- Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
Background: In recent years, the incidence of endometrial cancer (EC) has been rising. This meta-analysis aims to clarify the prognostic significance of serum CA-125 levels in EC.
Methods: Articles up to March 1, 2024, were systematically searched in EMBASE, Cochrane Library, PubMed, and Web of Science. This analysis pooled hazard ratios (HR) and 95% confidence intervals (CI) from qualifying studies to evaluate the association of CA-125 levels with overall survival (OS), progression-free survival (PFS), disease-free/relapse-free survival (DFS/RFS), and disease-specific survival (DSS).
Results: 25 studies involving 7,716 patients were included. The analysis revealed that elevated CA-125 levels correlate with poorer OS (HR = 1.848, 95% CI: 1.571-2.175, p < 0.001). This association persisted across various study regions and sample sizes, and was notably strong in subgroups with a CA-125 cut-off value of less than 35 (HR = 2.07, 95% CI: 1.13-3.80, p = 0.019) and equal to 35 (HR = 2.04, 95% CI: 1.49-2.79, p < 0.001), and among type II pathology patients (HR = 1.72, 95% CI: 1.07-2.77, p = 0.025). Similarly, high CA-125 levels were linked to reduced PFS, particularly in subgroups with a CA-125 cut-off value less than 35 (HR = 1.87, 95% CI: 1.15-3.04, p = 0.012) and equal to 35 (HR = 4.94, 95% CI: 2.56-9.54, p < 0.001), and in endometrioid endometrial cancer patients (HR = 2.28, 95% CI: 1.18-4.40, p = 0.014). Elevated CA-125 levels were also indicative of worse DFS/RFS (HR = 2.17, 95% CI: 1.444-3.262, p < 0.001) and DSS (HR = 2.854; 95% CI: 1.970-4.133, p < 0.001).
Conclusion: Serum CA-125 levels before treatment was highly associated with prognosis of EC patients.
1 Introduction
Endometrial cancer (EC) ranks among the top three malignant tumors in the female reproductive system, predominantly affecting perimenopausal and postmenopausal women, with rising global incidence over the past two decades (1). In 2020, there were over 417,000 new cases of EC worldwide, resulting in approximately 97,737 deaths (2). Major risk factors for EC include anthropometric indices, diet, physical activity, medical conditions, hormonal therapy, biochemical markers, gynecological history, and smoking (3–6). The primary treatment for EC, as per guidelines, involves comprehensive staging surgery, complemented by radiotherapy, chemotherapy, hormonal therapy, and targeted therapies (7, 8). Early-stage EC usually offers a favorable outlook with a recurrence rate between 10%-15%, whereas advanced-stage EC, particularly stage IV, has a poor prognosis with a five-year survival rate of only 15% (9).
CA-125, a macromolecular sugar chain antigen, is linked to tumorigenesis, cell proliferation, and metastasis (10–12) in various malignancies (13–15). Despite its sensitivity as a tumor marker, CA-125 levels can be elevated due to various factors such as menstrual periods, pregnancy, inflammation, radiation damage, benign ovarian tumors, and heart disease (16–18). CA-125 level correlates with EC clinicopathological features and predicts lymph node metastasis or extra-uterine spread in advanced cases (19–21). This study aims to elucidate the contentious role of pretreatment serum CA-125 levels in prognosticating EC, thereby contributing to the resolution of existing academic debates (22–24).
2 Materials and methods
2.1 Search strategy
This Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-compliant meta-analysis (CRD42023443479) (25) searched PubMed, Web of Science, Embase, and Cochrane Library up to March 1, 2024, using keywords related to EC and CA-125. (Endometrial Neoplasm OR Endometrial Carcinoma OR Endometrial Cancer OR Endometrium Cancer OR cancer of the endometrium OR Carcinoma of Endometrium OR Carcinoma of Endometrium OR Cancer of Endometrium OR Endometrium Cancers) AND (CA-125 OR CA 125 OR Carbohydrate antigen 125 OR Cancer antigen 125) AND (prognosis OR prediction)) (search details were showed in the Supplementary Tables 1–4). Additionally, references from selected articles and grey literature were reviewed to ensure inclusion of all relevant studies. Since the data used in this article were extracted from previous literature, no patient consent or ethical approval was required.
2.2 Eligibility criteria
Studies were included if they: (1) diagnosed EC pathologically or clinically; (2) measured pre-treatment serum CA-125 with a specified cut-off; (3) provided hazard ratios (HRs) and 95% confidence intervals (CIs) or sufficient data to calculate them; (4) reported survival outcomes [overall survival (OS), progression-free survival (PFS), disease-free survival (DFS), relapse-free survival (RFS), disease-specific survival (DSS), or cancer-specific survival (CSS)]; (5) were full-text; and (6) were in English. Exclusion criteria were: (1) non-original articles; (2) in vitro or animal studies; (3) duplicates; and (4) insufficient data.
2.3 Data collection and quality assessment
Data collection was performed by two independent investigators, with disputes resolved by a third investigator. Extracted information included study characteristics, patient demographics, and survival outcomes. HRs were sourced from either multivariate or univariate analyses. In cases where HRs and CIs were not directly provided, they were calculated using Kaplan-Meier survival curves (26). Due to a limited number of studies addressing RFS (only two included), survival data were grouped into OS, PFS, disease-free/relapse-free survival (DFS/RFS), and DSS categories for analysis to enhance statistical robustness. The Newcastle-Ottawa Scale (NOS) (27) evaluated the methodological quality of the studies, assigning scores from 0 to 9 based on criteria such as patient selection, comparability, follow-up, and outcome accuracy. Studies scoring 6 or higher were deemed high-quality.
2.4 Statistical analysis
Pooled HRs and 95% CIs evaluated the impact of pretreatment CA-125 on prognosis. Heterogeneity among studies was measured using the Chi-squared test and I2 value, with I2 > 50% indicating significant heterogeneity and prompting a random-effects model (28). A fixed-effects model was used for lower heterogeneity. Subgroup analyses were conducted to identify the sources of heterogeneity, considering variables such as study region, sample size, pathology classifications, CA-125 threshold values, and data origins. The integrity of the results was verified through sensitivity analysis, while Egger’s test investigated publication bias, with adjustments made using the trim-and-fill method where necessary (29). Statistical computations were conducted using STATA 15.0 with HR > 1 indicating poorer survival and significance at 95% CI not intersecting 1 and a p-value less than 0.05 in a two-sided test.
