- Department of Radiology, Heping Hospital Affiliated to Changzhi Medical College, Changzhi, Shanxi, China
Primary undifferentiated high-grade pleomorphic sarcoma (UPS) in the mediastinum is exceptionally rare. This paper reports a unique case of anterior mediastinal UPS in an 84-year-old Asian male presenting with recurrent upper respiratory tract infections and upper abdominal discomfort. Imaging via CT and MRI suggested an invasive thymoma, but postoperative pathology confirmed UPS. Despite radical surgery, local recurrence occurred within three months, and palliative radiotherapy was ineffective. This case provides the first comprehensive imaging data of UPS in the anterior mediastinum, aiming to improve diagnostic accuracy for clinicians and radiologists by summarizing imaging features across various modalities.
1 Introduction
Undifferentiated pleomorphic sarcoma (UPS) is a diagnosis of exclusion that requires the elimination of various other tumor types prior to confirmation. Radiology plays a crucial role in initial diagnosis, long-term follow-up, and the evaluation of many treatment-related complications. Undifferentiated pleomorphic sarcoma (UPS) is a rare and aggressive mesenchymal malignancy without a clear cell of origin, previously known as malignant fibrous histiocytoma. It is a high-grade sarcoma without any specific differentiation, appearing at any age but most commonly between 50 and 70 years, with a slight male predominance, primarily originating from the soft tissue of the limbs (1). Histologically, it is characterized by pleomorphic and spindle-shaped tumor cells and a spiral growth pattern (2). However, it lacks clear diagnostic criteria and is easily confused with other mediastinal tumors. To date, imaging characteristics of UPS at different sites have been documented to enhance diagnostic efficiency, but less so for mediastinal cases; we report a case of anterior mediastinal UPS with complete imaging data.
2 Case presentation
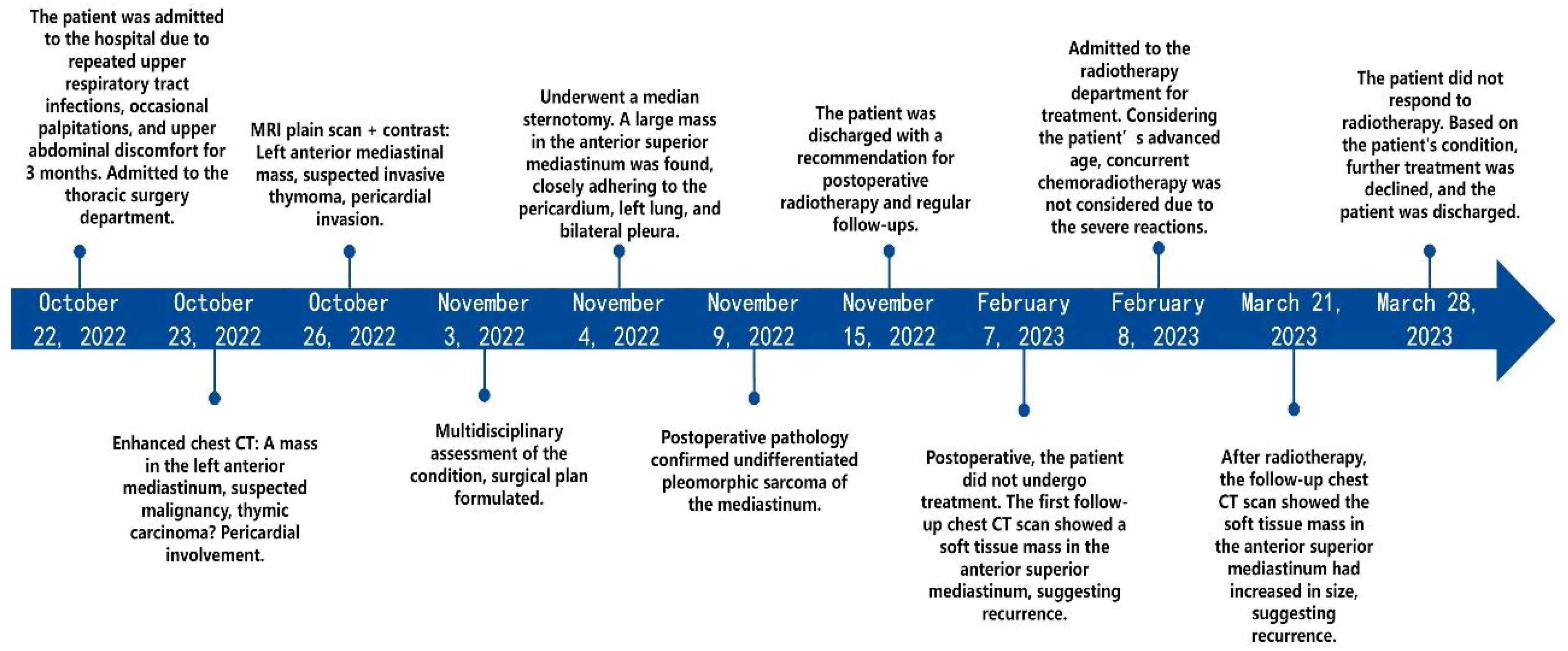
Patient’s medical history: An 84-year-old Asian male presented with recurring upper respiratory infections, occasional palpitations, and upper abdominal discomfort over the last month (Figure 1). He has a 7-year history of hypertension, with blood pressure reaching as high as 180/100 mmHg. He had no known history of smoking, alcohol use, or recreational drug use. Over ten years ago, he underwent laparoscopic surgery for a renal cyst. Physical examination upon admission showed jugular vein distention and diminished breath sounds in the upper right lung. Laboratory tests revealed: CD3 + 42.24% (reference range 56%–86%), helper/inducer T-cells (CD3+CD4+) 23.08% (reference range 33%–58%), and NK cells (CD3-CD56+) 38.75% (reference range 5%–26%). Blood tests indicated hemoglobin at 70 g/L (normal range 120–160 g/L), indicating moderate anemia.
2.1 Auxiliary examinations
Routine ultrasound identified a hypoechoic mass located near the pericardium, displaying indistinct boundaries with the anterior wall of the right ventricle and resulting in the posterior displacement of the heart (Figure 2). CT scan revealed an irregular mass in the left anterior mediastinum measuring approximately 16.5 cm × 9.6 cm, with heterogeneous enhancement and a CT value range of approximately 20 to 61 HU. The mass exhibited involvement of the pericardium with uncertain demarcations, leading to the displacement of major cardiac vessels and the presence of left pleural effusion (Figure 3). MRI imaging depicted an irregular mass in the anterior mediastinum with mixed T2 and T1 signals, measuring around 16.7 cm × 9.7 cm × 10.6 cm, and displaying well-defined margins. DWI demonstrated high signal diffusion, with an ADC value of 1.430 × 10-3 mm²/s, indicating areas of irregular liquefactive necrosis within the mass. DWI showed high signal diffusion, with an ADC value of 1.430 × 10-3 mm²/s, and irregular liquefied necrotic areas were seen. The boundary of the lesion with the pericardium was indistinct, and there was posterior displacement of the major cardiac vessels (Figure 4).
