- 1Department of Neurosurgery, The First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
- 2Department of Neurosurgery, The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
- 3Department of Neurology, Heilongjiang Province Hospital, Harbin, Heilongjiang, China
Intramedullary schwannomas in the conus medullaris are very rare and are usually not associated with syringomyelia. We report a unique case of intramedullary schwannoma in the conus medullaris with long-segment syringomyelia. The patient was a 60-year-old male, initially presenting with left dorsalgia, subsequently developing weakness in the right lower extremity. As the symptoms progressed, the patient exhibited ataxia in gait, accompanied by sphincter insufficiency and voiding dysfunction. Lumbar MRI revealed the presence of two tumors at the L3 and T11-L1 levels, accompanied by syringomyelia extending from T4 to T10. During surgery, it was determined that the tumor located at the T11-L1 vertebral level was intramedullary, whereas the tumor situated at the L3 level exhibited an extramedullary intradural configuration. Pathological examination conclusively identified both the intramedullary and extramedullary tumors as schwannomas. Although intramedullary schwannomas at the conus medullaris are very rare, schwannoma remains a diagnosis that cannot be ignored when facing patients with intramedullary tumors with syringomyelia. Intramedullary schwannoma can have a good neurological prognosis after surgical treatment.
Introduction
Schwannomas are one of the most common primary spinal tumors. It usually originates from Schwann cells of the peripheral nervous system, and most of them are solitary and accompanied by a capsule. Based on the different positional relationships with the spinal dura mater, spinal schwannomas can be classified into three types: epidural (8-32%), intradural-extramedullary (1-19%), and intradural(49-83%) (3, 4). The vast majority of schwannomas are located in the intradural space. Intradural schwannomas can be categorized into extramedullary intradural and intramedullary(IM) schwannomas. IM schwannomas are considered rare neoplasms, comprising only 1.1% of spinal schwannomas and 0.3% of all IM tumors (7). The most common site of IM schwannomas is the cervical spine and the level of the conus medullaris are less common, and those with syringomyelia are even rarer (11). We describe the first-ever instance to our knowledge of IM schwannoma of conus medullaris with long segment syringomyelia.
Case report
Anal sphincter weakness, urinary difficulties for two months, right lower limb weakness and walking instability for half a year, and left back pain for a year all are the symptoms which brought the 60-year-old male patient to the hospital. Neurological examination revealed sensory impairment below the inguinal plane, disappearance of abdominal wall reflex and cremasteric reflex. After admission, ultrasound examination showed that the residual urine volume in the bladder after urination was 530 ml. The patient did not have any previous major illness and had only taken oral pain medication in the past, this was his first visit to the hospital. Even with painkillers, his symptoms got worse. None of the patient’s family members had a history of schwannoma.
We conducted a comprehensive MRI scan of the patient’s entire spine, which included T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), and enhanced scanning following the injection of a contrast agent. The tumors showed mixed signals on MRI plain scan and heterogeneous enhancement on enhanced MRI.MRI examination showed two tumors at L3 level and T11-L1 level respectively, measuring 8 × 8 × 10 mm and 24 × 54 × 20 mm. At the same time, syringomyelia at T4-T10 level appeared above the tumor at T11-L1 level (Figure 1). Based on the patient’s clinical manifestations and MRI examination, the preoperative preliminary diagnosis was schwannoma or ependymoma. Other differential diagnoses included glioma, astrocytoma, calcified tuberculoma etc.
