- 1Division of Radiation Oncology, Department of Radiology, Far Eastern Memorial Hospital, New Taipei, Taiwan
- 2Department of Computer Science and Engineering, Yuan Ze University, Taoyuan, Taiwan
- 3School of Medicine, College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 4Head and Neck Cancer Surveillance and Research Group, Far Eastern Memorial Hospital, New Taipei, Taiwan
- 5Department of Otolaryngology Head and Neck Surgery, Far Eastern Memorial Hospital, New Taipei, Taiwan
- 6Graduate Institute of Medicine, Yuan Ze University, Taoyuan, Taiwan
- 7Department of Dentistry, Far Eastern Memorial Hospital, New Taipei, Taiwan
- 8General Education Center, Lunghwa University of Science and Technology, Taoyuan, Taiwan
- 9Graduate Institute of Clinical Dentistry, School of Dentistry, National Taiwan University, Taipei, Taiwan
- 10Division of Hematology and Oncology, Department of Internal Medicine, Far Eastern Memorial Hospital, New Taipei, Taiwan
- 11Department of Electrical Engineering, College of Electrical and Communication Engineering, Yuan Ze University, Taoyuan, Taiwan
- 12Institute of Toxicology, College of Medicine, National Taiwan University, Taipei, Taiwan
- 13Division of Gastroenterology and Hepatology, Department of Internal Medicine, Far Eastern Memorial Hospital, New Taipei, Taiwan
- 14College of Medicine, Fu Jen Catholic University, New Taipei, Taiwan
- 15Institute of Traditional Medicine, College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
Purpose: The present longitudinal study aimed to evaluate the potential impact of modern radiotherapy (RT) techniques on quality of life (QOL) in patients with head and neck (HNC) cancer.
Materials and methods: In this single-center prospective study, participants were asked to complete QOL questionnaires that included the EORTC QLQ-C30, QLQ-H&N 35 and utility score by time trade-off (TTO) at three time points (2 weeks, 3 months and 6 months) after completion of RT. All patients were treated by modern RT techniques [volumetric modulated arc therapy (VMAT) or helical tomotherapy (HT)]. Patients who developed recurrence or died before the 6-month follow-up were excluded. Linear mixed models with random intercepts for participants and restricted maximum likelihood estimates were used to assess the effect of our study variables (age, sex, primary site, cancer stage, treatment, radiation dose and radiation method). Overall changes in QOL, utility scores and symptom burdens at different time points were tested using paired t tests.
Results: A total of 45 patients were recruited from 2022 to 2023. Those who completed the surveys at 2 weeks with at least 1 follow-up (30 patients, 67%) were enrolled in the final analysis. The majority of these 30 patients were men (76.7%), had oral cancer (40%), had stage III or IV disease (60%), received surgical intervention (63%) and were treated with chemoradiation (80%). A curative total dose of 66 to 70 Gy was delivered to 23 (76.7%) patients, half of whom received HT. Patients who received chemotherapy had significantly lower global QoL scales (mean difference, 27.94; 95% CI, 9.33-46.55; p=0.005). Global QOL, physical function, symptoms of sticky saliva, cough, feelings of illness and weight loss improved significantly between 2 weeks and 3 months. There was no significant difference between 3 and 6 months. Interestingly, improvements in social function, social contact, pain and nutrition reached significance at 6 months. Subgroup analysis revealed greater pain relief over time for patients who underwent HT (p=0.030). Moreover, patients who participated in swallowing rehabilitation programs had a greater decrease in nausea and vomiting (p=0.036).
Conclusion: HNC patients treated with modern RT techniques experience improved QOL and physical function over time. The most significant improvement occurs between 2 weeks and 3 months, after which the improvement plateaus. However, social function, social contact, pain and nutrition may require longer recovery intervals after treatment. HT with daily image guidance could provide a therapeutic opportunity for improving pain relief in patients with HNC.
Background
Radiotherapy (RT) contributes to survival and locoregional control for patients with head and neck cancer (HNC) (1). With increasing survival rates and local control in HNC patients, long-term quality of life (QOL) after curative treatment has gradually become a topic of interest (2). Modern RT techniques aim to improve treatment results (3, 4) and treatment-related side effects (5) by delivering highly conformal dose distributions to the target tissue via image-guided techniques and minimizing dosage over normal adjacent structures. Recent literature on intensity-modulated radiotherapy (IMRT) has shown a significant reduction in xerostomia and improved QOL compared to three-dimensional conformal radiotherapy (3DCRT) (6). While studies have shown comparable results in patients treated with volumetric modulated arc therapy (VMAT) versus IMRT, there are still limited reports regarding QOL resulting from the use of arc-based radiation techniques (7–10).
Hammerlid et al. (11) reported the greatest change in health-related quality of life (HRQOL) for HNC patients within the first year after diagnosis and significant deterioration immediately after completing treatment. Despite successful eradication of disease, we observed a certain number of patients with decisional regrets upon the first few follow-up visits, mostly resulting from symptom burdens of dysphagia, pain and speech disturbance. Despite their awareness of side effects, patients still expect a prompt recovery and return to normal daily life at the time of treatment completion. Although most studies have focused on assessing QOL for more than 6 months, Elumalai et al. (12) reported a more rapid return to baseline QOL in HNC patients treated with VMAT-based radiotherapy.
We previously conducted a cross-sectional study to test factors that contribute to QOL and utility in HNC patients who completed treatment after 6 months (13). In the current study, we sought to assess the changes in QOL and utility in patients with HNC within 6 months and to identify factors that may impact QOL outcomes after radiation therapy in a longitudinal setting.
Materials and methods
Study design and patient selection
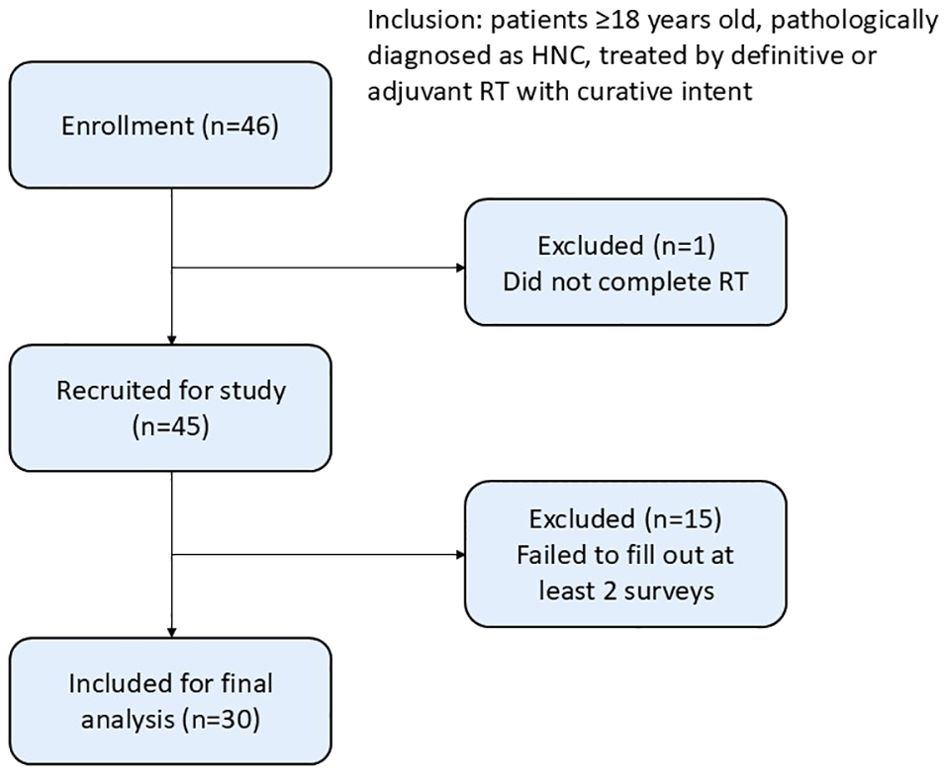
Patients with HNC who completed treatment between March 2022 and January 2023 were prospectively included in this single-center study. Patients who were more than 18 years old, pathologically diagnosed with HNC, treated with definitive or adjuvant arc-based modern radiotherapy (VMAT or helical tomotherapy, HT) with curative intent and were deemed disease free at the 6-month follow-up were eligible for inclusion. All participants were asked to complete QOL questionnaires, which included the EORTC QLQ-C30, QLQ-H&N 35 and utility score by time trade-off (TTO) at three time points (2 weeks, 3 months and 6 months) after RT completion. Patients with previous treatments, uncontrolled comorbid conditions, developed recurrence or died before the 6-month follow-up were excluded. Participants were followed on an outpatient basis, and those who failed to complete at least two surveys were also excluded (Figure 1). This study was approved by the Institutional Review Board of Far Eastern Memorial Hospital (reference number: FEMH 110183-E). Informed consent was obtained from all participants, and the results were analyzed and tabulated.
