
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 17 October 2024
Sec. Hematologic Malignancies
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1446482
 Barath Prashanth Sivasubramanian1†
Barath Prashanth Sivasubramanian1† Shashvat Joshi2†
Shashvat Joshi2† Diviya Bharathi Ravikumar3*†
Diviya Bharathi Ravikumar3*† Madhumithaa Jagannathan4†
Madhumithaa Jagannathan4† Sonia Babu5†
Sonia Babu5† Shanthi Reddy Sripathi6†
Shanthi Reddy Sripathi6† Avinash Javvaji7†
Avinash Javvaji7† Priyanshu Jain8†
Priyanshu Jain8† Dinesh Kumar Shanmugam9†
Dinesh Kumar Shanmugam9† Bharath Duraisamy Swami Kannan10†
Bharath Duraisamy Swami Kannan10† Raghavendra Tirupathi11
Raghavendra Tirupathi11 Rutul Dalal12
Rutul Dalal12Background: By 2023, COVID-19 had caused 6.8 million deaths in the United States. COVID-19 presents more severely in leukemia compared to solid tumors (OR 1.6, p<0.05). However, data on Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS) are limited. We investigated the mortality in AML and MDS patients with COVID-19.
Methods: Data from the 2020-2021 National Inpatient Sample was used to conduct a cross-sectional analysis. We identified AML and MDS patients with COVID-19 hospitalizations through ICD-10 codes. Analysis was done by propensity matching and multivariate regression with a p-value of ≤0.05.
Results: Of 28,028 AML admissions, 336 (1.2%) were admitted for COVID-19. AML-COVID-19 cohort had a lower hospitalization risk (aOR 0.3, p=0.000) and higher mortality (21.7% vs 8.7%; aOR 1.6, p=0.023) than AML patients admitted for other causes. AML patients post-HSCT (Hematopoietic Stem Cell Transplantation) had a higher risk of COVID-19 (20.2% vs 9.8%; aOR 2.6, p=0.000) and increased mortality (19.1% vs 6.7%; aOR 4.1, p=0.000) compared to other causes. Similarly, of 28,148 MDS patients, 769 (2.7%) were admitted for COVID-19. The MDS-COVID-19 cohort had a lower hospitalization risk (aOR 0.59, p=0.000) and higher mortality (19.6% vs 6.6%; aOR 2.2, p=0.000) compared to other causes. In MDS, HSCT did not alter the risk of COVID-19 hospitalizations (3% vs 3.9%; aOR 0.9, p=0.662), but these patients had higher mortality (17.4% vs 5.1%; aOR 4.0, p=0.032).
Conclusion: COVID-19 hospitalization was low in AML and MDS but carried a high mortality risk. Post-HSCT, the mortality is high, warranting research into understanding the underlying factors.
As of 2023 in the United States, the COVID-19 pandemic has caused over 6.8 million fatalities (1). Immunocompromised patients (those with HIV, transplants, and malignancies) have an increased risk of acquiring COVID-19 infections, developing prolonged infections, and potentially contributing to the emergence of concerning viral variants (2, 3). In hematological malignancies, specifically, those with leukemia are known to experience more severe COVID-19 outcomes compared to those with solid organ tumors (OR 1.57, p<0.0043) (4, 5). Additionally in hematological malignancies, male gender, pre-induction and induction phases, ICU admission, low levels of oxygen saturation at the onset of infection, Rhesus (RH) factor positivity, and higher fibrinogen levels have all been associated with increased mortality. The state of the current malignancy, recent administration of myeloablative chemotherapy or immunosuppressive therapies, recent surgery, and radiotherapy further impact the outcomes (5, 6).
Acute Myeloid Leukemia (AML) patients had a lower COVID-19 prevalence of 5.4% compared to Myelodysplastic Syndrome (MDS) with COVID-19 at 19.7% (6). In patients with AML and MDS, a heightened risk of mortality from COVID-19 is noted, with rates reaching up to 40% and 42.3%, respectively (7–9). Several underlying factors may exacerbate the severity of COVID-19 in hematological malignancies. These include the aggressive nature of their underlying disease, immune dysfunction (particularly involving neutrophils and T-cells), delayed seroconversion, and prolonged hospitalization (10–14). Additionally, treatment outcomes in MDS patients can be challenging, with a high risk of treatment failure (HR 3.564, p=0.041) observed when using antivirals and anti-spike monoclonal antibodies (MABs) for COVID-19 (15). In patients with AML and MDS, those undergoing hematopoietic stem cell transplant (HSCT) have shown mortality rates of 24.8% and 27% for allogeneic (allo-HSCT) and autologous (auto-HSCT) transplants, respectively, following COVID-19 infection (7). Furthermore, AML patients post-HSCT have shown several mutations in the virus, including substitution (D796H) in the S2 subunit, deletion [deltaH69/deltaV70] in the S1 N-terminal domain of the spike protein, E340K, K356R, R346T, and E484V). These mutations have led to viral evolution, a protracted period (4 months) for neutralizing antibodies to appear, and reduced sensitivity to neutralizing antibodies (16–19).
Currently, available studies on AML and COVID-19 are restricted to small cohorts and case reports/series (4). Moreover, evidence-based guidelines to aid clinicians in treatment decisions are scarce due to evolving recommendations for COVID-19 treatment. Additionally, crucial information regarding readmission rates and out-of-hospital mortality rates remains lacking (8, 20).
Our study aims to assess mortality in AML and MDS patients hospitalized with COVID-19. Furthermore, our secondary objective was to assess the outcomes of COVID-19 in AML and MDS patients who have received HSCT.
We queried the 2020-2021 National Inpatient Sample (NIS) database. A cross-sectional study was carried out using the NIS database. The NIS was developed by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ) (21). Its purpose was to generate regional and national estimates of inpatient utilization, expenditures, and outcomes in the United States. The document comprises various components, including patient demographics (such as age, sex, and race), diagnosis and procedure codes derived from the International Classification of Diseases, Tenth Revision, Clinical Modification/Procedure Coding System (ICD-10-CM/PCS), measures of severity and comorbidity, hospital characteristics, discharge status, and length of stay (LOS).
