- 1Department of Oncology, Shangrao People’s Hospital, Shangrao, China
- 2Department of Respiratory and Critical Care Medicine, Ganzhou People’s Hospital, Ganzhou, China
Background: Combining epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) with chemotherapy (ETC) offers more advantages for patients with EGFR-positive non-small cell lung cancer (NSCLC) than using EGFR TKIs alone (ET). However, whether this conclusion applies to patients with brain metastases (BM) remains controversial. This meta-analysis was performed to evaluate the benefits and risks of the two groups.
Methods: Six databases were systematically searched for relevant literatures comparing ETC versus ET in treating EGFR-positive NSCLC patients with BM. The primary outcome assessed was overall survival (OS), while secondary outcomes included progression-free survival (PFS), and central nervous system (CNS)-PFS, responses, progression status and safety.
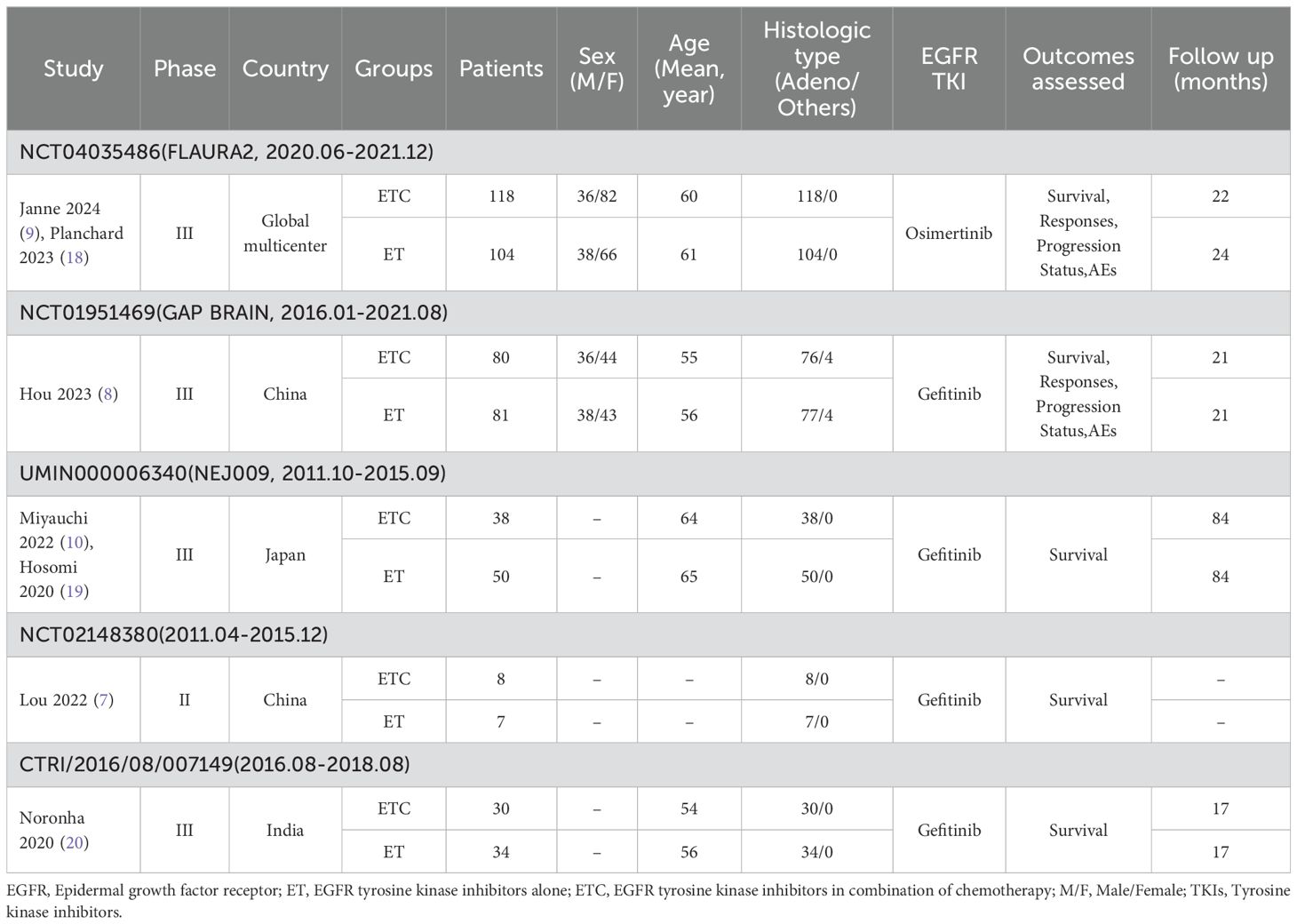
Results: Seven studies based on five randomized clinical trials with 550 patients were included. The ETC group exhibited better OS (hazard ratio [HR]: 0.64 [0.48, 0.87]), PFS (HR: 0.42 [0.34, 0.52]), and CNS-PFS (HR: 0.42 [0.31, 0.57]). The benefits in survival for OS, PFS, and CNS-PFS were validated in nearly all subgroups. Meanwhile, the overall objective response rate (ORR) (risk ratio [RR]: 1.25 [1.02, 1.52]) and CNS-ORR (RR: 1.19 [0.93, 1.51]) also tended to favor the ETC group. However, the addition of chemotherapy also brought about more grade 3-5/serious adverse events (AEs). The top five grade 3-5 AEs in the ETC group were alanine aminotransferase increase (11.25%), neutropenia (7.5%), nausea (7.5%), anorexia (5%), and diarrhea (5%).
Conclusions: ETC appears to be better than ET in treating EGFR-positive NSCLC patients with BM, with better OS, PFS, CNS-PFS, and responses. However, its poorer safety profile also needs to be taken into consideration.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024551073.
Introduction
Lung cancer is the foremost cause of both incidence and mortality among malignant tumors globally, with non-small cell lung cancer (NSCLC) making up about 90% of cases (1). Epidermal growth factor receptor (EGFR) mutations are the most common type among NSCLC cases, occurring in approximately 15% of Western NSCLC patients and 30-40% of Asian patients (2). For advanced EGFR-positive NSCLC, EGFR tyrosine kinase inhibitors (TKIs) significantly extend progression-free survival (PFS) and overall survival (OS) compared to traditional chemotherapy, while reducing the occurrence of adverse events (AEs) (3). The combination of chemotherapy with EGFR-TKIs (ETC) further improves patient outcomes (4). However, there remains clinical debate regarding whether this conclusion applies to EGFR-positive NSCLC patients with brain metastases (BM).
The National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) guidelines recommended both EGFR-TKI alone (ET) and ETC as first-line treatments for EGFR-positive NSCLC patients with BM (5, 6). Studies by Lou et al. and Hou et al. had demonstrated that ETC significantly improves patients’ OS and PFS (7, 8). Janne et al. also reported that ETC significantly enhances patients’ central nervous system (CNS) PFS (9). However, study by Miyauchi et al. indicated that ETC did not improve the OS of EGFR-positive NSCLC patients with BM and significantly increases the occurrence of AEs (10).
Addressing the clinical controversy outlined above, this meta-analysis compared the efficacy and safety of ETC and ET treatments in EGFR-positive NSCLC patients with BM.
Materials and methods
Selection criteria
Inclusion criteria: (1) Population: EGFR-positive NSCLC patients with BM; (2) Intervention and comparison: ETC versus ET; (3) Outcomes: survival, responses, progression status, and safety; (4) Study design: Randomized clinical trial (RCT).
Exclusion criteria: (1) Case reports, reviews, or meta-analyses; (2) Animal studies; (3) Studies with inaccessible full-text or from which useful data cannot be extracted.
Search strategy
A computerized search was conducted in PubMed, Scopus, EMBASE, ScienceDirect, Cochrane Library, and Web of Science, covering studies published up to August 27, 2024, that compared ETC and ET in treating EGFR-positive NSCLC patients with BM. The English search terms used were: “EGFR,” “Chemotherapy,” “Lung cancer,” and “Randomized” (Supplementary Table S1).
