- 1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Interventional Therapy, Peking University Cancer Hospital & Institute, Beijing, China
- 2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Hepatic & Biliary Surgery, Peking University Cancer Hospital & Institute, Beijing, China
Background and aim: The prognosis of microsatellite stable (MSS)-colorectal cancer liver metastasis (CRCLM) following failure of multi-line therapy remains dismal. The aim of this study is to evaluate the efficacy and safety of hepatic arterial infusion chemotherapy (HAIC) plus fruquintinib and tislelizumab (HAIC-F-T treatment) for MSS-CRCLM which failed from multiple-line therapy.
Methods: From February 2021 to June 2023, 45 patients with MSS-CRCLM after failure of multiple-line therapy who received HAIC combined with fruquintinib and tislelizumab (HAIC-F-T triple treatment) were enrolled. The combination therapy included HAIC regimens with oxaliplatin and 5-fluorouracil or irinotecan, oxaliplatin, and 5-fluorouracil on days 1-2, intravenous tislelizumab (200 mg) before HAIC on day 1, and oral fruquintinb (3 mg/d) on day 3-21, every 4 weeks. Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan-Meier method.
Results: The follow-up ended on June 22, 2024, with a median follow-up time of 17.5 months. The objective response rate was 42.2%, and the disease control rate was 82.2%. The median OS was 15.3 months (95% confidence interval [CI]:12.634-17.966), and the median PFS was 7.5 months (95% CI:5.318-9.682). The independent risk factors related to worse OS were previous PD-1 immunotherapy (P = 0.021) and the number of HAIC-F-T triple treatment cycles of ≤ 2 (P = 0.007). The incidence of grade 3 or higher adverse events (AEs) was 20%, with the most frequent grade 3 or higher AEs being abdominal pain (3/45, 6.7%).
Conclusion: HAIC combined with fruquintinib and tislelizumab may be an alternative salvage treatment for patients with MSS-CRCLM following failure of multiple-line therapy.
Introduction
Colorectal cancer (CRC) is the third most common malignancy and the second leading cause of cancer-related death worldwide (1). Approximately 20% of patients with CRC have synchronous liver metastasis, and approximately 50% of patients with CRC will develop liver metastasis (2, 3). Liver resection has been confirmed to significantly improve the prognosis of patients with colorectal cancer liver metastasis (CRCLM), whereas only 20% of patients with CRCLM are suitable for liver resection (4, 5).
For patients with unresectable metastatic CRC, systemic chemotherapy with or without targeted therapy (anti-vascular endothelial growth factor [VEGF] or anti-epidermal growth factor receptor [EGFR] therapy) has been demonstrated to prolong survival and is recommended as first- and second-line treatment for metastatic CRC. However, the prognosis of metastatic CRC that has failed standard second-line treatment remains dismal. Although regorafenib, TAS-102, and fruquintinib are recommended as third-line treatments, the median progression-free survival (PFS) and median overall survival (OS) ranged from 1.9 to 3.7 months and from 6.4 to 9.3 months, respectively, in multiple phase III trials (6–10).
Programmed death receptor 1 (PD-1)/programmed death receptor ligand-1 (PD-L1) inhibitors have shown great efficacy in CRC with microsatellite instability-high (MSI-H)/deficiency mismatch repair (dMMR) in some proof-of-concept studies and phase II trials, with an objective response rate (ORR) ranging from 31.1% to 65% (11–14). Nevertheless, in the KEYNOTE-016 and KEYNOTE-028 trial, the efficacy of PD-1/PD-L1 inhibitors for CRC with microsatellite-stable (MSS)/proficiency mismatch repair (pMMR), which accounts for 80-90% of patients with CRC, was dismal, with an ORR of 0% (11, 15, 16). This may due to the absent or inadequate T cell infiltration and an immunosuppressive tumor microenvironment presented by MSS tumor, which leads to the resistance to PD-1/PD-L1 inhibitors (17). However, anti-VEGF therapy and PD-1/PD-L1 inhibitors presented with synergistic effect in treating MSS-CRC in recent years, with an ORR ranging from 7.1% to 60% (18–20). Fruquintinib, an oral multi-kinase inhibitor targeting VEGF receptors 1-3, was recently demonstrated to enhance the efficacy of PD-1/PD-L1 inhibitors for MSS-CRC (21, 22). A median PFS ranging from 3.4 to 5.6 months was achieved after treatment of the combination of fruquintinib and PD-1/PD-L1 inhibitors in MSS-CRCLM in preliminary studies (23, 24).
Hepatic arterial infusion chemotherapy (HAIC) can deliver chemotherapeutic agents directly into the vessels supplying the tumor in the liver, reaching a high concentration of chemotherapeutic agents in the tumor and achieving great local tumor control in the liver (25). As HAIC has been shown to be beneficial to unresectable CRCLM for many years, it is recommended as a salvage treatment following failure of standard systemic treatment (26–29). In 2021, a meta-analysis showed that both the OS rate and ORR in the HAIC group were significantly higher than those in the systemic therapy group (30). The hazard ratio of the OS rate was 0.17 (P < 0.001) in the palliative treatment setting and 0.63 (P < 0.001) in the adjuvant setting, and the relative risk of ORR was 2.09 (P = 0.001) in the palliative treatment setting and 2.14 (P < 0.001) in the adjuvant setting.
Thus, this retrospective study aimed to evaluate the efficacy and safety of HAIC combined with fruquintinib and tislelizumab (a PD-1 inhibitor) for patients with MSS-CRCLM following failure of multiple-line therapy.
Materials and methods
Patients
This retrospective study was approved by the ethics committee of the Peking University Cancer Hospital, and the need for informed consent was waived. The study was performed in line with the principles of the Declaration of Helsinki. All patients with CRCLM who were treated by HAIC combined with fruquintinib and tislelizumab from February 2021 to June 2023 were reviewed.
The inclusion criteria were as follows: (1) 18-80 years old; (2) diagnosed with unresectable CRCLM by histopathology and confirmed by a multidisciplinary team; (3) after failure of multiple-line therapy; (4) at least one measurable lesion in the liver according to the RECIST 1.1 criteria; (5) microsatellite stable; and (6) at least one evaluation of the tumor response to the treatment. Patients with other concomitant malignancies were excluded, as were those with a lack of baseline or follow-up data and a follow-up time of < 6 months.
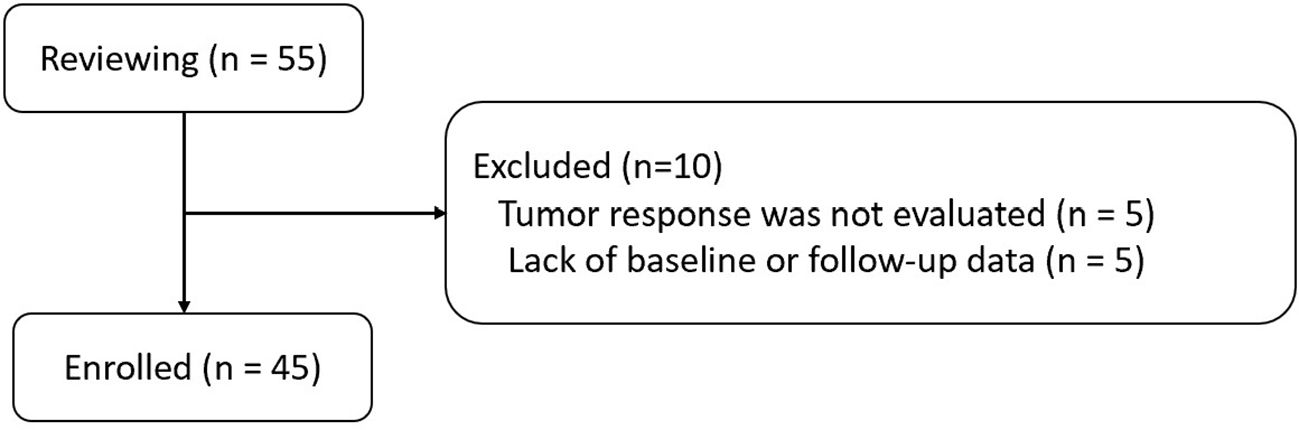
Searching for patients in the Hospital Information System-based Case Retrieval System via the keywords “CRCLM,” “fruquintinib,” and “tislelizumab,” 55 patients with CRCLM treated by HAIC combined with fruquintinib and tislelizumab were identified for the period from February 2021 to June 2023. Finally, 45 patients with MSS-CRCLM were enrolled in this study according to the inclusion and exclusion criteria (Figure 1).