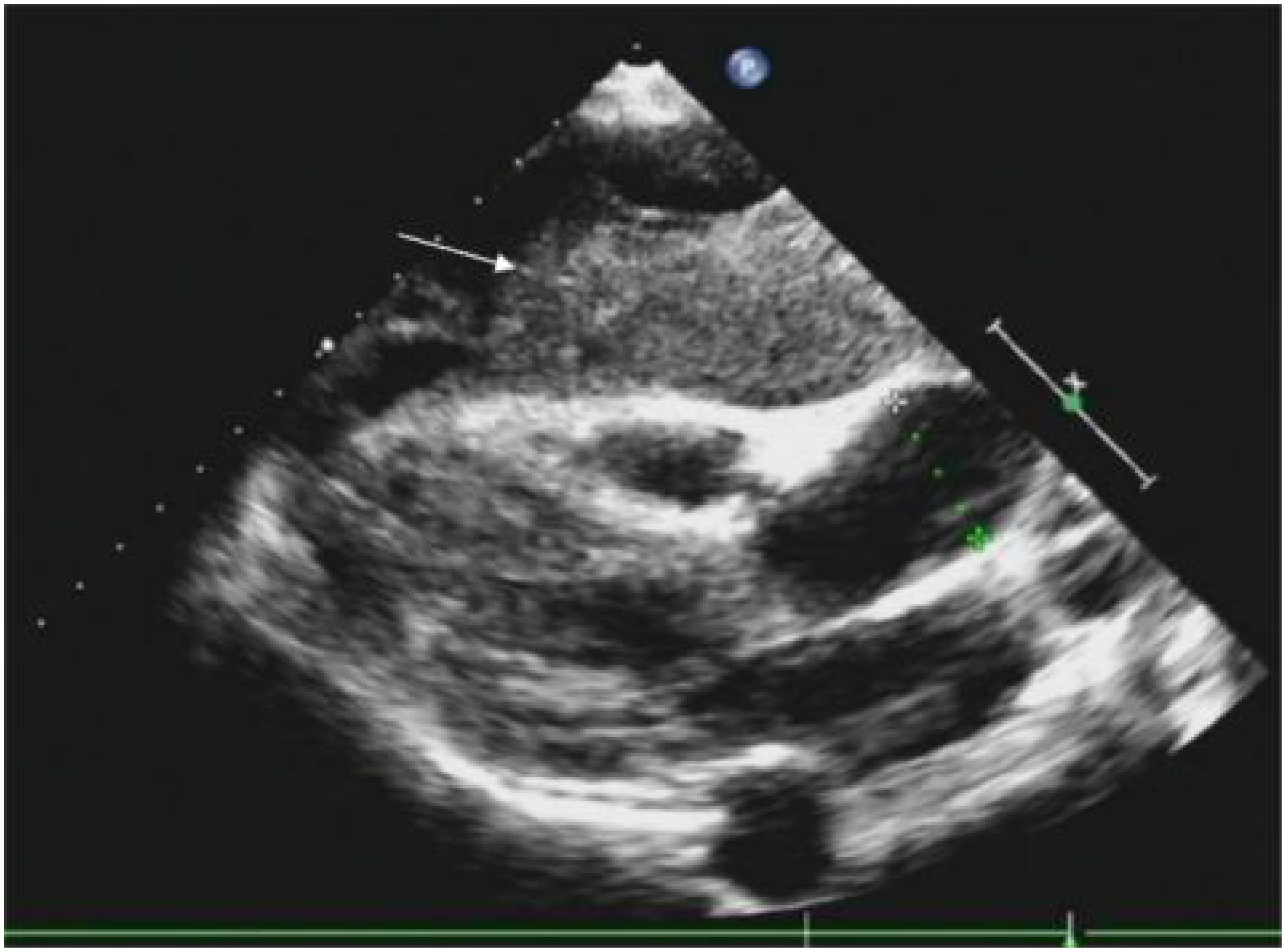
Figure 2. A hypoechoic mass is visible on the outer pericardium, with indistinct boundaries with the anterior wall of the right ventricle (indicated by a white arrow), causing posterior displacement of the heart.

Figure 3. (A, B) demonstrate enhanced chest CT scans in the coronal and transverse planes, respectively. An irregular mass is noted in the left anterior mediastinum (highlighted by white arrows), with an approximate cross-sectional size of 165mm×96mm, showing heterogeneous enhancement with CT values ranging approximately from 20 to 61 Hounsfield units (HU). (C) illustrates the lesion invading the pericardium with indistinct margins (denoted by black asterisks), accompanied by posterior displacement of the cardiac great vessels and presence of left pleural effusion (denoted by white asterisks).
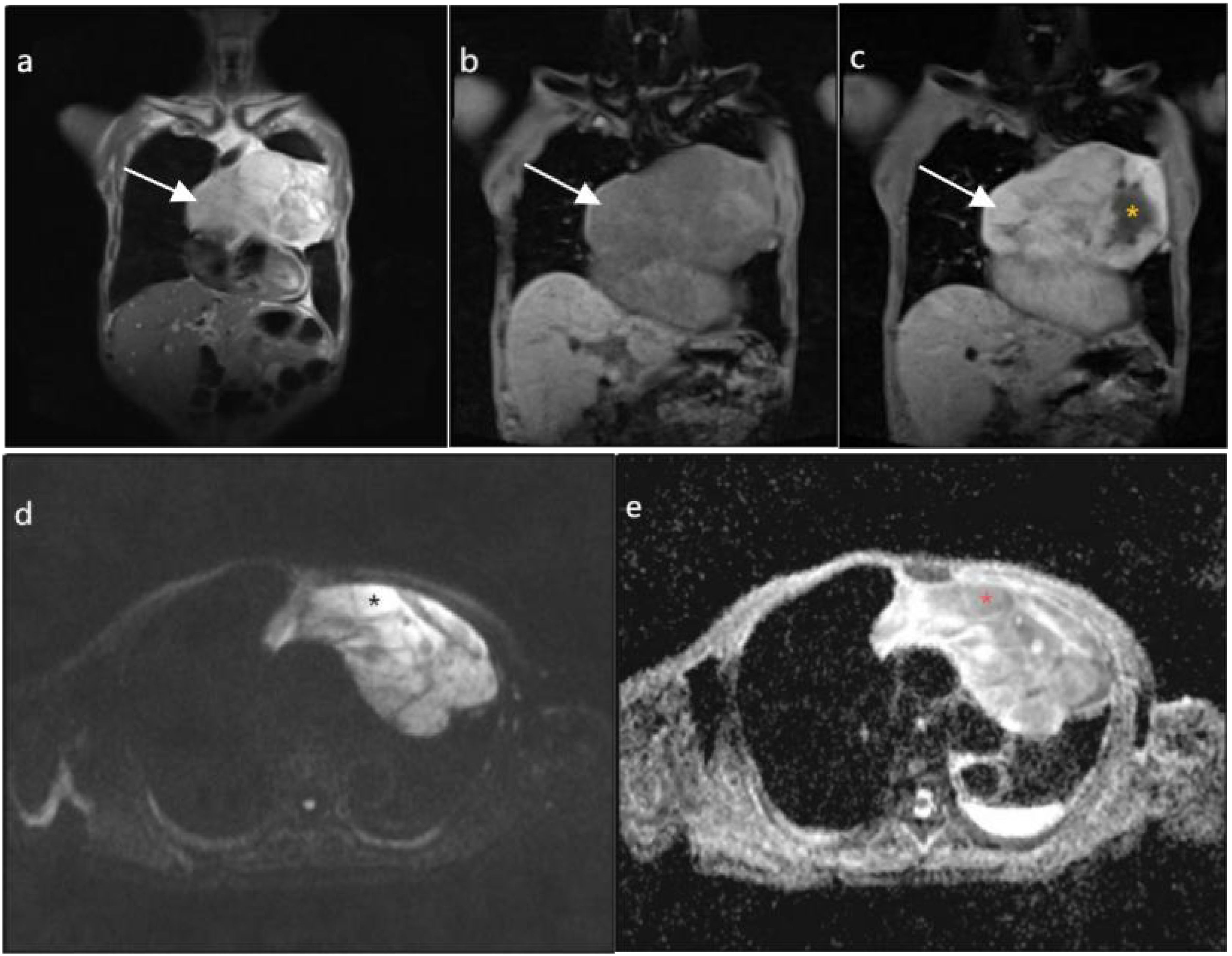
Figure 4. (A, B) depict coronal T2-weighted imaging (T2WI) and T1-weighted imaging (T1WI), respectively, demonstrating an irregular mass in the anterior mediastinum (highlighted by white arrows), displaying mixed T2 and T1 signal intensities with internal low signal septations on T2WI, measuring approximately 167mm×97mm×106mm with well-defined margins. (C), on coronal T1WI post-contrast imaging, reveals heterogeneous enhancement with irregular cystic necrotic areas within (denoted by yellow asterisks). (D) shows diffusion-weighted imaging (DWI) with a b value of 800, demonstrating diffusely high signal intensity in the solid component (denoted by black asterisks). (E) displays an apparent diffusion coefficient (ADC) value of 1.430×10-3mm2/s (denoted by red asterisks).
2.2 Surgical procedure
Via a median sternotomy, a large mass was observed in the upper anterior mediastinum, predominantly on the left side, measuring approximately 20 cm × 15 cm × 5.0 cm. The mass was firm in texture, lobulated, and partially tense, adhering closely to the pericardium, the upper lobe of the left lung, and bilateral mediastinal pleura. It was carefully dissected and completely excised along with portions of the mediastinal pleura and pericardium.
