- 1Department of Breast Surgery, Fujian Medical University Union Hospital, Fuzhou, Fujian, China
- 2Department of Breast Surgical Nursing, Fujian Medical University Union Hospital, Fuzhou, Fujian, China
- 3Department of General Surgery, Fujian Medical University Union Hospital, Fuzhou, Fujian, China
- 4Breast Cancer Institute, Fujian Medical University, Fuzhou, Fujian, China
- 5Department of Nursing, Fujian Medical University Union Hospital, Fuzhou, Fujian, China
Purpose: Breast cancer-related lymphedema (BCRL) is a common complication among breast cancer survivors. Most BCRL studies have focused on patients receiving adjuvant chemotherapy, with relatively little attention paid to BCRL in patients undergoing neoadjuvant chemotherapy (NAC). This study aimed to investigate the risk factors associated with BCRL in Chinese women undergoing NAC and axillary lymph node dissection (ALND).
Methods: At our institution, this cohort study collected data from 336 women with breast cancer and documented axillary nodal metastasis at diagnosis, who received NAC and ALND surgery between 2015 and 2020. BCRL was assessed through both objective limb circumference measurements and subjective self-reported symptoms. Multivariate logistic regression was employed to identify risk factors for BCRL, considering clinical, demographic, and lifestyle-related characteristics.
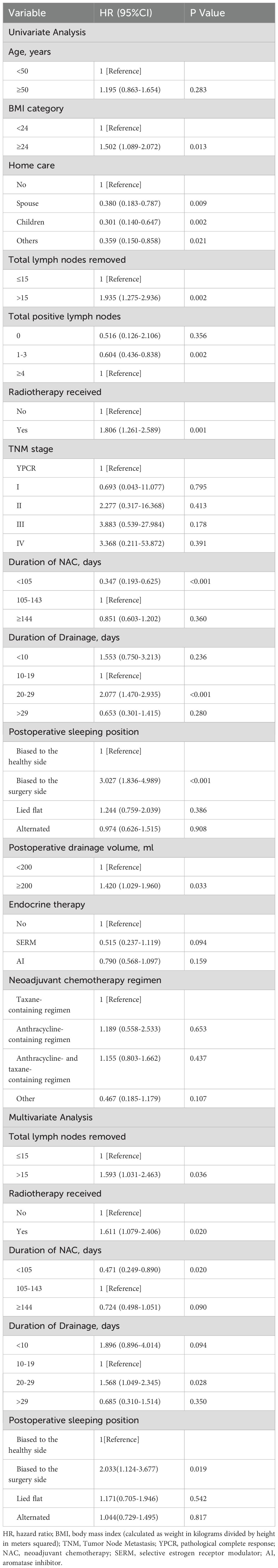
Results: The cumulative incidence of BCRL within 2.5 years was 43.75%. Factors independently associated with BCRL included radiotherapy (versus no radiotherapy; hazard ratio (HR) = 1.611; P = 0.020), NAC duration of 105 days or shorter (versus 105-143 days; HR = 0.471; P = 0.020), removal of more than 15 lymph nodes (versus 15 or fewer lymph nodes; HR = 1.593; P = 0.036), drainage duration of 20-29 days (versus 10-19 days; HR = 1.568; P = 0.028), and sleeping biased toward the affected arm (versus sleeping biased toward the healthy arm; HR = 2.033; P = 0.019).
Conclusion: This study identified several risk factors for BCRL in breast cancer patients following NAC and ALND. Patients presenting with one or more of these factors should be monitored closely for early detection and intervention. Further research is warranted to explore the impact of drainage duration and sleep position on the development of BCRL.
Introduction
Breast cancer survival rates have increased significantly in recent decades due to improvements in screening and advances in multidisciplinary treatment (1). Therefore, maintaining the quality of life and controlling treatment-related complications in long-term survivors has become an important goal (2–5). Breast cancer-related lymphedema (BCRL) is a common comorbidity of the upper extremity secondary to breast cancer treatment that occurs in approximately 22% of survivors (6). Additionally, a report indicates that BCRL might manifest anytime between the initial treatment and up to 20 years post-surgery, with the majority of cases occurring within the first 3 years (7). BCRL occurs when protein-rich fluid accumulates in the soft tissues caused by an interruption of lymphatic flow, which negatively affects the patient’s quality of life, both physically and psychosocially (8, 9).
