
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 01 July 2024
Sec. Head and Neck Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1420860
 Masanobu Sato1,2,3†
Masanobu Sato1,2,3† Tomohiro Enokida1†
Tomohiro Enokida1† Takao Fujisawa1
Takao Fujisawa1 Susumu Okano1
Susumu Okano1 Naohiro Takeshita1,4
Naohiro Takeshita1,4 Nobukazu Tanaka1
Nobukazu Tanaka1 Hideki Tanaka1
Hideki Tanaka1 Atsushi Motegi5
Atsushi Motegi5 Sadamoto Zenda5
Sadamoto Zenda5 Takeshi Shinozaki2
Takeshi Shinozaki2 Kazuto Matsuura2
Kazuto Matsuura2 Ryuichi Hayashi2
Ryuichi Hayashi2 Tetsuo Akimoto5
Tetsuo Akimoto5 Makoto Tahara1*
Makoto Tahara1*Background: The significance of induction chemotherapy (IC) in the treatment of squamous cell carcinoma of the head and neck (SCCHN) with unresectable locoregional recurrence after curative surgery has not been clarified. The aim of this study was to evaluate the efficacy of IC followed by chemoradiotherapy (CRT) in these patients.
Methods: Among patients with unresectable locoregional recurrent SCCHN who had not undergone prior irradiation and were eligible for cisplatin, we conducted a retrospective analysis of patients who received CRT following IC with paclitaxel, carboplatin, or cetuximab (IC-PCE group) and those who received CRT without prior IC (CRT group) between June 2013 and August 2021.
Result: Forty-two patients were included. The CRT group and IC-PCE group consisted of 15 and 27 patients, respectively. Primary site was the oral cavity (n=25), oropharynx (n=3), hypopharynx (n=13) and larynx (n=1). Objective response rate (ORR) with IC-PCE was 55.6%; 24 patients (88.9%) subsequently received CRT. ORR after completion of CRT was significantly better in the IC-PCE group (95.8% in the IC-PCE group vs. 66.7% in the CRT group, p=0.024). Progression-free survival (PFS) of the total population on median follow-up of 2.4 years (range: 0.8-7.3) tended to be better in the IC-PCE group (2-year PFS: 55.6% in the IC-PCE group vs. 33.3% in the CRT group, log-rank p=0.176), especially in oral cancer (2-year PFS: 37.5% in the IC-PCE group vs. 0% in the CRT group, log-rank p=0.015).
Conclusion: Therapeutic strategies including IC-PCE in patients with unresectable locoregional recurrent SCCHN after curative surgery may contribute to improved prognosis, especially in oral cancer.
Squamous cell carcinoma of the head and neck (SCCHN) occurs mainly in the oral cavity, pharynx, and larynx, and globally accounts for 8% of all epithelial malignancies. Approximately 60% of patients present with advanced disease (1). Locally advanced SCCHN (LA-SCCHN) is usually treated multimodally with surgery and radiotherapy or concurrent chemoradiotherapy (CRT), but local recurrence occurs at a rate of 50-60%.
Treatment of local recurrence after curative therapy is determined by the feasibility of surgical resection and the presence or absence of prior radiation therapy; according to NCCN guidelines, in the absence of prior irradiation, resection of the recurrent lesion ± postoperative adjuvant therapy (radiation therapy alone or concurrent CRT) is offered if applicable. However, when surgical resection is performed as prior curative treatment, especially with reconstruction procedures, further radical resection is often technically challenging. Also, some cases of recurrence are rapidly progressive, a situation which also hampers surgical intervention; examples include early locoregional recurrence detected by planned computed tomography (CT) or 18-fluorodeoxyglucose positron emission tomography with CT (PET/CT) performed for postoperative radiotherapy for patients at risk of recurrence after radical resection (2, 3). Overall, the percentage of patients able to receive salvage surgery for loco-regional recurrent lesions is 27-65%. Moreover, prognosis in patients who do not undergo surgical salvage but receive radiation therapy and chemotherapy is dismal, with one group reporting a mean survival of 7 months (4). A similarly poor prognosis is also reported for non-surgical treatment, including chemoradiotherapy, with a 3-year OS of 9-10% in patients with local recurrence of oral cancer after initial surgery (5, 6). These findings highlight the critical need for reliable non-surgical therapeutic strategies for this patient population.
Induction chemotherapy (IC) in head and neck cancer is generally defined as systemic chemotherapy performed prior to concurrent CRT as an initial curative therapy aimed at improving the survival prognosis. Historically, the combination of docetaxel, cisplatin (CDDP), and 5-fluorouracil (TPF) has been used and tested in this setting (7–10). To our knowledge, however, no study has investigated the significance of IC itself amongst patients with locoregional recurrence which is not suitable for surgical intervention. In this situation, we have adopted IC consisting of paclitaxel (PTX), carboplatin (CBDCA), and cetuximab (IC-PCE) followed by CRT (11) for patients with SCCHN who develop locoregional recurrence but have no prior irradiation history. IC-PCE prior to curative therapy is feasible and effective (12), and is recommended as a category 2B induction chemotherapy regimen in the NCCN guidelines. To date, however, its use in patients with recurrence has not been evaluated.
In this study, we assessed the potential clinical significance of this therapy by a comprehensive review of patients treated in our institution.