Data extraction
After independently screening the literature and extracting data, two researchers conducted a cross-check. The extracted data included baseline characteristics of studies (study design, number of patients, etc.), survival outcomes (OS, PFS, CNS-PFS, etc.), responses (ORR, DCR, etc.), progression status (total progression, CNS progression, etc.), and safety indicators (Total AEs, grade 3-5 AEs, etc. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events [NCI-CTCAE], version 4.0/5.0) (11, 12). In instances of discrepancies, a third researcher was consulted to make a decision.
Outcome assessments
The survival rates of PFS, OS, and CNS-PFS were analyzed at 6 to 60 months. Subgroup analyses of PFS, OS, and CNS-PFS were also conducted according to age, sex, ECOG PS, EGFR mutation type, extracranial metastases, and EGFR TKIs.
Quality assessment
The five-point Jadad scale was used to assess the quality of RCTs, which evaluates randomization, blinding, and patient accountability. Studies with scores of 3 points or higher were considered to be of high quality (13).
The Grades of Recommendations Assessment, Development, and Evaluation (GRADE) system was employed to evaluate the evidence categories of the results, considering five aspects: imprecision, risk of bias, indirectness, inconsistency, and publication bias. The evidence was divided into four categories: very low, low, moderate, and high (14).
Statistical analysis
The effect measures used included the risk ratio (RR) for binary data and the hazard ratio (HR) for survival data. All effect sizes were presented with 95% confidence intervals (CI). Prior to combining the effect sizes, a test for heterogeneity should be conducted. Heterogeneity among included studies will be assessed using the default Chi-square test. If the p-value is less than 0.1 and the I2 statistic is more than 50%, indicating significant heterogeneity. A fixed-effect model will be applied for data analysis if heterogeneity is non-significant. Otherwise, a random-effects model will be used. Funnel plots, Egger’s test, and Begg’s test were conducted to assess publication bias (15–17). REVMAN 5.3 and STATA 12.0 were used for data analysis. This study was conducted following the PRISMA guidelines and registered in PROSPERO (ID: CRD42024551073) (Supplementary Table S2).
Results
Search results
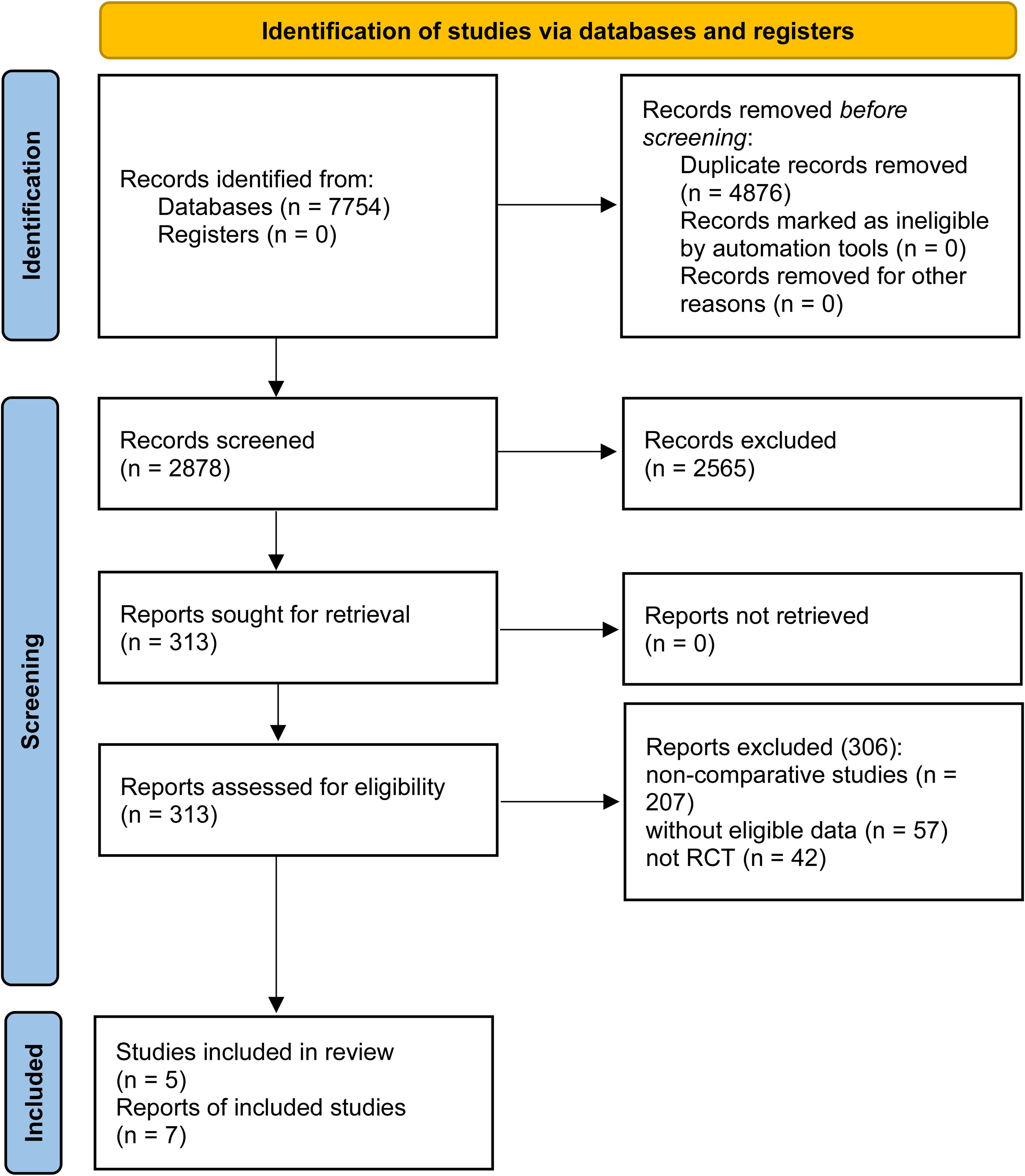
Seven studies based on 5 RCTs were included (274 patients were in the ETC group, while 276 were in the ET group) (Figure 1) (7–10, 18–20). Table 1 detailed the baseline characteristics of 5 RCTs. Four RCTs (7, 8, 10, 19, 20) were conducted in Asia and another one (9, 18) was global multicenter study. According to the quality assessment, all studies were of medium to high quality (Supplementary Table S3, Supplementary Figure S1). The quality of evidence for all results, as per the GRADE system, ranged from medium to high (Supplementary Table S4).