Current data suggest that the development of BCRL is multifactorial and influenced by three categories of factors: disease and treatment-related factors (such as tumor size, axillary lymph node dissection [ALND] surgery, chemotherapy, and radiotherapy), lifestyle factors (such as physical activity, body mass index [BMI], and preventive behaviors, and demographic factors (such as monthly income, marital status, and ethnicity) (9–15). In addition to established risk factors, our study identified two rarely reported independent factors: postoperative sleeping position and drainage duration (16). To the best of our knowledge, no previous studies have specifically explored the link between sleeping position and BCRL.
Neoadjuvant chemotherapy (NAC) is increasingly used in treating breast cancer because of its ability to downstage the primary tumor in the breast and the metastatic axillary lymph node (17). However, NAC has recently been recognized as an independent risk factor for BCRL (18). On the other hand, the advent of sentinel lymph node biopsy (SLNB) has resulted in lower lymphedema rates by avoiding unnecessary ALND (19). But ALND remains the standard of axillary surgery in patients with clinically positive lymph nodes or metastatic sentinel nodes (20). Therefore, women who have undergone NAC and ALND surgery are at significant risk of developing lymphedema. However, few studies have simultaneously investigated the demographic, disease and treatment-related, and lifestyle factors that predict the development of BCRL in this subset of patients.
Breast cancer survivors who have been provided with BCRL information have significantly reduced symptoms and increased knowledge of BCRL (21). Therefore, this study was carried out to identify potential risk factors for the occurrence of BCRL in breast cancer patients who received NAC and ALND, with the aim of optimizing lymphedema surveillance and improving patient education on BCRL.
Methods
Eligibility criteria
This cohort study enrolled 354 newly diagnosed breast cancer patients who had undergone primary breast cancer surgery from June 2015 to June 2020 at Fujian Medical University’s Union Hospital. The inclusion criteria were as follows: (1) 18 years of age or older, (2) AJCC clinical T0-4N1-2M0 breast cancer patients who underwent fine-needle aspiration or core needle biopsy of an axillary node with documented nodal metastasis at diagnosis, prior to neoadjuvant chemotherapy, (3) data available at baseline and at least one post-operative follow-up time point, (4) received NAC and subsequent ALND surgery. Exclusion criteria were as follows: (1) bilateral breast cancer, (2) existing arm edema before surgery, (3) presence of severe cardiac or renal disease, (4) local or systemic recurrence of breast cancer. Finally, all 336 patients who received NAC and subsequent breast surgery and ALND were successfully included in the cohort. The hospital ethics committee approved the protocol, and informed consent was obtained from all study patients.
Measurement and assessment of lymphedema
Breast cancer-related lymphedema is diagnosed by objective measurement of limb circumference and subjective assessment (self-reported symptom). Measurements were obtained at the following time points: before surgery (after completion of neoadjuvant chemotherapy) (baseline), and 1, 3, 6, 12, 18, 24, and 30 months after surgery. The professionally trained nurse measured the limb circumference of both arms with flexible tape at four anatomical locations: (1) metacarpal, (2) wrist, (3) 10 cm below the lateral condyle, and (4) 10 cm above the lateral condyle, with the patient in a standing position with the elbow extended and the forearm in flexion (22). Additionally, patients were asked at each follow-up visit whether they were currently experiencing swelling, heaviness, numbness, tightness, or pain in the affected arm.