We retrospectively reviewed the medical records of 53 patients with unresectable locoregional recurrent SCCHN treated with radiotherapy from June 2013 to August 2021 at the National Cancer Center Hospital East in Japan. Of these patients, those who met all the following eligibility criteria were selected as the target population: primary site of origin in the oral cavity, oropharynx, hypopharynx, or larynx; recurrence after curative resection; no evidence of distant metastasis; and planned to receive either IC-PCE followed by CRT consisting of cisplatin (CDDP) and RT or CRT alone. Unresectable locoregional recurrence was defined as meeting any of the following: (i) difficulty in surgical resection with enough surgical margin (e.g., carotid artery invasion, skull base invasion, prevertebral muscle invasion, and unreconstructible); or (ii) inability to control tumor progression by surgical resection (e.g., early and rapidly progressed locoregional recurrence revealed by planned CT before postoperative radiotherapy, and far advanced lymph node metastasis [retropharyngeal so-called “Rouviere”, mediastinal or supraclavicular lymph nodes]).
The decision to offer IC-PCE followed by CRT or upfront CRT was primarily determined in a multidisciplinary meeting in which medical oncologists, radiation oncologists, diagnostic radiologists, and head and neck surgeons discussed individual patients. Patients who received radiotherapy alone, who were intolerant to CDDP, who received induction chemotherapy other than PCE, or who had synchronous multiple cancers were excluded (Supplementary Figure 1). This study was approved by the Institutional Review Board of the National Cancer Center Hospital East (2016-243).
The induction PCE regimen consisted of CBDCA area under the plasma concentration-time curve (AUC) = 1.5, PTX 80 mg/m2, and cetuximab at an initial dose of 400 mg/m2 followed by 250 mg/m2 administered weekly for eight weeks. Following IC, concurrent CRT was started. During CRT, CDDP was administered intravenously at a dose of 80 mg/m2 every three weeks on days 1, 22, and 43 (Tri-CDDP) or at a dose of 20 mg/m2 on days 1-4, repeated three times at 3-week intervals (Fractionated tri-CDDP). As with radiotherapy, all patients were treated with intensity-modulated radiation therapy (IMRT). The planned total radiation dose was 70 Gy (2Gy per day, five days per week). Toxicity during treatment was graded using the Common Toxicity Criteria for Adverse Events (CTCAE version 5.0).
Clinical tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) v.1.1 via the review of computerized tomography (CT) or magnetic resonance (MRI) imaging and [18F]-fluorodeoxyglucose positron-emission tomography (PET)/CT. The first post-CRT tumor response assessment was performed 8–12 weeks (2-3 months) after the end of radiotherapy using a CT scan or MRI in all cases, considering the potential delayed tumor regression. In addition, as for the IC-PCE group, the evaluation for tumor response by IC-PCE was performed within 1-2 weeks of the last cycle performed. Optionally, an interim evaluation was planned when completing four cycles of PCE. Patients were continuously investigated for tumor progression and survival until death, loss to follow-up, or end of the cutoff period, whichever occurred first. Overall survival (OS) was defined as the time from the initiation of treatment to death for any reason. Progression-free survival (PFS) was defined as the time from initiation of treatment to initial disease recurrence, progression of disease, or death from any cause. OS and PFS were estimated by the Kaplan–Meier method (log-rank test). For patients who were treated with CRT, the proportion with CRT completion (%CRT completion) was also evaluated, defined by (a) completion of a cumulative CDDP dose ≥ 200mg/m2 and (b) completion of radiotherapy within two weeks after the planned completion date.
All statistical analyses were performed with EZR (version.1.51; Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria; v. 4.1.1). More precisely, it is a modified version of R commander which was designed to add statistical functions frequently used in biostatistics (13).
Of the 53 patients with unresectable locoregional recurrence after curative surgery and no previous irradiation, 42 patients who were planned to receive either CDDP + RT (CRT group, n=15) or IC-PCE followed by CDDP +RT (IC-PCE group, n=27) at the initiation of treatment were identified (Supplementary Figure 1). Table 1 and Supplementary Table 1 compare the main patient characteristics between the CRT group and IC-PCE group. There was no statistically significant difference in clinical background, except that the IC-PCE group had a higher proportion of patients with hypopharynx primary disease and advanced pathologically proven lymph node metastasis at the most recent surgery.
Figure 1 shows the actual treatment delivery in this patient cohort. In the CRT group, all patients were treated with CRT without prior administration of IC. In the IC-PCE group, patients received IC-PCE as initial treatment, followed by subsequent therapy according to tumor response as well as the patient’s condition at the completion or termination of IC-PCE.

Figure 1 Details of treatment delivery in the CRT and IC-PCE groups. *Two patients received palliative chemotherapy as subsequent systemic therapy for progressive disease. **One patient received palliative RT to prevent skeletal-related events due to bone metastases after PCE failure. CRT, chemoradiotherapy; RT, radiotherapy; tri-CDDP, tri-weekly cisplatin; IC, induction chemotherapy; PCE, paclitaxel + carboplatin + cetuximab; ST, systemic therapy.
