
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 06 November 2024
Sec. Cancer Molecular Targets and Therapeutics
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1441025
This article is part of the Research Topic Advances in the Management of Lung Cancer: From the Bench to the Bedside and Back View all 13 articles
Background: Non-small cell lung cancer (NSCLC) with human epidermal growth factor receptor 2 (HER2) alterations poses a substantial treatment challenge. Current HER2-targeted therapies offer limited efficacy. Antibody-drug conjugates (ADCs) targeting HER2 have emerged as a promising therapeutic strategy. This study aimed to evaluate the clinical response to a novel ADC drug Disitamab vedotin (RC48) in advanced NSCLC with HER2 alterations.
Methods: This study conducted a retrospective review of patients harboring HER2 alterations treated with RC48 in the real world. Clinical outcomes were evaluated in terms of objective response rate (ORR), disease control rate (DCR), and progression-free survival (PFS).
Results: Out of 22 patients, 21 (95.5%) received RC48 combination therapy, while one received RC48 monotherapy. The ORR of all patients reached 45.5%, and the DCR stood at 90.9%. The median PFS (mPFS) was 7.5 months. Among patients receiving RC48 combination therapy, the ORR was 47.7%, and the mPFS of 8.1 months. The combination of RC48 with platinum+/- bevacizumab resulted in the highest ORR of 71.4% (5 out of 7 patients), with HER2 TKI following at a 50.0% ORR (4 out of 8 patients). First-line (1L) treatment with RC48 showed an ORR of 62.5% (5 out of 8 patients), second-line (2L) treatments had a 57.1% ORR (4 out of 7 patients), and beyond second-line (>2L) treatments exhibited a 14.3% ORR (1 out of 7 patients). Patients with 1L, 2L, or >2L treatment had a mPFS of 8.1 months, 7.2 months, and 7.4 months, respectively. Patients with HER2 mutations or amplifications, and those with concurrent mutations and amplifications at baseline, showed mPFS of 8.1 months, 9.4 months, and 7.4 months, respectively. The mPFS was significantly longer in patients with HER2 amplification. The most common adverse events included hand-foot syndrome (54.5%), asthenia (50.0%), decreased white blood cell count (45.5%), and liver impairment (45.5%). Grade 3 adverse events occurred in one (4.5%) patient.
Conclusion: RC48, particularly in combination regimens, demonstrates promising efficacy in advanced NSCLC with HER2 alterations. These findings underscore the need for further research to validate RC48’s application in clinical practice.
Non-small cell lung cancer (NSCLC) with human epidermal growth factor receptor 2 (HER2) alterations mainly manifest as protein overexpression, gene amplification, or gene mutation (1–3). HER2 mutations are found in 1-4% of NSCLC and amplifications are found in 2–5% of cases (4, 5). In comparison to other oncogenic drivers, HER2 is a distinctive molecular with a poor prognosis (3, 6). The standard first-line treatment for advanced NSCLC with HER2 alterations, immune checkpoint inhibitor (ICI) therapy, has shown limited clinical activity with an objective response rate (ORR) ranging from 7.4% to 27.3% and median progression-free survival (mPFS) ranging from 1.9 to 2.5 months (7). Tyrosine kinase inhibitors (TKIs) are transformative agents for the treatment of NSCLC, especially in terms of epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK). However, HER2-targeted TKIs such as afatinib (8, 9), poziotinib (10, 11), and pyrotinib (12) had moderate efficacy as second- or later-line therapies, with ORRs of 19–30% and mPFS 4.0-6.9 months.
Regarding HER2-targeted monoclonal antibodies, previous studies have mostly focused on NSCLC with HER2 protein over-expression but they have shown limited efficacy (13–15). Antibody-drug conjugates (ADCs), consisting of a monoclonal antibody (mAb) carrying a high-activity cytotoxic drug (payload) via a chemical linker, are one of the fastest growing oncology therapeutics, and are now one of the potential options for lung cancer patients (16, 17). Currently, HER2 ADCs such as trastuzumab deruxtecan (T-DXd) and ado-trastuzumab emtansine (T-DM1) have shown considerable clinical benefits. Both agents have been recommended as options for HER2-mutant NSCLC after progressing with standard treatment by the National Comprehensive Cancer Network (NCCN) guidelines (18). A phase II basket trial of T-DM1 showed an ORR of 44% and mPFS of 5 months in 18 patients with advanced HER2-mutant NSCLC patients (19). Another clinical trial reported a 51% ORR for T-DM1 in 49 patients with HER2-amplified or -mutant lung cancers (20). However, the efficacy of T-DM1 has not been validated in large-scale samples and has not been approved by the Food and Drug Administration (FDA). The pivotal DESTINY-Lung 02 trial of T-DXd reported a 49% ORR, 9.9 months mPFS, and 19.5 months median overall survival (mOS) in HER2-mutant NSCLC (21). Based on this data, the FDA approved 5.4mg/kg T-DXd for the treatment of HER2-mutant locally advanced or metastasis NSCLC in August 2022. Nevertheless, 13% of patients treated with T-DXd developed adjudicated drug-related interstitial lung disease (2.0% grade >3), and one patient developed ILD at grade 5, which limits its widespread use in NSCLC patients.
Disitamab vedotin (RC48) emerges as an innovative therapeutic agent, consisting of a humanized anti-HER2 antibody linked to monomethyl auristatin E (MMAE) via a cleavable linker (22). The National Medical Products Administration of China (NMPA) has approved RC48 for patients with HER2-overexpressing metastatic gastric cancer/gastroesophageal junction (G/GEJ) adenocarcinoma after >2L of treatment, and HER2 IHC2+/3+ metastatic urothelial carcinoma post-platinum-based therapy. To date, RC48 has demonstrated promising antitumor activity and a manageable safety profile in clinical applications.
The purpose of this study is to explore the efficacy and safety of RC48 with unresectable locally advanced or metastatic NSCLC patients harboring HER2 mutations or amplifications.