The patient was diagnosed with BCRL if the circumference of the affected arm (arm on the side where the axillary dissection was performed) was greater than 2 cm at one or more anatomical locations compared to baseline and the contralateral arm. The formula for calculating the increase in arm circumference was as follows: (ipsilateral time point value - ipsilateral baseline value) - (contralateral time point value - contralateral baseline value) (23). Additionally, patients who reported at least one of four self-reported arm symptoms (swelling, heaviness, tightness, or numbness), but with less than a 2 cm interlimb difference, were also considered to have lymphedema (24). The time to develop BCRL was calculated from the date of the defined breast surgery to the date of BCRL diagnosis (25).
Statistical analysis
The aim of this analysis was to assess the risk factors associated with lymphedema. Categorical variables were presented as the number of patients (%) and differences between the two groups were assessed using the χ2 and Fisher’s exact tests. The cumulative risk of various factors was determined using univariate logistic regression analysis. Factors associated with the development of lymphedema were analyzed using univariate logistic regression, without considering the interference of other factors. Variables with p-values < 0.1 in univariate analysis were included in multivariate logistic regression. After adjusting for other factors, multivariate logistic regression was employed to identify statistically significant risk factors correlated with lymphedema development. A statistically significant difference was defined as a p-value less than 0.05 in multivariate analysis. Point estimates (e.g., percentage of patients, hazard ratio [HR], and 95% confidence interval [CI] were used to summarize variables and correlations. All statistical analyses were performed using SPSS software version 25.
Result
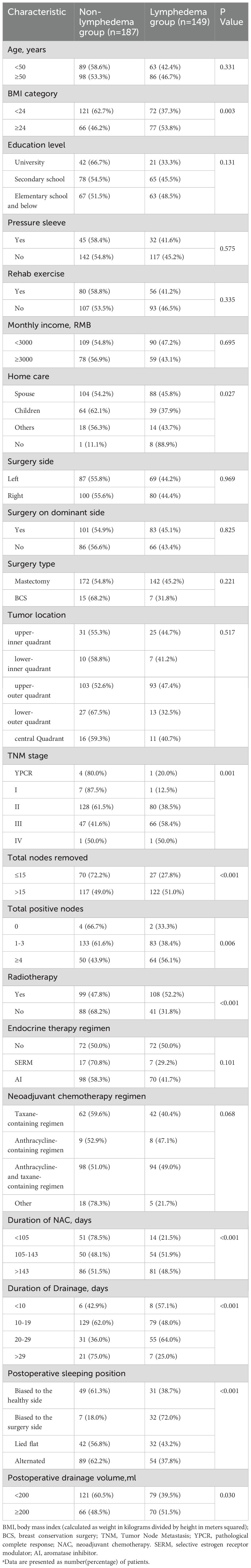
This study involved 336 patients with unilateral breast cancer, of whom 147 (43.75%) had BCRL. The comparison of demographics, clinical, and lifestyle characteristics between the BCRL and non-BCRL cohorts is presented in Table 1. Patients with BCRL exhibited higher BMI, more positive lymph nodes, and a greater number of lymph nodes removed. They were also more likely to undergo radiotherapy and longer NAC treatment compared to those without BCRL. Additionally, significant differences (P < 0.05) were observed in the following variables: home care, TNM staging, duration of drainage, postoperative sleeping position and postoperative drainage volume.
In the univariate analysis, ten factors were correlated with the development of BCRL and were included in the multivariate analysis (Table 2). The multivariate analysis identified five factors as independent predictors of BCRL (P < 0.05): (1) The BCRL rates significantly increased in relation to the total lymph nodes removed >15 (HR = 1.593; P = 0.036). (2) Radiotherapy was associated with a higher rate of BCRL (HR = 1.611; P = 0.020). (3) The BCRL rates were lower among patients who received NAC for 105 days or shorter (HR = 0.471; P = 0.020). (4) Women with drainage for 20-29 days were found to be at a significantly increased risk of developing BCRL compared to those with drainage for 10-19 days (HR = 1.568; P = 0.028). (5) The HR of BCRL for the postoperative sleeping position (biased to the surgery side versus biased to the healthy side) was 2.033 with P = 0.019. However, after adjusting for other variables, BMI, postoperative drainage volume, home care, total positive nodes, and endocrine therapy were not statistically significant in the multivariate analysis. The 2.5-year cumulative risk of BCRL according to the five independent risk factors that were found to be significantly different in both univariate and multivariate analyses (radiotherapy, total lymph nodes removed, duration of NAC, duration of drainage, and postoperative sleeping position) is presented in Figure 1.