In the IC-PCE group, the median number of administered PCE cycles was seven (range, 1-8), and 13 patients (48.1%) completed the eight cycles of IC as planned. The patients in the IC group who received PCE of 0-2 cycles, 3-4 cycles, 5-6 cycles, and 7-8 cycles were 1 (3.7%), 5 (18.5%), 5 (18.5%), and 16 (59.3%), respectively. The reasons for not completing full cycles of 8 weekly PCE therapy in 14 patients were either not apparent tumor shrinkage observed at interim response evaluation after completing four cycles of PCE (n=5), and any grade 3 adverse events (n=4), good tumor response after 7 cycles (n=2), and other reasons (n=3). The objective response rate (ORR) by IC-PCE was 55.6%, including six patients (22.2%) with complete response (CR), nine (33.3%) with partial response (PR), and four with progression disease (PD) (Table 2). The ORR for different cycles of IC-PCE was 0%, 60%, and 75% in 0-4 cycles, 5-6 cycles, and 7-8 cycles, respectively (Supplementary Table 2). Following IC-PCE, 24 of 27 patients (88.9%) proceeded to CRT, while the remaining three received other therapies; all patients received systemic therapy or palliative RT for disease progression with distant metastasis. The other one with PD, who experienced progression of local disease progression but without distant disease progression, received CRT following the IC phase. Regarding compliance with CRT, %CRT completion was 93.3% in the CRT group and 83.3% in the patients treated with IC-PCE followed by CRT, with no significant difference between them (p=0.631). The patients who received cumulative CDDP of 0-100 mg/m2, 100-200 mg/m2, and >200 mg/m2 were 0 (0%), 1 (6.7%), and 14 (93.3%) patients in the CRT group and 1 (4.2%), 3 (12.5%), and 20 (83.3%) in the IC-PCE group, respectively. In the CRT group, one patient did not complete CRT due to grade 4 hyponatremia; of four patients in the IC-PCE group, the reasons for failure to complete CRT were either grade 3/4 hyponatremia, hypokalemia, or hypercalcemia in two patients, febrile neutropenia in one patient, and pneumonia in the other patient. At completion of CRT, ORR was 66.7% (CR: 60.0%, PR: 6.7%) in the CRT group and 95.8% (CR: 91.7%, PR: 4.2%) in the IC-PCE group, with this difference being statistically significant (p=0.024) (Table 2).
With a median follow-up time of 31.2 months (range: 5.5 – 88.6) in the total population, the IC-PCE group showed a trend toward better PFS and OS than the CRT group (2-year PFS: 55.6% vs. 33.3%, log-rank p=0.176, 2-year OS: 92.6% vs. 79.0%, log-rank p=0.541) (Figures 2A, B). Next, we compared the subjects’ prognoses by clinical factors to extract the patient population which is most expected to benefit by adding IC-PCE to CRT. Among these, comparing prognoses in oral cancer and non-oral cancers in the CRT group revealed that the patients with oral cancer had a statistically significantly worse PFS and OS than those with non-oral cancers (Supplementary Figure 2). We then focused on analysis by tumor primary site (i.e., oral cancer vs. non-oral cancer), and as expected found that the significance of adding IC-PCE was more prominent and profound in patients with oral cancer, with the IC-PCE group showing better PFS than the CRT group (2-year PFS: 37.5% vs. 0%, log-rank p=0.015) (Figure 3A). On the other hand, the two groups showed an equally favorable prognosis for PFS in those patients with a non-oral primary cancer (Figure 3B). Regarding OS, the IC-PCE group showed a trend toward better survival than the CRT group (2-year OS: 87.5% vs. 63.5%, log-rank p=0.333) in those patients with oral cancer, versus no difference in those with non-oral cancer (2-year OS: 100% vs. 100%, log-rank p=1.000) (Supplementary Figure 3). Furthermore, we analyzed the impact of tumor responsiveness to IC on subsequent prognosis and found that the IC responders had more favorable PFS and OS than the non-responder group (2-year PFS: 80% vs. 18.2%, log-rank p=0.001, and 2-year OS: 100% vs. 81.8%, log-rank p=0.033), suggesting that the chemo-responsiveness of IC would be a prognostic factor in this clinical setting (Supplementary Figure 4).
