- 1Department of Radiation Medicine, Georgetown University Hospital, Washington, DC, United States
- 2Department of Urology, The University of Kansas Health System, Kansas City, KS, United States
- 3Biotechnology Research Institute, North Carolina Central University, Durham, NC, United States
Purpose: Following treatment for localized prostate cancer, a subset of men will develop recurrent disease in the abdominopelvic nodes. For radiation therapy (RT), the optimal treatment volume, fractionation schedule, and dose remain unanswered questions. We report early outcomes for patients treated with involved-field stereotactic body radiation therapy (SBRT) (IF-SBRT) for nodal oligo-recurrent (NOR) prostate cancer.
Methods: Between January 2018 and October 2023, 67 patients with a median age of 75 with NOR prostate cancer treated with 74 courses of IF-SBRT at Georgetown were eligible for this analysis. NOR was defined as any volume of disease that could be safely treated within an IF. All patients were treated with five-fraction IF-SBRT (27.5–35 Gy). The IF treatment volume was defined as the nodal basin containing the gross disease as well as the immediately adjacent basins. Disease progression was defined as a prostate-specific antigen (PSA) rise above the pretreatment baseline or initiation of a second treatment. Local control and progression-free survival were calculated using the Kaplan–Meier method.
Results: Detection of pre-SBRT NOR was ascertained by prostate-specific membrane antigen (PSMA) (38%), fluciclovine (50%), or MRI/CT (12%). Median follow-up was 50 months (1–262). The median pre-salvage PSA was 6.5 ng/mL (range, 0.1–335). The median number of involved nodes was 3 (range, 1–16). The local control at 1 and 2 years was 98% and 93%, respectively. The 1- and 2-year progression-free survival was 78% and 50%, respectively. Twenty percent of treatment courses were followed by acute Grade 2 gastrointestinal (GI) toxicity: diarrhea (9%) and/or nausea (14%). Two patients (3%) experienced late Grade 2 nausea. On univariate analysis, measures of disease volume such as hormone sensitivity (p = 0.03), increasing involved node number (p = 0.008), and abdominal treatment (p = 0.03) were significantly associated with GI toxicity.
Conclusions: With the widespread adoption of PSMA agents, NORs are likely to increase. The optimal combination of local and systemic therapy in this population is unknown. With a favorable toxicity profile, IF-SBRT represents a safe and convenient local therapy treatment option for an elderly patient population. Patient- and treatment-related factors such as a large number of involved nodes and/or abdominal treatment may be associated with an increased risk of GI toxicity.
Background
The management of nodal oligo-recurrent prostate cancer is an area of active clinical investigation. Prostate cancer has a tropism for lymph nodes, making it a common and, frequently the only, site of metastatic disease (1). The prognosis for men with nodal oligo-recurrent prostate cancer is superior to that for patients with bone metastases (2). In the past, patients were primarily treated with androgen deprivation therapy (ADT) alone (3). Patients receiving ADT with low-volume disease and isolated lymph node metastases fare particularly well with longer remissions (4).
All patients with nodal oligo-recurrences will ultimately progress on ADT despite the initial response. Data are emerging that local radiotherapy may be effective in treating these nodal oligo-recurrences (5). Studies have used several radiotherapy treatment approaches ranging from involved node (focal) stereotactic body radiation therapy (SBRT) to extended nodal conventionally fractionated radiotherapy (ENRT) (1). With the available data, no consensus treatment approach has emerged, with each approach having potential benefits and drawbacks (1).
For patients with disease limited to a few nodal basins, SBRT to the nodal oligo-recurrences may offer long-term local control and increased progression-free survival. SBRT accurately delivers high doses to the target volume while sparing adjacent normal tissues such as the small bowel. SBRT may improve tumor control and reduce treatment-related toxicity through improved target accuracy. This allows for high-dose (>5 Gy per fraction) extremely hypofractionated treatment courses (1–5 fractions) that may be more radiobiologically effective and are certainly more convenient for patients than conventionally fractionated radiotherapy (6).
Most studies until this point have focused on the involved node (focal) SBRT (5). Additional treatment volumes include involved site and involved-field radiotherapy (1). These volumes expand on involved nodes to include an expansion on the gross disease to cover adjacent microscopic diseases. The rationale for these approaches stems from the poor sensitivity of currently available imaging for small-volume nodal disease (<0.5 cm) (7–10), and the adjacent nodal failures are often seen when patients are treated with involved node SBRT (11). Prospective studies with long follow-up including GETUG P07 (12) and PET/CT-Choline-TT (13) have demonstrated very good local and progression-free survival with ENRT and hormonal therapy potentially with increased toxicity (11).
Involved-field radiotherapy involves an expansion to include the involved nodal basin as well as adjacent nodal basins (14). Given the radiobiological advantages of SBRT, it is reasonable to believe that when combined with an involved-field treatment volume with a boost to gross disease and utilization of ADT, the rate of local and distant control could improve (1).
Methods
Patient selection
Patients with nodal oligo-recurrent prostate cancer treated with involved-field SBRT at a MedStar Georgetown University Hospital were eligible for inclusion in this analysis. Patients with bone metastases were excluded. Institutional review board (IRB) approval was obtained for this prospective quality-of-life study (IRB 2012-1175).
