- 1West China School of Public Health, Sichuan University, Chengdu, Sichuan, China
- 2West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China
Objective: To systematically evaluate the efficacy and safety of PD-1 inhibitors in neoadjuvant therapy for locally advanced colorectal cancer (LACRC).
Method: Retrieved from PubMed, Embase, and the Cochrane Library, all relevant studies about PD-1 inhibitors for neoadjuvant treatment of LACRC were collected from inception to 31 December 2023. The efficacy was assessed by the rate of pathological complete response (PCR), clinical complete response (CCR), and major pathological response (MPR), and the safety was evaluated by the incidence of all adverse effects (TRAEs). Subgroup analysis was conducted by experimental design, types of PD-1 inhibitors, and disease types.
Result: A total of 803 patients were included in 21 studies. The results of the meta-analysis showed that the PCR rate of PD-1 inhibitors in the treatment of LACRC was 54% (95% CI: 43%–65%, P<0.05); the CCR of anti-PD-1 was 40% (95% CI: 26%–54%, P<0.05); the MPR was 66% (95% CI: 56%–76%, P<0.05); and the irAEs was 27% (95% CI: 17%–37%, P<0.05). Subgroup analysis showed that the PCRs in prospective studies and retrospective studies were 49% (95% CI: 32%–66%, P<0.05) and 57% (95% CI: 42%–73%, P<0.05), respectively. Among the 803 patients, 619 (77%) were diagnosed with rectal cancer (RC), and the PCR and MPR were 49% and 65%, respectively; 184 (23%) were diagnosed with colorectal cancer (CRC), and the PCR and MPR were both 67%. In our meta-analysis, types of PD-1 inhibitors, including sintilimab, toripalimab, camrelizumab, avelumab, pembrolizumab, and tislelizumab, and patients who received PD-1 inhibitors alone or in combination achieved good PCR rates.
Conclusion: Neoadjuvant therapy combined with a PD-1 inhibitor has a favorable PCR and relatively low incidences of irAEs for patients with LACRC, suggesting that this regimen including a PD-1 inhibitor is significantly effective and sufficiently safe.
Background
Colorectal cancer (CRC) is one of the most common malignant gastrointestinal cancers, and it ranks third in incidence and second in mortality worldwide among all cancers (1). CRC has an annual incidence of approximately 732,000 cases worldwide (2). Based on statistical data, the annual global mortality rate from the disease exceeds 800,000 individuals (3). The CRC poses a serious health threat to residents because CRC is a highly heterogeneous disease and not easy to detect; in addition, approximately 60% of patients with CRC have locally advanced diseases upon diagnosis (4), which accounts for a large proportion of rectal cancers.
The current standard treatment for patients with LACRC involves neoadjuvant therapy followed by total mesorectal excision, with the option of adjuvant chemotherapy (5). Neoadjuvant therapy has been shown to enhance prognosis by reducing local recurrence, inducing tumor regression, downstaging clinical presentation, and increasing the rate of sphincter preservation. However, neoadjuvant chemotherapy can cause postsurgical issues like anastomotic leakage, poor perineal wound healing, long-term problems such as urination and sexual dysfunction, and loss of anal sphincter function (6). Recently, immune checkpoint inhibitors (ICIs), including PD-1 inhibitors, have transformed cancer treatment with their superior efficacy. They are particularly effective for treating most microsatellite instability-high (MSI-H) and mismatch repair-deficient (dMMR) CRC (7). Several studies have demonstrated the efficacy of neoadjuvant therapy with PD-1 inhibitors, but with a small sample size (8–10). However, a comprehensive analysis of the efficacy and safety of neoadjuvant immunotherapy for patients with LACRC remains limited. Consequently, an extensive review of relevant literature was undertaken, succeeded by a meta-analysis, to rigorously evaluate the efficacy and safety of neoadjuvant immunotherapy utilizing PD-1 inhibitors for LACRC.
Methods
This systematic review and meta-analysis adhered to the reporting guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (11).
Search strategy and selection process
A systematic literature search of Embase, PubMed, and the Cochrane Library was conducted from the time when the database was established until 31 December 2023, limited to the English language. The key terms included “Neoadjuvant therapy,” “Colon Cancer,” “Colorectal Cancer,” “Rectal Cancer,” “PD-1 Inhibitors,” “Programmed cell death protein one inhibitor,” and “Immune checkpoint inhibitor”. We also searched the references to the literature that had been retrieved. Two reviewers independently reviewed the title and abstract of all papers and a full-text review of potentially eligible studies. A third reviewer adjudicated any discrepancies or conflicts.
