- 1Department of Nuclear Medicine, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China
- 2School of Clinical Medicine, Ningxia Medical University, Yinchuan, Ningxia, China
- 3Department of Nuclear Medicine, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China
Purpose: Fibroblast activation protein (FAP) is highly expressed in the mesenchyme of most malignant epithelial tumors, while its expression is low in normal tissues. FAP inhibitors (FAPIs) bind specifically to FAP and are used for tumor-targeted diagnosis and therapy. The aim of this study was to radiosynthesize a novel molecular probe 131I-FAPI and evaluate its in-vitro targeting and biological characteristics.
Methods: The structurally modified FAPI was labelled with 131I through the chloramine-T method. The radiolabeling rate was then detected by thin-layer chromatography (TLC). The stability of 131I-FAPI was determined at PBS (room temperature) and serum (37°C). Its hydrophilicity was calculated by measuring its lipid-water partition coefficient. Pancreatic cancer PANC-1 cell line and glioma U87 cell line were cultured in vitro. Cell uptake assay was used to show the binding ability of 131I-FAPI. The CCK-8 assay was used to calculate the inhibitory effects of 131I-FAPI at different time points (4h, 8h, 12h, 24h, 48h) after comparing with the 131I and FAPI. The before-and-after-24h scratch areas of the two cells were determined in order to verify the effect of 131I-FAPI on the migration ability of the cells.
Results: The radiolabeling rate was (84.9 ± 1.02) %. The radiochemical purity of 131I-FAPI remained over 80% in both 25°C PBS and 37°C serum. The value of the lipid-water partition coefficient was -0.869 ± 0.025, indicating the hydrophilic of the probe. The cellular uptake assay showed that U87 cells had a specific binding capacity for 131I-FAPI. In cell inhibition assays, the inhibitory effect of 131I-FAPI on U87 cells increased with time. The results of cell scratch assay showed that 131I-FAPI had the strongest inhibitory effect on the migratory ability of U87 cells compared with 131I and FAPI (P<0.001).
Conclusion: 131I-FAPI was synthesized with good in-vitro stability and hydrophilic properties. It can be specifically bound by U87 cells. The proliferation and migration of U87 cells can be effectively inhibited. 131I-FAPI is promising to become a therapeutic probe.
1 Introduction
Recently, the hotspot of tumor target is the tumor microenvironment (TME). Because tumors are not isolated masses in organs, but are caused by various complicated factors in TME (1). TME is a complex internal environment that consists of different cell types, vessels, lymphatic vessels, and some physical elements. Cancer-associated fibroblasts (CAFs) are one of the most important ingredients. As the main stromal cells, CAFs promote the invasion and migration of tumors through several mechanisms. Therefore, targeting to CAFs can suppress the growth and progress of tumors.
Fibroblasts upregulate the expression of certain molecules after activating to CAFs, such as, myostatin C, platelet-derived growth factor receptor α or β, fibroblast activating protein (FAP), and natriuretic peptide B and so on (2). Among them, FAP is highly specific for expression on 90% tumor stroma (e.g, primary and metastatic cancers of the breast, lung, and colorectal cancer), and almost not expressed in benign tumors and normal tissues. So FAP is regarded as a specific marker of CAFs. In other words, the expression level of FAP plays an important role in the diagnosis of malignant tumors, staging and recurrence. Most importantly, molecular probes targeting FAP can be specifically located in tumors to achieve tumor imaging and treatment.
In recent years, radionuclide labelled probes targeting FAP were widely used in tumor diagnosis and treatment. Based on the molecular imaging, the pathophysiological processes in molecular, cellular or subcellular level can be visualized and quantified. At the same time, the information of the structure and function will be obtained. Among radionuclide imaging devices, positron emission tomography/computed tomography (PET/CT) is more sensitive for the visualization of tumors. But it has not yet been fully popularized in the primary hospitals due to the high cost of positronic tracer. In this way, the use of single-photon radionuclides for research on single photon emission computed tomography/computed tomography (SPECT/CT) scan is extremely necessary.
Fibroblast activation protein inhibitor (FAPI) specifically binds with FAP and can be selectively expressed in FAP-positive tissues. It was previously used as a class of anticancer drugs (3). But now as a novel target, it has been developed to be labeled with nuclides for tumor nuclide imaging and therapy in nuclear medicine. 68Ga-labeled FAPI PET/CT imaging is the most widely used, and has the advantage of evaluation on primary and metastatic foci of a variety of tumors compared with 18F-FDG PET/CT imaging (4, 5). Subsequent studies on 18F/99mTc-labelled FAPI have also confirmed the feasibility of this type of molecular probe for imaging (6, 7). Our group synthesized a novel FAP-targeting SPECT/CT imaging molecular probe 99mTc-HYNIC-FAPI. It has a high radiolabeling rate and good in-vitro stability with specific uptake ability for tumor cells overexpressing FAP (8).
In order to explore the potential of FAPI-based radionuclide therapy, several teams developed therapeutic molecular probes. 177Lu-FAPI (9) has achieved preliminary results in targeted radioligand therapy (TRT) for tumors. Studies on 90Y (10), 64Cu, 225Ac (11), 153Sm (12) labeled FAPI were only case reports, but they showed the effectiveness in tumor therapy with the need of more verifications on their therapeutic efficacy and toxicity. However, the above nuclides are difficult to obtain and expensive and requires special equipment.
As the earliest and widely used single-photon nuclide, 131I emits β and γ rays with greater availability, longer biological half-lives, and greater operability. For primary hospitals, it plays vital roles on disease diagnosis, treatment, and biomedical researches as a labelled nuclide. The selection of 131I will be more conducive to promoting the application of FAPI research in treatment, and has good clinical translation value. However, there were few studies on 131I labeling FAPI (13).