SBRT treatment planning and delivery
Nodal oligo-recurrence was defined as any volume of nodal disease that could be safely treated within an involved field. The planning target volume (PTV) for the involved field was defined as the nodal basin containing the gross nodal disease as well as the immediately adjacent nodal basins (Figure 1) (1). Nodal basins were contoured per Radiation Therapy Oncology Group (RTOG) guidelines (15). All patients were treated with five-fraction involved-field SBRT to a dose of 27.5 Gy over 5 to 11 days. Using the linear-quadratic equation with an alpha/beta ratio of 1.5 Gy, the dose was equivalent to 55 Gy in 27–28 fractions. A subset of patients received a prostate-specific membrane antigen (PSMA)-guided simultaneous integrated boost (SIB) up to 30–35 Gy. The total dose to the involved nodes ranged from 27.5 to 35 Gy. In general, pelvic-only recurrences were treated with homogeneous volumetric modulated arc therapy (VMAT) with daily cone beam computed tomography (CBCT) guidance (RapidArc, Varian, Palo Alto, CA, USA), and para-aortic (PA)-only recurrences were treated with non-homogeneous robotic SBRT with continuous spine tracking (CyberKnife, Accuray, Sunnyvale, CA, USA) (16). The choice of RT modality, boost dose, and usage of ADT was dictated by the treating radiation oncologist (SPC).
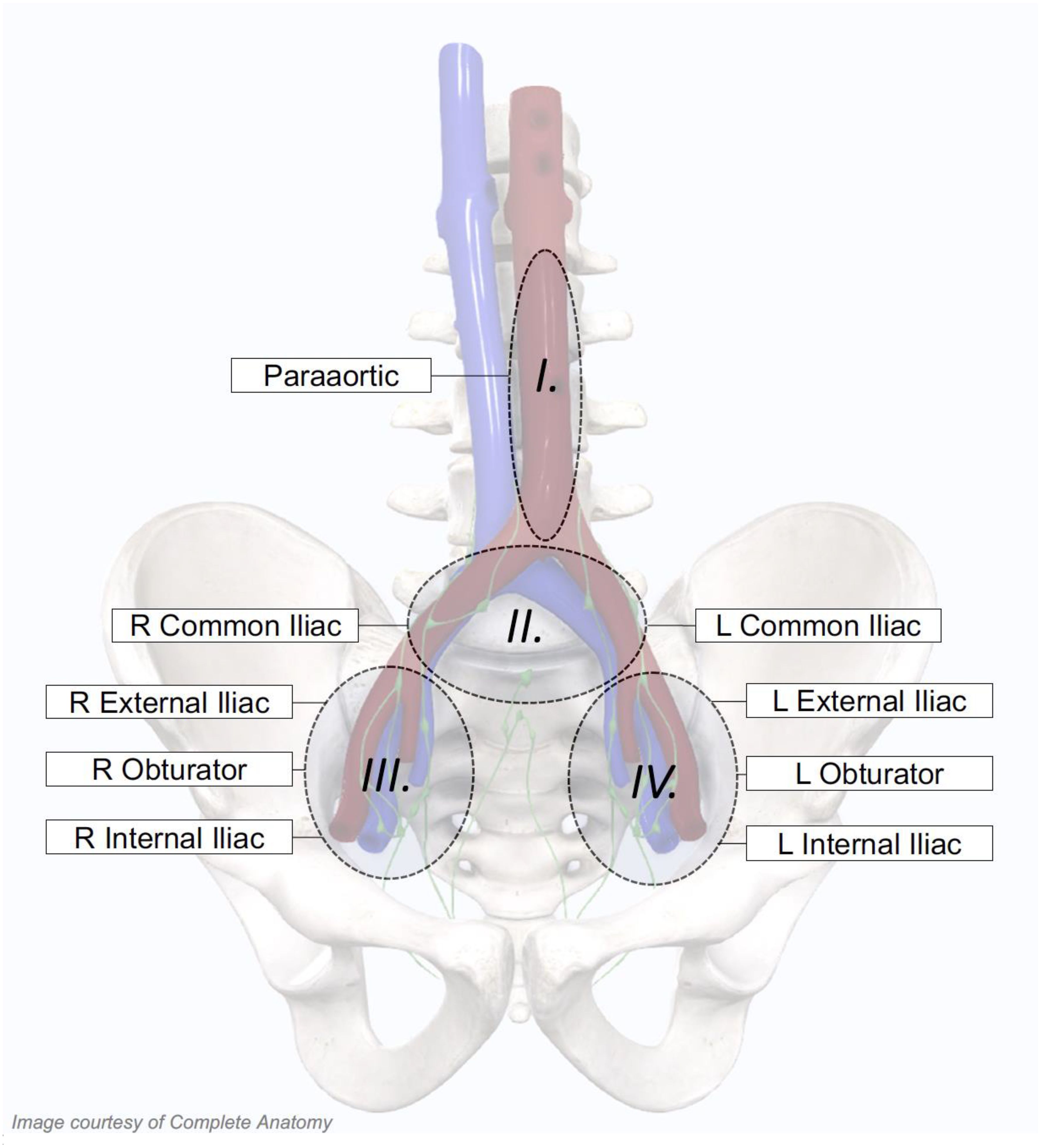
Figure 1. Treatment volumes for individualized involved-field stereotactic body radiation therapy (SBRT). Patient-specific involved fields were utilized, which included specific nodal basins depending on the location of the patients’ PET-positive nodes. For patients with isolated para-aortic involvement, the para-aortic nodal basin alone was treated. If either the left or right common iliac nodal basins were involved, the bilateral common iliac nodal basins were treated. Finally, if the obturator, external iliac, or internal iliac nodal basins were involved on either side, all three unilateral nodal stations were included in the involved field.