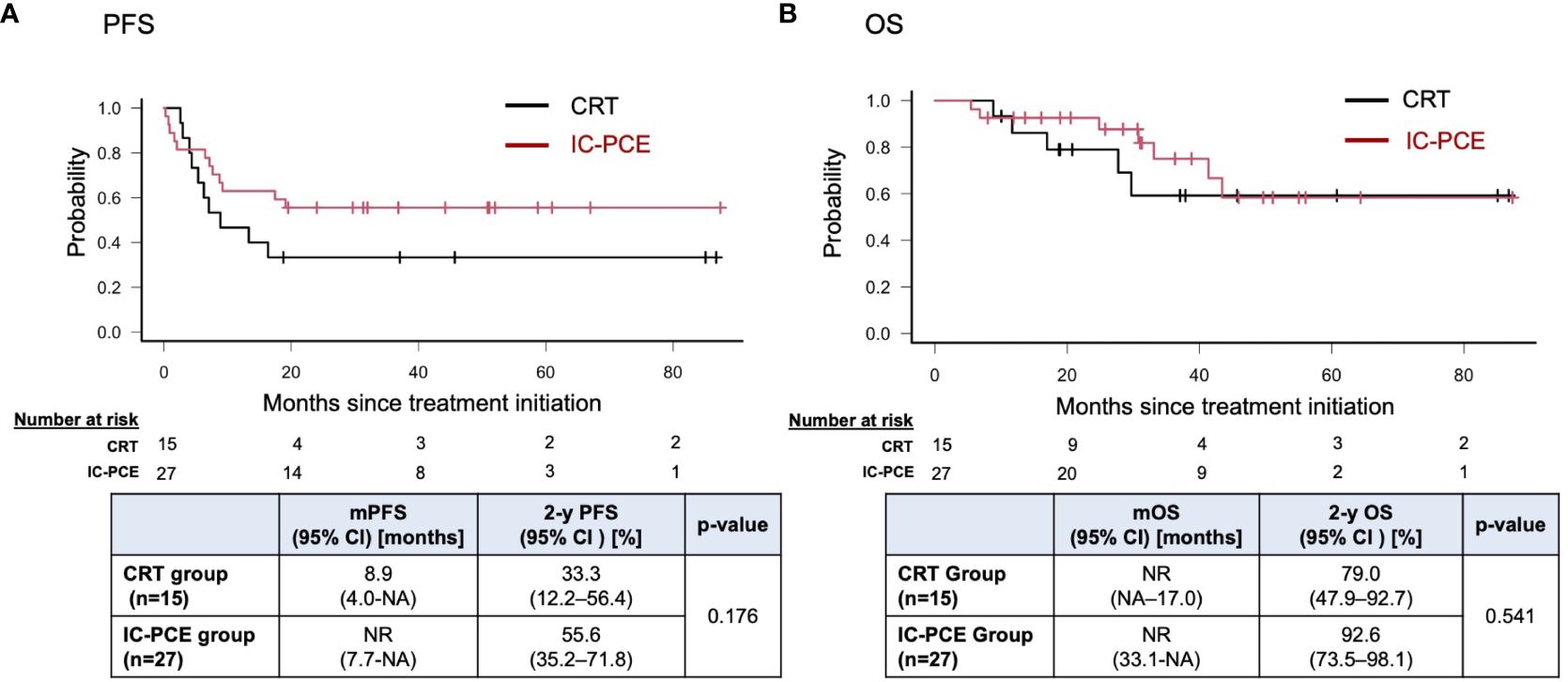
Figure 2 Progression-free survival (A) and overall survival (B) in the total population according to treatment. PFS, progression-free survival; mPFS, median PFS; OS, overall survival; mOS, median OS; NR, not reached; NA, not available.
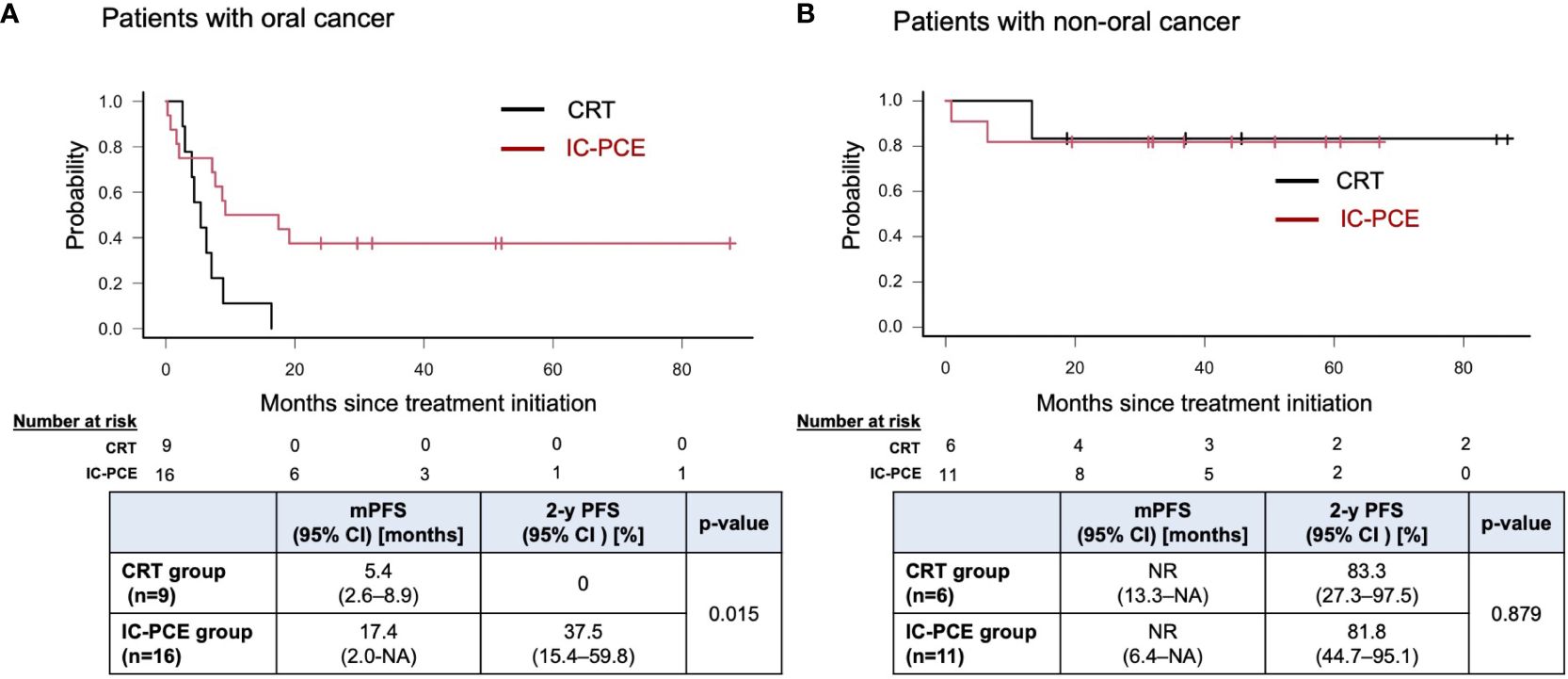
Figure 3 Progression-free survival in patients with oral cancer (A) and non-oral cancers (B) according to treatment. PFS, progression-free survival; mPFS, median PFS; NR, not reached; NA, not available.
