- 1Department of Neuropathology, Royal Prince Alfred Hospital, Sydney, NSW, Australia
- 2Hyphenated Mass Spectrometry Laboratory, School of Mathematical and Physical Sciences, University of Technology Sydney, Sydney, NSW, Australia
Toxic metals such as mercury, lead, and cadmium have multiple carcinogenic capacities, including the ability to damage DNA and incite inflammation. Environmental toxic metals have long been suspected to play a role in the pathogenesis of cancer, but convincing evidence from epidemiological studies that toxic metals are risk factors for common neoplasms has been difficult to gain. Another approach is to map the location of potentially toxic elements in normal human cells where common cancers originate, as well as in the cancers themselves. In this Perspective, studies are summarized that have used elemental biomapping to detect toxic metals such as mercury in human cells. Two elemental biomapping techniques, autometallography and laser ablation-inductively coupled-mass spectrometry imaging, have shown that multiple toxic metals exist in normal human cells that are particularly prone to developing cancer, and are also seen in neoplastic cells of breast and pancreatic tumors. Biomapping studies of animals exposed to toxic metals show that these animals take up toxic metals in the same cells as humans. The finding of toxic metals such as mercury in human cells prone to cancer could explain the increasing global incidence of many cancers since toxic metals continue to accumulate in the environment. The role of toxic metals in cancer remains to be confirmed experimentally, but to decrease cancer risk a precautionary approach would be to reduce emissions of mercury and other toxic metals into the environment from industrial and mining activities and from the burning of fossil fuels.
1 Introduction
Most cancers appear to result from interactions between genetic variations and injurious environmental agents (1). Advances have been made in identifying germline and somatic gene variants that increase cancer risk, but finding convincing cancer-promoting environmental toxic agents (toxicants) for most cancers has proven difficult. Reasons for this include: (1) the increasing number of potential environmental agents that could play a role in cancer pathogenesis, with over 350,000 chemicals and mixtures of chemicals registered (2); (2) exposure to toxic agents may have been years before the cancer developed, during which time the toxicant was removed from the tissue (a “hit and run” scenario); (3) multiple synergistically-acting toxic agents may need to be involved before cancer develops (3, 4), i.e., a “poly-environmental” combination of risk factors; (4) most people are unaware of which toxic agents they have been exposed to. All of these make studies looking for toxic metals as risk factors for cancer challenging to undertake and interpret (3, 5, 6).
Some groups have looked for toxicants within tumor samples, usually using bulk chemistry methods (7–12). The difficulty here is that tumors are often supplied by new blood vessels that are permeable to circulating toxicants, which would normally only have limited access to the original tissue. The finding of toxicants in tumor tissue may therefore be a secondary phenomenon not related to cancer initiation. Animal experiments have given insights as to how exposure to some toxicants could give rise to cancer (13, 14), but the number of toxicants tested has been small, and young genetically-identical animals are often employed. It is difficult to design animal studies to examine how exposure to multiple toxicants over many years, in a genetically variable population, could result in cancers affecting humans.
Metal toxicants suspected to be involved in cancer pathogenesis include lead, cadmium, mercury, arsenic, and chromium (3–6, 13, 15–19). These metals are found globally in air, water, and soil (20), and exhibit many of the complex mechanisms that underlie cancers. These mechanisms include somatic mutation-inducing DNA damage (13, 17, 18, 21–24), impaired DNA repair (13, 24–26), inflammation and oxidative stress (13, 17, 24, 27, 28), epigenetic changes (4, 24, 29–32), changes to apoptosis with increased cell survival and proliferation (14, 17, 33, 34), and damage to cellular organelles and membranes such as mitochondria (35, 36), the Golgi apparatus (37, 38), lysosomes (39, 40), and nuclear envelopes (37, 41). Other toxic metal cancer-promoting mechanisms include alterations to microtubules (13, 42), increased angiogenesis (43, 44), damaged RNA (45, 46), and immune changes (47, 48). Recently published reviews of the carcinogenic potentials of toxic metals emphasize that multiple injurious modes of these metals can act together, and that synergistic actions come into play when several toxic metals are present (Supplementary Table 1). A model of toxic metal-promoted carcinogenesis is proposed, illustrating the extensive range of mechanisms that could be involved (Figure 1). The sequence in which these events occur could be important. For example, it has been proposed that early epigenetic changes in pre-cancerous lesions that allow for cell proliferation would favor subsequent genetic mutations in these cells (32, 49).
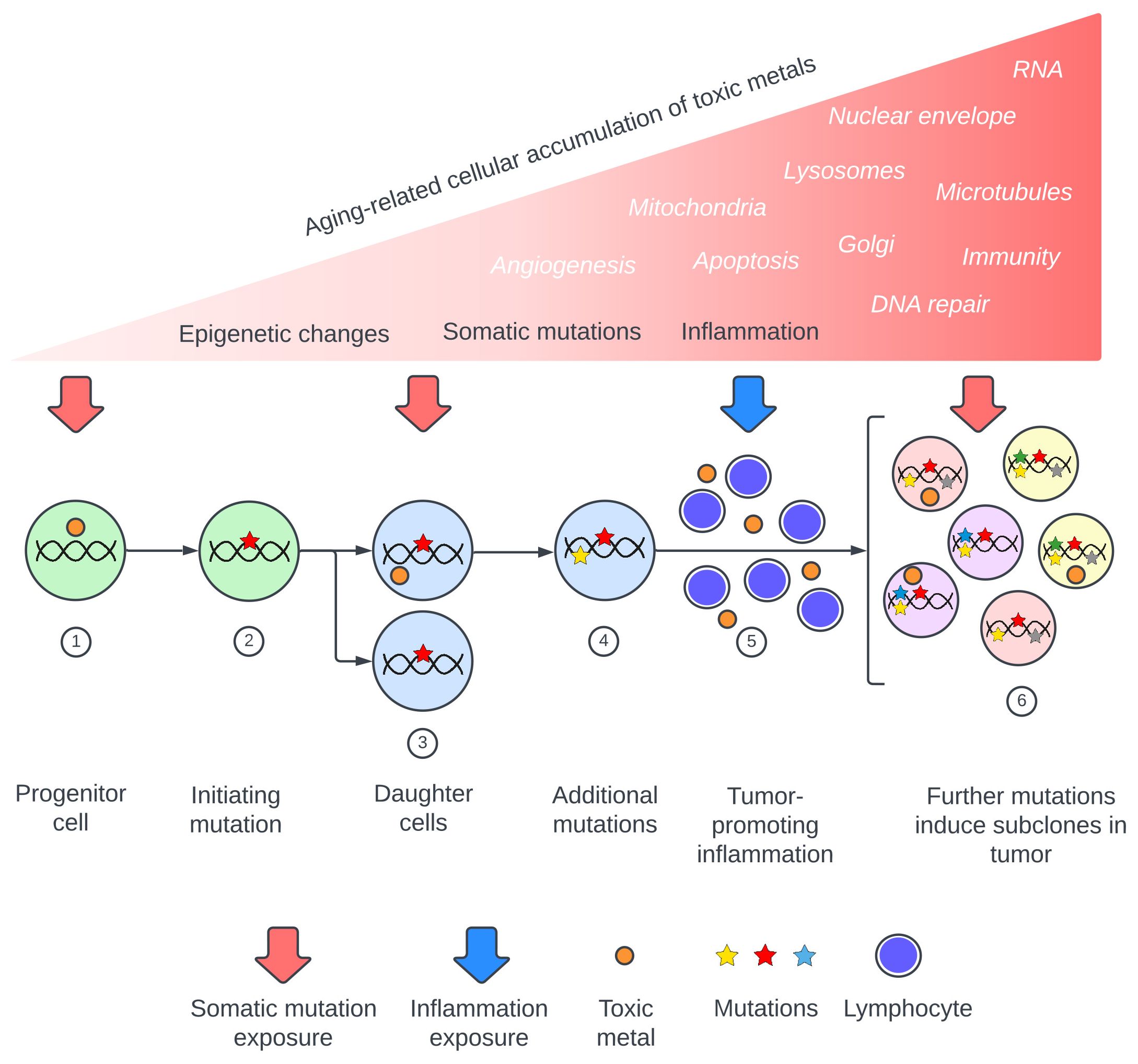
Figure 1 Proposed pathway of toxic metal exposure leading to cancer. Upper section: Toxic metal exposure arises from repeated episodes or from a constant source, with the cellular burden of toxic metals increasing during aging. Major consequences are somatic mutations, inflammation, and epigenetic changes, while toxic metal-induced alterations to intracellular processes and organelles (in white italics) can also promote carcinogenesis. Lower section: Examples of toxic metal exposures resulting in two of these mechanisms, somatic mutations and inflammation. (1) Toxic metals enter a progenitor cell and produce (2) a cancer-initiating mutation. (3) Daughter cells carrying the initiating mutation take up further toxic metals which produce driver mutations (4). (5) Circulating toxic metals initiate tumor-promoting inflammation. (6) Toxic metals within tumor cells produce subclone mutations.