Procedures and treatment regimens
All patients underwent abdominal contrast-enhanced CT/MRI within 1 month before the initiation of the treatment, and blood tests, such as blood routine examination, blood biochemistry, and tumor markers, were performed within 3 days before the initiation of the treatment.
The treatment, which was repeated every 4 weeks, consisted of HAIC, fruquintinib, and tislelizumab (HAIC-F-T triple treatment). The HAIC regimens, which depended on the decision of clinicians based on the previous regimens of systemic chemotherapy, were oxaliplatin (85 mg/m2, 0-2h, split into d1 and d2) and 5-fluorouracil (2 g/m2, 2-24h, split into d1 and d2) or irinotecan (100 mg/m2, 0-2 h, d1), oxaliplatin (65 mg/m2, 0-2 h, d2) and 5-fluorouracial (2 g/m2, 2-24 h, split into d1 and d2). Fruquintinib was administered orally after HAIC at a dosage of 3 mg on days 3-23, then suspended for 1 week. Tislelizumab was intravenously administered at a dosage of 200 mg before 24 h of HAIC.
HAIC was performed via a temporary indwelling hepatic artery catheter inserted as follows: After puncturing the femoral artery using the Seldinger technique, celiac and superior mesenteric angiographies were performed to detect variations of the hepatic artery. As described in previous published study, extrahepatic blood flow redistribution was performed via a 2.4/2.7F microcatheter to embolize the arteries that supply the extrahepatic organs, such as right gastric artery and accessory left gastric artery, with micro-coils; and intrahepatic blood flow redistribution were performed to convert the multiple hepatic arteries into one hepatic artery in cases of multiple hepatic artery variations such as accessory right/left hepatic artery arising from superior mesenteric artery or left gastric artery (31). The micro-catheter was then placed in the proper hepatic artery or common hepatic artery to ensure whole-liver perfusion via HAIC. HAIC was performed in the ward, and the catheter and sheath were removed following its completion. The same procedure was repeated for the next cycle of HAIC.
The combination of fruquintinib and tislelizumab was administered as maintenance treatment until tumor progression or patients died for patients who achieved liver tumor control after four to six cycles of the HAIC-F-T triple treatment.
Assessment and follow-up
Blood tests, such as blood routine examination, blood biochemistry, and tumor markers, were performed before every cycle of the treatment or every 3 months until tumor progression or death of patients, while abdominal contrast-enhanced CT/MRI was performed after every two cycles or every 3 months until tumor progression or death of patients. The CT/MRI images were analyzed by two radiologists with 12 and 17 years of experience in diagnostic imaging. Liver tumor burden was defined as the extent of liver metastasis proportional to the whole liver volume before the initiation of the combination therapy, and liver metastasis dominant was defined as a proportion of liver metastasis to systemic metastasis of ≥ 75% before the initiation of the combination treatment. The liver tumor burden and liver metastasis, irrespective of whether it was dominant or not, were independently assessed by radiologists, and a consensus was obtained.
Tumor response was evaluated using the RECIST 1.1 criteria. The ORR consisted of the complete response (CR) and partial response (PR), and the disease control rate (DCR) consisted of CR, PR, and stable disease (SD). Treatment related adverse events (AEs) were assessed using the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) Version 5.0.
Statistical analysis
Continuous variables are described as the mean ± standard deviation (SD), and were analyzed using chi-square tests or Fisher’s tests. Categorical variables are described as proportions, which were analyzed using Wilcoxon signed-rank tests. The OS and PFS were calculated using the Kaplan-Meier method and assessed using log-rank tests. The OS was calculated from the initiation of HAIC-F-T triple treatment to the time of the patients’ death or last follow-up, and the PFS was calculated from the initiation of HAIC-F-T triple treatment to the time of disease progression or patients’ death, whichever occurred first. Univariate and multivariate analyses were performed using the Cox proportional hazards regression method to detect risk factors related to worse survival, and characteristics with P < 0.1 in univariate analysis were included in the multivariate analysis. P < 0.05 was considered as statistically significant. The optimal cut-off values for continuous variables, such as the CEA level, CA 19-9 level, and time from liver metastasis to HAIC-F-T triple treatment, were determined using X-tile software (version 3.6.1, Yale University, New Haven, CT, USA). All other statistical analyses were performed using R software (R version 4.2.0, http://www.r-project.org).
Results
Patients
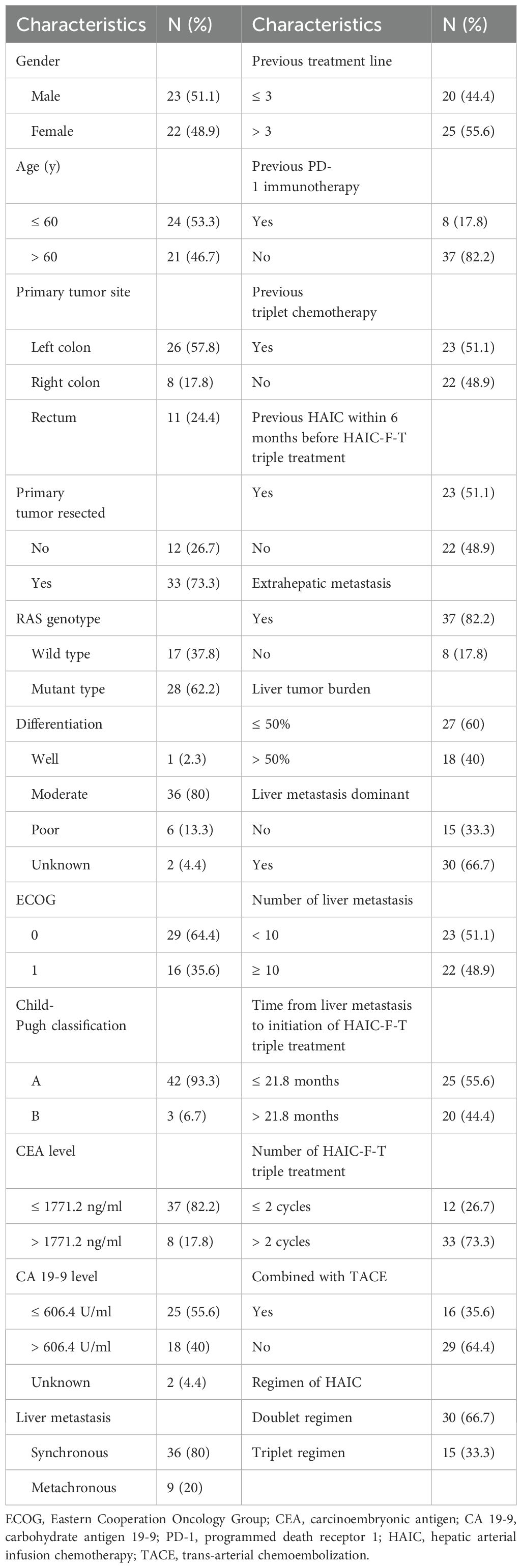
Of the 45 patients with MSS-CRCLM finally who were enrolled in this retrospective study, the median age was 58.73 ± 7.86 years, and 23 (51.1%) were male. Twenty-six patients (57.8%) were diagnosed with left colon cancer, 8 (17.8%) with right colon cancer, and 11 (24.4%) with rectal cancer. Most patients (73.3%) had primary tumors resected, and 82.2% had extrahepatic metastases. All patients had previously received second- or more-line oxaliplatin-based or irinotecan-based doublet or triplet chemotherapy with targeted treatment before HAIC-F-T triple treatment. Twenty-five patients (55.6%) had received more than three-line previous treatment, and 29 patients (64.4%) had received previous HAIC, with a mean of 3.06 ± 2.58 cycles, while got progression before HAIC-F-T triple treatment. The characteristics of the patients are detailed in Table 1.