We conducted a retrospective observational study at The First Affiliated Hospital of Nanjing Medical University (Jiangsu Provincial People’s Hospital) and Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & The Affiliated Cancer Hospital of Nanjing Medical University, from August 2021 to March 2023. Patients over 18, diagnosed pathologically with NSCLC of unresectable, locally advanced, or metastatic stage, and confirmed to have HER2 mutations or amplifications via PCR or NGS, were included. Data cutoff date of July 30th, 2023. Our investigation included a comprehensive review of clinicopathological characteristics, encompassing demographic data, smoking status, ECOG-PS score, cancer stage, and histological type, along with HER2 genomic alteration status. The specifics of the treatment combination therapies, such as the dosage, treatment cycles, and duration, were documented. Ethical approvals were obtained in Ethical Committees from both institutions.
Of the 40 patients initially screened, patients with incomplete medical records, lacking follow-up, or without documented HER2 genomic status were excluded. Eventually 22 eligible cases were enrolled in this study.
Anonymized data were evaluated for clinicopathologic characteristics and outcomes for RC48 treatment, focusing on ORR, disease control rate (DCR), and PFS. Objective responses were evaluated based on Response Evaluation Criteria in Solid Tumors (RECIST, v1.1), where ORR was defined as the percentage of patients achieving either a complete response (CR) or partial response (PR) to the treatment. DCR was calculated as the proportion of patients exhibiting a CR, PR, or stable disease (SD). PFS was defined as the duration from the onset of treatment to the occurrence of disease progression or death from any cause. Patients experiencing relapse within six months post-systemic anticancer therapy were subsequently classified as receiving second-line treatment for their advanced disease.
For continuous variables, medians and ranges were used to summarize the demographic and clinical characteristics of patients, whereas for categorical variables, frequencies and percentages were used to describe them. The Kaplan-Meier method was employed to analyze survival outcomes. To investigate the impact of different treatments on PFS among various patient subgroups, univariate analyses were conducted. The log-rank test was employed to assess the significance of differences in PFS, with a threshold of P < 0.05 for statistical significance. All analyses were performed using R software (version 3.5.1).