Survival
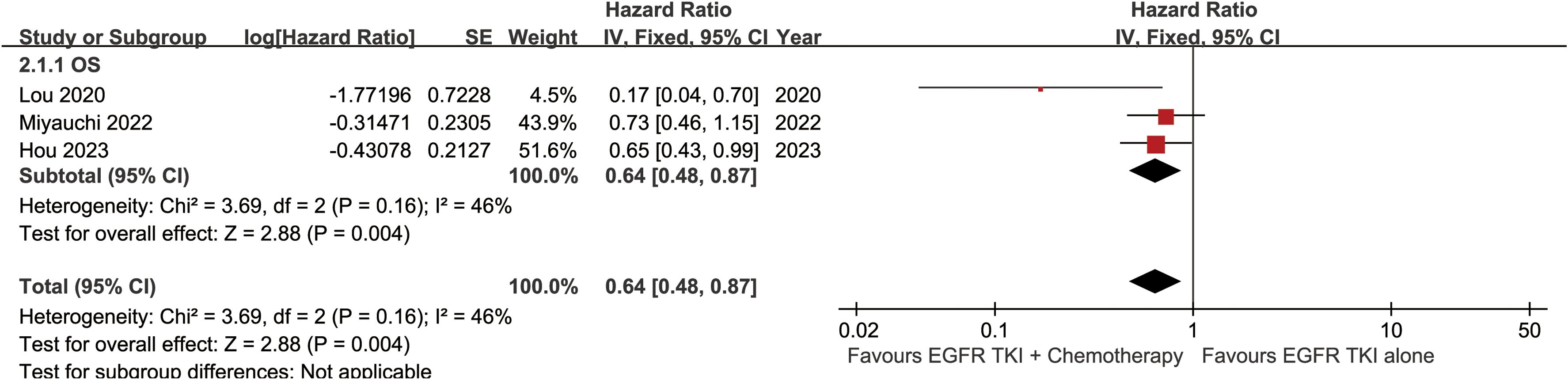
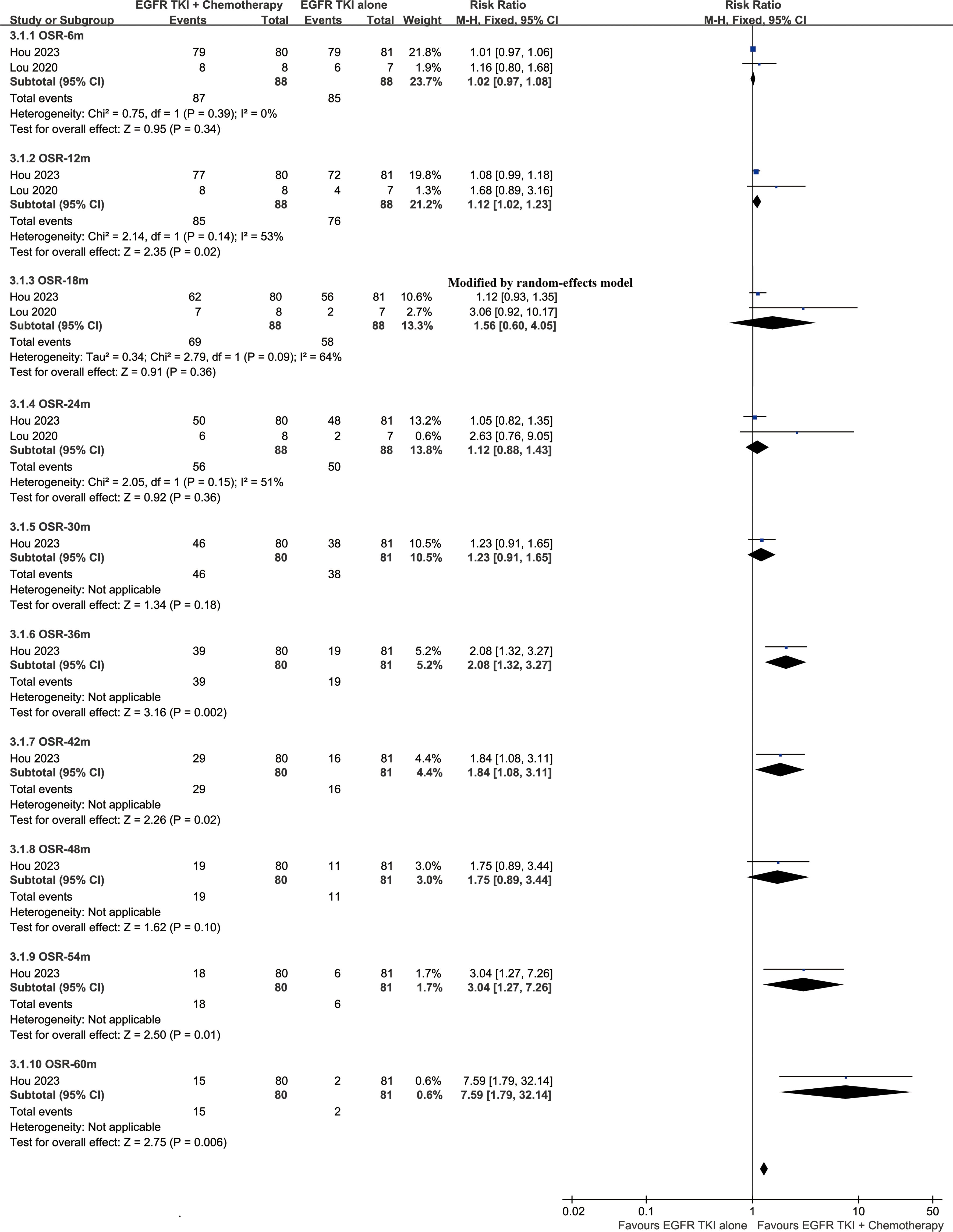
The OS was better in the ETC group (HR: 0.64 [0.48, 0.87]) (Figure 2). The overall survival rate (OSR) also tended to favor the ETC group at 12 to 60 months (Figure 3).
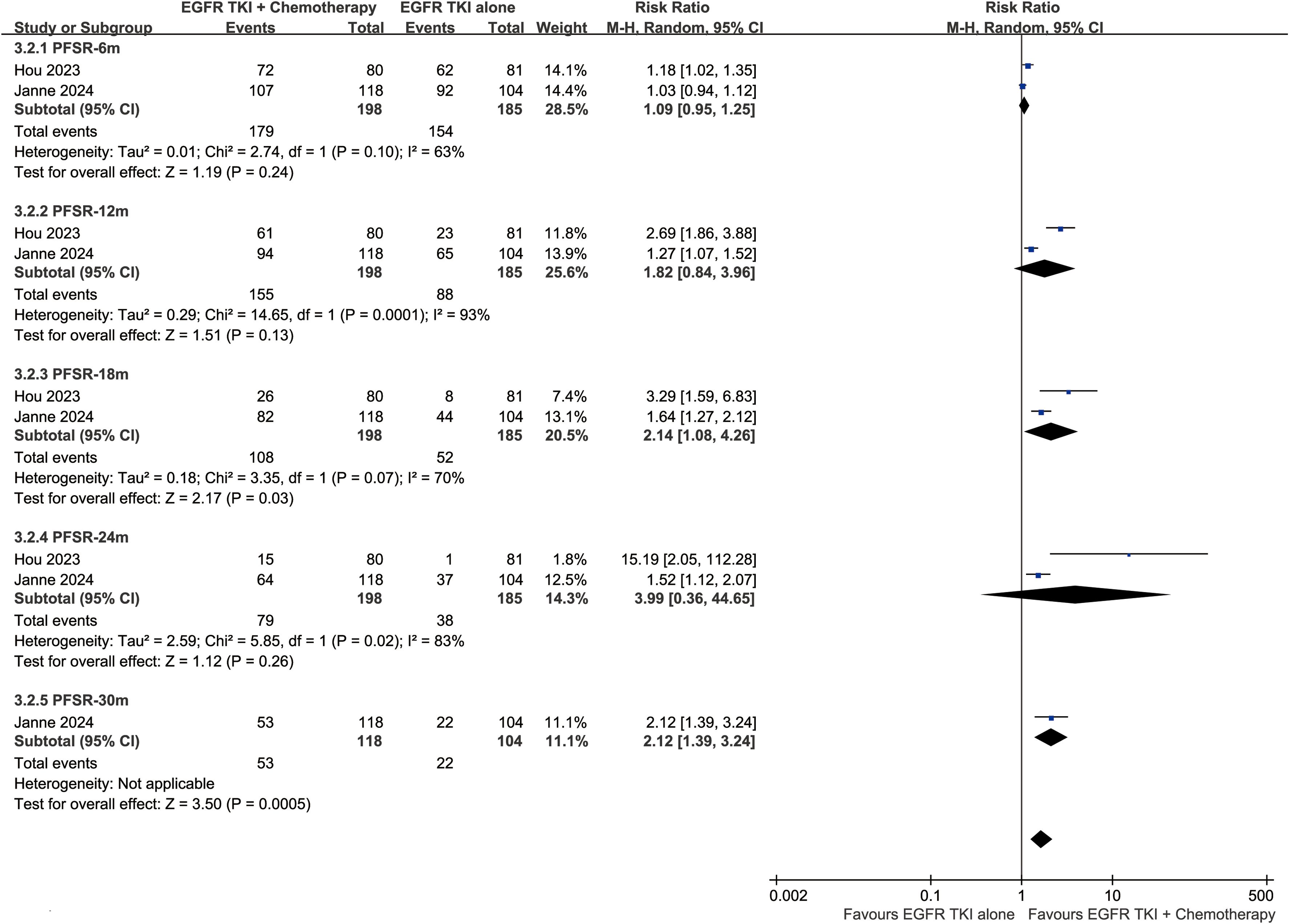
The PFS was better in the ETC group (HR: 0.42 [0.34, 0.52]) (Figure 4). The progression-free survival rate (PFSR) also tended to favor the ETC group at 6 to 30 months (Figure 5).