Treatment delivery
Radiotherapy delivery protocol for HNC in our institution is standardized according to modalities, namely VMAT and Tomotherapy. For VMAT, treatment is conducted using a linear accelerator (Eleckta Versa HD, Eleckta Oncology Systems Ltd., Crawley, UK), selecting 6 MV photon energy with treatment plans designed via the Pinnacle Treatment Planning System (version 9.8.1, Phillips Healthcare, Andover, MA), including at least two full arcs to ensure conformal dose distribution. For HT, treatment and planning design employs the TomoTherapy Hi Art Planning system (version 5.1.3, Tomotherapy, Inc., Madison, WI, USA). Prior to each HT treatment session, patient positioning and image guidance via MVCT is performed, with daily image adjustments ensuring high precision for treatment localization. Therefore, we create a 3 mm expansion from CTV to PTV considering HT planning, compared to a 5 mm expansion when using VMAT.
QOL questionnaires
The validated Taiwan Chinese version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) version 3 and the QLQ-H&N35 were used (13). Patients were asked to complete a form including content from the abovementioned questionnaires and 2 additional questions for utility assessment at 3 regular follow-up visits at 2 weeks, 3 months and 6 months after completion of the RT course. The scores of the QLQ-C30 and QLQ-H&N35 items are linearly transformed to scales from 0 to 100. For the functioning scales and global QOL scales, higher scores correlate with better levels of daily functioning. In contrast, for symptom scales, higher scores represent higher levels of symptoms or problems (14).
EORTC QLQ-C30 version 3
The QLQ-C30 is composed of both multi-item scales and single-item measures, including five functional scales, three symptom scales, a global health status/QOL scale, and six single items. All the scales range from 0 to 100. A high score on the functional scale represents a high level of functioning, and a high score on the symptom scale represents a high level of symptomatology. A high score on the global QOL represents a high general QOL. The manual contains scoring procedures for the QLQ-C30 version 3.0 and QLQ-C30 version 3.0, which are used in the current studies. All scales were scored in accordance with the EORTC scoring manual (15).
EORTC QLQ-H&N35
The QLQ-H&N35 is a module used for assessing QOL specifically in HNC patients. The QLQ-H&N35 included seven multiple-item scales and six single-item scales (13). The seven multiple-item scales assess the symptoms of pain, swallowing ability, sensation (taste/smell), speech, social eating, social contact, and sexuality. Six single-item scales survey the presence of symptomatic problems associated with the teeth, mouth opening, dry mouth (xerostomia), sticky saliva, coughing, and malaise. A high score on the symptom scale represents a high level of symptomatology.
Utility instrument
The time trade-off (TTO) has previously been used to assess laryngeal utility in several studies (16). We used TTO instead of EuroQol-5D (EQ-5D) as our measurement technique for HNC survivors given that TTO is a “choice task” rather than a “rating task”, while the latter are prone to scaling bias. TTO is recommended when performing cost-utility analysis using quality-adjusted life years as an outcome. The patients were first asked to imagine how many years they had left to live (X). Then, they could choose to give up some life years (Y) to live for a shorter period in perfect health. The utility would then be (X-Y)/X, according to the TTO method. The values are anchored at 1 (full health) and 0 (dead); higher values indicate greater health utility. Utility and QOL were assessed simultaneously in our study.
Statistical analysis
Patient characteristics were summarized with descriptive statistics. Categorical variables are presented as frequencies and percentages, whereas continuous variables are expressed as means with standard deviations (SDs). Linear mixed models with random intercepts for participants and restricted maximum likelihood estimates were used to assess the effects of variables (age, sex, primary site, cancer stage, treatment, radiation dose and radiation method). Participants with at least 1 follow-up measurement to examine changes in QOL and utility outcomes were enrolled for evaluation. In each model, the interaction between time (visit) and study variables was tested, while marginal post hoc estimates were calculated to test the association of variables with each outcome by visit. Overall changes in QOL and utility scores at different time points were tested using paired t tests.
All analyses were carried out in SPSS, version 21.0 (IBM, Armonk, NY, USA) and RStudio, version 4.0.3 (PBC, Boston, MA, USA). Two-tailed p values <0.05 were considered to indicate statistical significance.
Results
Patient characteristics
Of the 45 patients (46 patients who met the inclusion criteria were approached, and 45 [97.8%] patients were enrolled after they provided informed consent) recruited from March 2022 to January 2023, 30 (67%) patients completed the surveys at 2 weeks with at least 1 follow-up.
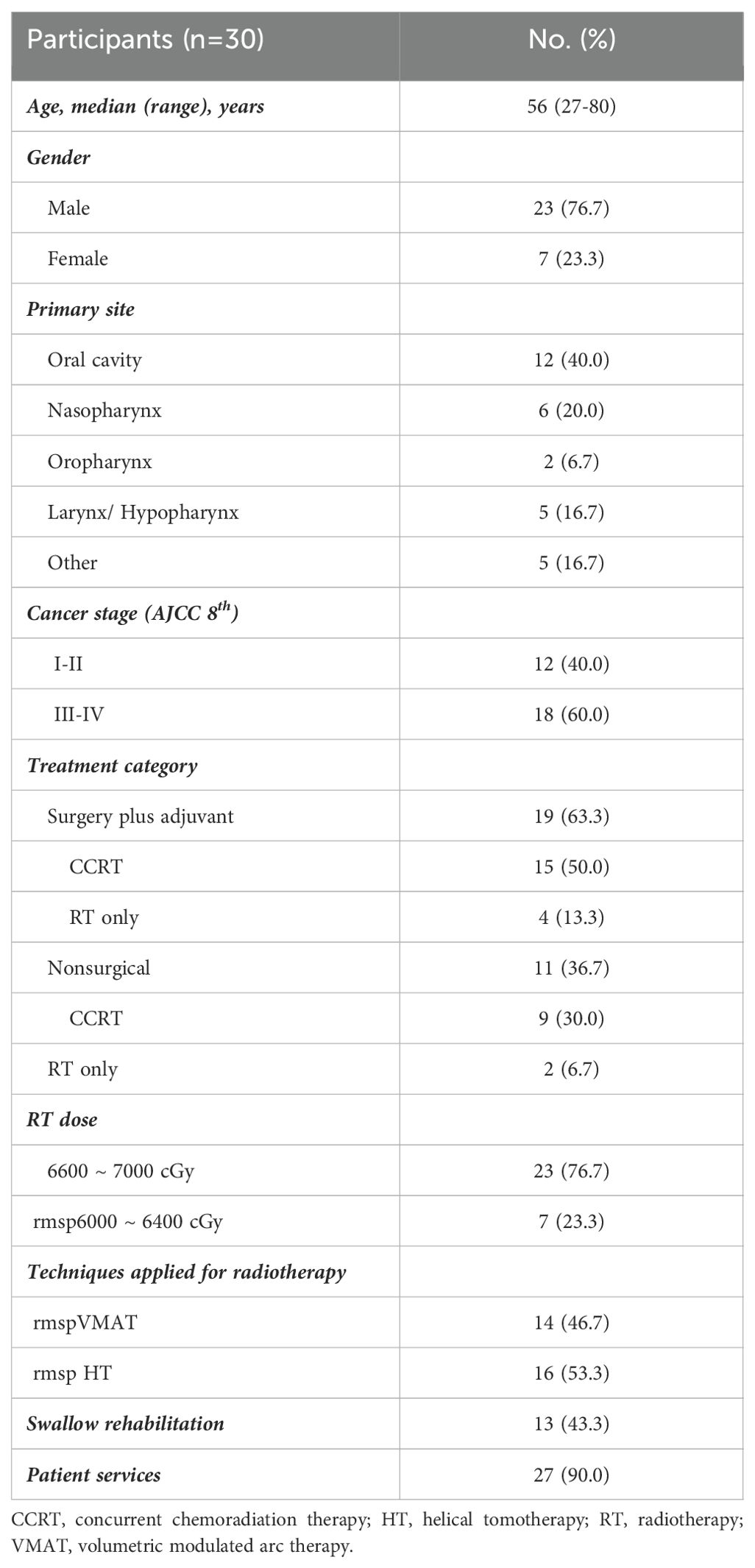
The demographic and clinical characteristics of the patients are shown in Table 1. Among the 30 patients included in the final analysis, 12 (40%) had oral cancer, 6 (20%) had nasopharyngeal carcinoma, 2 (6.7%) had oropharyngeal cancer, 5 (16.7%) had hypopharyngeal/laryngeal cancer, and 5 (16.7%) had cancer of other head and neck origins. The major characteristics of the patients in the study group were as follows: adult males (76.7%), stage III or IV disease (60%), surgical intervention (63%) and treatment with chemoradiation therapy (CCRT, 80%). In total, 66 to 70 Gy was delivered to 23 (76.7%) patients with curative intent, and half of them were treated with HT. Meanwhile, there is a higher proportion of patients with more advanced stage III or IV disease (81.3% vs. 35.7%) and received chemotherapy (93.8% vs. 64.3%) in the HT group compared to VMAT group.