A total of 22 patients with HER2-altered NSCLC receiving monotherapy or combination therapy with RC48 were enrolled from August 2021 and March 2023. A significant majority, representing 90.9% (20 out of 22), had adenocarcinoma histologically. Only a single patient (4.5%) was treated with RC48 as a monotherapy, whereas the remaining 21 (95.5%) received combination therapies. Detailed therapeutic regimens included 8 patients with TKIs, 7 with platinum with or without bevacizumab (3 only with platinum, 4 with platinum combined with bevacizumab), 4 with antiangiogenic drugs, 2 with PD-(L)1 inhibitor with or without bevacizumab. Further demographic and clinical characteristics of the patients are shown in Table 1. Notably, eight (36.4%) patients received RC48 as 1L treatment, while 2L or >2L treatments were received by 7 (31.8%) each. Molecular profiling performed at baseline disclosed 15 patients with HER2 mutation, 5 with HER2 amplifications, and 2 harboring both mutation and amplification simultaneously. Brain metastases were observed in 31.8% of the patients.
Of the 22 patients, 10 (45.5%) patients achieved PR, and 10 (45.5%) patients showed SD, with a confirmed investigator-assessed ORR of 45.5% (10 out of 22) and a DCR of 90.9% (20 out of 22). A waterfall plot for the best percentage change in target lesion size is shown in Figure 1. At the time of data cut-off, survival analysis was conducted on all 22 patients, with the mPFS of 7.5 months (95% CI, 6.6-8.4 months) and the estimated 6-month PFS rate and 12-month PFS rate of 77.9% and 24.4%, respectively (Figure 2A).
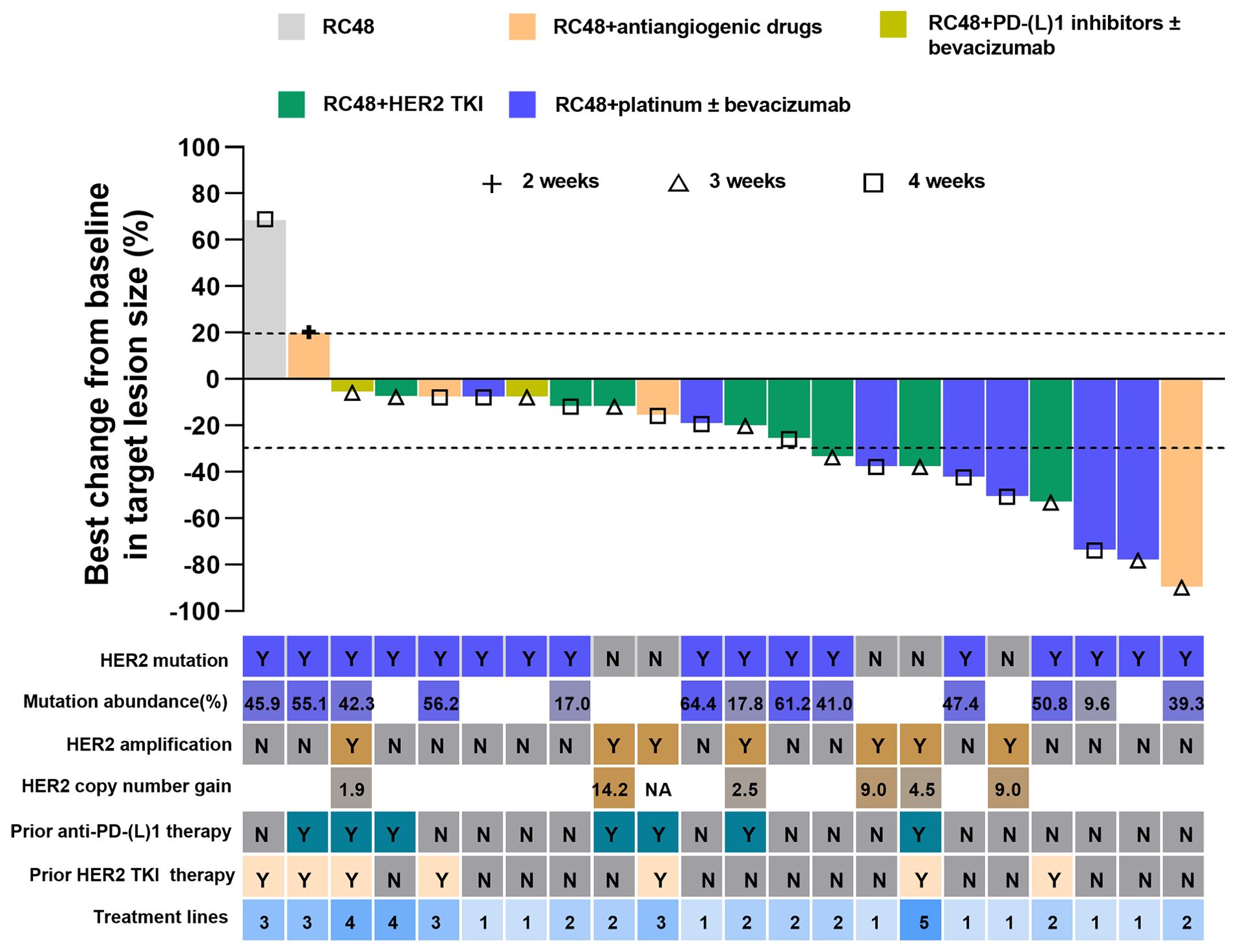
Figure 1. Best change from baseline in target lesion size by each patient. The line at -30% indicates a partial response. Alphabet in the HER2 mutation row or HER2 amplification row indicate status. Y, yes; N, no; HER2, human epidermal growth factor receptor 2; TKI, tyrosine kinase inhibitor; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand1; CI, Confidence interval.
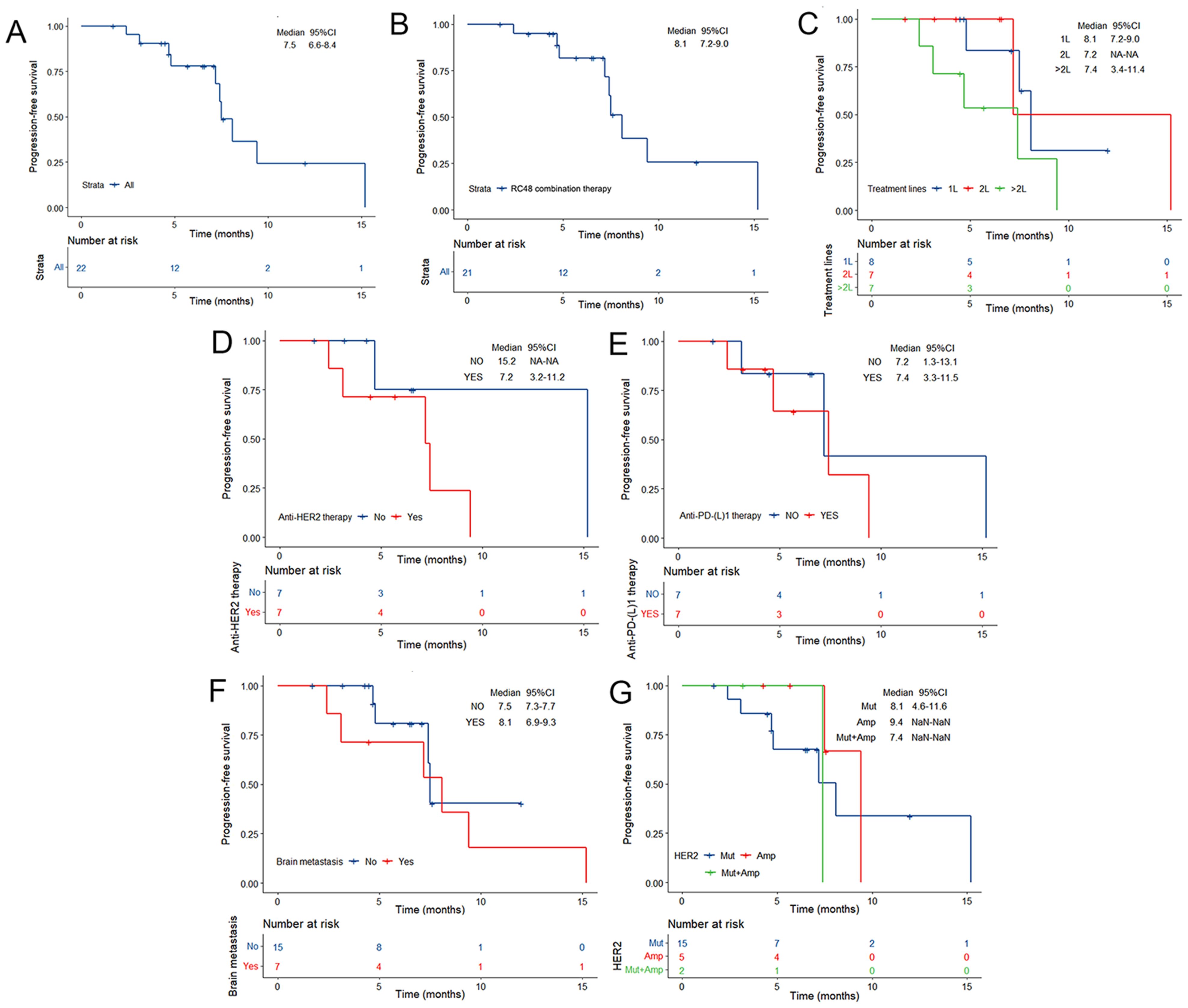
Figure 2. Kaplan–Meier estimates of PFS according to (A) the overall NSCLC population, (B) RC48 combination therapy, (C) RC48 treatment line, (D) prior anti-HER2 therapy, (E) prior anti-PD-(L)1 therapy, (F) brain metastases and (G) HER2 alteration status at baseline. TKI, tyrosine kinase inhibitor; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand1; CI, Confidence interval. Mut, mutation; Amp, amplification; Mut+ Amp, concurrent mutation and amplification; HER, human epidermal growth factor receptor 2; CI, Confidence interval.
