first three recurrent lung metastases. The image presents the changes in red cell folate levels against CEA (carcinoembryonic antigen) with detection of each new met (metastasis). The highlighted band depicts the standard reference range for red cell folate levels over this period.
Over the 20-month monitoring phase, the patient focused on his nutritional and health status by maintaining a healthy weight and avoiding alcohol, which could adversely affect his folate levels. He followed a diet plan that consisted of adequate lean animal protein (~1 g/kg body weight—predominantly chicken and fish, with limited red meat and no processed meats) to maintain his cell counts, vitamin B12, and iron status. Plant foods comprised approximately 75% of his meal plan with a consistent high intake of leafy greens to ensure he met his daily requirements for folate. He also followed a personalised aerobic and weight training exercise program devised by exercise physiologists intermittently from late August 2016.
During the 20-month “watch and wait” period, the disease was kept relatively stable. A CT scan in July 2016 reported no increase in the number of metastases, just a slight increase in size but these were all still sub-centimetre. A scan of the chest, abdomen, and pelvis 12 months later in July 2017 detected four small bilateral lung tumours, with a slight increase in size of the largest ones to 12 and 15 mm. Given the slowly progressive nature of the disease, the multidisciplinary team decided that these tumours would be treated by stereotactic radiation. This was scheduled according to size, anatomical position, and avidity of the tumours in each lung. This involved 60 Gy in eight fractions over a 2.5-week course of stereotactic radiation to two metastases in his right lung in August 2017. Between this and his next course of treatment 8 weeks later, the patient suffered from a dental abscess and subsequent tooth extraction. In late October 2017, he had 55 Gy in five fractions to one of his left lung metastases. Prior to the final course of stereotactic ablative radiotherapy to his remaining left lung metastasis comprising 60 Gy in eight fractions from mid-December 2017 to early January 2018, a CT and PET scan showed two new mildly avid metastases to his right middle (6 mm) and right lower lobes (7 mm). Up until this point in his medical management, the case had maintained good overall health. He had tolerated all previous chemotherapies, radiotherapies, and surgeries well, requiring only minimal use of any analgesia, anti-emetics, and anti-diarrhoeal medications throughout the course of these different modalities. However, during this treatment period, he had not only experienced a severe dental infection but also a bout of shingles, and a prolonged upper respiratory infection requiring antibiotics suggesting that he was severely immunocompromised over this time.
In mid-2018 during regular monitoring of the patient’s blood, a rapid increase in CEA was observed, and follow-up investigations by CT and PET scans identified multiple metastases in the patient’s liver in June 2018. The patient recommenced a regimen of capecitabine, irinotecan, and bevacizumab in late June 2018 for 6 months proceeded by a maintenance dose of capecitabine and bevacizumab only until early May 2019. Some disease progression was detected via CT scan and MRI in early May 2019, and after considering stereotactic radiation to the liver, the multidisciplinary team opted for selective internal radiation therapy (SIRT) at the end of May 2019.
During the SIRT procedure, there was some leakage of radioactive microspheres (Yttrium 90) to the patient’s duodenum and pancreas. As a result, the patient suffered from pancreatitis and ongoing duodenitis and experienced unintentional weight loss (~10 kg), along with a compromised nutritional status and reduced quality of life. Follow-up blood work and imaging identified further disease progression, and the case commenced a chemotherapy regimen of FOLFIRI (5-FU, leucovorin, and irinotecan) and bevacizumab in late June 2019. He subsequently experienced an episode of sepsis from an unknown source and was hospitalised in late August/early September 2019. Chemotherapy ceased in October 2019, and the patient suffered from ongoing reduced appetite, mobility, and overall quality of life.
The patient became jaundiced in December 2019 and suffered from choledocholithiasis, complicated by Enterococcus faecium bacteraemia. The patient developed a liver abscess in February 2020, which did not respond to intensive intravenous and oral antibiotic treatment. Repeated insults to the liver, including progression of metastatic disease, caused the patient to develop significant ascites requiring regular drainage over the final few months of his life. He died in early April 2020, 9 years and 9 months after his initial diagnosis.