Factors associated with overall global QOL and utility
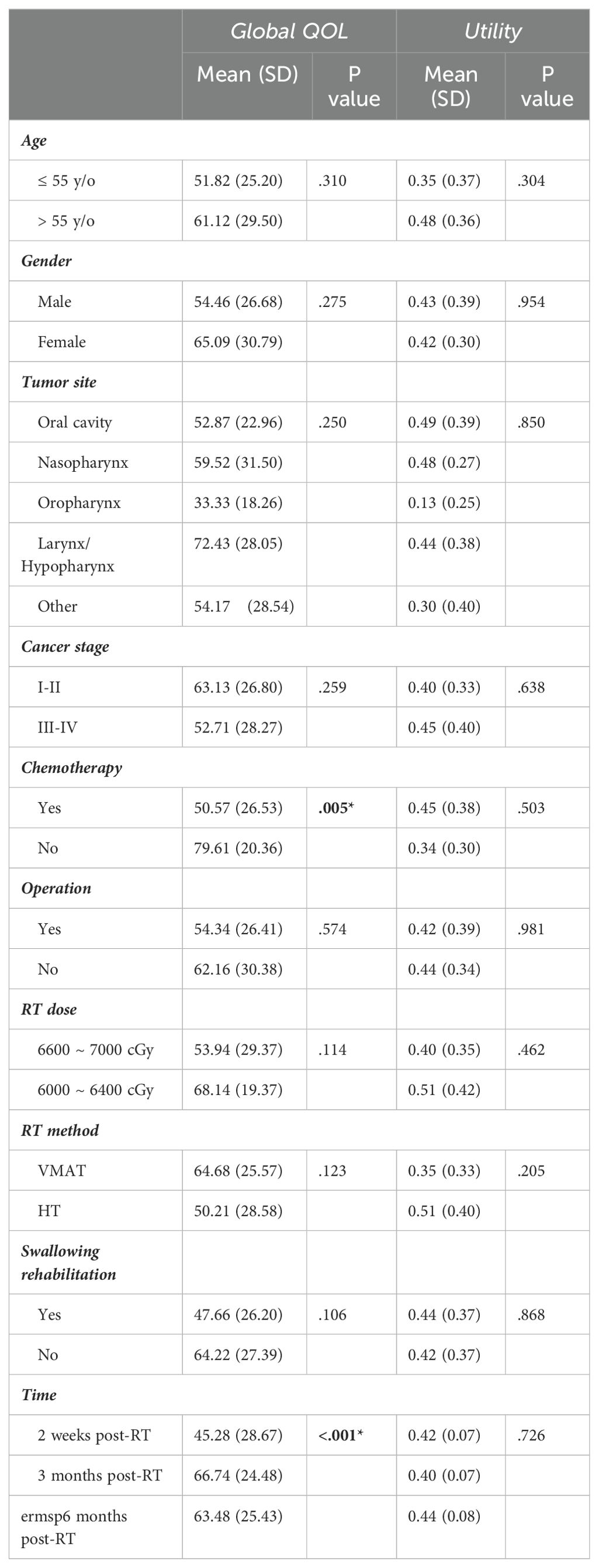
Chemotherapy and time after completion of therapy were predictive factors for overall global QOL. The mean global health status scale at 2 weeks after the completion of radiotherapy was 45.28 out of 100 for the 30 patients included in the analysis: 43.06 for oral cancer, 52.78 for NPC, 16.71 for oropharyngeal cancer, 63.33 for hypopharyngeal/laryngeal cancer, and 35 for other cancers (p = 0.373, one-way ANOVA). The overall mean utility index at 2 weeks after the completion of radiotherapy was 0.42 out of 1. The mean utility indices were 0.47 for oral cancer, 0.44 for NPC, 0.25 for oropharyngeal cancer, 0.36 for hypopharyngeal/laryngeal cancer, and 0.40 for thyroid cancer (p = 0.603, one-way ANOVA). According to our mixed model analysis, the global QOL was significantly lower in patients who received chemotherapy (mean difference, 27.94; 95% CI, 9.33-46.55; p=0.005). Aside from the effect of chemotherapy, the effect of time on QOL improvement was also prominent (mean difference, 9.71; 95% CI, 3.88-15.54, p<0.001), while no obvious difference was observed in the other remaining subgroups (Table 2). The effects of chemotherapy and time were not significant in the utility domain (Table 2).
Changes in QOL and symptom burden
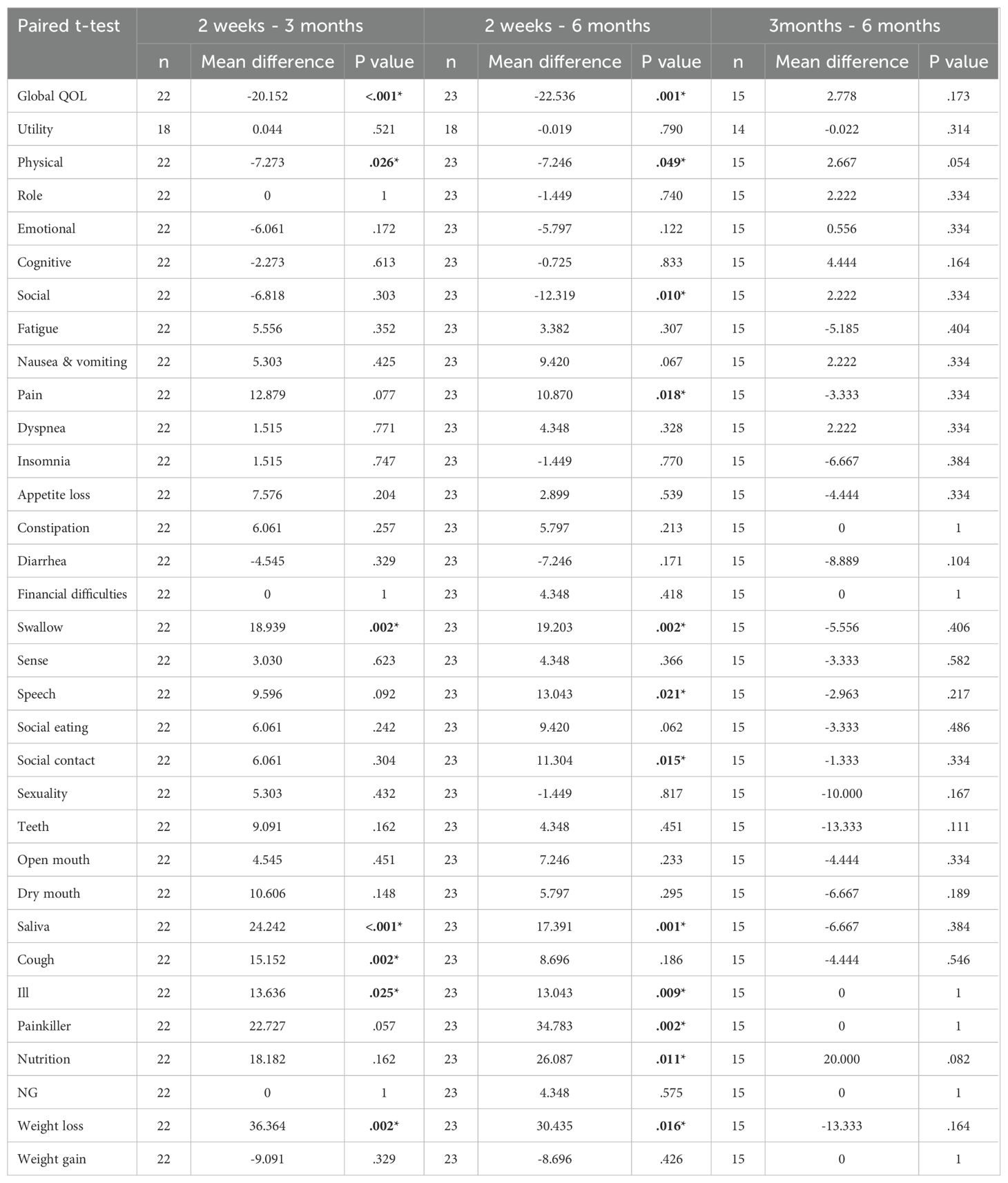
Global QOL, physical function, swallowing function, sticky saliva, cough, malaise and weight loss improved significantly between 2 weeks and 3 months after RT. No significant difference between any of the scales at 3 and 6 months was observed. Interestingly, the improvements in social function, social contact, speech, pain and nutrition were non-significant at 3 months but still reached significance at 6 months when compared to the scales at 2 weeks (Table 3).