3 Results
3.1 Study retrieval, selection, and characteristics
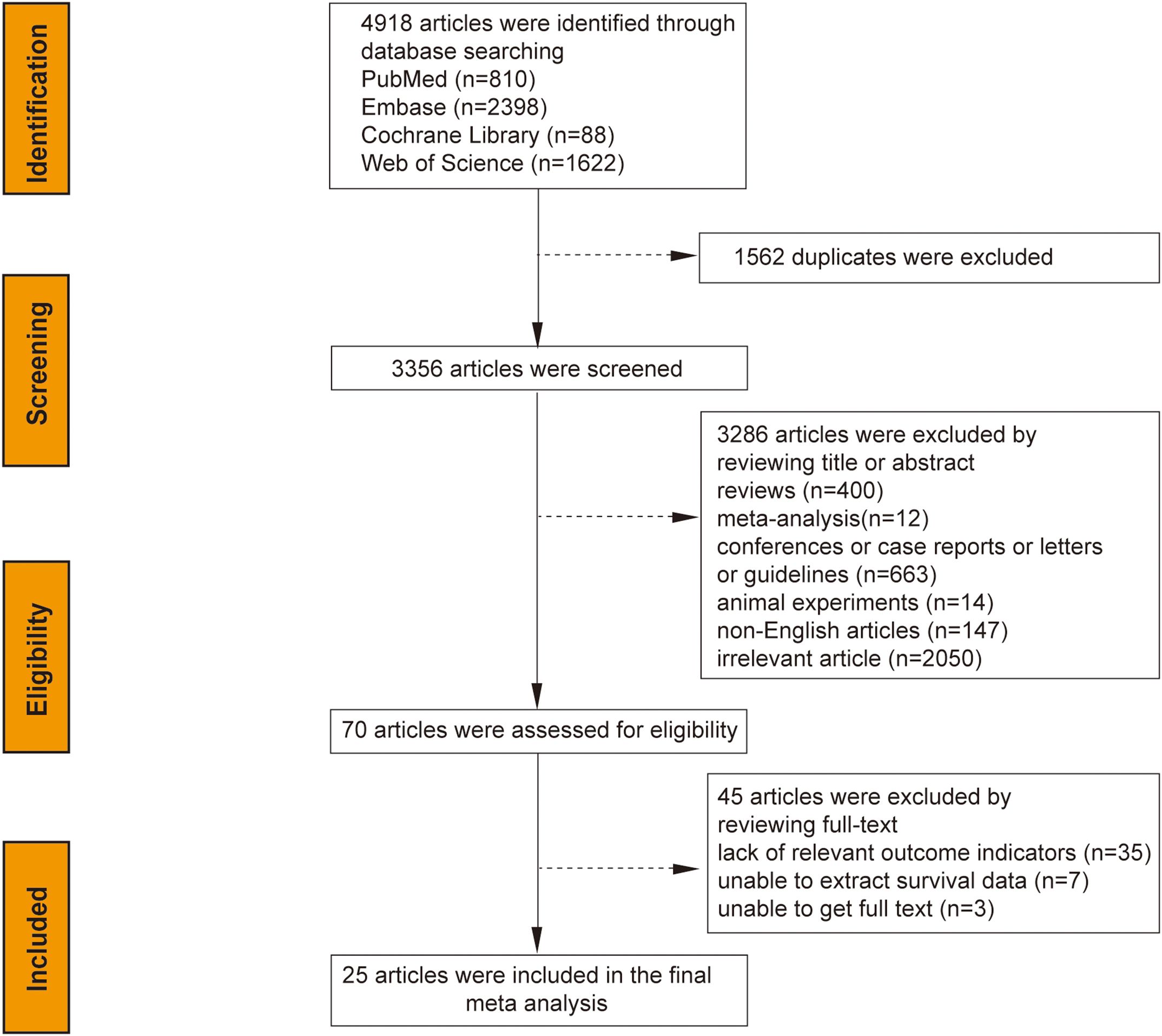
The initial search yielded 4,918 articles from the system database; after removing 1,562 duplicates and excluding 3,286 based on title/abstract analysis, 70 articles were fully reviewed. Of these, 45 were excluded for lacking relevant outcome indicators (35 articles), inability to extract survival data (7 articles), or unavailability of the full text (3 articles). Ultimately, 25 studies were selected for meta-analysis, as illustrated in the search flow chart (Figure 1). The 25 studies, spanning from 1997 to 2023, encompassed 7,716 patients with sample sizes ranging from 40 to 1,483. Study breakdown included 20 assessing the correlation between CA-125 levels and OS, 10 on DFS/RFS, six on PFS, and three on DSS. CA-125 cut-off values varied from 18 to 70.8 U/mL. Most studies (24) were retrospective, with one prospective study. Geographic distribution included 9 studies from Europe, 13 from Asia, and 3 from the Americas. Statistical analyses employed multivariate methods in 19 studies, univariate in two, and survival curves in 4. Specific cancer types analyzed were endometrioid endometrial cancer (EEC) in 2 studies and Type II EC, including uterine carcinosarcomas (UCSs), uterine papillary serous carcinoma (UPSC), and mixed Type II EC (G3 endometrioid and non-endometrioid cancers) in 5 studies. Seventeen studies covered mixed pathological types of both Type I and Type II EC. All studies achieved NOS scores between 6 and 9, indicating high quality (Table 1).
3.2 Correlation of pretreatment serum CA-125 level with OS in EC
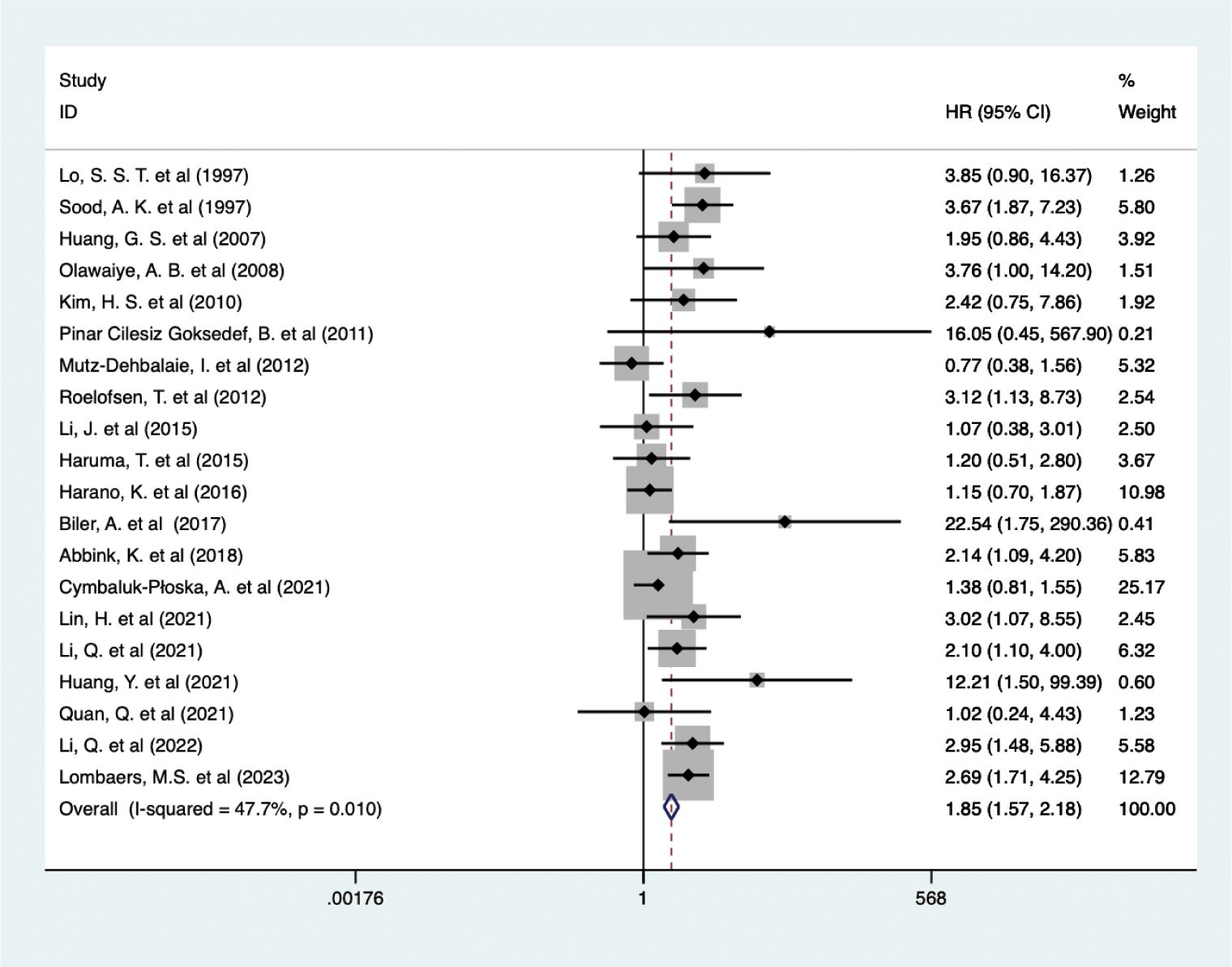
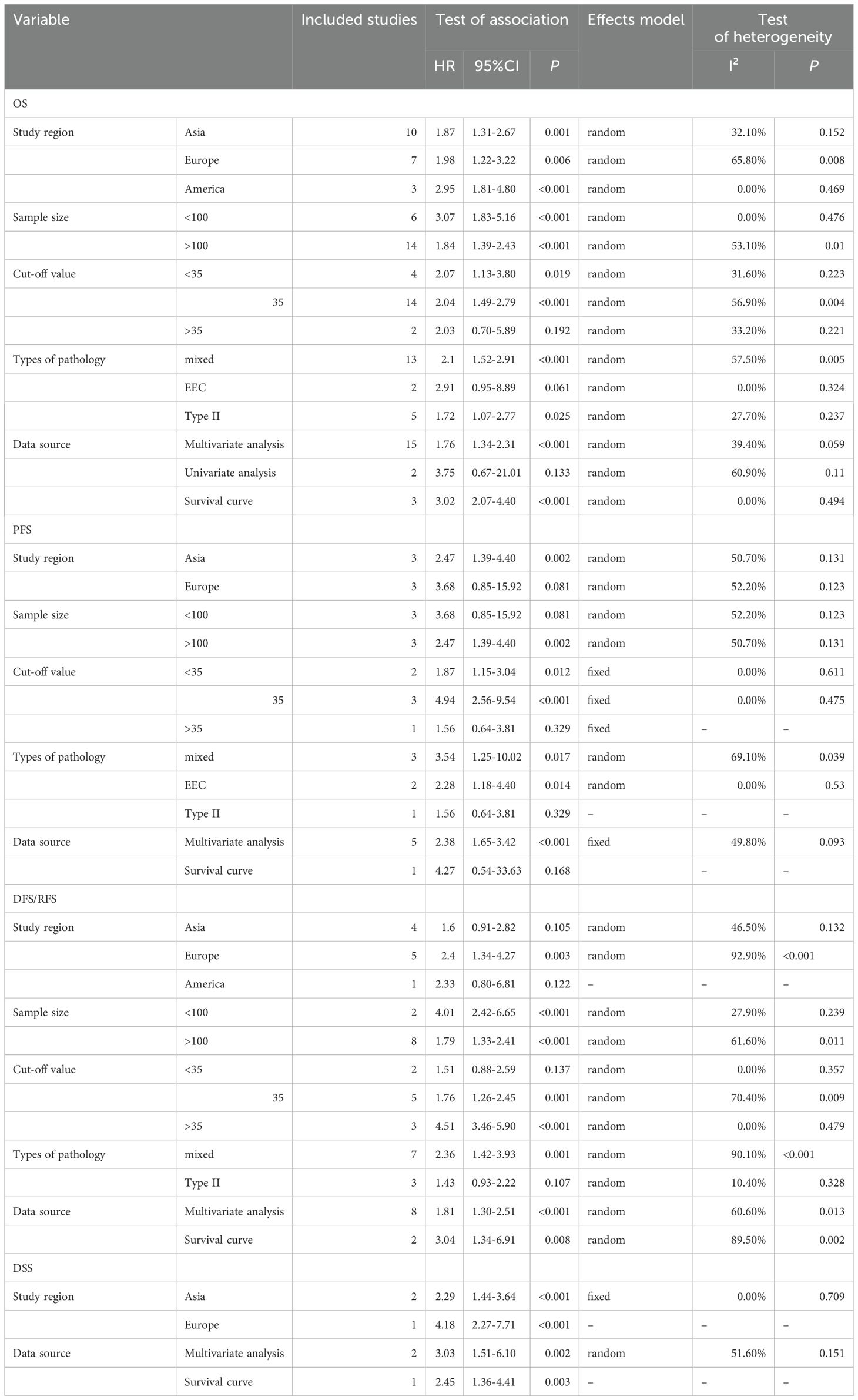