Figure 1. Lymphedema cumulative risk based on the independent risk factors. (A) The cumulative risk of lymphedema according to radiotherapy. (B) The cumulative risk of lymphedema according to the total number of nodes removed. (C) The cumulative risk of lymphedema according to the duration of drainage. (D) The cumulative risk of lymphedema according to the duration of NAC. (E) The cumulative risk of lymphedema according to the postoperative sleeping position. NAC, neoadjuvant chemotherapy; HR, hazard ratio; CI, confidence interval.
Discussion
The aim of this study was to explore potential risk factors for the development of BCRL in breast cancer patients who received NAC and ALND. Five independent risk factors were identified, including radiotherapy, duration of NAC, number of excised lymph nodes, duration of drainage, and postoperative sleeping positions. Our research encompasses a substantial dataset derived from a considerable patient cohort, coupled with a meticulously followed-up period. We believe that our findings could provide credible evidence prompting alterations in management strategies and enhancing patient care in this context.
The placement of a closed-suction drain in the mastectomy site and axilla after breast cancer surgery aims to decrease postoperative complications, particularly seroma formation (26–28). However, in our study, we found that patients with a longer duration of drainage were at a higher risk of BCRL. Similarly, Saadet et al. stated that the long duration of the axillary drain was a risk factor for BCRL (P = 0.045) (16). Two reasons may explain this phenomenon. Firstly, a longer duration of drainage reflects a higher degree of lymphatic vessel damage, supporting the notion that more extensive axillary surgery increases the incidence of BCRL (29). Secondly, patients with drainage tubes need to immobilize the affected limb to reduce drainage volume (30). However, carrying the drainage tube for a long time can lead to stiffness in the arm, causing the optimal time for postoperative limb rehabilitation exercises to be missed. A cross-sectional study of 775 patients showed that women who exercised their affected arm decreased the risk of developing BCRL through a potential mechanism called the “muscle pump” (15, 31, 32). At the same time, Our results showed that the >29 days drainage group had the lowest likelihood of lymphedema, which appears contradictory but is not statistically significant, likely due to the small sample size in this group.
A meta-analysis reported that the decision to remove drainage based on the amount of drainage would reduce the incidence of seroma, an independent risk factor for lymphedema, compared to short-term removal of drainage (33, 34). However, due to management regarding drain placement, the number of drains, and hospitalization varying widely between breast units (35), there are no widely applicable criteria for the removal of drainage. Therefore, future multicenter and larger cohort studies based on uniform criteria are required to better understand the relationship between drainage time and BCRL.
The relationship between postoperative sleeping position and BCRL has never been studied before. Our study found that sleeping biased towards the affected arm significantly increased the incidence of BCRL (HR = 2.033; p = 0.019). Prolonged compression of the affected limb impedes the return of lymphatic fluid, disrupting the morphology and function of the lymphatic system and ultimately leading to BCRL. Furthermore, prolonged compression of the limb leads to ischemia of the subcutaneous tissues, causing a reduction in subcutaneous fat and muscle atrophy, which further affects the functional recovery of the lymphatics. Patients often consciously avoid putting pressure on the affected arm in the early postoperative period. However, later in life, they may think they have recovered from breast cancer and may unconsciously sleep on the affected side. Additionally, postoperative sleeping position may be related to whether the surgery was performed on the dominant hand or not, but a chi-square test showed no statistical difference between the two (p = 0.252). This finding suggests that some breast cancer survivors overlook the negative impact of common lifestyle habits on BCRL, highlighting the importance of correcting postoperative sleep position.