2.3 Postoperative pathology findings
Postoperative Pathology Findings: Gross examination (Figure 5A): A mass of gray-white, gray-yellow, and gray-brown tissues, total volume 25 cm × 16 cm × 6.5 cm, with areas of gray-white translucency and others of gray-yellow and gray-brown, solid and of medium consistency. Pathological results suggested a soft tissue tumor, pending further immunohistochemical diagnosis. Immunohistochemistry (Figures 5B, C): Immunostaining showed positive results for vimentin Ki-67 (50%) and negative results for SMA, Des, MyoD1, Myogenin, S-100, MDM2, CKpan, CD34, SOX-10, HMB-45, EMA. CD163, Thus, the condition was diagnosed as undifferentiated pleomorphic sarcoma.
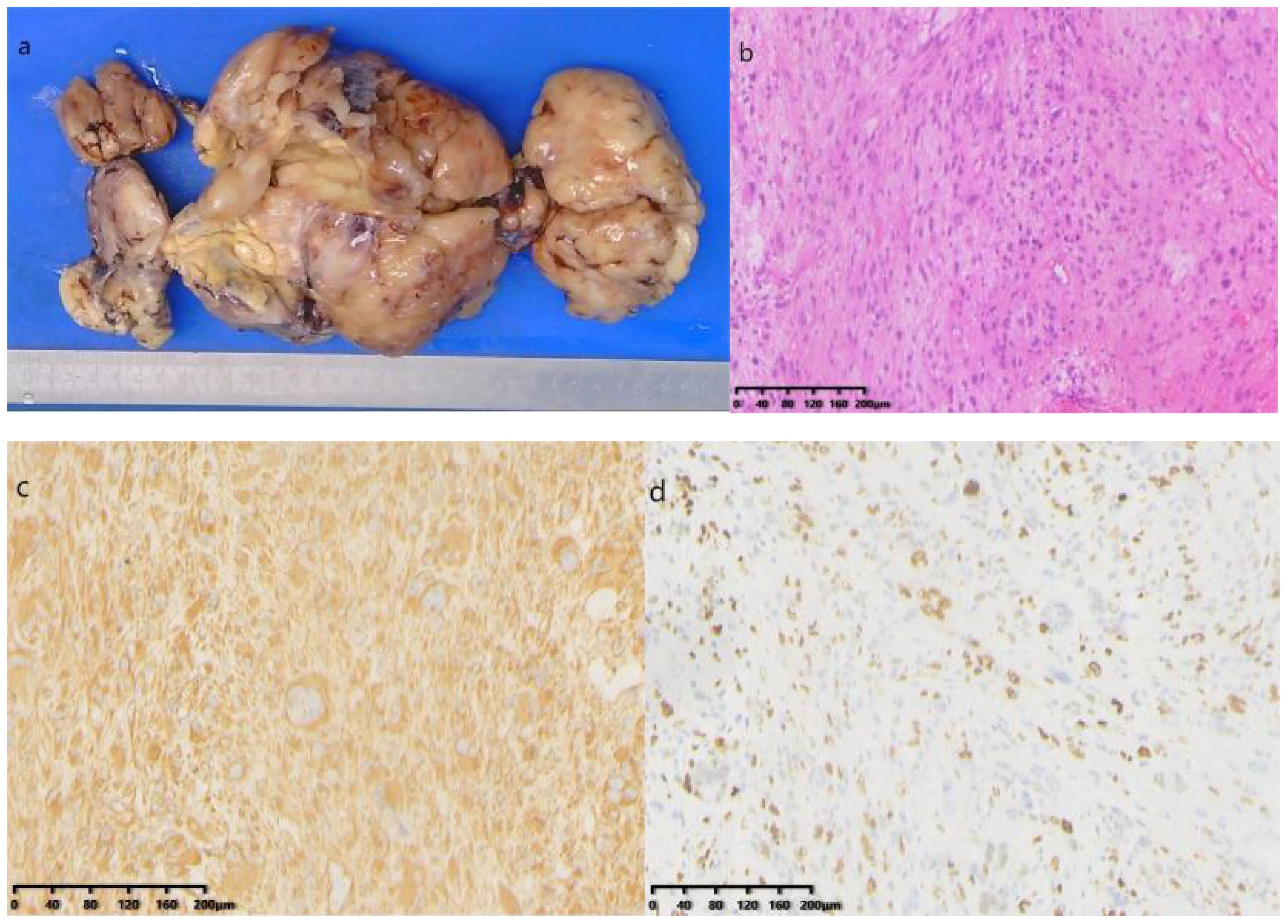
Figure 5. (A) Gross specimen: A collection of gray-white, gray-yellow, and gray-brown tissue; (B) Hematoxylin and Eosin staining microscopic examination: The tumor consists of morphologically diverse and large atypical cells, with large, deeply stained nuclei, prominent nucleoli, and occasional pathological mitotic figures and tumor giant cells; (C) Immunohistochemical staining shows positive for vimentin; (D) Ki-67 positive in 50% of cells (HE×200).
2.4 Postoperative follow-up
The patient recovered well post-surgery, with stable condition, and there was no particular discomfort. Postoperative radiotherapy and regular check-ups were recommended. Three months later, a follow-up CT scan of the chest revealed an irregular soft tissue density in the upper anterior mediastinum, measuring about 3.2 cm × 5.1 cm × 8.0 cm, suggesting a recurrence (Figure 6A). The patient was readmitted to our radiotherapy department. Chemotherapy was not considered for the time being due to the patient’s advanced age and the obvious side effects of receiving radiotherapy. A chest CT scan two months following radiation therapy revealed that the tumor had gotten bigger (Figure 6B). Given the tumor’s insensitivity to radiotherapy and poor therapeutic effects, the patient and family were informed, and he requested discharge after the completion of radiotherapy, refusing further treatment.
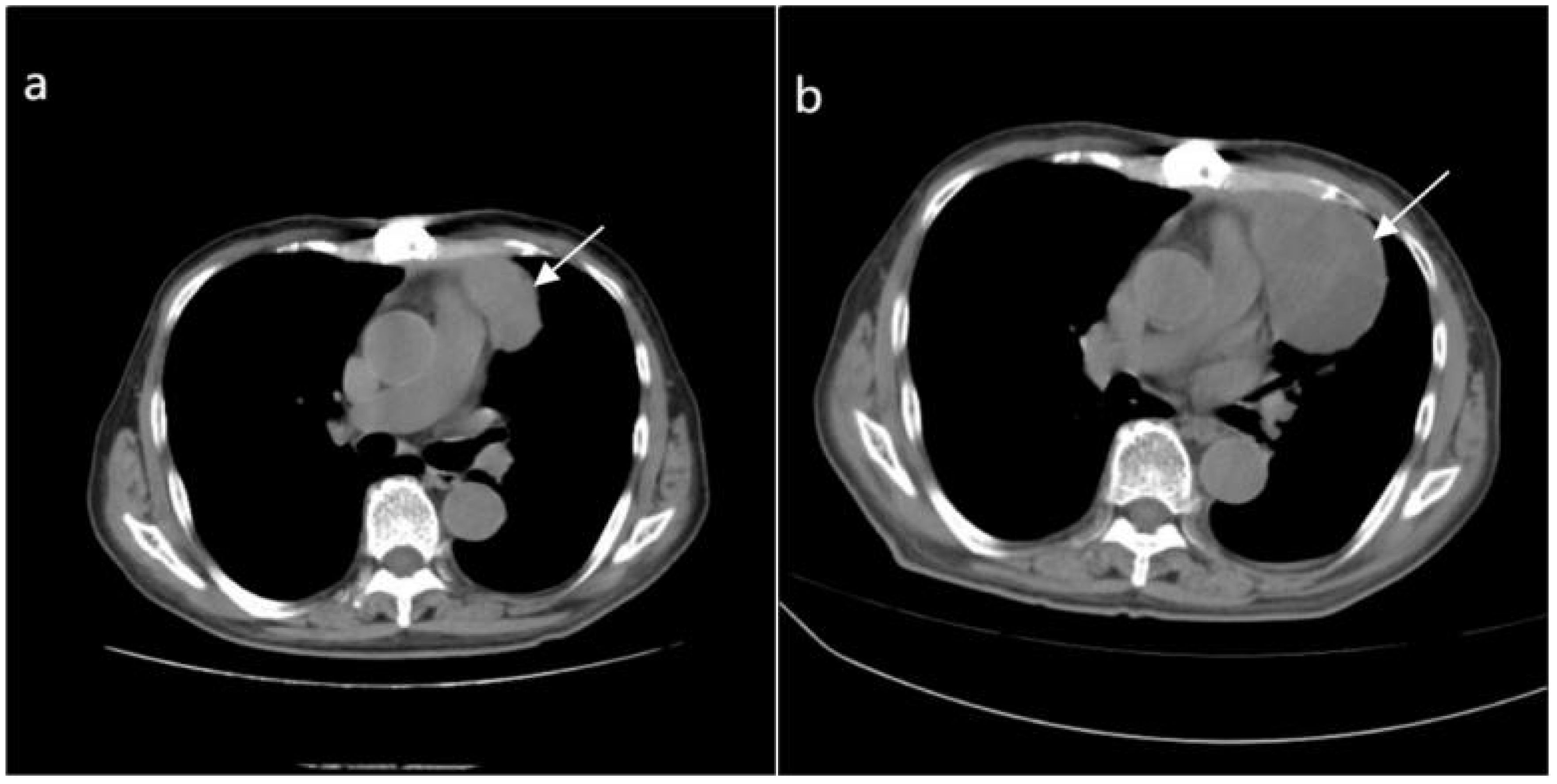
Figure 6. (A) Depicts an irregular soft tissue density shadow in the upper anterior mediastinum (highlighted by white arrows), approximately 32mmX51mmx80mm in size, observed 3 months after surgery for the anterior mediastinal mass. (B) Taken 2 months after radiotherapy, a larger irregular soft tissue density shadow is visible in the upper anterior mediastinum (highlighted by white arrows), measuring approximately 56mmx78mmx92mm, with uneven density and showing enlargement compared to the previous image.
