
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 14 November 2024
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1440131
Objectives: Endoscopic retrograde cholangiopancreatography(ERCP) with endoscopic metallic biliary endoprosthesis(EMBE) serves as a crucial palliative treatment for advanced malignant biliary obstruction(MBO). While endoscopic nasobiliary drainage(ENBD) effectively reduces post-ERCP cholangitis (PEC) incidence, its impact on PEC in MBO patients is unclear. This study evaluates ENBD’s effects on PEC in patients undergoing EMBE and identifies risk factors.
Methods: This retrospective cohort study at the First Hospital of Jilin University involved MBO patients who underwent EMBE from September 2011 to September 2022. Propensity score matching (PSM) was applied to minimize selection bias. Primary and secondary outcomes included the incidence and recovery rate/time of PEC, biliary drainage success, and hospitalization duration. Univariate, multivariate, and Lasso regression analyses identified independent risk factors.
Results: In this study of 1,008 patients, 730 were analyzed after PSM(365 each in the EMBE+ENBD and EMBE groups). No significant differences were observed in PEC incidence(10.7% vs 11.2%, p=0.9057) or recovery rates(48.7% vs 31.7%, p=0.1855). However, PEC recovery time was shorter in the EMBE+ENBD group(4.0 days [3.0, 6.0] vs 5.0 days [4.0, 7.5], p=0.0240), as was hospitalization duration(6.0 days [4.0, 8.0] vs 7.0 days [5.0, 10.0], p=0.0146), and a higher success rate of biliary drainage(54.0% vs 43.3%, p=0.0049). Tumor location(HR 1.10, 95% CI 1.00-1.20) and preoperative total bilirubin(HR 2.13, 95% CI 1.66-2.73) were identified as independent risk factors.
Conclusion: In this large-scale PSM study, ENBD did not reduce PEC incidence but expedited recovery and shortened hospital stays. Patients with hilar MBO of Bismuth III-IV or high preoperative bilirubin were more prone to PEC.
Malignant biliary obstruction (MBO) is caused by the invasion or compression of bile ducts by tumors such as cholangiocarcinoma, pancreatic carcinoma, gallbladder carcinoma, ampullary carcinoma, and metastatic diseases (1). Due to nonspecific early symptoms, MBO typically presents at advanced stages, with fewer than 20% of patients being candidates for curative resection (2). To relieve obstruction and prolong survival, Endoscopic Retrograde Cholangiopancreatography (ERCP) is commonly performed for biliary drainage (3, 4). Compared to plastic stents used in endoscopic retrograde biliary drainage (ERBD), endoscopic metallic biliary endoprosthesis (EMBE) with self-expandable metal stents offers longer patency (5), lower reintervention rates (6), and reduced risk of stent migration (5, 7), thereby improving quality of life.
Cholangitis, a common adverse event post-ERCP (PEC), occurs in 1% to 3% of cases (3, 8). Risk factors include invasive procedures like cholangiography (3) and cholangioscopy (9), which may cause bacterial infections, inadequate biliary drainage, and a history of primary sclerosing cholangitis (PSC) (10). In patients with MBO undergoing EMBE, PEC incidence ranges from 6% to 25.9% (6, 11–13), with poor general health and inadequate biliary drainage in hilar MBO patients being significant risk factors (10, 14).
Multiple guidelines state that endoscopic nasobiliary drainage (ENBD) reduces biliary pressure, minimizes intestinal reflux, and lowers the risk of PEC (3, 4, 15). However, there is no consensus on postoperative nasobiliary tube placement in MBO patients undergoing EMBE, and the impact of ENBD on PEC remains unclear. Limited clinical studies exist on the short-term benefits of combining EMBE with ENBD for MBO treatment (16). Thus, we designed a real-world study to retrospectively analyze the impact of ENBD on PEC in MBO patients with self-expandable metal stents.
Consecutive patients diagnosed with hepatobiliary cancer at the First Hospital of Jilin University from September 2011 to September 2022 were included in this study. Diagnoses followed the National Comprehensive Cancer Network Clinical Practice Guidelines for Hepatobiliary Cancers-Evidence Blocks (1). Patient treatments were determined through multidisciplinary discussions.
Inclusion criteria: a)Patients with diagnosed MBO, unsuitable for curative resection due to metastasis or poor health;b) Patients with symptoms of significant biliary obstruction (jaundice, abdominal pain, anorexia);c) Age≥18 years;d) Patients receiving the first ERCP and self-expandable metal stent. Exclusion criteria: a) Patients with concomitant cholelithiasis or choledocholithiasis;b) Patients who have undergone percutaneous transhepatic cholangial drainage (PTCD);c) Patients with preoperative acute cholangitis.
Patients were categorized into the EMBE group and the EMBE + ENBD group based on whether ENBD was performed. Clinical data were extracted from the Real World Data Platform of the First Hospital of Jilin University. Baseline characteristics and relevant variables were collected using clinical expertise and literature evidence (3, 8–10, 17, 18). This study received approval from the Ethics Committee of the First Hospital of Jilin University (2024-006) and was conducted according to the Declaration of Helsinki.
After obtaining ERCP informed consent, all patients received prophylactic antibiotics and non-steroidal anti-inflammatory drugs preoperatively. Procedures were performed under conscious sedation or general anesthesia by skilled endoscopists. ERCP was conducted in the prone position using a standard therapeutic duodenoscope (TJF-260V, Olympus, Tokyo, Japan). Successful cannulation of the common bile duct led to cholangiography, which delineated the location and extent of biliary strictures (Figure 1). For difficult cannulations, pre-cut sphincterotomy, endoscopic sphincterotomy (EST), or endoscopic papillary balloon dilation (EPBD) were employed (Supplementary Materials Figures 1, 2). Endoscopic radiofrequency ablation (RFA) was performed as needed (Supplementary Materials Figures 3, 4). Endoscopic retrograde pancreatic drainage (ERPD) was considered to prevent post-ERCP pancreatitis following pancreatic duct cannulation (Supplementary Materials Figure 5). Metal stents were optimally positioned under fluoroscopic guidance (Figures 2, 3). In the EMBE + ENBD group, a nasobiliary tube with a negative pressure device was inserted through the stenosis(Figure 4). Broad-spectrum antibiotics were administered postoperatively, with regular monitoring of blood biochemical markers, including liver and kidney functions. Nasobiliary tubes were removed after 24 hours if no PEC occurred.