Follow-up and statistical analysis
Prostate-specific antigen (PSA) and total testosterone levels were obtained before treatment and during routine follow-up visits every 3 months. PSA response was defined as any decrease in the PSA following treatment. Progression was defined as a follow-up PSA that was less than or equal to baseline or re-initiation of treatment. Local recurrence was defined as any new or growing lesion within the treatment field. Oligo-nodal recurrences outside the irradiated field were treated with a second course of involved-field SBRT.
Toxicity was prospectively documented at follow-up visits using the National Cancer Institute (NCI) Common Toxicity Criteria (CTC) version 4.0. Antidiarrheal and antiemetic use were documented at each follow-up. Acute and late toxicity data were defined as any treatment-related toxicity occurring ≤90 days and >90 days following treatment, respectively. The gastrointestinal toxicities analyzed were nausea and diarrhea. In general, Grade 1 toxicity represents minimal side effects not requiring medications for symptom control. Grade 2 toxicity indicates symptoms requiring a medication (i.e., antiemetic or antidiarrheal). Grade 3 indicates complications requiring minor surgical intervention. At each follow-up visit, toxicity events were scored independently for each of the different toxicity types, and the highest gastrointestinal (GI) toxicity was determined for each patient. Actuarial likelihood estimates for GI toxicities were determined using the Kaplan–Meier method. The highest GI toxicity available for each patient was evaluated for the actuarial analysis.
Results
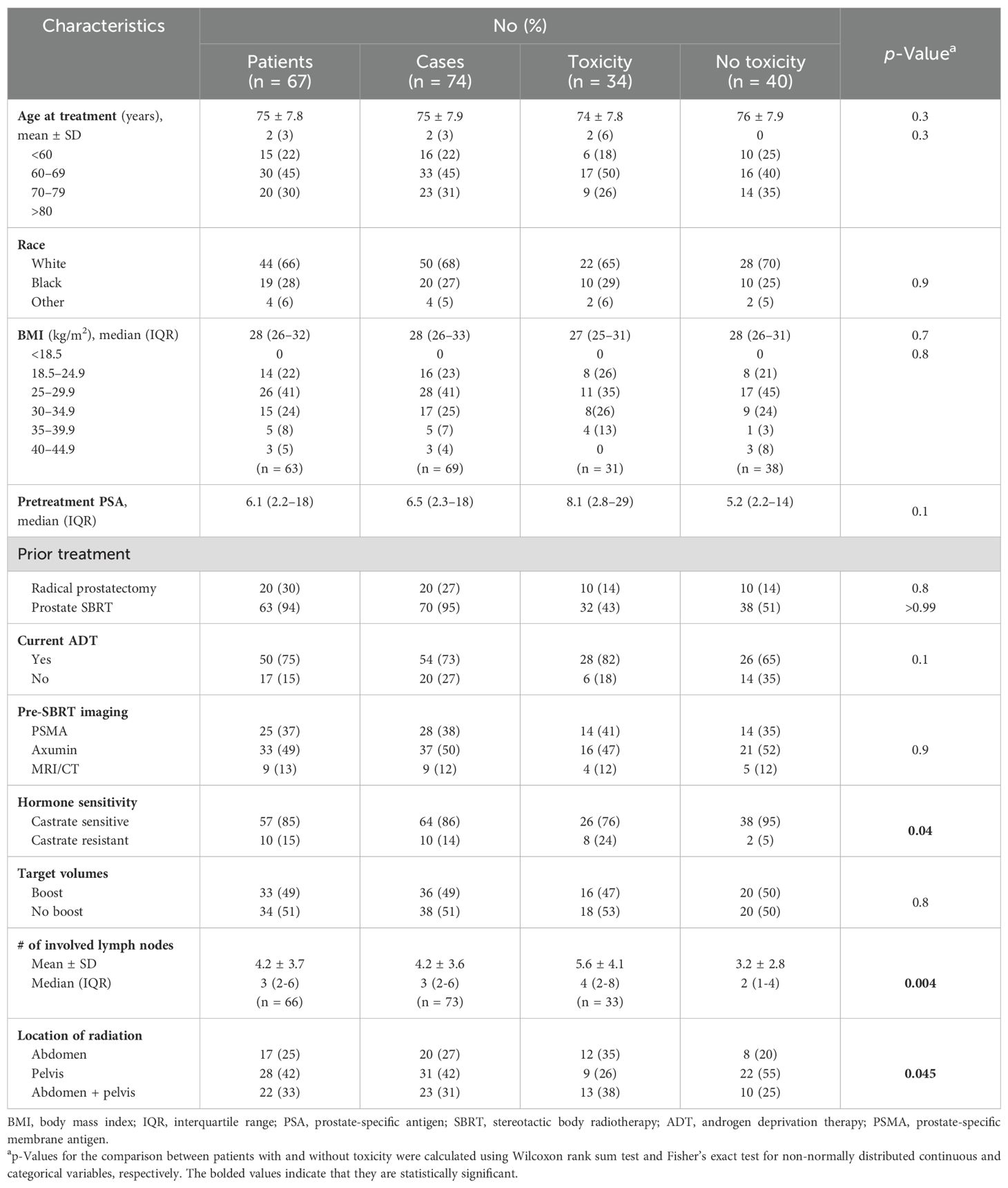
Patient demographic and clinical characteristics are reported in Table 1. Detection of pre-SBRT nodal oligo-recurrence was ascertained by imaging including PSMA (38%), fluciclovine F18 (50%), or MRI/CT (12%) following a PSA rise. The median pre-salvage PSA was 6.5 ng/mL (range, 0.1–335 ng/mL). Of the cases, 86% had hormone-sensitive prostate cancer. The median number of involved nodes was 3 (range, 1–16). In 49% of the cases, involved nodes were boosted to a higher dose. Lower abdominal nodes were included in 58% of treatment fields. Of the treatments, 73% were combined with concurrent hormone therapy. Of the patients, 10% completed a second course of IF-SBRT.