Inclusion and exclusion criteria
Inclusion criteria
1. Patients diagnosed with LACRC pathologically
2. Patients were administered PD-1 inhibitor therapy in conjunction with neoadjuvant treatment for LACRC
3. No history of ICIs or other experimental drug therapy
4. The literature provides the outcome indicators, including pathological complete response (PCR), clinical complete response (CCR), major pathological response (MPR), and incidence of all adverse effects (TRAEs), which can be extracted or calculated from the original research
6. Study design: randomized controlled trials, case–control studies, cohort studies
Exclusion criteria
1. Studies published as reviews, letters, case reports, and duplicate literature
2. Studies that did not report relevant outcome measures or for which relevant data were not available
3. The study is based on cell or animal experiments
4. Patients with other tumors
Data extraction
Two authors independently carried out the screening and extraction processes. A third reviewer adjudicated any discrepancies or conflicts. For the lack of information, we contacted the original author as much as possible. The following data elements were extracted from the included studies, including the first author, publication year, disease type, the number of patients, gender, age, the clinical stage, study design, types of research, treatment cycle, types of PD-1 inhibitors, median follow-up period, and the outcome measures data. The PCR/CCR/MPR evaluated the efficacy, and the adverse effects measured the safety.
Quality evaluation
The quality of included RCTs was evaluated using the Rob risk of bias quality assessment (12), and non-RCTs were carried out using the Newcastle–Ottawa Scale(NOS) (13).
Statistical analysis
Meta-analyses were conducted using STATA 15.0 software. The PCR, CCR, and MPR and the incidence of TRAEs with their 95% confidence interval (CIs) were evaluated for the studies included in the meta-analysis. Heterogeneity was assessed by the χ² test on Cochrane’s Q statistic and quantified by I² values. I² >50% and a P-value<0.05 indicated significant heterogeneity, and the random-effects model was used. Otherwise, the fixed-effects model was used. We performed subgroup analyses based on the type of experimental design, intervention PD-1 agent, and disease type. Sensitivity analysis was used to evaluate the stability and reliability of the results by the one-by-one elimination method. Funnel plots were generated to assess publication bias, and according to Egger’s test, P>0.05 manifested that there was no publication bias in the study.