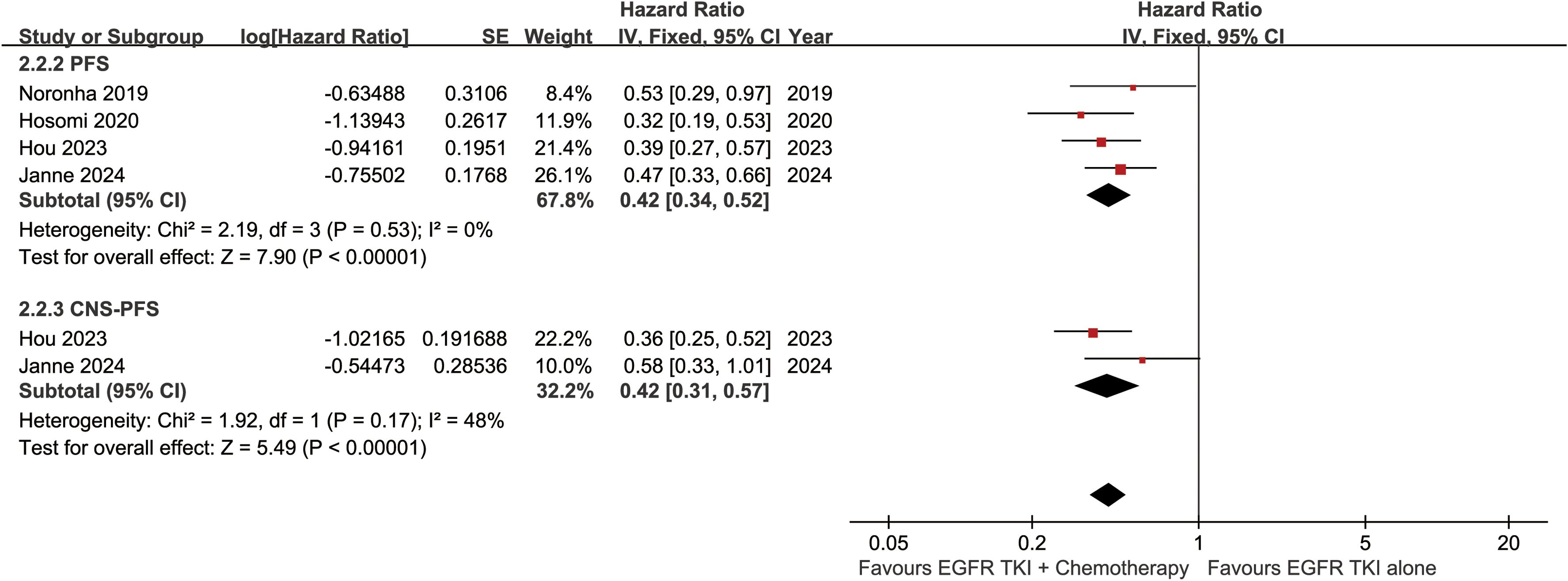
Figure 4. Forest plots of progression-free survival, and CNS-progression-free survival associated with ETC versus ET.
The CNS-PFS was better in the ETC group (HR: 0.42 [0.31, 0.57]) (Figure 4). The central nervous system progression-free survival rate (CNS-PFSR) also tended to favor the ETC group at 6 to 30 months (Supplementary Figure S2).
Subgroup analysis of survival
The survival advantages of OS, PFS, and CNS-PFS in the ETC group were confirmed in almost all subgroups according to age, sex, ECOG PS, EGFR mutation type, extracranial metastases, and EGFR TKIs. ECOG PS = 0, EGFR mutation - Ex19del, and a large intracranial tumor size < 20mm might be favorable factors for the ETC group (Table 2, Supplementary Figures S3–S5).
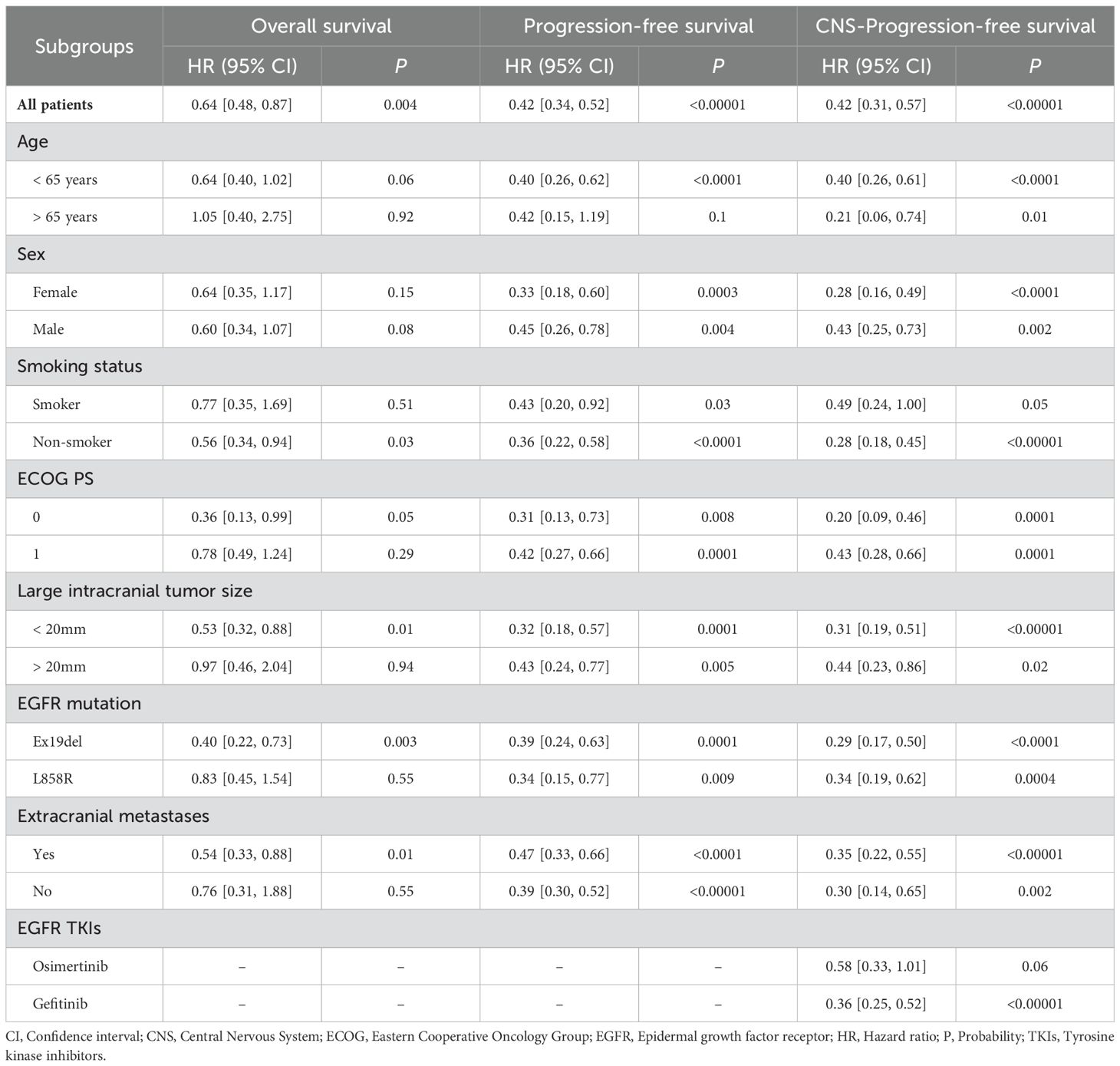
Table 2. Subgroup analysis of overall survival, progression-free survival, and CNS-progression-free survival.
Responses
In the analysis of overall responses, the overall response rate (ORR) (RR: 1.25 [1.02, 1.52]) and partial response (PR) (RR: 1.25 [1.02, 1.52]) were higher in the ETC group. The disease control rate (DCR) was similar between the two groups. The stable disease (SD) (RR: 0.49 [0.26, 0.90]) was higher in the ET group (Supplementary Figure S6).