Factors associated with changes in global QOL and symptom burdens
We analyzed values of QOL and symptom burden change separately according to different factors including age, gender, treatment, radiation dose, and radiation method. Due to the significant effect of chemotherapy on global QOL, we did not include chemotherapy as part of our subgroup analysis. According to the subgroup analysis, patients treated with helical tomotherapy (HT) experienced greater pain relief over time than did those treated with VMAT (mean difference, 21.78; 95% CI, 2.27-41.29; p=0.03, Figure 2, Supplementary Table S1.1). At 6 months after treatment, the group that received a lower RT dose (60-64 Gy) showed greater improvement in cognitive function (p=0.039) and sensory problems (p=0.030) than did the group treated with a higher RT dose (66-70 Gy). Patients who underwent surgery showed more significant improvements in cognitive function (p=0.048), emotion (p=0.023), pain (p=0.046) and swallowing difficulty (p=0.017). Moreover, patients who participated in swallowing rehabilitation programs had more obvious baseline symptoms and experienced more significant decreases in nausea and vomiting (mean difference, 24.07; 95% CI, 1.93-46.22; p=0.036, Figure 3, Supplementary Table S1.1).
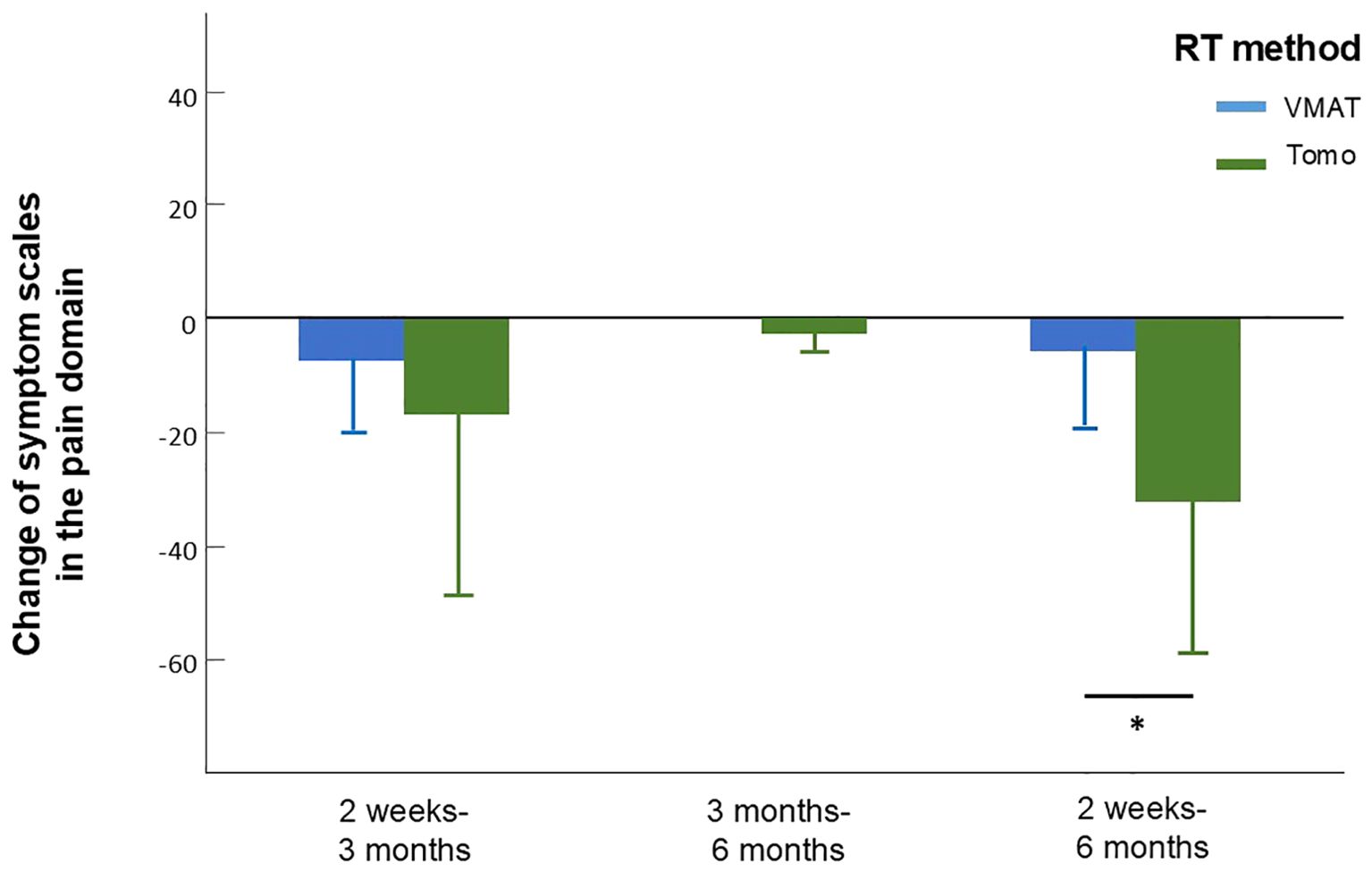
Figure 2. Change of symptom scales in the pain domain in patients who received treatment applying VMAT or tomotherapy assessed between different time intervals. Between 2 weeks to 6 months, patients who received tomotherapy experienced greater pain relief assessed by the reduce of EORTC QLQ-H&N35 pain scale. Error bars represent standard deviation (SD).