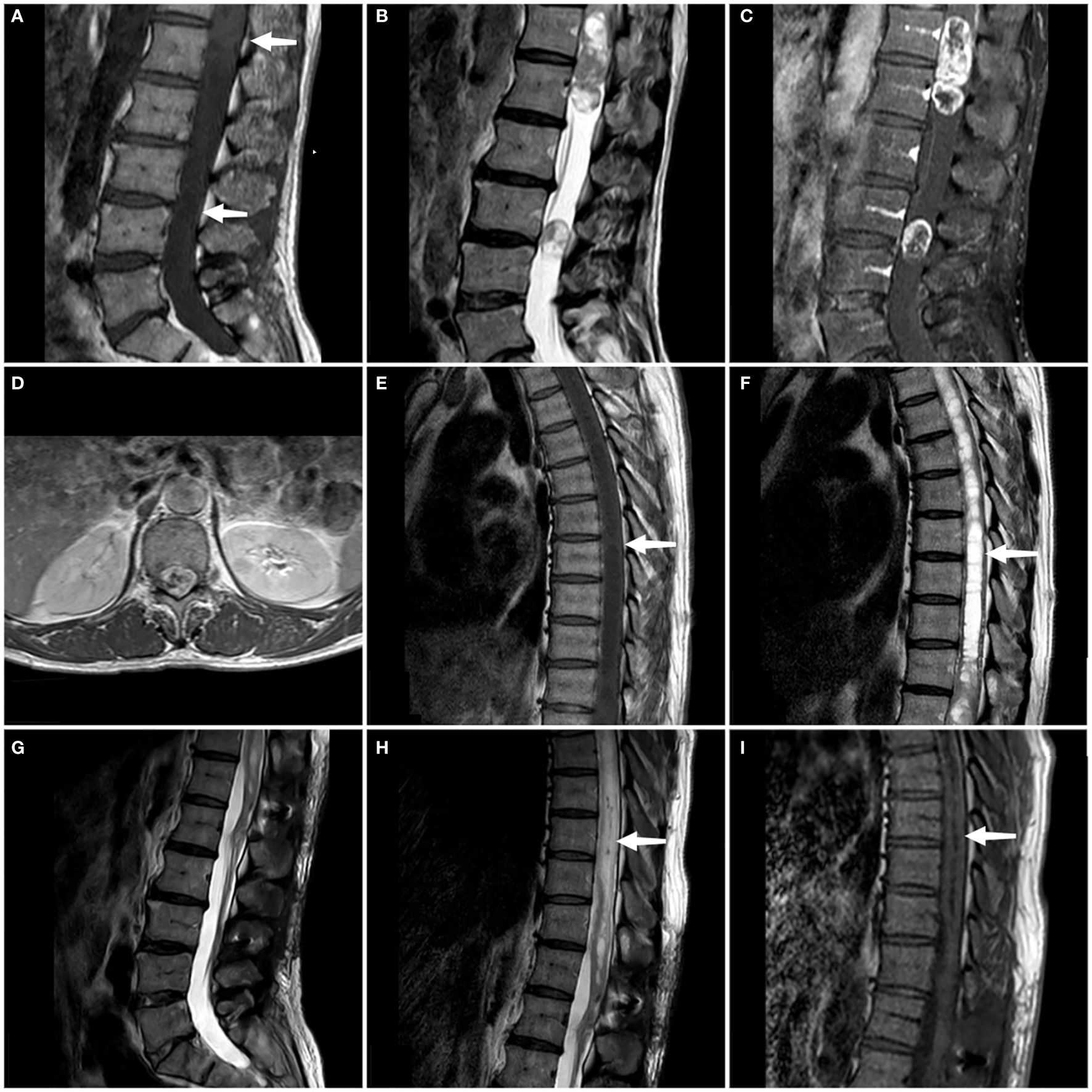
Figure 1. On the sagittal T1WI sequence, iso-signal occupying lesions (at the white arrow) are discernible separately within the T11-L1 segment and the L3 segment (A). On the sagittal T2WI sequence, both tumors exhibit mixed signals (B). Enhanced T1WI scans in both sagittal and axial planes reveal significant and heterogeneous enhancement of both lesions (C, D). A persistent intramedullary abnormal signal at T4-T10 was seen above the lesion (at the white arrow), showing hypointense on the T1WI and hyperintense on the T2WI (E, F). Follow-up MRI at 3 months confirmed complete tumor removal with no recurrence (G). Sagittal T1- and T2-weighted thoracic MRI images reveal a marked reduction in syringomyelia compared to preoperative findings (white arrow) (H, I).
Subsequently, both tumors were removed simultaneously. The patient was placed in the prone position and two incisions were made at T12-L1 and L3 spinous processes. The skin and fascia were dissected layer by layer, and the muscles on both sides of the spinous process were separated and pulled. The bilateral laminae of T12, L1 and L3 were opened to expose the tumors, revealing that the tumor at the T11-L1 level was IM, while the tumor at L3 was extramedullary intradural.
Both tumors were completely resected under the microscope, the patient’s neurological symptoms improved after the operation. Pathological examination showed that both tumors were schwannomas (Figure 2).