The NIS is a de-identified, publicly accessible database. The current investigation was not submitted to an institutional review board for approval. A predetermined sample size was not calculated for this study. Adults aged 18 or above admitted with an ICD10 code of hematological malignancies (acute myeloid leukemia and myelodysplastic syndrome) were included. We followed a method previously used to identify patients with a diagnosis of hematological malignancies (acute myeloid leukemia and myelodysplastic syndrome) and COVID-19 adequately. ICD-10-CM/PCS codes are provided in the Supplementary Table S1. We complied with the AHRQ’s data user agreement before accessing the NIS databases. The databases utilized adhere to the HIPAA (Health Insurance Portability and Accountability Act) Privacy Rule’s definition of limited data sets and do not comprise any explicit identifiers of patients.
Gender was delineated as male and female. Patient race was defined as White, African American, Hispanic, Asian or Pacific Islander, and Native American. Insurance status was defined by Medicare (referent), Medicaid, Private Insurance, and other/self-pay/No charge. The comorbidities were classified using ICD-10-CM codes. The codes encompass acute kidney injury, myocardial infarction, invasive ventilation, severe sepsis, acute respiratory failure, acute respiratory distress syndrome, acute heart failure, and vasopressor usage. The codes are provided in the Supplementary Table S1. The inpatient mortality, total hospital charges, and length of stay were acquired from the NIS database.
We conducted our analysis by examining continuous variables through means and t-tests, and qualitative variables were assessed using the chi-square test. We set a significance level of p≤ 0.05. Stata v18 was used to conduct the analysis. We used two different methods to adjust for confounders in our analysis: Propensity score matching and multivariate regression analysis. Propensity scores were used to match AML and MDS patients with COVID-19. A non parsimonious multivariate logistic regression model was developed to estimate the propensity score for developing mortality using the following variables: age, gender, and race. The double robust method was then used to generate treatment weights and the inverse probability of treatment weighting was used to match cases with controls using generalized linear models (22). The second analysis used multivariable regression analysis models to adjust the results for potential confounders. Multivariable regression models were built by including all confounders significantly associated with the outcome on univariate analysis with a cutoff p-value of 0.2. Variables that were deemed important determinants of the outcomes based on literature were added to the models.
Our primary objective was to determine the factors associated with in-hospital mortality among adults (≥18 years) with AML and MDS, primarily admitted for COVID-19. Our secondary objective was to understand the mortality of COVID-19 in patients who are HSCT recipients. Additionally, we studied the healthcare utilization (length of stay and total charges incurred) and the prevalence of comorbidities in hospitalized adult AML/MDS patients with COVID-19.
A total of 28,028 AML patients were identified, with 337 patients (1.2%) hospitalized for COVID-19. This rate was lower compared to a 4.6% hospitalization rate among 11 million non-AML cases (p<0.001). Supplementary Table S2 represents the sociodemographic profile of Acute Myeloid Leukemia Patients. The average age of patients did not differ between the two groups (62.3 ± SE 0.9 vs 61 ± SE 0.1, p=0.1919, non-significant). Table 1 represents the outcomes of Acute Myeloid Leukemia (AML) patients who were hospitalized for COVID-19. Supplementary Table S3 represents univariate logistic regression of AML patients and COVID-19 who did not survive. AML patients showed a decreased risk of COVID-19 hospitalization compared to AML patients admitted for other reasons (adjusted Odds Ratio aOR of 0.3; 95% CI 0.2-0.3; p<0.001) when adjusted for age over 65, gender, race, hospital characteristics (size, teaching status, and location), complications and comorbidities (stroke, acute respiratory distress syndrome, acute respiratory failure, sepsis, acute kidney injury, and myocardial infarction), and interventions (vasopressor usage and invasive ventilation).
The mortality rate in AMLCov was 22.7% (95%CI 16.4%-30.4%) while in All-cause-admission was only 8.8% (95%CI 8.3%-9.4%). Among these, patients aged ≥65 years showed a high mortality rate (Nonsurvivors 74% vs Survivors 48.1%, p=0.0001). Male patients experienced high mortality (Nonsurvivors 60.3% vs Survivors 49.4%, p=0.1028, non-significant). Additionally, based on a median household income of $1 - $49,999, we observed a higher number of nonsurvivors than survivors (33.3% vs 27.3%, p=0.5907, non-significant). We observed significantly higher occurrences of severe sepsis (26% vs 1.9%, p<0.0001), acute respiratory distress syndrome (27.4% vs 3%, p<0.0001), acute respiratory failure (65.7% vs 52.3%, p=0.0459), and myocardial infarction (12.3% vs 1.5%, p<0.0001) among nonsurvivors during hospitalization. Additionally, the usage of vasopressors (17.8% vs 1.1%, p<0.0001) and the necessity for invasive ventilation (2.7% vs 0.4%, p=0.056) were markedly higher among nonsurvivors.
Table 2 represents Comorbidities of Acute Myeloid Leukemia and Myelodysplastic Syndrome patients with COVID-19. After matching with age, gender, and race and adjusting the multivariate regression analysis for age over 65, gender, race, hospital characteristics (size, teaching status, and location), complications and comorbidities (stroke, ARDS, acute respiratory failure, sepsis, acute kidney injury, and myocardial infarction), and interventions (vasopressor usage, invasive ventilation, and HSCT status), COVID-19 showed an increased risk of mortality (aOR 1.6; 95% CI 1.1-2.6; p=0.028) compared to non-COVID-19 admissions. We identified several mortality predictors with p<0.05. Age ≥65 years (aOR 2.8; 95% CI 1.3-6.0), severe sepsis (aOR 6.7; 95% CI 1.5-29.5), ARDS (aOR 17.0; 95% CI 3.6-80.8), acute respiratory failure (aOR 4.5; 95% CI 1.5-13.7), myocardial Infarction (aOR 8.4; 95% CI 2.2-32.6), and invasive ventilation (aOR 9.8; 95% CI 1.3-74.9) were found to have a high risk of mortality.