Results
Literature search and study characteristics
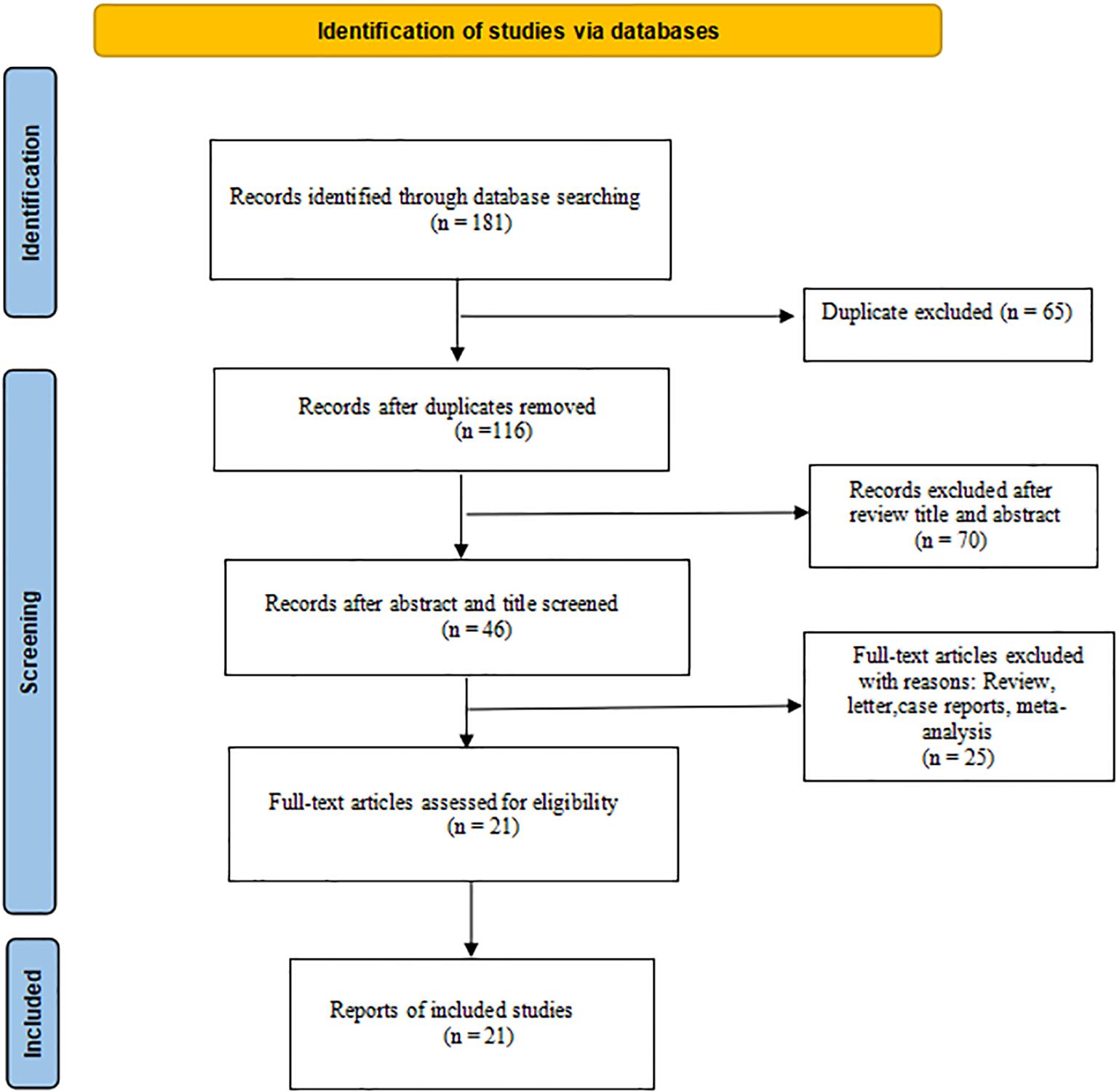
Initially, 181 types of literature were identified through searches of PubMed, Embase, and the Cochrane Library. Following the removal of duplicate studies, a total of 116 unique articles were included in the literature review. Subsequent screening of these articles resulted in 78 papers being assessed for potential inclusion. Further examination of the titles and abstracts led to the identification of 46 relevant records on the topic at hand; finally, 21 studies (7, 9, 14–32), including 803 patients, were identified for eligibility criteria in the survey via full-text review. The flowchart of the literature search procession is reported in Figure 1.
We incorporated studies spanning a 3-year period from 2021 to 2023. Within the selected literature, there were 17 cases of RC, encompassing 619 patients, and 4 cases of CRC, involving 184 patients. The reviewed studies comprised 12 prospective clinical trials and eight retrospective studies. The PD-1 inhibitors documented in the literature encompassed sintilimab, toripalimab, camrelizumab, dostarlimab, avelumab, pembrolizumab, tislelizumab, nivolumab, vudalimab, and bevacizumab. The detailed information is shown in Table 1.