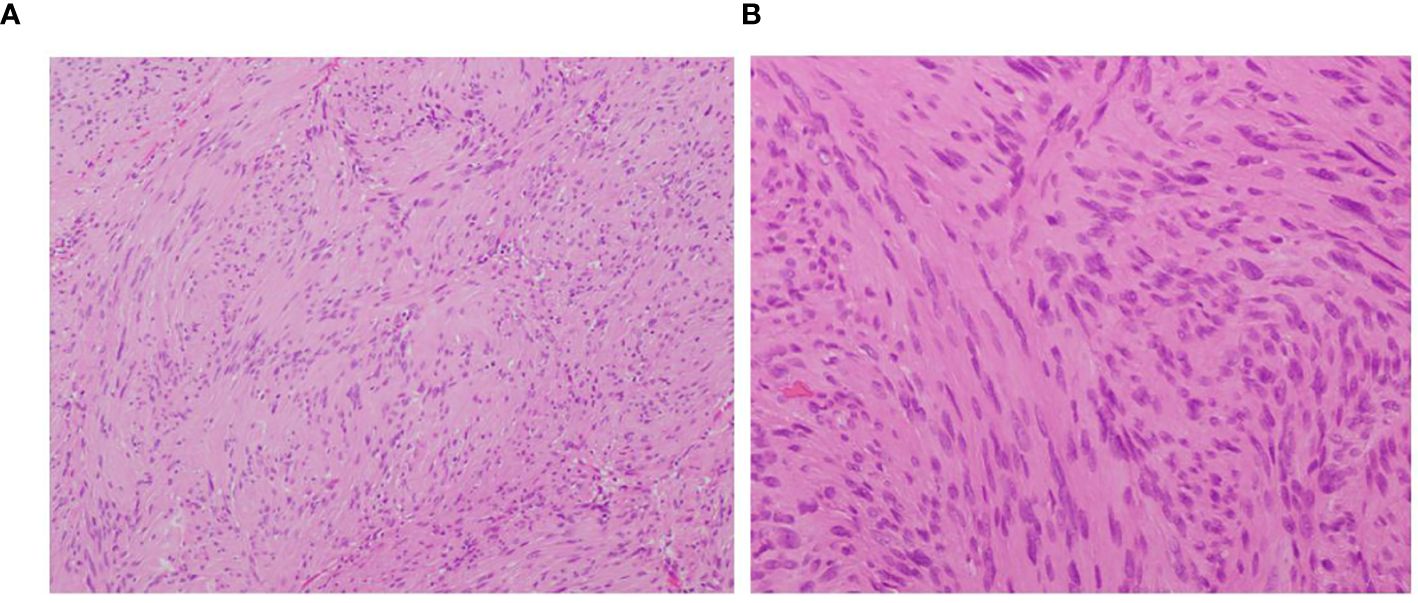
Figure 2. (A) observing under a microscope, the tumor shows cellular and hypocellular areas (H&E×200). (B) plumped spindle-shaped cells with palisading nuclei can be seen (H&E ×400).
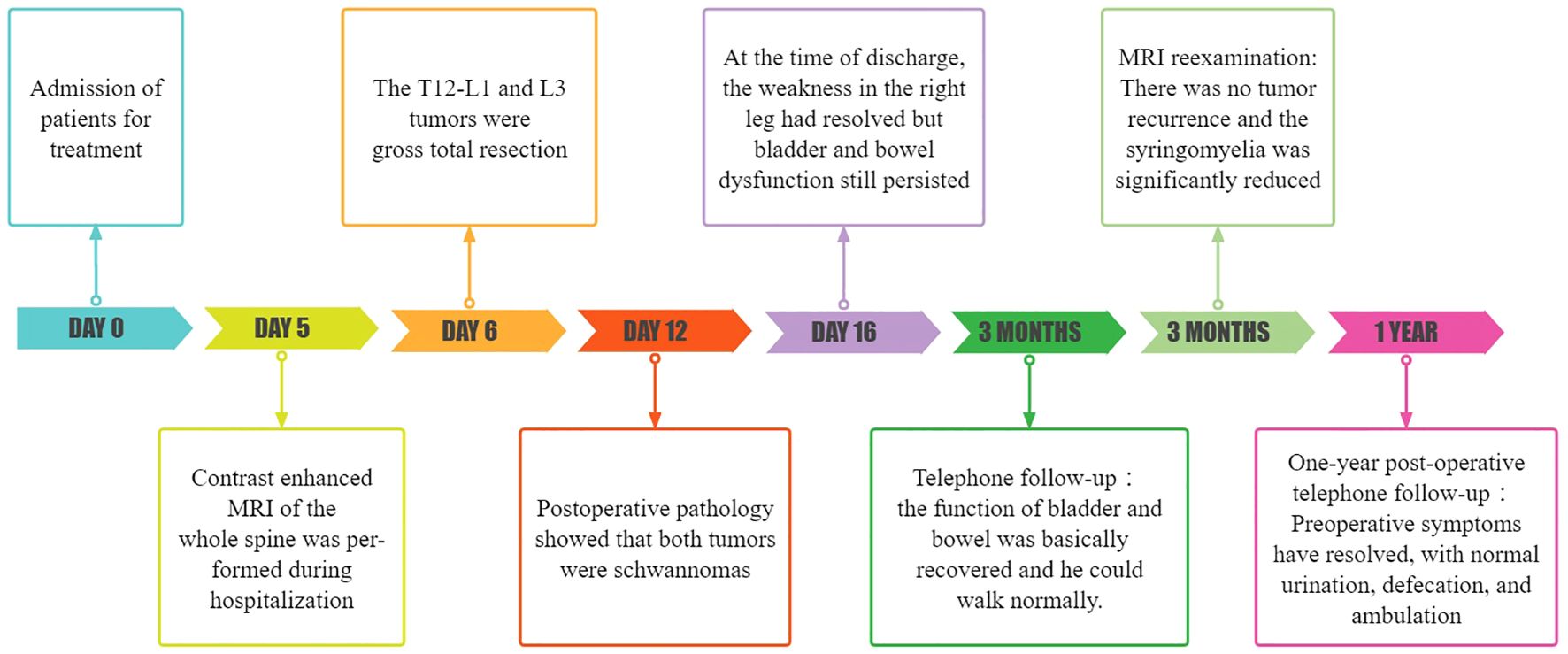
At the time of discharge, the patient’s condition was significantly better than that on admission, the weakness of the right lower limb was significantly relieved, and he could basically walk normally. He still had weakness of the anal sphincter and difficulty in bowel and defecation. The lumbar incision healed well. Physical examination showed grade 5 muscle strength in the right lower extremity and Babinski signs were negative. Three months later, the patient’s bladder and stool function was significantly improved compared with that before surgery, and he could walk completely independently. MRI reexamination showed that the tumor was completely removed and no recurrence was observed, and syringomyelia significantly shrank (Figure 1). During the one-year post-operative telephone follow-up, the patient is currently able to walk normally, with urinary and bowel functions having largely returned to normal, and the preoperative symptoms have essentially dissipated.The treatment timeline is shown in Figure 3.