Discussion
This case report demonstrates the benefit of a multidisciplinary approach to cancer management, including the assessment, monitoring, and management of key nutrients, folate, vitamin B12, and vitamin D in the patient’s ongoing battle with recurring rectal cancer. It also highlights tumour resistance to multimodalities and more aggressive forms of disease among some rectal cancer patients.
Although the scientific evidence is limited and equivocal at best for the role of folate in cancer prevention, progression, and survival, a particular pattern could be seen across the 8 years of monitoring this vitamin in our case. Sudden increases in the patient’s folate levels were observed, which were inconsistent with his dietary exposure at the time, e.g., when he had low dietary folate and high alcohol intake. Given that this dietary pattern should equate to low/suboptimal folate levels, it suggests that it may have reflected tumour metabolism rather than nutritional exposures.
We hypothesise that these elevated folate levels seen in the patient’s blood immediately preceding identification of a new distant recurrence possibly indicated hypermethylation and the silencing of tumour-suppressor genes (28). Although not statistically significant, van Engeland et al. reported that promoter hypermethylation was higher in colorectal cancers with low folate and high alcohol intakes (29). A mutation in the MTHFR (C677T) gene combined with a diet low in folate and high in alcohol has also been reported to increase the risk of colorectal cancer (30). This was a dietary pattern observed by this patient immediately preceding his initial diagnosis and subsequent first three distant metastatic recurrences. However, at the start of his 20-month “watch and wait” period, he commenced total abstinence from alcohol and followed a diet that was adequate in folate (mainly from leafy green vegetables), which coincided with fairly stable metastases and very limited growth over this period.
In terms of folate and DNA methylation, some studies have also conversely reported that low folate and high alcohol intakes are associated with global hypomethylation and increased risk of colorectal cancer (31). Sudden decreases in folate levels seen in our patient’s blood following the unexplained increases suggest possible hypomethylation resulting in chromosomal instability and further cancer development (10). Hypomethylation has also been associated with a higher proportion of colorectal cancers in younger patients with non-hereditary colorectal cancer, like our patient (9).
Based on our observations, both high and low folate levels appeared to reflect possible aberrant DNA methylation, and the combination of both hypermethylation and hypomethylation being involved in tumorigenesis and disease progression (32). A cross-sectional study of 189 colorectal cancer patients investigated the association between nutrients in the one-carbon metabolism pathway, particularly folate, along with a folate metabolism genetic polymorphism (MTHFR) and global DNA methylation in colorectal cancer (33). The authors of this cross-sectional study reported that serum folate levels were positively correlated with total dietary folate intake and global DNA methylation in the patients’ blood, and these measures influenced clinicopathological staging of disease (33).
While folate has a major role in DNA methylation, synthesis, and repair, and polymorphisms in the MTHFR gene involved in folate metabolism are reported to be predictive of cancer treatment outcomes (3, 11), the scientific evidence for optimal folate levels is critically lacking. Furthermore, the measurement of serum folate measures is not currently part of the standard range of blood tests for rectal cancer (34). Blood concentrations of folate can be affected by genetic predisposition, dietary exposure, food fortification, cancer treatments, and disease (3, 11, 28).
Another important consideration for monitoring folate status is the fact that wheat flour has been fortified with folic acid, a synthetic form of folate, for the past 15 years in Australia and longer in other parts of the world (35). While folate deficiency is relatively rare in the post fortification era, it is worth noting that both high and low levels of folate have been associated with risk of rectal cancer, and these levels are not currently being routinely checked in any population, including colorectal cancer patients (11). It was only by taking regular measures and tracking for trends that we could observe the unexpected significant changes in our patient’s folate levels across the course of his disease and modify his diet accordingly.
Recent studies have reported a positive association between vitamin D levels and colorectal cancer survival rates (36, 37). This patient had a history of low vitamin D levels for several years prior to diagnosis. Even though the patient continued taking his daily vitamin D supplement, his lower vitamin D levels toward the end of his life were most likely due to his impaired liver function, just like the elevation seen in his vitamin B12 levels at this time.