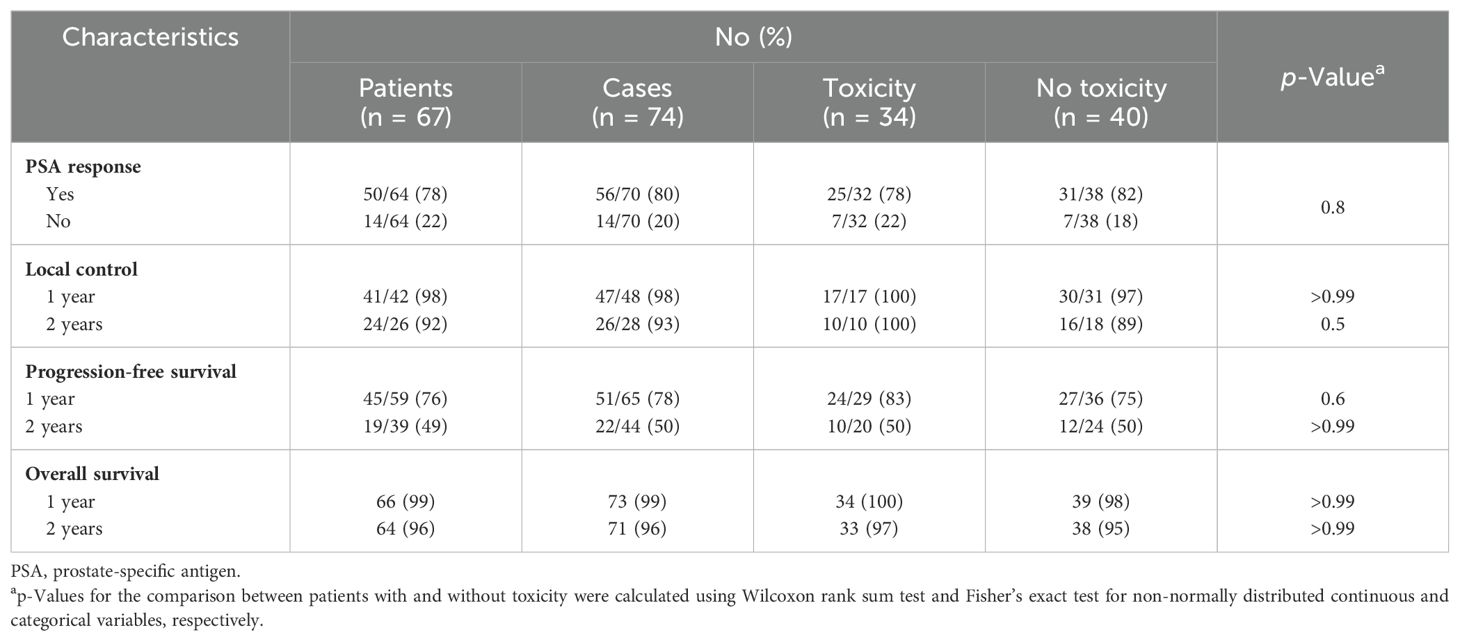
Cancer control outcomes are shown in Table 2. The median follow-up for all patients was 50 months (1–262 months). Of the cases, 80% had a PSA response following treatment. The local control at 1 and 2 years was 98% and 93%, respectively. The 1- and 2-year progression-free survival was 78% and 50%, retrospectively. In the 20 patients who did not receive ADT, the progression-free survival at 1 and 2 years was 56% and 31%, respectively. Of the 54 patients who were treated in the pelvis, there was one local recurrence and 14 distance recurrences. Two of the distant recurrences were in the contralateral pelvis. Of the 43 patients who were treated in the abdomen, there were no local failures and 11 distant recurrences, including one in the upper abdomen. The overall survival at 1 and 2 years was 99% and 96%, respectively. Three patients died from prostate cancer.