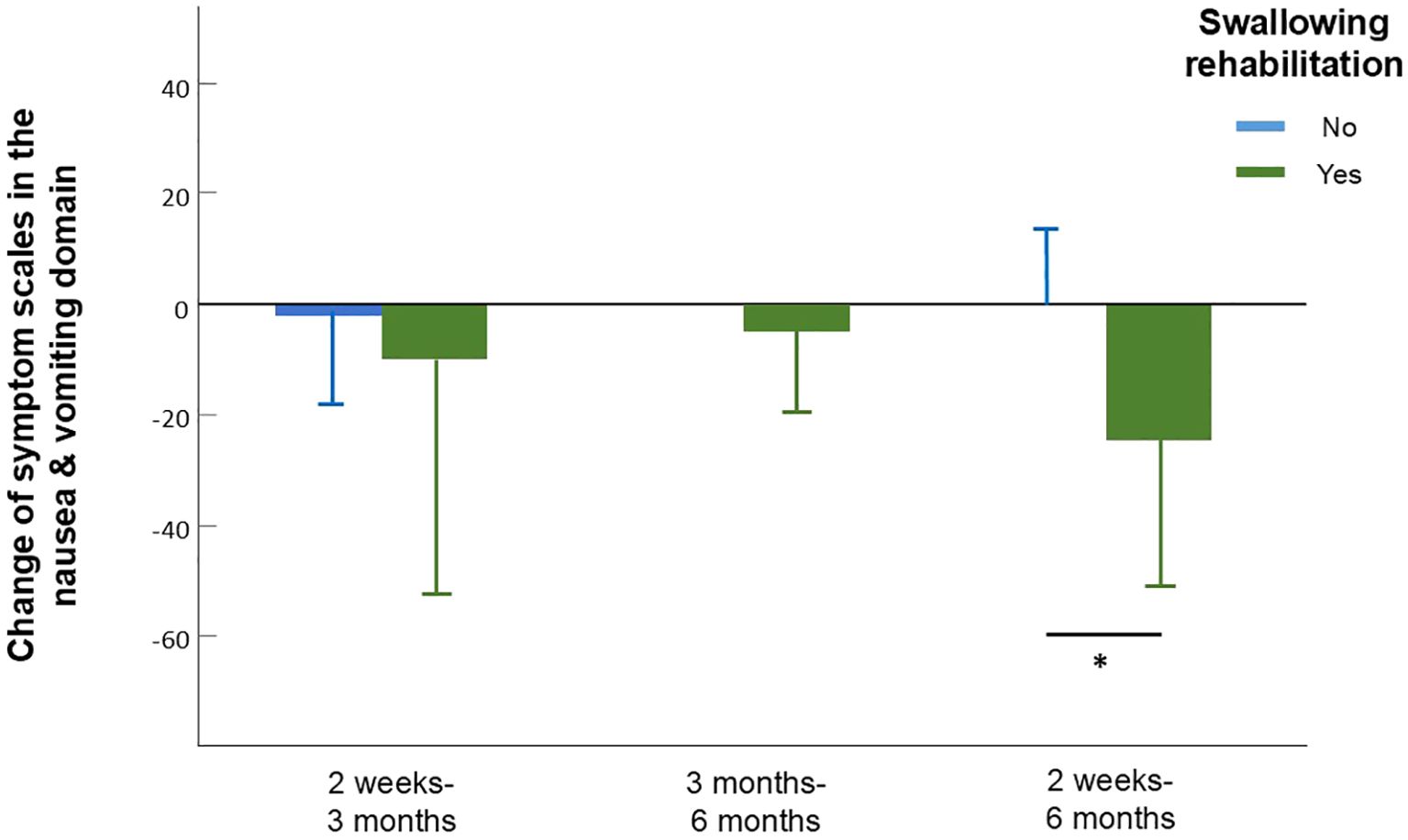
Figure 3. Change of symptom scales in the nausea and vomiting domain in patients who did and did not participate in swallowing rehabilitation programs assessed between different time intervals. Between 2 weeks to 6 months, patients who participated in swallowing rehabilitation programs had a more obvious decrease in symptoms of nausea and vomiting assessed by the reduce of EORTC QLQ-C30 nausea and vomiting scale. Error bars represent standard deviation (SD).
Longitudinal analysis on patients with data at all three time points
Among the 30 patients analyzed, only 15 had complete data at 2 weeks, 3 months, and 6 months. To validate our results as much as possible, we also conducted additional analysis focusing on the 15 patients with data at all three time points.
Among the 15 patients, the majority was male (66.7%), underwent operation (66.7%) and received a higher dose of RT of 66 to 70 Gy (80%). In 8 (53.3%) of the patients, VMAT was applied while the other 7 (46.7%) patients were treated with tomotherapy. 6 (40%) patients participated in swallowing rehabilitation. Chemotherapy and time after completion of therapy were repeatably predictive factors for overall global QOL.
Global QOL, physical function, social function, fatigue, pain, swallowing function, speech, sticky saliva, cough, malaise and weight loss improved significantly between 2 weeks and 3 months after RT. No significant difference between any of the scales at 3 and 6 months was observed (Figure 4). Improvements in social contact, painkiller use and nutrition were non-significant at 3 months but reached significance at 6 months when compared to the scales at 2 weeks. (Supplementary Table S2)
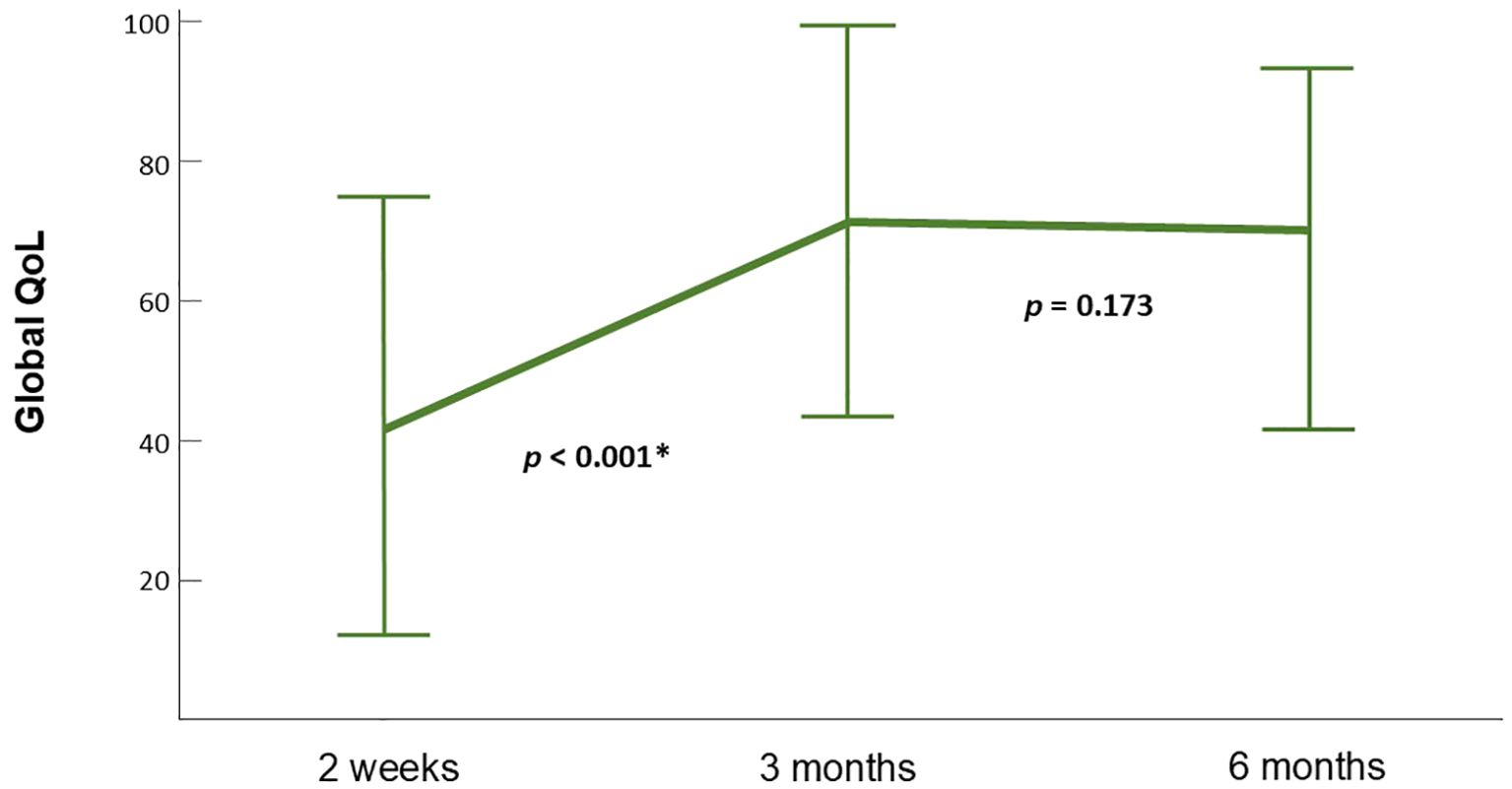
Figure 4. Dynamic change of global QOL scales. Global QOL improved significantly between 2 weeks and 3 months and reached a plateau between 3 and 6 months. The figure is illustrated according to patients that contain data at all time points (n=15). Error bars represent standard deviation (SD).
In subgroup analysis of factors effecting QOL change, patients treated with HT experienced greater pain relief over time compared to VMAT but failed to reach statistical significance (p=0.087, Supplementary Table S1.2). Patients who participated in swallowing rehabilitation programs experienced more significant decreases in nausea and vomiting (p=0.013, Supplementary Table S1.2).
Discussion
This is a novel study that longitudinally evaluated global QOL, function, symptom scales and utility in Asian HNC patients. We found that HNC survivors treated with modern RT techniques experience post-treatment improvements in QOL and physical function over time.
Compared to 3DCRT, a significantly shorter duration of feeding tube placement has been reported in HNC patients who receive IMRT (17). Vergeer et al. (18) also demonstrated statistically significant reductions in xerostomia with IMRT compared with 3DCRT. At 6 months post-treatment, 67% and 41% of patients treated with 3DCRT and IMRT, respectively, reported moderate or severe xerostomia. Moreover, IMRT minimizes radiation to surrounding tissues, which possibly results in a better outcome in multiple QOL domains in comparison with conventional RT (18–20). These data suggest that significant QOL benefits are gained by applying IMRT in patients with HNC.