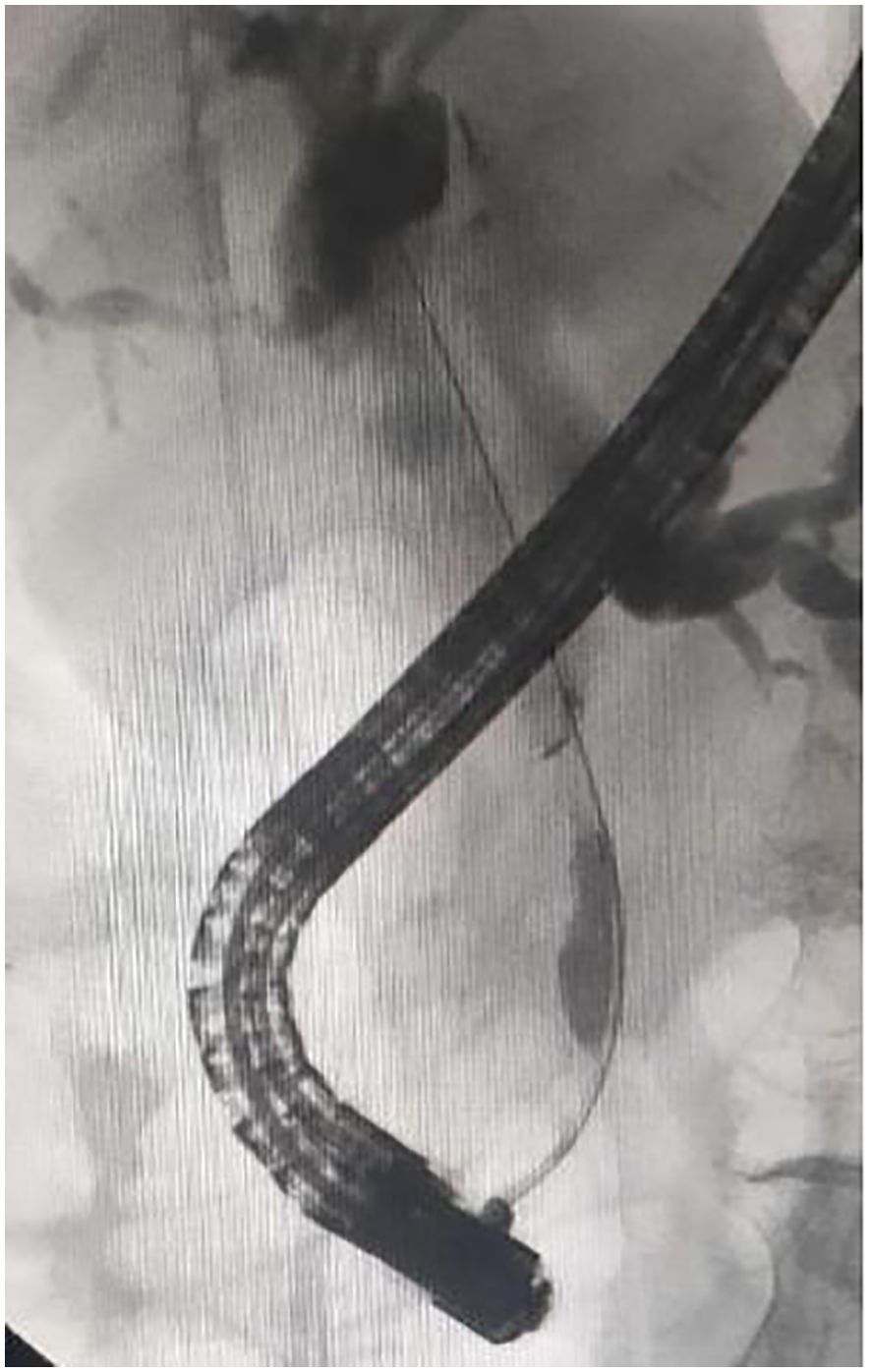
Figure 1. Cholangiography showing malignant biliary obstruction involving in the middle of common bile duct.
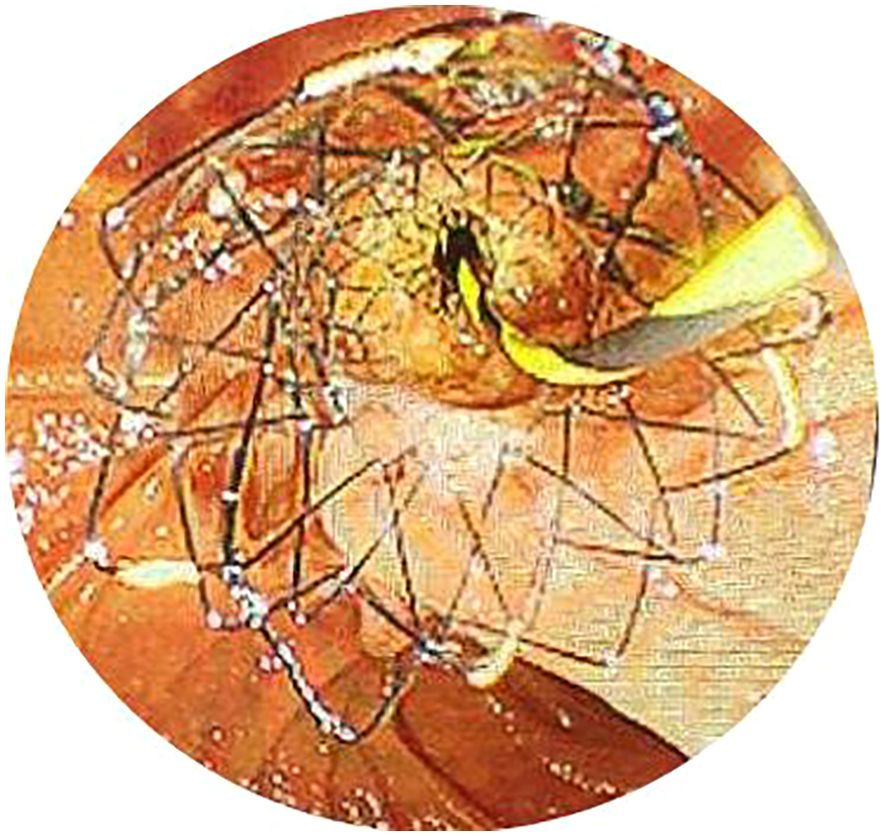
Figure 3. The metal stent was placed in the bile duct with the end located in the intestine under endoscopy.