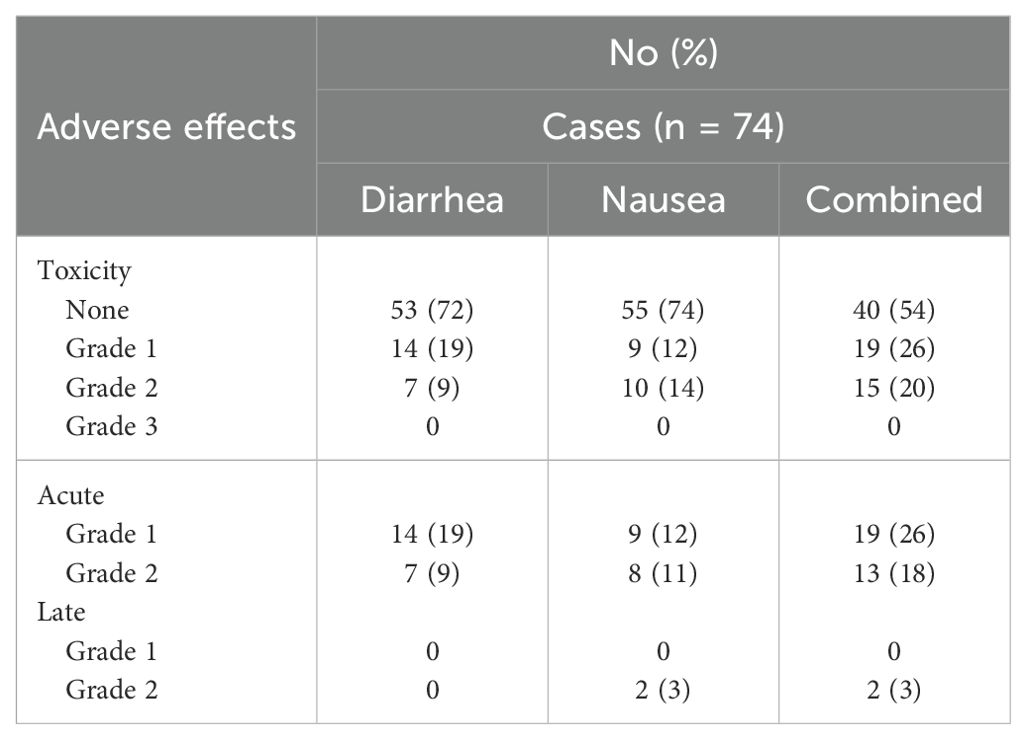
Common Terminology Criteria for Adverse Events (CTCAE)-graded GI toxicities are shown in Table 3. Of the treatment courses, 20% were followed by acute Grade 2 GI toxicity: diarrhea (9%) and/or nausea (14%). Two patients (3%) experienced late Grade 2 nausea. No Grade 3+ side effects occurred at any time following treatment. On univariate analysis (Table 4), measures of disease volume such as hormone sensitivity, increasing involved node number, and abdominal treatment were significantly associated with GI toxicity. No demographic or clinical characteristics were significant in the multivariate analysis (Table 5).
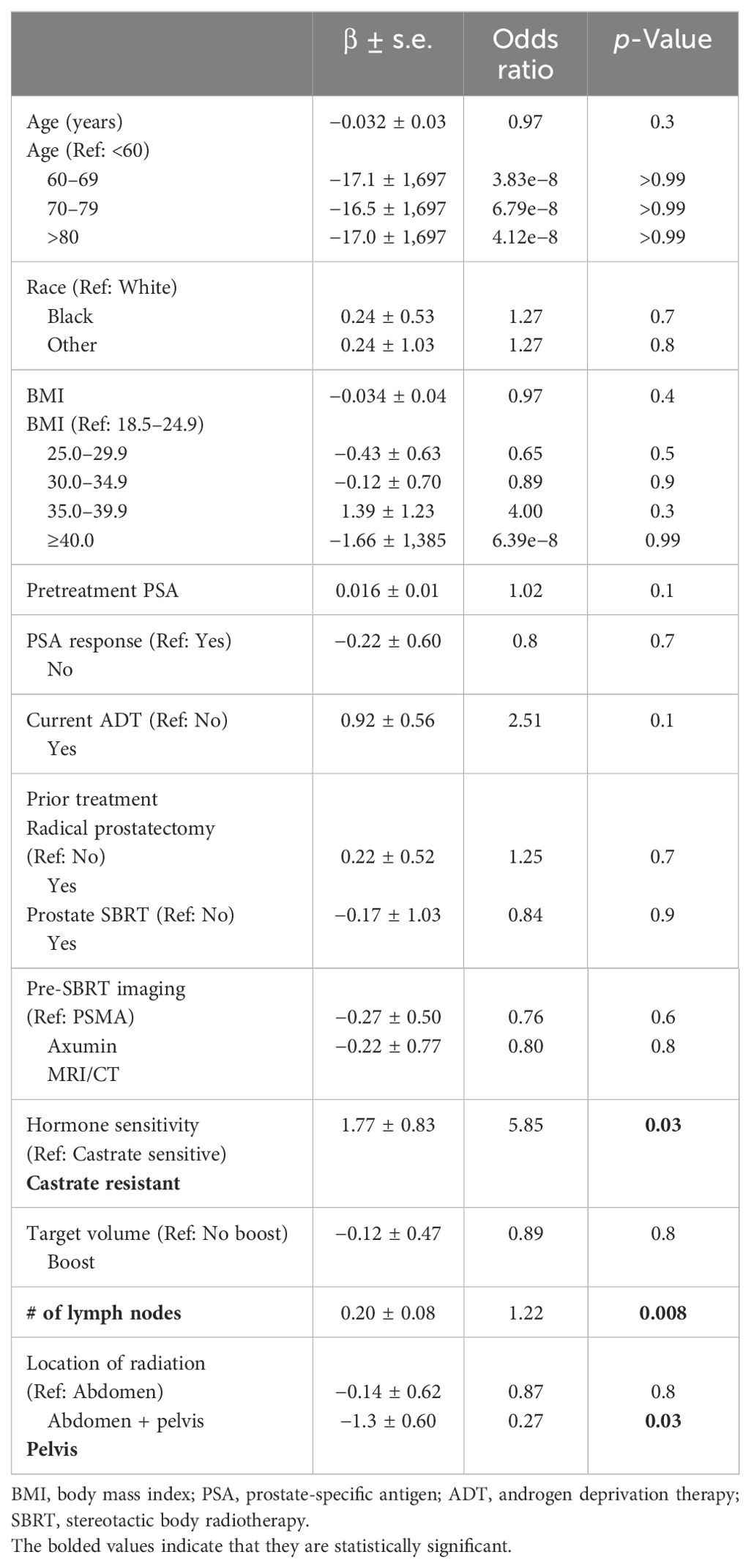
Table 4. Univariate logistic regression model predicting the risk of oligometastatic prostate cancer with toxicity vs. no toxicity.