The University of Michigan designed a prospective study of oropharyngeal carcinoma patients treated by IMRT with specific sparing of uninvolved swallowing organs, which resulted in absent to minimal dysphagia (21, 22). Without specific sparing of swallowing structures, however, oropharyngeal carcinoma patients treated with IMRT demonstrated a 7% rate of feeding-tube dependence at 1 year (23). These results indicated that targeted sparing of swallowing structures may provide additional benefits in preventing long-term dysphagia and improving QOL.
A growing body of evidence shows that VMAT is superior or noninferior to IMRT in terms of dosimetry (8, 24). While previous literature reported a return of QOL to baseline at 6-12 months in HNC patients treated with IMRT (11, 25–30), in our current study, when VMAT or HT was applied, the global QOL reached a plateau at 3 months after the completion of RT, regardless of age, sex, cancer site, stage, treatment method or total radiation dose. Loorents et al. (31) reported that most symptoms and functions deteriorated significantly by the end of RT for HNC patients, improved gradually by 3 months and reached baseline levels at 12 months after RT completion. In a study conducted by Periasamy et al., the QOL in oropharyngeal, laryngeal and hypopharyngeal patients treated with VMAT returned to baseline values by 3 months post-treatment, which is consistent with our results (32). The accelerated symptom recovery after RT may be due to the greater dose-sparing effect achieved by the VMAT technique or to advancements in medical treatment, such as new medications that prevent or alleviate the side effects of cancer treatment (33, 34).
Over time, patients who underwent surgery had a more significant improvement in swallowing and pain, which are likely the initial consequences of postoperative inflammation and nerve overstimulation (35, 36). A meta-analysis of 82 studies evaluating pain in HNC patients treated according to various schemes estimated that pain occurred in 57% of patients before treatment and in 42% of patients after treatment (37). In the present study, the improvement in pain over time was more significant in patients treated with helical tomotherapy (HT) than in patients treated with VMAT. Although the degree of pain relief between the two groups was comparable between 2 weeks and 3 months, patients in the HT group had continue improvement on pain between 3 months and 6 months while patients in the VMAT group seemed to remain constant. Therefore, despite having higher pain scales at baseline, HT group patients managed to reach almost equal results as VMAT group at 6 months. Compared with those of VMAT and IMRT, better conformation numbers, healthy tissue conformity indices and homogeneity have been achieved by HT in HNC patients (38). Moreover, image-guided radiotherapy (IG IGRT) is associated with significantly greater overall survival and locoregional survival and lower toxicity after daily position correction than is non-IG IMRT (5). For HNC patients treated with HT in our facility, daily image guidance techniques are routinely applied with a lesser margin to form the planning target volume (PTV), which could result in a smaller treatment volume and decreased toxicity. The results from the abovementioned IMRT and VMAT studies as well as our current study indicate that IGRT, such as HT, may provide more QOL-related benefits than IMRT and VMAT. Longer follow up will be needed to support our point of view.
The Radiation Therapy Oncology Group and the Head and Neck Intergroup (RTOG 91-11) confirmed that patients receiving radiotherapy with chemotherapy had greater chemotherapy-related toxic effects and increased rates of severe radiation-related toxic effects (39). According to Barker et al. (40), HNC patients who were asymptomatic at baseline reported a prompt worsening of QOL following CCRT. Growing evidence also shows a trend toward worse QOL in patients receiving combined chemoradiotherapy (CCRT) than in those receiving RT alone (33, 41–43). Similarly, the QOL and symptom-related scales were worse in patients who received chemotherapy in our study. Further studies should focus on new chemotherapy regimens and more precise RT modalities to decrease side effects and improve QOL with equal clinical effects.
Swallowing dysfunction after HNC therapy contributes to reduced patient QOL, increased morbidity, and increased mortality. Goepfert et al. (27) reported that the decisional regrets of oropharyngeal cancer patients were mainly associated with symptom burdens focused on swallowing difficulty, depression and pain. The incidence of aspiration for liquids in patients with oropharyngeal cancer treated with CCRT was 24% at 12 months (22) and 7% at 6 months for those treated with accelerated radiotherapy (44). Patterson et al. (45) reported that there was a significant reduction in swallowing scores for HNC patients treated with CCRT from pretreatment to 3 months posttreatment and no improvement in scores from 3 to 12 months post-CCRT. Notably, earlier intervention potentially helped achieve better responses in terms of diet and QOL. Van Daele et al. (46) reported that starting a swallowing therapy program within one year of RT completion of improved QOL and diet performance to a greater extent than did starting such a program later. Other studies have shown that the addition of swallowing rehabilitation to the post-radiation period for a longer duration may further benefit swallowing outcomes, particularly in patients with negative predictors (46–49). In our study, patients who received early rehabilitation intervention had more obvious baseline symptoms. Although they did not seem to show significant improvement in swallowing dysfunction, they had a more obvious decrease in symptoms of nausea and vomiting (Figure 4, p=0.036), which potentially reduces the risk and severity of liquid aspiration. During follow up period, 6 of our patients developed aspiration-related events; 3 of them had participated in swallowing rehabilitation programs and 3 did not. Upon further investigation, we found that the 3 patients which received swallowing rehabilitation programs but still developed aspiration-related events had advanced stage disease and primary site of oropharynx or hypopharynx. To be noted, all of them recovered after medical treatment. The other 3 that developed aspiration-related events which did not participate in swallowing rehabilitation programs before were oral cancer patients and 2 of them had early-stage disease. One of them with advanced stage disease eventually failed to recover after aspiration-related event.
Although Nallani et al. (50) reported a positive correlation between anxiety and decision regret at the 3-month follow-up and lasting to the 6-month follow-up, a low incidence of emotional problems was noted at 2 weeks and at 3 and 6 months posttreatment in our study. As women with HNC tend to have more emotional problems than men do according to previous literature (51), we speculate that the low incidence of emotional problems observed may have been because there were more men (77%) included in our study, which is consistent with results reported by Loorents et al. (31)
Furthermore, there was no difference in the QOL change between patients who did and did not receive patient services (including psychologist, nutritionist and social worker consultations). However, we still believe that such services play an important role in social function and the long-term recovery of nutritional status because 90% of our patients had patient service records.
In our additional analysis focusing on the 15 patients with data at all three time points, the characteristics were similar and had the same predictive factors for overall global QOL. The trend of dynamic change was consistent with our original group, showing significant improvement of global QOL, physical function and certain symptom burdens between 2 weeks and 3 months after RT followed by a plateau from 3 months to 6 months. Although improvement of social function and pain were also detected at 3 months in this group, the significance was increased at 6 months while significant improvements of social contact, painkiller use and nutrition were only reached at 6 months after RT. The advantage of HT over VMAT on pain relief was still observed but did not reach statistical significance in this group presumably due to an even smaller sample size.
Our current results should be interpreted cautiously in light of several limitations. First, despite the prospective nature of our study, the sample size included in the final analysis was small (n = 30) and only half of which had complete data at all three time points. Thus, the generalizability of the research results is limited. In our previous study published in 2019 (13), we analyzed various factors associated with QOL of head and neck cancer survivors 6 months after completing definitive treatment including education level, marriage status, socioeconomic class and occupation status. Lower annual family income was found to be associated with generally lower QOL and utility scores. However, the trend was not found in this longitudinal study which may also be relevant to our relatively smaller sample size. Second, our baseline was set at 2 weeks after the last course of radiation therapy. Therefore, we did not collect questionnaires from these patients before treatment delivery. Third, although the samples were all obtained from HNC survivors, the various cancers included oral cancer, oropharyngeal cancer, nasopharyngeal cancer, hypopharyngeal cancer, and laryngeal cancer. In addition, some patients needed to undergo surgery prior to CCRT, and some did not. Therefore, the symptoms or side effects might not be the same and could affect the prediction of QOL changes. Therefore, the results need further validation from larger longitudinal investigations, and it is recommended that future studies enroll patients diagnosed with a specific type of HNC to increase the homogeneity. In a practical point of view, the original design of EORTC questionnaires is precise and comprehensive but contain numerous items which may interfere with subject’s attention and compliance. Therefore, we will consider focusing on questions limited to a certain function domain or symptom burden in the future to ensure better compliance and data integrity during longitudinal analysis.