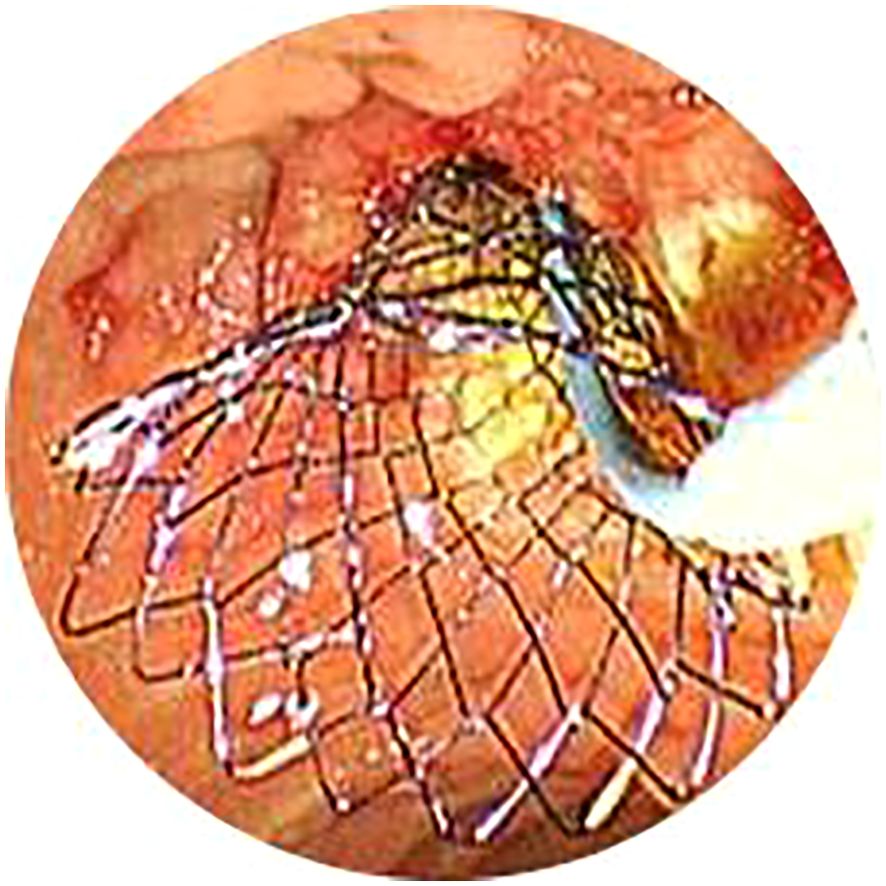
Figure 4. In the EMBE+ENBD group, one nasobiliary catheter with negative pressure drainage device was deployed after insertion of metal stents. EMBE, endoscopic metallic biliary endoprosthesis; ENBD, endoscopic nasobiliary drainage.
Primary Outcome: PEC is defined as the presence of one item from group a, one from group d, and one from either group b or c (19). a)Systemic Inflammation after ERCP (a-1: Fever(body temperature> 38°C) and/or shaking chills;a-2: Laboratory data showing evidence of inflammatory response(white blood cell(WBC): < 4*109/L or > 10 *109/L)); b)Cholestasis (b-1: Jaundice(total bilirubin≥34.2µmol/L);b-2: Abnormal liver function tests (ALP, GGT, AST or ALT: > 1.5 times the standard deviation of the normal range)); c) Imaging evaluation(c-1: Biliary dilatation; c-2: Evidence of the etiology on imaging); d)Exclusion of post-ERCP pancreatitis and other infections.
Secondary Outcomes: Biliary drainage success (20) is defined as a return to normal or a decrease of over 25% in total bilirubin levels within two weeks. Hospitalization duration is the number of days from admission to discharge. PEC recovery is achieved when successful biliary drainage coincides with the normalization of clinical symptoms and inflammatory markers post-treatment (19). The observation period for evaluating biliary drainage is also set at two weeks.
Adverse events: Adverse events are classified according to the American Society for Gastrointestinal Endoscopy (ASGE) lexicon for endoscopic adverse events (21), including post-ERCP pancreatitis, bleeding, perforation and death.
A study by Xinjian Wan et al. involving 378 patients reported PEC rates of 11.9% in the EMBE group and 2.4% in the EMBE+ENBD group (16), suggesting that combining EMBE with ENBD could reduce incidence rates. Literature review showed the lowest PEC incidence after EMBE was 6% (6, 11–13). Since this rate did not distinguish between cases with or without ENBD, it may underestimate PEC incidence in the EMBE group, aiding in a more moderate sample size calculation. This study assumes a PEC incidence of 6% for EMBE and 2.4% for EMBE+ENBD. Targeting an 80% statistical power and a 0.1 type I error (one-sided), 560 cases are needed (280 per group), adjusted to 672 cases (336 per group) to account for a 20% dropout rate.
Statistical analysis was conducted using R version 4.1.1. P values of less than 0.05 were considered significant. Results were summarized by frequency and percentage for categorical variables, means and standard deviations for normally distributed continuous variables, and medians with interquartile ranges for skewed continuous variables. Chi-square or Fisher’s exact tests were used for categorical variables, while t-tests or Mann-Whitney U tests were used for continuous variables, depending on their distribution. The Kaplan-Meier method and log-rank tests analyzed PEC recovery time. Propensity score matching (PSM) minimized selection bias using the “MatchIt” R package, with scores calculated from a logistic model including six predictors: EPBD, RFA, operative duration, type of cancer, preoperative AST, and TB. Predictors were selected based on baseline imbalances and literature support. Patients were matched using the nearest neighbor method with a caliper width of 0.1, without replacement.
Univariate and multivariate analyses were conducted on the pre-matching dataset to assess risk factors for PEC. Univariate logistic regression identified clinical variables impacting outcomes using the “autoReg” R package. Variables with p-values < 0.1 were included in the multivariate logistic regression; those statistically significant were deemed independent risk factors. Lasso regression was employed to validate the multivariate analysis results using the “glmnet” R package.
From September 2011 to September 2022, 1,973 MBO patients with hepatobiliary cancer were admitted to our hospital. Among them, 1,008 were enrolled in the study: 420 (41.7%) in the EMBE group and 588 (58.3%) in the EMBE+ENBD group (Figure 5). Demographic and clinical characteristics are shown in Table 1. The median age was 67 years [59.0, 74.0], with 619 males (61.4%). The most frequent MBO causes were cholangiocarcinoma (637, 63.2%) and pancreatic carcinoma (297, 29.5%). Obstruction locations were Bismuth I-II (180, 17.9%), Bismuth III-IV (281, 27.9%), and non-hilar (547, 54.3%). Interventions included EST (855, 84.8%), EPBD (895, 88.8%), ERPD (287, 28.5%), and RFA (34, 3.4%), with a median operative duration of 0.7 hours [0.4, 1.1].