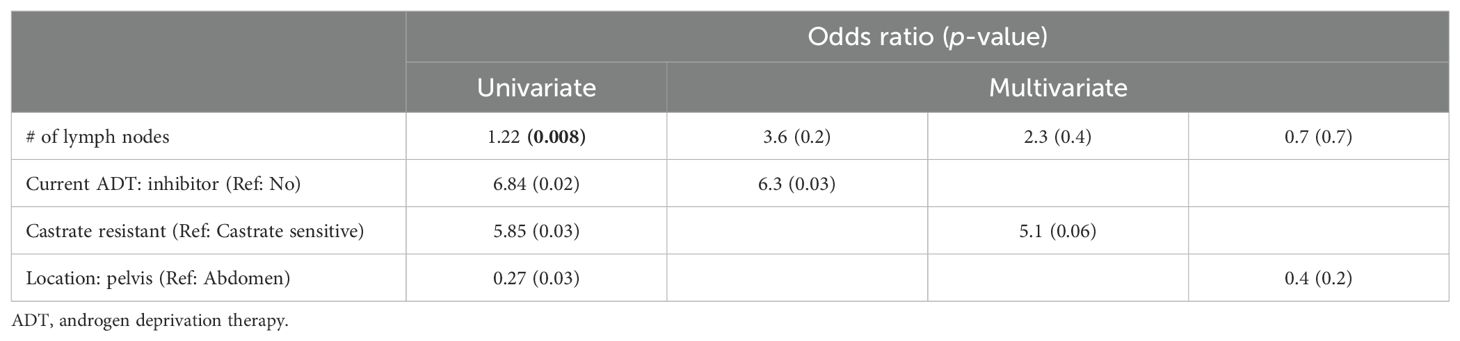
Table 5. Multivariate logistic regression model predicting the risk of oligometastatic prostate cancer with toxicity vs. no toxicity.
Discussion
Our institutional experience adds to the growing body of evidence supporting the safety and effectiveness of SBRT for nodal oligo-recurrent prostate cancer. Our early PSA outcomes have been favorable with low rates of early local recurrence considering the inclusion of patients with castrate-resistant disease and relatively low biologically effective dose (BED) (17). As with other SBRT series and other radiation therapy modalities, recurrences were common in higher nodal stations (18). Considering that our series included a low rate of pretreatment PSMA imaging and a relatively high baseline PSA/nodal burden, our suboptimal 50% actuarial 2-year biochemical failure-free survival rate is not unexpected. It is hoped that with salvage at lower baseline PSAs with pretreatment PSMA staging, this percentage would increase.
Toxicity following lower abdominal/upper pelvic SBRT was similar to that following conventionally fractionated radiation therapy at these sites (11). Acute and late Grade 2 GI toxicity were observed in 20% and 3%, respectively. Antiemetic or antidiarrheal utilization was the most common Grade 2 toxicity. There were no Grade 3 toxicities. Knowledge of these GI toxicities and their resolution with conservative management will enable clinicians to relieve patient concerns. Our outcomes are similar to those of para-aortic nodes treated with conventionally fractionated radiation therapy and pelvic nodes treated with SBRT (11, 19–22).
We argue against proposing a strict numerical cut-off for the number of nodes to qualify for nodal oligo-recurrent nodal disease. Increased nodal involvement was associated with increased GI toxicity. However, GI toxicity was low-grade and short-lived in most cases. Instead, we advocate for any volume of nodal disease that can be safely treated with curative intent. This approach preserves the rationale for treating nodal recurrent disease, which is to halt or delay widespread disease progression while maximizing the number of patients who may benefit. Because the rationale for treatment of nodal disease is diminished if cancer has already spread past the lymph nodes, one must be relatively certain that no other systemic disease is present. For this reason, we recommend oligo-recurrence only be defined with the use of advanced PET imaging, preferably PSMA PET, if available, which has been shown to outperform other agents in this clinical setting (23).
Our study has several limitations. First, it is limited by the retrospective nature of the analysis. However, subjects were accrued consecutively to a prospective quality of life (QOL) study, and all data were collected prospectively in a centralized database, therefore limiting selection and reporting bias present in pure retrospective studies. Second, the study was initiated prior to the widespread availability of PSMA PET scans in the United States. Therefore, not all patients had a pretreatment PSMA scan, so the extent of the disease was likely underestimated. If all patients had received a pretreatment PSMA PET, it is likely that cancer control would have been improved (24).