Many previous studies have looked at the possible roles of toxic metals in cancer; a recent review found 820 studies on heavy metals and cancer risk published between 2000 and 2022 alone (6). What remains lacking to support the toxic metal hypothesis for cancer is evidence that metal toxicants are present early in the cells in which neoplasms originate. In this Perspective, an overview of selected elemental bioimaging studies of human tissues is presented, concentrating on normal tissues (to look for underlying predispositions to cancer initiation) and including some neoplasms (to look for factors promoting tumor progression). Mercury is mainly used throughout the Perspective as an example of a toxicant that could play a role in carcinogenesis, since human exposure to mercury is common from both inhaled mercury vapor and ingested methylmercury (especially from eating large predatory fish) (50), the tissue distribution of mercury in experimentally-exposed animals has been well studied (51–56), both mercury vapor and methylmercury are metabolized in the body to toxic divalent mercury cations that accumulate in cells (57), mercury has many of the toxic effects suspected to underlie carcinogenesis (13, 18, 21), and mercury is the major toxic metal detected by autometallography, the technique that is used for elemental mapping of large numbers of tissue samples (58–60). Therefore, in this Perspective we will focus on studies that have looked at the cellular distribution of mercury (as a typical toxic metal) in human cells to see if these give clues to cancer pathogenesis.
2 Elemental biomapping of human tissues
2.1 Elemental biomapping techniques
Two techniques that can be used to examine the distribution of toxic elements in human cells are autometallography (AMG) and laser ablation-inductively coupled-mass spectrometry imaging (LA-ICP-MSI). (1) Autometallography is a physical development amplification technique (based on that first used in photography) that enables inorganic mercury, silver, or bismuth bound to selenides or sulfides in tissues to convert added silver ions (from silver nitrate or lactate) into black metallic silver, which then visibly coats even a few atoms of these metals (58–63) (referred to here as AMGTM). Autometallography can be combined with immunohistochemistry (64–67) or electron microscopy (58, 61) to detect AMGTM in specific cells. (2) LA-ICP-MSI is a multi-elemental imaging technique that uses a laser to sample histological sections, with the ablation plume swept into an ICP-MS (68). When analyzed alongside matrix-matched calibration standards, quantitative images are reconstructed from the data. LA-ICP-MSI is a powerful technique for detecting the roles metals play in cancer (69), however is not as sensitive as AMG, the detection limit being 0.05–0.81 μg per g (70). Other elemental imaging techniques are not covered in this review due to their specialized requirements. For example, synchrotron X-ray fluorescence microscopy measures concentrations of elements within cells but is limited to very small areas of frozen tissue (71). NanoSIMS is a sensitive high resolution imaging technique, but the ultra-thin samples need a high vacuum and cannot be applied to stored histological sections (72).
2.2 Human cells containing metal toxicants
Studies of autopsy-sampled tissues of 170 people with a variety of clinicopathological conditions [one exposed to mercury (73)], as well as surgical samples of normal tissue adjacent to neoplastic tissue, show that toxic metals are commonly present in selective groups of human cells (64–67, 74–79). Human cells that contained toxic metals were seen most often in the kidney, pancreas, thyroid, nervous system, anterior pituitary, breast, ovary, adrenal gland, liver, retina, and in endothelial cells in many organs.
2.2.1 Kidney
Autometallography of human kidneys showed AMGTM predominantly in the cortex in renal proximal tubule cells (sparing glomeruli and distal tubule cells) (Figures 2A, B), and in the medulla in Henle thin loops (not in collecting ducts) (66, 80). Renal tubule cell AMGTM started appearing in the third decade of life (in 66% of people aged 21–40 years) and peaked at 84% of people aged 61–80 years (77, 80). LA-ICP-MSI indicated that the AMGTM most commonly present was mercury, and demonstrated mixtures of cadmium, lead, nickel, and silver in kidneys (Figure 2C) (66). Clear cell carcinoma, the most common type of renal cancer, is thought to arise from the proximal tubules (81), the cells in the kidney that most often harbored toxic metals.
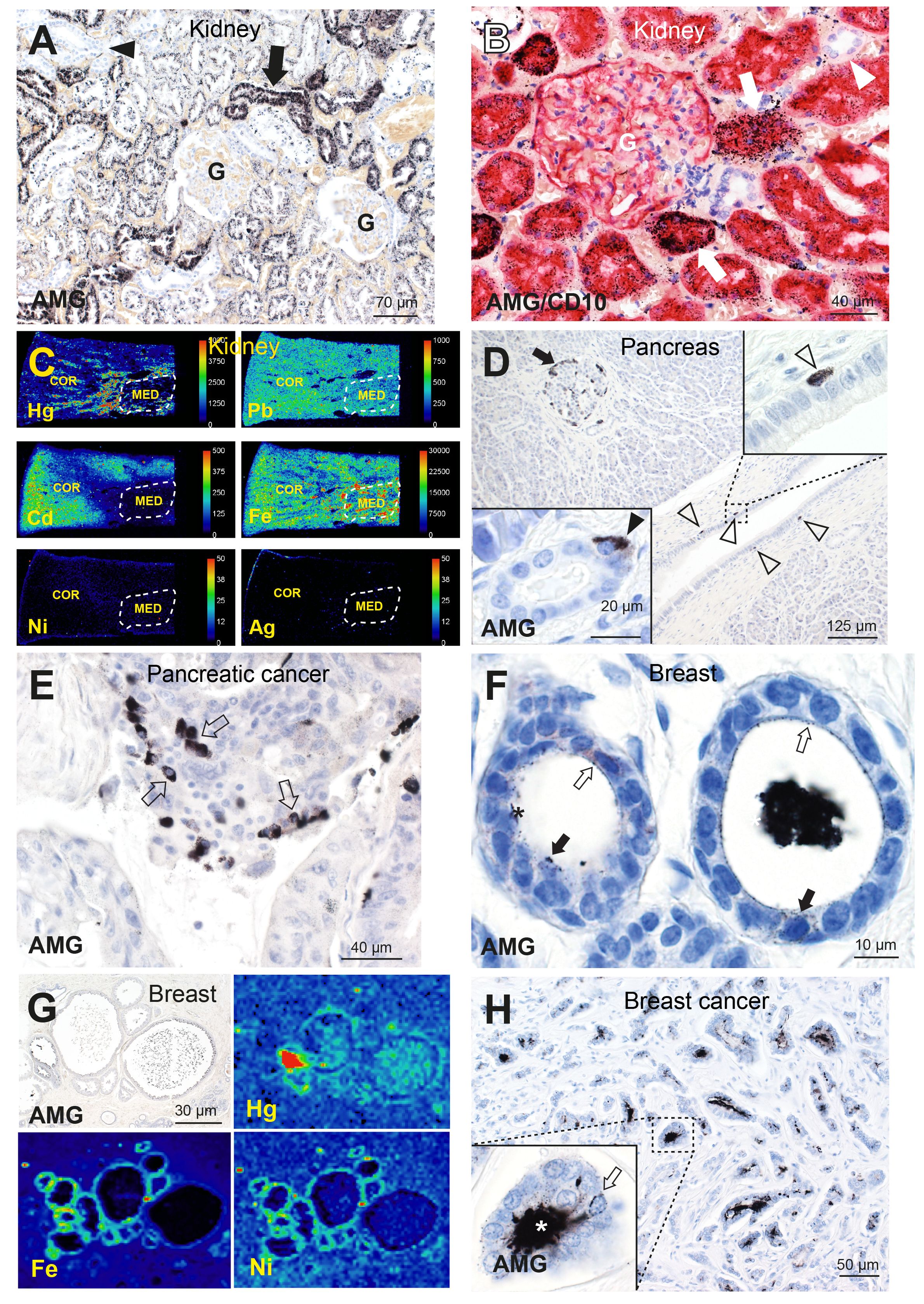
Figure 2 Toxic metals in the human kidney, pancreas, and breast. (A) A normal kidney has abundant AMGTM in proximal tubule cells (arrow). No mercury is seen in glomeruli (G) or in distal tubules (arrowhead). AMG/hematoxylin (66). (B) AMG with CD10 immunostaining shows red proximal tubule cells containing black mercury grains (arrows). No mercury is seen in CD10-negative distal tubules (arrowhead), or in a glomerulus (G) whose cells stain lightly with CD10. AMG/CD10/hematoxylin (66). (C) LA-ICP-MSI shows mercury, lead, cadmium and iron in kidney cortex or medulla, but not nickel or silver (66). Scale = counts per second (proportional to abundance). CO: cortex, ME: medulla (within dashed outlines). (D) A pancreas with AMGTM in peripheral (arrow) and internal islet cells. Scattered periductal cells (open arrowheads), one enlarged in the upper right inset, contain AMGTM. Lower left inset: an acinar cell contains AMGTM (closed arrowhead). AMG/hematoxylin (65). (E) Individual and groups of pancreatic carcinoma cells contain AMGTM (arrows). AMG/hematoxylin. (F) Normal breast tissue, with fine grains of AMGTM (open arrows) attached to the luminal surface of lobule epithelial cells, and particulate AMGTM in scattered epithelial cells (closed arrows). The lumen of one lobule (right) contains black AMGTM-stained secretion (artefactually shrunken); in the left lobule the secretion has fallen out during processing. AMG/hematoxylin (76). (G) LA-ICP-MSI of (AMGTM-containing) normal breast lobules showing mercury (red/green) in the luminal secretion and epithelium, and iron and nickel (green) in the epithelium (76). (H) Breast cancer with numerous neoplastic ductules containing black luminal AMGTM. Enlarged view shows AMGTM in neoplastic duct cells, with AMGTM grains (arrow) attached to the nuclear membrane. AMGTM is present in the ductule lumen (asterisk). AMG/hematoxylin (76).