The analysis of data from 6,380 EC patients across 20 studies revealed that higher pretreatment serum CA-125 levels detrimentally influenced OS (HR = 1.848, 95% CI: 1.571-2.175, p < 0.001) (Figure 2) (24, 30–48). A fixed-effects model (I2 = 47.7%, p = 0.010), confirmed that higher CA-125 levels adversely affected OS across all study regions and sample sizes. Subgroup analyses revealed a significant negative association between CA-125 and OS for cut-off values ≤35 (HR = 2.07, 95% CI: 1.13-3.80, p = 0.019; HR = 2.04, 95% CI: 1.49-2.79, p < 0.001, respectively) but not >35 (HR = 2.03, 95% CI: 0.70-5.89, p = 0.192). High CA-125 levels were linked with worse OS in type II (HR = 1.72, 95% CI: 1.07-2.77, p = 0.025) and mixed pathology types (HR = 2.10, 95% CI: 1.52-2.91, p < 0.001) but not in EEC (HR = 2.91, 95% CI: 0.95-8.89, p = 0.061). Data source subgroups from multiple analyses (HR = 1.76, 95% CI: 1.34-2.31, p < 0.001) and survival curves (HR = 3.02, 95% CI: 2.07-4.40, p < 0.001) also showed an association between high CA-125 and poorer OS. These detailed findings are summarized in Table 2.
3.3 Correlation of pretreatment serum CA-125 level with PFS in EC
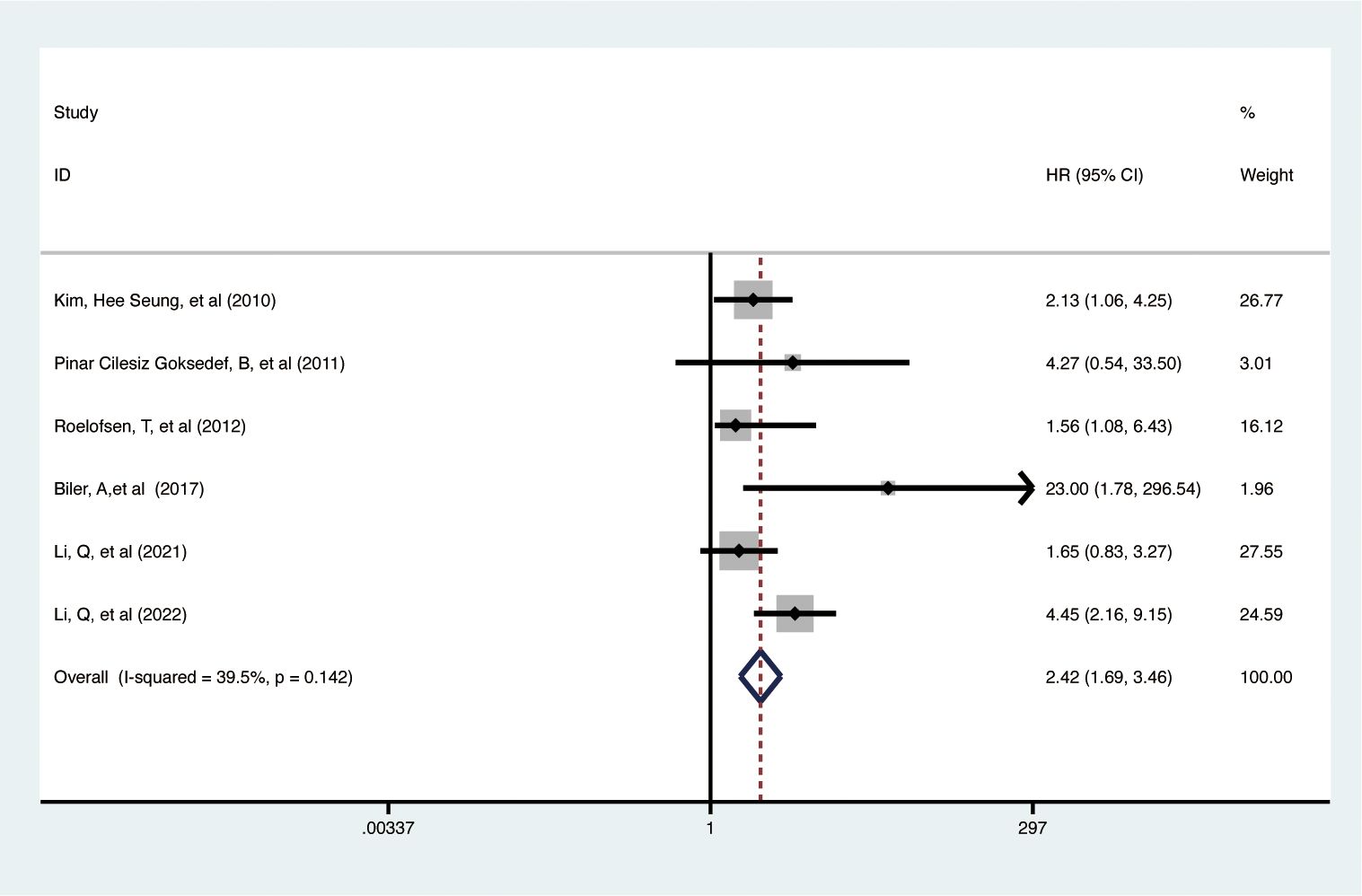
Data from 6 studies with 3,138 participants showed that elevated pretreatment serum CA-125 levels were significantly linked to poorer PFS in EC patients (HR = 2.42, 95% CI: 1.692–3.463, p < 0.001) (Figure 3) (32, 35, 37, 41, 45, 46). A fixed-effects model was used (I2 = 39.5%, p = 0.142). Subgroup analysis revealed that higher CA-125 levels correlated with poorer PFS in Asian EC patients, studies with sample sizes >100 (HR = 2.47, 95% CI: 1.39-4.40, p = 0.002), and cut-off values ≤35 (HR = 1.87, 95% CI: 1.15-3.04, p = 0.012; HR = 4.94, 95% CI: 2.56-9.54, p < 0.001, respectively) but not >35 (HR = 1.56, 95% CI: 0.64-3.81, p = 0.329). Elevated CA-125 was associated with worse PFS in EEC (HR = 2.28, 95% CI: 1.18-4.40, p = 0.014) and mixed pathology types (HR = 3.54, 95% CI: 1.25-10.02, p = 0.017) but not in type II EC (HR = 1.56, 95% CI: 0.64-3.81, p = 0.329). High CA-125 also associated worse PFS in studies with data from multiple analyses (HR = 2.38, 95% CI: 1.65-3.42, p < 0.001). Details of these findings are available in Table 2.