Discussion
IM schwannomas are very rare, pathologist James Kernohan first reported IM schwannoma in 1931 (17). The last possible literature review was done in 2021 by V. M. Swiatek et al. that listed 166 IM schwannoma instances that were documented at the time (11). The cervical spine accounted for 63% of recorded cases of IM schwannoma, with the thoracic spine of the spinal cord coming in second at 26% and the lumbar spine at 11% (21). We conducted a systematic review of the literature in PubMed up to January 1, 2023 using the keywords “intramedullary” and “schwannoma” to retrieve all relevant studies and case reports of IM schwannoma. Inclusion criteria were as follows (1): at least one histologically confirmed IM schwannoma was reported (2), the tumor was located at the site of the conus medullaris, and (3) clinical information on the patient was available. We did not include the cases with neurofibromatosis. Only 15 cases—not including this one—of IM schwannoma in the spinal cord’s conus have ever been documented in the literature (1, 2, 5, 6, 8–10, 12, 13, 15, 16, 18–20, 22) (Table 1).
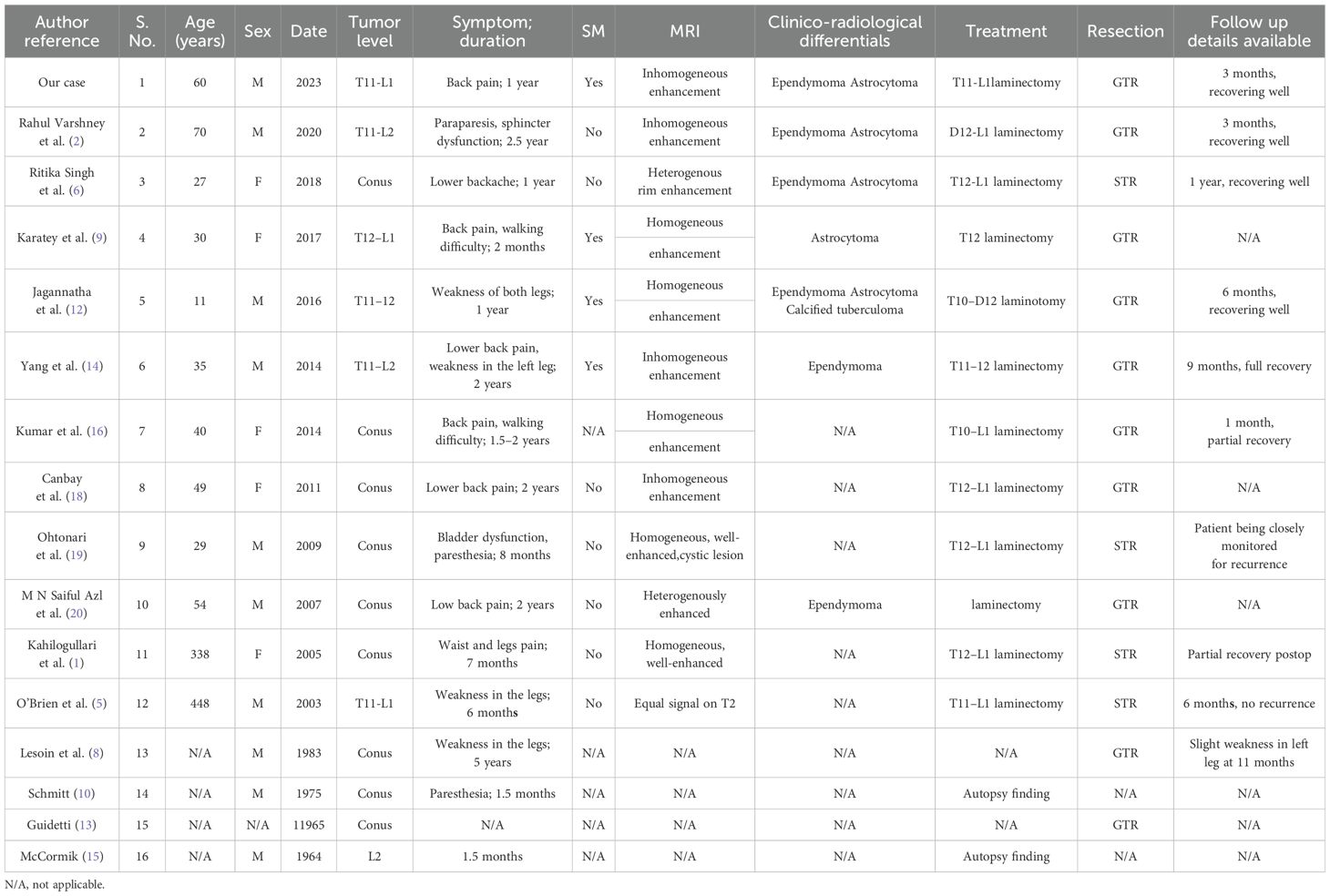
Table 1. Summary of conus intramedullary schwannomas (in non-neurofibromatosis patients) reported to date.
By reviewing 16 cases with IM schwannomas in the conus of the spinal cord including our case, the age range was found to be 11–70 years old, with an average age of 40.1 ± 16.2 years. In a 2:1 ratio, men are more affected than women, which is consistent with the previous literature (11). The mean interval between the symptom onset and diagnosis was 16.9 months. This may be because IM schwannoma often grows slowly and infrequently exhibits clinical signs in its early stages. Among the 16 cases, the main symptom of about 57% was low back pain, 43% had movement disorders, 21% had paresthesia, 14% had sphincter dysfunction, and 1 patient had hypolibido or sexual dysfunction. Depending on where the tumor is located, the symptomatology of schwannomas might include neurological impairments as well as signs and symptoms (6). Prior research focusing on the clinical characteristics and surgical outcomes of patients with IM schwannomas has consistently demonstrated that pain or sensory disturbance constitutes the most prevalent initial symptom, followed by motor dysfunction, with sphincter dysfunction ultimately manifesting in the late stages (14).
Since there are no Schwann cells in the central nervous system’s white matter, the pathophysiology of IM schwannomas is up for discussion (23). Intramural Schwann cells arising from the embryonal neural tube; Schwann cells transforming from neuroectoderm into pial cells; Schwann cells migrating into the cord in response to cord trauma; Schwann cells proliferating in the spinal artery nerve fibers; Schwann cells extending into cord from the region where spinal nerve roots enter pia mater are some of the theories