Risk-of-bias assessment
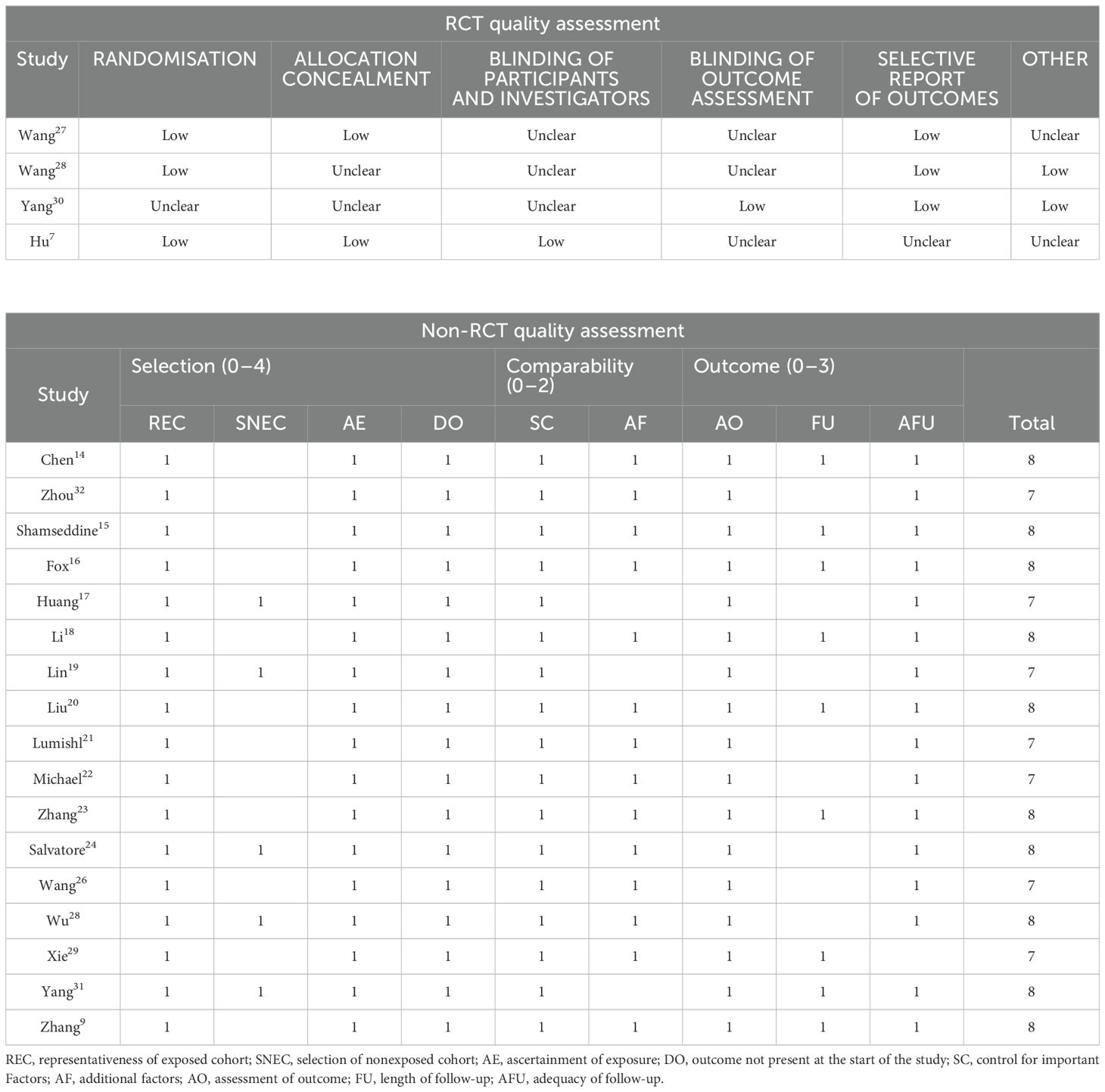
The NOS was employed to evaluate the quality of the included non-RCTs. There were 10 studies that received a score of eight points, whereas seven studies scored seven points, indicating a medium to high quality of the non-RCTs. For RCTs, the ROB tool was utilized for quality assessment in four studies. The most prevalent risks identified included the lack of blinding of participants and investigators, as well as the blinding of outcome assessment.
Table 2 shows detailed information for each study.
Efficacy
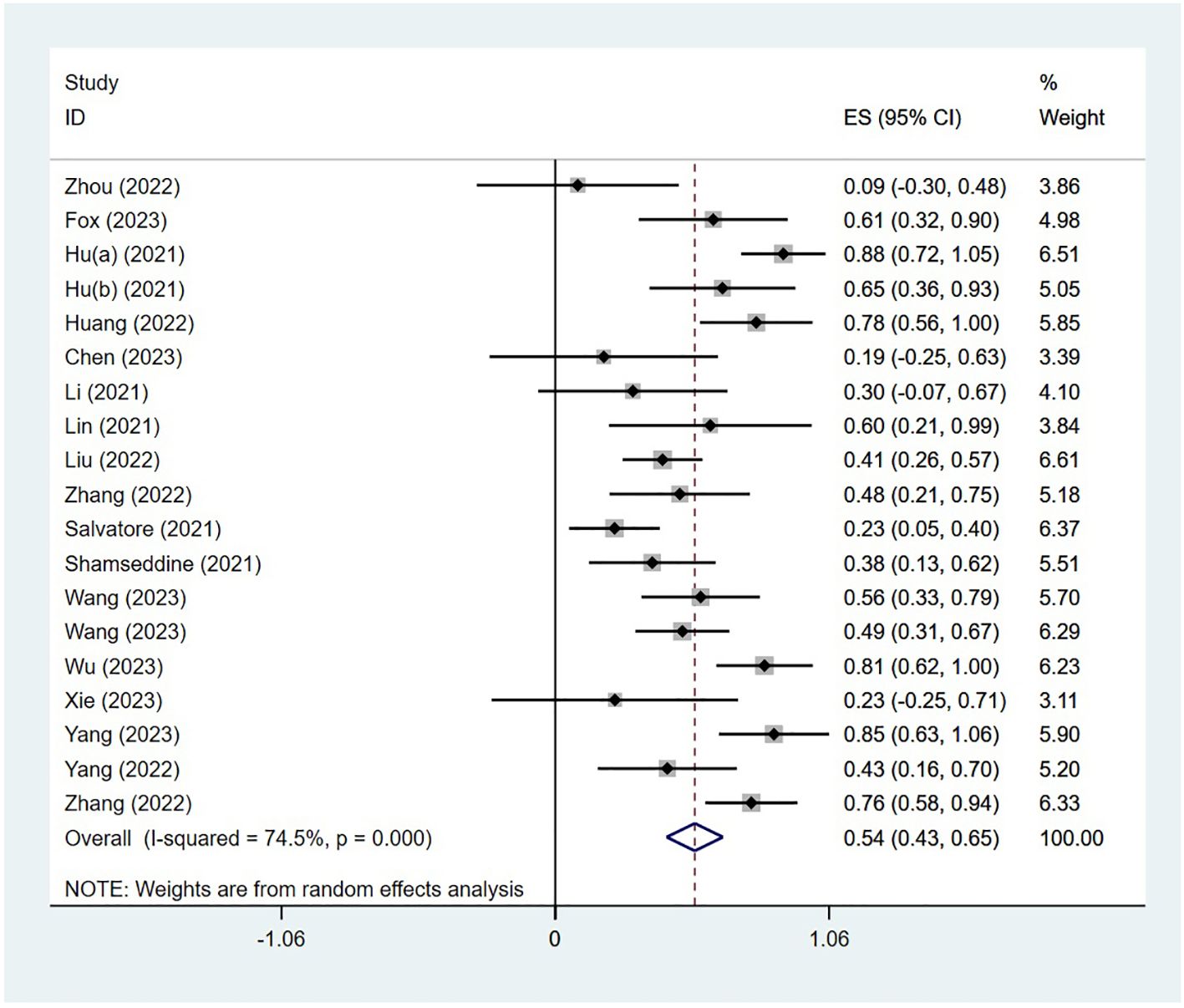
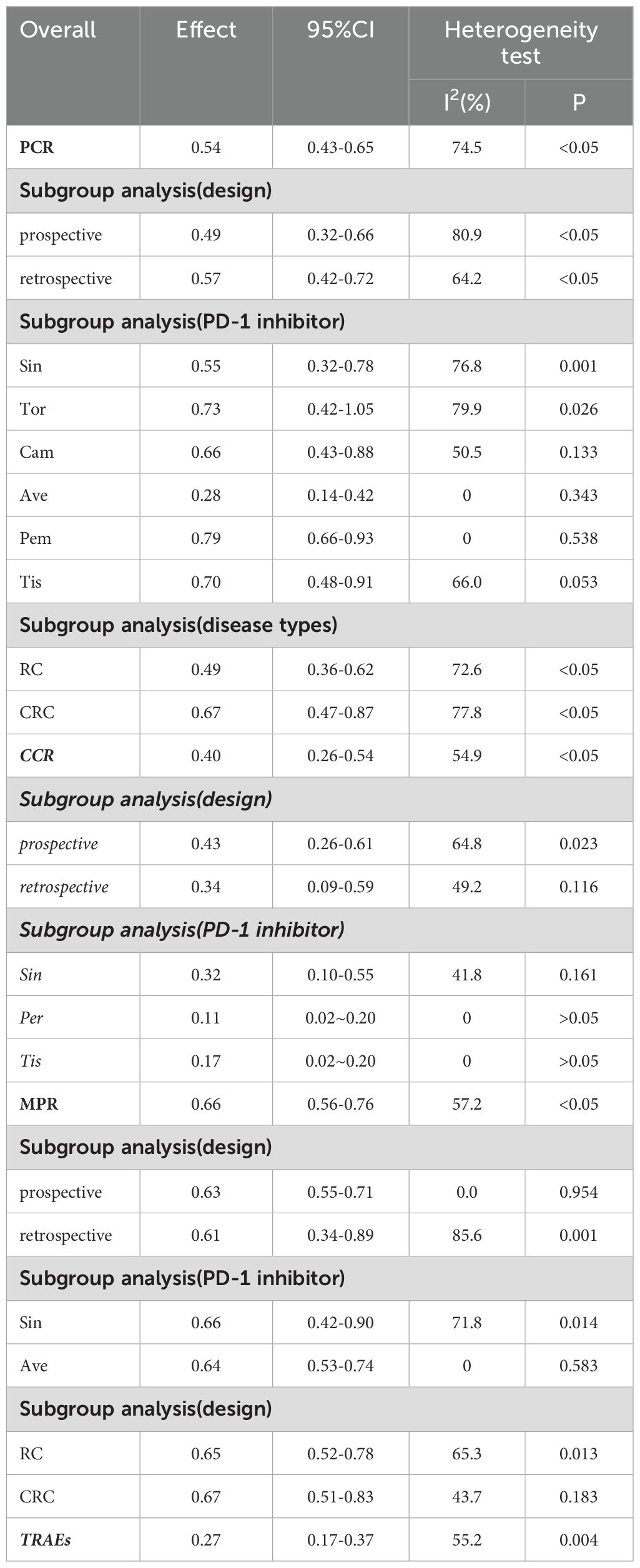
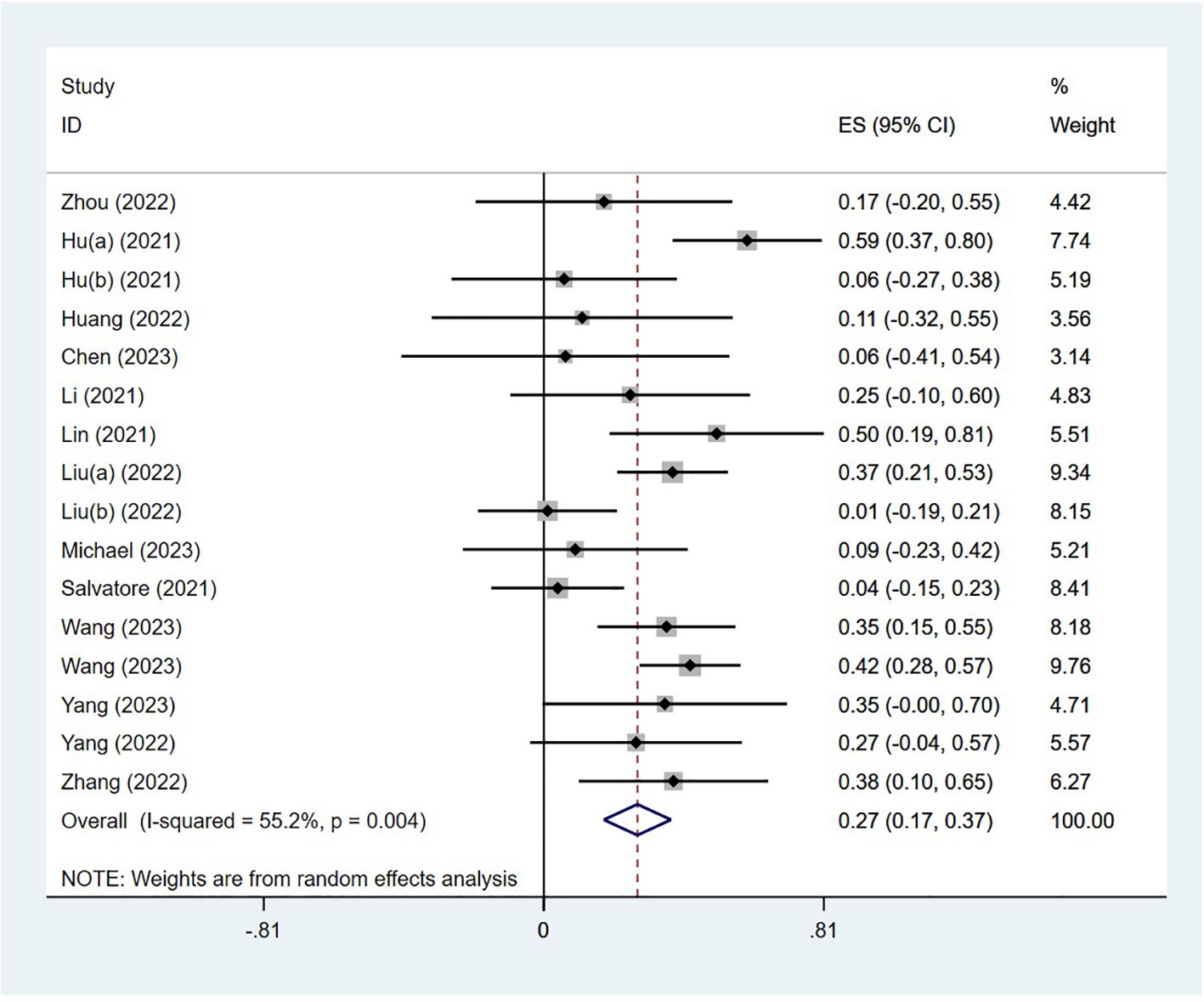
The PCR was reported in 18 studies, and the PCR of PD-1 inhibitors in neoadjuvant treatment of LACRC was 54% (95% CI: 43%–65%, P<0.05). The forest plot of PCR is shown in Figure 2. The CCR was reported in nine studies, and the CCR was 40% (95% CI: 26%–54%, P<0.05). The MPR was reported in eight studies, and the MPR was 66% (95% CI: 56%–76%, P<0.05). The results are shown in Table 3.
Subgroup analysis