3.4 Correlation of pretreatment serum CA-125 level with DFS/RFS in EC
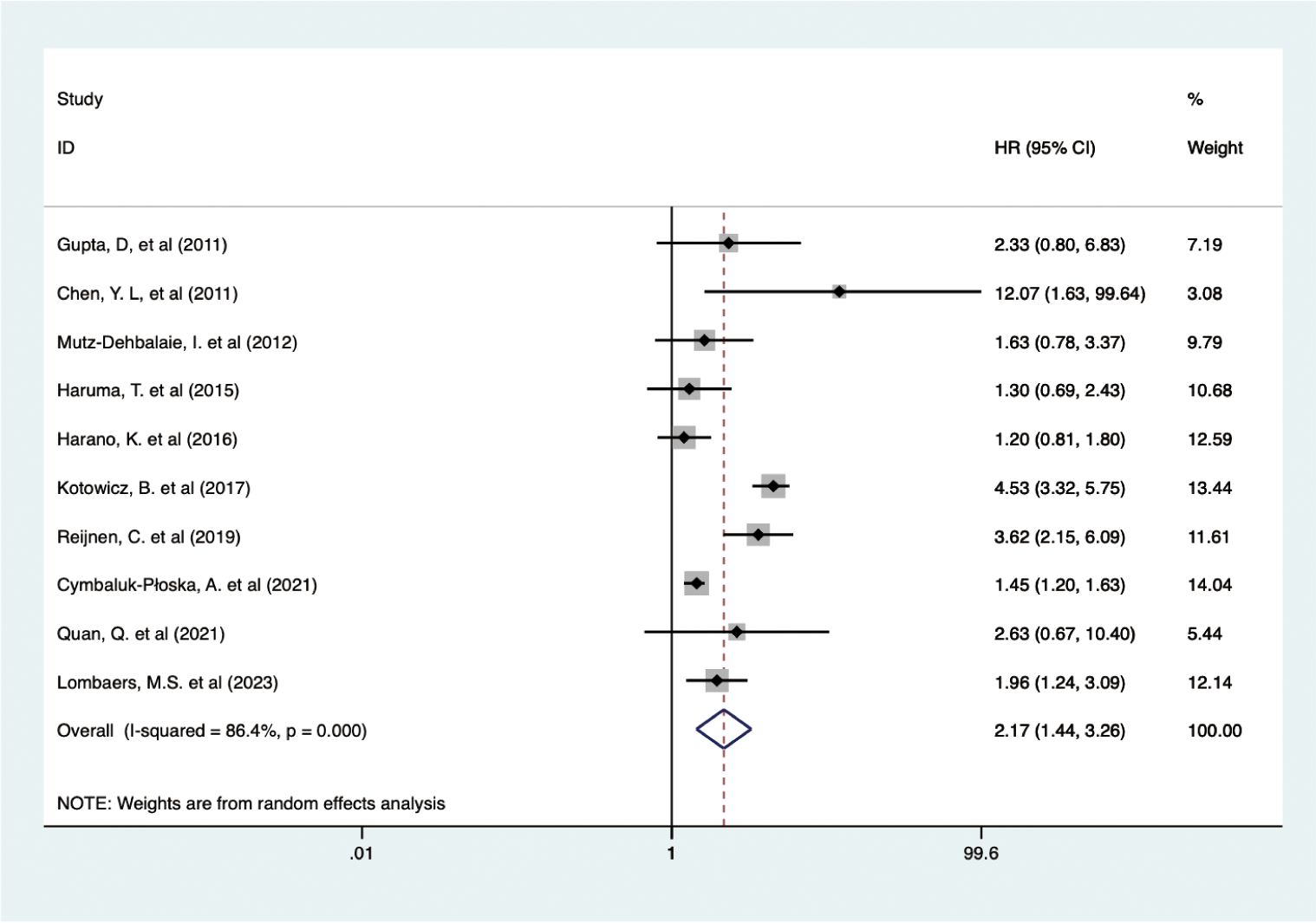
Data from 10 studies with 2,438 patients showed that higher pretreatment serum CA-125 levels were associated with poorer DFS/RFS outcomes in EC (HR = 2.170, 95% CI: 1.444–3.262, p < 0.001) (19, 23, 34, 38, 39, 42, 47–50) (Figure 4). A random-effects model was used (I2 = 86.4%, p < 0.001). Subgroup analysis demonstrated that elevated CA-125 adversely affected DFS/RFS in European patients (HR = 2.40, 95% CI: 1.34–4.27, p = 0.003), subgroups with CA-125 cutoff values ≥35 (HR = 4.51, 95% CI: 3.46–5.90, p < 0.001; HR = 1.76, 95% CI: 1.26–2.45, p = 0.001, respectively), and patients with mixed pathology types (HR = 2.36, 95% CI: 1.42–3.93, p = 0.001). The negative correlation between high CA-125 levels and poor DFS/RFS was consistent across varying sample sizes and data sources. These results are detailed in Table 2.
3.5 Correlation of pretreatment serum CA-125 level with DSS in EC
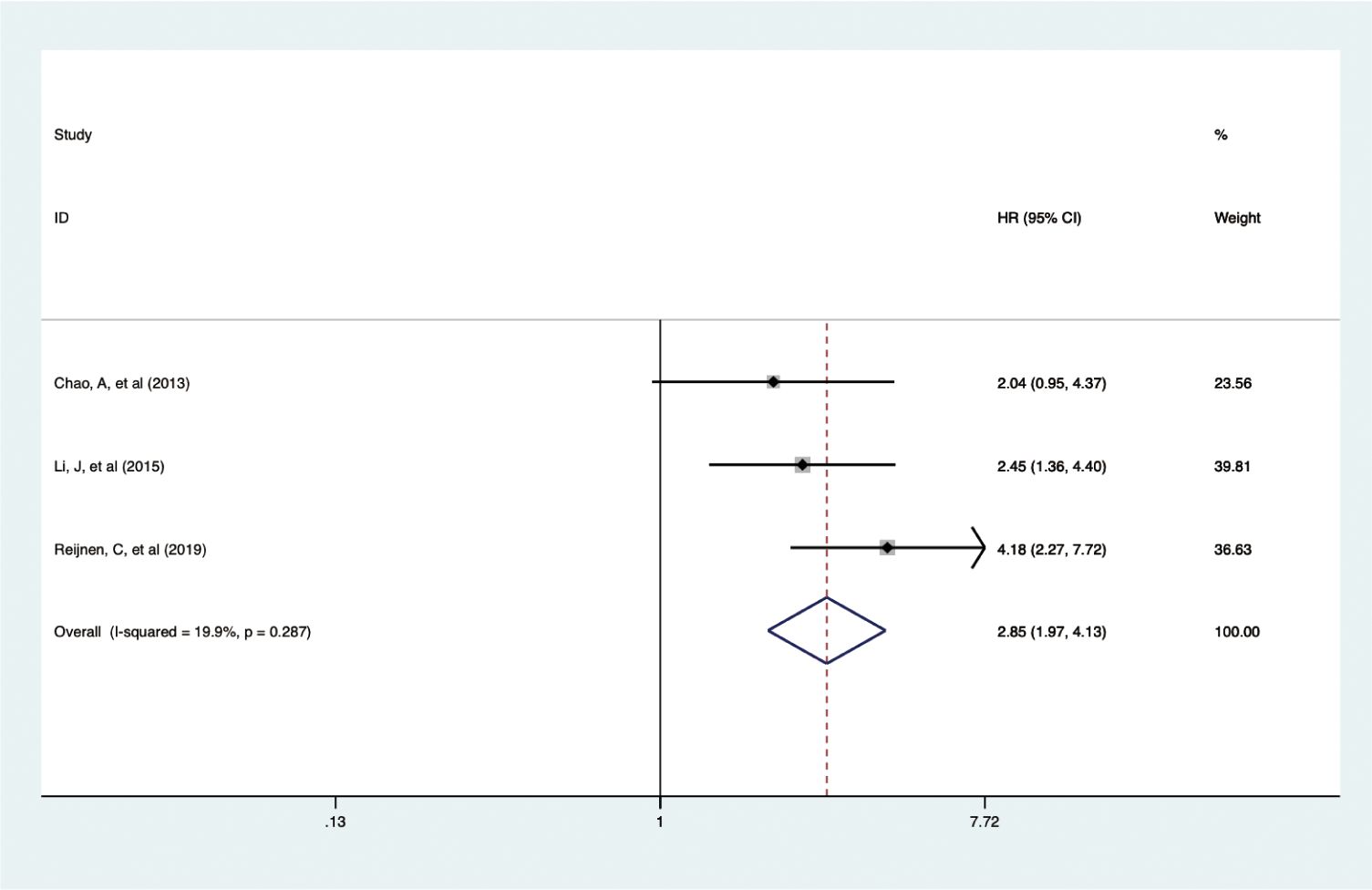
In a smaller cohort, three studies with 1,372 patients showed that elevated pretreatment serum CA-125 levels were linked to poorer DSS outcomes in EC (HR = 2.854, 95% CI: 1.970-4.133, p < 0.001) (19, 22, 36) (Figure 5). A fixed-effects model was used (I2 = 19.9%, p = 0.287). Subgroup analysis indicated that the adverse effects of high CA-125 levels on DSS were consistent across different study regions and data sources, as summarized in Table 2.