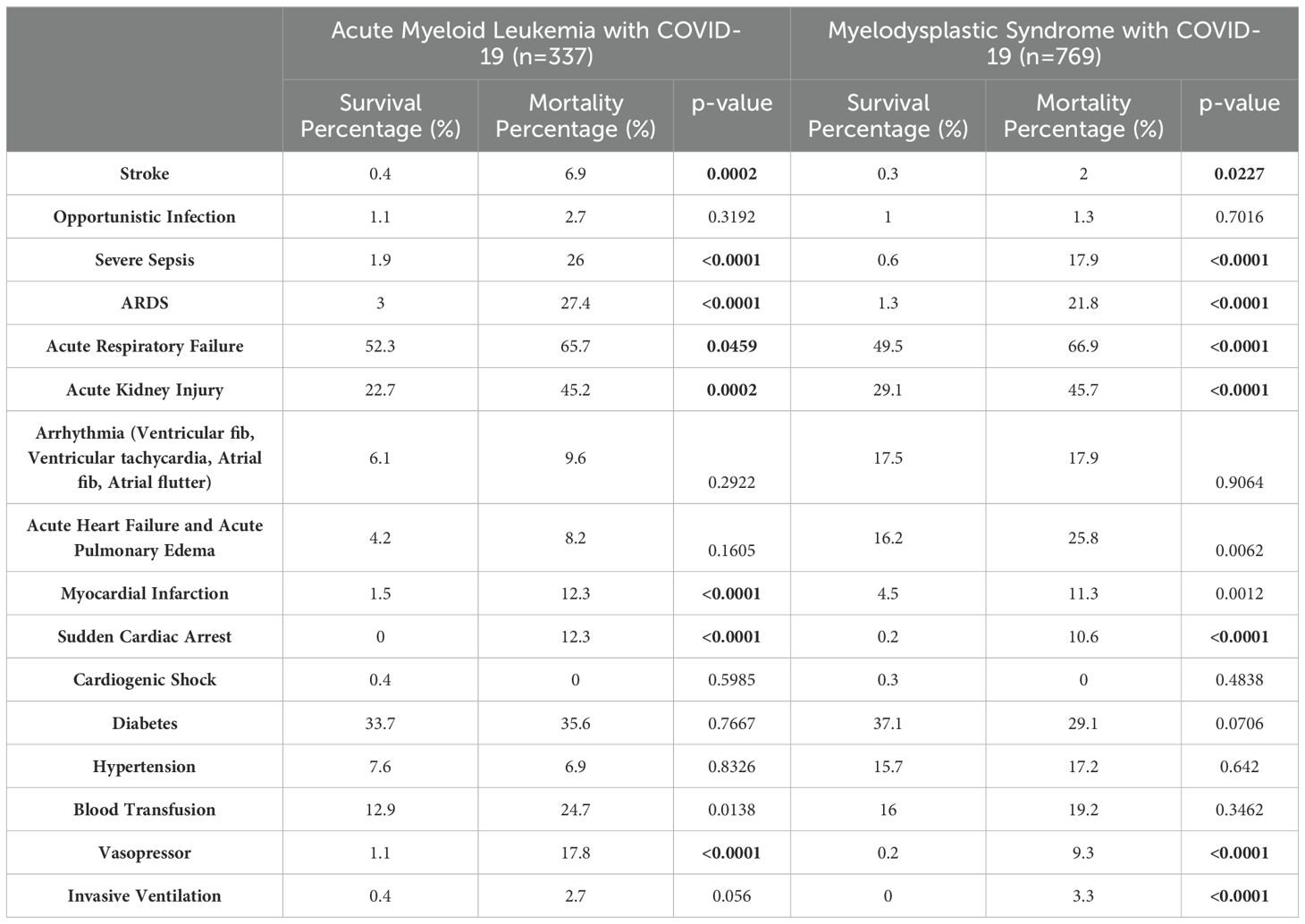
Table 2. Comorbidities of Acute Myeloid Leukemia and Myelodysplastic Syndrome patients with COVID-19.
On analyzing the outcomes in AML patients post-hematopoietic stem cell transplantation (HSCT), we found that a higher proportion of patients were admitted for COVID-19 compared to those admitted for other causes (20.2% vs 9.8%; p<0.001). After matching with age over 65 years, gender, and race and adjusting the multivariate regression analysis for age over 65, gender, race, and hospital characteristics (size, teaching status, and location), the hospitalization risk in the HSCT patients was higher with an aOR of 2.6 (95% CI 1.9-3.4; p<0.001). The mortality rate for COVID-19 was higher (19.1%; 95% CI 11.3%-30.4%) than the non-COVID-19 group (6.7%; 95% CI 5.8%-7.7%). After matching and adjusting with the same variables as before, multivariate regression showed a high mortality risk in COVID-19 with an aOR of 4.1 (95% CI 2-8.7; p<0.001). Table 3 represents the results of the multivariate regression analysis used to study the predictors of mortality in AML patients with COVID-19.