3 Discussion
Undifferentiated sarcoma, previously known as malignant fibrous histiocytoma (MFH), is a rare mesenchymal-origin soft tissue malignancy clinically. The concept of undifferentiated sarcoma was introduced in the 2013 WHO classification of soft tissue tumors, distinguishing it into four subtypes under microscopy: pleomorphic, spindle cell, round cell, and epithelioid (3). The WHO classification was updated in 2020, categorizing it into three subtypes: undifferentiated pleomorphic sarcoma, undifferentiated spindle cell sarcoma, and undifferentiated round cell sarcoma (4). Weiss et al (5). analyzed 200 cases of MFH, reporting it most commonly occurs in males and individuals over 40, with an incidence rate of one per 100,000. Due to the small number of published case reports, diagnosing UPS remains challenging.
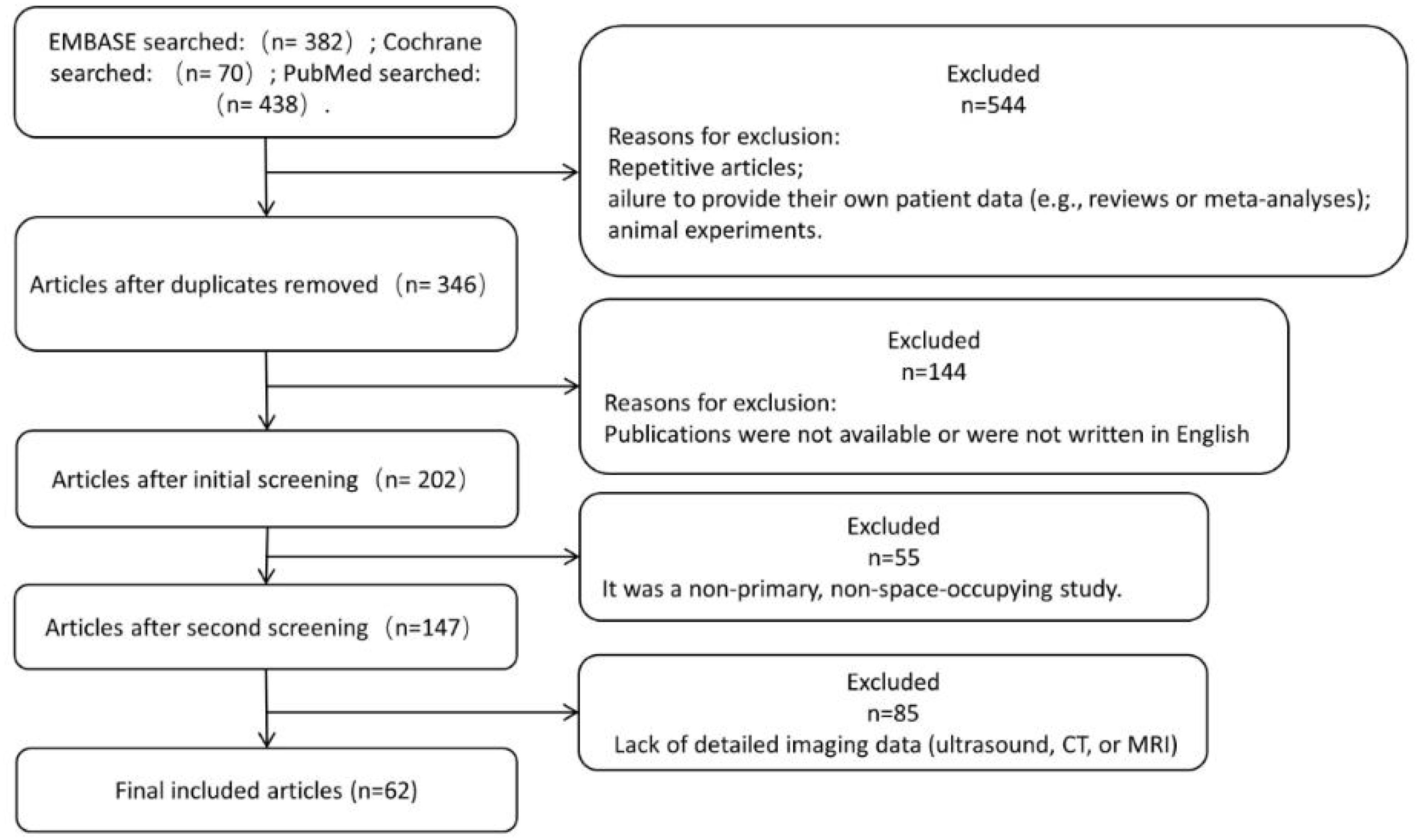
To further explore UPS, we searched the literature in Embase, Cochrane, and PubMed, with a date range of 1 January 2013 to 21 February 2024 considered for the studies. The search criteria were as follows: undifferentiated pleomorphic sarcoma [Title/Abstract]. Notably, the World Health Organization has chosen to replace this term with a neutral and more accurate label (i.e. UPS) (3). Studies were included based on the following criteria: (1) primary UPS, histologically confirmed; (2) detailed imaging data; (3) case reports. Detailed imaging data were defined as textual information given according to at least 2 of the following criteria: tumor size, borders, aggressiveness, hemorrhage, necrosis, calcification, contrast enhancement (CT or MRI), MR signal behavior (T1W or T2W), and ultrasound performance.
A total of 382 EMBASE, 70 Cochrane and 438 PubMed studies published between 1 January 2013 and 21 February 2024 were identified. Duplicate articles, failure to provide own patient data (e.g. reviews or meta-analyses), animal studies were excluded; publications that were not accessible, or not written in English were excluded from our analyses; non-primary, non-occupying UPS was not considered for further analysis; the lack of detailed imaging data (CT or MRI) in publications led to exclusion, and ultimately 62 publications were eligible for further analysis (Figure 7).
After searching and screening, UPS was distributed in different parts of the body, with a total of 62 articles (Figure 8): soft tissues of the limbs and trunk (13 cases), thyroid (4 cases), gastrointestinal tract (5 cases), heart (6 cases), pancreas (3 cases), spleen (3 cases), breast (2 cases), maxillofacial area (5 cases), retroperitoneum (5 cases), lungs (3 cases), head and neck (3 cases), reproductive organs (3 cases), skin (1 case), and mediastinum (6 cases). Diagnostic examinations included X-rays (13 cases); CT scans (48 cases); MRI scans (34 cases); ultrasound examinations (26 cases); PET-CT scans (17 cases).