3.6 Sensitivity analysis
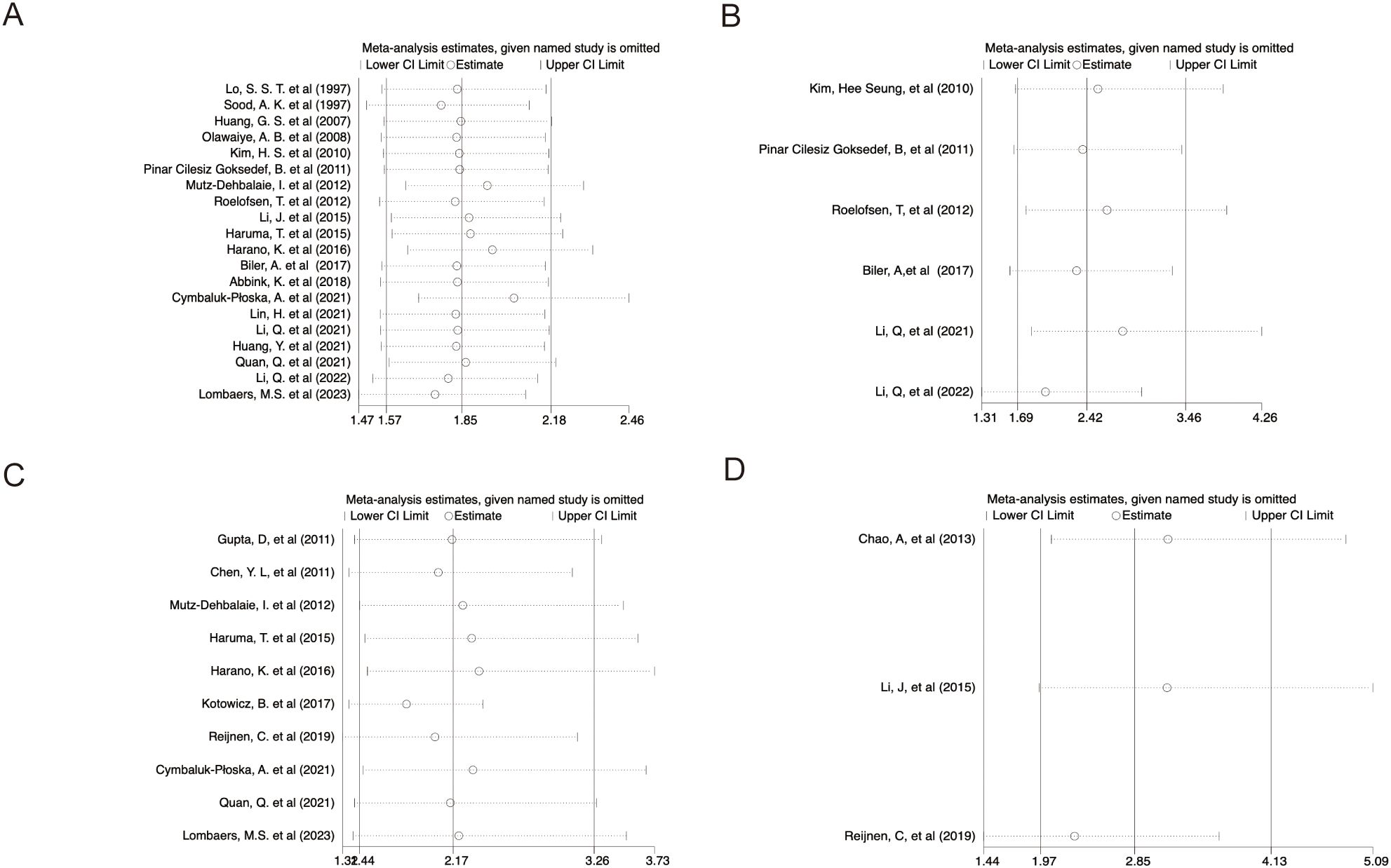
Sensitivity analyses using the leave-one-out method demonstrated that excluding any single study from the pool did not significantly alter HRs for survival outcomes, suggesting the meta-analysis results were stable and reliable (Figures 6A–D).
3.7 Publication bias
Egger’s test detected bias in the OS analysis (p = 0.031) (Supplementary Figure 1A). The trim-and-fill method, introducing six hypothetical studies, produced an adjusted HR for OS (HR = 1.762, 95% CI: 1.502-2.067, p < 0.001) (Supplementary Figure 1B). The adjusted outcome indicated no significant alteration in the overall effect size, suggesting that the observed bias did not compromise the conclusions. For PFS, DFS/RFS, and DSS, Egger’s tests indicated no significant publication bias (PFS: p = 0.271; DFS/RFS: p = 0.424; DSS: p = 0.670) (Supplementary Figures 1C–E).
4 Discussion
EC represents a significant gynecologic malignancy within the female reproductive system. Given the rising morbidity and mortality among high-risk and advanced EC patients, identifying prognostic factors is crucial (51). Prior research has highlighted the significance of various surgical and pathological features in prognosticating EC, including FIGO stage, tumor grade, histopathological type, lymph vascular space infiltration, myometrial infiltration, and cervical involvement (52, 53). A preoperative HE4 was associated with tumor’s features and has a good performance in prognosis and monitoring of EC (34, 40, 54). Moreover, molecular characteristics such as DNA mismatch repair deficiency (dMMR), CTNNB1 exon-3 mutation, TP53 mutation, and aberrant p53 expression patterns on IHC have been identified as poor prognostic indicators based on recent TCGA molecular typing (55–60). Additionally, factors like estrogen receptors (ERs), progesterone receptors (PRs), bcl-2, c-erb-B2 (HER2/neu), and proliferation markers (PCNA, Ki-67, MIB-1) are also associated with poor survival in EC (61–63).
CA-125, a well-established biomarker in gynecological malignancies, is crucial for diagnosing, predicting clinical outcomes, and monitoring treatment response in ovarian cancer (OC) (64–66). However, its prognostic value in EC remains contentious. Some studies report that elevated pretreatment serum CA-125 levels correlate with poor EC prognosis (31, 33, 37, 40, 41, 43, 45), while others have produced inconclusive or non-significant findings (22–24, 34, 35, 38, 39, 42, 48). These discrepancies may stem from variations in sample size, patient characteristics, pathological types, and CA-125 cut-off values. To address these inconsistencies, this meta-analysis synthesized data from 25 studies involving 7,716 patients to evaluate the impact of pretreatment serum CA-125 levels on EC survival outcomes, including OS, PFS, DFS/RFS, and DSS.
Elevated pretreatment serum CA-125 levels have been substantially associated with adverse prognostic indicators in EC patients, affirming the marker’s effectiveness in prognostication, similar to findings in OC (67) and other malignancies such as bladder urothelial carcinoma (68), pancreatic ductal adenocarcinoma (69), and renal cell carcinoma (70). This meta-analysis corroborates the pivotal prognostic value of CA-125 in EC, suggesting its potential to enhance prediction of clinical outcomes and guide effective treatment strategies to reduce mortality. Subgroup analyses examining variables such as study region, sample size, cut-off values, pathological types, and data sources revealed no significant differences, reinforcing the consistency of CA-125’s prognostic capacity across diverse clinical settings.
CA-125 is also known to relate closely with clinical pathological characteristics in EC. Higher CA-125 levels are typically linked with extrauterine tumor spread, advanced disease stages (24, 71), and are indicative of lymph node metastasis and greater myometrial invasion depth (72, 73). Furthermore, CA-125 levels vary with different pathological types of EC, being more prevalent in type II EC (48). Subgroup analysis focused on pathological types showed that heightened CA-125 levels significantly correlate with poorer prognosis in both EEC and type II EC, although the studies focusing on a single pathological type were limited.