Efficacy
A total of 165 cycles (mean ± SD: 3.7 ± 1.7 cycles) of HAIC-F-T triple treatment were performed in this study. The follow-up procedures were continued until June 22, 2024, with a median follow-up time of 17.5 months.
None of the patients achieved CR in this study, whereas PR, SD, and progressive disease (PD) were achieved in 19 (42.2%), 18 (40%), and 8 (17.8%) patients, respectively, according to the RECIST 1.1 criteria. The ORR was 42.2%, and the DCR was 82.2%.
The median OS was 15.3 months (95% confidence interval [CI]:12.634-17.966), and the median PFS was 7.5 months (95% CI:5.318-9.682) (Figure 2). The intrahepatic PFS, which was calculated from the initiation of the combination therapy to progression of intrahepatic lesions or patients’ death, whichever occurred first, was 7.9 months (95% CI:6.931-8.869), whereas the extrahepatic PFS, which was calculated from the initiation of the combination therapy to progression of extrahepatic lesions or patients’ death, whichever occurred first, was 5.7 months (95% CI:4.124-7.276).
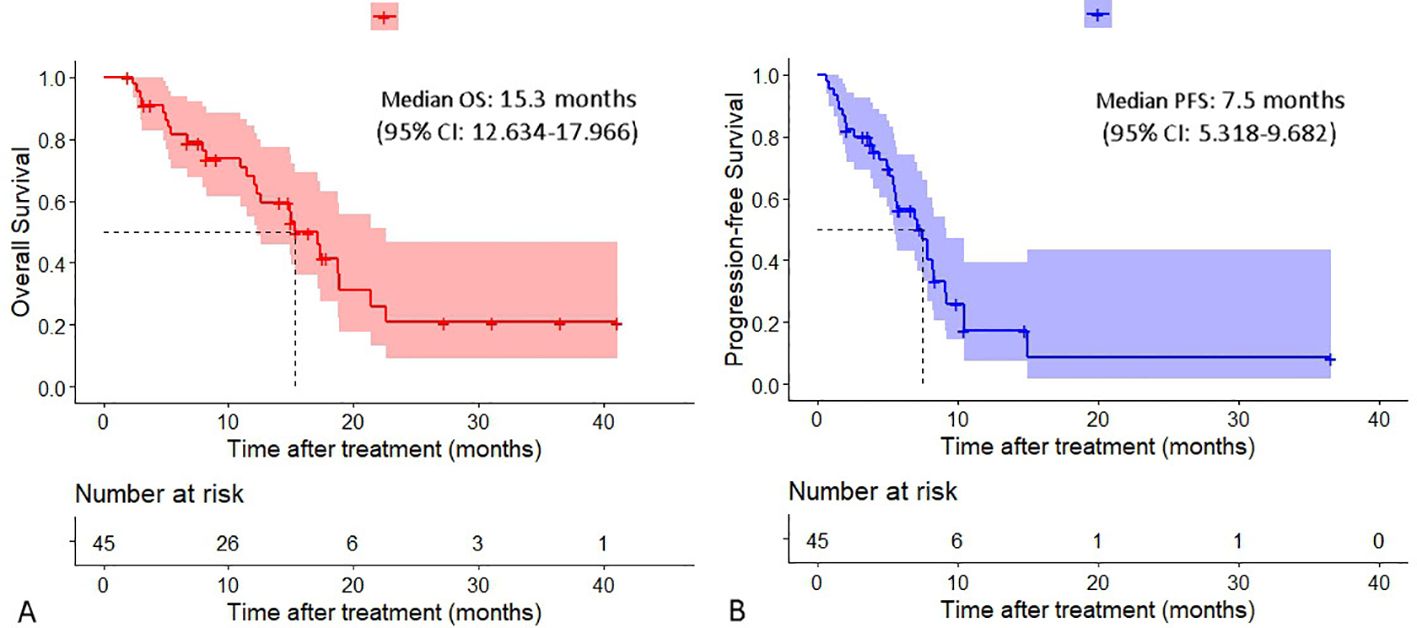
Figure 2. Cumulative curves of overall survival and progression-free survival. (A) Overall survival. (B) Progression-free survival.
In patients with two or three lines of previous treatment, the median OS was 18.8 months (95% CI:15.401-22.199), which was longer than that in patients whose previous treatment line was > 3 (12.3 months [95% CI:10.714-13.886], P = 0.056), and a tendency for prolonged median PFS was also observed (8.2 vs. 5.6 months, P = 0.158). In patients who did not receive previous PD-1 immunotherapy, both the median PFS and OS were significantly longer than those in patients who received previous PD-1 immunotherapy, with a median PFS of 7.9 vs. 2.0 months (P = 0.003) and a median OS of 17.3 vs. 5.4 months (P < 0.001), respectively (Figure 3).
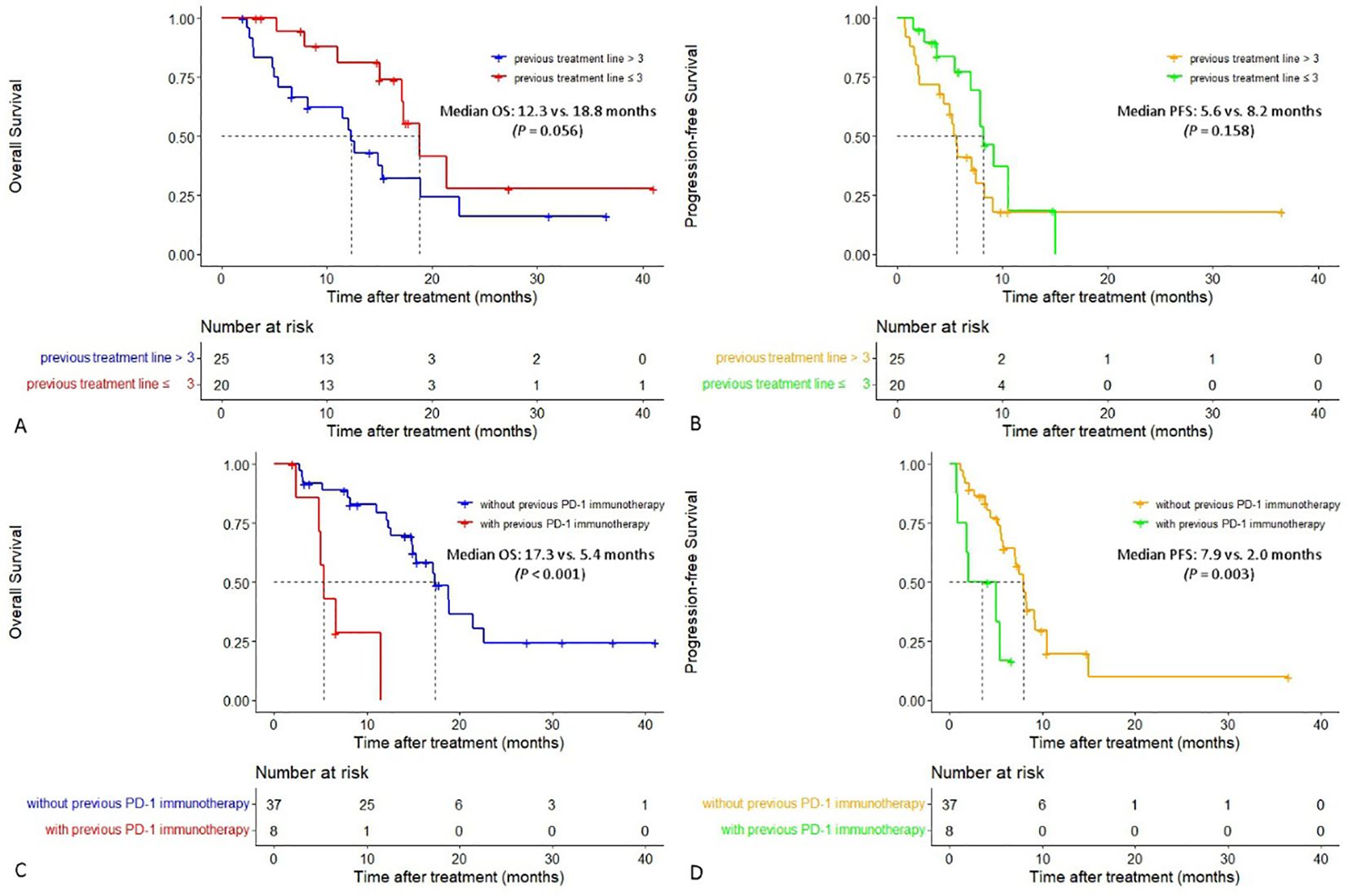
Figure 3. Cumulative curves of overall survival and progression-free survival in different subgroups. (A, B) Cumulative curves of overall survival and progression-free survival in patients with different previous treatment lines. (C, D) Cumulative curves of overall survival and progression-free survival in patients with or without previous PD-1 immunotherapy.