The association between radiotherapy and BCRL is well documented in the literature (24, 25, 36, 37). In our study, patients who received radiotherapy were 1.8 times more likely to develop BCRL than those who did not. A retrospective study of 7,617 patients showed that patients with more extensive radiation fields were at greater risk of lymphedema; compared with no radiation or breast/chest wall radiation alone, regional lymph node irradiation (RNI) increased the risk of BCRL by 2-4 times (24). Additionally, the total number of lymph nodes removed is another well-known independent risk factor for BCRL (6, 9, 38, 39). Hwa Kyung Byun et al. reported that the 3-year cumulative BCRL rates were 3.0%, 10.0%, 20.2%, and 24.4% in patients with 0 to 5, 6 to 10, 11 to 15, and >15 lymph nodes removed, respectively (P < 0.001) (24). Interestingly, several reports suggest that the combination of ALND and radiotherapy has a synergistic effect on the development of BCRL (9, 25, 36). However, the relationship between radiotherapy regimens and the number of lymph nodes removed has rarely been studied, which may be helpful in developing individualized radiotherapy regimens for breast cancer patients receiving axillary dissection to reduce the incidence of BCRL.
In recent years, increasing attention has focused on studying the risk factors for BCRL in the NAC setting. Giacomo Montagna et al. found that NAC was an independent risk factor for BCRL (OR = 2.10; 95%CI = 1.16-3.95; P = 0.01) (40). Our study suggests a lower incidence of BCRL in patients with a shorter duration of NAC, which is in line with a study reporting that a longer NAC duration was correlated with increased BCRL incidence (18). In general, two possible factors contribute to this result. Firstly, the number of cycles of chemotherapy infusion in the ipsilateral arm was reported as an independent risk factor for developing BCRL by José Luiz B. Bevilacqua et al. (41). Secondly, regarding the specific toxicity of chemotherapy agents, many studies showed that taxane-based chemotherapy could result in BCRL by increasing extracellular fluid accumulation (42–44). Therefore, more attention should be paid to patients with a longer duration of NAC and those treated with taxane-based chemotherapy.
The strength of our study lies in the collection of bilateral arm measurements at multiple time points over a 30-month period, including preoperative measurements, which helps control for potential size differences between the dominant and non-dominant arms. However, the study has the following limitations: Firstly, in the absence of a gold standard, the diagnosis of BCRL in our study was based on a 2-cm difference in circumference and self-reported symptoms, which are inherently flawed and diagnostically imprecise, potentially leading to misdiagnosis. Secondly, we only looked at whether radiotherapy was given and did not analyze the effect of different radiotherapy regimens, which may limit the applicability of our results to more recently established treatments. However, many studies have shown that RNI is associated with a higher risk of developing BCRL than radiation to the breast or chest wall alone (24, 25). Finally, the overall rate of BCRL may be underestimated due to loss to follow-up, as some participants did not complete all scheduled assessments, leading to incomplete data. Future studies with more comprehensive follow-up strategies are necessary to mitigate the impact of loss to follow-up and provide a more accurate estimate of BCRL rates.
Conclusion
In conclusion, our research showed that more than 40% of breast cancer patients who received NAC and ALND suffered from BCRL. We identified five independent risk factors associated with the development of BCRL: radiotherapy, duration of NAC, number of lymph nodes removed, duration of drainage, and postoperative sleeping position. Healthcare workers should focus on monitoring patients with one or more of these factors to enable early detection and intervention. Further research is needed to investigate the effects of drainage time and sleep position on the development of BCRL.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Fujian Medical University Union Hospital ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JF: Conceptualization, Data curation, Investigation, Project administration, Writing – original draft. RC: Data curation, Formal Analysis, Methodology, Software, Writing – original draft. LH: Data curation, Investigation, Resources, Writing – original draft. LB: Project administration, Software, Validation, Writing – original draft. ZL: Methodology, Project administration, Software, Writing – original draft. WJ: Data curation, Software, Supervision, Writing – original draft. JZ: Project administration, Supervision, Writing – review & editing. CW: Validation, Visualization, Writing – review & editing, Supervision. YL: Conceptualization, Funding acquisition, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Fujian Provincial Department of Finance (2022CZ013).