Previous research often used a CA-125 range of 0–35 IU/mL to determine normal levels. In our meta-analysis, among the 25 studies, 15 studies selected 35 as the cut-off value. The number of studies involving other specific cut-off values is too small to form a subgroup. Therefore, we could only conduct subgroup analysis according to cut-off value equal to 35, greater than 35, and less than 35. Our analysis suggested that when the cut-off value was equal to 35 U/mL, elevated CA125 was associated with all the poor survival outcomes, including OS, PFS, DFS/RFS, DSS. When the cut-off value was greater than 35, elevated CA125 was associated with poor DFS/RFS. When the cut-off value was less than 35, elevated CA125 was associated with poor OS and PFS. According to previous studies, elevated CA-125 was found associated with advanced stages in EC patients, a universal cut-off value of 35 might not accurately reflect disease severity or evaluate prognosis across different EC stages. Because the study objects in all the included articles were patients with EC from stage I to stage IV, future studies should include more detailed stage-specific analyses to refine the prognostic utility of CA-125 in EC.
Serum CA-125, a well-studied tumor biomarker in EC, reflects the expression levels of MUC16, the largest known transmembrane mucin, which is highly expressed in various epithelial cancers (74). The molecular dynamics of CA-125 as a prognostic biomarker are closely linked to the abnormal, high expression of MUC16 in tumor cells, facilitating oncogenesis, proliferation, and metastasis (75–77). Additionally, elevated MUC16 expression is associated with increased chemotherapeutic resistance, metabolic alterations, immune surveillance evasion, and pro-inflammatory signaling (78–80). Mutations in MUC16 also correlate with EC prognosis by enhancing the infiltration of cytotoxic T lymphocytes, which play a critical role in antitumor immunity (81). With numerous clinical trials currently exploring MUC16 as a therapeutic target in OC (82–84), there is growing optimism that targeting MUC16 may similarly improve prognostic outcomes in EC patients.
This meta-analysis identified some intriguing outcomes but also faced several limitations. First, the number of studies analyzing the relationship between CA-125 and various survival outcomes was limited. Second, the predominance of retrospective studies could introduce selection bias. Third, extracting HRs from univariate analyses and survival curves might have resulted in overestimated effects. Additionally, a lack of detailed staging prevented subgroup analyses across different FIGO stages as the studies encompassed all stages collectively. Moreover, the lack of data on menopausal status prevented stratification of analyses. Despite these limitations, the study underscores the potential of CA-125. Cancer’s multifactorial nature often diminishes the accuracy of individual markers (85). A combined approach, integrating various biomarkers with clinicopathological features, is likely to yield more precise and sensitive prognostic assessments (86).
5 Conclusions
Generally, this study substantiates the association between elevated CA-125 levels and adverse prognosis in EC, supporting its prospective role as a pivotal molecular biomarker.
Author contributions
ZY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft. YS: Data curation, Formal analysis, Software, Writing – original draft. CG: Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1442814/full#supplementary-material
Supplementary Figure 1 | Publication bias assessment of included studies. (A) Egger’s test for OS; (B) Filled funnel plot using trim and fill method for OS; (C) Egger’s test for PFS; (D) Egger’s test for DFS/RFS; (E) Egger’s test for DSS.
Supplementary Table 1 | Search strategy details and results in PubMed.
Supplementary Table 2 | Search strategy details and results in Web of Science.
Supplementary Table 3 | Search strategy details and results in Embase.
Supplementary Table 4 | Search strategy details and results in Cochrane Library.
Abbreviations
EC, endometrial cancer; HR, Hazard ratio; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses; CI, Confidence interval; OS, overall survival; PFS, progression-free survival; DFS, disease-free survival; RFS, relapse free survival; DSS, disease-specific survival; DFS/RFS, disease-free/relapse-free survival.
References
1. Gu B, Shang X, Yan M, Li X, Wang W, Wang Q, et al. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990-2019. Gynecol Oncol. (2021) 161:573–80. doi: 10.1016/j.ygyno.2021.01.036
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int J Cancer. (2019) 145:1719–30. doi: 10.1002/ijc.31961
4. Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol. (2016) 34:4225–30. doi: 10.1200/JCO.2016.69.4638
5. Clarke MA, Long BJ, Del Mar Morillo A, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of endometrial cancer risk with postmenopausal bleeding in women: A systematic review and meta-analysis. JAMA Intern Med. (2018) 178:1210–22. doi: 10.1001/jamainternmed.2018.2820
6. Allen NE, Key TJ, Dossus L, Rinaldi S, Cust A, Lukanova A, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer. (2008) 15:485–97. doi: 10.1677/ERC-07-0064
7. Abu-Rustum N, Yashar C, Arend R, Barber E, Bradley K, Brooks R, et al. Uterine neoplasms, version 1.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2023) 21:181–209. doi: 10.6004/jnccn.2023.0006
8. Colombo N, Creutzberg C, Amant F, Bosse T, Gonzalez-Martin A, Ledermann J, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. (2016) 27:16–41. doi: 10.1093/annonc/mdv484
9. Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. (2019) 69:258–79. doi: 10.3322/caac.21561
10. Bast RC Jr., Spriggs DR. More than a biomarker: CA125 may contribute to ovarian cancer pathogenesis. Gynecol Oncol. (2011) 121:429–30. doi: 10.1016/j.ygyno.2011.04.032
11. Chen SH, Hung WC, Wang P, Paul C, Konstantopoulos K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci Rep. (2013) 3:1870. doi: 10.1038/srep01870
12. Theriault C, Pinard M, Comamala M, Migneault M, Beaudin J, Matte I, et al. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol Oncol. (2011) 121:434–43. doi: 10.1016/j.ygyno.2011.02.020
13. Bottoni P, Scatena R. The role of CA 125 as tumor marker: biochemical and clinical aspects. Adv Exp Med Biol. (2015) 867:229–44. doi: 10.1007/978-94-017-7215-0_14
14. Sun A, tumor markers SCC. NSE, CA 125, CA 19-9, and CYFRA 21-1 in patients with lung cancer. Lab Med. (2023) 54:638–45. doi: 10.1093/labmed/lmad020
15. Luo J, Xiao J, Yang Y, Chen G, Hu D, Zeng J. Strategies for five tumour markers in the screening and diagnosis of female breast cancer. Front Oncol. (2022) 12:1055855. doi: 10.3389/fonc.2022.1055855
16. Sjovall K, Nilsson B, Einhorn N. The significance of serum CA 125 elevation in Malignant and nonmalignant diseases. Gynecol Oncol. (2002) 85:175–8. doi: 10.1006/gyno.2002.6603
17. Falcao F, de Oliveira FRA, da Silva M, Sobral Filho DC. Carbohydrate antigen 125: a promising tool for risk stratification in heart diseases. biomark Med. (2018) 12:367–81. doi: 10.2217/bmm-2017-0452
18. Miralles C, Orea M, Espana P, Provencio M, Sanchez A, Cantos B, et al. Cancer antigen 125 associated with multiple benign and Malignant pathologies. Ann Surg Oncol. (2003) 10:150–4. doi: 10.1245/aso.2003.05.015
19. Reijnen C, IntHout J, Massuger L, Strobbe F, Kusters-Vandevelde HVN, Haldorsen IS, et al. Diagnostic accuracy of clinical biomarkers for preoperative prediction of lymph node metastasis in endometrial carcinoma: A systematic review and meta-analysis. Oncologist. (2019) 24:e880–e90. doi: 10.1634/theoncologist.2019-0117