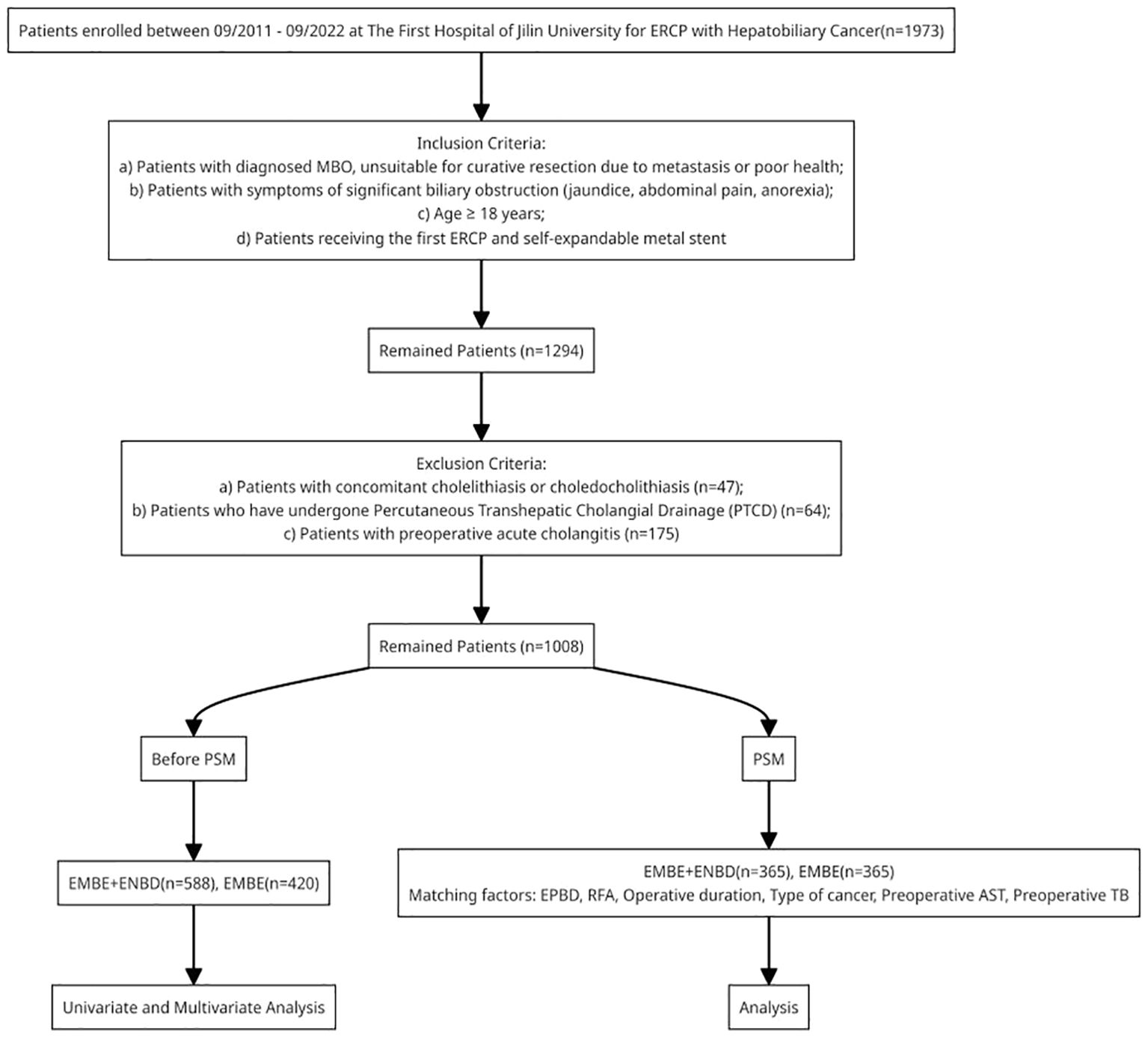
Figure 5. Flowchart of patients inclusion. MBO, malignant biliary obstruction; PSM, propensity score matching; ERCP, endoscopic retrograde cholangiopancreatography; EMBE, endoscopic metallic biliary endoprosthesis; ENBD, endoscopic nasobiliary drainage.
Statistical differences were observed between the EMBE and EMBE+ENBD groups in nine factors: type of cancer (p<0.001), tumor location (p=0.026), preoperative AFP (p<0.001), gamma-glutamyl transpeptidase (p<0.001), aspartate amino transferase (p<0.001), TB (p=0.002), EPBD (p<0.001), RFA (p<0.001), and operative duration (p<0.001). Based on literature (3, 8–10, 17) and expert opinions, six factors were selected for PSM: EPBD, RFA, operative duration, type of cancer, preoperative AST, and TB. After PSM, there were 365 patients in each group, showing no significant differences in baseline characteristics (Table 1). We assessed PSM effectiveness by plotting density graphs for each covariate before and after matching (R package “cobalt”). Results showed that matching made the factors more consistent between groups, enhancing the study’s methodological quality and reliability (Supplementary Materials Figure 6).
In the matched cohort of 730 patients, the incidence of PEC was similar between the EMBE+ENBD group (39, 10.7%) and the EMBE group (41, 11.2%), showing no significant difference (p=0.9057) (Table 2). Over a 2-week observation period, PEC recovery rates showed no statistical difference (19 (48.7%) vs. 13 (31.7%), p=0.1855). PEC recovery time was documented. If outcomes were not observed within the observation period due to discharge or other reasons, these were noted and categorized as lost to follow-up. Median recovery times were 4.0 days [3.0, 6.0] for EMBE+ENBD and 5.0 days [4.0, 7.5] for EMBE, showing a significant difference (p=0.0240, log-rank test, Figure 6). Additionally, biliary drainage success (197 (54.0%) vs. 158 (43.3%), p=0.0049) and hospitalization duration (6.0 days [4.0, 8.0] vs. 7.0 days [5.0, 10.0], p=0.0146) were superior in the EMBE+ENBD group.