regarding the origin of IM schwannomas (5, 24, 25). The Neurospinal Society of Japan conducted a nationwide analysis on IM Schwannoma of the spinal cord (26). Two primary categories of tumor location and extension can be distinguished: intramedullary to extramedullary extension and fully intramedullary extension. Each type may have a distinct developing pathway in light of these mechanisms and the location of the tumor. The shape of the tumor may depend on where in the spinal cord the aforementioned mechanisms take place. For instance, it is believed that tumors arising in more central parts of the spinal cord have intramedullary lesions, whereas tumors arising at the surface of the spinal cord have exophytic lesions.
Preoperative diagnosis might be challenging. MRI is the modality of choice for diagnosing IM schwannomas. On T1-weighted images, these IM schwannomas often show low to intermediate signal strength. They may be heterogeneous on T2-weighted imaging, showing collagen deposition, hemorrhage, and focal areas of hyper- and hypo-intensity (18). Strong contrast enhancement is typically seen in schwannomas, most likely as a result of open-gap junctions, which are short, straight, patent, and readily communicate with a sizable extracellular space (9). Of the 15 cases we reviewed, MRI data of 11 patients could be obtained, and the other 4 patients only had the relevant description of myelography. Uniform or nodular strong contrast enhancement was seen in all but one case (no T1 enhanced scan was performed), which may be due to the abundant blood supply of the schwannoma. Of them, syringomyelia was present in just 3 cases, whereas 3 cases had uneven enhancement and 7 cases had uniform enhancement. In our case, both lesions showed isointensity on sagittal T1-weighted images, with a long strip of hypointense shadow above the lesion at T11-L1. A heterogeneously low-signal lesion at T11-L1 with a noticeable high signal extending upward is visible on the sagittal T2-weighted MRI, which is consistent with syringomyelia. Both lesions showed significant uneven enhancement and well-delineated masses on coronal enhanced T1-weighted images. Differential diagnosis typically encompasses all IM lesions with contrast enhancement, such as ependymomas, astrocytomas, hemangioblastomas, and metastatic tumors, which generally exhibit unclear tumor boundaries and are associated with spinal cord edema and tumor cysts (27). While a well-defined enhancement pattern can serve as a distinguishing feature between IM schwannomas and other IM tumors, our review identified that 27% (3/11) of the cases did not conform to this pattern, manifesting as heterogeneous or rim enhancement. Peritumoral edema or tumor cysts are also prevalent in IM schwannomas, as observed in our cases. In conclusion, it is challenging to establish a definitive preoperative diagnosis of IM schwannoma solely based on MRI findings.