Acknowledgments
The authors thank all of the women who participated in the study and the assistance of Fujian Medical University’s Union Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin. (2019) 69:438–51. doi: 10.3322/caac.21583
2. Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women's Health Study. J Clin Oncol. (2008) 26:5689–96. doi: 10.1200/jco.2008.16.4731
3. Rief W, Bardwell WA, Dimsdale JE, Natarajan L, Flatt SW, Pierce JP. Long-term course of pain in breast cancer survivors: a 4-year longitudinal study. Breast Cancer Res Treat. (2011) 130:579–86. doi: 10.1007/s10549-011-1614-z
4. Khan F, Amatya B, Pallant JF, Rajapaksa I. Factors associated with long-term functional outcomes and psychological sequelae in women after breast cancer. Breast. (2012) 21:314–20. doi: 10.1016/j.breast.2012.01.013
5. Bakar Y, Tuğral A, Üyetürk Ü. Measurement of local tissue water in patients with breast cancer-related lymphedema. Lymphat Res Biol. (2018) 16:160–4. doi: 10.1089/lrb.2016.0054
6. Shen A, Lu Q, Fu X, Wei X, Zhang L, Bian J, et al. Risk factors of unilateral breast cancer-related lymphedema: an updated systematic review and meta-analysis of 84 cohort studies. Support Care Cancer. (2022) 31:18. doi: 10.1007/s00520-022-07508-2
7. Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. (2001) 92:1368–77. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9
8. Chachaj A, Małyszczak K, Pyszel K, Lukas J, Tarkowski R, Pudełko M, et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology. (2010) 19:299–305. doi: 10.1002/pon.1573
9. Kim M, Kim SW, Lee SU, Lee NK, Jung SY, Kim TH, et al. A model to estimate the risk of breast cancer-related lymphedema: combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys. (2013) 86:498–503. doi: 10.1016/j.ijrobp.2013.02.018
10. Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. (2010) 116:5138–49. doi: 10.1002/cncr.25458
11. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. (2013) 14:500–15. doi: 10.1016/s1470-2045(13)70076-7
12. Jung SY, Shin KH, Kim M, Chung SH, Lee S, Kang HS, et al. Treatment factors affecting breast cancer-related lymphedema after systemic chemotherapy and radiotherapy in stage II/III breast cancer patients. Breast Cancer Res Treat. (2014) 148:91–8. doi: 10.1007/s10549-014-3137-x
13. Leysen L, Beckwée D, Nijs J, Pas R, Bilterys T, Vermeir S, et al. Risk factors of pain in breast cancer survivors: a systematic review and meta-analysis. Support Care Cancer. (2017) 25:3607–43. doi: 10.1007/s00520-017-3824-3
14. Rockson SG. Lymphedema after breast cancer treatment. N Engl J Med. (2018) 379:1937–44. doi: 10.1056/NEJMcp1803290
15. Liu YF, Liu JE, Zhu Y, Mak YW, Qiu H, Liu LH, et al. Development and validation of a nomogram to predict the risk of breast cancer-related lymphedema among Chinese breast cancer survivors. Support Care Cancer. (2021) 29:5435–45. doi: 10.1007/s00520-021-06122-y
16. Ugur S, Arıcı C, Yaprak M, Mescı A, Arıcı GA, Dolay K, et al. Risk factors of breast cancer-related lymphedema. Lymphat Res Biol. (2013) 11:72–5. doi: 10.1089/lrb.2013.0004
17. Wang H, Mao X. Evaluation of the efficacy of neoadjuvant chemotherapy for breast cancer. Drug Des Devel Ther. (2020) 14:2423–33. doi: 10.2147/dddt.S253961
18. Armer JM, Ballman KV, McCall L, Ostby PL, Zagar E, Kuerer HM, et al. Factors associated with lymphedema in women with node-positive breast cancer treated with neoadjuvant chemotherapy and axillary dissection. JAMA Surg. (2019) 154:800–9. doi: 10.1001/jamasurg.2019.1742