The purpose of this study was to explore a new molecular probe, 131I-FAPI. The stability and lipophilic properties of the probe in vitro were evaluated. The specific binding ability of the probe on tumor cells was verified through cell uptake experiments. The cell proliferation toxicity assays and cell scratch assays were used to confirm the inhibition of the probe on tumor cell proliferation and migration, which laid a foundation for therapeutic assessment based on tumor-bearing models.
2 Materials and methods
2.1 Preparation of the ligand
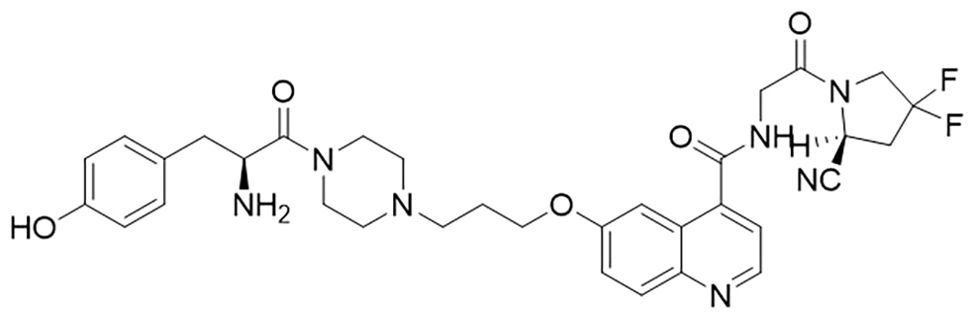
H-Tyr-FAPI ligand was designed and synthesized from Ganzhou Probe Biopharmaceutical Co., Ltd (Jiangxi, China). Figure 1 is the structure of the FAPI, which was modified with tyrosine based on FAPI-04. Its purity was >95% determined by high–performance liquid chromatography (HPLC). The peptide was diluted in phosphate-buffered saline (PBS) at the concentration of 2mg/ml before being stored at -20°C.
2.2 Radiolabeling of FAPI with 131I
In this study, the radiolabeling method was Chloramine T method. 131I and chloramine-T trihydrate and Sodium metabisulfite were obtained from Sichuan CNNC Qualcomm Pharmaceutical Co., Ltd. and Shanghai Macklin Biochemical Co., Ltd., respectively. The varying mass of FAPI (50μg, 60μg, 70μg, 80μg and 90μg), the concentration of Chloramine T (1mg/ml, 1.5mg/ml, 2mg/ml, 2.5mg/ml, 3mg/ml, 4mg/ml and 5mg/ml) and the reaction time (2min, 5min, 10min, 15min and 30min) were used to obtain optimal conditions of radiolabeling.
Thin layer chromatography (TLC) was used for the determination of radiolabeling rate.
2.3 Stability of 131I-FAPI
131I-FAPI was mixed with different medium, 25°C PBS and 37°C fetal calf serum that mimics the human environment. At different time points (0h, 4h, 8h, 24h, 48h, 72h), the radiochemical purity and the stability of the probe were determined with paper chromatography. The results were calculated in GraphPad Prism v9.
2.4 Partition coefficient
The hydrophilicity or lipophilicity of 131I-FAPI was obtained by the following steps. 50µl 131I-FAPI, 500µl PBS and 500µl n-octanol were added into a centrifugal tube. The tube was vibrated on a vortex mixer (Thermo Scientific, USA) for 2 min and then centrifugated at 13000 rpm for 5 min. Samples were separately collected from lipid phase and aqueous phase. The radioactivity was counted with automatic gamma counter (PerkinElmer Instruments Inc., USA).
2.5 Cell culture
The human glioblastoma cell line (U87MG) and the pancreatic cell line (PANC-1) (Suzhou Haixing Biological Technology Co., Ltd, China) were cultivated in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin streptomycin at 37°C under 5% CO2.
2.6 Cell uptake essays
Three groups were divided, including 131I-FAPI group, 131I group and blocking group (FAPI was added 30min before adding 131I-FAPI). U87 cells were seeded in 24-well plates (5×104 cells/well) for 24h to 80%-90% density (3 wells per group). They were incubated in FBS-free DMEM containing the three different agents for another 24h. The medium and cells were separately collected before measuring their radioactivity counts with γ counter. Cell uptake rate=100% × radioactivity counting of intracellular/(intracellular + extracellular). The uptake of 131I-FAPI on PANC-1 cells was verified using the above same process. The experiment was done in triplicate.
2.7 Cell proliferation and cytotoxicity essays
Cells were seeded in 5 96-well plates at the concentration of 8×103 cells per well and cultured for 24 hours. Then the medium was replaced with 100µl FBS-free DMEM containing 131I-FAPI, 131I, FAPI or PBS (3 wells per group). Cells were cultivated for 5 time points, 4h, 8h, 12h, 24h, 48h at 37°C. At each time point, 10µl Cell counting Kit-8 (CCK8) was added into each well. The optical density (OD) at 450 nm was measured after two-hour incubation of medium with CCK8 in the dark place. The survival rate of cells was then calculated in GraphPad Prism v9.
2.8 Cell scratching assay
Cells were seeded in 6-well plates. After being cultivated for 24h, cells were washed, scratched and then incubated in FBS-free DMEM containing 131I-FAPI, 131I, FAPI or PBS for 24h at 37°C. Cell growth was recorded at 0h and 24h after cells were scratched using a 200μl plastic pipette tip.
2.9 Statistical analysis
Data analysis was performed using SPSS (IBM, version 26.0). The quantitative data were presented as mean ± standard deviation (SD). The differences between groups were compared using Student’s t-test and one-way ANOVA. P < 0.05 were considered statistically significant.