20. Shawn LyBarger K, Miller HA, Frieboes HB. CA125 as a predictor of endometrial cancer lymphovascular space invasion and lymph node metastasis for risk stratification in the preoperative setting. Sci Rep. (2022) 12:19783. doi: 10.1038/s41598-022-22026-1
21. Nicklin J, Janda M, Gebski V, Jobling T, Land R, Manolitsas T, et al. The utility of serum CA-125 in predicting extra-uterine disease in apparent early-stage endometrial cancer. Int J Cancer. (2012) 131:885–90. doi: 10.1002/ijc.26433
22. Chao A, Tang YH, Lai CH, Chang CJ, Chang SC, Wu TI, et al. Potential of an age-stratified CA125 cut-off value to improve the prognostic classification of patients with endometrial cancer. Gynecologic Oncol. (2013) 129:500–4. doi: 10.1016/j.ygyno.2013.02.032
23. Gupta D, Gunter MJ, Yang K, Lee S, Zuckerwise L, Chen LM, et al. Performance of serum CA125 as a prognostic biomarker in patients with uterine papillary serous carcinoma. Int J Gynecological Cancer. (2011) 21:529–34. doi: 10.1097/IGC.0b013e31821091b5
24. Huang GS, Chiu LG, Gebb JS, Gunter MJ, Sukumvanich P, Goldberg GL, et al. Serum CA 125 predicts extrauterine disease and survival in uterine carcinosarcoma. Gynecologic Oncol. (2007) 107:513–7. doi: 10.1016/j.ygyno.2007.08.060
25. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. (2009) 3:e123–30. doi: 10.7717/peerj.7905/fig-1
26. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
27. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
28. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
29. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x
30. Lo SST, Cheng DKL, Ng TY, Wong LC, Ngan HYS. Prognostic significance of tumour markers in endometrial cancer. Tumor Biol. (1997) 18:241–9. doi: 10.1159/000218037
31. Sood AK, Buller RE, Burger RA, Dawson JD, Sorosky JI, Berman M. Value of preoperative CA 125 level in the management of uterine cancer and prediction of clinical outcome. Obstetrics Gynecology. (1997) 90:441–7. doi: 10.1016/S0029-7844(97)00286-X
32. Kim HS, Park C-Y, Lee J-M, Lee J-K, Cho C-H, Kim S-M, et al. Evaluation of serum CA-125 levels for preoperative counseling in endometrioid endometrial cancer: A multi-center study. Gynecologic Oncol. (2010) 118:283–8. doi: 10.1016/j.ygyno.2010.04.018
33. Olawaiye AB, Rauh-Hain JA, Withiam-Leitch M, Rueda BR, Goodman A, del Carmen MG. Utility of pre-operative serum CA-125 in the management of uterine papillary serous carcinoma. Gynecologic Oncol. (2008) 110:293–8. doi: 10.1016/j.ygyno.2008.05.027
34. Mutz-Dehbalaie I, Egle D, Fessler S, Hubalek M, Fiegl H, Marth C, et al. HE4 is an independent prognostic marker in endometrial cancer patients. Gynecologic Oncol. (2012) 126:186–91. doi: 10.1016/j.ygyno.2012.04.022
35. Pinar Cilesiz Goksedef B, Gorgen H, Baran SY, Api M, Cetin A. Preoperative serum CA 125 level as a predictor for metastasis and survival in endometrioid endometrial cancer. J Obstetrics Gynaecology Canada. (2011) 33:844–50. doi: 10.1016/S1701-2163(16)34988-X
36. Li J, Lin J, Luo Y, Kuang M, Liu Y. Multivariate analysis of prognostic biomarkers in surgically treated endometrial cancer. PloS One. (2015) 10(6):e0130640. doi: 10.1371/journal.pone.0130640
37. Roelofsen T, Mingels M, Hendriks JCM, Samlal RA, Snijders MP, Aalders AL, et al. Preoperative CA-125 predicts extra-uterine disease and survival in uterine papillary serous carcinoma patients. Int J Biol Markers. (2012) 27:e263–e71. doi: 10.5301/JBM.2012.9346
38. Harano K, Hirakawa A, Yunokawa M, Nakamura T, Satoh T, Nishikawa T, et al. Prognostic factors in patients with uterine carcinosarcoma: a multi-institutional retrospective study from the Japanese Gynecologic Oncology Group. Int J Clin Oncol. (2016) 21:168–76. doi: 10.1007/s10147-015-0859-7
39. Haruma T, Nakamura K, Nishida T, Ogawa C, Kusumoto T, Seki N, et al. Pre-treatment neutrophil to lymphocyte ratio is a predictor of prognosis in endometrial cancer. Anticancer Res. (2015) 35:337–44. doi: 10.1200/jco.2015.33.15_suppl.e16555
40. Abbink K, Zusterzeel PL, Geurts-Moespot AJ, Herwaarden AEV, Pijnenborg JM, Sweep FC, et al. HE4 is superior to CA125 in the detection of recurrent disease in high-risk endometrial cancer patients. Tumour Biol. (2018) 40:1010428318757103. doi: 10.1177/1010428318757103
41. Biler A, Solmaz U, Erkilinc S, Gokcu M, Bagci M, Temel O, et al. Analysis of endometrial carcinoma in young women at a high-volume cancer center. Int J Surgery. (2017) 44:185–90. doi: 10.1016/j.ijsu.2017.06.083
42. Cymbaluk-Płoska A, Gargulińska P, Bulsa M, Kwiatkowski S, Chudecka-Głaz A, Michalczyk K. Can the determination of he4 and ca125 markers affect the treatment of patients with endometrial cancer? Diagnostics. (2021) 11(4):626. doi: 10.3390/diagnostics11040626
43. Huang Y, Chen Y, Zhu Y, Wu Q, Yao C, Xia H, et al. Postoperative systemic immune-inflammation index (SII): A superior prognostic factor of endometrial cancer. Front Surg. (2021) 8:704235. doi: 10.3389/fsurg.2021.704235
44. Lin H, Fu H-C, Kung F-T, Chen Y-J, Chien C-CC, Wu C-H, et al. A comparison of laparoscopic and open surgery for early stage endometrial cancer with analysis of prognostic factors: a propensity score matching study. Eur J Gynaecological Oncol. (2021) 42:703–10. doi: 10.31083/j.ejgo4204107
45. Li Q, Cong R, Wang Y, Kong F, Ma J, Wu Q, et al. Naples prognostic score is an independent prognostic factor in patients with operable endometrial cancer: Results from a retrospective cohort study. Gynecologic Oncol. (2021) 160:91–8. doi: 10.1016/j.ygyno.2020.10.013
46. Li Q, Kong F, Ma J, Wang Y, Wang C, Yang H, et al. Nomograms based on fibrinogen, albumin, neutrophil-lymphocyte ratio, and carbohydrate antigen 125 for predicting endometrial cancer prognosis. Cancers. (2022) 14(22):5632. doi: 10.3390/cancers14225632
47. Kotowicz B, Fuksiewicz M, Jonska-Gmyrek J, Wagrodzki M, Kowalska M. Preoperative serum levels of YKL 40 and CA125 as a prognostic indicators in patients with endometrial cancer. Eur J Obstetrics Gynecology Reprod Biol. (2017) 215:141–7. doi: 10.1016/j.ejogrb.2017.06.021
48. Quan Q, Liao Q, Yin W, Zhou S, Gong S, Mu X. Serum HE4 and CA125 combined to predict and monitor recurrence of type II endometrial carcinoma. Sci Rep. (2021) 11:21694. doi: 10.1038/s41598-021-01263-w
49. Chen YL, Huang CY, Chien TY, Huang SH, Wu CJ, Ho CM. Value of pre-operative serum CA125 level for prediction of prognosis in patients with endometrial cancer. Aust New Z J Obstetrics Gynaecology. (2011) 51:397–402. doi: 10.1111/j.1479-828X.2011.01325.x
50. Lombaers MS, Cornel KMC, Visser NCM, Bulten J, Küsters-Vandevelde HVN, Amant F, et al. Preoperative CA125 significantly improves risk stratification in high-grade endometrial cancer. Cancers (Basel). (2023) 15(9):2605. doi: 10.3390/cancers15092605
51. Restaino S, Paglietti C, Arcieri M, Biasioli A, Della Martina M, Mariuzzi L, et al. Management of patients diagnosed with endometrial cancer: comparison of guidelines. Cancers (Basel). (2023) 15(4):1091. doi: 10.3390/cancers15041091
52. Prat J. Prognostic parameters of endometrial carcinoma. Hum Pathol. (2004) 35:649–62. doi: 10.1016/j.humpath.2004.02.007
53. Balaraj KS, Shanbhag NM, Bin Sumaida A, Hasnain SM, El-Koha OA, Puratchipithan R, et al. Endometrial carcinoma: A comprehensive analysis of clinical parameters, treatment modalities, and prognostic outcomes at a tertiary oncology center in the UAE. Cureus. (2023) 15:e48689. doi: 10.7759/cureus.48689
54. Fanfani F, Restaino S, Cicogna S, Petrillo M, Montico M, Perrone E, et al. Preoperative serum human epididymis protein 4 levels in early stage endometrial cancer: A prospective study. Int J Gynecol Cancer. (2017) 27:1200–5. doi: 10.1097/IGC.0000000000001015