Conclusions
Involved-field SBRT for nodal oligo-recurrent prostate cancer is well-tolerated. Local recurrence in the radiation field was uncommon. As shown previously, out-of-field recurrences were common in superior nodal stations but potentially treatable (25, 26). Rates of late GI toxicity are comparable to those of alternative radiation therapy. Patient- and treatment-related factors such as a large number of involved nodes and/or abdominal treatment may be associated with an increased risk of GI toxicity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Georgetown-MedStar Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MJK (1st author): Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Funding acquisition. PP: Data curation, Writing – review & editing. MJK (3rd author): Formal Analysis, Writing – review & editing. M-KL: Writing – review & editing. TO’C: Writing – review & editing. AZ: Writing – review & editing. MD: Writing – review & editing. DK: Writing – review & editing. SS: Writing – review & editing. MCa: Writing – review & editing. SC: Writing – original draft, Writing – review & editing. MC: Conceptualization, Validation, Resources, Visualization, Software, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by The James and Theodore Pedas Family Foundation. SC and DK acknowledge the grant R01MD012767 from the National Institute on Minority Health and Health Disparities.
Conflict of interest
SC serves as a clinical consultant to Accuray, Inc., Blue Earth Diagnostics, Pfizer, and Sumitomo Pharma America, Inc. The Department of Radiation Medicine at Georgetown University Hospital receives a grant from Accuray to support a research coordinator.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADT, androgen deprivation therapy; CTC, Common Toxicity Criteria; GI, gastrointestinal; MDT, metastasis-directed therapy; PET, positron emission tomography; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; SBRT, stereotactic body radiation therapy; VMAT, volumetric modulated arc therapy.
References
1. Carrasquilla M, Creswell ML, Pepin AN, Wang E, Forsthoefel M, McGunigal M, et al. Rationale for involved field stereotactic body radiation therapy-enhanced intermittent androgen deprivation therapy in hormone-sensitive nodal oligo-recurrent prostate cancer following prostate stereotactic body radiation therapy. Front Oncol. (2021) 10:606260. doi: 10.3389/fonc.2020.606260
2. Gandaglia G, Karakiewicz PI, Briganti A, Passoni NM, Schiffmann J, Trudeau V, et al. Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur Urol. (2015) 68:325–34. doi: 10.1016/j.eururo.2014.07.020
3. Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. (2006) 7:472–9. doi: 10.1016/S1470-2045(06)70700-8
4. Abrahamsson P-A. Intermittent androgen deprivation therapy in patients with prostate cancer: Connecting the dots. Asian J Urol. (2017) 4:208–22. doi: 10.1016/j.ajur.2017.04.001
5. Miszczyk M, Rajwa P, Yanagisawa T, Nowicka Z, Shim SR, Laukhtina E, et al. The efficacy and safety of metastasis-directed therapy in patients with prostate cancer: A systematic review and meta-analysis of prospective studies. Eur Urol. (2024) 85:125–38. doi: 10.1016/j.eururo.2023.10.012
6. Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncologica. (2005) 44:265–76. doi: 10.1080/02841860410002824
7. Hope TA, Eiber M, Armstrong WR, Juarez R, Murthy V, Lawhn-Heath C, et al. Diagnostic accuracy of 68Ga-PSMA-11 PET for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. (2021) 7:1635–42. doi: 10.1001/jamaoncol.2021.3771
8. Pienta KJ, Gorin MA, Rowe SP, Carroll PR, Pouliot F, Probst S, et al. A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with 18F-DCFPyL in prostate cancer patients (OSPREY). J Urol. (2021) 206:52–61. doi: 10.1097/ju.0000000000001698
9. Budäus L, Leyh-Bannurah S-R, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. (2016) 69:393–6. doi: 10.1016/j.eururo.2015.06.010
10. Surasi DS, Eiber M, Maurer T, Preston MA, Helfand BT, Josephson D, et al. Diagnostic performance and safety of Positron emission tomography with 18F-rhPSMA-7.3 in patients with newly diagnosed unfavourable intermediate- to very-high-risk prostate cancer: Results from a Phase 3, Prospective, Multicentre Study (Lighthouse). Eur Urol. (2023) 84:361–70. doi: 10.1016/j.eururo.2023.06.018
11. De Bleser E, Jereczek-Fossa BA, Pasquier D, Zilli T, Van As N, Siva S, et al. Metastasis-directed therapy in treating nodal oligorecurrent prostate cancer: A multi-institutional analysis comparing the outcome and toxicity of stereotactic body radiotherapy and elective nodal radiotherapy. Eur Urol. (2019) 76:732–9. doi: 10.1016/j.eururo.2019.07.009
12. Vaugier L, Morvan C, Pasquier D, Buthaud X, Magné N, Beckendorf V, et al. Long-term outcomes and patterns of relapse following high-dose elective salvage radiotherapy and hormone therapy in oligorecurrent pelvic nodes in prostate cancer: Oligopelvis (GETUG-P07). Eur Urol. (2024). doi: 10.1016/j.eururo.2024.02.013