In the analysis of CNS responses, the CNS-ORR (RR: 1.19 [0.93, 1.51]) and CNS-CR (RR: 1.31 [1.02, 1.70]) were higher in the ETC group. The CNS-DCR, CNS-PR, and CNS-SD were similar between the two groups (Supplementary Figure S7).
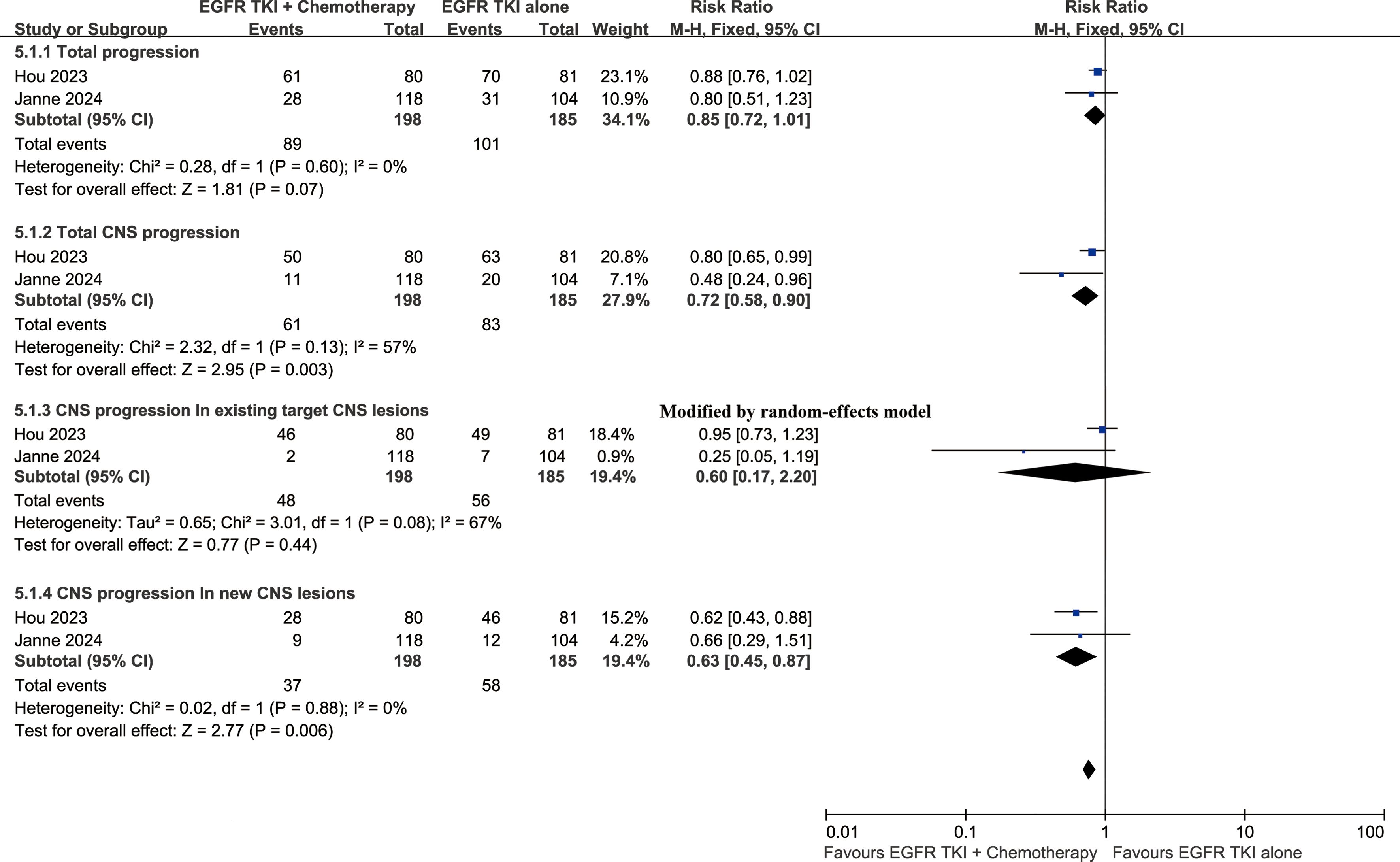
Progression status
At the cutoff time of the studies, the total progression (RR: 0.85 [0.72, 1.01]) and CNS progression (RR: 0.72 [0.58, 0.90]) tended to favor the ETC group. The addition of chemotherapy was particularly effective in controlling newly developed intracranial lesions (RR: 0.63 [0.45, 0.87]) (Figure 6).
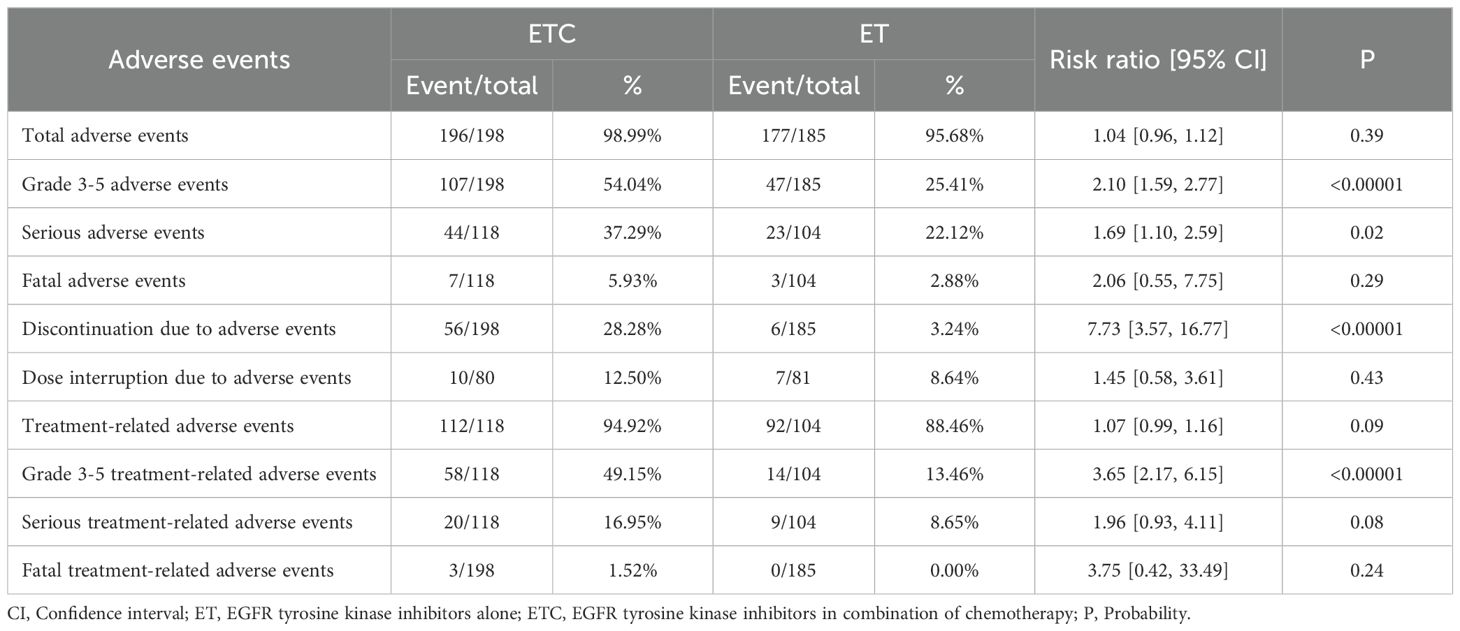
Safety
The rates of grade 3-5 AEs (RR: 2.10 [1.59, 2.77]), serious AEs (RR: 1.69 [1.10, 2.59]), discontinuation due to AEs (RR: 7.73 [3.57, 16.77]), and grade 3-5 treatment-related AEs (TRAEs) (RR: 3.65 [2.17, 6.15]) were higher in the ETC group. The total AEs, fatal AEs, dose interruption due to AEs, total TRAEs, serious TRAEs, and fatal TRAEs tended to favor the ET group without statistical differences (Table 3, Supplementary Figure S8).