We conducted subgroup analysis from three aspects: experimental design, PD-1 inhibitor interventions, and disease types. The identified studies included 12 prospective clinical trials (13–33), and the pooled PCR of PD-1 inhibitors in the prospective clinical trials of LACRC was 49% (95% CI: 32%–66%, P<0.05); the rate of PCR in eight retrospective studies was 57% (95% CI: 42%–72%, P<0.05).
The patients receiving sintilimab had a PCR rate of 55%. The patients who were administered toripalimab as a monotherapy exhibited a PCR of 73%, whereas those receiving camrelizumab demonstrated a PCR rate of 66%. Patients treated with avelumab displayed a PCR of 28%, those receiving Pembrolizumab had a PCR rate of 79%, and individuals administered tislelizumab had a PCR rate of 70%. In contrast, patients receiving pembrolizumab showed a CCR rate of 11%, whereas those treated with tislelizumab had a CCR rate of 17%. Additionally, patients receiving avelumab had an MPR rate of 64%. Among the 803 patients enrolled in the study, 619 (77%) were diagnosed with rectal cancer (RC), exhibiting PCR and MPR rates of 49% and 65%, respectively. The remaining 184 patients (23%) were diagnosed with CRC, with both PCR and MPR rates recorded at 67%. The detailed results of the subgroup analysis are shown in Table 3.