After analyzing preoperative MRI characteristics of IM schwannomas that had previously been documented in the literature, Ho et al. (7) came to the conclusion that the lack of syringomyelia was a diagnostic MRI sign of IM schwannomas. However, some cases with IM schwannomas also have syringomyelia associated with them. Nine of the twenty instances with IM schwannoma reported by Yang et al. (22) had syringomyelia. The study conducted by V. M. Swiatek et al. (11) reported that 20.9% of the 165 IM schwannomas patients were found to have association with syringomyelia. In our case, the MRI showed very long segment syringomyelia at the level of T4-T10. The review of 16 cases, including the present one, resulted in the exclusion of 2 cases due to missing imaging data and the identification of 4 cases associated with syringomyelia (9, 12, 22). We speculate that a tumor that obstructed the flow of cerebrospinal fluid in the central canal produced the syringomyelia. As a result, our conclusion is that the absence of syringomyelia, although prevalent, lacks specificity. For tumors such as IM ependymoma, astrocytoma, and hemangioblastoma, which require differentiation from IM schwannoma, the presence of syringomyelia also lacks specificity. Notably, ependymoma, due to its location at the center of the spinal cord, is often accompanied by syringomyelia (28). Therefore, we propose that the absence of syringomyelia may have a certain role in the diagnosis of IM schwannoma, albeit lacking specificity. Pathological diagnosis constitutes an indispensable component in confirming the diagnosis of schwannomas. Histopathological examination typically reveals the tumor tissue arranged in alternating hypercellular and hypocellular areas (Antoni A and B), with tumor cells dispersed within a loose, mucinous stroma. These cells display an oval to spindle shape and form palisading patterns (Figure 2). Immunohistochemical staining often demonstrates positive expression for S-100 protein and Leu-7 (4).
As schwannoma is benign, gross total resection (GTR) is the preferred treatment for IM schwannoma. Nonetheless, not every patient may be able to achieve GTR. A well-defined tumor-spinal cord anatomical plane is critical for obtaining GTR; therefore, an ill-defined anatomical plane may mean that the tumor is difficult to achieve GTR even if it is benign. STR may also be necessary to prevent deterioration of neurological function if the lesion is adherent to the neural tissue (21). Furthermore, the residual tumor may be removed with a second surgery (29). After reporting 20 cases with IM schwannomas, 16 of which had GTR and 4 of which had subtotal resection (STR), Yang et al. (22) came to the conclusion that GTR or STR could result in a favorable clinical outcome. By reviewing reported 14 IM schwannoma cases (2 cases were excluded because surgical information was not available), GTR was achieved in 79% (11/14) of patients. In the study conducted by Yang et al. (14), the syrinx shrank in 77.8% (7/9) of patients with IM schwannoma accompanied by syringomyelia who underwent tumor resection only, with no syrinx enlargement observed. In our case, the shrinkage of syringomyelia was also observed during the radiological reassessment conducted three months post-surgery. Therefore, for syringomyelia secondary to IM schwannoma, additional drainage of the syrinx may not be necessary, as the syrinx may collapse following tumor resection (28). Adjuvant treatment modalities, such as radiotherapy and chemotherapy, have failed to exhibit favorable therapeutic outcomes in the context of IM spinal cord schwannomas. Conversely, adjuvant radiotherapy and chemotherapy may be utilized in the management of partially resected or recurrent IM spinal cord tumors, encompassing gliomas and ependymomas.Research is ongoing to develop novel therapeutic strategies for these patients, including targeted drug delivery and nanomedicine technologies (30).
Recurrence of IM schwannoma tumors is rare, even in cases with STR (14). M. Swiatek et al. (11) reported 165 patients with IM schwannomas with a mean follow-up of 34 months, and tumor recurrence was observed in only 4% of cases. Of the 15 cases of conical IM schwannomas we collected, follow-up information was not available in 6 cases. Tumor recurrence was not observed in the remaining 9 cases, even though 4 of them only achieved STR. During the follow-up period with an average duration of 6 months, almost all cases exhibited improved neurological function post-operatively, including 4 patients who underwent STR. In our case, the tumor achieved GTR because both tumors had clear boundaries with the surrounding tissue. Three months following surgery, a follow-up MRI revealed no signs of tumor recurrence and a considerable reduction in the edema surrounding the tumor. In conclusion, our analysis suggests that the prompt surgical management of symptomatic IM schwannomas, irrespective of the surgical approach employed (either GTR or STR), holds the potential to elicit substantial improvements in patient prognosis.