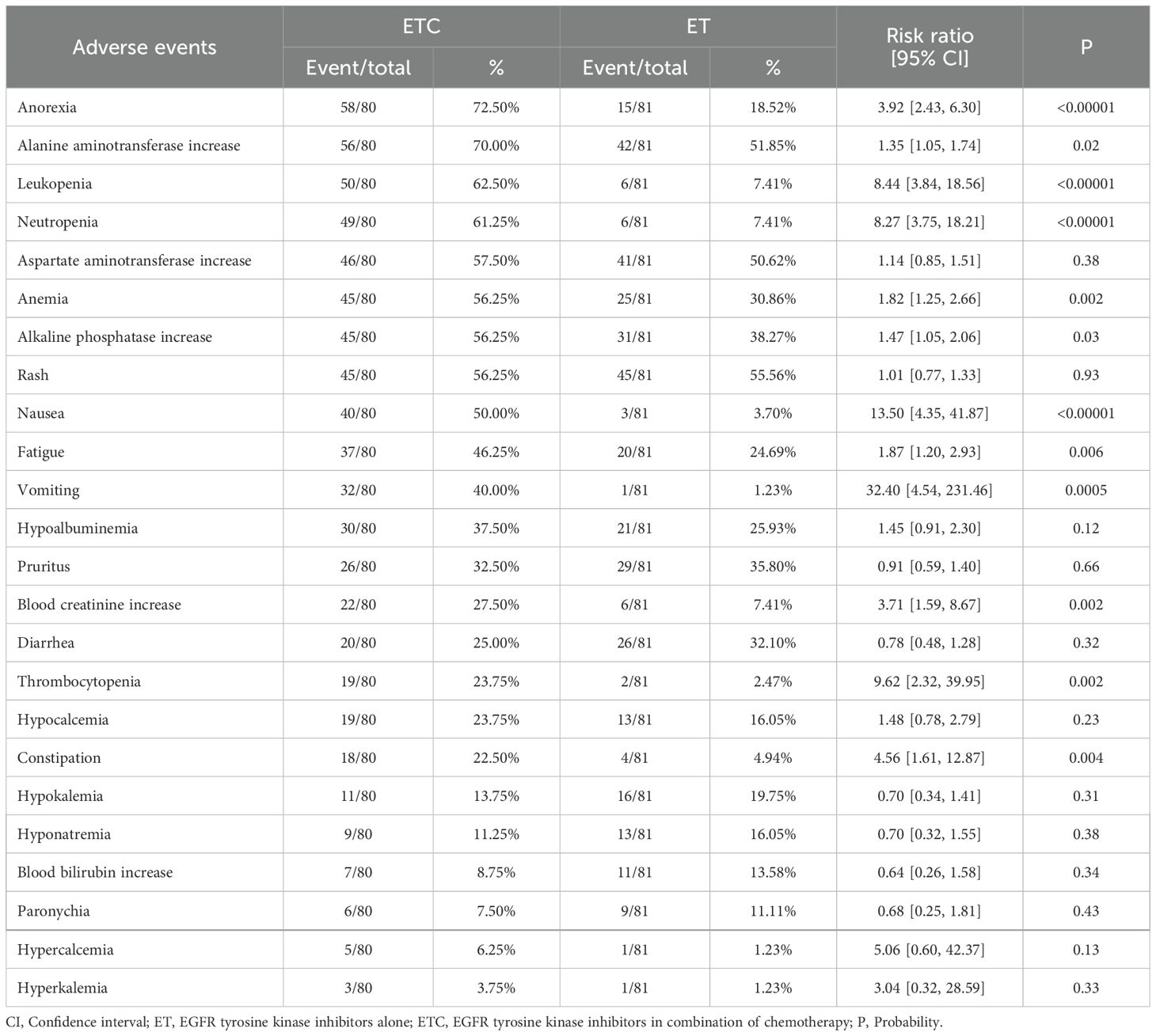
In the analysis of any grade AEs, more cases of anorexia, alanine aminotransferase increase, neutropenia, alkaline phosphatase increase, nausea, fatigue, vomiting, blood creatinine increase, thrombocytopenia, and constipation were found in the ETC group (Table 4, Supplementary Figure S9).
In the analysis of grade 3-5 AEs, most AEs tended to favor the ET group without statistical differences. The top 5 grade 3-5 AEs in the ETC group were alanine aminotransferase increase (11.25%), neutropenia (7.5%), nausea (7.5%), anorexia (5%), and diarrhea (5%) (Supplementary Table S5, Supplementary Figure S10).
Sensitivity analysis
Sensitivity analyses of OS and PFS were performed, demonstrating that excluding any single study had no impact on the credibility of the results (Supplementary Figure S11).
Publication bias
Funnel plots of survival, OSR, CNS responses, and safety summary were constructed. It was observed that studies were evenly distributed on both sides of the funnel plot, with almost all falling within its confines. This suggested minimal publication bias in this study (Figure 7). Egger’s and Begg’s tests based on OS and PFS also showed no significant publication bias (Supplementary Figure S11).
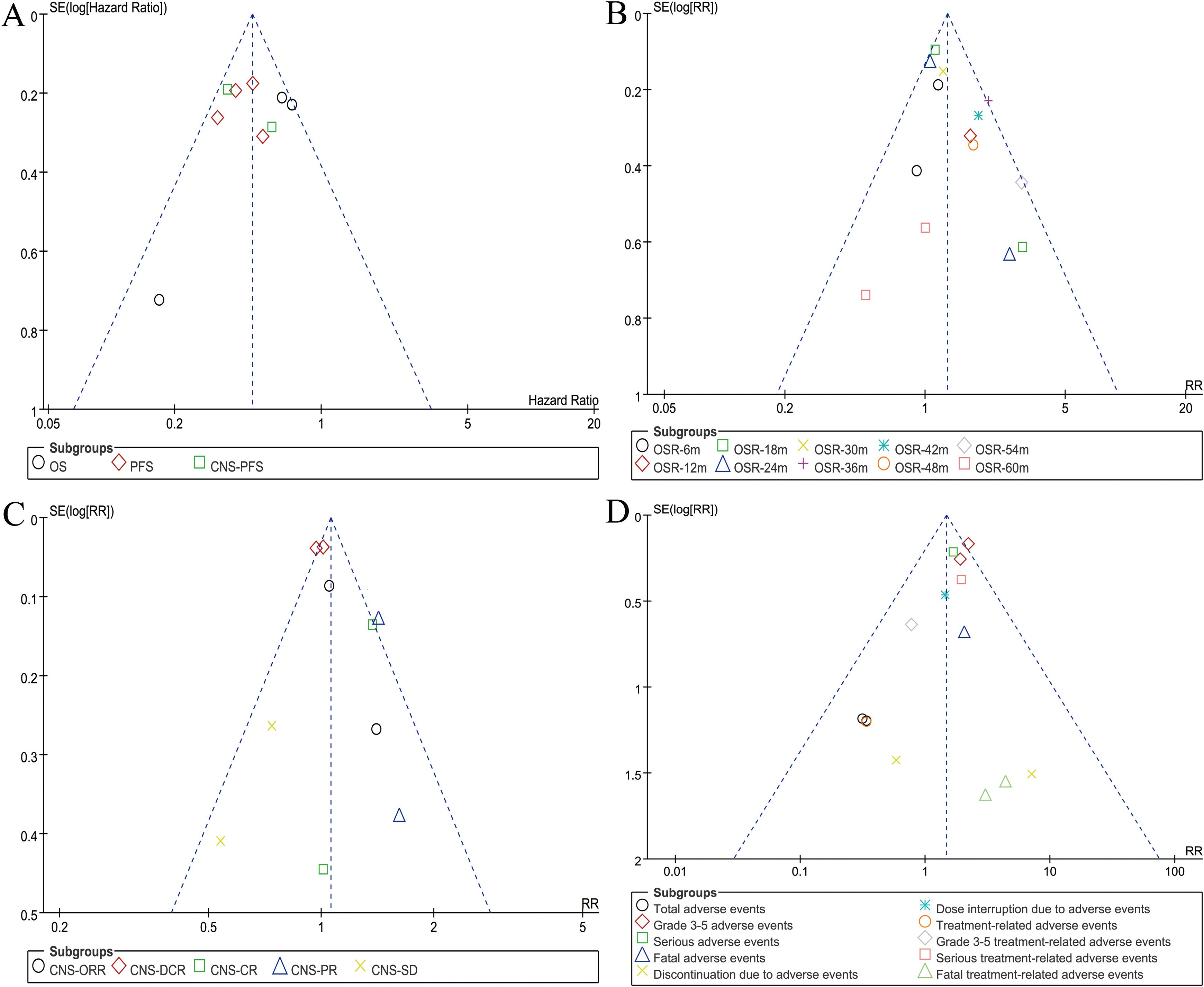
Figure 7. Funnel plots of overall survival (A), overall survival rate (B), CNS responses (C), and safety summary (D).