2.2.2 Pancreas
Pancreatic islets, and nearby acinar and ductal cells, readily take up xenobiotics due to their high blood flow and fenestrated capillaries (82). Autometallography showed AMGTM in normal pancreatic islet cells, often in peripheral islet cells and those adjacent to microvessels (Figure 2D), in 16% of people without pancreatic cancer and in 53% with pancreatic cancer (65). AMGTM in islets was confined to insulin-producing ß-cells (77), the cell of origin of insulinomas. AMGTM was also seen in acinar cells (Figure 2D) in 24% of people with pancreatic cancer, but not in people without pancreatic cancer. Periductal cells containing AMGTM (Figure 2D) were present in 11% of people with pancreatic cancer, but not in people without tumors. Islet cells, periductal cells and acinar cells are all potential candidates for pancreatic progenitor cells (83, 84) where mutations could initiate pancreatic cancer. LA-ICP-MSI showed the AMGTM in the pancreas was most often mercury (Supplementary Figure 1A). Other metals detected in the pancreas were cadmium, chromium, lead, and nickel (65). AMGTM was seen in some groups of pancreatic cancer cells (Figure 2E), and so could promote further mutations leading to tumor clones.
2.2.3 Thyroid
Autometallography showed AMGTM in the cytoplasm of thyroid follicle epithelial cells (Supplementary Figure 1B) of 4% of people aged 1–29 years, 9% aged 30–59 years, and 38% aged 60–104 years (85). The density of AMGTM varied both within and between samples. No thyroid cancer was present in any sample. LA-ICP-MSI indicated that mercury was likely to be the cause of most AMGTM positivity (Supplementary Figure 1C). Other metals seen in thyroid follicle epithelium were cadmium, lead, and nickel (85). Most thyroid carcinomas are derived from thyroid follicle epithelium (81).
2.2.4 Nervous system
Cells in the human nervous system that contained AMGTM were astrocytes, oligodendrocytes, neurons, pericytes, pinealocytes, choroid plexus cells, and white blood cells (67, 74, 75, 80, 86, 87). Astrocytes were the cells most frequently containing AMGTM, often with dense staining of cell bodies and astrocytic processes (Supplementary Figure 1D), and involving all four types of astrocytes. Astrocyte-derived tumors (astrocytoma and glioblastoma multiforme) are the most common glial tumors (81). Oligodendrocytes often contained AMGTM (Supplementary Figure 1D), especially those in grey matter. Oligodendrogliomas are the second most common glial tumor. Neurons in the locus ceruleus in the brain stem had a marked age-related tendency to take up and retain toxic metals, with AMGTM starting to be seen in the 20–29 years group (22% of people), and peaking at 67% of people in the 60–69 years group, indicating that toxic metals are commonly taken up by adult brains (71, 88). The locus ceruleus helps maintain the blood-brain-barrier, so toxic metals in this nucleus could impair this barrier and allow other toxicants to pass into the central nervous system to initiate tumors. Other neurons in the brain were less likely than glial cells to take up AMGTM, and neuronal tumors in adults are less common than glial tumors (81). Other nervous system cells that contained AMGTM (and their associated tumors) were pericytes (Supplementary Figure 1D) (hemangiopericytoma), pinealocytes (pineal gland tumor), choroid plexus cells (papilloma), and white blood cells (primary CNS lymphoma). LA-ICP-MSI indicated that mercury and silver were likely to be the metals responsible for the nervous system AMGTM, though only one person had a known exposure to mercury. LA-ICP-MSI of the locus ceruleus showed neurons harbor different combinations of toxic metals (88), which enables this nucleus to be used to estimate previous exposures to metal toxicants.
2.2.5 Anterior pituitary
Autometallography showed AMGTM in anterior pituitary cells (Supplementary Figure 1E) in 33% of people aged 2–20 years, and increased in frequency on aging, reaching a peak of 88% of people in the 61–80 years group (64). Growth-hormone containing somatotrophs were the cells most often containing AMGTM (Supplementary Figure 1F). LA-ICP-MSI showed mercury in regions of the pituitary that had AMGTM (Supplementary Figure 1G), with no other toxic metals being seen in the pituitary (64). Growth hormone-secreting somatotroph adenomas are a common type of pituitary tumor (81).
2.2.6 Breast
Breast cancer samples removed at surgery showed AMGTM in normal breast tissue apart from the tumor, with AMGTM seen in intraductal secretions and luminal epithelial cells in 55% of samples (76) (Figure 2F). LA-ICP-MSI detected mercury in samples that contained AMGTM (Figure 2G), and found other metals such as nickel, iron, aluminum, chromium and cadmium in some samples (76). Neoplastic cells containing AMGTM (Figure 2H) were seen in 23% of breast cancers. The female breast may be particularly susceptible to cancer because metal transporters ferry toxicants like mercury from the circulation through breast epithelial cells to enter luminal secretions (76). Epithelial progenitor cells undergoing mitoses during an episode of mercury exposure would be at risk for genotoxic damage from mercury, and mercury-rich luminal secretions would expose epithelial cells to mercury long-term (76).
2.3.7 Other organs
In the ovary AMGTM was seen attached to nuclei of epithelial cells and in the zona pellucida (Supplementary Figure 1H) (77). Fallopian tube tissue [where some ovarian cancers arise (81)] was not available for analysis. Other human organs where AMGTM or LA-ICP-MSI detected toxic metals have been found (and common tumors arising at these sites) are the liver (77) hepatocytes (hepatocellular carcinoma) and portal tracts (biliary carcinoma), the adrenal gland (78) cortex (adrenal adenoma) and medulla (phaeochromocytoma), and the retina (79) (retinoblastoma).