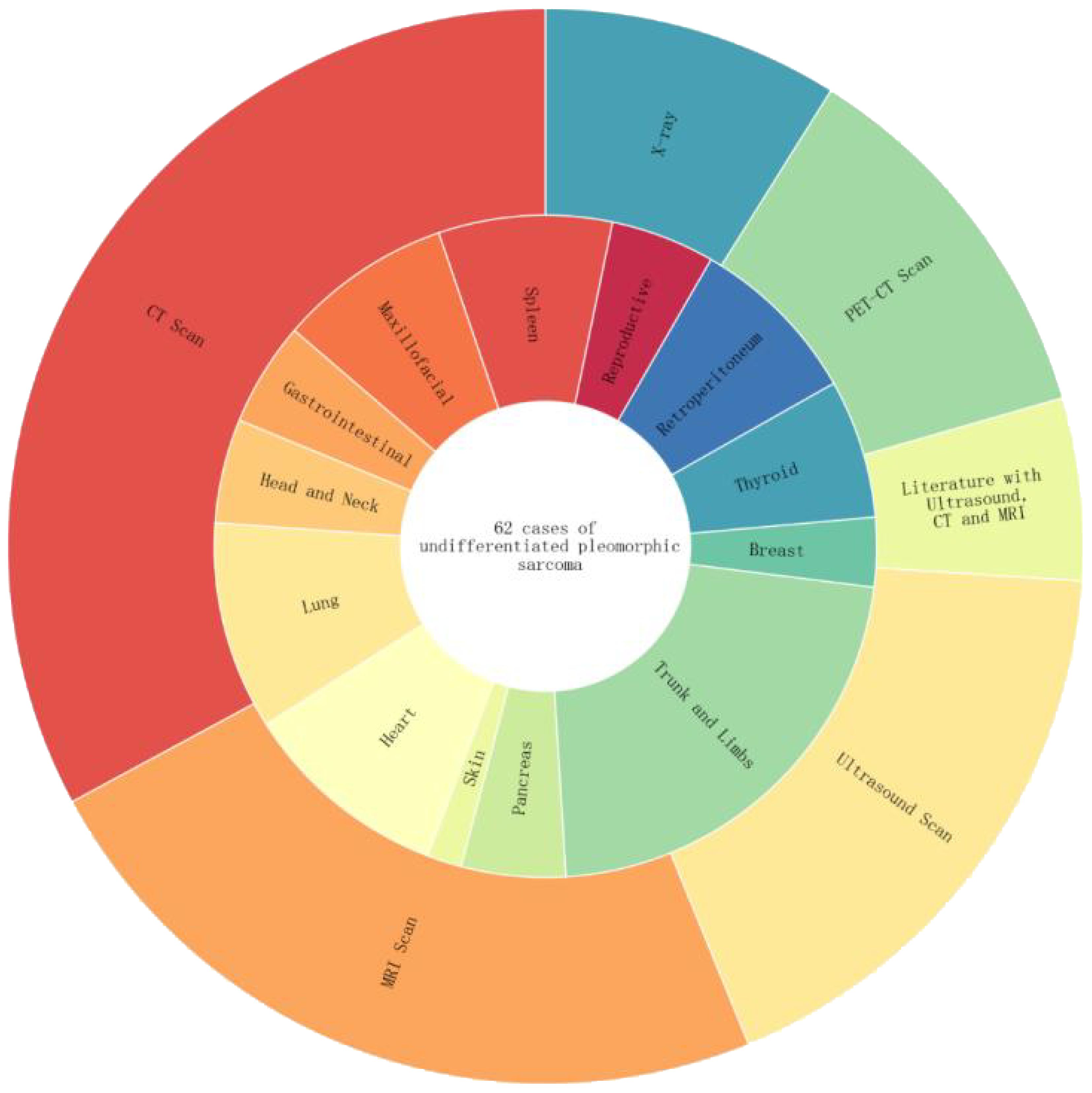
Figure 8. The inner circle represents the percentage distribution of UPS cases in different body regions. For example, the trunk and limbs have the highest proportion at 13/62. The outer circle represents the distribution of imaging examination techniques among the 62 UPS cases, including CT, MRI, ultrasound, PET-CT, and cases utilizing a combination of ultrasound, CT, and MRI imaging techniques.
From initial diagnosis, staging, prognosis and stratification, to identifying locally invasive disease and distant metastases, radiological investigations are crucial for patients with sarcomas. X-rays are usually used for initial evaluation (especially in the extremities, chest) and can show calcification, translucency (corresponding to fat) and associated bone changes. Ultrasound is the preferred initial method for superficial lesions due to its accessibility and excellent spatial and contrast resolution, allowing differentiation between parenchymal and cystic lesions and revealing internal morphology and vascularity. CT is commonly used to assess lesions in the head and neck, mediastinum and retroperitoneal regions, and contrast-enhanced scans providing better delineation of tumor vasculature and malignant potential. Any lesion that is indeterminate or suspicious by ultrasound or CT, deep tumor, superficial tumor invading the fascia, incompletely visible tumor by ultrasound, tumor >5cm should be further investigated using magnetic resonance imaging (MRI) (6).
Contrast-enhanced MRI is considered the optimal modality for characterizing soft tissue tumors (STTs). MRI can delineate tumor contents (or matrix), tumor margins, and surrounding tissues, especially for heterogeneous tumors, necrotic content, and hemorrhagic components. Following successful surgical resection with clear margins, patients should undergo continuous monitoring by CT or MRI for recurrence or late metastasis (7). Although PET imaging is not routinely used in the initial staging of pleomorphic sarcomas (8), PET/CT, as a metabolic imaging modality, can identify necrotic areas of high-grade sarcomas because of their increased FDG uptake can determine the systemic impact of UPS. It can also assess the systemic impact of undifferentiated pleomorphic sarcoma (UPS), which may be difficult to detect with conventional CT and MRI, thereby predicting overall survival and disease-free survival in patients (9, 10).
Non-mediastinal UPS tumors (56 cases) median diameter (max-min) 6.4 cm (1.8-2.4 cm).16% (9/56) tumors were hypoechoic ultrasonographically. On CT they were usually heterogeneous in appearance.17% (10/56) tumors were lobulated and 26% (15/56) The tumor contained areas of necrosis or cystic degeneration and hemorrhage within the tumor, with calcification in a few areas, most commonly in the thyroid gland.17% (10/56) of the tumors had unclear borders, and tumor enhancement on enhancement was heterogeneous or peripherally enhanced.3% (10/56) of the tumors had a heterogeneous appearance on CT. On MRI, the signal intensity varied according to the internal components of the tumour.10% (6/56) showed predominantly low or intermediate signal on T1WI, with a few high signals, and 16% (9/56) showed predominantly high or mixed signals on T2WI, with intratumorally cystic necrotic areas of varying sizes. The one case of a lesion in the pancreas demonstrated low-signal segregation of T2 signals, and most of them showed inhomogeneous enhancement on enhancement. The one case of UPS in the soft tissue of the tibia showed enhancement tailing forward along the fascia. Only 3% (2/56) of the literature mentioned restricted diffusion-weighted imaging (DWI), with one instance providing an apparent diffusion coefficient (ADC) value of 1.210 × 10^−3 mm^2/s (11). PET-CT exhibited high uptake in 48% (27/56) of cases, with specific numerical values mentioned in 5 instances (Figure 9).
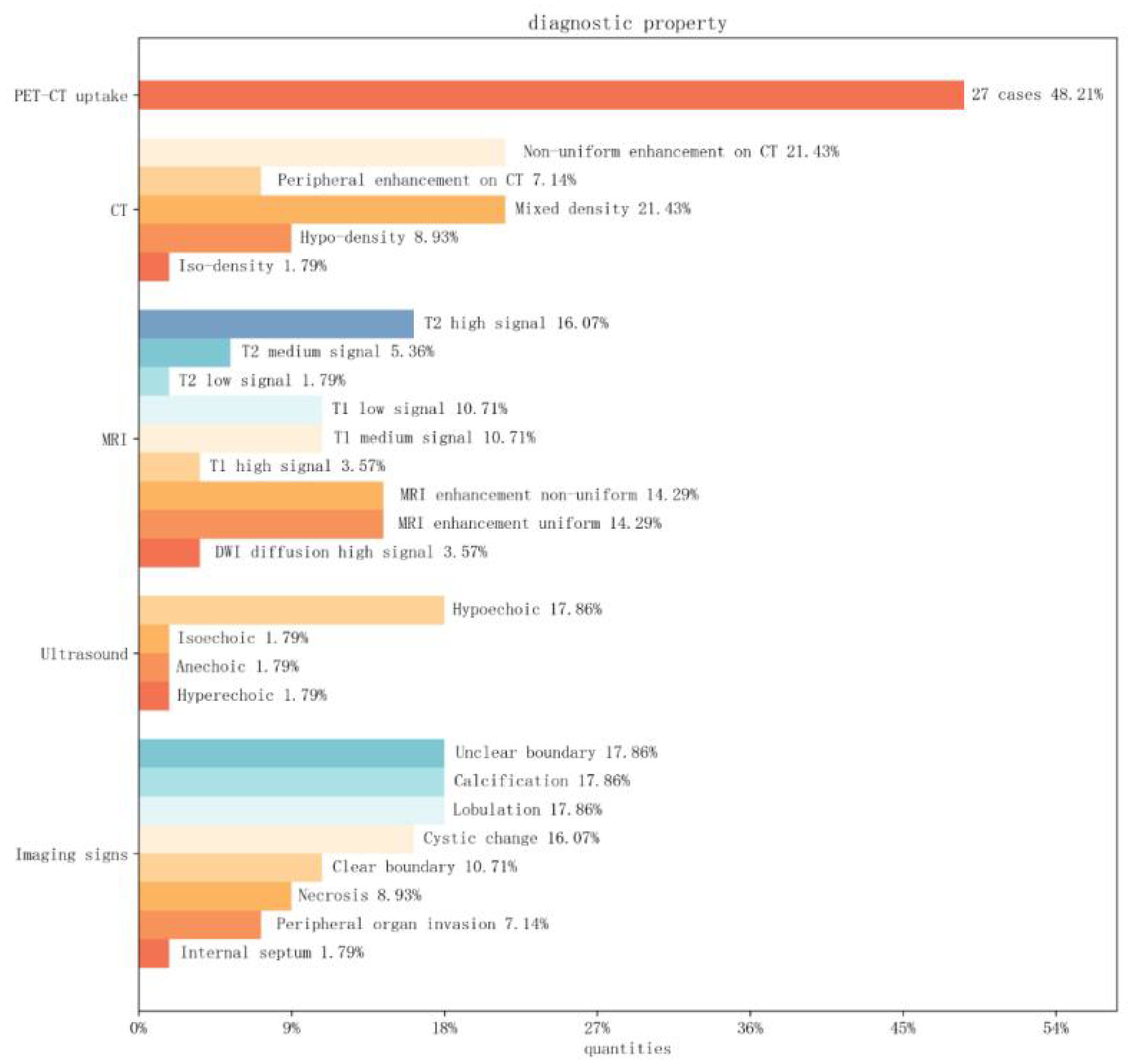
Figure 9. Image Feature Bar Chart: The vertical axis represents PET-CT, CT, MRI, ultrasound findings, and special imaging characteristics. The horizontal axis represents the percentage distribution among cases of non-mediastinal UPS.