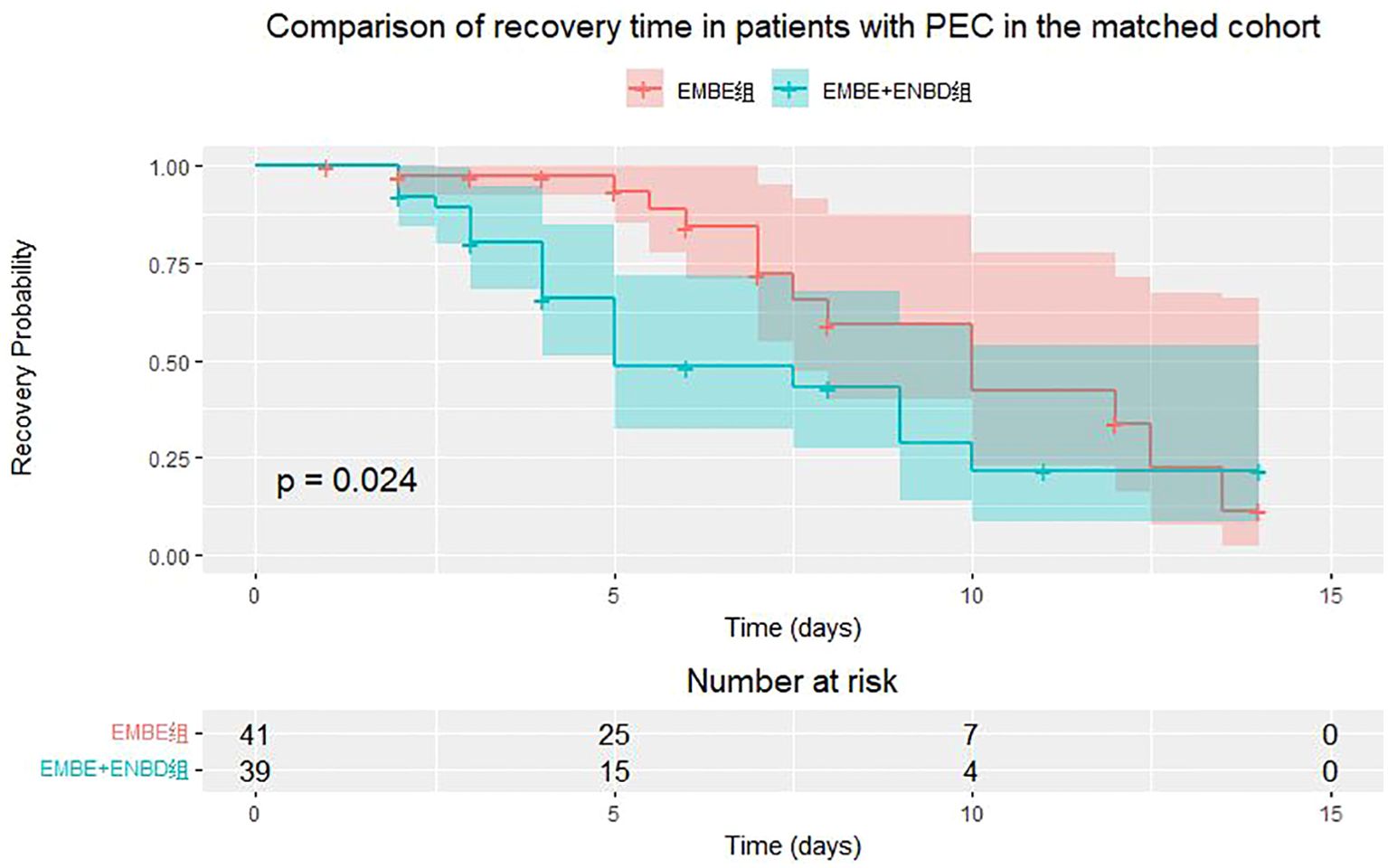
Figure 6. Kaplan-Meier curve for post-ERCP cholangitis (PEC) recovery time in EMBE+ENBD and EMBE groups.
Regarding ERCP-related adverse events (Table 3), the post-ERCP pancreatitis rate was 3.3% overall (2.5% EMBE+ENBD vs. 4.1% EMBE, p=0.2994). Bleeding was similar at 1.9% for EMBE+ENBD and 2.2% for EMBE (p=1.0000). Perforation occurred in 0.5% of EMBE+ENBD and 0.3% of EMBE patients (p=1.0000). There was no significant difference in mortality between the groups, each at 0.3% (p=1.0000). Similar adverse event outcomes were noted in the cohort before matching.
Based on references and clinical practice, and to prevent multicollinearity, we identified 10 potential risk factors for PEC (Table 4). Continuous variables were converted to categorical ones to categorize risk levels. In univariate analysis, significant variables included tumor location (p<0.001), preoperative TB (p<0.001), EPBD (p=0.058), and operative duration (p=0.061). Multivariate analysis revealed significant predictors: tumor location (OR, 1.10; 95% CI, 1.00–1.20; p=0.048) and preoperative TB (OR, 2.13; 95% CI, 1.66–2.73; p<0.001). We calculated the odds ratios for each independent risk factor based on categorical indicators to assess their clinical significance (Table 5).
Risk factors were also screened using Lasso regression, with the coefficient variation characteristics of these variables shown in Figure 7. A 10-fold cross-validation method optimized the model to ensure minimal variables and excellent performance, resulting in a λ of 0.01112489 (Figure 8). The results of Lasso regression are consistent with those of multivariate analysis, strongly confirming the reliability of the variable selection outcome.
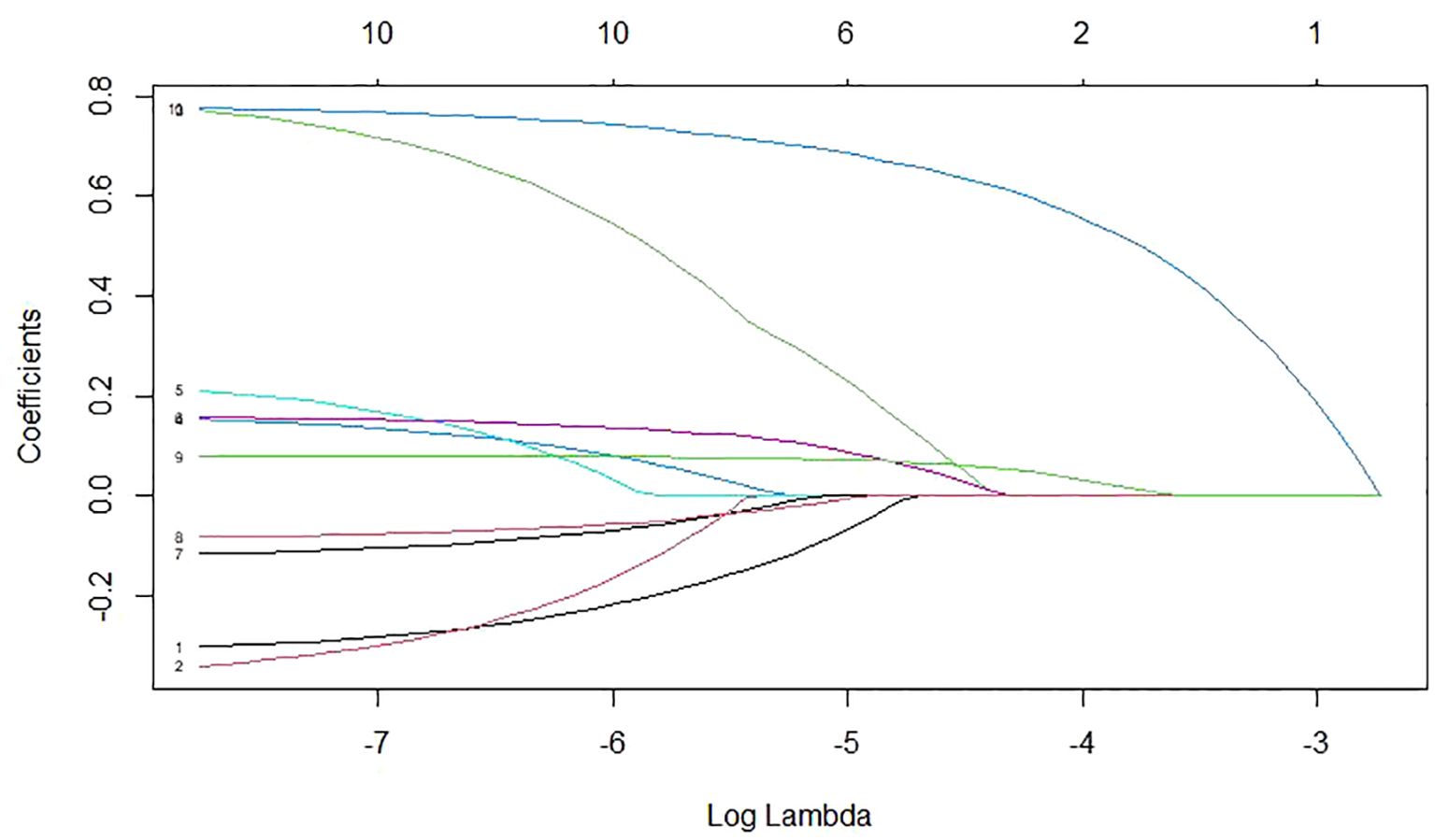
Figure 7. The coefficient variation characteristics of the variables in Lasso regression variable screening.