While findings from our study regarding folate levels is novel and warrants further investigation, a major limitation of this case report is that it only contains data collected from one case making it more hypothesis generating and unable to provide statistical validation. Also, as nutritional assessment and management were only instigated after detection of the first metastatic recurrence in 2012, nutritional biomarkers were unavailable earlier, i.e., prior to and at diagnosis. Reporter bias is another potential limitation; however, this should have been minimised by reporting data from different independent and objective sources including multiple blood measures of key nutrients of interest, which we could cross-check with nutrigenomic test results, dietary exposures, laboratory investigations, and medical reports over 8 of the 10 years of the patient’s history of disease.
As an essential nutrient involved in multiple molecular pathways, this case report demonstrates the potential advantages of including folate assessment in the multidisciplinary management of rectal cancer patients. Future research should be directed towards identifying those who may benefit from higher or lower levels of folate via a transdisciplinary approach and include nutrigenomics and folate status in large molecular/genomic, epidemiological, and clinical studies (38).
Conclusion
In the age of precision medicine, nutritional profiling and management of certain subsets of rectal cancer patients, such as those with aggressive disease resistant to standard treatments, have potential to be an effective, low cost, and non-invasive addition to the multidisciplinary management of these patients. The use of nutrigenomic testing to identify potential metabolic issues and nutritional deficiencies is an area worthy of further investigation for its possible translation into evidenced-based clinical practice. Larger follow-up studies are essential to determine the role of folate status and metabolism in methylation, and carcinogenesis, and to confirm if regular monitoring and management of folate levels may be clinically beneficial to help improve patient outcomes and survival rates of rectal cancer (39).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access the datasets should be directed to bWFyZWUuYnJpbmttYW5AbmJyaS5jb20uYXU=.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MB: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. SC: Conceptualization, Investigation, Writing – review & editing. HG: Conceptualization, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This case report was fully funded by Nutrition Biomed Research Institute (NBRI).
Acknowledgments
We would like to thank the patient and his family who generously allowed us to use his de-identified data for this case report, and Emily Higgs and Dr. Bridget Jones who provided their expertise and guidance with the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. (2023) 73:233–54. doi: 10.3322/caac.21772
2. Mondaca S, Yaeger R. Genetics of rectal cancer and novel therapies: primer for radiologists. Abdom Radiol (NY). (2019) 44:3743–50. doi: 10.1007/s00261-019-02051-x
3. De Mattia E, Roncato R, Palazzari E, Toffoli G, Cecchin E. Germline and somatic pharmacogenomics to refine rectal cancer patients selection for neo-adjuvant chemoradiotherapy. Front Pharmacol. (2020) 11:897. doi: 10.3389/fphar.2020.00897
4. Park H. Predictive factors for early distant metastasis after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. World J Gastrointest Oncol. (2021) 13:252–64. doi: 10.4251/wjgo.v13.i4.252
5. Ouyang Y, Zhu Y, Chen H, Li G, Hu X, Luo H, et al. Case Report: Long-term survival of a patient with advanced rectal cancer and multiple pelvic recurrences after seven surgeries. Front Oncol. (2023) 13:1169616. doi: 10.3389/fonc.2023.1169616
6. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. (2020) 70:145–64. doi: 10.3322/caac.21601
7. De Mattia E, Polesel J, Mezzalira S, Palazzari E, Pollesel S, Toffoli G, et al. Predictive and prognostic value of oncogene mutations and microsatellite instability in locally-advanced rectal cancer treated with neoadjuvant radiation-based therapy: A systematic review and meta-analysis. Cancers (Basel). (2023) 15:1469. doi: 10.3390/cancers15051469