Reports on all cases in the mediastinum (12–17) indicate that UPS patients ranged in age from 50-84 years, with an average age of 68.1 years, and a male to female ratio of 4:3 (Table 1). Five cases were located in the anterior mediastinum, including one concurrent with thymoma and another in the middle mediastinum. The average tumor diameter was 12.5 cm. CT scans showed soft tissue density in six cases, irregular morphology in five cases, uneven enhancement in two cases, and invasion of peripheral tissues in four cases. Five tumors caused pleural and pericardial effusions. This case is the only preoperative MRI examination in the mediastinal cases, showing mixed T2 and T1 signals, with internal low signal separations on T2WI, high signal on DWI, and an ADC value of 1.430×10-3 mm2/s, consistent with the MRI characteristics of UPS in the pancreas, retroperitoneum, and stomach (9, 18, 19).
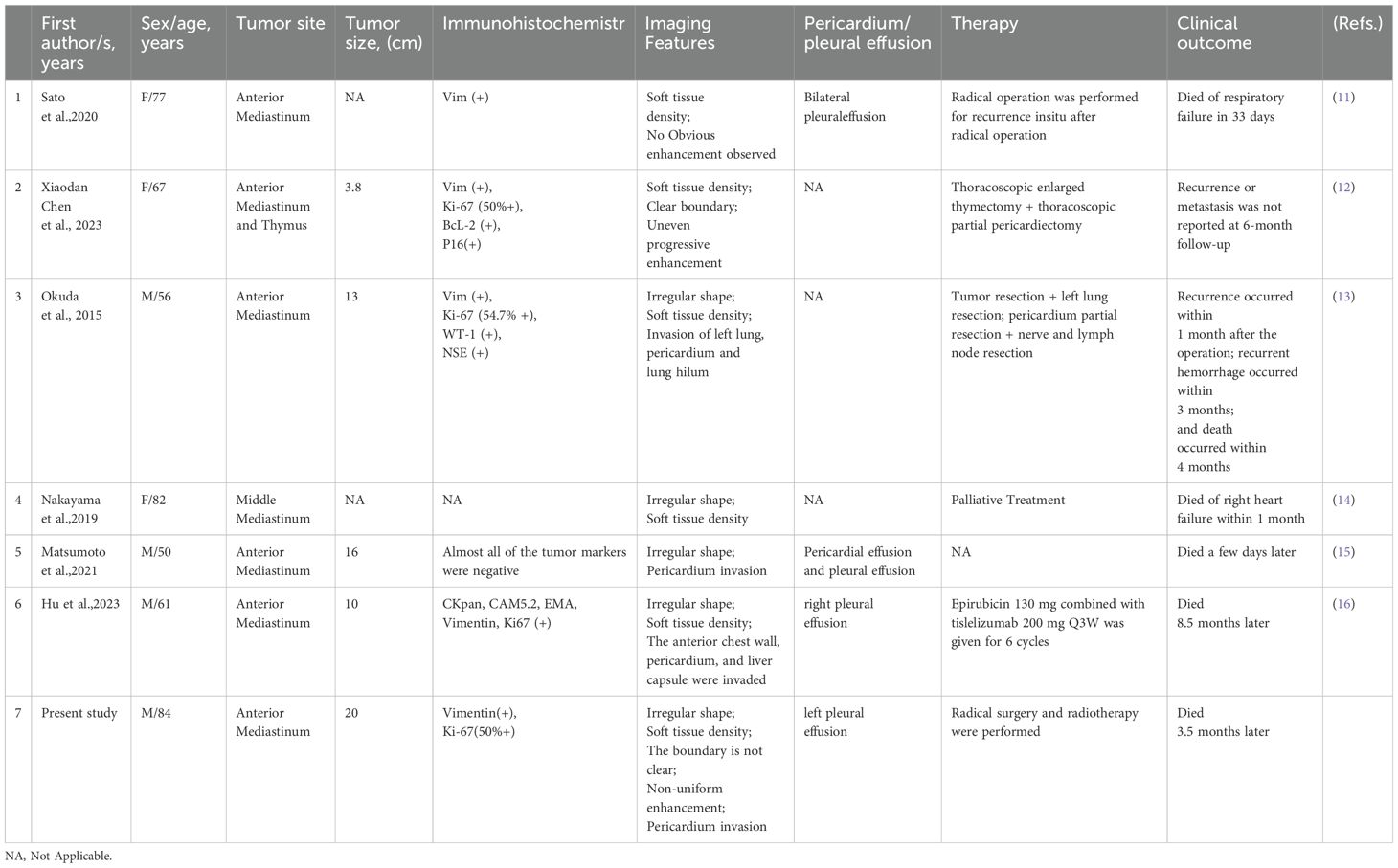
Table 1. Clinical and imaging data of seven patients with pleomorphic undifferentiated sarcoma in the mediastinal site.
Tumors in the mediastinum requiring differential diagnosis include thymic epithelial tumors, germ cell tumors, lymphomas, and neuroendocrine carcinomas (20). Thymic epithelial tumors, most commonly thymomas, appear on CT as well-demarcated round or oval soft tissue masses within the thymus region. The tumors typically present with soft tissue attenuation and mild to moderate contrast enhancement, CT shows lobulated or irregular contours or heterogeneous enhancement suggestive of infiltrating thymoma or thymic carcinoma (21). Occasionally, focal areas of low attenuation are found within the tumor reflecting hemorrhage, necrosis or cyst formation, and linear or circumferential calcifications are occasionally seen in pericyclic thymomas and invasive thymomas (22). On MRI, thymoma shows moderate signal intensity slightly higher than muscle on T1-weighted images and high signal intensity on T2-weighted images (23). Additionally, the absence of a strong capsule on MRI strongly suggests tumor invasion of surrounding tissues. Jeong et al. (24) reported that major vessel infiltration is an important characteristic of thymic tumors as it is only seen in thymic carcinoma. In this study case, the smooth contour of the anterior mediastinal irregular mass, the presence of septations, and the absence of vascular infiltration serve as differential points from thymoma/carcinoma, although both exhibit malignant signs similar to those of surrounding tissue structures, making differentiation challenging.
Lymphoma, a malignancy originating in the lymph nodes or lymphatic tissue, typically presents on CT as a large, irregular-shaped or conglomerate nodular soft tissue mass in the anterior mediastinum with unclear margins and uniform density. Mediastinal lymph node involvement is common, and approximately half of the cases have pleural and/or pericardial effusions (25). After enhancement, the mass often exhibits moderate, uniform enhancement and commonly envelops surrounding blood vessels without invading the vascular structures, presenting a “vascular encasement sign,” a characteristic imaging feature useful for differential diagnosis. Before treatment, mediastinal lymphomas typically do not show calcifications, an important consideration in differential diagnosis.