Of note, the efficacy of the RC48 combination treatment group showed better performance when compared with monotherapy (Table 2). The mPFS of patients who received RC48 combination therapy was 8.1 months (95% CI, 7.2-9.0 months; Figure 2B). The subgroup receiving RC48 in combination with platinum-based chemotherapy (with or without bevacizumab) achieved an impressive ORR of 71.4% (95%CI: 29.0-96.3%) and the mPFS was not reached. As shown in Table 2, the group of patients treated with RC48 plus HER2 TKIs achieved a favorable outcome with an ORR of 50.0% (95%CI: 15.7-84.3%) and a mPFS of 7.2 months (95% CI, 3.6-10.8 months; Supplementary Figure S1). Patients receiving RC48 as a first-line treatment (n=8) showed the best efficacy, with an ORR of 62.5% (95% CI, 24.5-91.5%) and a mPFS of 8.1 months (95% CI,7.2-9.0 months). Patients undergoing second-line treatment (n=7) achieved an ORR of 57.1% (95% CI, 18.4-90.1%), and a mPFS of 7.2 months (95% CI, NA-NA). Patients in the >2L treatment group (n=7) had a mPFS of 7.4 months (95% CI, 3.4-11.4 months), although showing a lower ORR of 14.3% (0.4-57.9%), (Table 2, Figures 2C, 3).
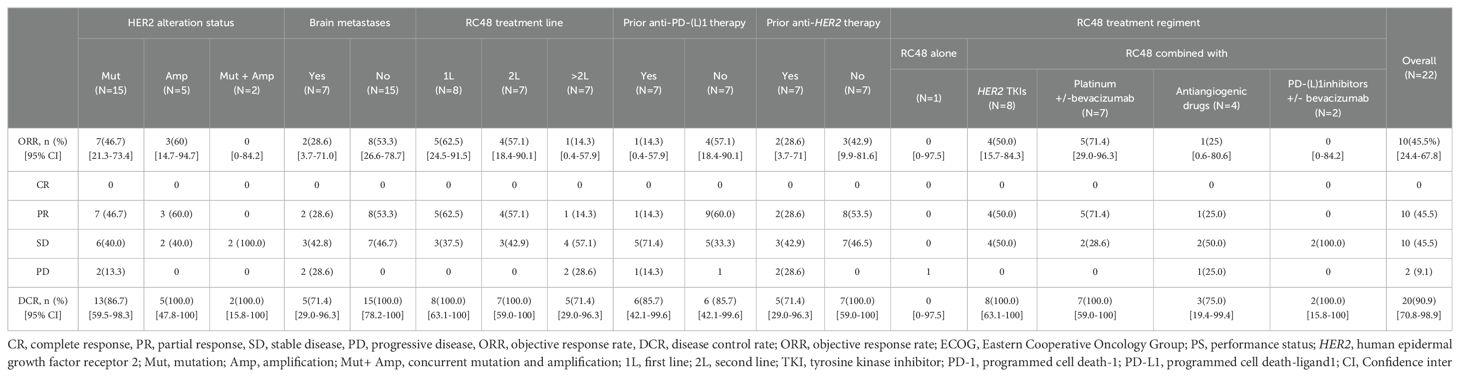
Table 2. Clinical response to RC48 in the overall HER2 alterations NSCLC population and subgroups population.
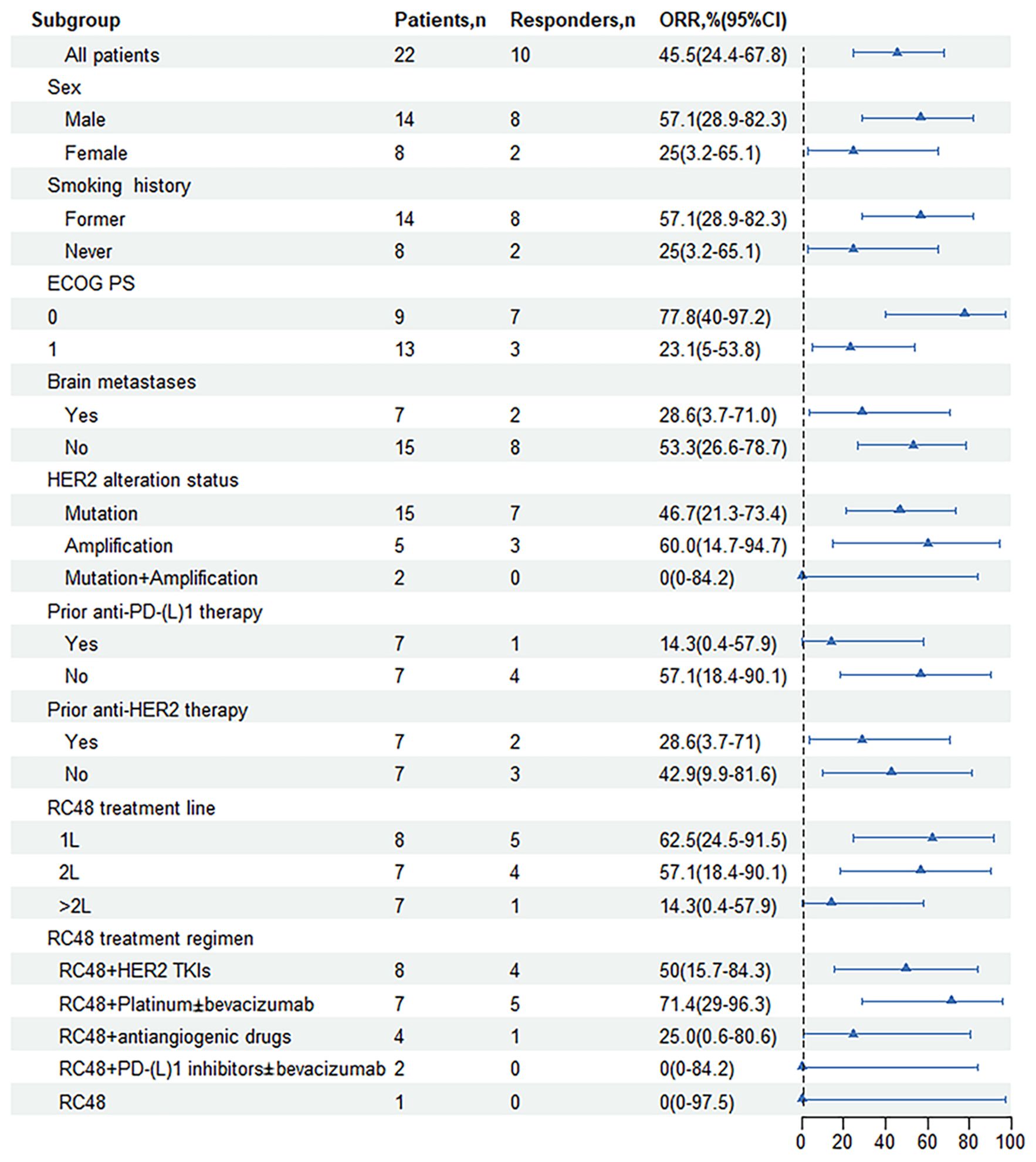
Figure 3. Forest plot of subgroup analysis of objective response rates by baseline demographic and disease characteristics. ORR, objective response rate; ECOG, Eastern Cooperative Oncology Group; PS, performance status; HER2, human epidermal growth factor receptor 2; Mutation + Amplification, concurrent mutation and amplification; 1L, first line; 2L, second line; TKI, tyrosine kinase inhibitor; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand1; CI, Confidence interval.
Patients with prior anti-HER2 therapy (n=7) responded to subsequent RC48-based anti-HER2 treatment with an ORR of 28.6% (95% CI, 3.7-71%) and the mPFS of 7.2 months (95% CI, 3.2-11.2 months; Figure 2D). Among those previously treated with anti-PD-(L)1 inhibitors (n=7), the median treatment line was 3.5 (2-5 line), the ORR was 14.3% (95% CI, 0.4-57.9%), and the mPFS was 7.4 months (95% CI, 3.3-11.5 months; Figures 2E, 3).
In patients present with baseline brain metastases, the ORR was 28.6% (95% CI, 3.7-71.0%), the DCR was 71.4% (95% CI, 29.0-96.3%; Table 2). The mPFS was 8.1 months (95% CI, 6.9-9.3 months) for patients presenting with baseline brain metastases, compared to 7.5 months (95% CI, 7.3-7.7 months) for those without baseline brain metastases. This comparison revealed no significant difference in mPFS between the two groups (P=0.503; Figures 2F, 3).