3 Discussion
There are two key points of this Perspective. (1) Many types of normal human cells contain toxic metals such as mercury, which increase during aging. (2) The human cell types containing toxic metals are those susceptible to neoplasia. The finding of toxic metals in human cells can help explain the increasing incidence of some cancers, the increase of cancer incidence with aging, and multiple cancers.
Studies of age-adjusted cancer incidences over time indicate that some cancers are increasing in incidence (89, 90). Furthermore, the incidence of colorectal, breast, kidney, pancreas, and uterine cancer is increasing in younger age groups (91, 92). One possible reason for increases in cancer incidence could be increasing atmospheric, water and soil pollution with carcinogenic toxic elements (2). For example, increased atmospheric pollution with mercury from burning fossil fuels and artisanal gold mining (20, 93) leads to increased human mercury intake, both with mercury vapor inhalation and with methylmercury ingestion of mercury-contaminated fish (94), with global fish consumption now outstripping human population growth (95).
The incidence of most adult cancers increases with increasing age (81), so it is of interest that the prevalence of people with AMGTM in cells of the kidney, thyroid, anterior pituitary, pancreas, adrenal medulla, and brain neurons also increases during aging (77). Another potential reason for the later age of onset of most adult cancers is that cellular methylmercury is slowly demethylated in the body to more toxic inorganic mercury (96), so the genotoxic potential of intracellular mercury increases with increasing age. A puzzling phenomenon is the decrease in mortality for many cancers in advanced older age (97, 98), which could contribute to the plateau of mortality in advanced age (99, 100). One reason for this could be that the proportion of people having detectable AMGTM in cancer-prone cells falls over the age of 80 years (77), so people who have been exposed to less metal toxicant during their lives could be less likely to develop cancer later in life.
People with AMGTM in one organ usually have toxic metals in several other organs as well (77). This raises the possibility that exposure to metal toxicants could contribute to the occurrence of concurrent multiple primary tumors, in conjunction with genetic susceptibilities to these cancers (101).
Exposing experimental animals to toxic metals (usually mercury) and then biomapping the metal location with autometallography has given valuable insights into the types of cells taking up toxic metals. (1) Autometallography has shown AMGTM within the same cell types in animals as contain AMGTM in humans (Supplementary Table 2). For example, in animals exposed to mercury, silver or bismuth, AMGTM was seen in the same pattern as human cells in the kidney (63, 102–109), pancreas (62, 102, 104, 109), thyroid (103, 104, 110), nervous system (37, 63, 102–104, 109, 111–114), pituitary (102, 104, 109, 110, 115, 116), ovary (62, 104), liver (47, 102–106, 117), adrenal gland (58, 62, 102, 104), retina (111), white cells (47, 62, 102, 104), and endothelial cells (47, 58, 103, 104, 112, 118, 119). Even exposure to low levels of mercury from a few dental amalgam fillings in non-human primates resulted in the widespread cellular uptake of mercury (102). (2) Autometallography combined with electron microscopy showed AMGTM from mercury or silver exposure in lysosomes, mitochondria, Golgi apparatus, endothelial basement membrane, and nuclear membrane, as well as within nuclear euchromatin and nucleoli (37, 47, 58, 62, 63, 87, 102, 104, 112, 114, 116, 120–128), all subcellular sites implicated in neoplasia (129) (Supplementary Table 2). (3) Mercury vapor and methylmercury pass readily through the placenta and enter the developing fetus (52, 55). After gestational exposure to mercury vapor, AMGTM was found in neonatal mouse renal tubule cells, liver periportal cells, synovial cells, chondrocytes, retinal cells, optic nerve glial cells, and fibroblast progenitor cells (111, 117), indicating toxic metals can be taken up preferentially by developing cells (Supplementary Table 2). These findings suggest that prenatal toxic metal exposure could plant the seed for later carcinogenesis, either via mutations or epigenetic changes in developing cells, and could contribute to the pathogenesis of early-onset cancers such as retinoblastoma, optic nerve glioma, and soft tissue sarcomas (81).
Some human autopsy tissue is not available for elemental analysis because of limited routine organ sampling. Here small animal autoradiography using radiolabeled isotopes is useful, since it has shown the widespread organ uptake (including the colon, esophagus, lacrimal and salivary glands, bone marrow, fat, and muscle) of different forms of mercury, and the length of time mercury persists within organs (51–55) (Supplementary Table 2). Autoradiography can map the routes inhaled mercury vapor takes through the body (via the lungs, blood, kidneys, liver, bile duct, and gastrointestinal tract), and that ingested methylmercury takes (via the gastrointestinal tract, blood, liver, bile duct, and colon) (56). Mercury therefore passes through many of the organs where cancers frequently arise (Supplementary Figure 2).
Future projects on the role of toxic metals in cancer could be: (1) Since toxic metals can be transported across the placenta into the fetus (130), animal studies could be used to find if AMGTM is present in a wide range of fetal stem or progenitor cells (131). (2) Elemental biomapping of biopsied tissues not routinely sampled at autopsy, such as the fallopian tubes, could be undertaken. (3) DNA damage has been reported for metals other than mercury, including cadmium (132, 133) and silver (134). Toxicity synergy exists for several metals (3, 135) so exposure to multiple toxic metals may be more potent in promoting carcinogenesis than single metals alone (4). Future experimental studies therefore need to examine the carcinogenic effect of multiple metal toxicants. (4) Toxicologists could take a leaf out of the genetics playbook (where whole genome or exome sequencing has largely replaced searches for individual gene variants) by greatly expanding the range of potentially toxic elements to be biomapped in tissues. (5) Potentially-toxic metals acting alone are unlikely to be the sole cause of most cancers, since in humans many organs contain AMGTM in later adult life (77), whereas cancers arise in only a proportion of these. It is likely that genetic susceptibilities to environmental toxicants are present in a majority of cancers, so combined next-generation genetic analyses and extensive toxic element biomapping will be needed to uncover these interactions (1).
In conclusion, human elemental biomapping shows that potentially-carcinogenic toxic metals are present in many of cells from which common tumors arise. The increasing global incidence of many tumors could be associated with increasing toxic metal pollution from anthropogenic toxic metal pollution of the atmosphere, water and soil. More work is needed to confidently assign the roles of toxic metals to common cancers, in particular looking for gene-toxic metal interactions. However, a precautionary approach to reduce the incidence of cancers would be to reduce toxic metal-emitting industrial and mining activities and the burning of fossil fuels.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Human Ethics Committee, Sydney Local Health District (Royal Prince Alfred Branch). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from previous studies for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
RP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. DPB: Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. RP was supported by the Aimee Stacey Memorial and Ignacy Burnett Bequests. DPB was supported by the Australian Research Council Discovery Project grant DP230101740.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1420451/full#supplementary-material
Supplementary Figure 1 | Toxic metals in the human pancreas, thyroid, brain, pituitary, and ovary. (A) LA-ICP-MSI shows the location of pancreatic islets demonstrated by zinc imaging (left panel). Mercury (red/green) is seen in the same islets (right panel) that contained AMGTM (65). (B) A normal thyroid with dense AMGTM in most thyroid follicle epithelial cells. Inset: magnified view showing AMGTM (arrows) within the cytoplasm of thyroid follicle epithelial cells. AMG/hematoxylin (85). (C) LA-ICP-MSI shows mercury (red/green) in thyroid follicle epithelium that contained AMGTM (85). (D) Cerebral white matter where the cell bodies of brown-stained fibrous astrocytes contain dense black AMGTM (open arrowheads). AMGTM grains are seen in astrocyte processes (closed arrowheads) which end on blood vessels (BV). Right inset: a cortical oligodendrocyte contains perinuclear AMGTM (arrowhead). Left inset: AMGTM in brain pericytes (closed arrowheads) and an endothelial cell (open arrowhead). AMG/glial fibrillary acidic protein (AMG/Luxol fast blue in left inset) (67, 75). (E) Anterior pituitary cells containing AMGTM granules. AMG/hematoxylin (64). (F) Anterior pituitary growth hormone-stained (red) somatotrophs contain AMGTM granules (closed arrows), but not cells without growth hormone (open arrows). AMG/growth hormone/hematoxylin (64). (G) LA-ICP-MSI shows mercury (red/yellow) in a region of the anterior pituitary with AMGTM-containing cells (right lower panel), but not in an AMGTM-free region (right upper panel) (64). Scale = counts per second (proportional to abundance). (H) Normal ovarian follicle with AMGTM grains attached to nuclei of epithelial cells (arrowhead in enlargement), in the zona pellucida (arrow), and in round profiles (asterisk) in the antrum. AMG/hematoxylin (77).