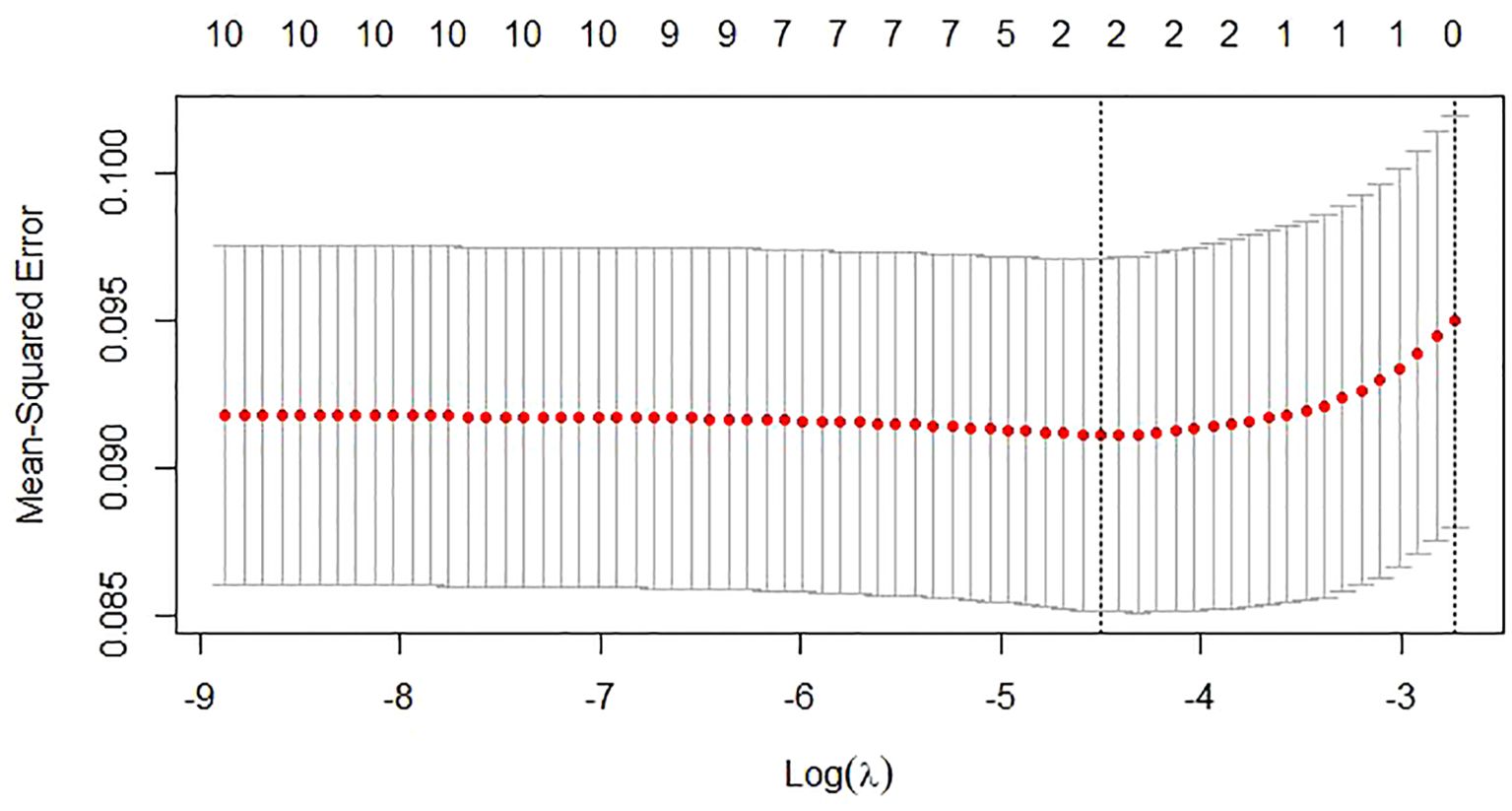
Figure 8. The selection process of the optimum value of the parameter λ in the Lasso regression model by cross-validation.
For unresectable MBO, biliary drainage through ERCP is a commonly used and effective treatment (3, 4). EMBE is widely used in clinical practice due to its advantages of prolonged patency (5), low reintervention rates (6), and reduced risk of stent migration (5, 7). PEC is a common postoperative complication that can affect patient recovery, potentially prolong hospital stays and increase medical costs (3, 8, 18). Invasive procedures such as cholangiography (3) and cholangioscopy (9), incomplete biliary drainage, and a history of PSC (10) are recognized risk factors. A retrospective study of 4,324 cases identified hilar obstruction, age ≥60 years, and a history of prior ERCP as independent risk factors, while complete removal of biliary stones was protective (22). Both PSC and hilar obstruction, which cause incomplete biliary drainage, are related to PEC, though no controlled studies are available (10). Another retrospective study of 4,214 ERCPs found that cholangioscopy increased the risk of PEC (9). Some factors are not risk factors for PEC: cirrhosis (23), operator experience (24), and periampullary diverticulum (25).
The mechanisms underlying PEC include bacterial infection and increased biliary pressure (26, 27). The sphincter of Oddi prevents duodenal reflux and ascending bacterial infections. Bile’s flushing action and antibacterial activity of bile salts maintain bile duct sterility. Secretory IgA and biliary mucus act as anti-adhesion factors, preventing bacterial colonization. Invasive procedures introduce bacteria and impair defenses, increasing PEC risk (9). Cholangiography can increase biliary pressure, affecting host defenses, bile flow, and IgA production. This may increase bile duct wall permeability, facilitating bacterial entry into systemic circulation and raising sepsis risk.