Conclusion
HNC survivors treated with modern RT techniques experience improvements in QOL and physical function over time. The most significant improvement occurs between 2 weeks and 3 months, after which it substantially reaches a plateau. Social function, social contact, pain and nutrition may require longer recovery intervals after treatment. HT with daily image guidance could provide a therapeutic opportunity for improving pain relief in patients with HNC. Further replicative results could eventually guide us toward better clinical management.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Far Eastern Memorial Hospital (reference number: FEMH 110183-E). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
EC: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Methodology, Visualization. P-YH: Data curation, Supervision, Writing – review & editing. P-WS: Resources, Supervision, Writing – review & editing. W-CL: Resources, Supervision, Writing – review & editing. P-YL: Resources, Supervision, Writing – review & editing. S-CL: Supervision, Writing – review & editing. P-HW: Resources, Supervision, Writing – review & editing. J-GJ: Resources, Supervision, Writing – review & editing. C-SC: Supervision, Writing – review & editing. C-XH: Resources, Supervision, Writing – review & editing. D-YK: Investigation, Supervision, Writing – review & editing. Y-FL: Resources, Supervision, Writing – review & editing. L-JL: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. C-HH: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Far Eastern Memorial Hospital (FEMH-2024-C-033) for the design of the study; collection, analysis, and interpretation of the data; and writing of the manuscript.
Acknowledgments
We are thankful for the help from all our colleagues in the Head and Neck Cancer Surveillance & Research Group, Far Eastern Memorial Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1424034/full#supplementary-material
References
1. Anderson G, Ebadi M, Vo K, Novak J, Govindarajan A, Amini A. An updated review on head and neck cancer treatment with radiation therapy. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13194912
2. Rogers SN, Ahad SA, Murphy AP. A structured review and theme analysis of papers published on 'quality of life' in head and neck cancer: 2000-2005. Oral Oncol. (2007) 43:843–68. doi: 10.1016/j.oraloncology.2007.02.006
3. Vordermark D. Ten years of progress in radiation oncology. BMC Cancer. (2011) 11:503. doi: 10.1186/1471-2407-11-503
4. Hsieh C-H, Shueng P-W, Wang L-Y, Huang Y-C, Liao L-J, Lo W-C, et al. Impact of postoperative daily image-guided intensity-modulated radiotherapy on overall and local progression-free survival in patients with oral cavity cancer. BMC Cancer. (2016) 16:139. doi: 10.1186/s12885-016-2165-9
5. Nien H-H, Wang L-Y, Liao L-J, Lin P-Y, Wu C-Y, Shueng P-W, et al. Advances in image-guided radiotherapy in the treatment of oral cavity cancer. Cancers. (2022) 14(19):4630. doi: 10.3390/cancers14194630
6. Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicenter randomized controlled trial. Lancet Oncol. (2011) 12:127–36. doi: 10.1016/S1470-2045(10)70290-4
7. Afrin KT, Ahmad S. 3D conformal, IMRT and VMAT for the treatment of head and neck cancer: a brief literature review. J Radiotherapy Pract. (2022) 21:259–62. doi: 10.1017/S1460396920001053
8. Buciuman N, Marcu LG. Dosimetric justification for the use of volumetric modulated arc therapy in head and neck cancer-A systematic review of the literature. Laryngoscope Investig Otolaryngol. (2021) 6:999–1007. doi: 10.1002/lio2.642
9. Huang TL, Tsai MH, Chuang HC, Chien CY, Lin YT, Tsai WL, et al. Quality of life and survival outcome for patients with nasopharyngeal carcinoma treated by volumetric-modulated arc therapy versus intensity-modulated radiotherapy. Radiat Oncol. (2020) 15:84. doi: 10.1186/s13014-020-01532-4
10. Osborn J. Is VMAT beneficial for patients undergoing radiotherapy to the head and neck? Radiography (Lond). (2017) 23:73–6. doi: 10.1016/j.radi.2016.08.008
11. Hammerlid E, Silander E, Hörnestam L, Sullivan M. Health-related quality of life three years after diagnosis of head and neck cancer—A longitudinal study. Head Neck. (2001) 23:113–25. doi: 10.1002/(ISSN)1097-0347
12. Elumalai T, Mukherji A, Vijayaprabhu N, Periasamy K, Yadala A. The patient-reported outcome measures in oropharyngeal, laryngeal and hypopharyngeal cancer patients treated with Volumetric Modulated Arc based simultaneous integrated boost radiotherapy. Tech Innov Patient Support Radiat Oncol. (2021) 18:1–7. doi: 10.1016/j.tipsro.2021.02.007
13. Liao LJ, Hsu WL, Lo WC, Cheng PW, Shueng PW, Hsieh CH. Health-related quality of life and utility in head and neck cancer survivors. BMC Cancer. (2019) 19:425. doi: 10.1186/s12885-019-5614-4
14. Bjordal K, Hammerlid E, Ahlner-Elmqvist M, de Graeff A, Boysen M, Evensen JF, et al. Quality of life in head and neck cancer patients: validation of the european organization for research and treatment of cancer quality of life questionnaire-H&N35. J Clin Oncol. (1999) 17:1008–8. doi: 10.1200/JCO.1999.17.3.1008
15. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. the european organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. JNCI: J Natl Cancer Institute. (1993) 85:365–76. doi: 10.1093/jnci/85.5.365
16. Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. (2010) 96:5–21. doi: 10.1093/bmb/ldq033
17. Beadle BM, Liao K-P, Giordano SH, Garden AS, Hutcheson KA, Lai SY, et al. Reduced feeding tube duration with intensity-modulated radiation therapy for head and neck cancer: A Surveillance, Epidemiology, and End Results-Medicare Analysis. Cancer. (2017) 123:283–93. doi: 10.1002/cncr.30350
18. Vergeer MR, Doornaert PA, Rietveld DH, Leemans CR, Slotman BJ, Langendijk JA. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys. (2009) 74:1–8. doi: 10.1016/j.ijrobp.2008.07.059
19. Ge X, Liao Z, Yuan J, Mao D, Li Y, Yu E, et al. Radiotherapy-related quality of life in patients with head and neck cancers: a meta-analysis. Supportive Care Cancer. (2020) 28:2701–12. doi: 10.1007/s00520-019-05077-5
20. Hawkins PG, Lee JY, Mao Y, Li P, Green M, Worden FP, et al. Sparing all salivary glands with IMRT for head and neck cancer: Longitudinal study of patient-reported xerostomia and head-and-neck quality of life. Radiotherapy Oncol. (2018) 126:68–74. doi: 10.1016/j.radonc.2017.08.002
21. Eisbruch A, Kim HM, Feng FY, Lyden TH, Haxer MJ, Feng M, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat OncologyBiologyPhysics. (2011) 81:e93–9. doi: 10.1016/j.ijrobp.2010.12.067
22. Feng FY, Kim HM, Lyden TH, Haxer MJ, Worden FP, Feng M, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. (2010) 28:2732–8. doi: 10.1200/JCO.2009.24.6199
23. Setton J, Lee NY, Riaz N, Huang S-H, Waldron J, O'Sullivan B, et al. A multi-institution pooled analysis of gastrostomy tube dependence in patients with oropharyngeal cancer treated with definitive intensity-modulated radiotherapy. Cancer. (2015) 121:294–301. doi: 10.1002/cncr.29022
24. Buciuman N, Marcu LG. Is there a dosimetric advantage of volumetric modulated arc therapy over intensity modulated radiotherapy in head and neck cancer? Eur Arch Oto-Rhino-Laryngology. (2022) 279:5311–21. doi: 10.1007/s00405-022-07452-1
25. Curran D, Giralt J, Harari PM, Ang KK, Cohen RB, Kies MS, et al. Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol. (2007) 25:2191–7. doi: 10.1200/JCO.2006.08.8005