Acute toxicities experienced during IC-PCE or CRT in the two groups are listed in Tables 3 and 4, respectively. Common grade 3/4 adverse events during IC-PCE were neutropenia (18.6%), leukopenia (3.7%), and acneiform dermatitis (11.1%). Notably, one patient experienced a grade 3 infusion reaction due to cetuximab; this patient could not continue PCE and was moved to CRT. The total frequency of grades 3/4 toxicity in the period of IC was 44.4% (Table 3), while the toxicities did not hamper proceeding to CRT in all cases. Regarding toxicities observed during CRT, mucositis (CRT group vs. IC-PCE group: 46.7% vs. 33.3%), leukopenia (20.0% vs. 20.8%), neutropenia (6.7% vs. 20.8%), and anemia (13.3 vs. 20.8%) were the most frequently observed grade 3/4 adverse events during CRT. The total frequency of grade 3/4 toxicity during CRT in the CRT and IC-PCE groups was 66.7% and 62.5%, respectively (Table 4). There was no treatment-related death throughout treatment in any case. As for late toxicity, no treatment-related deaths, including fatal bleeding, were observed.
The current study evaluated the significance of IC in patients with unresectable locoregional recurrent SCCHN after curative surgery by comparing patients receiving IC followed by CRT with those receiving CRT alone. The results revealed that adding IC-PCE to CRT provided a promising improvement in prognosis, particularly in patients with oral cancer (2-year PFS: 37.5% vs. 0%, log-rank p=0.015), without any apparent increase in toxicity during CRT.
Among head and neck cancers, oral cancer has been recognized as a surgical disease, if applicable, primarily due to its relatively low sensitivity to chemotherapy and radiotherapy (14–17). In the clinical setting of initial curative treatment, the prognosis of patients with locally advanced oral cancer who harbor resectable disease but receive CRT is poor compared with that of patients treated with surgery plus postoperative (chemo) radiotherapy (17–19). A report based on the National Cancer Database of the United States revealed that surgery with postoperative radiotherapy provided a better prognosis than CRT in a propensity score-matched cohort (3-year OS: 51.8% vs. 39.3%) (20). This phenomenon was similarly seen in a population which developed local recurrence after curative surgery: the prognosis of patients who did not undergo salvage surgery was again reportedly poor (median OS: 5 months (5), 2-year OS: 0-20% (6, 21)), similar to the results seen in our patients with oral cancer treated with CRT alone (2-year PFS of 0% and 2-year OS of 63.5% in Supplementary Figure 2). Thus, a novel approach to improving the dismal prognosis of this population has been warranted.
Induction chemotherapy, which is usually prescribed prior to definitive CRT in attempting to improve prognosis in patients whose prognosis is strongly expected to be unsatisfactory with CRT alone, has been tested in locally advanced head and neck cancer, including oral cavity cancer. However, the representative triplet regimen (i.e., TPF) has failed to show any value, at least in the initial curative setting (8–10). Allowing that the percentage of patients with oral cavity cancer in these previous studies was generally low (14-21%), no study has yet evaluated the significance of IC in patients with unresectable local recurrence after curative surgery. Against this background, we believe our current findings of the potential clinical significance of IC-PCE prior to CRT would be of benefit in the patient population whose prognosis is disappointingly poor with CRT alone (Supplementary Figure 2).
As for the reported efficacy of IC-PCE in the current study, 55.6% of ORR was relatively lower than previously reported (91%) in patients with untreated locally advanced SCCHN (22). We speculate that this is likely due to the highly aggressive disease feature causing recurrence after curative resection. Since no patient received chemotherapy even as postoperative chemoradiotherapy before treatment, we could not assess the potential impact of pretreatment systemic therapy on the observed relatively low ORR. On the other hand, although approximately 20% of patients obtained CR after IC-PCE in the current study, chemoradiotherapy, not surgery, was given as planned. Indeed, several reports suggested that the chemotherapy responder may have a favorable prognosis by subsequent surgery and postoperative chemoradiotherapy in unresectable but previously untreated head and neck cancer (23, 24). However, in the population with unresectable loco-regional recurrence after curative surgery, even if radiological CR is obtained, complete surgical resection is usually technically challenging, and we thus believe chemoradiotherapy should be practical and reasonable in this situation.
Concerning prognosis, we must consider systemic therapy, a usually selected treatment for patients with unresectable recurrent squamous cell carcinoma of the head and neck, as a competitive one to the RT-containing strategy discussed in the current study. Especially pembrolizumab-containing systemic therapy has been recognized as standard systemic therapy for platinum-sensitive recurrent or metastatic disease based on the results of Keynote-048 (25). In this setting, we recognized that the data on PFS, which is one of the most prioritized outcomes, is numerically favored toward IC-CRT reported in the current study when compared with pembrolizumab plus chemotherapy in Keynote-048 (2y-PFS: 55.6% vs.10.7%). Moreover, OS as an ultimate outcome is also better in the IC-CRT (2y-OS: 92.6% vs. 29%). Based on these findings, although we cannot reach a decisive conclusion, we believe the RT-containing strategy, especially IC-CRT, should be a promising therapeutic option in anticipating better clinical outcomes, including cure, if applicable.