Safety
The adverse effects were reported in 14 studies. The incidence of AEs was 27% (95% CI: 17%–37%, P<0.05). The forest plot of AEs is shown in Figure 3. The occurrence of grade 1–2 adverse events was 35.0% (95% CI: 19.7%–50.3%), whereas the incidence of grade 3–4 adverse events was 20.5% (95% CI: 7.8%–33.2%). The most common treatment-related AEs were mainly gastrointestinal reactions and skin adverse reactions, including fatigue, diarrhea, pruritus, nausea, pyrexia, abdominal pain, decreased appetite, bowel obstruction, hyperthyroidism, increased ALT, increased AST, thrombocytopenia, pneumonia, and neutropenia. Most grade 1–2 adverse effects can be improved through symptomatic treatment, and patients can continue to take medication such as vomiting. Only a small number of grade 3–4 treatment-related AEs have the potential to lead patients to discontinue the drug, indicating that the neoadjuvant regimen involving single-agent PD-1 inhibitors was generally deemed safe.
Disease-free survival and overall survival
One multiple-center, cohort study including 20 patients reported (30) that the 2-year disease-free survival and overall survival in each group was 100%.
Publication bias analysis and sensitivity analysis
The funnel plot was a traditional method used to assess the presence of publication bias in meta-analyses, whereas Egger’s test provides a quantitative evaluation of such bias. In this meta-analysis, the results of Egger’s test (P = 0.152) and Begg’s test (P = 0.274) indicated an absence of publication bias in the included studies. Furthermore, the sensitivity analysis demonstrated that the findings of the study were generally stable. Refer to Figure 4 for further details.
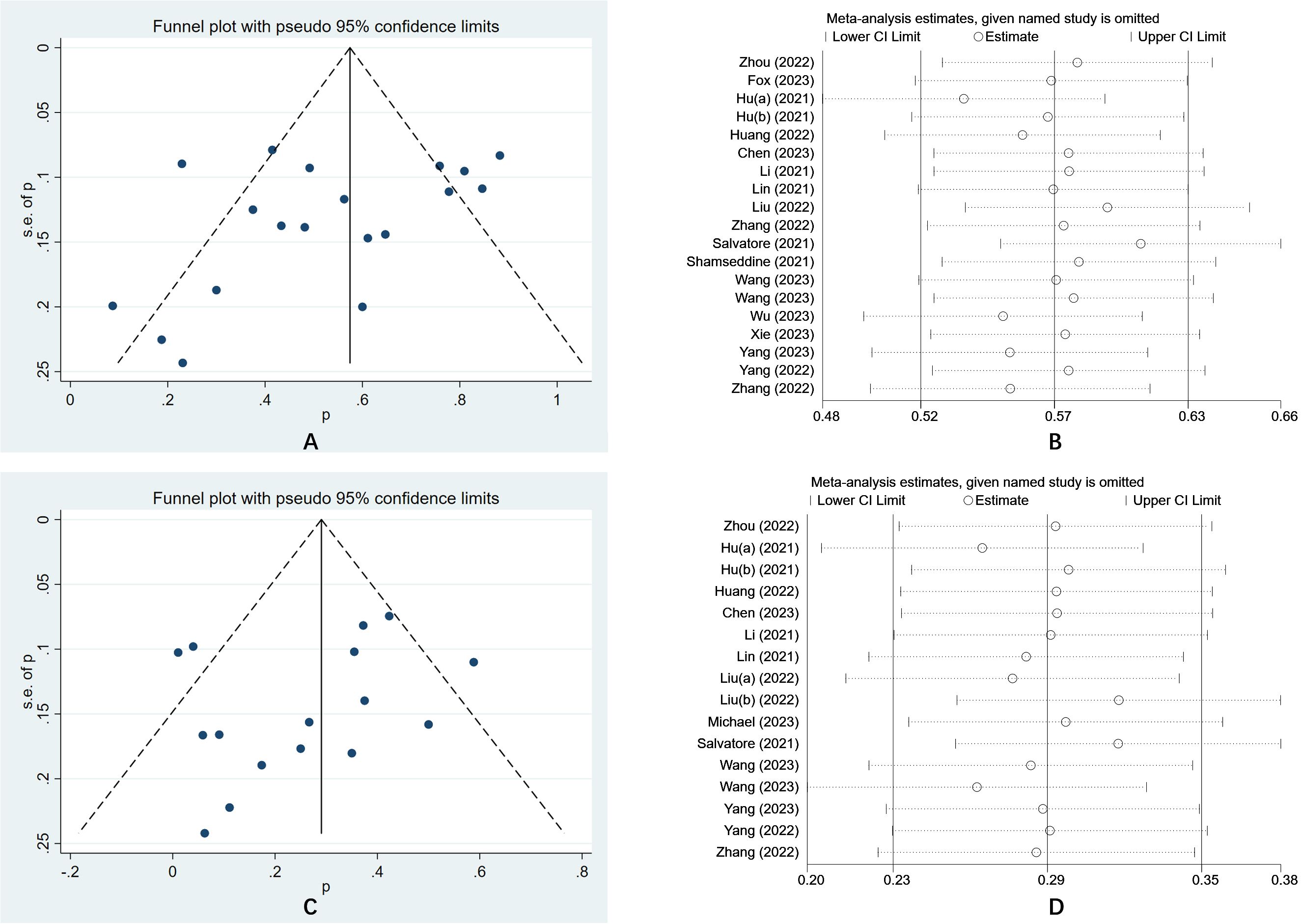
Figure 4. (A) The funnel plot of a meta-analysis of the PCR; (B) The sensitivity analysis of a meta-analysis of the PCR; (C) The funnel plot of a meta-analysis of the irAEs; (D) The funnel plot of a meta-analysis of the irAEs.