The median PFS in patients with liver metastasis dominant was significantly longer than that in patients with liver metastasis non-dominant (8.2 vs. 5.2 months, P = 0.016). The extrahepatic PFS in patients with liver metastasis dominant and patients with liver metastasis non-dominant was 6.6 vs. 5.2 months (P = 0.066). Additionally, both median OS and intrahepatic PFS in the former group were longer than that in the latter group (median OS: 17.1 vs. 8.2 months, median intrahepatic PFS: 8.3 vs. 7.1 months), although the differences were not statistically significant (P = 0.302 and P = 0.100) (Figure 4).
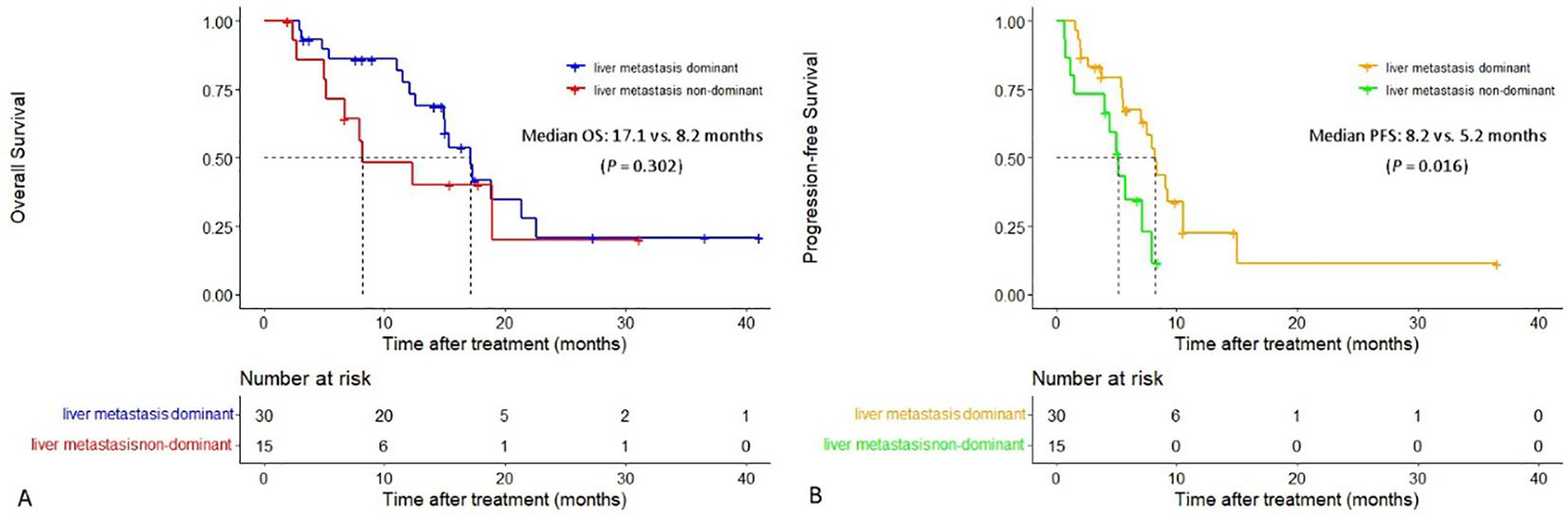
Figure 4. Cumulative curves of overall survival and progression-free survival in patients with liver metastasis dominant and those with liver metastasis non-dominant. (A) Overall survival. (B) Progression-free survival.
Univariate and multivariate analyses of factors related to OS
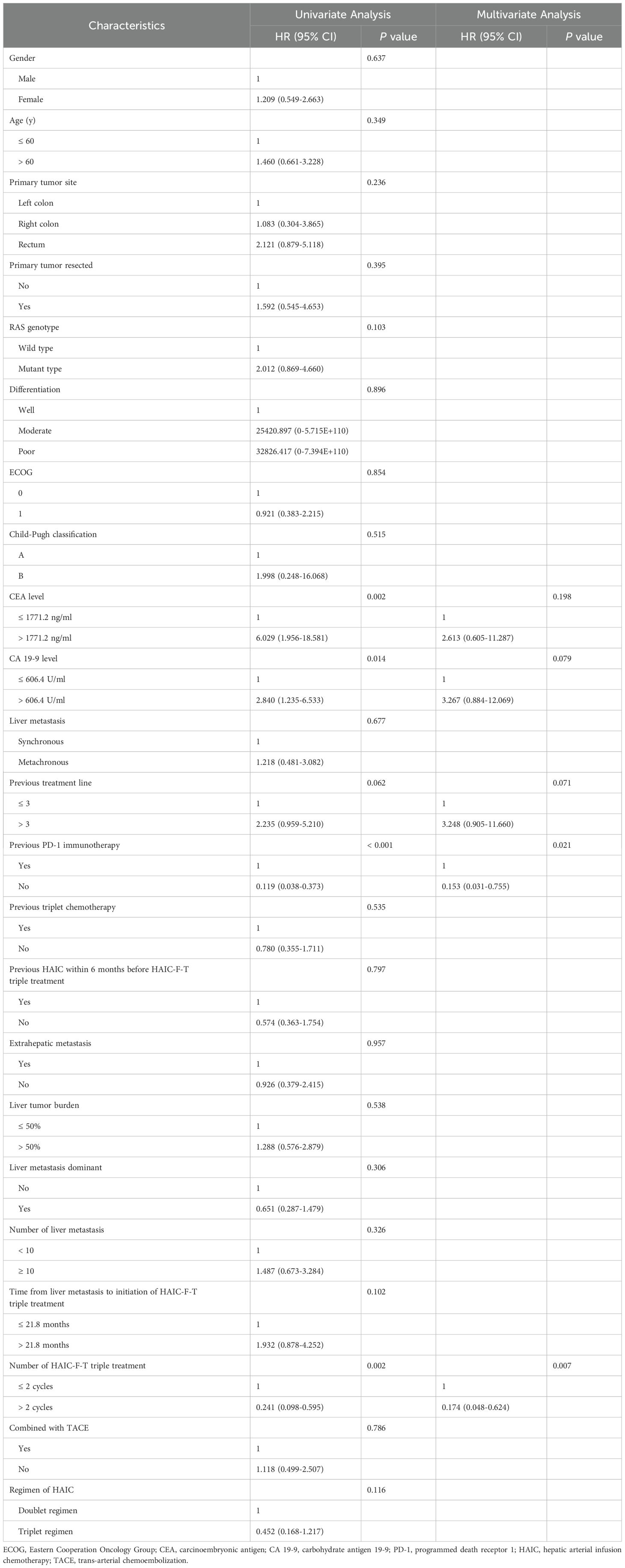
In the univariate analysis, CEA > 1771.2 ng/ml (P = 0.002), CA 19-9 > 606.4 U/ml (P = 0.014), previous treatment line > 3 (P = 0.062), previous immunotherapy (P < 0.001), and the number of HAIC-F-T triple treatment cycles of ≤ 2 (P = 0.002) were found to be risk factors associated with worse OS. After multivariate analysis, previous immunotherapy (P = 0.021) and the number of HAIC-F-T triple treatment cycles of ≤ 2 (P = 0.007) were identified as independent risk factors related to worse OS (Table 2).