Among NSCLC patients harboring HER2 mutations (n=15), the RC48 treatment regimen had an ORR of 46.7% and a DCR of 86.7%. The HER2-amplified subgroup (n=5) showed an ORR of 60.0% and a DCR of 100.0%. In rare cases of concurrent amplification and mutation, the DCR reached 100.0% in both patients (Table 2, Figure 3). The mPFS of patients with HER2 mutations, amplifications, and concurrent HER2 mutation and amplification was 8.1 months (95% CI, 4.6-11.6 months), 9.4 months (95% CI, NA-NA) and 7.4 months (95% CI, NA-NA), respectively (Figure 2G). Median PFS was significantly prolonged in HER2-amplified patients, and no significant difference in mPFS was observed (P=0.73).
The duration of RC48 treatment ranged from 2 to 19 months with a median treatment period of 5.5 months. Importantly, none of the patients were found to have reduced or discontinued their medication due to side effects during treatment. Adverse events are detailed in Table 3. All patients reported at least one AE. The most common adverse events included hand-foot syndrome (54.5%), asthenia (50.0%), decreased white blood cell count (45.5%), and liver impairment (45.5%). Grade 3 adverse events occurred in one (4.5%) patient.
HER2-targeted therapeutics have shown favorable antitumor efficacy, including tyrosine kinase inhibitors (afatinib, lapatinib, neratinib, and tucatinib), monoclonal antibodies (mAbs) (trastuzumab, pertuzumab, initumumab), and bispecific antibodies (23). mAbs precisely targets tumor surface antigens. However, its clinical efficacy is often inadequate because its lethality against cancer cells is not inadequate when using mAbs alone. ADC drugs are monoclonal antibodies loaded with a small toxin molecule which specifically targeting cancer cells and then produce a potent toxic effect. ADC drugs make up for the limitations of HER2-targeted therapies. Moreover, the ability to exert cytotoxic activity against antigen-negative cells of ADC drugs, also called the bystander effect, allows to overcome tumor heterogeneity (24). RC48 is a novel ADC drug comprised of disitamab coupling with the cytotoxic agent MMAE via a cleavable linker. It was well tolerated and showed promising efficacy in several HER2-positive cancers such as breast cancer (25), gastric cancer (26), and urothelial carcinoma (27).
To our knowledge, this is the first study conducted in a real-world setting to report the efficacy and safety of RC48 combination therapy in patients with advanced HER2-altered NSCLC. Our findings reveal that RC48 therapy yield a favorable clinical response with an ORR of 45.5%, a DCR of 90.9%, and a mPFS of 7.5 months among HER2-altered NSCLC. The combination therapy, particularly, showed enhanced effectiveness with an ORR of 47.6% and a mPFS of 8.1 months, underscoring the significant clinical benefits RC48 may offer to patients with HER2-altered NSCLC.