3 Results
3.1 Synthesis and radiochemistry
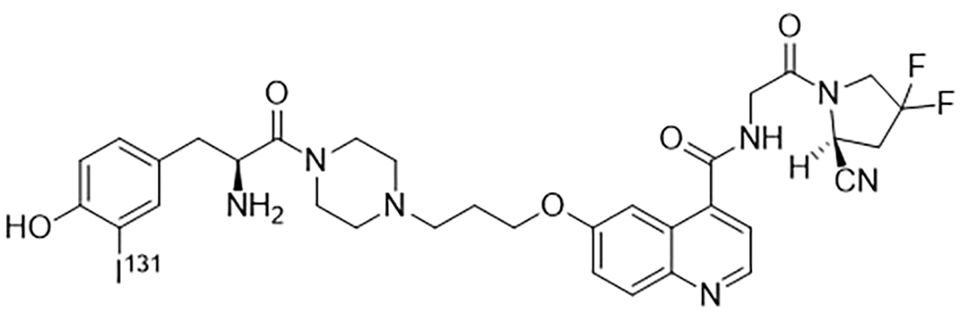
By single variable control method, optimal radiolabeling condition was determined: 37MBq fresh iodine was dropped into 35µl 70µg FAPI solution. Then 100µl 1.5 mg/ml freshly prepared Chloramine T and 50µl PBS were added. The mixture was vortexed for 15 min before the reaction was terminated by 100µl 1mg/ml Sodium metabisulfite. The structure of 131I-FAPI was shown in Figure 2.
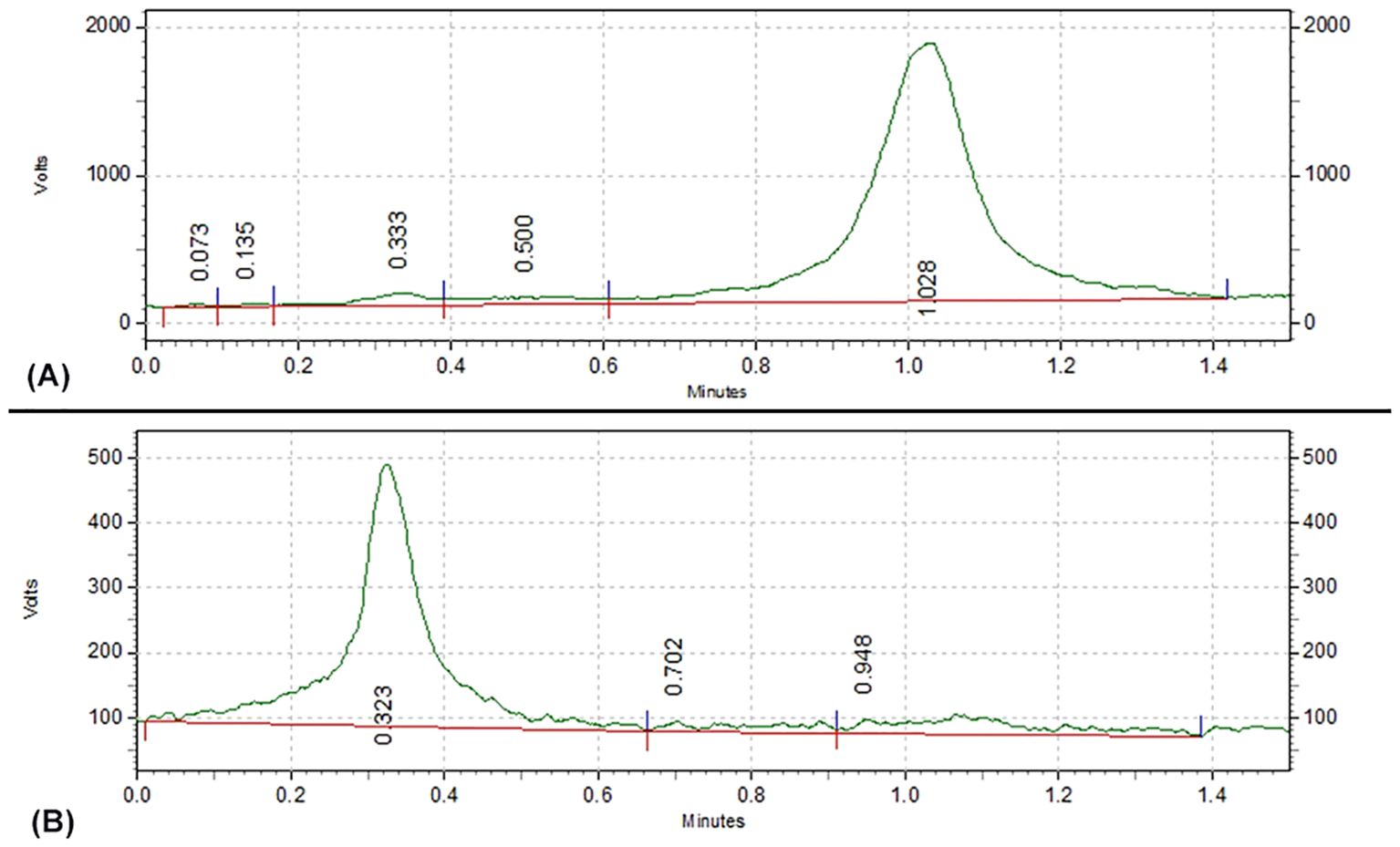
When NaCl was used as the mobile phase, the radiolabeling rate of 131I-FAPI was (84.9 ± 1.02) %. As shown in Figure 3, 131I-FAPI and 131I were expanded at totally different position.