13. Fodor A, Brombin C, Deantoni CL, Giannini L, Ferrario F, Villa SL, et al. Extended nodal radiotherapy for prostate cancer relapse guided with [11C]-choline PET/CT: Ten-year results in patients enrolled in a prospective trial. Eur J Nucl Med Mol Imaging. (2024) 51:590–603. doi: 10.1007/s00259-023-06445-4
14. Soldatov A, von Klot CAJ, Walacides D, Derlin T, Bengel FM, Ross TL, et al. Patterns of progression after 68Ga-PSMA-ligand PET/CT-guided radiation therapy for recurrent prostate cancer. Int J Radiat Oncol Biol Physics. (2019) 103:95–104. doi: 10.1016/j.ijrobp.2018.08.066
15. Harris VA, Staffurth J, Naismith O, Esmail A, Gulliford S, Khoo V, et al. Consensus guidelines and contouring Atlas for pelvic node delineation in prostate and pelvic node intensity modulated radiation therapy. Int J Radiat Oncol Biol Physics. (2015) 92:874–83. doi: 10.1016/j.ijrobp.2015.03.021
16. Napieralska A, Miszczyk L, Stąpór-Fudzińska M. CyberKnife stereotactic ablative radiotherapy as an option of treatment for patients with prostate cancer having oligometastatic lymph nodes. Technol Cancer Res Treat. (2016) 15:661–73. doi: 10.1177/1533034615595945
17. Zilli T, Achard V, Dal Pra A, Schmidt-Hegemann N, Jereczek-Fossa BA, Lancia A, et al. Recommendations for radiation therapy in oligometastatic prostate cancer: An ESTRO-ACROP Delphi Consensus. Radiother Oncol. (2022) 176:199–207. doi: 10.1016/j.radonc.2022.10.005
18. Deek MP, van der Eecken K, Sutera P, Deek RA, Fonteyne V, Mendes AA, et al. Long-term outcomes and genetic predictors of response to metastasis-directed therapy versus observation in oligometastatic prostate cancer: Analysis of STOMP and ORIOLE trials. J Clin Oncol. (2022) 40:3377–82. doi: 10.1200/jco.22.00644
19. Rich BJ, Montoya C, Jin WH, Spieler BO, Mahal BA, Delgadillo R, et al. Para-aortic radiation therapy for oligorecurrent prostate cancer. Int J Radiat Oncol Biol Physics. (2022) 114:718–24. doi: 10.1016/j.ijrobp.2022.05.040
20. Mohamad O, Zamboglou C, Zilli T, Murthy V, Aebersold DM, Loblaw A, et al. Safety of ultrahypofractionated pelvic nodal irradiation in the definitive management of prostate cancer: systematic review and meta-analysis. Int J Radiat Oncol Biol Physics. (2023) 118:998–1010. doi: 10.1016/j.ijrobp.2023.09.053
21. Glicksman RM, Loblaw A, Morton G, Szumacher E, Chung HT, Vesprini D, et al. Elective pelvic nodal irradiation with a simultaneous hypofractionated integrated prostate boost for localized high risk prostate cancer: Long term results from a prospective clinical trial. Radiother Oncol. (2021) 163:21–31. doi: 10.1016/j.radonc.2021.07.018
22. Tang C, Sherry AD, Haymaker C, Bathala T, Liu S, Fellman B, et al. Addition of metastasis-directed therapy to intermittent hormone therapy for oligometastatic prostate cancer: the EXTEND phase 2 randomized clinical trial. JAMA Oncol. (2023) 9:825–34. doi: 10.1001/jamaoncol.2023.0161
23. Savir-Baruch B, Choyke PL, Rowe SP, Schuster DM, Subramaniam RM, Jadvar H. Role of 18F-fluciclovine and prostate-specific membrane antigen PET/CT in guiding management of oligometastatic prostate cancer: ajr expert panel narrative review. Am J Roentgenol. (2021) 216:851–9. doi: 10.2214/ajr.20.24711
24. Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. (2020) 6:650–9. doi: 10.1001/jamaoncol.2020.0147
25. Spratt DE, Vargas HA, Zumsteg ZS, Golia Pernicka JS, Osborne JR, Pei X, et al. Patterns of lymph node failure after dose-escalated radiotherapy: Implications for extended pelvic lymph node coverage. Eur Urol. (2017) 71:37–43. doi: 10.1016/j.eururo.2016.07.043
Keywords: prostate cancer, SBRT, CyberKnife, involved field, common toxicity criteria (CTC), nodal oligo-recurrence
Citation: Koh MJ, Pilkington P, Koh MJ, Lawlor M-K, Creswell M, O’Connor T, Zwart A, Danner M, Kumar D, Suy S, Carrasquilla M and Collins SP (2024) Safety and early efficacy of involved-field SBRT for nodal oligo-recurrent prostate cancer. Front. Oncol. 14:1434504. doi: 10.3389/fonc.2024.1434504
Received: 17 May 2024; Accepted: 16 September 2024;
Published: 17 October 2024.
Edited by:
Nadia Gisella Di Muzio, Vita-Salute San Raffaele University, ItalyReviewed by:
Andrei Fodor, IRCCS San Raffaele Scientific Institute, ItalyDonald Blake Fuller, Genesis Healthcare Partners, United States
Copyright © 2024 Koh, Pilkington, Koh, Lawlor, Creswell, O’Connor, Zwart, Danner, Kumar, Suy, Carrasquilla and Collins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sean P. Collins, Q29sbGluc3MxOUB1c2YuZWR1
 Min Ji Koh
Min Ji Koh Padraig Pilkington
Padraig Pilkington Min Jung Koh
Min Jung Koh Mary-Kate Lawlor
Mary-Kate Lawlor Michael Creswell
Michael Creswell Timothy O’Connor1
Timothy O’Connor1 Malika Danner
Malika Danner Deepak Kumar
Deepak Kumar Simeng Suy
Simeng Suy Michael Carrasquilla
Michael Carrasquilla Sean P. Collins
Sean P. Collins