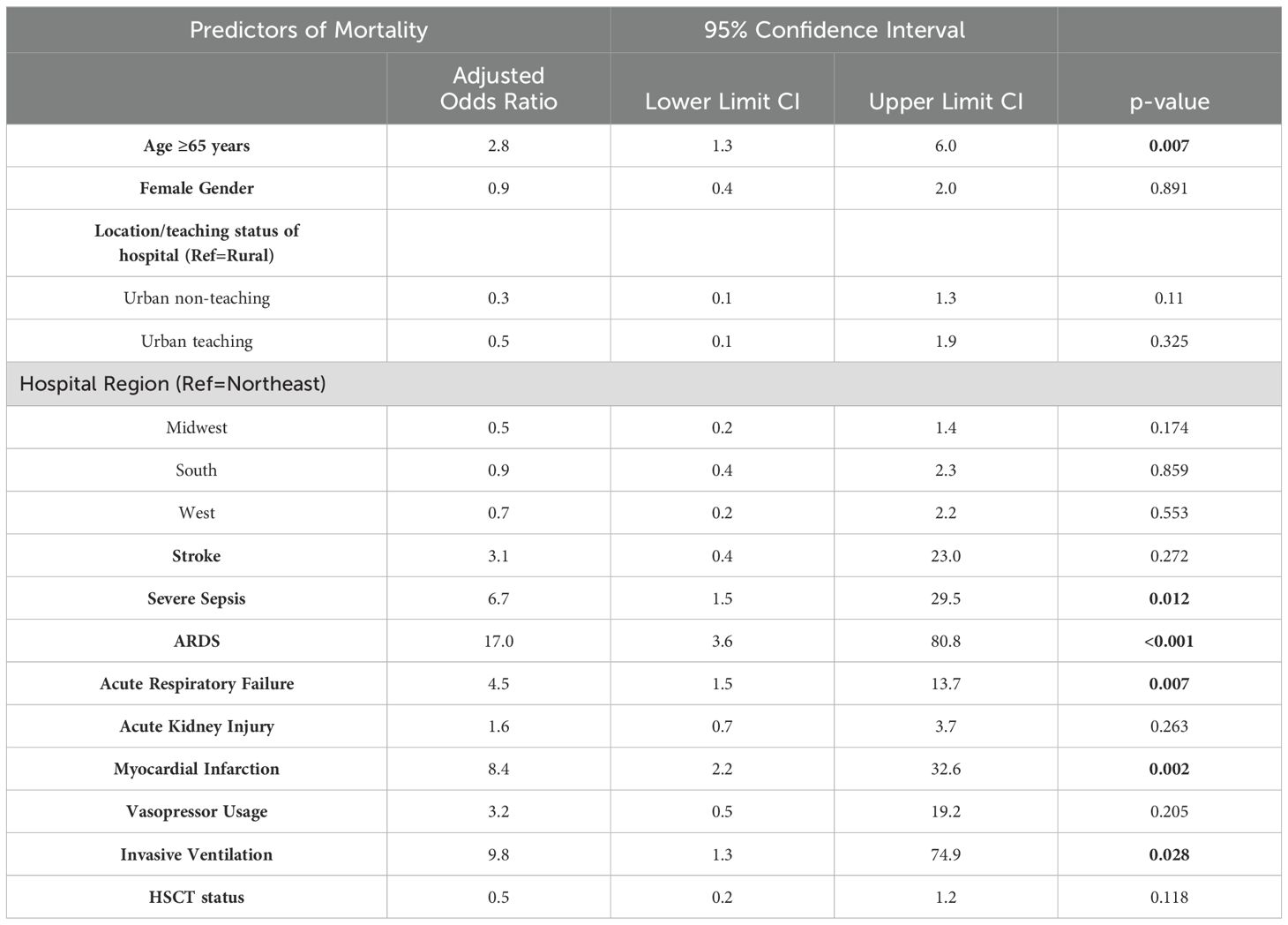
Table 3. Multivariate Logistic Regression to determine predictors of mortality in Acute Myeloid Leukemia patients with COVID-19.
Table 4 shows hospital utilization in Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS) patients who were admitted for COVID-19. After matching and adjusting with age, gender, race, and hospital characteristics (size, teaching status, and location), we identified the length of hospital stay and hospital charges for AML patients hospitalized for COVID-19. Both of these were lower in COVID-19 compared to non-COVID-19 admissions (Hospital Stay: Coefficient -1.7 days, 95% CI -2.9 to -0.4 days, p=0.011; Charges: Coefficient $ -34,200; 95% CI $-62,200 to $-6,163; p= 0.017).
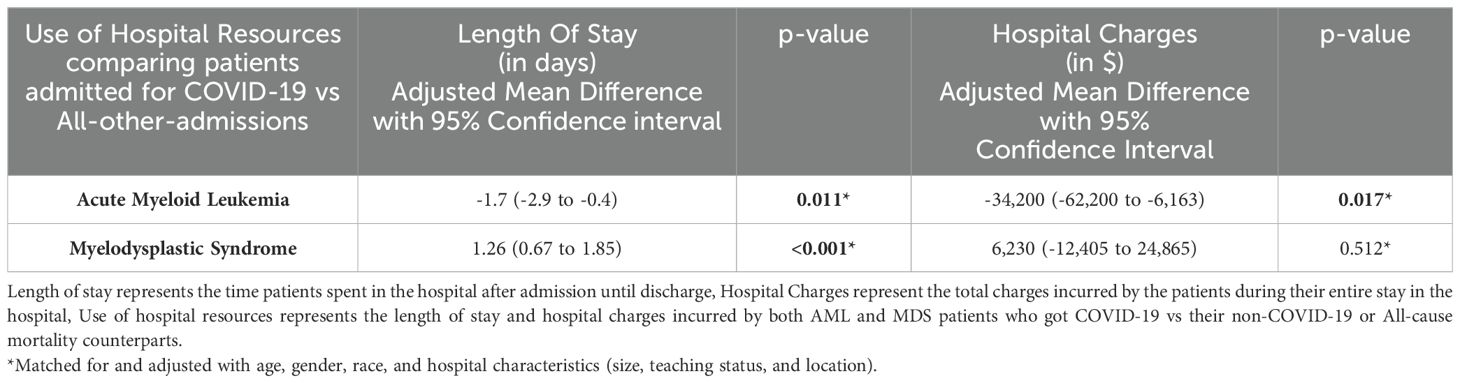
Table 4. Hospital Utilization by Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS) patients who were hospitalized for COVID-19.
In MDS patients (n=28,148), we identified 769 COVID-19 admissions (MDSCov 2.7%), and the remaining were admitted for other reasons. In the non-MDS population of 11 million, we identified 500,000 COVID-19 admissions (4.6%) with a p-value of 0.000. Supplementary Table S4 represents the sociodemographic profile of patients with Myelodysplastic Syndrome.