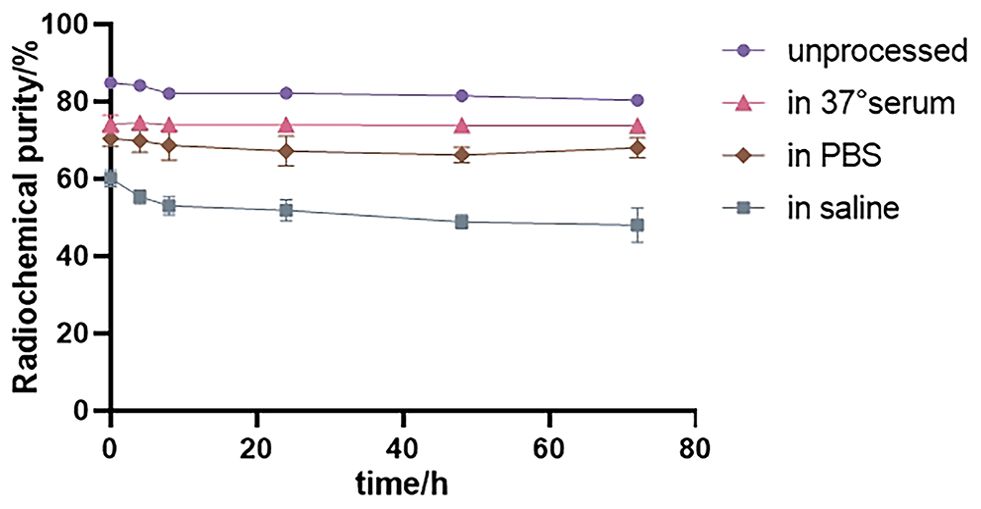
As shown in Figure 4, the radiochemical purity of 131I-FAPI was still more than 80% over time, but it decreased slightly in different environments. It was higher in 37°C serum than in other conditions. In saline, the radiochemical purity was the lowest.
The partition coefficient (log P) value of 131I-FAPI was -0.869 ± 0.025, indicating the hydrophily of the probe.
3.2 Cellular uptake of 131I-FAPI
After 24-hour drug intervention in each group, the uptake rate of U87 cells was the highest in the 131I-FAPI group, which was (1.18 ± 0.29) %. The uptake rates in the blocking group and the 131I group were (0.68 ± 0.26) % and (0.22 ± 0.12) %, respectively. As shown in Figure 5, there was a significant difference between the 131I-FAPI group and the 131I group (P<0.001). For PANC-1 cells, the uptake rates in 131I-FAPI group, blocking group and 131I group were (0.99 ± 0.12)%、(0.84 ± 0.14)% and (0.36 ± 0.12)%, respectively. A statistical difference was only shown in 131I-FAPI group and 131I-FAPI group (P<0.01).

Figure 5. (A) cell uptake rate of U87 cells; (B) cell uptake rate of PANC-1 cells. *, P<0.05; **, P<0.01; ***, P<0.001.
3.3 Inhibition of 131I-FAPI on cell proliferation and migration
After measuring the OD value at 450 nm, we found that in all groups, the viability of U87 cells decreased over time (Figure 6). The inhibition of 131I-FAPI on the cell viability was the highest. In addition, after drug intervene of 12h, 24h, 48h, cell survival rate significantly lowered in 131I-FAPI group compared with 131I group (P<0.01). But for PANC-1 cells, the cell survival rate fluctuated.
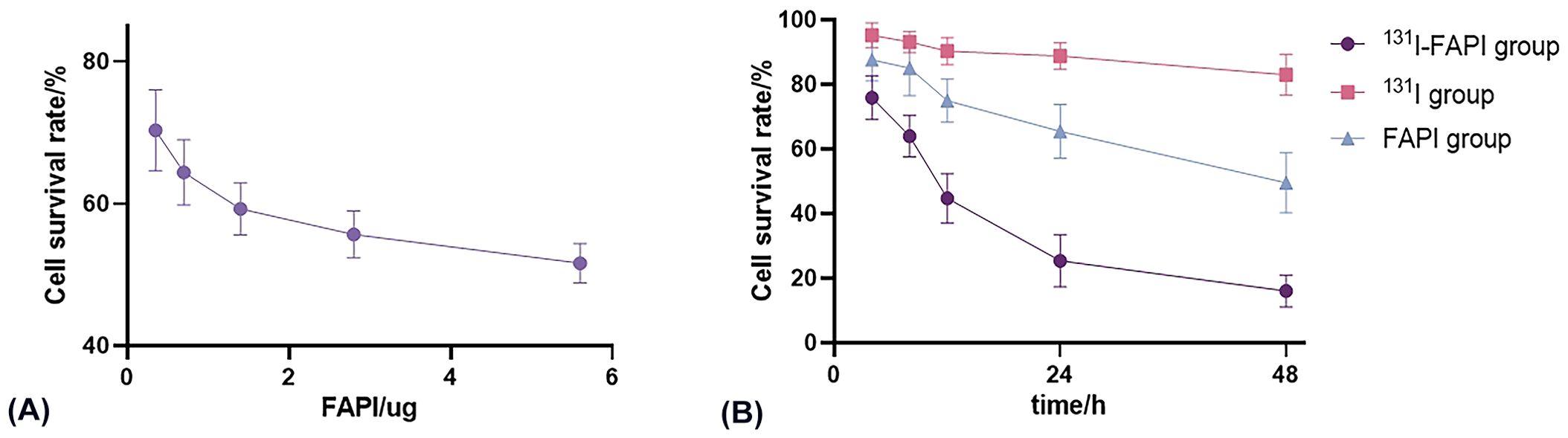
Figure 6. (A) Effect of concentration of FAPI on the viability of U87 cells. (B) Effect of incubation time with 131I-FAPI, 131I, FAPI on the viability of U87 cells.
As shown in Figure 7, on cell scratching assay, cell migration was apparently inhibited on 131I-FAPI with cell migration rate (28.38 ± 7.35) %. There were significant differences in the mobility rate between the 131I-FAPI group and the 131I group ((62.75 ± 3.01) %) or FAPI group ((13.09 ± 3.10) %) the PBS group ((67.02 ± 1.36) %). The migration ability of PANC-1 cells was (29.89 ± 4.52) %, which was significantly lower than that of U87 cells (67.02 ± 1.36) % (P<0.001). For PANC-1 cells, migration rate between 131I-FAPI group and PBS group has statistical difference (P<0.05).