Supplementary Figure 2 | Cellular pathways in the body of mercury vapor (Hg0) and methylmercury (MeHg+). (A) Inhaled Hg0 is taken up by the lungs, and absorbed by plasma, red blood cells, and cells in multiple organs, where conversion to toxic inorganic mercury (Hg2+) takes place. Excretion of Hg2+ is via the kidney and urine, and via the liver, bile duct, and gastrointestinal tract (GIT) and excreted through the feces. (B) Ingestion of dietary MeHg+ by the GIT leads to MeHg+ being taken up by the blood and then either (i) passes into cells of multiple organs where it is slowly converted to Hg2+, or (ii) is re-circulated into the GIT by the hepato-biliary pathway. MeHg+ in the colon is rapidly converted to Hg2+ by colonic flora and excreted in the feces. In both pathways toxic Hg2+ is deposited or passes through many of the cells that are prone to cancer.
References
1. Simonds NI, Ghazarian AA, Pimentel CB, Schully SD, Ellison GL, Gillanders EM, et al. Review of the gene-environment interaction literature in cancer: what do we know? Genet Epidemiol. (2016) 40:356–65. doi: 10.1002/gepi.21967
2. Wang Z, Walker GW, Muir DCG, Nagatani-Yoshida K. Toward a global understanding of chemical pollution: A first comprehensive analysis of national and regional chemical inventories. Environ Sci Technol. (2020) 54:2575–84. doi: 10.1021/acs.est.9b06379
3. Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp Suppl. (2012) 101:133–64. doi: 10.1007/978–3-7643–8340-4_6
4. Chen QY, DesMarais T, Costa M. Metals and mechanisms of carcinogenesis. Annu Rev Pharmacol Toxicol. (2019) 59:537–54. doi: 10.1146/annurev-pharmtox-010818–021031
5. Parida L, Patel TN. Systemic impact of heavy metals and their role in cancer development: a review. Environ Monit Assess. (2023) 195:766. doi: 10.1007/s10661-023-11399-z
6. Budi HS, Catalan Opulencia MJ, Afra A, Abdelbasset WK, Abdullaev D, Majdi A, et al. Source, toxicity and carcinogenic health risk assessment of heavy metals. Rev Environ Health. (2024) 39:77–90. doi: 10.1515/reveh-2022–0096
7. Yaman M. Comprehensive comparison of trace metal concentrations in cancerous and non-cancerous human tissues. Curr Med Chem. (2006) 13:2513–25. doi: 10.2174/092986706778201620
8. Yaman M, Kaya G, Yekeler H. Distribution of trace metal concentrations in paired cancerous and non-cancerous human stomach tissues. World J Gastroenterol. (2007) 13:612–8. doi: 10.3748/wjg.v13.i4.612
9. Sohrabi M, Gholami A, Azar MH, Yaghoobi M, Shahi MM, Shirmardi S, et al. Trace element and heavy metal levels in colorectal cancer: comparison between cancerous and non-cancerous tissues. Biol Trace Elem Res. (2018) 183:1–8. doi: 10.1007/s12011–017-1099–7
10. Olaiya DO, Alatise OI, Oketayo OO, Abiye OE, Obianjunwa EI, Balogun FA. Trace element analysis of cancerous and non-cancerous breast tissues of African women in Southwest Nigeria using particle-induced X-ray emission technique. Breast Cancer (Auckl). (2019) 13:1178223419840694. doi: 10.1177/1178223419840694
11. Mulware SJ. Comparative trace elemental analysis in cancerous and noncancerous human tissues using PIXE. J Biophys. (2013) 2013:192026. doi: 10.1155/2013/192026
12. Coradduzza D, Congiargiu A, Azara E, Mammani IMA, De Miglio MR, Zinellu A, et al. Heavy metals in biological samples of cancer patients: a systematic literature review. Biometals. (2024). doi: 10.1007/s10534–024-00583–4
13. Crespo-Lopez ME, Macedo GL, Pereira SI, Arrifano GP, Picanco-Diniz DL, do Nascimento JL, et al. Mercury and human genotoxicity: critical considerations and possible molecular mechanisms. Pharmacol Res. (2009) 60:212–20. doi: 10.1016/j.phrs.2009.02.011
14. Skalny AV, Aschner M, Sekacheva MI, Santamaria A, Barbosa F, Ferrer B, et al. Mercury and cancer: Where are we now after two decades of research? Food Chem Toxicol. (2022) 164:113001. doi: 10.1016/j.fct.2022.113001
15. Grzesiakowska A, Kasprowicz MJ, Kuchta-Gladysz M, Rymuza K, Szeleszczuk O. Genotoxicity of physical silver nanoparticles, produced by the HVAD method, for Chinchilla lanigera genome. Sci Rep. (2021) 11:18473. doi: 10.1038/s41598–021-97926–9
16. Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol. (2021) 12:643972. doi: 10.3389/fphar.2021.643972
17. Kim HS, Kim YJ, Seo YR. An overview of carcinogenic heavy metal: molecular toxicity mechanism and prevention. J Cancer Prev. (2015) 20:232–40. doi: 10.15430/JCP.2015.20.4.232
18. Nersesyan A, Kundi M, Waldherr M, Setayesh T, Misik M, Wultsch G, et al. Results of micronucleus assays with individuals who are occupationally and environmentally exposed to mercury, lead and cadmium. Mutat Res. (2016) 770:119–39. doi: 10.1016/j.mrrev.2016.04.002
19. Bonfiglio R, Sisto R, Casciardi S, Palumbo V, Scioli MP, Palumbo A, et al. The impact of toxic metal bioaccumulation on colorectal cancer: Unravelling the unexplored connection. Sci Total Environ. (2024) 906:167667. doi: 10.1016/j.scitotenv.2023.167667
20. Streets DG, Devane MK, Lu Z, Bond TC, Sunderland EM, Jacob DJ. All-time releases of mercury to the atmosphere from human activities. Environ Sci Technol. (2011) 45:10485–91. doi: 10.1021/es202765m
21. Costa M, Christie NT, Cantoni O, Zelikoff JT, Xin WW, Rossman TG. DNA damage by mercury compounds: an overview. In: Suzuki T, Imura N, Clarkson TW, editors. Advances in mercury toxicology. Plenum Press, New York (1991). p. 255–73.