Germ cell tumors are composed of three types: teratomas, seminomas, and non-seminomatous malignant germ cell tumors. Over 80% of germ cell tumors are benign, with the majority being mature teratomas (26). Seminomas, the most common malignant subtype, display well-defined, lobulated or multinodular masses with relatively uniform signal intensity on MR images. Cystic changes, necrosis, and hemorrhage are visible but usually localized, with thin septations within the mass. On contrast-enhanced MRI, seminomas typically show uniform enhancement of the mass. Mediastinal teratomas vary in appearance on CT and MRI based on their contents. On CT, teratomas most commonly appear as well-demarcated unilocular or multilocular cystic lesions containing fluid, soft tissue, and fat attenuation. Various forms of calcification might also be present, occasionally including teeth or bone. However, the capsules of teratomas are characteristically thickened, whereas the capsules of other mediastinal cystic lesions are usually thin (27).
Thymic neuroendocrine tumors are commonly found in the anterior superior mediastinum and often present on CT as large soft tissue masses with cystic changes and necrosis, the extent of which is typically proportional to the tumor diameter. The radiological features on CT and MRI do not significantly differ from those of thymomas (28). Shimamoto et al. (29) reviewed the CT and MRI findings of 11 cases of thymic carcinoids (3 typical and 8 atypical carcinoids), reporting that most lesions had irregular margins and lacked a peripheral capsule. The masses were heterogeneous with necrotic or cystic changes and hemorrhage, and septa within the mass were rare.
The primary differential diagnoses for UPS are the multiple subtypes of STS. The most common differential diagnoses considered for UPS are Lipoma, Liposarcoma, Angiosarcoma, Angioma, Leiomyosarcoma, Osteosarcoma, and Dermatofibrosarcoma Protuberans. Tumor metastases from other sites are also considered (8).
The cornerstone for the management of UPS occurring in the trunk, head, neck, and limbs is complete and utter surgical resection with adequate free margins. This can be successfully accomplished via wide local resection of a free margin of 2 cm. Radiotherapy implemented after surgical excision is applied either when the free margins are distanced <1 cm from the lesion, the lesion invaded the bony structures, or when there are significant vascular or nerve entities involved (30). Furthermore, chemotherapy can also be considered for advanced UPS which has an advanced stage that hinders surgical resection or spread out in the body.
The gold standard for establishing a final diagnosis relies on competent histopathological analysis. Upon examination of the UPS specimens under the light microscope, it portrays vivid cellular atypia, prominent mitotic structures, and pleomorphic cellular components. The malignant lesion of UPS could also demonstrate a sheet-like, storiform, or fascicular tissue organization inside a fibrous-based stroma (31).
Nevertheless, the ultimate diagnosis is defined via thorough immunohistochemical analysis (32). Some of the IHC markers used to differentiate UPS from other STS include CD68, CD30, S100, SMA, Kinases, Desmin, Vimentin, Ki-67 proliferation index, and p53 (33, 34).
In recent years, genomic approaches have significantly advanced the identification of predictive biomarkers in soft tissue sarcomas (STS), improving differential diagnosis, prognosis assessment, and targeted therapy. Next-generation sequencing (NGS), as a pivotal tool, enables comprehensive tumor genomic profiling, providing the foundation for personalized treatment strategies. For instance, NGS can detect critical mutations and gene fusions that are essential for the treatment of various STS subtypes. Gounder et al (32). found through targeted gene sequencing that up to 31.7% of patients harbor actionable genetic alterations, which are of significant value in diagnosis and treatment decision-making. Simon et al (35). further analyzed the genomic characteristics of undifferentiated pleomorphic sarcoma (UPS) using NGS, identifying tumor mutational burden (TMB), microsatellite instability (MSI), and DNA damage repair defects as biomarkers that could predict responses to immunotherapy or targeted therapy. Moreover, NGS can identify high-risk genetic variants associated with cancer susceptibility, aiding in early screening and monitoring. Vanni et al (36). emphasized that combining genomics with pharmacology can assist in identifying predictive biomarkers, particularly for differential diagnosis, prognosis assessment, and targeted therapy in UPS. RNA sequencing analysis revealed that matrix metalloproteinase 13 (MMP13) and WNT7B genes are upregulated in UPS, which are linked to enhanced cell migration and tumor aggressiveness. Additionally, the immune system plays a crucial role in UPS chemosensitivity, suggesting potential directions for future immunotherapy. While it remains challenging to implement NGS for every sarcoma patient, the application of NGS in genetically complex sarcomas with limited therapeutic options offers the potential for precision medicine in patient subgroups, especially through emerging therapies such as immune checkpoint inhibitors.
This study had some limitations. First, undifferentiated pleomorphic sarcoma (UPS) in the anterior mediastinum is an extremely rare malignancy with a low incidence rate and typically lacks characteristic clinical presentations. Second, definitive diagnosis still relies on histopathology and immunohistochemistry for an exclusionary comprehensive diagnosis. Third, although genomic approaches, such as next-generation sequencing (NGS), have shown promise in identifying potential biomarkers and molecular targets for treatment, the rarity of UPS limits the availability of large genomic datasets specific to this malignancy. As a result, comprehensive genomic profiling and the identification of specific actionable mutations or pathways remain challenging. This limitation hinders the development of targeted therapies, and personalized treatment strategies for UPS based on genomic data are still in their early stages.
Regular surveillance for affected patients should be conducted thoroughly to ensure that there aren’t any relapses, recurrences, or metastases. With regards to clinical examination, it must be carried out at three-to-six-month intervals for the first 2 years postoperatively and then performed at an annual rate (37).
4 Conclusion
Primary undifferentiated high-grade pleomorphic sarcoma (UPS) in the anterior mediastinum is extremely rare, and imaging and pathological diagnosis are crucial. Although genomic approaches show potential, personalized treatment for UPS is still in its early stages, and future research and data collection will drive therapeutic advancements.
5 Patient perspective
The patient and their family, after careful consideration and on the advice of the doctors, jointly decided to proceed with radiation therapy. Due to the patient’s advanced age and the progression of the disease, they opted to forgo chemotherapy. The family expressed that their hope was to ensure the patient’s comfort and dignity in their final days, providing the best possible care and support. aggressive nature.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Heping Hospital Affiliated to Changzhi Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LW: Writing – original draft. AX: Conceptualization, Investigation, Methodology, Visualization, Writing – review & editing. LK: Conceptualization, Investigation, Methodology, Writing – review & editing. PJ: Conceptualization, Visualization, Writing – review & editing. YL: Conceptualization, Investigation, Visualization, Writing – review & editing. XG: Data curation, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Geneva, Switzerland: World Health Organization (2013).
2. O'Brien JE, Stout AP. Malignant fibrous xanthomas. Cancer. (1964) 17:1445–56. doi: 10.1002/1097-0142(196411)17:11<1445::AID-CNCR2820171112>3.0.CO;2-G
3. Fletcher CD. The evolving classification of soft tissue tumours–an update based on the new 2013 WHO classification. Histopathology. (2014) 64:2–11. doi: 10.1111/his.2013.64.issue-1
4. Murphey MD, Kransdorf MJ. Staging and classification of primary musculoskeletal bone and soft-tissue tumors according to the 2020 WHO update, from the AJR Special Series on Cancer Staging. Am J Roentgenol. (2021) 217:1038–52. doi: 10.2214/AJR.21.25658
5. Weiss SW, Enzinger FM. Malignant fibrous histiocytoma. Anal 200 cases. Cancer. (1978) 41:2250–66. doi: 10.1002/1097-0142(197806)41:6<2250::AID-CNCR2820410626>3.0.CO;2-W
6. Noebauer-Huhmann IM, Weber MA, Lalam RK, Trattnig S, Bohndorf K, Vanhoenacker F, et al. Soft tissue tumors in adults: ESSR-approved guidelines for diagnostic imaging. In: Seminars in Musculoskeletal Radiology, vol. 19. Thieme Medical Publishers (2015). p. 475–82.