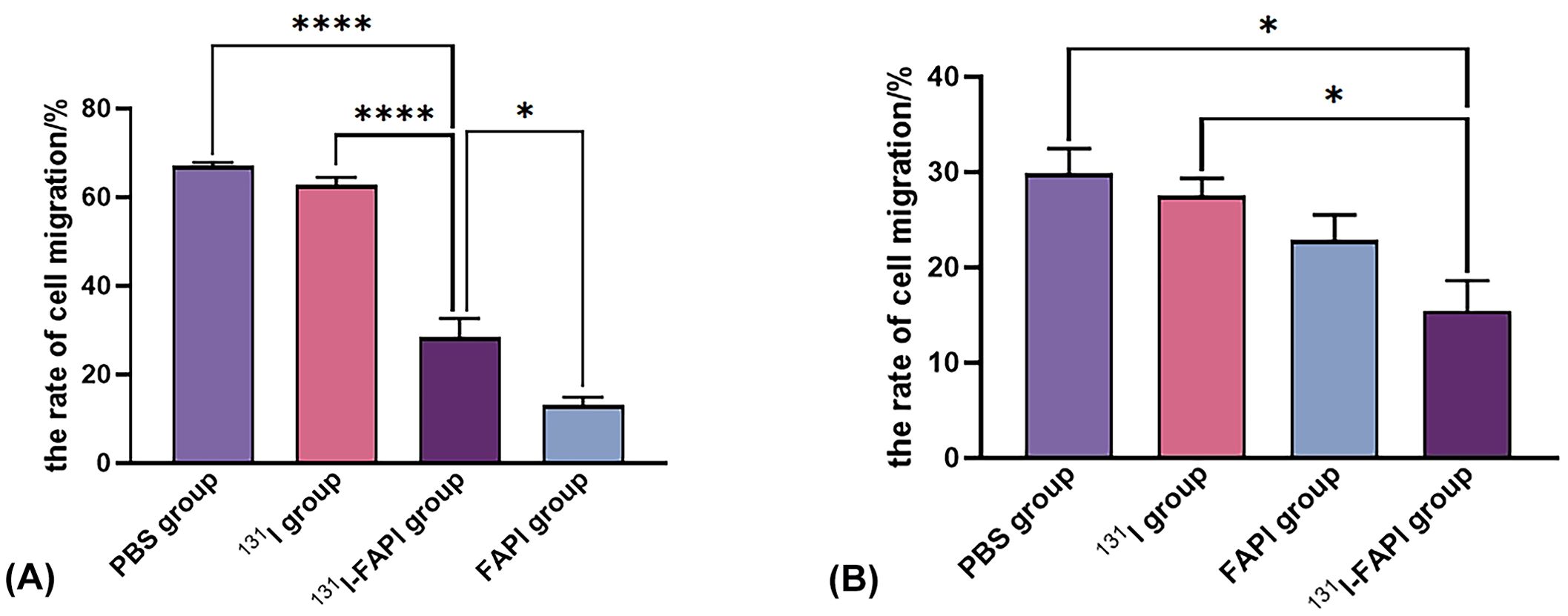
Figure 7. The rate of cell migration on U87 cells (A) and PANC-1 cells (B) after interventions with 131I, 131I-FAPI, FAPI and PBS. *, P<0.05; ****, P<0.0001.
4 Discussion
Tumor microenvironment (TME) plays an important role in tumor development, especially in tumorigenesis and metastasis. Fibroblasts are thought to greatly influence the TME (14). The appearance of cancer-associated fibroblast (CAFs) may be responsible to the crosstalk between tumorigenic cells and fibroblasts (15). CAFs are highly heterogenous and can enhance cell migration, alter the metabolism of epithelial tumor cells (16), promote angiogenic cytokine signal (17, 18), regulate the plasticity of cancer cells (19).
FAP derives from CAFs of tumor stroma. It highly expresses in more than 90% epithelial tumors with low or no expression in begin tumors and normal tissues. Molecular probes targeting FAP could achieve high image contrast and low uptake of non-target tissues. In a word, radiolabeled molecule targeting FAP can be specifically used nuclide imaging and treatment of tumors.
FAPI and its analogues, small molecule inhibitors targeting FAP, have been developed and utilized. Molecular probe targeting FAPI are currently used in clinical practice to diagnose and manage a variety of malignances and the metastases. FAPI labeled with 68Ga and 18F had good diagnostic performances in cancers or sarcoma of head, digestive system, breast and so on. Compared with 18F-FDG, these probes are more sensitive in identifying primary tumors and lymph node, bone and visceral metastases (20–22). 99mTc-FAPI is more accessible due to the low cost of 99mTc, and this type of probes is a promising option when PET imaging is not available (7). A series of evaluations on FAPI variants labeled with therapeutic radionuclides, such as 131I, 90Y, 177Lu and 225Ac have shown great clinical value on targeted therapy of tumors. The evaluations showed that 131I-FAPI-04 and 177Lu-EB-FAPI-B1can significantly inhibit U87 glioma (13, 23), 177Lu-FAPI-46 and 225Ac-FAPI-46/04 had tumor growth inhibition in tumor-bearing models of pancreatic cancer (11, 24). The therapeutic effects were produced through ionizing radiation of radiolabeled FAPIs and resulted in indirect damage to cancer cells adjacent to CAF through crossfire effect (25). Therefore, further research is of great significance to improve the therapeutic efficacy of FAP-targeted radionuclides, including optimizing the chemical structure of FAPI vectors, increasing the dose of therapeutic radionuclides, and combining with other types of therapies (e.g., immunotherapy, external beam radiotherapy, and molecularly targeted therapy).