22. Simpson R. Association constants of methylmercuric and mercuric ions with nucleosides. J Am Chem Soc. (1964) 86:2059–65. doi: 10.1021/ja01064a029
23. Li Y, Jiang Y, Yan XP. Probing mercury species-DNA interactions by capillary electrophoresis with on-line electrothermal atomic absorption spectrometric detection. Anal Chem. (2006) 78:6115–20. doi: 10.1021/ac060644a
24. Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol. (2008) 21:28–44. doi: 10.1021/tx700198a
25. Morales ME, Derbes RS, Ade CM, Ortego JC, Stark J, Deininger PL, et al. Heavy metal exposure influences double strand break DNA repair outcomes. PloS One. (2016) 11:e0151367. doi: 10.1371/journal.pone.0151367
26. Groelly FJ, Fawkes M, Dagg RA, Blackford AN, Tarsounas M. Targeting DNA damage response pathways in cancer. Nat Rev Cancer. (2023) 23:78–94. doi: 10.1038/s41568–022-00535–5
27. Pollard KM, Cauvi DM, Toomey CB, Hultman P, Kono DH. Mercury-induced inflammation and autoimmunity. Biochim Biophys Acta Gen Subj. (2019) 1863:129299. doi: 10.1016/j.bbagen.2019.02.001
28. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
29. Yang C, Wang Z. The epitranscriptomic mechanism of metal toxicity and carcinogenesis. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms231911830
30. Manic L, Wallace D, Onganer PU, Taalab YM, Farooqi AA, Antonijevic B, et al. Epigenetic mechanisms in metal carcinogenesis. Toxicol Rep. (2022) 9:778–87. doi: 10.1016/j.toxrep.2022.03.037
31. Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. (2012) 150:12–27. doi: 10.1016/j.cell.2012.06.013
32. Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. (2006) 6:107–16. doi: 10.1038/nrc1799
33. Unoki T, Abiko Y, Toyama T, Uehara T, Tsuboi K, Nishida M, et al. Methylmercury, an environmental electrophile capable of activation and disruption of the Akt/CREB/Bcl-2 signal transduction pathway in SH-SY5Y cells. Sci Rep. (2016) 6:28944. doi: 10.1038/srep28944
34. Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. (2020) 17:395–417. doi: 10.1038/s41571-020-0341-y
35. Grasso D, Zampieri LX, Capeloa T, Van de Velde JA, Sonveaux P. Mitochondria in cancer. Cell Stress. (2020) 4:114–46. doi: 10.15698/cst2020.06.221
36. Galvan DL, Green NH, Danesh FR. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. (2017) 92:1051–7. doi: 10.1016/j.kint.2017.05.034
37. Chang LW, Hartmann HA. Electron microscopic histochemical study on the localization and distribution of mercury in the nervous system after mercury intoxication. Exp Neurol. (1972) 35:122–37. doi: 10.1016/0014–4886(72)90064–7
38. Del Giudice S, De Luca V, Parizadeh S, Russo D, Luini A, Di Martino R. Endogenous and exogenous regulatory signaling in the secretory pathway: role of golgi signaling molecules in cancer. Front Cell Dev Biol. (2022) 10:833663. doi: 10.3389/fcell.2022.833663
39. Verity MA, Reith A. Effect of mercurial compounds on structure-linked latency of lysosomal hydrolases. Biochem J. (1967) 105:685–90. doi: 10.1042/bj1050685
40. Tang T, Yang ZY, Wang D, Yang XY, Wang J, Li L, et al. The role of lysosomes in cancer development and progression. Cell Biosci. (2020) 10:131. doi: 10.1186/s13578-020-00489-x
41. Chow KH, Factor RE, Ullman KS. The nuclear envelope environment and its cancer connections. Nat Rev Cancer. (2012) 12:196–209. doi: 10.1038/nrc3219
42. Parker AL, Kavallaris M, McCarroll JA. Microtubules and their role in cellular stress in cancer. Front Oncol. (2014) 4:153. doi: 10.3389/fonc.2014.00153
43. Saghiri MA, Orangi J, Asatourian A, Sorenson CM, Sheibani N. Functional role of inorganic trace elements in angiogenesis part III: (Ti, Li, Ce, As, Hg, Va, Nb and Pb). Crit Rev Oncol Hematol. (2016) 98:290–301. doi: 10.1016/j.critrevonc.2015.10.004
44. Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. (2020) 77:1745–70. doi: 10.1007/s00018–019-03351–7
45. Kuznetsov DA, Richter V. Modulation of messenger RNA metabolism in experimental methyl mercury neurotoxicity. Int J Neurosci. (1987) 34:1–17. doi: 10.3109/00207458708985935
46. Goodall GJ, Wickramasinghe VO. RNA in cancer. Nat Rev Cancer. (2021) 21:22–36. doi: 10.1038/s41568–020-00306–0
47. Christensen MM. Histochemical localization of autometallographically detectable mercury in tissues of the immune system from mice exposed to mercuric chloride. Histochem J. (1996) 28:217–25. doi: 10.1007/BF02331446
48. Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. (2021) 21:345–59. doi: 10.1038/s41568-021-00347-z
49. Gronbaek K, Hother C, Jones PA. Epigenetic changes in cancer. APMIS. (2007) 115:1039–59. doi: 10.1111/j.1600-0463.2007.apm_636.xml.x
50. Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. (2006) 36:609–62. doi: 10.1080/10408440600845619
51. Khayat A, Dencker L. Whole body and liver distribution of inhaled mercury vapor in the mouse: influence of ethanol and aminotriazole pretreatment. J Appl Toxicol. (1983) 3:66–74. doi: 10.1002/jat.2550030203
52. Khayat A, Dencker L. Organ and cellular distribution of inhaled metallic mercury in the rat and Marmoset monkey (Callithrix jacchus): influence of ethyl alcohol pretreatment. Acta Pharmacol Toxicol (Copenh). (1984) 55:145–52. doi: 10.1111/j.1600-0773.1984.tb01977.x
53. Berlin M, Ullberg S. Accumulation and retention of mercury in the mouse. III. An autoradiographic comparison of methylmercuric dicyandiamide with inorganic mercury. Arch Environ Health. (1963) 6:610–6. doi: 10.1080/00039896.1963.10663449
54. Berlin M, Ullberg S. Accumulation and retention of mercury in the mouse. II. An autoradiographic comparison of phenylmercuric acetate with inorganic mercury. Arch Environ Health. (1963) 6:602–9. doi: 10.1080/00039896.1963.10663448
55. Berlin M, Ullberg S. Accumulation and retention of mercury in the mouse. I. An autoradiographic study after a single intravenous injection of mercuric chloride. Arch Environ Health. (1963) 6:589–601. doi: 10.1080/00039896.1963.10663447
56. Clarkson TW, Vyas JB, Ballatori N. Mechanisms of mercury disposition in the body. Am J Ind Med. (2007) 50:757–64. doi: 10.1002/ajim.20476
57. Clarkson TW. The toxicology of mercury. Crit Rev Clin Lab Sci. (1997) 34:369–403. doi: 10.3109/10408369708998098
58. Danscher G, Moller-Madsen B. Silver amplification of mercury sulfide and selenide: a histochemical method for light and electron microscopic localization of mercury in tissue. J Histochem Cytochem. (1985) 33:219–28. doi: 10.1177/33.3.2579122
59. Stoltenberg M, Danscher G. Histochemical differentiation of autometallographically traceable metals (Au, Ag, Hg, Bi, Zn): protocols for chemical removal of separate autometallographic metal clusters in Epon sections. Histochem J. (2000) 32:645–52. doi: 10.1023/a:1004115130843
60. Danscher G, Stoltenberg M, Juhl S. How to detect gold, silver and mercury in human brain and other tissues by autometallographic silver amplification. Neuropathol Appl Neurobiol. (1994) 20:454–67. doi: 10.1111/j.1365-2990.1994.tb00996.x
61. Danscher G. Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electronmicroscopy. Histochemistry. (1981) 71:1–16. doi: 10.1007/BF00592566
62. Danscher G. Light and electron microscopic localization of silver in biological tissue. Histochemistry. (1981) 71:177–86. doi: 10.1007/bf00507822
63. Danscher G, Stoltenberg M, Kemp K, Pamphlett R. Bismuth autometallography: protocol, specificity, and differentiation. J Histochem Cytochem. (2000) 48:1503–10. doi: 10.1177/002215540004801107