Studies indicate that PEC incidence following EMBE can be as high as 6% to 25.9% (6, 11–13). PEC is often reported as an adverse event, with higher incidence rates than conventional ERCP. High-risk factors include poor health and suboptimal biliary drainage in patients with hilar MBO (10, 14). Guidelines suggest that ENBD can reduce PEC occurrence (3, 4, 15), but there’s no consensus on its benefits for EMBE with MBO. Some scholars argue for ENBD to manage temporary biliary obstruction post-EMBE, while others cite increased discomfort and costs (28). Few studies explore this. Xinjian Wan et al’s research shows ENBD is safe and feasible for unresectable hilar MBO, but non-hilar cases may not need ENBD (16). Therefore, it is essential to study the impact of ENBD on the occurrence and recovery of cholangitis post-EMBE to guide clinical practice.
To our knowledge, this is the largest and first retrospective clinical study using PSM to focus on PEC development following EMBE. We evaluated 1008 patients, and after PSM, 730 patients (365 per group) were included in the analysis. The overall incidence of PEC was higher than that following conventional ERCP, with no significant difference between the EMBE and EMBE+ENBD groups. ENBD does not appear to protect against PEC in MBO patients, differing from Wan et al.’s findings. Several reasons may explain this: advanced cancer impairs biliary barrier function and reduces local resistance to bacteria, while EMBE can cause mechanical damage to the bile duct mucosa and increase infection risks (26). Stent expansion might compress local blood vessels, causing ischemia and making the bile duct more susceptible to bacterial attack (17). Tumor thrombi and necrotic tissue can cause temporary stent blockages, leading to poor biliary drainage (8). Hilar MBO may not achieve complete drainage even with stent placement, and procedures like intraoperative cholangiography can increase biliary pressure, contributing to PEC (2, 3). Given these factors, PEC incidence may be higher than with conventional ERCP, and factors causing PEC are present during surgery; optimal biliary drainage with ENBD cannot fully prevent cholangitis. Our study’s large sample size and use of PSM enhance result reliability.
Subsequently, we assessed PEC recovery in both groups based on recovery rate, recovery time, biliary drainage success rate, and hospitalization duration. The EMBE+ENBD group showed shorter PEC recovery time, shorter hospitalization duration, and higher biliary drainage success rates, despite similar recovery rates. This similarity may be due to comparable PEC incidences and aggressive symptomatic treatment. Continuous and unobstructed biliary drainage relieves preoperative biliary obstruction and aids in the recovery of jaundice and PEC. ENBD allows for the assessment of bile quality and quantity, facilitates bacterial culture, and guides antibiotic use, thereby shortening recovery time (29, 30). Adverse event incidence was similar in both groups, indicating that ENBD is safe and does not increase risk.
For the unmatched patient cohort, we analyzed risk factors for PEC. Results indicated that tumor location and preoperative total bilirubin are significant risk factors. Hilar obstructions are more likely to cause cholangitis than distal ones, with Bismuth III-IV obstructions increasing the risk by 107% especially. Hilar MBO affects more bile duct branches, making adequate drainage difficult even after stent placement (5, 14). Surgical manipulations and aggressive tumors increase bacterial infection risk. Moderate and severe jaundice, compared to mild jaundice, increased cholangitis incidence by 212% and 419%. Higher preoperative bilirubin levels, associated with impaired liver function, reduce immune capacity and increase infection risk. Cholestasis enhances bacterial growth, damages mucosal barriers, and facilitates bacterial invasion (31). LASSO regression confirmed these conclusions.
Our study has limitations, including potential biases from its retrospective design and single-center nature. We minimized selection bias through PSM and conducted multivariable analysis to limit confounding factors. However, residual confounding and selection bias may still exist. Further prospective and randomized controlled trials are needed to validate our conclusions.
In conclusion, our large-scale study found no evidence that ENBD after EMBE reduces PEC incidence in MBO patients. However, the EMBE+ENBD group had higher biliary drainage success rates, shorter PEC recovery and hospitalization times, without increased adverse events. Tumor location and preoperative total bilirubin levels were identified as independent risk factors. This suggests that patients with high preoperative bilirubin levels and hilar MBO of Bismuth III-IV are at higher risk for PEC, and nasobiliary tube placement may be beneficial for these patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Ethics Committee of the First Hospital of Jilin University, The First Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because All medical records/biological information in this study were from the real world data application platform of the First Hospital of Jilin University, and there was no need to re-collect medical records/biological specimens information. This clinical study will not directly contact the subjects and the results will not be used for the diagnosis of the subjects and will not have any adverse effects on the subjects. This study does not utilize medical records and specimens that the patient/subject has explicitly refused to use in the past.
HJ: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. CF: Investigation, Methodology, Writing – review & editing. XS: Investigation, Visualization, Writing – review & editing. CqF: Investigation, Visualization, Writing – review & editing. JC: Conceptualization, Data curation, Writing – review & editing. HZ: Visualization, Writing – original draft. KL: Conceptualization, Supervision, Writing – original draft. HX: Conceptualization, Data curation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The clinical data for this study comes from the Real-World Data Platform of the First Hospital of Jilin University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1440131/full#supplementary-material
1. Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:541–65. doi: 10.6004/jnccn.2021.0022
2. Pietrzak J, Przybyłkowski A. Endoscopic treatment of Malignant hilar biliary obstruction. Cancers (Basel). (2023) 15(24):5819 . doi: 10.3390/cancers15245819
4. Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, et al. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. (2018) 50:910–30. doi: 10.1055/a-0659-9864
5. Kulpatcharapong S, Piyachaturawat P, Mekaroonkamol P, Angsuwatcharakon P, Ridtitid W, Kongkam P, et al. Efficacy of multi-hole self-expandable metal stent compared to fully covered and uncovered self-expandable metal stents in patients with unresectable Malignant distal biliary obstruction: a propensity analysis. Surg Endosc. (2024) 38:212–21. doi: 10.1007/s00464-023-10541-9
6. Cheng JLS, Bruno MJ, Bergman JJ, Rauws EA, Tytgat GN, Huibregtse K. Endoscopic palliation of patients with biliary obstruction caused by nonresectable hilar cholangiocarcinoma: Efficacy of self-expandable metallic Wallstents. Gastrointestinal Endoscopy. (2002) 56:33–9. doi: 10.1067/mge.2002.125364
7. Irisawa A, Katanuma A, Itoi T. Otaru consensus on biliary stenting for unresectable distal Malignant biliary obstruction. Dig Endosc. (2013) 25 Suppl 2:52–7. doi: 10.1111/den.12069
8. Dumonceau JM, Kapral C, Aabakken L, Papanikolaou IS, Tringali A, Vanbiervliet G, et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. (2020) 52:127–49. doi: 10.1055/a-1075-4080
9. Sethi A, Chen YK, Austin GL, Brown WR, Brauer BC, Fukami NN, et al. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: a single-center experience. Gastrointest Endosc. (2011) 73:251–6. doi: 10.1016/j.gie.2010.08.058
10. Navaneethan U, Jegadeesan R, Nayak S, Lourdusamy V, Sanaka MR, Vargo JJ, et al. ERCP-related adverse events in patients with primary sclerosing cholangitis. Gastrointest Endosc. (2015) 81:410–9. doi: 10.1016/j.gie.2014.06.030
11. Paik WH, Park YS, Hwang JH, Lee SH, Yoon CJ, Kang SG, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. (2009) 69:55–62. doi: 10.1016/j.gie.2008.04.005
12. Li J, Li T, Sun P, Yu Q, Wang K, Chang W, et al. Covered versus uncovered self-expandable metal stents for managing Malignant distal biliary obstruction: A meta-analysis. PloS One. (2016) 11:e0149066. doi: 10.1371/journal.pone.0149066
13. Wu CH, Wang SF, Lee MH, Tsou YK, Lin CH, Chang LL, et al. Efficacy of Fully Covered Self-Expandable Metal Stents for Distal Biliary Obstruction Caused by Pancreatic Ductal Adenocarcinoma: Primary Metal Stent vs. Metal Stent following Plastic Stent. Cancers (Basel). (2023) 15(11):3001. doi: 10.3390/cancers15113001
14. Mosconi C, Renzulli M, Giampalma E, Galuppi A, Balacchi C, Brandi G, et al. Unresectable perihilar cholangiocarcinoma: multimodal palliative treatment. Anticancer Res. (2013) 33:2747–53.