In this study, tyrosine-modified FAPI was labeled with 131I. 131I-FAPI was identified as having high stability and hydrophily. In addition, it can be accumulated by tumor cells and inhibit the proliferation and migration of tumor cells through in-vitro cell uptake experiments, cell proliferation and cytotoxicity essays and scratching assay. In a word, 131I-FAPI was a promising theranostics molecular probe.
The structure of FAPI-04 was selectively modified to promote reaction with 131I. FAPI-04 has an N-(4-quinolinyl)-Gly-(2-cyanopyrrolidine) structure that exhibits high nanomolar affinity for FAP and high selectivity for dipeptidyl peptidase and prolyl oligopeptidase (26, 27). The characteristics lead to strong accumulation at tumor sites, low uptake in normal tissues and rapid clearance from circulation of FAPI-04 and its derivatives (28, 29), which are the requisite of high-contrast images.
The chloramine T method was used to label FAPI with 131I. The chloramine T method is a commonly direct labeling method. The will-be-used reagents are inexpensive and easy to be obtained, and the steps are highly repeatable. Chloramine T acts as an oxidizing agent and turns the iodine anion into an active iodine molecule. The radiolabeling with 131I relies on oxidation reaction. The tyrosine of peptide is essential in this reaction (the widely used method is Chloramine T method). In the radiolabeling reaction, the iodine anion is turned to an active iodine molecule through an oxidizing agent, Chloramine T. Then the hydrogen at the hydroxyl site of tyrosine phenylphene will be replaced by the iodine molecule, resulting in the formation of carbon-iodine chemical bond. In this study, the following conditions are necessary for stable labeling rate at more than 80%. ① The iodine source is fresh, the activity is 5-10 mCi/50 μl. ② Chloramine T is sealed and stored in the dark, and dissolved when it will be used. ③ The volume of iodination reaction is controlled at 250-350μl. We found that the labeling rate of 131I-FAPI also increased with the increase of FAPI amount. However, the radiochemical purity of the probe after 24h of the labeling was much lower than that of 0 h when FAPI amount greater than 70μg. Therefore, we selected a FAPI amount with a relatively stable labeling rate for subsequent probe synthesis, i.e., 70μg. Under optimal labeling conditions, the radiochemical purity varied in different environments. The labeling rate in normal saline was significantly lower than that in PBS, so in subsequent experiments, we used PBS for the dilution of 131I-FAPI. In the stability test, we found that the probe degraded, because the carbon-iodine chemical bond is not stable and the bond is fragile in different environments.
The lipid-water partition coefficient of the probe was -0.869 ± 0.025, which means that the probe has hydrophilic properties. The result was similar to those FAPIs labeled with 177Lu (30), 68Ga (31) and 99mTc (32) and slightly different from the lipophilic properties of FAPI labeled with 131I (13). In this study, the labeling rate of 131I-FAPI was (84.9% ± 1.02) %. When the lipid-water partition coefficient was determined, the freely unlabeled iodine molecules were dissolved in PBS, which increased the radioactivity count in the aqueous phase and correspondingly decreased the value of logP. The hydrophilicity of 131I-FAPI referred to that the probe was excreted from urinary system and less bound to plasma proteins, which was conducive to further tumor target therapy (13).
In order to evaluate the retention capacity of 131I-FAPI in tumors, we measured the cell uptake of this molecular probe within tumor cells after 24h. U87MG has been used to evaluate the biocharacteristics of FAPIs labeled with 99mTc (32), 18F (33) and 177Lu (30). And U87MG cell lines was proved to be FAP-positive (33). In our study, U87 cells had the highest uptake to 131I-FAPI, which was much greater than that in the blocking group and the 131I group (P<0.05). In blocking group, FAPI can bind to the FAP receptors that are originally bound with 131I-FAPI, which resulted in that the cell uptake rate of blocking group is much lower than that of 131I-FAPI group. The statistically significant differences of cell uptake rate between 131I-FAPI group and any other groups indicated that 131I-FAPI could be specifically accumulated by U87 tumor cells. Compared to 131I-FAPl groups, FAPl group had a higher impact on the migration of u87 cells, we sorted out several factors: Firstly, the uptake of the radiolabeled probe might be different from that of the non-radiolabeled peptide, possibly affecting signaling pathways involved in cell migration. Secondly, it is possible that the FAPl remains higher affinity to its target receptors on U87 cells, thus having a more effect on migration. Thirdly, we also consider the possibility of differences in cell culture conditions and timing, which may contribute to the observed differences. PANC-1 cells are a type of epithelial tumor of human pancreatic cancer and our group previously verified the negative expression of FAP in this type of cell lines (8). 177Lu-FAPI-46 and 225Ac-FAPI-46 could be specifically accumulated by FAP-transfected PANC-1 cells (24). In this study, PANC-1 cells have a certain binding ability to free Na131I, and FAPI failed to block the uptake of 131I-FAPI by receptors, which indirectly indicates that 131I-FAPI has low specificity for PANC-1 cells.
Therapeutic effect of 131I-FAPI on tumors was also speculated in this study. The inhibitory effect of the probe on cell proliferation and migration was verified by CCK8 assay and scratching assay. CCK8 assay showed that the proliferation of U87 cells were significantly inhibited in 131I-FAPI group and the inhibitory effect gradually increased with time. However, we found that the OD value was abnormally increased after 72 h of drug intervention (not listed in the results). We analyzed that cell damage increased with the intervention of reagents over time, then the cell membrane was broken and the. Or the space and nutrition in the wells were no longer enough to support the cells to proliferate, which resulted in the rupture of cells. The above-mentioned damage brought about the drainage of intracellular dehydrogenase that reacted with CCK8 reagent to produce brownish-yellow formazan. The OD values of PANC-1 cells were irregular because that 131I-FAPI was accumulated with low specificity. Cell scratching assay showed that the change of scratching area after 24h incubation was apparently shown in U87 cells. We analyzed that the shape of U87 cells is epithelial cell-like, spindle-shaped with long synapsis, while the shape of PANC-1 cells is oval with limited migration range. The inhibition of 131I-FAPI and FAPI on the migration of U87 cells was also obviously shown, which exhibited the specificity of 131I-FAPI.