Conclusion
In conclusion, although IM schwannomas in the conus medullaris are rare tumors, they should still be an option when diagnosing intramedullary tumors, especially with syringomyelia. As a benign tumor, intramedullary schwannoma can achieve good neurological outcome after surgical treatment.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of First Affiliated Hospital of Harbin Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HG: Writing – original draft, Writing – review & editing. YW: Writing – original draft, Writing – review & editing. LW: Writing – original draft, Writing – review & editing. DH: Writing – review & editing. XM: Writing – review & editing. XC: Writing – original draft, Writing – review & editing. QM: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Heilongjiang Welfare Fund Organization of Disabled Persons (HJ2022-1), the Postdoctoral Science Foundation of Heilongjiang Province (LBH-Z19076), and the National Natural Science Foundation of China (82360366) provided funding for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kahilogullari G, Aydin Z, Ayten M, Attar A, Erdem A. Schwannoma of the conus medullaris. J Clin Neurosci. (2005) . 12:80–1. doi: 10.1016/j.jocn.2004.02.016
2. Varshney R, Bharadwaj P, Choudhary A, Paliwal P, Kaushik K. A rare case of intramedullary schwannoma at conus medullaris: A case report with review of literature. Surg Neurol Int. (2020) . 11:454. doi: 10.25259/sni_718_2020
3. Conti P, Pansini G, Mouchaty H, Capuano C, Conti R. Spinal neurinomas: retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature. Surg Neurol. (2004) . 61:34–43. doi: 10.1016/s0090-3019(03)00537-8
4. Seppälä MT, Haltia MJ, Sankila RJ, Jääskeläinen JE, Heiskanen O. Long-term outcome after removal of spinal schwannoma: a clinicopathological study of 187 cases. J Neurosurg. (1995) . 83:621–6. doi: 10.3171/jns.1995.83.4.0621
5. O’Brien DF, Farrell M, Fraher JP, Bolger C. Schwann cell invasion of the conus medullaris: case report. Eur Spine J. (2003) . 12:328–31. doi: 10.1007/s00586-002-0484-9
6. Singh R, Chaturvedi S, Pant I, Singh G, Kumari R. Intramedullary schwannoma of conus medullaris: rare site for a common tumor with review of literature. Spinal Cord Ser Cases. (2018) . 4:99. doi: 10.1038/s41394-018-0134-z
7. Ho T, Tai KS, Fan YW, Leong LL. Intramedullary spinal schwannoma: case report and review of preoperative magnetic resonance imaging features. Asian J Surg. (2006) . 29:306–8. doi: 10.1016/s1015-9584(09)60108-1
8. Lesoin F, Delandsheer E, Krivosic I, Clarisse J, Arnott G, Jomin M, et al. Solitary intramedullary schwannomas. Surg Neurol. (1983) . 19:51–6. doi: 10.1016/0090-3019(83)90211-2
9. Karatay M, Koktekir E, Erdem Y, Celik H, Sertbas I, Bayar MA. Intramedullary schwannoma of conus medullaris with syringomyelia. Asian J Surg. (2017) . 40:240–42. doi: 10.1016/j.asjsur.2014.04.004
10. Schmitt HP. Epi-” and intramedullary neurilemmoma of the spinal cord with denervation atrophy in the related skeletal muscles. J Neurol. (1975) . 209:271–8. doi: 10.1007/bf00314366
11. Swiatek VM, Stein KP, Cukaz HB, Rashidi A, Skalej M, Mawrin C, et al. Spinal intramedullary schwannomas-report of a case and extensive review of the literature. Neurosurg Rev. (2021) . 44:1833–52. doi: 10.1007/s10143-020-01357-5
12. Jagannatha AT, Joshi KC, Rao S, Srikantha U, Varma RG, Mahadevan A. Paediatric calcified intramedullary schwannoma at conus: A common tumor in a vicarious location. J Pediatr Neurosci. (2016) . 11:319–21. doi: 10.4103/1817-1745.199474
13. Guidetti B. Intramedullary tumours of the spinal cord. Acta Neurochir (Wien). (1967) . 17:7–23. doi: 10.1007/bf01670413
14. Yang T, Wu L, Deng X, Yang C, Xu Y. Clinical features and surgical outcomes of intramedullary schwannomas. Acta Neurochir (Wien). (2014) . 156:1789–97. doi: 10.1007/s00701-014-2168-8
15. McCormick WF. Intramedullary spinal cord schwannoma. A unique case. Arch Pathol. (1964) . 77:378–82.
16. Kumar R, Mittal RS. Intramedullary schwannoma of conus medullaris: case report and review of the literature. World Spinal Column J. (2014), 1–5.