The average age of MDSCov (n=769) was higher than those admitted for all other causes (77.3 ± SE 0.4 years vs 75.3 ± SE 0.1 years, p<0.0001). Table 5 represents outcomes in Myelodysplastic syndrome (MDS) patients who were hospitalized for COVID-19. Supplementary Table S5 represents univariate logistic regression of Myelodysplastic Syndrome patients and COVID-19 who did not survive. The MDS population had a decreased risk of hospitalization for COVID-19 with aOR of 0.59 (95% 0.54-0.63; p<0.001) compared to those MDS patients admitted for other reasons when adjusted for age over 65 years, gender, race, and hospital characteristics (size, teaching status, and location), comorbidities and complications (stroke, sepsis, ARDS, acute respiratory failure, acute kidney injury, and acute heart failure), and vasopressor usage.
The mortality rate due to COVID-19 (19.6%; 95% CI 17%-22.6%) was higher than non-COVID-19 admissions (6.6%; 95% CI 6.3%-7%). Patients aged ≥65 years had a higher mortality rate (Nonsurvivors 90.1% vs Survivors 88.2%, p=0.5107, non-significant). Male patients also experienced a high mortality rate (Nonsurvivors 64.9% vs Survivors 53.9%, p=0.015). Additionally, among those with a median household income of $65,000 - $85,999, the mortality was high (28.5% vs. 25%, p=0.7966, non-significant). Table 2 represents Comorbidities that occurred in Acute Myeloid Leukemia and Myelodysplastic Syndrome (MDS) patients with COVID-19.
We observed a significantly higher prevalence of stroke (2% vs 0.3%, p=0.0227), severe sepsis (17.9% vs 0.6%, p<0.0001), acute respiratory distress syndrome (21.8% vs 1.3%, p<0.0001), acute respiratory failure (66.9% vs 49.5%, p=0.0001), and sudden cardiac arrest (10.6% vs 0.2%, p<0.0001) among nonsurvivors. Additionally, the usage of vasopressors (9.3% vs 0.2%, p<0.0001) was markedly higher among nonsurvivors.
After matching the patients based on age, gender, and race and conducting a multivariate regression analysis, which was adjusted for age over 65 years, gender, race, hospital characteristics (size, teaching status, and location), comorbidities and complications (stroke, sepsis, ARDS, acute respiratory failure, acute kidney injury, and acute heart failure), and interventions (vasopressor usage and HSCT), we found an increased risk of mortality due to COVID-19 with an aOR 2.2 (95% CI 1.7-2.9; p<0.001) compared to non-COVID-19 admissions. Following this, we identified several predictors of mortality, such as female gender (aOR=0.6, 95% CI 0.4-1.0, p=0.031), severe sepsis (aOR=9.9, 95% CI 3.3-30, p<0.001), ARDS (aOR=35.1, 95% CI 12.00-102.4, p<0.001), acute respiratory failure (aOR=4.2, 95% CI 2.5-7.00, p<0.001), and vasopressor usage (aOR=7.7, 95% CI 1-60.6, p=0.051). While Age ≥65 years, stroke, Acute Kidney Injury, and Acute Heart Failure did not contribute to mortality. Table 6 represents the results of the multivariate regression analysis used to study the predictors of mortality in Myelodysplastic syndrome patients with COVID-19.
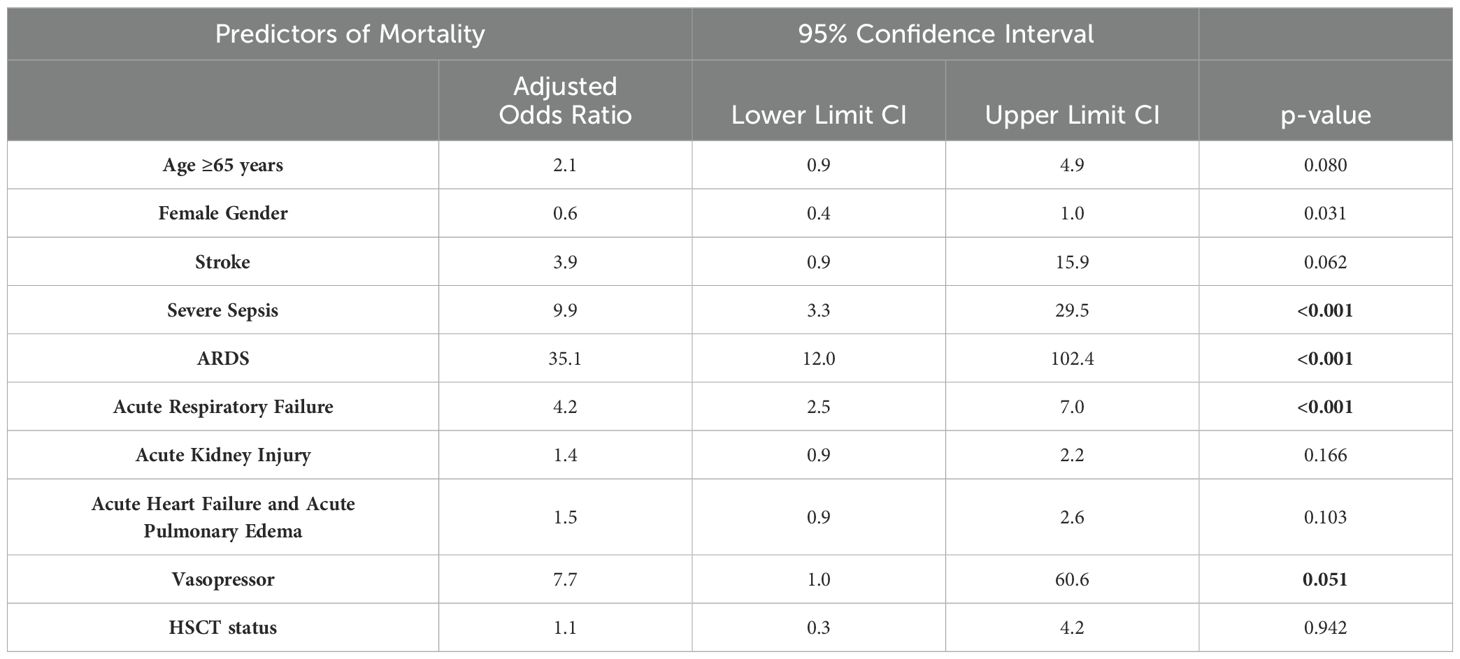
Table 6. Multivariate Logistic Regression to determine predictors of mortality in patients with Myelodysplastic Syndrome and COVID-19.