Discussion
In recent years, for advanced NSCLC patients with EGFR mutations, EGFR-TKI has become the standard first-line treatment, replacing chemotherapy. The antitumor mechanisms of EGFR-TKI and chemotherapy differ, and relevant preclinical and clinical studies have confirmed the potential of combination therapy (21, 22). Numerous studies have demonstrated that combination therapy can achieve better OS and PFS for advanced EGFR-positive NSCLC (23, 24). The survival advantage of combination therapy has also been confirmed by numerous meta-analyses, not only compared to chemotherapy, but also compared to EGFR-TKI monotherapy (Supplementary Table S6). However, whether this conclusion applies to patients with BM remains controversial in clinical practice. This meta-analysis, for the first time, compared the ETC and ET treatments in EGFR-positive NSCLC patients with BM based on RCTs. The results showed that the ETC group exhibited better survival, which was confirmed across almost all subgroups. Additionally, the overall objective response rate (ORR) and CNS-ORR tended to favor the ETC group. However, the addition of chemotherapy also led to more grade 3-5/serious AEs.
The greatest advantage of ETC over the ET group lies in its superior survival outcomes (OS, PFS, and CNS-PFS). This conclusion was supported by evidence from studies by Lou et al. and Hou et al. (7, 8). Preclinical studies had found that the ETC exerted a synergistic inhibitory effect on EGFR-sensitive cells (25, 26), as confirmed in trials such as CALGB30406, FASTACT-2, and NEJ005/TCOG0902 (27–29). NEJ005 also indicated a significant advantage in OS for EGFR-TKI combined with chemotherapy compared to sequential treatment, although the difference in PFS between patients was not significant (29). The enhanced efficacy of ETC might be related to the reduction of EGFR T790M mutation, which could promote resistance to EGFR TKIs (30). Another reason was the better drug response observed in the ETC group. Our study indicated that the ETC group exhibits superior ORR and CNS-ORR. The survival advantages of OS, PFS, and CNS-PFS in the ETC group were confirmed across almost all subgroups, particularly in patients with ECOG performance status = 0, EGFR mutation - Ex19del, and large intracranial tumor size < 20mm. In conclusion, due to its superior systemic and intracranial efficacy, we believed that combination therapy should be considered as the preferred treatment for EGFR-positive NSCLC patients with BM.
The main concern among clinical physicians regarding the ETC regimen is the potential for chemotherapy to induce more severe AEs (31, 32). Our study indicated that the rates of grade 3-5 AEs, serious AEs, discontinuation due to AEs, and grade 3-5 TRAEs were higher in the ETC group. The top 5 grade 3-5 AEs in the ETC group were alanine aminotransferase increase (11.25%), neutropenia (7.5%), nausea (7.5%), anorexia (5%), and diarrhea (5%). Studies by Janne et al. and Hou et al. had also found a significant increase in AEs occurrence in the ETC group, primarily concentrated in any grade AEs (8, 9). Although most grade 3-5 AEs tended to favor the ET group, they did not reach statistical significance. Therefore, we believe that the combined use of EGFR TKIs and chemotherapy, while potentially increasing the occurrence of AEs, remains within an acceptable range in terms of incidence and severity.
Limitations of this meta-analysis include: 1. The inclusion criteria were limited to English-published studies, potentially reducing the comprehensiveness of the analysis. 2. Some survival data were collected from subgroup analyses of large-scale RCTs, where differences in baseline characteristics among patients might affect the reliability of the data. 3. The number of studies included in some result analyses was small, compromising the clinical guidance value of the final results. 4. The majority of studies included in the analysis were conducted in Asia, potentially limiting the applicability of the data analysis conclusions to patients in other regions. 5. The included studies used different evaluation criteria to assess AEs, which would increase the heterogeneity of AEs analysis.
Conclusion
ETC appears to outperform ET in treating EGFR-positive NSCLC patients with BM, showing improvements in OS, PFS, CNS-PFS, and responses. The survival advantages of OS, PFS, and CNS-PFS in the ETC group were observed across nearly all subgroups, particularly in those with ECOG performance status = 0, EGFR mutation - Ex19del, and large intracranial tumor size < 20mm. However, the poorer safety profile of ETC should also be considered. Given the aforementioned limitations, it is essential to conduct additional high-quality randomized controlled trials to validate these conclusions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
ZC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XF: Conceptualization, Data curation, Formal Analysis, Writing – original draft. LZ: Conceptualization, Data curation, Formal analysis, Writing – original draft. XW: Conceptualization, Data curation, Formal analysis, Writing – original draft. SZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank professor Wenxiong Zhang, MD (Department of Thoracic Surgery, The second affiliated hospital of Nanchang University) for his data collection and statistical advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1448336/full#supplementary-material
Supplementary Figure 1 | Cochrane Risk Assessment.
Supplementary Figure 2 | Forest plots of CNS-PFSR at 3-30 months associated with ETC versus ET.
Supplementary Figure 3 | Forest plots of subgroup analysis of overall survival associated with ETC versus ET.
Supplementary Figure 4 | Forest plots of subgroup analysis of progression-free survival associated with ETC versus ET.
Supplementary Figure 5 | Forest plots of subgroup analysis of CNS-progression-free survival associated with ETC versus ET.
Supplementary Figure 6 | Forest plots of overall responses associated with ETC versus ET.
Supplementary Figure 7 | Forest plots of CNS responses associated with ETC versus ET.
Supplementary Figure 8 | Forest plots of safety summary associated with ETC versus ET.
Supplementary Figure 9 | Forest plots of any grade adverse events associated with ETC versus ET.
Supplementary Figure 10 | Forest plots of grade 3-5 adverse events associated with ETC versus ET.
Supplementary Figure 11 | Sensitivity analysis of overall survival (A) and progression-free survival (B).
Supplementary Figure 12 | Egger’s and Begg’s tests based on OS (A) and PFS (B).