7. Allen AH. Large undifferentiated pleomorphic sarcoma of the posterior thigh. Am J Case Rep. (2019) 20:318. doi: 10.12659/AJCR.914079
8. Roberge D, Vakilian S, Alabed YZ, Turcotte RE, Freeman CR, Hickeson M. FDG PET/CT in initial staging of adult soft-tissue sarcoma. Sarcoma. (2012) 2012:960194. doi: 10.1155/2012/960194
9. Oguri Y, Cho H, Oohinata R, Onoyama H, Takada R, Motoi T. Aggressive undifferentiated pleomorphic sarcoma of the stomach involving long-term survival: A case report and literature review. Mol Clin Oncol. (2018) 9:661–5. doi: 10.3892/mco.2018.1739
10. Li YJ, Dai YL, Cheng YS, Zhang WB, Tu CQ. Positron emission tomography (18) F-fluorodeoxyglucose uptake and prognosis in patients with bone and soft tissue sarcoma: a meta-analysis. Eur J Surg Oncol (EJSO). (2016) 42:1103–14. doi: 10.1016/j.ejso.2016.04.056
11. Iwakawa Y, Nishi M, Wada Y, Yoshikawa K, Takasu C, Shimada M, et al. Pleomorphic type undifferentiated gastric sarcoma, report of a case. Clin J Gastroenterology. (2023) 16:20–5. doi: 10.1007/s12328-022-01729-y
12. Sato M, Inoue S, Arao T, Igarashi A, Yamauchi K, Sato K, et al. Undifferentiated pleomorphic sarcoma in the anterior mediastinum with a rapidly progressive course: A case report. EXCLI J. (2020) 19:1161. doi: 10.17179/excli2020-2694
13. Chen X, Yang J, Huang B, Liu H, Chen L. Pleomorphic undifferentiated sarcoma of the mediastinal thymus: A case report and literature review. Oncol Lett. (2023) 26:1–7. doi: 10.3892/ol.2023.13892
14. Okuda K, Yano M, Moriyama S, Haneda H, Tatematsu T, Suzuki A, et al. A case of mediastinum undifferentiated high grade pleomorphic sarcoma. Int J Clin Exp Med. (2015) 8:19566.
15. Nakayama T, Numasawa Y, Hashimoto K, Hirano K. Undifferentiated high-grade pleomorphic sarcoma of the mediastinum Vol. 2019. Oxford, UK: Oxford Medical Case Reports (2019). p. omz086.
16. Matsumoto K, Nakamura Y, Inagaki Y, Taniguchi Y, Tamiya A, Matsuda Y, et al. Mediastinal undifferentiated pleomorphic sarcoma with pleural effusion cytopathologically misdiagnosed as epithelial Malignant pleural mesothelioma: An autopsy case report. Thorac Cancer. (2021) 12:1137–40. doi: 10.1111/1759-7714.13898
17. Yang H, Qin Z, He X, Xue Q, Zhou H, Sun J, et al. Tislelizumab immunotherapy combined with chemotherapy in the treatment of a patient with primary anterior mediastinal undifferentiated pleomorphic sarcoma with high PD-L1 expression: A case report and literature review. Front Oncol. (2023) 13:1110997. doi: 10.3389/fonc.2023.1110997
18. Liang Z, Han J, Tuo H, Xue D, Yu H, Peng Y. Undifferentiated pleomorphic sarcoma of the pancreas: a rare case report and literature review. World J Surg Oncol. (2022) 20:55. doi: 10.1186/s12957-022-02525-1
19. Nakamura M, Yamanaka K, Kato T, Hatano K, Kakuta Y, Kawashima A, et al. A retroperitoneal primary undifferentiated pleomorphic sarcoma. Urol Case Rep. (2024) 53:102664. doi: 10.1016/j.eucr.2024.102664
20. Priola AM, Priola SM, Cardinale L, Cataldi A, Fava C. The anterior mediastinum: diseases. La radiologia medica. (2006) 111:312–42. doi: 10.1007/s11547-006-0032-5
21. Bakan S, Kandemirli SG, Dikici AS, Erşen E, Yıldırım O, Samancı C, et al. Evaluation of anterior mediastinal solid tumors by CT perfusion: a preliminary study. Diagn Interventional Radiol. (2017) 23:10. doi: 10.5152/dir.2016.16093
22. Sadohara J, Fujimoto K, Müller NL, Kato S, Takamori S, Ohkuma K, et al. Thymic epithelial tumors: comparison of CT and MR imaging findings of low-risk thymomas, high-risk thymomas, and thymic carcinomas. Eur J Radiol. (2006) 60:70–9. doi: 10.1016/j.ejrad.2006.05.003
23. Kushihashi T, Fujisawa H, Munechika H. Magnetic resonance imaging of thymic epithelial tumors. Crit Rev Diagn Imaging. (1996) 37:191–259.
24. Jeong YJ, Lee KS, Kim J, Shim YM, Han J, Kwon OJ. Does CT of thymic epithelial tumors enable us to differentiate histologic subtypes and predict prognosis? Am J Roentgenol. (2004) 183:283–9. doi: 10.2214/ajr.183.2.1830283
25. Tateishi U, Müller NL, Johkoh T, Onishi Y, Arai Y, Satake M, et al. Primary mediastinal lymphoma: characteristic features of the various histological subtypes on CT. J Comput Assisted Tomography. (2004) 28:782–9. doi: 10.1097/00004728-200411000-00009
26. Drevelegas A, Palladas P, Scordalaki A. Mediastinal germ cell tumors: a radiologic–pathologic review. Eur Radiol. (2001) 11:1925–32. doi: 10.1007/s003300000725
27. Moeller KH, Rosado-de-Christenson ML, Templeton PA. Mediastinal mature teratoma: imaging features. AJR. Am J Roentgenol. (1997) 169:985–90. doi: 10.2214/ajr.169.4.9308448
28. Chaer R, Massad MG, Evans A, Snow NJ, Geha AS. Primary neuroendocrine tumors of the thymus. Ann Thorac Surgery. (2002) 74:1733–40. doi: 10.1016/S0003-4975(02)03547-6
29. Shimamoto A, Ashizawa K, Kido Y, Hayashi H, Nagayasu T, Kawakami A, et al. CT and MRI findings of thymic carcinoid. Br J Radiol. (2017) 90:20150341. doi: 10.1259/bjr.20150341
30. Roberge D, Vakilian S, Alabed YZ, Turcotte RE, Freeman CR, Hickenson M, et al. NCCN guidelines insights: soft tissue sarcoma, version 1.2021: featured updates to the NCCN guidelines. J Natl Compr Cancer Network. (2020) 18:1604–12. doi: 10.6004/jnccn.2020.0058
31. Nascimento AF, Raut CP. Diagnosis and management of pleomorphic sarcomas (so-called “MFH”) in adults. J Surg Oncol. (2008) 97:330–9. doi: 10.1002/jso.20972
32. Hornick JL. Subclassification of pleomorphic sarcomas: How and why should we care? Ann Diagn Pathol. (2018) 37:118–24. doi: 10.1016/j.anndiagpath.2018.10.006
33. Henderson MT, Hollmig ST. Malignant fibrous histiocytoma: changing perceptions and management challenges. J Am Acad Dermatol. (2012) 67:1335–41. doi: 10.1016/j.jaad.2012.04.013
34. Roland CL, May CD, Watson KL, Al Sannaa GA, Dineen SP, Feig R, et al. Analysis of clinical and molecular factors impacting oncologic outcomes in undifferentiated pleomorphic sarcoma. Ann Surg Oncol. (2016) 23:2220–8. doi: 10.1245/s10434-016-5115-5
35. Gounder MM, Agaram NP, Trabucco SE. Clinical genomic profiling in the management of patients with soft tissue and bone sarcoma. Nat Commun. (2022) 13:3406. doi: 10.1038/s41467-022-30496-0
36. Vyse S, Thway K, Huang PH, Jones RL. Next-generation sequencing for the management of sarcomas with no known driver mutations. Curr Opin Oncol. (2021) 33:315–22. doi: 10.1097/CCO.0000000000000741
Keywords: undifferentiated sarcoma, anterior mediastinal tumor, computed tomography, magnetic resonance imaging, diagnosis
Citation: Wang L, Xie A, Ke L, Jia P, Li Y and Guo X (2024) Undifferentiated pleomorphic sarcoma in the anterior mediastinum: a case report and literature review. Front. Oncol. 14:1445149. doi: 10.3389/fonc.2024.1445149
Received: 06 June 2024; Accepted: 30 September 2024;
Published: 15 October 2024.
Edited by:
Alessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyCopyright © 2024 Wang, Xie, Ke, Jia, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing Guo, Z3VveGluZ0Bjem1jLmVkdS5jbg==
 Luyao Wang
Luyao Wang Pingfan Jia
Pingfan Jia Xing Guo
Xing Guo