We are unable to explain the improvement in results with the addition of IC-PCE in the oral cavity population. One possible explanation may be cetuximab’s targeting of EGFR as a part of PCE and the characteristic nature of oral cavity cancer relating to EGFR. An analysis of the gene expression profile of head and neck cancer found that a phenotype called the basal type, in which gene expression of the pathway related to EGFR is activated, is particularly common in oral cancer (26). Further, in vitro studies have shown that EGFR is abundantly expressed on oral cancer cells and that cetuximab-mediated antibody-dependent cellular cytotoxicity (ADCC) is a crucial process associated with the therapeutic efficacy of cetuximab (27–29).
Clinically, the EXTREME trial, which evaluated the effect of platinum–fluorouracil plus cetuximab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck, suggested that cetuximab was preferentially effective in the oral cancer population, with a PFS-hazard ratio (HR) favoring chemotherapy plus cetuximab (PFS-HR: 0.34 [95%CI: 0.21-0.55]) compared with chemotherapy plus placebo (30). Moreover, two randomized trials (SPECTRUM and ZALUTE) which evaluated different anti-EGFR monoclonal antibodies (panitumumab and zalutumumab, respectively) also showed preferential antitumor effects compared with their control arms in patients with primary oral cancer (PFS-HRs: 0.70 [95%CI: 0.51-0.96] and 0.54 [0.31-0.93], respectively) (31–33).
A limitation of this study is that it was a retrospective evaluation of a small number of patients from a single institution. No prospective comparison with CRT alone has yet appeared, however. Confirmation of the role of PCE as induction chemotherapy in these patients requires a multicenter case series or prospective randomized controlled trial with a large sample size. Besides, because the current study focuses on patients who did not receive postoperative (chemo)radiotherapy as part of consecutive initial curative treatment, we could not address whether the current proposed treatment strategy with IC-PCE followed by CRT truly overcome the dismal prognosis, which might be avoided by performing postoperative (chemo)radiotherapy. Also, our present study did not assess whether other IC, such as TPF as a non-cetuximab-containing regimen, would provide similar clinical significance. Notably, there has been discussion on developing a practical and feasible IC regimen because its toxicity often compromises compliance with subsequent local therapy here in CRT. For example, a modified TPF regimen in which relatively low administrative doses are adopted retained favorable ORR (89.6%) and more manageable safety profiles than conventional TPF in the initial treatment setting (34). Another example is IC with Docetaxel + CDDP + Cmab (TPEx), characterized by replacing 5-FU with Cmab, which also showed reliable ORR (72.2%) in the same setting (35). Thus, this unanswered question should also be addressed in future studies seeking ideal IC regimens as non-surgical curative treatment.
In patients with squamous cell carcinoma of the head and neck with unresectable locoregional recurrence after curative surgery and no history of radiation, induction chemotherapy with PCE before CRT may improve prognosis, especially in patients with oral cancer.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Institutional Review Board of the National Cancer Center Hospital East (2016-243). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
MS: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. TE: Conceptualization, Writing – original draft, Writing – review & editing, Data curation, Formal analysis. TF: Conceptualization, Data curation, Writing – review & editing. SO: Data curation, Writing – review & editing. NaT: Data curation, Writing – review & editing. NoT: Data curation, Writing – review & editing. HT: Data curation, Writing – review & editing. AM: Data curation, Writing – review & editing. SZ: Data curation, Writing – review & editing. TS: Data curation, Writing – review & editing. KM: Data curation, Writing – review & editing. RH: Data curation, Writing – review & editing. TA: Data curation, Writing – review & editing. MT: Conceptualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1420860/full#supplementary-material
Supplementary Figure 1 | Patient extraction process. *One patient who received IC-PCE followed by RT alone was excluded from the study because of intolerance to cisplatin due to renal dysfunction prior to the start of treatment. RT, radiotherapy; 5-FU, 5-fluorouracil; CBDCA, carboplatin; TPS, docetaxel + cisplatin + S-1; CDDP, cisplatin; PCE, paclitaxel + carboplatin + cetuximab; CRT, chemoradiotherapy; IC, induction chemotherapy.
Supplementary Figure 2 | Progression-free survival (A) and overall survival (B) in the CRT group according to treatment. PFS, progression-free survival; mPFS, median PFS; OS, overall survival; mOS, median OS; 2-y OS, two-year OS; NR, not reached; NA, not available.
Supplementary Figure 3 | Overall survival in patients with oral cancer (A) and non-oral cancers (B). OS, overall survival; mOS, median OS; NR, not reached; NA, not available.
Supplementary Figure 4 | Progression-free survival (A) and overall survival (B) in the IC-PCE group according to response to IC. PFS, progression-free survival; mPFS, median PFS; OS, overall survival; mOS, median OS; 2-y OS, two-year OS; NR, not reached; NA, not available.