In summary, this study synthesized a novel molecular probe targeting FAP. 131I-FAPI has good stability in vitro and has hydrophilic properties. In vitro cell experiments showed that the probe had good targeting properties and inhibitions on tumor cell proliferation and migration.
There are still some shortcomings and limitations in this study, which were mainly reflected in: ① The properties of the probe was only identified at the cellular level. ② Only two tumor cells were used to identify the target specificity of 131I-FAPI. In the future, we will further use other tumor cells and perfect animal experiments in order to develop a therapeutic probe that is more suitable for primary hospitals.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
YT: Conceptualization, Methodology, Project administration, Writing – original draft. YJ: Methodology, Resources, Writing – original draft. PM: Formal analysis, Software, Writing – original draft. XM: Formal analysis, Writing – original draft. LD: Supervision, Writing – original draft. FW: Data curation, Writing – original draft. XY: Visualization, Writing – original draft. QZ: Writing – review & editing, Formal analysis, Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Ningxia Natural Science Foundation (2022AAC05059).
Acknowledgments
This study would not have been possible without the help of all the clinicians participating in our department.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Amend SR, Pienta KJ. Ecology meets cancer biology: the cancer swamp promotes the lethal cancer phenotype. Oncotarget. (2015) 6:9669–78. doi: 10.18632/oncotarget.v6i12
2. Lawrenson K, Grun B, Lee N, Mhawech-Fauceglia P, Kan J, Swenson S, et al. NPPB is a novel candidate biomarker expressed by cancer-associated fibroblasts in epithelial ovarian cancer. Int J Cancer. (2015) 136:1390–401. doi: 10.1002/ijc.29092
3. Poplawski SE, Lai JH, Li Y, Jin Z, Liu Y, Wu W, et al. Identification of selective and potent inhibitors of fibroblast activation protein and prolyl oligopeptidase. J Med Chem. (2013) 56:3467–77. doi: 10.1021/jm400351a
4. Koerber SA, Staudinger F, Kratochwil C, Adeberg S, Haefner MF, Ungerechts G, et al. The role of (68)Ga-FAPI PET/CT for patients with Malignancies of the lower gastrointestinal tract: first clinical experience. J Nucl Med. (2020) 61:1331–6. doi: 10.2967/jnumed.119.237016
5. Liu H, Yang X, Liu L, Qu G, Chen Y. Comparison of 18 F-FDG and 68 ga-FAPI-04 uptake in postoperative re-evaluation of gastric, duodenal, and colorectal cancers. Clin Nucl Med. (2023) 48:304–8. doi: 10.1097/RLU.0000000000004604
6. Hu K, Wang L, Wu H, Huang S, Tian Y, Wang Q, et al. [(18)F]FAPI-42 PET imaging in cancer patients: optimal acquisition time, biodistribution, and comparison with [(68)Ga]Ga-FAPI-04. Eur J Nucl Med Mol Imaging. (2022) 49:2833–43. doi: 10.1007/s00259-021-05646-z
7. Lindner T, Altmann A, Krämer S, Kleist C, Loktev A, Kratochwil C, et al. Design and development of (99m)Tc-labeled FAPI tracers for SPECT imaging and (188)Re therapy. J Nucl Med. (2020) 61:1507–13. doi: 10.2967/jnumed.119.239731
8. Jiang Y, Tian Y, Feng B, Zhao T, Du L, Yu X, et al. A novel molecular imaging probe [(99m)Tc]Tc-HYNIC-FAPI targeting cancer-associated fibroblasts. Sci Rep. (2023) 13:3700. doi: 10.1038/s41598-023-30806-6
9. Eryilmaz K, Kilbas B. Correction to: Fully-automated synthesis of 177Lu labelled FAPI derivatives on the module modular lab-Eazy. EJNMMI Radiopharm Chem. (2021) 6:17. doi: 10.1186/s41181-021-00134-z
10. Ferdinandus J, Costa PF, Kessler L, Weber M, Hirmas N, Kostbade K, et al. Initial clinical experience with (90)Y-FAPI-46 radioligand therapy for advanced-stage solid tumors: A case series of 9 patients. J Nucl Med. (2022) 63:727–34. doi: 10.2967/jnumed.121.262468
11. Watabe T, Liu Y, Kaneda-Nakashima K, Shirakami Y, Lindner T, Ooe K, et al. Theranostics targeting fibroblast activation protein in the tumor stroma: (64)Cu- and (225)Ac-labeled FAPI-04 in pancreatic cancer xenograft mouse models. J Nucl Med. (2020) 61:563–9. doi: 10.2967/jnumed.119.233122
12. Kratochwil C, Giesel FL, Rathke H, Fink R, Dendl K, Debus J, et al. [(153)Sm]Samarium-labeled FAPI-46 radioligand therapy in a patient with lung metastases of a sarcoma. Eur J Nucl Med Mol Imaging. (2021) 48:3011–3. doi: 10.1007/s00259-021-05273-8
13. Ma H, Li F, Shen G, Cai H, Liu W, Lan T, et al. Synthesis and preliminary evaluation of (131)I-labeled FAPI tracers for cancer theranostics. Mol Pharm. (2021) 18:4179–87. doi: 10.1021/acs.molpharmaceut.1c00566
14. Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. (2004) 4:806–13. doi: 10.1038/nrc1456
15. Moore-Smith LD, Isayeva T, Lee JH, Frost A, Ponnazhagan S. Silencing of TGF-β1 in tumor cells impacts MMP-9 in tumor microenvironment. Sci Rep. (2017) 7:8678. doi: 10.1038/s41598-017-09062-y