Accompanying and subsequent treatment
Drug-eluting trans-arterial chemoembolization was performed in 16 patients (35.6%) who had multiple lesions in the bi-lobe of the liver, with a mean of 1.2 ± 2.7 cycles before HAIC treatment. Among the five patients who achieved successful liver metastasis downstaging conversion, two (4.4%) received ablation and two (4.4%) received radiation therapy. Another patient underwent surgical resection after the HAIC-F-T triple treatment, and histopathological examination showed a pathological complete response in both primary tumor and liver metastasis (Figure 5). After tumor progression, 12 patients (26.7%) received HAIC with other regimens, 17 patients (37.8%) received systemic chemotherapy, and 9 patients (20%) received regorafenib, based on the progression of intrahepatic or extrahepatic metastasis.
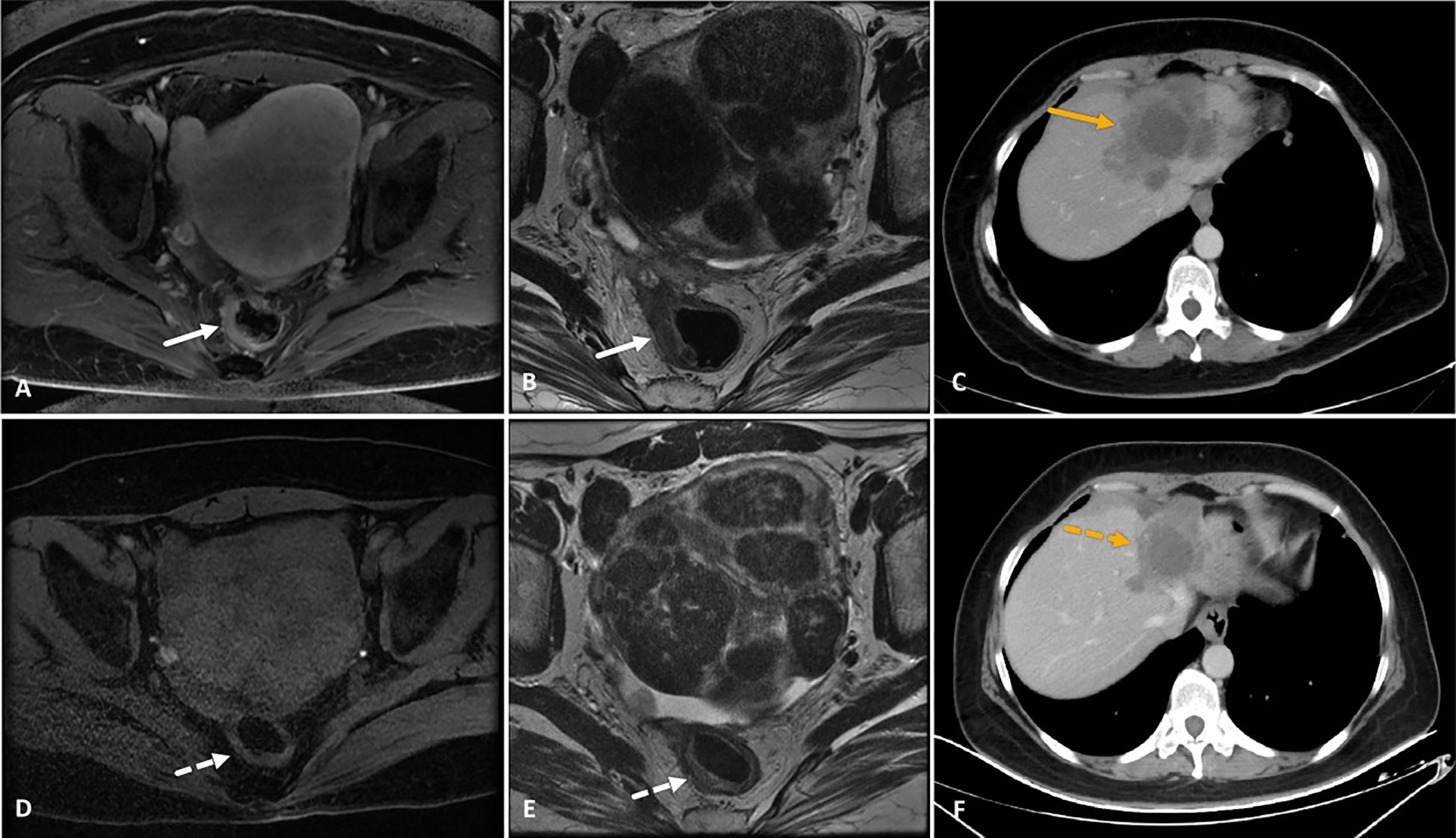
Figure 5. A 39-year-old female patient with microsatellite stable-rectal cancer liver metastasis and RAS wild type who received HAIC (irinotecan, oxaliplatin, and 5-fluorouracil) combined with fruquintinib and tislelizumab as third-line treatment. (A–C) MRI images showed a thickened rectal wall with significant enhancement (white arrows in images A and B), and CT image showed progress of liver metastasis (orange arrow in image C) after two lines of systemic chemotherapy and three cycles of HAIC. (D–F) After six consecutive cycles of HAIC-F-T triple treatment, the rectal wall exhibited significant thinning, with a significantly attenuated degree of enhancement (white dotted arrows in images D and E). Correspondingly, liver metastasis significantly decreased in both size and enhancement degree (orange dotted arrow in image F). The tumor response was partial response based on the RECIST 1.1 criteria. The patient underwent partial liver resection and radical rectal cancer resection subsequently, and histopathological examination showed a pathological complete response in both primary tumor and liver metastasis. Although PET-CT detected active lymph nodes in the retroperitoneal space 8.4 months after the surgery, this was relieved after subsequent radiation therapy. Up to the last follow-up, the patient was still alive, with an overall survival of 41.1 months after the HAIC-F-T triple treatment.
Safety
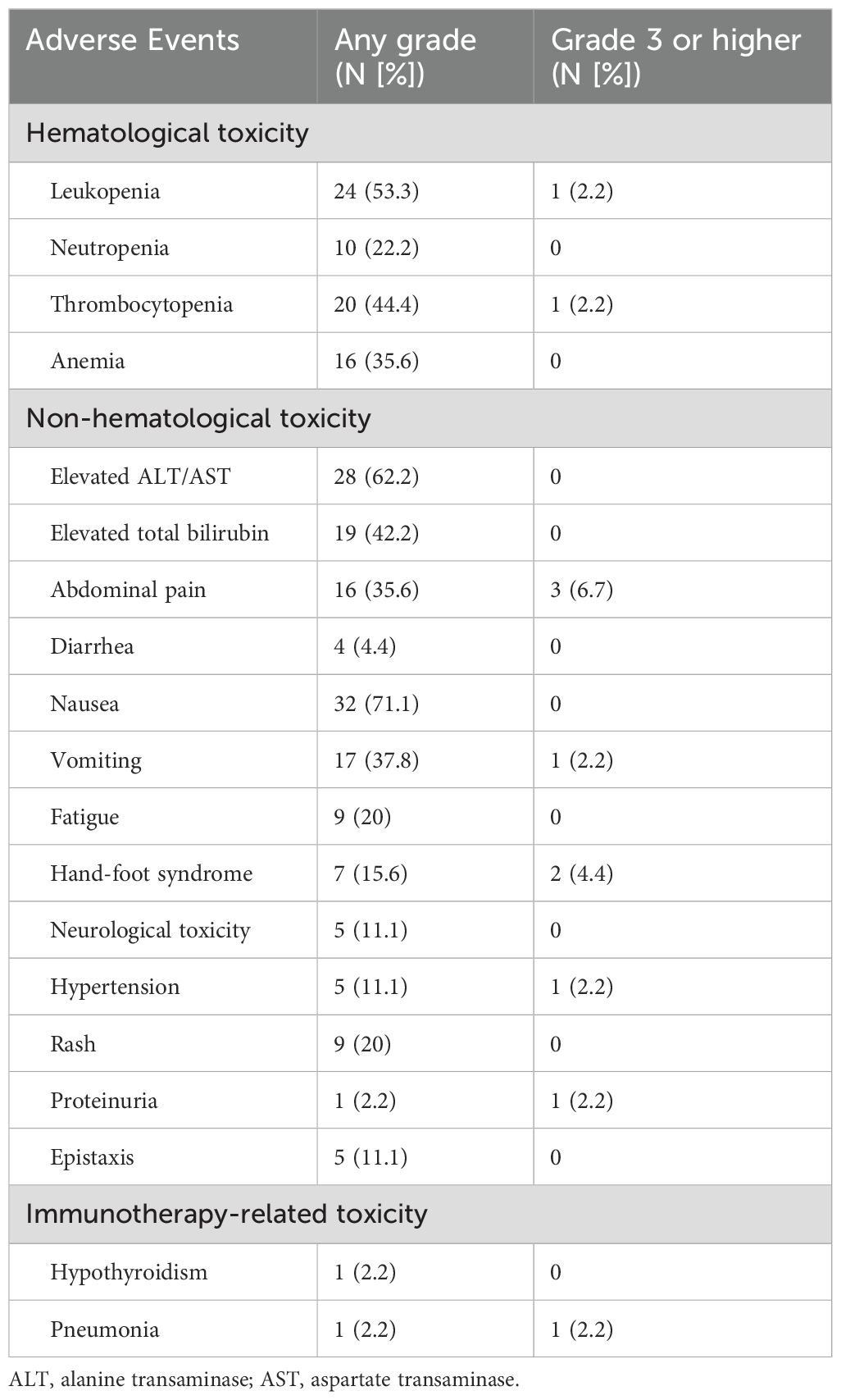
No treatment-related deaths occurred in this study, and none of the patients had catheter-related complications during HAIC. Treatment-related AEs, which occurred in all patients, were manageable, and most of them had recovered to normal before the next cycle of study treatment without any medical intervention. Grade 3 or higher AEs were observed in only 9 patients (20%), and the most frequent grade 3 or higher AEs were abdominal pain (3/45, 6.7%) and hand-foot syndrome (2/45, 4.4%). One patient suspended the administration of fruquintinib because of grade 3 proteinuria, while got progressed because of extrahepatic metastasis during the suspension. All treatment-related AEs are detailed in Table 3.