1. Seiwert TY, Cohen EE. State-of-the-art management of locally advanced head and neck cancer. Br J Cancer. (2005) 92:1341–8. doi: 10.1038/sj.bjc.6602510
2. Kibe Y, Nakamura N, Kuno H, Hiyama T, Hayashi R, Zenda S, et al. Frequency and predictors of detecting early locoregional recurrence/disease progression of oral squamous cell carcinoma with high-risk factors on imaging tests before postoperative adjuvant radiotherapy. Int J Clin Oncol. (2019) 24:1182–9. doi: 10.1007/s10147-019-01479-x
3. Yu Y, Schoder H, Zakeri K, Chen L, Kang JJ, McBride SM, et al. Post-operative PET/CT improves the detection of early recurrence of squamous cell carcinomas of the oral cavity. Oral Oncol. (2023) 141:106400. doi: 10.1016/j.oraloncology.2023.106400
4. Wong LY, Wei WI, Lam LK, Yuen AP. Salvage of recurrent head and neck squamous cell carcinoma after primary curative surgery. Head Neck. (2003) 25:953–9. doi: 10.1016/j.oraloncology.2023.106400
5. Contrera KJ, Zafereo ME, Yaniv D, Roberts DB, Gillenwater AM, Hanna EY, et al. Outcomes for recurrent oral cavity squamous cell carcinoma. Oral Oncol. (2022) 134:106127. doi: 10.1016/j.oraloncology.2022.106127
6. Tam S, Araslanova R, Low TH, Warner A, Yoo J, Fung K, et al. Estimating survival after salvage surgery for recurrent oral cavity cancer. JAMA Otolaryngol Head Neck Surg. (2017) 143:685–90. doi: 10.1001/jamaoto.2017.0001
7. Budach W, Bolke E, Kammers K, Gerber PA, Orth K, Gripp S, et al. Induction chemotherapy followed by concurrent radio-chemotherapy versus concurrent radio-chemotherapy alone as treatment of locally advanced squamous cell carcinoma of the head and neck (HNSCC): A meta-analysis of randomized trials. Radiother Oncol. (2016) 118:238–43. doi: 10.1016/j.radonc.2015.10.014
8. Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. (2014) 32:2735–43. doi: 10.1200/JCO.2013.54.6309
9. Haddad R, O'Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. (2013) 14:257–64. doi: 10.1016/S1470-2045(13)70011-1
10. Hitt R, Grau JJ, Lopez-Pousa A, Berrocal A, Garcia-Giron C, Irigoyen A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. (2014) 25:216–25. doi: 10.1093/annonc/mdt461
11. Enokida T, Ogawa T, Homma A, Okami K, Minami S, Nakanome A, et al. A multicenter phase II trial of paclitaxel, carboplatin, and cetuximab followed by chemoradiotherapy in patients with unresectable locally advanced squamous cell carcinoma of the head and neck. Cancer Med. (2020) 9:1671–82. doi: 10.1002/cam4.2852
12. Kies MS, Holsinger FC, Lee JJ, William WN Jr., Glisson BS, Lin HY, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. (2010) 28:8–14. doi: 10.1200/JCO.2009.23.0425
13. Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transpl. (2013) 48:452–8. doi: 10.1038/bmt.2012.244
14. Meng X, Lou QY, Yang WY, Wang YR, Chen R, Wang L, et al. The role of non-coding RNAs in drug resistance of oral squamous cell carcinoma and therapeutic potential. Cancer Commun (Lond). (2021) 41:981–1006. doi: 10.1002/cac2.12194
15. Ishigami T, Uzawa K, Higo M, Nomura H, Saito K, Kato Y, et al. Genes and molecular pathways related to radioresistance of oral squamous cell carcinoma cells. Int J Cancer. (2007) 120:2262–70. doi: 10.1002/ijc.22561
16. Lee SY, Park HR, Cho NH, Choi YP, Rha SY, Park SW, et al. Identifying genes related to radiation resistance in oral squamous cell carcinoma cell lines. Int J Oral Maxillofac Surg. (2013) 42:169–76. doi: 10.1016/j.ijom.2012.10.022
17. Gore SM, Crombie AK, Batstone MD, Clark JR. Concurrent chemoradiotherapy compared with surgery and adjuvant radiotherapy for oral cavity squamous cell carcinoma. Head Neck. (2015) 37:518–23. doi: 10.1002/hed.23626
18. Iyer NG, Tan DS, Tan VK, Wang W, Hwang J, Tan NC, et al. Randomized trial comparing surgery and adjuvant radiotherapy versus concurrent chemoradiotherapy in patients with advanced, nonmetastatic squamous cell carcinoma of the head and neck: 10-year update and subset analysis. Cancer. (2015) 121:1599–607. doi: 10.1002/cncr.29251
19. Elbers JBW, Al-Mamgani A, Paping D, van den Brekel MWM, Jozwiak K, de Boer JP, et al. Definitive (chemo)radiotherapy is a curative alternative for standard of care in advanced stage squamous cell carcinoma of the oral cavity. Oral Oncol. (2017) 75:163–8. doi: 10.1016/j.oraloncology.2017.11.006
20. Spiotto MT, Jefferson G, Wenig B, Markiewicz M, Weichselbaum RR, Koshy M. Differences in survival with surgery and postoperative radiotherapy compared with definitive chemoradiotherapy for oral cavity cancer: A national cancer database analysis. JAMA Otolaryngol Head Neck Surg. (2017) 143:691–9. doi: 10.1001/jamaoto.2017.0012