On analyzing the outcomes in MDS patients post-HSCT, we found that 3% were hospitalized for COVID-19, while 3.9% had non-COVID-19 admissions. HSCT did not alter the risk of COVID-19-related hospitalization (aOR 0.9; 95% CI 0.6-1.4; p=0.662, non-significant) when adjusted for age over 65 years, gender, race, and hospital characteristics (size, teaching status, and location). The mortality rate for COVID-19 was 17.4% (95% CI 6.7%-38.2%) while for non-COVID-19 was 5.1% (95% CI 4%-6.5%). After matching the patients based on age over 65, gender, and race and conducting a multivariate regression analysis, adjusted for age over 65 years, gender, race, and hospital characteristics (size and location), we found that COVID-19 was associated with an increased risk of mortality in this population (aOR 4; 95% CI 1.1-14.3; p=0.032) compared to non-COVID-19 admissions.
Table 4 shows hospital utilization by Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS) patients hospitalized for COVID-19. The length of stay and hospital charges were calculated by matching based on age, gender, and race and adjusted for age, gender, race, and hospital characteristics (size, teaching status, and location). COVID-19 patients had a longer stay with a Coefficient of 1.26 days (95% CI 0.67-1.85 days); p<0.001 than non COVID-19 related admissions. The hospital charges did not differ between the two groups (Coefficient $6,230; $-12,405 to $24,865; p=0.512,non-significant).
Our study investigated mortality rates among patients with Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS) who were hospitalized for COVID-19. We found that both AML and MDS patients had significantly higher mortality rates compared to the rest of the hospital population. We demonstrated that severe sepsis (aOR of 6.7 in AML; aOR of 9.9 in MDS), acute respiratory distress syndrome (aOR of 17 in AML; aOR of 35.1 in MDS), and acute respiratory failure (aOR of 4.5 in AML; aOR of 4.2 in MDS) had higher mortality with a significant p-value of less than 0.05. Notably, socioeconomic factors such as household income, insurance model, and hospital size did not contribute to mortality. Additionally, patients who underwent hematopoietic stem cell transplantation (HSCT) also had higher mortality rates with COVID-19 in both malignancies.
We found AML patients had higher COVID-19 mortality in those above 65 years compared to survivors (74% vs 48.1%, p=0.0001). This could be attributed to a relatively lower immune status with increasing age and factors like more than three comorbidities (non-survivors 72.6% vs survivors 66.7%). A study conducted by the European Hematology Association Survey (EPICOVIDEHA), also supports our findings in which they found patients with more comorbidities had higher mortality (7). Mortality in male AML patients with COVID-19 was higher than in females but the results did not attain statistical significance (60.3% vs 39.7%, p=0.0989). Jin et al. also reported males in the general population had 2.4 times higher mortality from COVID-19 compared to females of the same age groups (23). The reason for this gender disparity is not fully understood but ACE2 expression is higher in males than in females (23). We found that HSCT had increased COVID-19 hospitalizations (aOR 2.6; 95% CI 1.9-3.4; p<0.001) and increased mortality (aOR 4.1; 95% CI 2-8.7; p<0.001) in AML patients compared to patients admitted for other reasons. Leukemic patients post-HSCT struggle to develop immunity following both natural illness and vaccination (24). We encourage future research in this area, to get a better understanding of the effects of HSCT in COVID-19.
Among patients with Myelodysplastic Syndrome and COVID-19, males had higher mortality than females (64.9% vs 35.1%, p=0.015). This could be due to the same reason as seen in AML patients, i.e. higher protein expression of ACE2 receptors particularly in males, correlating to organ failures (23). We found the in-hospital mortality for MDS was 19.6% (95% CI 17%-22.6%), whereas Fernandez-Cruz et al. reported 38% in those with hematological malignancies (p=0.002), with only 14% of the study population having MDS (25). Complications such as severe sepsis (17.9%), acute kidney injury (45.7%), acute heart failure (25.8%), and ARDS (21.8%) were significantly higher in those with mortality (p<0.0001). COVID-19 can cause endothelial injury, microthrombus formation, and pulmonary cell hyperplasia (26). Complications such as ARDS due to direct viral damage, neutropenia, or immunosuppression increase the risk of sepsis, and a hypercoagulable state due to both viral inflammation and malignancy have led to severe outcomes in MDS patients (25, 27–30). COVID-19 is associated with lower gamma globulin levels and patients with hematological malignancies are prone to have neutropenia and immature granulocytosis. Both these factors lead to increased mortality in this cohort (31, 32). Vasopressors and mechanical ventilation were commonly administered to non-surviving MDSCoV patients, as evidenced by the higher mortality rates among them (nonsurvivors 9.3% vs. survivors 0.2%, p<0.0001). This trend aligns with findings from a study which concluded that critically ill patients with COVID-19 requiring vasopressors were associated with significantly higher mortality (33). Invasive mechanical ventilation was also used in nonsurvivors (3.3%, p<0.0001). However, due to our study design, we couldn’t ascertain the duration of mechanical ventilation or if the patients developed ventilator-associated lung injury (34). We identified that 3% of post-HSCT patients were hospitalized for COVID-19, and 17.4% of those did not survive. This mortality rate is higher compared to post-HSCT patients hospitalized for other reasons (5.1%). HSCT has been shown to retain anti-SARS-CoV-2 IgG antibodies in patients with hematological malignancies (35). However, the higher mortality rate in this subgroup warrants investigation.
Our research faced several limitations, primarily due to its design. The values we reported pertain only to the hospitalized population and the exact prevalence of COVID-19 from the general population could not be determined. We were unable to follow up with survivors or compare results across different waves of COVID-19. Additionally, laboratory results of patients at admission were unavailable, preventing us from determining or monitoring trends in various complications. Furthermore, we could not account for the newer management guidelines that have evolved since the start of the pandemic, nor did we account for coding errors and duplication of data.