Immunotherapy-related AEs were observed in two patients (4.4%). Hypothyroidism was detected in one patient after two cycles of the study treatment, and levothyroxine was administered without suspension or interruption of tislelizumab administration. However, another patient was diagnosed with immunotherapy-related pneumonia after two cycles of the HAIC-F-T triple treatment, which required the interruption of tislelizumab administration, hospitalization for best supportive care, and the administration of adrenocortical hormone agents.
Discussion
The survival benefits of the recommended third-line treatment remain limited, with a median PFS of up to 3.7 months and a median OS of up to 9.3 months (6–10). In the present study, HAIC combined with fruquintinib and tislelizumab (HAIC-F-T triple treatment) showed great efficacy in patients with MSS-CRCLM following failure of standard second- or more-line therapies. The ORR was 42.2%, and the DCR was 82.2%, with a median OS and median PFS of 15.3 and 7.5 months, respectively, all of which were better than the standard third-line treatment of CRCLM.
Fruquintinb is a multi-kinase inhibitor that targets at VEGF receptors 1-3. Recently, some preclinical studies showed that fruquintinib could enhance the anti-tumor efficacy of PD-1/PD-L1 inhibitors for treating MSS-metastatic CRC by decreasing angiogenesis, reprograming the structure of vessels, and increasing the infiltration of CD8+ T cells, CD8+TNFα+ T cells, and CD8+IFNγ+ T cells in the tumor microenvironment (21, 22). Additionally, the combination of fruquintinib and PD-1/PD-L1 inhibitors showed great efficacy for patients with MSS-metastatic CRC who failed standard treatment in some retrospective studies and phase II trials, with an ORR, median PFS, and median OS ranging from 7.1% to 21.05%, 5.4 to 9.6 months, and 11.1 to 13.7 months, respectively (18, 23, 24, 32). However, the survival benefits were compromised in patients presenting with liver metastasis, with the median PFS ranging from 3.4 to 5.6 months (23, 24).
HAIC has been explored for the treatment of patients with CRCLM since 1987, and has shown survival benefits as a first-line and adjuvant treatment for patients with CRCLM in some phase III trials (27, 33–36). Recently, HAIC, using double or triple agents, with or without systemic chemotherapy were investigated and showed high local tumor control and survival benefits as a salvage treatment for patients with CRCLM following failure of systemic chemotherapy, with an ORR ranging from 22.4% to 36% and median OS ranging from 13.1 to 32.8 months, respectively (28, 37–39). However, systemic therapy had been demonstrated to relate to greater tumor control for extrahepatic tumor than HAIC (27). Thus, it was suggested to combine HAIC with systemic therapy for treating patients who had both intrahepatic and extrahepatic metastases.
The synergistic effect of the triple combination of HAIC, anti-VEGF, and PD-1 inhibitors involved in this study protocol has been explored previously. A decade ago, anti-VEGF therapy was demonstrated to normalize the vessels and improve their permeability, thus showing a synergistic effect with chemotherapy (40, 41). Meanwhile, chemotherapy has been demonstrated to be synergistic with immunotherapy by directly stimulating the immune system, modulating the immunosuppressive microenvironment, and enhancing immunogenicity (42–45). Recently, the synergistic effects of the triple combination, which includes HAIC, anti-angiogenesis therapy, and immunotherapy for treating malignancies, have been increasingly explored and have shown great efficacy in hepatocellular carcinoma and biliary tract cancer as first-line treatment in some phase II trials (46, 47). In the European Society of Medical Oncology Congress 2023, Wang et al. reported the interim analysis of a phase II trial, which evaluated the efficacy and safety of HAIC combined with fruquintinib and tislelizumab for advanced CRCLM, with an ORR and DCR of 27.59% and 93.1%, respectively; however, neither the median OS nor the median PFS has been reached yet (48). To the best of our knowledge, to date, the present study, which uses this triple regimen for the treatment of CRCLM, is the earliest to start enrolling, with the largest sample size and completed survival data. In the present study, even 64.4% of patients had already received HAIC, 55.6% received more than three lines of treatment before HAIC-F-T triple treatment, and 82.2% had extrahepatic metastasis, the median OS and PFS of 15.3 and 7.5 months, respectively, were achieved, which suggested that the HAIC-F-T triple treatment is a reasonable treatment choice for previous heavily treated patients with CRCLM, even for those previously treated with HAIC and those had extrahepatic metastasis.
Notably, the subgroup analysis showed that the median OS in patients whose previous treatment line 2-3 was longer than that in patients whose previous treatment line was > 3 (18.8 vs. 12.3 months), and both the median PFS and OS in patients who did not receive previous PD-1 immunotherapy were significantly longer than those in patients who received previous PD-1 immunotherapy (median PFS: 7.9 vs. 2.0 months, P = 0.003; median OS: 17.3 vs. 5.4 months, P < 0.001). The stronger the previous chemotherapy, the more severe the chemotherapy resistance and damage to the immune function of the whole body. The earlier application of HAIC could lead to better local tumor response and the release of more tumor antigens from chemotherapy-induced immunogenic death, thereby producing stronger synergistic therapeutic effects with PD-1 inhibitors. Thus, it has been suggested that the HAIC-F-T triple treatment may be used earlier in patients with advanced MSS-CRCLM. Moreover, multivariate analysis showed that the number of HAIC-F-T triple treatment cycles ≤ 2 was an independent risk factor related to worse OS (P = 0.007), indicating that more cycles of HAIC are crucial to the HAIC-F-T triple treatment.
Both the median PFS and OS in patients with liver metastasis dominant were longer than those in patients with liver metastasis non-dominant (8.2 vs. 5.2 months and 17.1 vs. 8.2 months, respectively), indicating the patients with liver metastasis dominant are more likely to benefit from this HAIC-F-T triple treatment. This result may suggest that tumors in patients with liver metastasis non-dominant presented with higher spatiotemporal heterogeneity, which was deemed to be associated with worse prognosis, than tumors in patients with liver metastasis dominant (49). Accordingly, liver metastasis dominant without obvious extrahepatic metastasis is suggested as the optimal indication of this HAIC-F-T triple treatment. Furthermore, it is speculated that immunogenic death of liver metastasis caused by HAIC is insufficient to stimulate a systemic immune response and synergistically inactivate the widespread systemic metastasis for patients with liver metastasis non-dominant.