In our study, we observed that the combination therapy (RC48 with platinum-based chemotherapy, with or without bevacizumab) showed an encouraging ORR of 71.4%. Chemotherapy, as cytotoxic partners of ADCs, can not only interfere with the cell cycle but also modulate the expression of surface antigen targeted by ADCs. Platinum agents, which cause S phase cell cycle arrest and subsequent G2/M phase accumulation, seem to have a synergistic effect with microtubule inhibitors like MMAE within RC48 (28). This combination potential has also been illustrated by carboplatin with mirvetuximb soravtansine (Folate receptor (FR)a-DM4) (29). Furthermore, the well-balanced DAR design of 4 in RC48 also demonstrated a milder toxicity, making it an appealing and flexible companion of platinum in clinical settings (28, 30). As platinum-based chemotherapy still plays a fundamental role in NSCLC treatment, ADCs have the potential to enable the development of highly potent and safe combinations by replacing cytotoxic regimens based on a better understanding of mechanisms. Moreover, antiangiogenic agents may synergistically enhance the delivery of ADCs to tumor cells by normalizing the vasculature and improving treatment sensitivity (6, 31). The combination of anetumab ravtansine with bevacizumab has shown potent effects in ovarian cancer model (32). However, as far as we know, such a combination design has not been tested in clinical trials in NSCLC. Therefore, our data provides real-world evidence for future ADC clinical trial designs of similar combination schemes with antiangiogenesis in NSCLC.
The efficacy of RC48 in combination with HER2-TKIs also merits attention, with an ORR of 50.0% and a mPFS of 7.2 months observed in our study. The addition of HER2-ADC to pan-HER irreversible inhibitor HER2-TKIs, predominantly pyrotinib in our study, demonstrates synergistic efficacy in terms of ORR. In previous studies, pyrotinib monotherapy was shown to have an ORR of 30% as well as an mPFS of 6.9 months in HER2-altered NSCLC (12, 33). Co-administration of these agents may enhance the internalization of HER2-ADC, eliciting robust antitumor activity. Sub-therapeutic doses of TKI could be adequate for enhancing ADC-dependent cell death and tumor shrinkage, thereby reducing the adverse effects associated with the daily use of these agents (20). A concern with pyrotinib is its toxicity, which limits its clinical dosage. The most common TRAE observed with a dose of 400 mg of pyrotinib was diarrhea (92.6%), and the severity was positively in line with the dosage (20, 34). In our study, seven patients received pyrotinib, which was initiated at a low dose of 240 mg, and the dose was increased to 320 mg if no adverse reactions were observed. This combined regimen showed a manageable safety profile with discrete dose management based on patient tolerance. These data also suggest that the combination of RC48 with pyrotinib may be a promising therapeutic approach for HER2-altered NSCLC and warrants further comprehensive clinical evaluation.
Our study also differentiated the efficacy of RC48 among various HER2 alterations and slight differences in efficacy were observed. For HER2-mutant NSCLC, the combination treatments exhibited an ORR of 46.7% and a median PFS of 8.1 months, comparable to current HER2 ADCs like T-DXd and T-DM1 (19, 21). Those data suggest that RC48 presents a potential treatment option in patients with HER2-mutated NSCLC. In cases of HER2 amplification, RC48 combination strategies showed promising results, with an ORR of 60.0% and a mPFS of 9.4 months. A preclinical study suggests that T-DXd could effectively inhibit the proliferation of HER2-amplified cells in vitro and in vivo (35). Other anti-HER2 therapies include HER2-amplified NSCLC patients, such as T-DM1, which shows an ORR of 55% in 14 HER2-amplified patients enrolled in a phase II basket trial and pyrotinib, which also showed an ORR of 22.2% and a mPFS of 6.3 months in 22 patients (20, 34). These results suggest that HER2 amplification may also be a target for anti-HER2 therapy in NSCLC. However, there still requires large sample size research to prospectively identify optimal amplification cut-off value to target patients who can benefit most from anti-HER2 therapies.
It is important to note that there was no statistically significant differences in our results, particularly in the mPFS comparisons between patients with and without baseline brain metastases and among different HER2 alteration subgroups. It might be because the small sample size reduces the statistical power and may not represent a broader patient population
There are several limitations in this study. First, the retrospective nature of the study makes bias inevitable, and prospective studies are needed to validate these results. Second, although this study provided a comprehensive evaluation of all available treatment options and RC48 showed excellent antitumor activity in HER2-altered NSCLC, the small sample size reduces the statistical power and caution should be exercised in interpreting these results. Thirdly, this study was conducted during the COVID-19 pandemic, which may also have influenced outcomes, including delays in patient’s access to medical care, and delays in RC48 treatment. At last, the retrospective nature of the study may result in underreporting or recall bias in reporting AEs. Therefore, future prospective studies with more rigorous safety monitoring are needed to provide a more comprehensive understanding of the safety profile of RC48.
Despite the small sample size, this investigation introduces a viable therapeutic alternative for patients with advanced HER2-altered NSCLC, particularly through a regimen incorporating RC48 in conjunction with platinum-based chemotherapy, with or without bevacizumab. RC48-based therapies pave the way for new treatment in the case of HER2-amplified patients. Overall, the safety profile was well tolerated, and no dose reduction or discontinuation of treatment was found due to side effects. However, further studies with larger sample sizes are needed to confirm these preliminary findings. Future research on HER2-targeted ADCs should primarily focus on combination treatment strategies with other treatment modalities, to further improve patients’ outcomes.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by both the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (Jiangsu Provincial People’s Hospital) and the Ethics Committee of Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & The Affiliated Cancer Hospital of Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this retrospective study did not involve direct intervention or interaction with study participants.