Abbreviations
AEs, Adverse effects; BM, Brain metastases; CI, Confidence interval; CNS, Central Nervous System; CR, Complete response; CT, Cohort study; ECOG, Eastern Cooperative Oncology Group; EGFR, Epidermal growth factor receptor; ET, EGFR tyrosine kinase inhibitors alone; ETC, EGFR tyrosine kinase inhibitors in combination of chemotherapy; ESMO, European Society for Medical Oncology; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; HR, Hazard ratio; LC, Lung cancer; M/F, male/female; NCCN, National Comprehensive Cancer Network; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse; NSCLC, Non-small cell lung cancer; ORR, Objective response rate; OS, Overall survival; OSR, Overall survival rate; P, Probability; PFS, Progression-free survival; PICOS, Participants, Intervention, Control, Outcome and Study design; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; RCT, randomized controlled trial; RR, Risk ratio; TKIs, Tyrosine kinase inhibitors; TRAEs, Treatment-related adverse effects.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Ma J, Song YD, Bai XM. Global, regional, and national burden and trends of early-onset tracheal, bronchus, and lung cancer from 1990 to 2019. Thorac Cancer. (2024) 15:601–13. doi: 10.1111/1759-7714.15227
3. Kanagalingam S, Ul Haq Z, Victory N Srinivasan, Khan AI, Mashat GD, Hazique M, et al. Comparing gefitinib and traditional chemotherapy for better survival in patients with non-small cell lung cancer: A systematic review. Cureus. (2023) 15:e33691. doi: 10.7759/cureus.33691
4. Zhu CM, Lian XY, Zhang HY, Bai L, Yun WJ, Zhao RH, et al. EGFR tyrosine kinase inhibitors alone or in combination with chemotherapy for non-small-cell lung cancer with EGFR mutations: A meta-analysis of randomized controlled trials. J Cancer Res Ther. (2021) 17:664–70. doi: 10.4103/jcrt.JCRT_195_20
5. Riely GJ, Wood DE, Ettinger DS, Aisner DL, Akerley W, Bauman JR, et al. Non-small cell lung cancer, version 4.2024. J Natl Compr Canc Netw. (2024) 22:249–74. doi: 10.6004/jnccn.2204.0023
6. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2023) 34:339–57. doi: 10.1016/j.annonc.2022.12.009
7. Lou Y, Xu J, Zhang Y, Lu J, Chu T, Zhang X, et al. Chemotherapy plus EGFR-TKI as first-line treatment provides better survival for advanced EGFR-positive lung adenocarcinoma patients: updated data and exploratory in vitro study. Target Oncol. (2020) 15:175–84. doi: 10.1007/s11523-020-00708-y
8. Hou X, Li M, Wu G, Feng W, Su J, Jiang H, et al. Gefitinib plus chemotherapy vs gefitinib alone in untreated EGFR-mutant non-small cell lung cancer in patients with brain metastases: the GAP BRAIN open-label, randomized, multicenter, phase 3 study. JAMA Netw Open. (2023) 6:e2255050. doi: 10.1001/jamanetworkopen.2022.55050
9. Jänne PA, Planchard D, Kobayashi K, Cheng Y, Lee CK, Valdiviezo N, et al. CNS efficacy of osimertinib with or without chemotherapy in epidermal growth factor receptor-mutated advanced non-small-cell lung cancer. J Clin Oncol. (2024) 42:808–20. doi: 10.1200/JCO.23.02219
10. Miyauchi E, Morita S, Nakamura A, Hosomi Y, Watanabe K, Ikeda S, et al. Updated analysis of NEJ009: gefitinib-alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated EGFR. J Clin Oncol. (2022) 40:3587–92. doi: 10.1200/JCO.21.02911
11. Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol. (2012) 67:1025–39. doi: 10.1016/j.jaad.2012.02.010
12. National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 (2017). U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES. Available online at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (Accessed August 27, 2024).
13. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
14. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. (2011) 64:380–2. doi: 10.1016/j.jclinepi.2010.09.011
15. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
16. Langhorne P. Bias in meta-analysis detected by a simple, graphical test. Prospectively identified trials could be used for comparison with meta-analyses. BMJ. (1998) 316:471.
17. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
18. Planchard D, Jänne PA, Cheng Y, Yang JC, Yanagitani N, Kim SW, et al. Osimertinib with or without chemotherapy in EGFR-mutated advanced NSCLC. N Engl J Med. (2023) 389:1935–48. doi: 10.1056/NEJMoa2306434
19. Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. (2020) 38:115–23. doi: 10.1200/JCO.19.01488
20. Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. (2020) 38:124–36. doi: 10.1200/JCO.19.01154
21. Halmos B, Rai P, Min J, Hu X, Chirovsky D, Shamoun M, et al. Real-world outcomes on platinum-containing chemotherapy for EGFR-mutated advanced nonsquamous NSCLC with prior exposure to EGFR tyrosine kinase inhibitors. Front Oncol. (2024) 14:1285280. doi: 10.3389/fonc.2024.1285280
22. Zhang Q, Wang R, Xu L. Clinical advances in EGFR-TKI combination therapy for EGFR-mutated NSCLC: a narrative review. Transl Cancer Res. (2023) 12:3764–78. doi: 10.21037/tcr
23. Cui X, Li X, Lv C, Yan S, Wang J, Wu N. Efficacy and safety of adjuvant EGFR TKI alone and in combination with chemotherapy for resected EGFR mutation-positive non-small cell lung cancer: A Bayesian network meta-analysis. Crit Rev Oncol Hematol. (2023) 186:104010. doi: 10.1016/j.critrevonc.2023.104010
24. Xue J, Li B, Wang Y, Huang Z, Liu X, Guo C, et al. Efficacy and safety of epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor combination therapy as first-line treatment for patients with advanced EGFR-mutated, non-small cell lung cancer: A systematic review and bayesian network meta-analysis. Cancers (Basel). (2022) 14:4894. doi: 10.3390/cancers14194894
25. Okabe T, Okamoto I, Tsukioka S, Uchida J, Iwasa T, Yoshida T, et al. Synergistic antitumor effect of S-1 and the epidermal growth factor receptor inhibitor gefitinib in non-small cell lung cancer cell lines: role of gefitinib-induced down-regulation of thymidylate synthase. Mol Cancer Ther. (2008) 7:599–606. doi: 10.1158/1535-7163.MCT-07-0567
26. La Monica S, Madeddu D, Tiseo M, Vivo V, Galetti M, Cretella D, et al. Combination of gefitinib and pemetrexed prevents the acquisition of TKI resistance in NSCLC cell lines carrying EGFR-activating mutation. J Thorac Oncol. (2016) 11:1051–63. doi: 10.1016/j.jtho.2016.03.006
27. Wang S, Gao A, Liu J, Sun Y. First-line therapy for advanced non-small cell lung cancer with activating EGFR mutation: is combined EGFR-TKIs and chemotherapy a better choice? Cancer Chemother Pharmacol. (2018) 81:443–53. doi: 10.1007/s00280-017-3516-1
28. Mok T, Ladrera G, Srimuninnimit V, Sriuranpong V, Yu CJ, Thongprasert S, et al. Tumor marker analyses from the phase III, placebo-controlled, FASTACT-2 study of intercalated erlotinib with gemcitabine/platinum in the first-line treatment of advanced non-small-cell lung cancer. Lung Cancer. (2016) 98:1–8. doi: 10.1016/j.lungcan.2016.04.023
29. Sugawara S, Oizumi S, Minato K, Harada T, Inoue A, Fujita Y, et al. Randomized phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutations: NEJ005/TCOG0902. Ann Oncol. (2015) 26:888–94. doi: 10.1093/annonc/mdv063
30. Menzel M, Kirchner M, Kluck K, Ball M, Beck S, Allgäuer M, et al. Genomic heterogeneity at baseline is associated with T790M resistance mutations in EGFR-mutated lung cancer treated with the first-/second-generation tyrosine kinase inhibitors. J Pathol Clin Res. (2024) 10:e354. doi: 10.1002/cjp2.354
31. Gendarme S, Zebachi S, Corre R, Greillier L, Justeau G, Bylicki O, et al. Predictors of three-month mortality and severe chemotherapy-related adverse events in patients aged 70 years and older with metastatic non-small-cell lung cancer: A secondary analysis of ESOGIA-GFPC-GECP 08-02 study. J Geriatr Oncol. (2023) 101506. doi: 10.1016/j.jgo.2023.101506
32. Herbach E, O’Rorke MA, Carnahan RM, McDowell BD, Allen B, Grumbach I, et al. Cardiac adverse events associated with chemo-radiation versus chemotherapy for resectable stage III non-small-cell lung cancer: A surveillance, epidemiology and end results-medicare study. J Am Heart Assoc. (2022) 11:e027288. doi: 10.1161/JAHA.122.027288
Keywords: EGFR, tyrosine kinase inhibitors, chemotherapy, non-small cell lung cancer, brain metastases, meta-analysis
Citation: Chen Z, Fu X, Zhu L, Wen X and Zhang S (2024) The benefit and risk of addition of chemotherapy to EGFR tyrosine kinase inhibitors for EGFR-positive non-small cell lung cancer patients with brain metastases: a meta-analysis based on randomized controlled trials. Front. Oncol. 14:1448336. doi: 10.3389/fonc.2024.1448336
Received: 13 June 2024; Accepted: 02 October 2024;
Published: 21 October 2024.
Edited by:
Lin Zhou, Sichuan University, ChinaReviewed by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesSong Xu, Tianjin Medical University General Hospital, China
Copyright © 2024 Chen, Fu, Zhu, Wen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shihao Zhang, MTU2MDc5NzIwMDJAMTYzLmNvbQ==
 Zhigang Chen1
Zhigang Chen1 Shihao Zhang
Shihao Zhang