Regarding the treatment-related AEs in this study, most of treatment-related AEs was grade 1-2, and most had recovered to normal before the initiation of the next combination treatment; however, in one patient who developed immunotherapy-related pneumonia, it was necessary to interrupt the administration of tislelizumab. Notably, only 20% of patients experienced grade 3 or higher AEs in this study. Compared with the CORRECT, CONCUR, RECOURSE, FRESCO, and FRESCO-2 trials, the incidence of grade 3 or higher AEs in this study was more acceptable (20% vs. 54% vs. 61.2% vs. 63% vs. 69%), which may be due to the following reasons: first, the exposure of the whole body to chemotherapeutic agents was less during HAIC due to the first-pass effect in the liver; second, the dosage of fruquintinib administered in this study was less than the standard dosage in the FRESCO and FRECSO-2 trial (6–10).
However, this study has some limitations that warrant discussion. First, the results might be biased due to the retrospective data collection. Second, the sample size was small, with only 45 patients enrolled. Third, 82.2% of patients with extrahepatic metastasis and 33.3% with liver metastasis non-dominant were enrolled in this study, which may have compromised the efficacy of this HAIC-F-T triple treatment. Fourth, the multivariate analysis showed that the number of HAIC-F-T triple treatment cycles of ≤ 2 was one of the independent risk factors related to worse OS (P = 0.001); thus, the mean of 3.6 ± 1.6 cycles might limit the optimal survival benefits of the HAIC-F-T triple treatment. At last, this study is preliminary, prospective study is ongoing to confirm the efficacy and safety of the HAIC-F-T triple treatment for MSS-CRCLM.
Conclusion
HAIC combined with fruquintinib and tislelizumab may serve as an alternative treatment method for MSS-CRCLM that has failed multiple-line therapy, because of its high efficacy and acceptable safety. The number of treatment cycles is closely related to the survival efficacy of this HAIC-F-T triple treatment. The HAIC-F-T triple treatment may be used earlier in patients with MSS-CRCLM and liver metastasis dominant. The results of this study should be verified further in prospective trials with large number of patients with MSS-CRCLM.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the XW on reasonable request.
Ethics statement
This retrospective study was approved by the ethics committee of the Peking University Cancer Hospital, and the need for informed consent was waived because this was a retrospective study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KZ: Data curation, Formal analysis, Investigation, Writing – original draft. XZ: Data curation, Writing – review & editing. LX: Data curation, Writing – review & editing. GC: Data curation, Writing – review & editing. CN: Data curation, Writing – review & editing. XY: Data curation, Writing – review & editing. DX: Data curation, Writing – review & editing. WL: Data curation, Writing – review & editing. QB: Data curation, Writing – review & editing. LW: Data curation, Writing – review & editing. KW: Data curation, Writing – review & editing. BX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. XW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Natural Science Foundation of China (No. 82172039), Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (No. ZYLX202117), and Beijing Natural Science Foundation (No. 7212198).
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Keck J, Gaedcke J, Ghadimi M, Lorf T. Surgical therapy in patients with colorectal liver metastases. Digestion. (2022) 103:245–52. doi: 10.1159/000524022
3. Väyrynen V, Wirta EV, Seppälä T, Sihvo E, Mecklin JP, Vasala K, et al. Incidence and management of patients with colorectal cancer and synchronous and metachronous colorectal metastases: a population-based study. BJS Open. (2020) 4:685–92. doi: 10.1002/bjs5.50299
4. Taylor A, Primrose J, Langeberg W, Kelsh M, Mowat F, Alexander D, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. (2012) 4:283-301. doi: 10.2147/CLEP
5. Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. (2014) 14:810. doi: 10.1186/1471-2407-14-810
6. Grothey A, Cutsem EV, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. (2013) 381:303–12. doi: 10.1016/S0140-6736(12)61900-X
7. Li J, Qin S, Xu R, Yau TCC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2015) 16:619–29. doi: 10.1016/S1470-2045(15)70156-7
8. Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. New Engl J Med. (2015) 372:1909–19. doi: 10.1056/NEJMoa1414325
9. Li J, Qin S, Xu R-H, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. (2018) 319:2486–96. doi: 10.1001/jama.2018.7855
10. Dasari A, Lonardi S, Garcia-Carbonero R, Elez E, Yoshino T, Sobrero A, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet. (2023) 402:41–53. doi: 10.1016/S0140-6736(23)00772-9
11. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. New Engl J Med. (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
12. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz H-J, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. (2017) 18:1182–91. doi: 10.1016/S1470-2045(17)30422-9
13. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
14. André T, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142☆. Ann Oncol. (2022) 33:1052–60. doi: 10.1016/j.annonc.2022.06.008
15. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. New Engl J Med. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
16. Yamamoto N, Nokihara H, Yamada Y, Shibata T, Tamura Y, Seki Y, et al. Phase I study of Nivolumab, an anti-PD-1 antibody, in patients with Malignant solid tumors. Investigational New Drugs. (2017) 35:207–16. doi: 10.1007/s10637-016-0411-2
17. Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold tumors: A therapeutic challenge for immunotherapy. Front Immunol. (2019) 10. doi: 10.3389/fimmu.2019.00168
18. Sun L, Huang S, Li D, Mao Y, Wang Y, Wu J. Efficacy and safety of fruquintinib plus PD-1 inhibitors versus regorafenib plus PD-1 inhibitors in refractory microsatellite stable metastatic colorectal cancer. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.754881
19. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol. (2020) 38:2053–61. doi: 10.1200/JCO.19.03296
20. Lenz H-J, Parikh A, Spigel DR, Cohn AL, Yoshino T, Kochenderfer M, et al. Modified FOLFOX6 plus bevacizumab with and without nivolumab for first-line treatment of metastatic colorectal cancer: phase 2 results from the CheckMate 9X8 randomized clinical trial. J ImmunoTherapy Cancer. (2024) 12:e008409. doi: 10.1136/jitc-2023-008409
21. Wang Y, Wei B, Gao J, Cai X, Xu L, Zhong H, et al. Combination of fruquintinib and anti–PD-1 for the treatment of colorectal cancer. J Immunol. (2020) 205:2905–15. doi: 10.4049/jimmunol.2000463
22. Li Q, Cheng X, Zhou C, Tang Y, Li F, Zhang B, et al. Fruquintinib enhances the antitumor immune responses of anti-programmed death receptor-1 in colorectal cancer. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.841977
23. Zhang W, Zhang Z, Lou S, Li D, Ma Z, Xue L. Efficacy, safety and predictors of combined fruquintinib with programmed death-1 inhibitors for advanced microsatellite-stable colorectal cancer: A retrospective study. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.929342
24. Zhao W, Lei J, Ke S, Chen Y, Xiao J, Tang Z, et al. Fecal microbiota transplantation plus tislelizumab and fruquintinib in refractory microsatellite stable metastatic colorectal cancer: an open-label, single-arm, phase II trial (RENMIN-215). eClinicalMedicine. (2023) 66:102315. doi: 10.1016/j.eclinm.2023.102315