55. Kurnit KC, Kim GN, Fellman BM, Urbauer DL, Mills GB, Zhang W, et al. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod Pathol. (2017) 30:1032–41. doi: 10.1038/modpathol.2017.15
56. Moroney MR, Davies KD, Wilberger AC, Sheeder J, Post MD, Berning AA, et al. Molecular markers in recurrent stage I, grade 1 endometrioid endometrial cancers. Gynecol Oncol. (2019) 153:517–20. doi: 10.1016/j.ygyno.2019.03.100
57. Myers A, Barry WT, Hirsch MS, Matulonis U, Lee L. beta-Catenin mutations in recurrent FIGO IA grade I endometrioid endometrial cancers. Gynecol Oncol. (2014) 134:426–7. doi: 10.1016/j.ygyno.2014.06.010
58. Yano M, Ito K, Yabuno A, Ogane N, Katoh T, Miyazawa M, et al. Impact of TP53 immunohistochemistry on the histological grading system for endometrial endometrioid carcinoma. Mod Pathol. (2019) 32:1023–31. doi: 10.1038/s41379-019-0220-1
59. He Y, Wang T, Li N, Yang B, Hu Y. Clinicopathological characteristics and prognostic value of POLE mutations in endometrial cancer: A systematic review and meta-analysis. Med (Baltimore). (2020) 99:e19281. doi: 10.1097/MD.0000000000019281
60. Ryan NAJ, Glaire MA, Blake D, Cabrera-Dandy M, Evans DG, Crosbie EJ. The proportion of endometrial cancers associated with Lynch syndrome: a systematic review of the literature and meta-analysis. Genet Med. (2019) 21:2167–80. doi: 10.1038/s41436-019-0536-8
61. Wik E, Raeder MB, Krakstad C, Trovik J, Birkeland E, Hoivik EA, et al. Lack of estrogen receptor-alpha is associated with epithelial-mesenchymal transition and PI3K alterations in endometrial carcinoma. Clin Cancer Res. (2013) 19:1094–105. doi: 10.1158/1078-0432.CCR-12-3039
62. Ross DS, Devereaux KA, Jin C, Lin DY, Zhang Y, Marra A, et al. Histopathologic features and molecular genetic landscape of HER2-amplified endometrial carcinomas. Mod Pathol. (2022) 35:962–71. doi: 10.1038/s41379-021-00997-2
63. Nordstrom B, Strang P, Bergstrom R, Nilsson S, Tribukait B. A comparison of proliferation markers and their prognostic value for women with endometrial carcinoma. Ki-67, proliferating cell nuclear antigen, and flow cytometric S-phase fraction. Cancer. (1996) 78:1942–51. doi: 10.1002/(sici)1097-0142(19961101)78:9<1942::aid-cncr15>3.3.co;2-e
64. Gadducci A, Cosio S, Carpi A, Nicolini A, Genazzani AR. Serum tumor markers in the management of ovarian, endometrial and cervical cancer. BioMed Pharmacother. (2004) 58:24–38. doi: 10.1016/j.biopha.2003.11.003
65. Esselen KM, Cronin AM, Bixel K, Bookman MA, Burger RA, Cohn DE, et al. Use of CA-125 tests and computed tomographic scans for surveillance in ovarian cancer. JAMA Oncol. (2016) 2:1427–33. doi: 10.1001/jamaoncol.2016.1842
66. Wang Y, Han C, Teng F, Bai Z, Tian W, Xue F. Predictive value of serum HE4 and CA125 concentrations for lymphatic metastasis of endometrial cancer. Int J Gynaecol Obstet. (2017) 136:58–63. doi: 10.1002/ijgo.12010
67. Wang Q, Feng X, Liu X, Zhu S. Prognostic value of elevated pre-treatment serum CA-125 in epithelial ovarian cancer: A meta-analysis. Front Oncol. (2022) 12:868061. doi: 10.3389/fonc.2022.868061
68. Laukhtina E, Pradere B, Mori K, Schuettfort VM, Quhal F, Mostafaei H, et al. Prognostic blood-based biomarkers in patients treated with neoadjuvant chemotherapy for urothelial carcinoma of the bladder: A systematic review. Urol Oncol. (2021) 39:471–9. doi: 10.1016/j.urolonc.2021.03.005
69. Mehta S, Bhimani N, Gill AJ, Samra JS, Sahni S, Mittal A. Serum biomarker panel for diagnosis and prognosis of pancreatic ductal adenocarcinomas. Front Oncol. (2021) 11:708963. doi: 10.3389/fonc.2021.708963
70. Bamias A, Chorti M, Deliveliotis C, Trakas N, Skolarikos A, Protogerou B, et al. Prognostic significance of CA 125, CD44, and epithelial membrane antigen in renal cell carcinoma. Urology. (2003) 62:368–73. doi: 10.1016/s0090-4295(03)00264-4
71. Modarres-Gilani M, Vaezi M, Shariat M, Zamani N, Nourizadeh R. The prognostic role of preoperative serum CA125 levels in patients with advanced endometrial carcinoma. Cancer biomark. (2017) 20:135–41. doi: 10.3233/CBM-160529
72. Atguden Z, Yildiz A, Aksut H, Yalcin SE, Yalcin Y, Uysal D, et al. The value of preoperative CA 125 levels in prediction of myometrial invasion in patients with early-stage endometrioid- type endometrial cancer. Asian Pac J Cancer Prev. (2016) 17:497–501. doi: 10.7314/apjcp.2016.17.2.497
73. Guo X, Lin C, Zhao J, Tang M. Development of a novel predictive model for lymph node metastasis in patients with endometrial endometrioid carcinoma. BMC Cancer. (2022) 22:1333. doi: 10.1186/s12885-022-10437-2
74. Xiang X, Feng M, Felder M, Connor JP, Man YG, Patankar MS, et al. HN125: A novel immunoadhesin targeting MUC16 with potential for cancer therapy. J Cancer. (2011) 2:280–91. doi: 10.7150/jca.2.280
75. Haridas D, Ponnusamy MP, Chugh S, Lakshmanan I, Seshacharyulu P, Batra SK. MUC16: molecular analysis and its functional implications in benign and Malignant conditions. FASEB J. (2014) 28:4183–99. doi: 10.1096/fj.14-257352
76. Sousa AM, Rei M, Freitas R, Ricardo S, Caffrey T, David L, et al. Effect of MUC1/beta-catenin interaction on the tumorigenic capacity of pancreatic CD133(+) cells. Oncol Lett. (2016) 12:1811–7. doi: 10.3892/ol.2016.4888
77. Giannakouros P, Comamala M, Matte I, Rancourt C, Piche A. MUC16 mucin (CA125) regulates the formation of multicellular aggregates by altering beta-catenin signaling. Am J Cancer Res. (2015) 5:219–30.
78. Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. (2010) 29:2893–904. doi: 10.1038/onc.2010.87
79. Bhatia R, Gautam SK, Cannon A, Thompson C, Hall BR, Aithal A, et al. Cancer-associated mucins: role in immune modulation and metastasis. Cancer Metastasis Rev. (2019) 38:223–36. doi: 10.1007/s10555-018-09775-0
80. Morgado M, Sutton MN, Simmons M, Warren CR, Lu Z, Constantinou PE, et al. Tumor necrosis factor-alpha and interferon-gamma stimulate MUC16 (CA125) expression in breast, endometrial and ovarian cancers through NFkappaB. Oncotarget. (2016) 7:14871–84. doi: 10.18632/oncotarget.7652
81. Hu J, Sun J. MUC16 mutations improve patients’ prognosis by enhancing the infiltration and antitumor immunity of cytotoxic T lymphocytes in the endometrial cancer microenvironment. Oncoimmunology. (2018) 7:e1487914. doi: 10.1080/2162402X.2018.1487914
82. Koneru M, O’Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med. (2015) 13:102. doi: 10.1186/s12967-015-0460-x
83. Liu JF, Moore KN, Birrer MJ, Berlin S, Matulonis UA, Infante JR, et al. Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody-drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann Oncol. (2016) 27:2124–30. doi: 10.1093/annonc/mdw401
84. Aithal A, Rauth S, Kshirsagar P, Shah A, Lakshmanan I, Junker WM, et al. MUC16 as a novel target for cancer therapy. Expert Opin Ther Targets. (2018) 22:675–86. doi: 10.1080/14728222.2018.1498845
85. Coll-de la Rubia E, Martinez-Garcia E, Dittmar G, Gil-Moreno A, Cabrera S, Colas E. Prognostic biomarkers in endometrial cancer: A systematic review and meta-analysis. J Clin Med. (2020) 9(6):1900. doi: 10.3390/jcm9061900
Keywords: endometrial cancer, CA-125, prognosis, survival, meta – analysis
Citation: Yu Z, Sun Y and Guo C (2024) Evaluating pretreatment serum CA-125 levels as prognostic biomarkers in endometrial cancer: a comprehensive meta-analysis. Front. Oncol. 14:1442814. doi: 10.3389/fonc.2024.1442814
Received: 03 June 2024; Accepted: 28 August 2024;
Published: 27 September 2024.
Edited by:
Philip Rosenberg, National Cancer Institute (NIH), United StatesReviewed by:
Stefano Restaino, Ospedale Santa Maria della Misericordia di Udine, ItalyBoya Guo, Fred Hutchinson Cancer Center, United States
Copyright © 2024 Yu, Sun and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuishan Guo, Z3VvY3M4ODA0QDEyNi5jb20=
 Zhong Yu
Zhong Yu Cuishan Guo
Cuishan Guo