19. Wilke LG, McCall LM, Posther KE, Whitworth PW, Reintgen DS, Leitch AM, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. (2006) 13:491–500. doi: 10.1245/aso.2006.05.013
20. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. Jama. (2017) 318:918–26. doi: 10.1001/jama.2017.11470
21. Fu MR, Chen CM, Haber J, Guth AA, Axelrod D. The effect of providing information about lymphedema on the cognitive and symptom outcomes of breast cancer survivors. Ann Surg Oncol. (2010) 17:1847–53. doi: 10.1245/s10434-010-0941-3
22. Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. (2005) 3:208–17. doi: 10.1089/lrb.2005.3.208
23. Armer JM. The problem of post-breast cancer lymphedema: impact and measurement issues. Cancer Invest. (2005) 23:76–83. doi: 10.1081/cnv-200048707
24. Byun HK, Chang JS, Im SH, Kirova YM, Arsene-Henry A, Choi SH, et al. Risk of lymphedema following contemporary treatment for breast cancer: an analysis of 7617 consecutive patients from a multidisciplinary perspective. Ann Surg. (2021) 274:170–8. doi: 10.1097/sla.0000000000003491
25. Nguyen TT, Hoskin TL, Habermann EB, Cheville AL, Boughey JC. Breast cancer-related lymphedema risk is related to multidisciplinary treatment and not surgery alone: results from a large cohort study. Ann Surg Oncol. (2017) 24:2972–80. doi: 10.1245/s10434-017-5960-x
26. Oertli D, Laffer U, Haberthuer F, Kreuter U, Harder F. Perioperative and postoperative tranexamic acid reduces the local wound complication rate after surgery for breast cancer. Br J Surg. (1994) 81:856–9. doi: 10.1002/bjs.1800810621
27. Talbot ML, Magarey CJ. Reduced use of drains following axillary lymphadenectomy for breast cancer. ANZ J Surg. (2002) 72:488–90. doi: 10.1046/j.1445-2197.2002.02456.x
28. Troost MS, Kempees CJ, de Roos MAJ. Breast cancer surgery without drains: no influence on seroma formation. Int J Surg. (2015) 13:170–4. doi: 10.1016/j.ijsu.2014.11.050
29. Goldberg JI, Wiechmann LI, Riedel ER, Morrow M, Van Zee KJ. Morbidity of sentinel node biopsy in breast cancer: the relationship between the number of excised lymph nodes and lymphedema. Ann Surg Oncol. (2010) 17:3278–86. doi: 10.1245/s10434-010-1155-4
30. Browse DJ, Goble D, Jones PA. Axillary node clearance: who wants to immobilize the shoulder? Eur J Surg Oncol. (1996) 22:569–70. doi: 10.1016/s0748-7983(96)92164-2
31. Stanton AW, Modi S, Mellor RH, Levick JR, Mortimer PS. Recent advances in breast cancer-related lymphedema of the arm: lymphatic pump failure and predisposing factors. Lymphat Res Biol. (2009) 7:29–45. doi: 10.1089/lrb.2008.1026
32. Hashemi HS, Fallone S, Boily M, Towers A, Kilgour RD, Rivaz H. Assessment of mechanical properties of tissue in breast cancer-related lymphedema using ultrasound elastography. IEEE Trans Ultrason Ferroelectr Freq Control. (2019) 66:541–50. doi: 10.1109/tuffc.2018.2876056
33. Droeser RA, Frey DM, Oertli D, Kopelman D, Baas-Vrancken Peeters MJ, Giuliano AE, et al. Volume-controlled vs no/short-term drainage after axillary lymph node dissection in breast cancer surgery: a meta-analysis. Breast. (2009) 18:109–14. doi: 10.1016/j.breast.2009.02.003
34. Toyserkani NM, Jørgensen MG, Haugaard K, Sørensen JA. Seroma indicates increased risk of lymphedema following breast cancer treatment: A retrospective cohort study. Breast. (2017) 32:102–4. doi: 10.1016/j.breast.2017.01.009