15. Hu B, Sun B, Cai Q, Wong Lau JY, Ma S, Itoi T, et al. Asia-Pacific consensus guidelines for endoscopic management of benign biliary strictures. Gastrointest Endosc. (2017) 86:44–58. doi: 10.1016/j.gie.2017.02.031
16. Wan X, Chen S, Zhao Q, Li T, Luo S, Cai X, et al. The efficacy of temporary placement of nasobiliary drainage following endoscopic metal stenting to prevent post-ERCP cholangitis in patients with cholangiocarcinoma. Saudi J Gastroenterol. (2018) 24:348–54. doi: 10.4103/sjg.SJG_94_18
17. Zhou Y, Zhang YQ, Huang SJ, Liang Y, Liang X, Wali M, et al. Urgent one-stage endoscopic treatment for choledocholithiasis related moderate to severe acute cholangitis: A propensity score-matched analysis. World J Gastroenterol. (2024) 30:2118–27. doi: 10.3748/wjg.v30.i15.2118
18. Buxbaum J, Qumseya B, Wani S. Toward an evidence-based approach for cholangitis diagnosis. Gastrointest Endosc. (2021) 94:297–302.e2. doi: 10.1016/j.gie.2021.04.016
19. Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. (2018) 25:17–30. doi: 10.1002/jhbp.2018.25.issue-1
20. Candido K, Bouchard S, Hansen-Barkun C, Huang DC, Chatterjee A, Menard C, et al. Aspirin exposure and its association with metal stent patency in Malignant distal biliary obstruction: a large international multicenter propensity score-matched study. Gastrointest Endosc. (2024) 99:557–65. doi: 10.1016/j.gie.2023.11.004
21. Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. (2010) 71:446–54. doi: 10.1016/j.gie.2009.10.027
22. Chen M, Wang L, Wang Y, Wei W, Yao YL, Ling TS, et al. Risk factor analysis of post-ERCP cholangitis: A single-center experience. Hepatobiliary Pancreat Dis Int. (2018) 17:55–8. doi: 10.1016/j.hbpd.2018.01.002
23. Park CH, Jung JH, Nam E, Kim EH, Kim MG, Kim JH, et al. Comparative efficacy of various endoscopic techniques for the treatment of common bile duct stones: a network meta-analysis. Gastrointest Endosc. (2018) 87:43–57.e10. doi: 10.1016/j.gie.2017.07.038
24. Keswani RN, Qumseya BJ, O'Dwyer LC, Wani S. Association between endoscopist and center endoscopic retrograde cholangiopancreatography volume with procedure success and adverse outcomes: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2017) 15:1866–75.e3. doi: 10.1016/j.cgh.2017.06.002
25. Jayaraj M, Mohan BP, Dhindsa BS, Mashiana HS, Radhakrishnan G, Dhir V, et al. Periampullary diverticula and ERCP outcomes: A systematic review and meta-analysis. Dig Dis Sci. (2019) 64:1364–76. doi: 10.1007/s10620-018-5314-y
26. Liu L, Zhao Z, Hou X, Wu J. Effect of sphincter of Oddi dysfunction on the abundance of biliary microbiota (biliary microecology) in patients with common bile duct stones. Front Cell Infect Microbiol. (2022) 12:1001441. doi: 10.3389/fcimb.2022.1001441
27. Susak YM. The impact of biliary drainage mode on bacteriobilia occurrence in patients with Hilar Malignant obstruction. Biotechnologia Acta. (2022) 15(4):44–6. doi: 10.15407/biotech15.04.044
28. Lin H, Li S, Liu X. The safety and efficacy of nasobiliary drainage versus biliary stenting in malignant biliary obstruction: A systematic review and meta-analysis. Medicine (Baltimore). (2016) 95(46):e5253. doi: 10.1097/MD.0000000000005253
29. Stathopoulos P, Lerner P, Astheimer P, Breitling LP, Zumblick M, Pararas M, et al. Endoscopic retrograde cholangiopancreatography-obtained bile culture in acute cholangitis: retrospective analysis of bile cultures and risk factors in a tertiary care center. J Gastroenterol Hepatol. (2024) 39:935–41. doi: 10.1111/jgh.16492
30. Reiter FP, Obermeier W, Jung J, Denk G, Mahajan UM, De Toni EN, et al. Prevalence, resistance rates, and risk factors of pathogens in routine bile cultures obtained during endoscopic retrograde cholangiography. Dig Dis. (2021) 39:42–51. doi: 10.1159/000509289
Keywords: cholestasis, endoscopic metallic biliary endoprosthesis, endoscopic nasobiliary drainage, cholangitis, recovery
Citation: Jin H, Fu C, Sun X, Fan C, Chen J, Zhou H, Liu K and Xu H (2024) The assessment of postoperative cholangitis in malignant biliary obstruction: a real-world study of nasobiliary drainage after endoscopic placement of self-expandable metal stent. Front. Oncol. 14:1440131. doi: 10.3389/fonc.2024.1440131
Received: 29 May 2024; Accepted: 29 October 2024;
Published: 14 November 2024.
Edited by:
Airazat M. Kazaryan, Østfold Hospital, NorwayReviewed by:
Khachatur Badalyan, Head of the Department of Surgery and Modern Surgical Technologies, ArmeniaCopyright © 2024 Jin, Fu, Sun, Fan, Chen, Zhou, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongji Xu, eHVfaG9uZ2ppQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.