25. Bartkowski R Fau - Berger MR, Berger Mr Fau - Aguiar JL, Aguiar Jl Fau - Henne TH, Henne Th Fau - Dörsam J, Dörsam J Fau - Geelhaar GH, Geelhaar Gh Fau - Schlag P, et al. Experiments on the efficacy and toxicity of locoregional chemotherapy of liver tumors with 5-fluoro-2’-deoxyuridine (FUDR) and 5-fluorouracil (5-FU) in an animal model. J Cancer Res Clin Oncol. (1986) 111:42–6. doi: 10.1007/BF00402774
26. Kemeny N, Seiter K Fau - Niedzwiecki D, Niedzwiecki D Fau - Chapman D, Chapman D Fau - Sigurdson E, Sigurdson E Fau - Cohen A, Cohen A Fau - Botet J, et al. A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasone versus fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer. (1992) 69:327–34. doi: 10.1002/(ISSN)1097-0142
27. Kemeny NE, Niedzwiecki D, Hollis DR, Lenz H-J, Warren RS, Naughton MJ, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: A randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. (2006) 24:1395–403. doi: 10.1200/JCO.2005.03.8166
28. Cercek A, Boucher TM, Gluskin JS, Aguiló A, Chou JF, Connell LC, et al. Response rates of hepatic arterial infusion pump therapy in patients with metastatic colorectal cancer liver metastases refractory to all standard chemotherapies. J Surg Oncol. (2016) 114:655–63. doi: 10.1002/jso.24399
29. Datta J, Narayan RR, Kemeny NE, D’Angelica MI. Role of hepatic artery infusion chemotherapy in treatment of initially unresectable colorectal liver metastases: A review. JAMA Surg. (2019) 154:768–76. doi: 10.1001/jamasurg.2019.1694
30. Zhang Y, Wang K, Yang T, Cao Y, Liang W, Yang X, et al. Meta-analysis of hepatic arterial infusion for liver metastases from colorectal cancer. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.628558
31. Hu J, Zhu X, Wang X, Cao G, Wang X, Yang R. Evaluation of percutaneous unilateral trans-femoral implantation of side-hole port-catheter system with coil only fixed-catheter-tip for hepatic arterial infusion chemotherapy. Cancer Imaging. (2019) 19:15–5. doi: 10.1186/s40644-019-0202-z
32. Ma S, Chen R, Duan L, Li C, Yang T, Wang J, et al. Efficacy and safety of toripalimab with fruquintinib in the third-line treatment of refractory advanced metastatic colorectal cancer: results of a single-arm, single-center, prospective, phase II clinical study. J Gastrointest Oncol. (2023) 14:1052–63. doi: 10.21037/jgo
33. Kemeny N, Daly J, Reichman B, Geller N, Botet J, Oderman P. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. Ann Internal Med. (1987) 107:459–65. doi: 10.7326/0003-4819-107-4-459
34. Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. New Engl J Med. (1999) 341:2039–48. doi: 10.1056/NEJM199912303412702
35. House MG, Kemeny NE, Gönen M, Fong Y, Allen PJ, Paty PB, et al. Comparison of adjuvant systemic chemotherapy with or without hepatic arterial infusional chemotherapy after hepatic resection for metastatic colorectal cancer. Ann Surg. (2011) 254:851–6. doi: 10.1097/SLA.0b013e31822f4f88
36. Kusano M, Honda M, Okabayashi K, Akimaru K, Kino S, Tsuji Y, et al. Randomized controlled Phase III study comparing hepatic arterial infusion with systemic chemotherapy after curative resection for liver metastasis of colorectal carcinoma: JFMC 29-0003. J Cancer Res Ther. (2017) 13:84–90. doi: 10.4103/0973-1482.184524
37. Feng A-W, Guo J-H, Gao S, Kou F-X, Liu S-X, Liu P, et al. A randomized phase II trial of hepatic arterial infusion of oxaliplatin plus raltitrexed versus oxaliplatin plus 5-fluorouracil for unresectable colorectal cancer liver metastases. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.913017
38. Dhir M, Jones HL, Shuai Y, Clifford AK, Perkins S, Steve J, et al. Hepatic arterial infusion in combination with modern systemic chemotherapy is associated with improved survival compared with modern systemic chemotherapy alone in patients with isolated unresectable colorectal liver metastases: A case–control study. Ann Surg Oncol. (2017) 24:150–8. doi: 10.1245/s10434-016-5418-6
39. Lévi F, Karaboué A, Gorden L, Innominato PF, Saffroy R, Giacchetti S, et al. Cetuximab and circadian chronomodulated chemotherapy as salvage treatment for metastatic colorectal cancer (mCRC): safety, efficacy and improved secondary surgical resectability. Cancer Chemotherapy Pharmacol. (2011) 67:339–48. doi: 10.1007/s00280-010-1327-8
40. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. (2005) 307:58–62. doi: 10.1126/science.1104819
41. Kano MR, Komuta Y, Iwata C, Oka M, Shirai Y-t, Morishita Y, et al. Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor-β receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer Sci. (2009) 100:173–80. doi: 10.1111/j.1349-7006.2008.01003.x
42. de Biasi AR, Villena-Vargas J, Adusumilli PS. Cisplatin-induced antitumor immunomodulation: A review of preclinical and clinical evidence. Clin Cancer Res. (2014) 20:5384–91. doi: 10.1158/1078-0432.CCR-14-1298
43. Longo V, Brunetti O, Azzariti A, Galetta D, Nardulli P, Leonetti F, et al. Strategies to improve cancer immune checkpoint inhibitors efficacy, other than abscopal effect: A systematic review. Cancers (Basel). (2019) 11:539. doi: 10.20944/preprints201903.0256.v1
44. Yu H, Chen P, Cai X, Chen C, Zhang X, He L, et al. Efficacy and safety of PD-L1 inhibitors versus PD-1 inhibitors in first-line treatment with chemotherapy for extensive-stage small-cell lung cancer. Cancer Immunology Immunotherapy. (2022) 71:637–44. doi: 10.1007/s00262-021-03017-z
45. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
46. Zhang T-Q, Geng Z-J, Zuo M-X, Li J-B, Huang J-H, Huang Z-L, et al. Camrelizumab (a PD-1 inhibitor) plus apatinib (an VEGFR-2 inhibitor) and hepatic artery infusion chemotherapy for hepatocellular carcinoma in Barcelona Clinic Liver Cancer stage C (TRIPLET): a phase II study. Signal Transduction Targeted Ther. (2023) 8:413. doi: 10.1038/s41392-023-01663-6
47. Wang SFX, Zheng K, Cao G, Xu L, Yang R, Zhu X, et al. A phase II trial of hepatic arterial infusion chemotherapy and bevacizumab in combination with toripalimab for advanced biliary tract cancers: Interim report. Ann Oncol. (2022) 33:S19–26. doi: 10.1016/annonc/annonc1036
48. Wang L, Zhang T, Zhao Y, Pan Q, Mao A, Zhu W, et al. A phase II study to evaluate the efficacy and safety of fruquintinib combined with tislelizumab and Hepatic Artery Infusion Chemotherapy (HAIC) for advanced Colorectal Cancer Liver Metastases (CRLM). Ann Oncol. (2023) 34:S451–1. doi: 10.1016/j.annonc.2023.09.1827
Keywords: colorectal cancer liver metastasis, microsatellite-stable, hepatic arterial infusion chemotherapy, fruquintinib, tislelizumab
Citation: Zheng K, Zhu X, Xu L, Cao G, Niu C, Yan X, Xu D, Liu W, Bao Q, Wang L, Wang K, Xing B and Wang X (2024) Efficacy and safety of hepatic arterial infusion chemotherapy combined with fruquintinib and tislelizumab for patients with microsatellite stable colorectal cancer liver metastasis following failure of multiple-line therapy. Front. Oncol. 14:1420956. doi: 10.3389/fonc.2024.1420956
Received: 21 April 2024; Accepted: 29 July 2024;
Published: 20 August 2024.
Edited by:
Marta Goglia, Sapienza University of Rome, ItalyReviewed by:
Silvia Stefanelli, Sapienza University of Rome, ItalySara Claudia Barone, Sapienza University of Rome, Italy
Copyright © 2024 Zheng, Zhu, Xu, Cao, Niu, Yan, Xu, Liu, Bao, Wang, Wang, Xing and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodong Wang, eGlhb2Rvbmd3NzVAeWFob28uY29t; Baocai Xing, eGluZ2Jhb2NhaTg4QHNpbmEuY29t
 Kanglian Zheng
Kanglian Zheng Xu Zhu1
Xu Zhu1 Guang Cao
Guang Cao Da Xu
Da Xu Lijun Wang
Lijun Wang Kun Wang
Kun Wang Baocai Xing
Baocai Xing Xiaodong Wang
Xiaodong Wang