16. Tommelein J, Verset L, Boterberg T, Demetter P, Bracke M, De Wever O. Cancer-associated fibroblasts connect metastasis-promoting communication in colorectal cancer. Front Oncol. (2015) 5:63. doi: 10.3389/fonc.2015.00063
17. Drebert Z, MacAskill M, Doughty-Shenton D, De Bosscher K, Bracke M, Hadoke PWF, et al. Colon cancer-derived myofibroblasts increase endothelial cell migration by glucocorticoid-sensitive secretion of a pro-migratory factor. Vascul Pharmacol. (2017) 89:19–30. doi: 10.1016/j.vph.2016.10.004
18. Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. (2005) 121:335–48. doi: 10.1016/j.cell.2005.02.034
19. Lau EY, Lo J, Cheng BY, Ma MK, Lee JM, Ng JK, et al. Cancer-Associated Fibroblasts Regulate Tumor-Initiating Cell Plasticity in Hepatocellular Carcinoma through c-Met/FRA1/HEY1 Signaling. Cell Rep. (2016) 15:1175–89. doi: 10.1016/j.celrep.2016.04.019
20. Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. (2020) 47:1820–32. doi: 10.1007/s00259-020-04769-z
21. Yao Y, Tan X, Yin W, Kou Y, Wang X, Jiang X, et al. Performance of (18) F-FAPI PET/CT in assessing glioblastoma before radiotherapy: a pilot study. BMC Med Imaging. (2022) 22:226. doi: 10.1186/s12880-022-00952-w
22. Mu X, Huang X, Jiang Z, Li M, Jia L, Lv Z, et al. [(18)F]FAPI-42 PET/CT in differentiated thyroid cancer: diagnostic performance, uptake values, and comparison with 2-[(18)F]FDG PET/CT. Eur J Nucl Med Mol Imaging. (2023) 50:1205–15. doi: 10.1007/s00259-022-06067-2
23. Wen X, Xu P, Shi M, Liu J, Zeng X, Zhang Y, et al. Evans blue-modified radiolabeled fibroblast activation protein inhibitor as long-acting cancer therapeutics. Theranostics. (2022) 12:422–33. doi: 10.7150/thno.68182
24. Liu Y, Watabe T, Kaneda-Nakashima K, Shirakami Y, Naka S, Ooe K, et al. Fibroblast activation protein targeted therapy using [(177)Lu]FAPI-46 compared with [(225)Ac]FAPI-46 in a pancreatic cancer model. Eur J Nucl Med Mol Imaging. (2022) 49:871–80. doi: 10.1007/s00259-021-05554-2
25. Calais J. FAP: the next billion dollar nuclear theranostics target? J Nucl Med. (2020) 61:163–5. doi: 10.2967/jnumed.119.241232
26. Jansen K, Heirbaut L, Verkerk R, Cheng JD, Joossens J, Cos P, et al. Extended structure-activity relationship and pharmacokinetic investigation of (4-quinolinoyl)glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J Med Chem. (2014) 57:3053–74. doi: 10.1021/jm500031w
27. Jansen K, Heirbaut L, Cheng JD, Joossens J, Ryabtsova O, Cos P, et al. Selective inhibitors of fibroblast activation protein (FAP) with a (4-quinolinoyl)-glycyl-2-cyanopyrrolidine scaffold. ACS Med Chem Lett. (2013) 4:491–6. doi: 10.1021/ml300410d
28. Pang Y, Zhao L, Luo Z, Hao B, Wu H, Lin Q, et al. Comparison of (68)Ga-FAPI and (18)F-FDG uptake in gastric, duodenal, and colorectal cancers. Radiology. (2021) 298:393–402. doi: 10.1148/radiol.2020203275
29. Ruan P, Tao Z, Tan A. Low expression of miR-30a-5p induced the proliferation and invasion of oral cancer via promoting the expression of FAP. Biosci Rep. (2018) 38:BSR20171027. doi: 10.1042/BSR20171027
30. Li H, Ye S, Li L, Zhong J, Yan Q, Zhong Y, et al. (18)F- or (177)Lu-labeled bivalent ligand of fibroblast activation protein with high tumor uptake and retention. Eur J Nucl Med Mol Imaging. (2022) 49:2705–15. doi: 10.1007/s00259-022-05757-1
31. Zhong X, Guo J, Han X, Wu W, Yang R, Zhang J, et al. Synthesis and preclinical evaluation of a novel FAPI-04 dimer for cancer theranostics. Mol Pharm. (2023) 20:2402–14. doi: 10.1021/acs.molpharmaceut.2c00965
32. Ruan Q, Feng J, Jiang Y, Zhang X, Duan X, Wang Q, et al. Preparation and bioevaluation of (99m)Tc-labeled FAP inhibitors as tumor radiotracers to target the fibroblast activation protein. Mol Pharm. (2022) 19:160–71. doi: 10.1021/acs.molpharmaceut.1c00712
Keywords: cancer-associated fibroblasts, FAPI, molecular probe, 131I, identification
Citation: Tian Y, Jiang Y, Ma P, Ma X, Du L, Wang F, Yu X and Zhao Q (2024) Radiosynthesis and in-vitro identification of a molecular probe 131I-FAPI targeting cancer-associated fibroblasts. Front. Oncol. 14:1442601. doi: 10.3389/fonc.2024.1442601
Received: 02 June 2024; Accepted: 15 August 2024;
Published: 30 August 2024.
Edited by:
Iju Yeh, Indiana University, United StatesReviewed by:
Shaobo Yao, First Affiliated Hospital of Fujian Medical University, ChinaSukhen Ghosh, University of Texas Health Science Center at Houston, United States
Copyright © 2024 Tian, Jiang, Ma, Ma, Du, Wang, Yu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Zhao, Y2VjaWxpYV9oaEAxMjYuY29t
 Yaxin Tian
Yaxin Tian Yanghongyan Jiang
Yanghongyan Jiang Ping Ma1
Ping Ma1 Qian Zhao
Qian Zhao