In conclusion, our study indicates that AML and MDS patients with COVID-19, have higher inpatient mortality with sepsis and respiratory complications emerging as significant contributors to this outcome. Additionally, patients post-HSCT had higher mortality in COVID-19 within both AML and MDS. These findings underscore the need for close monitoring and implementation of preventive strategies. Future research is warranted to identify optimal treatment approaches and risk mitigation strategies specifically for AMLand MDS patients.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
BS: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software. SJ: Writing – original draft, Writing – review & editing, Project administration, Resources, Supervision, Validation, Visualization. DR: Writing – original draft, Writing – review & editing, Formal Analysis, Investigation, Validation, Visualization. MJ: Writing – original draft, Writing – review & editing, Visualization. SB: Writing – review & editing. SS: Writing – review & editing. AJ: Writing – review & editing. PJ: Writing – review & editing. DK: Writing – review & editing. BS: Writing – review & editing. RT: Writing – review & editing, Conceptualization, Supervision, Validation, Visualization. RD: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1446482/full#supplementary-material
Supplementary Table 1 | ICD 10 Codes used in the making of the study -This table includes all the ICD codes that were used in our study.
1. Johns Hopkins Coronavirus Resource Center. COVID-19 Map (2024). Available online at: https://coronavirus.jhu.edu/map.html. (Accessed March 25, 2024)
2. Raglow Z, Surie D, Chappell JD, Zhu Y, Martin ET, Kwon JH, et al. SARS-CoV-2 shedding and evolution in patients who were immunocompromised during the omicron period: a multicentre, prospective analysis. Lancet Microbe. (2024) 5:e235–46. doi: 10.1016/S2666-5247(23)00336-1
3. Ketkar A, Willey V, Pollack M, Glasser L, Dobie C, Wenziger C, et al. Assessing the risk and costs of COVID-19 in immunocompromised populations in a large United States commercial insurance health plan: the EPOCH-US Study. Curr Med Res Opin. (2023) 39:1103–18. doi: 10.1080/03007995.2023.2233819
4. Marchesi F, Salmanton-García J, Emarah Z, Piukovics K, Nucci M, López-García A, et al. COVID-19 in adult acute myeloid leukemia patients: a long-term follow-up study from the European Hematology Association survey (EPICOVIDEHA). Haematologica. (2023) 108:22–33. doi: 10.3324/haematol.2022.280847
5. Azhdari Tehrani H, Ramezaninejad S, Mardani M, Shokouhi S, Darnahal M, Hakamifard A. Hematologic Malignancies and COVID-19 infection: A monocenter retrospective study. Health Sci Rep. (2022) 5:e638. doi: 10.1002/hsr2.638
6. Yigenoglu TN, Ata N, Altuntas F, Bascı S, Dal MS, Korkmaz S, et al. The outcome of COVID-19 in patients with hematological Malignancy. J Med Virol. (2021) 93:1099–104. doi: 10.1002/jmv.26404
7. Pagano L, Salmanton-García J, Marchesi F, Busca A, Corradini P, Hoenigl M, et al. COVID-19 infection in adult patients with hematological Malignancies: a European Hematology Association Survey (EPICOVIDEHA). J Hematol Oncol. (2021) 14:168. doi: 10.1186/s13045-021-01177-0
8. Buyuktas D, Acar K, Sucak G, Toptas T, Kapucu I, Bekoz H, et al. COVID-19 infection in patients with acute leukemia; Istanbul experience. Am J Blood Res. (2021) 11:427–37.
9. Leston M, Elson W, Ordóñez-Mena JM, Kar D, Whitaker H, Joy M, et al. Disparities in COVID-19 mortality amongst the immunosuppressed: A systematic review and meta-analysis for enhanced disease surveillance. J Infect. (2024) 88:106110. doi: 10.1016/j.jinf.2024.01.009
10. García-Suárez J, de la Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V, et al. Impact of hematologic Malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. (2020) 13:133. doi: 10.1186/s13045-020-00970-7
11. Rawson T, Iosa M, Ricci F, Kozlakidis Z, Su L, Ropert C, et al. Coronavirus disease (COVID-19): pathophysiology, epidemiology, clinical management and public health response, volume II (volume I.B). Front Media SA. (2023), 815 p. doi: 10.3389/978-2-88976-465-5
12. Guo W, Zheng Y, Feng S. Omicron related COVID-19 prevention and treatment measures for patients with hematological Malignancy and strategies for modifying hematologic treatment regimes. Front Cell Infect Microbiol. (2023) 13:1207225. doi: 10.3389/fcimb.2023.1207225
13. Paul S, Rausch CR, Jain N, Kadia T, Ravandi F, DiNardo CD, et al. Treating leukemia in the time of COVID-19. Acta Haematol. (2021) 144:132–45. doi: 10.1159/000508199
14. Abouzeid W, Mirza N, Bellafiore P, Kiwan C, Paige A, Suleiman A, et al. Surviving the storm: cardiac tamponade and effusive constrictive pericarditis complicated by pericardial decompression syndrome induced by COVID-19 infection in the setting of newly diagnosed acute myeloid leukemia (AML). Cureus. (2024) 16:e56710. doi: 10.7759/cureus.56710
15. Mikulska M, Testi D, Russo C, Balletto E, Sepulcri C, Bussini L, et al. Outcome of early treatment of SARS-CoV-2 infection in patients with haematological disorders. Br J Haematol. (2023) 201:628–39. doi: 10.1111/bjh.18690
16. Kemp SA, Collier DA, Datir RP, Ferreira IATM, Gayed S, Jahun A, et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature. (2021) 592:277–82. doi: 10.1038/s41586-021-03291-y
17. Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, et al. Persistence and evolution of SARS-coV-2 in an immunocompromised host. N Engl J Med. (2020) 383:2291–3. doi: 10.1056/NEJMc2031364
18. Quaranta EG, Fusaro A, Giussani E, D’Amico V, Varotto M, Pagliari M, et al. SARS-CoV-2 intra-host evolution during prolonged infection in an immunocompromised patient. Int J Infect Dis. (2022) 122:444–8. doi: 10.1016/j.ijid.2022.06.023
19. Kaya H, Tani H, Inasaki N, Yazawa S, Itamochi M, Higashi D, et al. Virus evolution and reduced viral viability during treatment of persistent COVID-19 Omicron BA.5 infection in an immunocompromised host. Int J Infect Dis. (2023) 136:146–8. doi: 10.1016/j.ijid.2023.09.010
20. Fox TA, Troy-Barnes E, Kirkwood AA, Chan WY, Day JW, Chavda SJ, et al. Clinical outcomes and risk factors for severe COVID-19 in patients with haematological disorders receiving chemo- or immunotherapy. Br J Haematol. (2020) 191:194–206. doi: 10.1111/bjh.17027
21. HCUP-US NIS overview (2024). Available online at: https://hcup-us.ahrq.gov/nisoverview.jsp. (Accessed April 20, 2024)
22. Dugoff EH, Schuler M, Stuart EA. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res. (2014) 49:284–303. doi: 10.1111/1475-6773.12090
23. Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. (2020) 8:152. doi: 10.3389/fpubh.2020.00152
24. Strasfeld L. COVID-19 and HSCT (Hematopoietic stem cell transplant). Best Pract Res Clin Haematol. (2022) 35:101399. doi: 10.1016/j.beha.2022.101399
25. Fernández-Cruz A, Puyuelo A, Núñez Martín-Buitrago L, Sánchez-Chica E, Díaz-Pedroche C, Ayala R, et al. Higher mortality of hospitalized haematologic patients with COVID-19 compared to non-haematologic is driven by thrombotic complications and development of ARDS: An age-matched cohorts study. Clin Infect Pract. (2022) 13:100137. doi: 10.1016/j.clinpr.2022.100137
26. Lu S, Huang X, Liu R, Lan Y, Lei Y, Zeng F, et al. Comparison of COVID-19 induced respiratory failure and typical ARDS: similarities and differences. Front Med. (2022) 9:829771. doi: 10.3389/fmed.2022.829771
27. Koçak Tufan Z, Kayaaslan B, Mer M. COVID-19 and sepsis. Turk J Med Sci. (2021) 51:3301–11. doi: 10.3906/sag-2108-239
28. Spring J, Munshi L. Hematology emergencies in adults with critical illness: Malignant hematology. Chest. (2022) 162:120–31. doi: 10.1016/j.chest.2022.02.004
29. Rhee CK, Kang JY, Kim YH, Kim JW, Yoon HK, Kim SC, et al. Risk factors for acute respiratory distress syndrome during neutropenia recovery in patients with hematologic Malignancies. Crit Care. (2009) 13:R173. doi: 10.1186/cc8149
30. Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. (2021) 21:319–29. doi: 10.1038/s41577-021-00536-9
31. Michot JM, Hueso T, Ibrahimi N, Pommeret F, Willekens C, Colomba E, et al. Severe COVID-19 in patients with hematological cancers presenting with viremia. Ann Oncol. (2021) 32:1297–300. doi: 10.1016/j.annonc.2021.07.002
32. Alay GH, Tatlisuluoglu D, Bulut K, Fikri BI, Oztas A, Turan G. The relationship between immature granulocyte count and mortality in ARDS Due to COVID-19. Niger J Clin Pract. (2022) 25:1301–7. doi: 10.4103/njcp.njcp_118_22
33. Sunnaa M, Kerolos M, Ruge M, Gill A, Du-Fay-de-Lavallaz JM, Rabin P, et alAssociation between number of vasopressors and mortality in COVID-19 patients. Am Heart J Plus: Cardiol Res Practice. (2023) 34. doi: 10.1016/j.ahjo.2023.100324
34. Khedr A, Al Hennawi H, Rauf I, Khan MK, Mushtaq HA, Lodhi HS, et al. Differential mortality with COVID-19 and invasive mechanical ventilation between high-income and low-and middle-income countries: a systematic review, meta-analysis, and meta-regression. Infez Med. (2022) 30:51–8. doi: 10.53854/liim-3001-6
Keywords: AML, myelodysplastic syndrome, COVID-19, severe sepsis, ARDS, acute respiratory failure, MDS, acute myeloid leukemia and HSCT
Citation: Sivasubramanian BP, Joshi S, Ravikumar DB, Madhumithaa Jagannathan, Babu S, Sripathi SR, Javvaji A, Jain P, Kumar Shanmugam D, Swami Kannan BD, Tirupathi R and Dalal R (2024) COVID-19 in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS): a propensity matched analysis (2020-2021). Front. Oncol. 14:1446482. doi: 10.3389/fonc.2024.1446482
Received: 09 June 2024; Accepted: 30 September 2024;
Published: 17 October 2024.
Edited by:
Roberto Crocchiolo, Niguarda Ca’ Granda Hospital, ItalyReviewed by:
Marianna Rossi, San Matteo Hospital Foundation (IRCCS), ItalyCopyright © 2024 Sivasubramanian, Joshi, Ravikumar, Madhumithaa Jagannathan, Babu, Sripathi, Javvaji, Jain, Kumar Shanmugam, Swami Kannan, Tirupathi and Dalal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diviya Bharathi Ravikumar, ZGl2aXlhYmhhcmF0aGkyNjEyQGdtYWlsLmNvbQ==
†ORCID: Barath Prashanth Sivasubramanian, orcid.org/0000-0002-6018-5647
Shashvat Joshi, orcid.org/0009-0009-0783-9662
Diviya Bharathi Ravikumar, orcid.org/0000-0002-9151-5960
Madhumithaa Jagannathan, orcid.org/0009-0009-6073-2362
Sonia Babu, orcid.org/0009-0003-6348-5498
Shanthi Reddy Sripathi, orcid.org/0009-0006-9534-0092
Avinash Javvaji, orcid.org/0009-0005-8126-9750
Priyanshu Jain, orcid.org/0009-0003-7128-3714
Dinesh Kumar Shanmugam, orcid.org/0009-0006-7754-1595
Bharath Duraisamy Swami Kannan, orcid.org/0009-0007-2828-1800
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.