35. Gupta R, Pate K, Varshney S, Goddard J, Royle GT. A comparison of 5-day and 8-day drainage following mastectomy and axillary clearance. Eur J Surg Oncol. (2001) 27:26–30. doi: 10.1053/ejso.2000.1054
36. Gross JP, Whelan TJ, Parulekar WR, Chen BE, Rademaker AW, Helenowski IB, et al. Development and validation of a nomogram to predict lymphedema after axillary surgery and radiation therapy in women with breast cancer from the NCIC CTG MA. 20 Randomized Trial. Int J Radiat Oncol Biol Phys. (2019) 105:165–73. doi: 10.1016/j.ijrobp.2019.05.002
37. Konishi T, Tanabe M, Michihata N, Matsui H, Nishioka K, Fushimi K, et al. Risk factors for arm lymphedema following breast cancer surgery: a Japanese nationwide database study of 84,022 patients. Breast Cancer. (2023) 30:36–45. doi: 10.1007/s12282-022-01395-5
38. Togawa K, Ma H, Sullivan-Halley J, Neuhouser ML, Imayama I, Baumgartner KB, et al. Risk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort study. Breast Cancer Res. (2014) 16:414. doi: 10.1186/s13058-014-0414-x
39. Kilbreath SL, Refshauge KM, Beith JM, Ward LC, Ung OA, Dylke ES, et al. Risk factors for lymphoedema in women with breast cancer: A large prospective cohort. Breast. (2016) 28:29–36. doi: 10.1016/j.breast.2016.04.011
40. Montagna G, Zhang J, Sevilimedu V, Charyn J, Abbate K, Gomez EA, et al. Risk factors and racial and ethnic disparities in patients with breast cancer-related lymphedema. JAMA Oncol. (2022) 8:1195–200. doi: 10.1001/jamaoncol.2022.1628
41. Bevilacqua JL, Kattan MW, Changhong Y, Koifman S, Mattos IE, Koifman RJ, et al. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol. (2012) 19:2580–9. doi: 10.1245/s10434-012-2290-x
42. Swaroop MN, Ferguson CM, Horick NK, Skolny MN, Miller CL, Jammallo LS, et al. Impact of adjuvant taxane-based chemotherapy on development of breast cancer-related lymphedema: results from a large prospective cohort. Breast Cancer Res Treat. (2015) 151:393–403. doi: 10.1007/s10549-015-3408-1
43. Penn IW, Chang YC, Chuang E, Chen CM, Chung CF, Kuo CY, et al. Risk factors and prediction model for persistent breast-cancer-related lymphedema: a 5-year cohort study. Support Care Cancer. (2019) 27:991–1000. doi: 10.1007/s00520-018-4388-6
Keywords: breast cancer, lymphedema, risk factors, ALND, neoadjuvant chemotherapy
Citation: Fu J, Chen R, He L, Bao L, Lin Z, Jiang W, Zhang J, Wang C and Lin Y (2024) Factors affecting lymphedema after neoadjuvant chemotherapy and axillary dissection in female breast cancer patients: a retrospective cohort study based on the Chinese population. Front. Oncol. 14:1436748. doi: 10.3389/fonc.2024.1436748
Received: 22 May 2024; Accepted: 08 October 2024;
Published: 29 October 2024.
Edited by:
San-Gang Wu, First Affiliated Hospital of Xiamen University, ChinaReviewed by:
Junlin Liao, The University of Iowa, United StatesFrancesca Piccotti, Scientific Clinical Institute Maugeri (ICS Maugeri), Italy
Copyright © 2024 Fu, Chen, He, Bao, Lin, Jiang, Zhang, Wang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Zhang, zjie1979@gmail.com; Chuan Wang, dr_chuanwang@fjmu.edu.cn; Yanjuan Lin, fjxhyjl@163.com
†These authors have contributed equally to this work and share first authorship
 Jianqin Fu
Jianqin Fu Ruiliang Chen
Ruiliang Chen Lijuan He1,2,3
Lijuan He1,2,3 Chuan Wang
Chuan Wang Yanjuan Lin
Yanjuan Lin