8. Martin-Carnicero A, Ramalle-Gomara E, Rubio-Mediavilla S, Alonso-Lago M, Zorrilla-Larraga M, Manrique-Abos I, et al. Prognostic and predictive biomarkers in patients with locally advanced rectal cancer (LARC) treated with preoperative chemoradiotherapy. J Clin Med. (2022) 11:6091. doi: 10.3390/jcm11206091
9. Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. (2020) 158:341–53. doi: 10.1053/j.gastro.2019.07.055
10. Mahmoud AM, Ali MM. Methyl donor micronutrients that modify DNA methylation and cancer outcome. Nutrients. (2019) 11:608. doi: 10.3390/nu11030608
11. Pieroth R, Paver S, Day S, Lammersfeld C. Folate and its impact on cancer risk. Curr Nutr Rep. (2018) 7:70–84. doi: 10.1007/s13668-018-0237-y
12. Hubner RA, Houlston RS. Folate and colorectal cancer prevention. Br J Cancer. (2009) 100:233–9. doi: 10.1038/sj.bjc.6604823
13. Kim YI, Salomon RN, Graeme-Cook F, Choi SW, Smith DE, Dallal GE, et al. Dietary folate protects against the development of macroscopic colonic neoplasia in a dose responsive manner in rats. Gut. (1996) 39:732–40. doi: 10.1136/gut.39.5.732
14. Le Leu RK, Young GP, McIntosh GH. Folate deficiency reduces the development of colorectal cancer in rats. Carcinogenesis. (2000) 21:2261–5. doi: 10.1093/carcin/21.12.2261
15. Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. (2007) 297:2351–9. doi: 10.1001/jama.297.21.2351
16. Wu K, Platz EA, Willett WC, Fuchs CS, Selhub J, Rosner BA, et al. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma. Am J Clin Nutr. (2009) 90:1623–31. doi: 10.3945/ajcn.2009.28319
17. Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR, uk CAPTG. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. (2008) 134:29–38. doi: 10.1053/j.gastro.2007.10.014
18. de Vogel S, Dindore V, van Engeland M, Goldbohm RA, van den Brandt PA, Weijenberg MP. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. (2008) 138:2372–8. doi: 10.3945/jn.108.091157
19. Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S, Japan Public Health Center-based Prospective Study G. Plasma folate and risk of colorectal cancer in a nested case-control study: the Japan Public Health Center-based prospective study. Cancer Causes Control. (2008) 19:67–74. doi: 10.1007/s10552-007-9071-z
20. Weinstein SJ, Albanes D, Selhub J, Graubard B, Lim U, Taylor PR, et al. One-carbon metabolism biomarkers and risk of colon and rectal cancers. Cancer Epidemiol Biomarkers Prev. (2008) 17:3233–40. doi: 10.1158/1055-9965.EPI-08-0459
21. Gibson TM, Weinstein SJ, Pfeiffer RM, Hollenbeck AR, Subar AF, Schatzkin A, et al. Pre- and postfortification intake of folate and risk of colorectal cancer in a large prospective cohort study in the United States. Am J Clin Nutr. (2011) 94:1053–62. doi: 10.3945/ajcn.110.002659
22. Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer. (2005) 113:825–8. doi: 10.1002/ijc.20648
23. La Vecchia C, Negri E, Pelucchi C, Franceschi S. Dietary folate and colorectal cancer. Int J Cancer. (2002) 102:545–7. doi: 10.1002/ijc.10720
24. Lee JE, Willett WC, Fuchs CS, Smith-Warner SA, Wu K, Ma J, et al. Folate intake and risk of colorectal cancer and adenoma: modification by time. Am J Clin Nutr. (2011) 93:817–25. doi: 10.3945/ajcn.110.007781
25. Konings EJ, Goldbohm RA, Brants HA, Saris WH, van den Brandt PA. Intake of dietary folate vitamers and risk of colorectal carcinoma: results from The Netherlands Cohort Study. Cancer. (2002) 95:1421–33. doi: 10.1002/cncr.10866
26. Roswall N, Olsen A, Christensen J, Dragsted LO, Overvad K, Tjonneland A. Micronutrient intake and risk of colon and rectal cancer in a Danish cohort. Cancer Epidemiol. (2010) 34:40–6. doi: 10.1016/j.canep.2009.12.012
27. Brink M, Weijenberg MP, de Goeij AF, Roemen GM, Lentjes MH, de Bruine AP, et al. Dietary folate intake and k-ras mutations in sporadic colon and rectal cancer in The Netherlands Cohort Study. Int J Cancer. (2005) 114:824–30. doi: 10.1002/ijc.20775
28. Sanchez H, Hossain MB, Lera L, Hirsch S, Albala C, Uauy R, et al. High levels of circulating folate concentrations are associated with DNA methylation of tumor suppressor and repair genes p16, MLH1, and MGMT in elderly Chileans. Clin Epigenet. (2017) 9:74. doi: 10.1186/s13148-017-0374-y
29. van Engeland M, Weijenberg MP, Roemen GM, Brink M, de Bruine AP, Goldbohm RA, et al. Effects of dietary folate and alcohol intake on promoter methylation in sporadic colorectal cancer: the Netherlands cohort study on diet and cancer. Cancer Res. (2003) 63:3133–7.