21. Koo BS, Lim YC, Lee JS, Choi EC. Recurrence and salvage treatment of squamous cell carcinoma of the oral cavity. Oral Oncol. (2006) 42:789–94. doi: 10.1016/j.oraloncology.2005.11.016
22. Seiwert TY, Melotek JM, Blair EA, Stenson KM, Salama JK, Witt ME, et al. Final results of a randomized phase 2 trial investigating the addition of cetuximab to induction chemotherapy and accelerated or hyperfractionated chemoradiation for locoregionally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. (2016) 96:21–9. doi: 10.1016/j.ijrobp.2016.04.030
23. Wang HM, Lin CY, Hsieh CH, Hsu CL, Fan KH, Chang JT, et al. Induction chemotherapy with dose-modified docetaxel, cisplatin, and 5-fluorouracil in Asian patients with borderline resectable or unresectable head and neck cancer. J Formos Med Assoc. (2017) 116:185–92. doi: 10.1016/j.jfma.2016.03.005
24. Patil VM, Prabhash K, Noronha V, Joshi A, Muddu V, Dhumal S, et al. Neoadjuvant chemotherapy followed by surgery in very locally advanced technically unresectable oral cavity cancers. Oral Oncol. (2014) 50:1000–4. doi: 10.1016/j.oraloncology.2014.07.015
25. Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. (2019) 394:1915–28. doi: 10.1016/S0140-6736(19)32591-7
26. Keck MK, Zuo Z, Khattri A, Stricker TP, Brown CD, Imanguli M, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. (2015) 21:870–81. doi: 10.1158/1078-0432.CCR-14-2481
27. Fujiwara T, Eguchi T, Sogawa C, Ono K, Murakami J, Ibaragi S, et al. Carcinogenic epithelial-mesenchymal transition initiated by oral cancer exosomes is inhibited by anti-EGFR antibody cetuximab. Oral Oncol. (2018) 86:251–7. doi: 10.1016/j.oraloncology.2018.09.030
28. Nakamura H, Tamaki S, Yagyuu T, Yamakawa N, Hatake K, Kirita T. Relationship between EGFR expression in oral cancer cell lines and cetuximab antibody-dependent cell-mediated cytotoxicity. Anticancer Res. (2019) 39:1275–82. doi: 10.21873/anticanres.13238
29. Namboodiri AM, Pandey JP. Differential inhibition of trastuzumab- and cetuximab-induced cytotoxicity of cancer cells by immunoglobulin G1 expressing different GM allotypes. Clin Exp Immunol. (2011) 166:361–5. doi: 10.1111/j.1365-2249.2011.04477.x
30. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. (2008) 359:1116–27. doi: 10.1056/NEJMoa0802656
31. Vermorken JB, Stohlmacher-Williams J, Davidenko I, Licitra L, Winquist E, Villanueva C, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. (2013) 14:697–710. doi: 10.1016/S1470-2045(13)70181-5
32. Machiels JP, Subramanian S, Ruzsa A, Repassy G, Lifirenko I, Flygare A, et al. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial. Lancet Oncol. (2011) 12:333–43. doi: 10.1016/S1470-2045(11)70034-1
33. Szturz P, Vermorken JB. Management of recurrent and metastatic oral cavity cancer: Raising the bar a step higher. Oral Oncol. (2020) 101:104492. doi: 10.1016/j.oraloncology.2019.104492
34. Hsieh CY, Lein MY, Yang SN, Wang YC, Lin YJ, Lin CY, et al. Dose-dense TPF induction chemotherapy for locally advanced head and neck cancer: a phase II study. BMC Cancer. (2020) 20:832. doi: 10.1186/s12885-020-07347-6
35. Zenda S, Ota Y, Kiyota N, Okano S, Fujii M, Kitamura M, et al. A multicenter phase II trial of docetaxel, cisplatin, and cetuximab (TPEx) followed by cetuximab and concurrent radiotherapy for patients with local advanced squamous cell carcinoma of the head and neck (CSPOR HN01: ECRIPS study). Front Oncol. (2019) 9:6. doi: 10.3389/fonc.2019.00006
Keywords: unresectable squamous cell carcinoma of the head and neck, induction chemotherapy, PCE, cetuximab, chemoradiotherapy
Citation: Sato M, Enokida T, Fujisawa T, Okano S, Takeshita N, Tanaka N, Tanaka H, Motegi A, Zenda S, Shinozaki T, Matsuura K, Hayashi R, Akimoto T and Tahara M (2024) Induction chemotherapy with paclitaxel, carboplatin, and cetuximab (PCE) followed by chemoradiotherapy for unresectable locoregional recurrence after curative surgery in patients with squamous cell carcinoma of the head and neck. Front. Oncol. 14:1420860. doi: 10.3389/fonc.2024.1420860
Received: 21 April 2024; Accepted: 18 June 2024;
Published: 01 July 2024.
Edited by:
Guopei Zhu, Shanghai Jiao Tong University, ChinaReviewed by:
Xin Zhou, Fudan University, ChinaCopyright © 2024 Sato, Enokida, Fujisawa, Okano, Takeshita, Tanaka, Tanaka, Motegi, Zenda, Shinozaki, Matsuura, Hayashi, Akimoto and Tahara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Makoto Tahara, bWF0YWhhcmFAZWFzdC5uY2MuZ28uanA=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.