17. Kernohan JW, Woltman HW, Adsion AW. Intramedullary tumors of the spinal cord: a review of fifty-one cases, with an attempt at histologic classification. Arch Neurol Psychiatry. (1931) . 25:679–701. doi: 10.1001/aurpsyc.1931.02230040013001
18. Canbay S, Hasturk AE, Markoc F, Caglar S. Schwannoma of the conus medullaris: a rare case. Chin J Cancer. (2011) . 30:867–70. doi: 10.5732/cjc.011.10213
19. Ohtonari T, Nishihara N, Ota T, Ota S, Koyama T. Intramedullary schwannoma of the conus medullaris complicated by dense adhesion to neural tissue. Neurol Med Chir (Tokyo). (2009) . 49:536–8. doi: 10.2176/nmc.49.536
20. Saiful Azli MN, Abd Rahman IG, Md Salzihan MS. Ancient schwannoma of the conus medullaris. Med J Malaysia. (2007) . 62:256–8.
21. Navarro Fernández JO, Monroy Sosa A, Cacho Díaz B, Arrieta VA, Ortíz Leyva RU, Cano Valdez AM, et al. Cervical intramedullary schwannoma: case report and review of the literature. Case Rep Neurol. (2018) . 10:18–24. doi: 10.1159/000467389
22. Yang T, Wu L, Yang C, Deng X, Xu Y. Coexisting intramedullary schwannoma with an ependymal cyst of the conus medullaris: A case report. Oncol Lett. (2015) . 9:903–06. doi: 10.3892/ol.2014.2786
23. Kodama Y, Terae S, Hida K, Chu BC, Kaneko K, Miyasaka K. Intramedullary schwannoma of the spinal cord: report of two cases. Neuroradiology. (2001) . 43:567–71. doi: 10.1007/s002340100540
24. Wood WG, Rothman LM, Nussbaum BE. Intramedullary neurilemoma of the cervical spinal cord. Case Rep J Neurosurg. (1975) . 42:465–8. doi: 10.3171/jns.1975.42.4.0465
25. Nayak R, Chaudhuri A, Chattopadhyay A, Ghosh SN. Thoracic intramedullary schwannoma: A case report and review of literature. Asian J Neurosurg. (2015) . 10:126–8. doi: 10.4103/1793-5482.145155
26. Hara T, Mizuno M, Hida K, Sasamori T, Miyoshi Y, Uchikado H, et al. Intramedullary schwannoma of the spinal cord: A nationwide analysis by the neurospinal society of Japan. Neurospine. (2023) . 20:747–55. doi: 10.14245/ns.2346376.188
27. Lee SE, Chung CK, Kim HJ. Intramedullary schwannomas: long-term outcomes of ten operated cases. J Neurooncol. (2013) . 113:75–81. doi: 10.1007/s11060-013-1091-9
28. Wang K, Zhao J, Zhang Y, Su Y. Pediatric intramedullary schwannoma with syringomyelia: a case report and literature review. BMC Pediatr. (2018) . 18:374. doi: 10.1186/s12887-018-1341-2
29. Hida K, Yano S, Iwasaki Y. Staged operation for huge cervical intramedullary schwannoma: report of two cases. Neurosurgery. (2008) 62:ONS456–60. doi: 10.1227/01.neu.0000326035.54714.cc
Keywords: intramedullary schwannoma, syringomyelia, conus medullaris, surgical treatment, case report
Citation: Guo H, Wang Y, Wang L, Han D, Meng X, Mu Q and Chen X (2024) Intramedullary schwannoma of conus medullaris with syringomyelia: a case report and literature review. Front. Oncol. 14:1422958. doi: 10.3389/fonc.2024.1422958
Received: 26 April 2024; Accepted: 11 November 2024;
Published: 02 December 2024.
Edited by:
Cheng-Ying Ho, Johns Hopkins Medicine, United StatesReviewed by:
Sheng-Che Hung, University of North Carolina at Chapel Hill, United StatesThomas Zaikos, University of California, Irvine, United States
Copyright © 2024 Guo, Wang, Wang, Han, Meng, Mu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingchun Mu, bXVxQHN1ZGEuZWR1LmNu; Xiaofeng Chen, Y2hlbnhpYW9mZW5nMjAwOEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Hua Guo
Hua Guo Yao Wang
Yao Wang Liankun Wang3†
Liankun Wang3† Xiangyi Meng
Xiangyi Meng Qingchun Mu
Qingchun Mu Xiaofeng Chen
Xiaofeng Chen