MZ: Data curation, Formal analysis, Investigation, Writing – original draft. LW: Data curation, Formal analysis, Investigation, Writing – original draft. QW: Data curation, Formal analysis, Investigation, Writing – original draft. JY: Methodology, Supervision, Visualization, Writing – review & editing. WP: Methodology, Supervision, Visualization, Writing – review & editing. XL: Writing – review & editing, Resources. MS: Conceptualization, Methodology, Writing – review & editing, Funding acquisition. KL: Conceptualization, Methodology, Writing – review & editing, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant No. 82172708).
The authors would like to thank the patients and their families, clinical researchers, and their teams and hospitals that have participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1441025/full#supplementary-material
ADC, antibody-drug conjugate; ALK, anaplastic lymphoma kinase; CR, complete response; DCR, disease control rate; EGFR, epidermal growth factor receptor; FDA, Food and Drug Administration; G/GEJ, gastric cancer/gastroesophageal junction; HER2, human epidermal growth factor receptor 2; ICI, immune checkpoint inhibitor; mAb, monoclonal antibody; MMAE, monomethyl auristatin E; mOS, median overall survival; mPFS, median progression-free survival; NCCN, National Comprehensive Cancer Network; NMPA, National Medical Products Administration of China; NSCLC, non-small cell lung cancer; ORR, objective response rate; PR, partial response; RC48, disitamab vedotin; SD, stable disease; T-DM1, ado-trastuzumab emtansine; T-DXd, trastuzumab deruxtecan; TKIs, tyrosine kinase inhibitors.
1. Passaro A, Jänne PA, Peters S. Antibody-drug conjugates in lung cancer: Recent advances and implementing strategies. J Clin Oncol. (2023) 41:3747–61. doi: 10.1200/jco.23.00013
2. Song Z, Yu X, Shi Z, Zhao J, Zhang Y. HER2 mutations in Chinese patients with non-small cell lung cancer. Oncotarget. (2016) 7:78152–8. doi: 10.18632/oncotarget.11313
3. Zhang S, Wang W, Xu C, Zhang Y, Cai X, Wang Q, et al. Chinese expert consensus on the diagnosis and treatment of HER2-altered non–small cell lung cancer. Thorac Cancer. (2022) 14:91–104. doi: 10.1111/1759-7714.14743
4. Nützinger J, Bum Lee J, Li Low J, Ling Chia P, Talisa Wijaya S, Chul Cho B, et al. Management of HER2 alterations in non-small cell lung cancer - the past, present, and future. Lung Cancer. (2023) 186:107385. doi: 10.1016/j.lungcan.2023.107385
5. Jebbink M, de Langen AJ, Boelens MC, Monkhorst K, Smit EF. The force of HER2 - a druggable target in NSCLC? Cancer Treat Rev. (2020) 86:101996. doi: 10.1016/j.ctrv.2020.101996
6. Chen J, Xu C, Wang Q, Lv J, Lu W, Zhang Y, et al. Exploration on the first-line treatment of ERBB2-altered advanced non-small cell lung cancer: A multicenter retrospective study. Lung Cancer. (2023) 183:107315. doi: 10.1016/j.lungcan.2023.107315
7. Vokes NI, Pan K, Le X. Efficacy of immunotherapy in oncogene-driven non-small-cell lung cancer. Ther Adv Med Oncol. (2023) 15:17588359231161409. doi: 10.1177/17588359231161409
8. Peters S, Curioni-Fontecedro A, Nechushtan H, Shih JY, Liao WY, Gautschi O, et al. Activity of afatinib in heavily pretreated patients with ERBB2 mutation-positive advanced NSCLC: Findings from a global named patient use program. J Thorac Oncol. (2018) 13:1897–905. doi: 10.1016/j.jtho.2018.07.093
9. Dziadziuszko R, Smit EF, Dafni U, Wolf J, Wasąg B, Biernat W, et al. Afatinib in NSCLC with HER2 mutations: Results of the prospective, open-label phase ii niche trial of european thoracic oncology platform (ETOP). J Thorac Oncol. (2019) 14:1086–94. doi: 10.1016/j.jtho.2019.02.017
10. Le X, Cornelissen R, Garassino M, Clarke JM, Tchekmedyian N, Goldman JW, et al. Poziotinib in non-small-cell lung cancer harboring HER2 exon 20 insertion mutations after prior therapies: Zenith20-2 trial. J Clin Oncol. (2022) 40:710–8. doi: 10.1200/jco.21.01323
11. Elamin YY, Robichaux JP, Carter BW, Altan M, Gibbons DL, Fossella FV, et al. Poziotinib for patients with HER2 exon 20 mutant non-small-cell lung cancer: Results from a phase II trial. J Clin Oncol. (2022) 40:702–9. doi: 10.1200/jco.21.01113
12. Zhou C, Li X, Wang Q, Gao G, Zhang Y, Chen J, et al. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: A multicenter, open-label, single-arm, phase ii study. J Clin Oncol. (2020) 38:2753–61. doi: 10.1200/jco.20.00297
13. Kinoshita TG I, Watanabe K, Maemondo M, Oizumi S, Amano T, Hatanaka Y, et al. A phase II study of trastuzumab monotherapy in pretreated patients with non-small cell lung cancers (NSCLCs) harboring HER2 alterations: HOT1303-B trial. Ann Oncol (2018). doi: 10.1093/annonc/mdy292.112
14. Lara PN Jr., Laptalo L, Longmate J, Lau DH, Gandour-Edwards R, Gumerlock PH, et al. Trastuzumab plus docetaxel in HER2/neu-positive non-small-cell lung cancer: A california cancer consortium screening and phase II trial. Clin Lung Cancer. (2004) 5:231–6. doi: 10.3816/clc.2004.n.004
15. Langer CJ, Stephenson P, Thor A, Vangel M, Johnson DH. Trastuzumab in the treatment of advanced non-small-cell lung cancer: Is there a role? Focus on eastern cooperative oncology group study 2598. J Clin Oncol. (2004) 22:1180–7. doi: 10.1200/jco.2004.04.105
16. Chau CH, Steeg PS, Figg WD. Antibody-drug conjugates for cancer. Lancet. (2019) 394:793–804. doi: 10.1016/s0140-6736(19)31774-x
17. Coleman N, Yap TA, Heymach JV, Meric-Bernstam F, Le X. Antibody-drug conjugates in lung cancer: Dawn of a new era? NPJ Precis Oncol. (2023) 7:5. doi: 10.1038/s41698-022-00338-9