64. Pamphlett R, Kum Jew S, Doble PA, Bishop DP. Elemental analysis of aging human pituitary glands implicates mercury as a contributor to the somatopause. Front Endocrinol (Lausanne). (2019) 10:419. doi: 10.3389/fendo.2019.00419
65. Pamphlett R, Colebatch AJ, Doble PA, Bishop DP. Mercury in pancreatic cells of people with and without pancreatic cancer. Int J Environ Res Public Health. (2020) 17. doi: 10.3390/ijerph17238990
66. Pamphlett R, Doble PA, Bishop DP. The prevalence of inorganic mercury in human kidneys suggests a role for toxic metals in essential hypertension. Toxics. (2021) 9. doi: 10.3390/toxics9030067
67. Pamphlett R, Kum Jew S. Inorganic mercury in human astrocytes, oligodendrocytes, corticomotoneurons and the locus ceruleus: implications for multiple sclerosis, neurodegenerative disorders and gliomas. Biometals. (2018) 31:807–19. doi: 10.1007/s10534–018-0124–4
68. Doble PA, de Vega RG, Bishop DP, Hare DJ, Clases D. Laser ablation-inductively coupled plasma-mass spectrometry imaging in biology. Chem Rev. (2021) 121:11769–822. doi: 10.1021/acs.chemrev.0c01219
69. Paul B, Kysenius K, Hilton JB, Jones MWM, Hutchinson RW, Buchanan DD, et al. An integrated mass spectrometry imaging and digital pathology workflow for objective detection of colorectal tumours by unique atomic signatures. Chem Sci. (2021) 12:10321–33. doi: 10.1039/D1SC02237G
70. Nunes MA, Voss M, Corazza G, Flores EM, Dressler VL. External calibration strategy for trace element quantification in botanical samples by LA-ICP-MS using filter paper. Anal Chim Acta. (2016) 905:51–7. doi: 10.1016/j.aca.2015.11.049
71. Pamphlett R, Mak R, Lee J, Buckland ME, Harding AJ, Kum Jew S, et al. Concentrations of toxic metals and essential trace elements vary among individual neurons in the human locus ceruleus. PloS One. (2020) 15:e0233300. doi: 10.1371/journal.pone.0233300
72. De Samber B, De Rycke R, De Bruyne M, Kienhuis M, Sandblad L, Bohic S, et al. Effect of sample preparation techniques upon single cell chemical imaging: A practical comparison between synchrotron radiation based X-ray fluorescence (SR-XRF) and Nanoscopic Secondary Ion Mass Spectrometry (nano-SIMS). Anal Chim Acta. (2020) 1106:22–32. doi: 10.1016/j.aca.2020.01.054
73. Pamphlett R, Waley P. Uptake of inorganic mercury by the human brain. Acta Neuropathol. (1996) 92:525–7. doi: 10.1007/s004010050556
74. Pamphlett R, Bishop DP. Mercury is present in neurons and oligodendrocytes in regions of the brain affected by Parkinson's disease and co-localises with Lewy bodies. PloS One. (2022) 17:e0262464. doi: 10.1371/journal.pone.0262464
75. Pamphlett R, Buckland ME, Bishop DP. Potentially toxic elements in the brains of people with multiple sclerosis. Sci Rep. (2023) 13:655. doi: 10.1038/s41598–022-27169–9
76. Pamphlett R, Satgunaseelan L, Kum Jew S, Doble PA, Bishop DP. Elemental bioimaging shows mercury and other toxic metals in normal breast tissue and in breast cancers. PloS One. (2020) 15:e0228226. doi: 10.1371/journal.pone.0228226
77. Pamphlett R. The prevalence of inorganic mercury in human cells increases during aging but decreases in the very old. Sci Rep. (2021) 11:16714. doi: 10.1038/s41598–021-96359–8
78. Pamphlett R, Kum Jew S, Doble PA, Bishop DP. Mercury in the human adrenal medulla could contribute to increased plasma noradrenaline in aging. Sci Rep. (2021) 11:2961. doi: 10.1038/s41598-021-82483-y
79. Pamphlett R, Cherepanoff S, Too LK, Kum Jew S, Doble PA, Bishop DP. The distribution of toxic metals in the human retina and optic nerve head: Implications for age-related macular degeneration. PloS One. (2020) 15:e0241054. doi: 10.1371/journal.pone.0241054
80. Pamphlett R, Kum Jew S. Uptake of inorganic mercury by human locus ceruleus and corticomotor neurons: implications for amyotrophic lateral sclerosis. Acta Neuropathol Commun. (2013) 1:13. doi: 10.1186/2051–5960-1–13
81. Kumar V, Abbas AK, Aster JC eds. Neoplasia. In: Robbins & Cotran pathologic basis of disease, 10th ed. Elsevier, Philadelphia, PA. p. 267–338.
82. Jansson L, Barbu A, Bodin B, Drott CJ, Espes D, Gao X, et al. Pancreatic islet blood flow and its measurement. Ups J Med Sci. (2016) 121:81–95. doi: 10.3109/03009734.2016.1164769
83. Yamaguchi J, Liss AS, Sontheimer A, Mino-Kenudson M, Castillo CF, Warshaw AL, et al. Pancreatic duct glands (PDGs) are a progenitor compartment responsible for pancreatic ductal epithelial repair. Stem Cell Res. (2015) 15:190–202. doi: 10.1016/j.scr.2015.05.006
84. Pour PM, Pandey KK, Batra SK. What is the origin of pancreatic adenocarcinoma? Mol Cancer. (2003) 2:13. doi: 10.1186/1476–4598-2–13
85. Pamphlett R, Doble PA, Bishop DP. Mercury in the human thyroid gland: Potential implications for thyroid cancer, autoimmune thyroiditis, and hypothyroidism. PloS One. (2021) 16:e0246748. doi: 10.1371/journal.pone.0246748
86. Pamphlett R, Bishop DP. The toxic metal hypothesis for neurological disorders. Front Neurol. (2023) 14:1173779. doi: 10.3389/fneur.2023.1173779
87. Stoltenberg M, Hogenhuis JA, Hauw JJ, Danscher G. Autometallographic tracing of bismuth in human brain autopsies. J Neuropathol Exp Neurol. (2001) 60:705–10. doi: 10.1093/jnen/60.7.705
88. Pamphlett R, Bishop DP, Kum Jew S, Doble PA. Age-related accumulation of toxic metals in the human locus ceruleus. PloS One. (2018) 13:e0203627. doi: 10.1371/journal.pone.0203627
89. Lin L, Li Z, Yan L, Liu Y, Yang H, Li H. Global, regional, and national cancer incidence and death for 29 cancer groups in 2019 and trends analysis of the global cancer burden, 1990–2019. J Hematol Oncol. (2021) 14:197. doi: 10.1186/s13045-021-01213-z
90. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
91. Ugai T, Sasamoto N, Lee HY, Ando M, Song M, Tamimi RM, et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol. (2022) 19:656–73. doi: 10.1038/s41571–022-00672–8
92. di Martino E, Smith L, Bradley SH, Hemphill S, Wright J, Renzi C, et al. Incidence trends for twelve cancers in younger adults-a rapid review. Br J Cancer. (2022) 126:1374–86. doi: 10.1038/s41416-022-01704-x
93. Streets DG, Lu Z, Levin L, Ter Schure AFH, Sunderland EM. Historical releases of mercury to air, land, and water from coal combustion. Sci Total Environ. (2018) 615:131–40. doi: 10.1016/j.scitotenv.2017.09.207
94. Schartup AT, Thackray CP, Qureshi A, Dassuncao C, Gillespie K, Hanke A, et al. Climate change and overfishing increase neurotoxicant in marine predators. Nature. (2019) 572:648–50. doi: 10.1038/s41586–019-1468–9
95. Myers J. We are eating more fish than ever. Can our oceans cope. Cologny, Switzerland: World Economic Forum (2016). Available at: https://www.weforum.org/agenda/2016/07/3-charts-that-help-explain-the-future-of-fishing/.
96. Friberg L, Mottet NK. Accumulation of methylmercury and inorganic mercury in the brain. Biol Trace Elem Res. (1989) 21:201–6. doi: 10.1007/BF02917253
97. Monnet-Tschudi F, Zurich MG, Boschat C, Corbaz A, Honegger P. Involvement of environmental mercury and lead in the etiology of neurodegenerative diseases. Rev Environ Health. (2006) 21:105–17. doi: 10.1515/reveh.2006.21.2.105
98. Harding C, Pompei F, Lee EE, Wilson R. Cancer suppression at old age. Cancer Res. (2008) 68:4465–78. doi: 10.1158/0008–5472.CAN-07–1670
99. Barbi E, Lagona F, Marsili M, Vaupel JW, Wachter KW. The plateau of human mortality: Demography of longevity pioneers. Science. (2018) 360:1459–61. doi: 10.1126/science.aat3119
100. Gampe J. Mortality of supercentenarians: estimates from the updated IDL. In: Maier H, Jeune B, Vaupel JW, editors. Exceptional lifespans. Springer, Cham, Switzerland (2021). p. 29–35.
101. Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open. (2017) 2:e000172. doi: 10.1136/esmoopen-2017–000172
102. Danscher G, Horsted-Bindslev P, Rungby J. Traces of mercury in organs from primates with amalgam fillings. Exp Mol Pathol. (1990) 52:291–9. doi: 10.1016/0014-4800(90)90070-T
103. Hansen JC, Reske-Nielsen E, Thorlacius-Ussing O, Rungby J, Danscher G. Distribution of dietary mercury in a dog. Quantitation and localization of total mercury in organs and central nervous system. Sci Total Environ. (1989) 78:23–43. doi: 10.1016/0048-9697(89)90020-X
104. Hansen JC, Danscher G. Quantitative and qualitative distribution of mercury in organs from arctic sledgedogs: an atomic absorption spectrophotometric and histochemical study of tissue samples from natural long-termed high dietary organic mercury-exposed dogs from Thule, Greenland. Pharmacol Toxicol. (1995) 77:189–95. doi: 10.1111/j.1600-0773.1995.tb01011.x
105. Woshner VM, O'Hara TM, Eurell JA, Wallig MA, Bratton GR, Suydam RS, et al. Distribution of inorganic mercury in liver and kidney of beluga and bowhead whales through autometallographic development of light microscopic tissue sections. Toxicol Pathol. (2002) 30:209–15. doi: 10.1080/019262302753559542
106. Stoltenberg M, Larsen A, Kemp K, Bloch D, Weihe P. Autometallographic tracing of mercury in pilot whale tissues in the Faroe Islands. Int J Circumpolar Health. (2003) 62:182–9. doi: 10.3402/ijch.v62i2.17552
107. Rodier PM, Kates B, Simons R. Mercury localisation in mouse kidney over time: autoradiography versus silver staining. Toxicol Appl Pharmacol. (1988) 92:235–45. doi: 10.1016/0041-008X(88)90383-3
108. Pamphlett R, Danscher G, Rungby J, Stoltenberg M. Tissue uptake of bismuth from shotgun pellets. Environ Res. (2000) 82:258–62. doi: 10.1006/enrs.1999.4016
109. Danscher G, Zimmer J. An improved Timm sulphide silver method for light and electron microscopic localization of heavy metals in biological tissues. Histochemistry. (1978) 55:27–40. doi: 10.1007/BF00496691
110. Thorlacius-Ussing O, Moller-Madsen B, Danscher G. Intracellular accumulation of mercury in the anterior pituitary of rats exposed to mercuric chloride. Exp Mol Pathol. (1985) 42:278–86. doi: 10.1016/0014–4800(85)90034–6
111. Pamphlett R, Kum Jew S, Cherepanoff S. Mercury in the retina and optic nerve following prenatal exposure to mercury vapor. PloS One. (2019) 14:e0220859. doi: 10.1371/journal.pone.0220859
112. Moller-Madsen B. Localization of mercury in CNS of the rat. V. Inhalation exposure to metallic mercury. Arch Toxicol. (1992) 66:79–89. doi: 10.1007/bf02342499
113. Warfvinge K. Mercury distribution in the neonatal and adult cerebellum after mercury vapor exposure of pregnant squirrel monkeys. Environ Res. (2000) 83:93–101. doi: 10.1006/enrs.1999.4013
114. Rungby J, Danscher G. Localization of exogenous silver in brain and spinal cord of silver exposed rats. Acta Neuropathol. (1983) 60:92–8. doi: 10.1007/BF00685352
115. Thorlacius-Ussing O, Rungby J. Ultrastructural localization of exogenous silver in the anterior pituitary gland of the rat. Exp Mol Pathol. (1984) 41:58–66. doi: 10.1016/0014-4800(84)90007-8
116. Moller-Madsen B, Thorlacius-Ussing O. Accumulation of mercury in the anterior pituitary of rats following oral or intraperitoneal administration of methyl mercury. Virchows Arch B Cell Pathol Incl Mol Pathol. (1986) 51:303–11. doi: 10.1007/BF02899039
117. Pamphlett R, Kum Jew S. Mercury is taken up selectively by cells involved in joint, bone, and connective tissue disorders. Front Med (Lausanne). (2019) 6:168. doi: 10.3389/fmed.2019.00168
118. Pamphlett R, Kum Jew S, Doble PA, Bishop DP. Elemental imaging shows mercury in cells of the human lateral and medial geniculate nuclei. PloS One. (2020) 15:e0231870. doi: 10.1371/journal.pone.0231870
119. Warfvinge K. Mercury distribution in the mouse brain after mercury vapour exposure. Int J Exp Pathol. (1995) 76:29–35.
120. Rungby J, Danscher G. Neuronal accumulation of silver in brains of progeny from argyric rats. Acta Neuropathol. (1983) 61:258–62. doi: 10.1007/BF00691995
121. Rungby J. Experimental argyrosis: ultrastructural localization of silver in rat eye. Exp Mol Pathol. (1986) 45:22–30. doi: 10.1016/0014–4800(86)90003–1
122. Moller-Madsen B. Localization of mercury in CNS of the rat. II. Intraperitoneal injection of methylmercuric chloride (CH3HgCl) and mercuric chloride (HgCl2). Toxicol Appl Pharmacol. (1990) 103:303–23. doi: 10.1016/0041–008x(90)90232-j
123. Moller-Madsen B. Localization of mercury in CNS of the rat. III. Oral administration of methylmercuric chloride (CH3HgCl). Fundam Appl Toxicol. (1991) 16:172–87.
124. Warfvinge K, Hua J, Berlin M. Mercury distribution in the rat brain after mercury vapor exposure. Toxicol Appl Pharmacol. (1992) 117:46–52. doi: 10.1016/0041-008X(92)90215-E
125. Chang LW, Hartmann HA. Ultrastructural studies of the nervous system after mercury intoxication. II. Pathological changes in the nerve fibers. Acta Neuropathol. (1972) 20:316–34. doi: 10.1007/BF00691749
126. Ernst E, Moller-Madsen B, Danscher G. Ultrastructural demonstration of mercury in Sertoli and Leydig cells of the rat following methyl mercuric chloride or mercuric chloride treatment. Reprod Toxicol. (1991) 5:205–9. doi: 10.1016/0890-6238(91)90052-H
127. Ernst E, Rungby J, Baatrup E. Ultrastructural localization of silver in rat testis and organ distribution of radioactive silver in the rat. J Appl Toxicol. (1991) 11:317–21. doi: 10.1002/jat.2550110504
128. Rungby J. Exogenous silver in dorsal root ganglia, peripheral nerve, enteric ganglia, and adrenal medulla. Acta Neuropathol. (1986) 69:45–53. doi: 10.1007/bf00687038
129. Zhao G. Editorial: the role of different subcellular organelles in DNA damage response. Front Cell Dev Biol. (2021) 9:760023. doi: 10.3389/fcell.2021.760023
130. Clarkson TW, Nordberg GF, Sager PR. Reproductive and developmental toxicity of metals. Scand J Work Environ Health. (1985) 11:145–54. doi: 10.5271/sjweh.2239
131. Liu HG, Chen C, Yang H, Pan YF, Zhang XH. Cancer stem cell subsets and their relationships. J Transl Med. (2011) 9:50. doi: 10.1186/1479–5876-9–50
132. Hossain Z, Huq F. Studies on the interaction between Cd(2+) ions and DNA. J Inorg Biochem. (2002) 90:85–96. doi: 10.1016/s0162–0134(02)00412–9
133. Hossain Z, Huq F. Studies on the interaction between Cd(2+) ions and nucleobases and nucleotides. J Inorg Biochem. (2002) 90:97–105. doi: 10.1016/s0162–0134(02)00411–7
134. Hossain Z, Huq F. Studies on the interaction between Ag(+) and DNA. J Inorg Biochem. (2002) 91:398–404. doi: 10.1016/s0162–0134(02)00454–3
Keywords: cancer, toxic metals, mercury, neoplasia, carcinogenesis, pathogenesis, human tissue, elemental biomapping
Citation: Pamphlett R and Bishop DP (2024) Elemental biomapping of human tissues suggests toxic metals such as mercury play a role in the pathogenesis of cancer. Front. Oncol. 14:1420451. doi: 10.3389/fonc.2024.1420451
Received: 20 April 2024; Accepted: 06 June 2024;
Published: 21 June 2024.
Edited by:
Anastasia De Luca, University of Rome Tor Vergata, ItalyReviewed by:
Patrizia Zavattari, University of Cagliari, ItalyCopyright © 2024 Pamphlett and Bishop. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roger Pamphlett, cm9nZXIucGFtcGhsZXR0QGhlYWx0aC5uc3cuZ292LmF1
 Roger Pamphlett
Roger Pamphlett David P. Bishop
David P. Bishop