26. O'Neill M, Heron DE, Flickinger JC, Smith R, Ferris RL, Gibson M. Posttreatment quality-of-life assessment in patients with head and neck cancer treated with intensity-modulated radiation therapy. Am J Clin Oncol. (2011) 34(5):478–82. doi: 10.1097/COC.0b013e3181f4759c
27. Goepfert RP, Fuller CD, Gunn GB, Hanna EY, Lewin JS, Zaveri JS, et al. Symptom burden as a driver of decisional regret in long-term oropharyngeal carcinoma survivors. Head Neck. (2017) 39:2151–8. doi: 10.1002/hed.24879
28. Tsan Y-H, Wung S-H, Lin M-W, Lo W-L, Wang Y-J. Predictors of quality of life change in head-and-neck cancer survivors during concurrent chemoradiotherapy: A prospective study. Asia-Pacific J Oncol Nurs. (2021) 8:237–45. doi: 10.4103/2347-5625.311132
29. Astrup GL, Rustøen T, Hofsø K, Gran JM, Bjordal K. Symptom burden and patient characteristics: Association with quality of life in patients with head and neck cancer undergoing radiotherapy. Head Neck. (2017) 39:2114–26. doi: 10.1002/hed.v39.10
30. Amit M, Hutcheson K, Zaveri J, Lewin J, Kupferman ME, Hessel AC, et al. Patient-reported outcomes of symptom burden in patients receiving surgical or nonsurgical treatment for low-intermediate risk oropharyngeal squamous cell carcinoma: A comparative analysis of a prospective registry. Oral Oncol. (2019) 91:13–20. doi: 10.1016/j.oraloncology.2019.01.020
31. Loorents V, Rosell J, Salgado Willner H, Börjeson S. Health-related quality of life up to 1 year after radiotherapy in patients with head and neck cancer (HNC). SpringerPlus. (2016) 5:669. doi: 10.1186/s40064-016-2295-1
32. Yüce Sarı S. Validation of the EORTC-QLQ-HN35 questionnaire in turkish head and neck cancer patients. Turkish J Oncol. (2020) 35(2):144–9. doi: 10.5505/tjo.2019.2195
33. Allen-Ayodabo CO, Eskander A, Davis LE, Zhao H, Mahar AL, Karam I, et al. Symptom burden among head and neck cancer patients in the first year after diagnosis: Association with primary treatment modality. Oral Oncol. (2019) 99:104434. doi: 10.1016/j.oraloncology.2019.09.026
34. Karabajakian A, Gau M, Reverdy T, Neidhardt E-M, Fayette J. Induction chemotherapy in head and neck squamous cell carcinoma: A question of belief. Cancers (Basel). (2018) 11(1):15. doi: 10.3390/cancers11010015
35. Borsook D, Kussman BD, George E, Becerra LR, Burke DW. Surgically induced neuropathic pain: understanding the perioperative process. Ann Surg. (2013) 257:403–12. doi: 10.1097/SLA.0b013e3182701a7b
36. Yang T, Velagapudi R, Terrando N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol. (2020) 21:1319–26. doi: 10.1038/s41590-020-00812-1
37. Macfarlane TV, Wirth T, Ranasinghe S, Ah-See KW, Renny N, Hurman D. Head and neck cancer pain: systematic review of prevalence and associated factors(2012). Available online at: http://www.ncbi.nlm.nih.gov/pubmed/24422003http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3886092http://dx.doi.org/10.5037/jomr.2012.3101http://www.ejomr.org/JOMR/archives/2012/1/e1/v3n1e1ht.pdf (Accessed 2012/04/01/Jan-Mar).
38. Pigorsch SU, Kampfer S, Oechsner M, Mayinger MC, Mozes P, Devecka M, et al. Report on planning comparison of VMAT, IMRT and helical tomotherapy for the ESCALOX-trial pre-study. Radiat Oncol. (2020) 15:253. doi: 10.1186/s13014-020-01693-2
39. Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. New Engl J Med. (2003) 349:2091–8. doi: 10.1056/NEJMoa031317
40. Barker CL, Price GJ, Lee LW, McPartlin A. Baseline MD anderson symptom inventory score is strongly associated with patient-reported acute and late toxicity following (Chemo) radiotherapy for head and neck cancers. Clin Oncol. (2022) 34:683–9. doi: 10.1016/j.clon.2022.05.018
41. Terrell JE, Ronis DL, Fowler KE, Bradford CR, Chepeha DB, Prince ME, et al. Clinical predictors of quality of life in patients with head and neck cancer. Arch Otolaryngology–Head Neck Surg. (2004) 130:401–8. doi: 10.1001/archotol.130.4.401
42. Sharma Y, Mishra G, Parikh V. Quality of life in head and neck cancer patients. Indian J Otolaryngol Head Neck Surg. (2019) 71:927–32. doi: 10.1007/s12070-019-01620-2
43. Klein J, Livergant J, Ringash J. Health related quality of life in head and neck cancer treated with radiation therapy with or without chemotherapy: A systematic review. Oral Oncol. (2014) 50:254–62. doi: 10.1016/j.oraloncology.2014.01.015
44. Cartmill B, Cornwell P, Ward E, Davidson W, Porceddu S. Long-term functional outcomes and patient perspective following altered fractionation radiotherapy with concomitant boost for oropharyngeal cancer. Dysphagia. (2012) 27:481–90. doi: 10.1007/s00455-012-9394-0
45. Patterson JM, McColl E, Carding PN, Hildreth AJ, Kelly C, Wilson JA. Swallowing in the first year after chemoradiotherapy for head and neck cancer: Clinician-and patient-reported outcomes. Head Neck. (2014) 36:352–8. doi: 10.1002/hed.23306
46. Van Daele DJ, Langmore SE, Krisciunas GP, Lazarus CL, Pauloski BR, McCulloch TM, et al. The impact of time after radiation treatment on dysphagia in patients with head and neck cancer enrolled in a swallowing therapy program. Head Neck. (2019) 41:606–14. doi: 10.1002/hed.25344
47. Carmignani I, Locatello LG, Desideri I, Bonomo P, Olmetto E, Livi L, et al. Analysis of dysphagia in advanced-stage head-and-neck cancer patients: impact on quality of life and development of a preventive swallowing treatment. Eur Arch Oto-Rhino-Laryngology. (2018) 275:2159–67. doi: 10.1007/s00405-018-5054-9
48. Yifru TA, Kisa S, Dinegde NG, Atnafu NT. Dysphagia and its impact on the quality of life of head and neck cancer patients: institution-based cross-sectional study. BMC Res Notes. (2021) 14:11. doi: 10.1186/s13104-020-05440-4
49. Vermaire JA, Raaijmakers CPJ, Monninkhof EM, Leemans CR, de Jong RJB, Takes RP, et al. The course of swallowing problems in the first 2 years after diagnosis of head and neck cancer. Support Care Cancer. (2022) 30:9527–38. doi: 10.1007/s00520-022-07322-w
50. Nallani R, Smith JB, Penn JP, Bur AM, Kakarala K, Shnayder Y, et al. Decision regret 3 and 6 months after treatment for head and neck cancer: Observational study of associations with clinicodemographics, anxiety, and quality of life. Head Neck. (2022) 44:59–70. doi: 10.1002/hed.26911
Keywords: head and neck cancer, quality of life, radiation therapy (radiotherapy), VMAT (volumetric modulated arc therapy), tomotherapy, arc radiation therapy
Citation: Chuang EY-H, Hou P-Y, Shueng P-W, Lo W-C, Lin P-Y, Lin S-C, Wu P-H, Jiang J-G, Chung C-S, Hsu C-X, Kuo D-Y, Lu Y-F, Liao L-J and Hsieh C-H (2024) Improved quality of life in head and neck cancer patients treated with modern arc radiotherapy techniques – A prospective longitudinal analysis. Front. Oncol. 14:1424034. doi: 10.3389/fonc.2024.1424034
Received: 27 April 2024; Accepted: 03 September 2024;
Published: 23 September 2024.
Edited by:
Gunnar Wichmann, University Hospital Leipzig, GermanyReviewed by:
Xu Liu, Sun Yat-sen University Cancer Center (SYSUCC), ChinaFeng-Ming Hsu, National Taiwan University Hospital, Taiwan
Copyright © 2024 Chuang, Hou, Shueng, Lo, Lin, Lin, Wu, Jiang, Chung, Hsu, Kuo, Lu, Liao and Hsieh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Jen Liao, ZGVuaXJvQG1haWwyMDAwLmNvbS50dw==; Chen-Hsi Hsieh, Y2hlbmNpYWJAZ21haWwuY29t
†These authors have contributed equally to this work
 Eva Yu-Hsuan Chuang
Eva Yu-Hsuan Chuang Pei-Yu Hou
Pei-Yu Hou Pei-Wei Shueng
Pei-Wei Shueng Wu-Chia Lo
Wu-Chia Lo Ping-Yi Lin4,7,8,9
Ping-Yi Lin4,7,8,9 Shih-Chiang Lin
Shih-Chiang Lin Chen-Shuan Chung
Chen-Shuan Chung Deng-Yu Kuo
Deng-Yu Kuo Chen-Hsi Hsieh
Chen-Hsi Hsieh