18. NCCN clinical practice guidelines in oncology (NCCN guidelines®)non-small cell lung cancer. (Version 1.2024).
19. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: Results from a phase ii basket trial. J Clin Oncol. (2018) 36:2532–7. doi: 10.1200/jco.2018.77.9777
20. Li BT, Michelini F, Misale S, Cocco E, Baldino L, Cai Y, et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discovery. (2020) 10:674–87. doi: 10.1158/2159-8290.Cd-20-0215
21. Goto K, Goto Y, Kubo T, Ninomiya K, Kim SW, Planchard D, et al. Trastuzumab deruxtecan in patients with HER2-mutant metastatic non-small-cell lung cancer: Primary results from the randomized, phase II destiny-lung02 trial. J Clin Oncol. (2023) 41:4852–63. doi: 10.1200/jco.23.01361
22. Li H, Yu C, Jiang J, Huang C, Yao X, Xu Q, et al. An anti-HER2 antibody conjugated with monomethyl auristatin e is highly effective in HER2-positive human gastric cancer. Cancer Biol Ther. (2016) 17:346–54. doi: 10.1080/15384047.2016.1139248
23. Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. (2020) 17:33–48. doi: 10.1038/s41571-019-0268-3
24. Wu JM, Lin MT. Effects of specific nutrients on immune modulation in patients with gastrectomy. Ann Gastroenterol Surg. (2020) 4:14–20. doi: 10.1002/ags3.12299
25. Li C, Sun L, Liu Z, Sun H, Wang X, Yu Q, et al. Efficacy and safety of disitamab vedotin after trastuzumab for HER2 positive breast cancer: A real-world data of retrospective study. Am J Cancer Res. (2024) 14:869–79. doi: 10.62347/emik7909
26. Nie C, Xu W, Guo Y, Gao X, Lv H, Chen B, et al. Immune checkpoint inhibitors enhanced the antitumor efficacy of disitamab vedotin for patients with HER2-positive or HER2-low advanced or metastatic gastric cancer: A multicenter real-world study. BMC Cancer. (2023) 23:1239. doi: 10.1186/s12885-023-11735-z
27. Chen M, Yao K, Cao M, Liu H, Xue C, Qin T, et al. HER2-targeting antibody-drug conjugate rc48 alone or in combination with immunotherapy for locally advanced or metastatic urothelial carcinoma: A multicenter, real-world study. Cancer Immunol Immunother. (2023) 72:2309–18. doi: 10.1007/s00262-023-03419-1
28. Fuentes-Antrás J, Genta S, Vijenthira A, Siu LL. Antibody-drug conjugates: In search of partners of choice. Trends Cancer. (2023) 9:339–54. doi: 10.1016/j.trecan.2023.01.003
29. Ponte JF, Ab O, Lanieri L, Lee J, Coccia J, Bartle LM, et al. Mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, potentiates the activity of standard of care therapeutics in ovarian cancer models. Neoplasia. (2016) 18:775–84. doi: 10.1016/j.neo.2016.11.002
30. Shi F, Liu Y, Zhou X, Shen P, Xue R, Zhang M. Disitamab vedotin: A novel antibody-drug conjugates for cancer therapy. Drug Delivery. (2022) 29:1335–44. doi: 10.1080/10717544.2022.2069883
31. Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. (2001) 7:987–9. doi: 10.1038/nm0901-987
32. Quanz M, Hagemann UB, Zitzmann-Kolbe S, Stelte-Ludwig B, Golfier S, Elbi C, et al. Anetumab ravtansine inhibits tumor growth and shows additive effect in combination with targeted agents and chemotherapy in mesothelin-expressing human ovarian cancer models. Oncotarget. (2018) 9:34103–21. doi: 10.18632/oncotarget.26135
33. Liu SM, Tu HY, Wei XW, Yan HH, Dong XR, Cui JW, et al. First-line pyrotinib in advanced HER2-mutant non-small-cell lung cancer: A patient-centric phase 2 trial. Nat Med. (2023) 29:2079–86. doi: 10.1038/s41591-023-02461-x
34. Song Z, Lv D, Chen SQ, Huang J, Li Y, Ying S, et al. Pyrotinib in patients with HER2-amplified advanced non-small cell lung cancer: A prospective, multicenter, single-arm trial. Clin Cancer Res. (2022) 28:461–7. doi: 10.1158/1078-0432.Ccr-21-2936
Keywords: HER2 mutation, HER2 amplification, target, non-small cell lung cancer, RC48
Citation: Zhang M, Wang L, Wang Q, Yang J, Peng W, Li X, Shi M and Lu K (2024) Efficacy of disitamab vedotin in non-small cell lung cancer with HER2 alterations: a multicenter, retrospective real-world study. Front. Oncol. 14:1441025. doi: 10.3389/fonc.2024.1441025
Received: 30 May 2024; Accepted: 16 September 2024;
Published: 06 November 2024.
Edited by:
Alessandro Leonetti, University Hospital of Parma, ItalyCopyright © 2024 Zhang, Wang, Wang, Yang, Peng, Li, Shi and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaihua Lu, bHVrYWlodWFAbmptdS5lZHUuY24=; Meiqi Shi, c2hpbWVpcWkxOTYzQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.