30. Iacopetta B, Heyworth J, Girschik J, Grieu F, Clayforth C, Fritschi L. The MTHFR C677T and DeltaDNMT3B C-149T polymorphisms confer different risks for right- and left-sided colorectal cancer. Int J Cancer. (2009) 125:84–90. doi: 10.1002/ijc.24324
31. Schernhammer ES, Giovannucci E, Kawasaki T, Rosner B, Fuchs CS, Ogino S. Dietary folate, alcohol and B vitamins in relation to LINE-1 hypomethylation in colon cancer. Gut. (2010) 59:794–9. doi: 10.1136/gut.2009.183707
32. Sahnane N, Magnoli F, Bernasconi B, Tibiletti MG, Romualdi C, Pedroni M, et al. Aberrant DNA methylation profiles of inherited and sporadic colorectal cancer. Clin Epigenet. (2015) 7:131. doi: 10.1186/s13148-015-0165-2
33. Ferrari A, Torrezan GT, Carraro DM, Aguiar Junior S. Association of folate and vitamins involved in the 1-carbon cycle with polymorphisms in the methylenetetrahydrofolate reductase gene (MTHFR) and global DNA methylation in patients with colorectal cancer. Nutrients. (2019) 11:1368. doi: 10.3390/nu11061368
34. Ismail O, Chin-Yee I, Gob A, Bhayana V, Rutledge A. Reducing red blood cell folate testing: a case study in utilisation management. BMJ Open Qual. (2019) 8:e000531. doi: 10.1136/bmjoq-2018-000531
35. van der Pols JC, Baade P, Spencer LB. Colorectal cancer incidence in Australia before and after mandatory fortification of bread flour with folic acid. Public Health Nutr. (2021) 24:1989–92. doi: 10.1017/S1368980021000562
36. Bao Y, Li Y, Gong Y, Huang Q, Cai S, Peng J. Vitamin D status and survival in stage II-III colorectal cancer. Front Oncol. (2020) 10:581597. doi: 10.3389/fonc.2020.581597
37. Maalmi H, Walter V, Jansen L, Boakye D, Schottker B, Hoffmeister M, et al. Association between blood 25-hydroxyvitamin D levels and survival in colorectal cancer patients: an updated systematic review and meta-analysis. Nutrients. (2018) 10:896. doi: 10.3390/nu10070896
38. Ulrich CM. Nutrigenetics in cancer research–folate metabolism and colorectal cancer. J Nutr. (2005) 135:2698–702. doi: 10.1093/jn/135.11.2698
Keywords: rectal cancer, recurrence, metastasis, folate, nutrigenomics
Citation: Brinkman MT, Crofts S and Green H (2024) The use of nutrigenomics and nutritional biomarkers with standard care of long-term recurrent metastatic rectal cancer: a case report. Front. Oncol. 14:1451675. doi: 10.3389/fonc.2024.1451675
Received: 19 June 2024; Accepted: 07 November 2024;
Published: 02 December 2024.
Edited by:
Nobu Oshima, Kyoto University Graduate School of Medicine, JapanReviewed by:
Zuheir Alshehabi, Tishreen University, SyriaLaiba Arshad, Forman Christian College, Pakistan
Copyright © 2024 Brinkman, Crofts and Green. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maree T. Brinkman, bWFyZWUuYnJpbmttYW5AbmJyaS5jb20uYXU=
 Maree T. Brinkman
Maree T. Brinkman Sam Crofts
Sam Crofts Hayden Green
Hayden Green