Discussion
The objective of our meta-analysis was to examine the efficacy and safety of neoadjuvant monotherapy with PD-1 blockade in individuals diagnosed with LACRC. Efficacy was assessed using PCR, resulting in a 54% PCR, whereas safety was evaluated based on AEs, yielding a 27% AE rate.
Neoadjuvant therapy presents several benefits, including increased radical resection rates, decreased local recurrence rates, reduced tumor regression, and improved quality of life (33). However, the efficacy of neoadjuvant therapy in treating dMMR/MSI-H LACRC remains limited (34). Recently, ICIs targeting the PD-1/PD-L1 pathway have provided a new treatment option for malignant tumors. Neoadjuvant therapy can specifically bind to PD-1 or PD-L1 to block the PD-1/PD-L1 signaling pathway so that T cells can restore the immune response against tumors, thereby increasing the killing of tumor cells (35, 36). PCR was defined as tumors without any viable tumor cells in the resected primary tumor sample and all sampled regional lymph nodes. Recent results of clinical trials indicated that neoadjuvant therapy based on ICIs holds great potential in the treatment of LACRC. However, the current studies were limited by small sample sizes. To obtain more reliable results, we synthesized the published articles using a meta-analysis approach. This meta-analysis conducted in the present study revealed a pooled PCR rate of 54%. Previous studies have reported that the range of PCR rates was very large. For instance, the Ave-rectal trial (22) performed SCRT followed by six cycles of mFOLFOX6 and avelumab and found that 37.5% of the patients achieved PCR. The ANAVA study (37) used six cycles of avelumab from the beginning of nCRT and reached a PCR of 23%. While another study demonstrated a PCR rate of 48.1% (13/27) (19). Furthermore, in the study (NCT04304209) conducted by Gong Chen (14), four cycles of neoadjuvant sintilimab therapy were performed, and the PCR rate was 50% in patients with LACRC. In the PICC study, six cycles of neoadjuvant toripalimab with or without celecoxib resulted in high PCRs (65% and 88%) in patients (38). In reality, the PCR rate of neoadjuvant immunotherapy varies, but through our research, the overall PCR rate was approximately 54%. However, the current sample size was still relatively small, and further verification was needed.
Moreover, because the efficacy of various PD-1 blockade drugs may vary, resulting in differential PCR rates, we performed a subgroup analysis stratified by specific drugs. Within this analysis, patients treated with pembrolizumab, toripalimab, and tislelizumab exhibited higher PCR rates of 79%, 73%, and 70%, respectively. However, the current number of included studies is still relatively small, and further validation is needed. Our research findings indicate that neoadjuvant immunotherapy has yielded benefits for certain patients by enhancing PCR rates. However, a subset of patients did not experience these benefits. Future efforts should focus on refining patient selection criteria to identify those most likely to benefit, thereby advancing the objectives of precision medicine.
Furthermore, we evaluated the safety profile of PD-1 blockade by analyzing AEs and identified an incidence rate of 27%. Previous studies reported that the adverse reactions of neoadjuvant immunotherapy were diverse. For example, the common treatment-emergent AEs of any grade were leukopenia, reactive cutaneous capillary endothelial proliferation, and radiation proctitis (19). The common grade 3–4 treatment-emergent AEs were neutropenia and anemia (19). The ANAVA study (23) incorporated six cycles of avelumab commencing at the initiation of CRT. Among the 96 patients who were eligible for pathological assessment, 22 individuals (23%) attained a PCR, whereas 59 patients (61.5%) exhibited pathological regression. Notably, the incidence of grade 3–4 immune-related toxicities was limited to 4% (37). The incidence of adverse effects observed in the aforementioned clinical trials was comparatively low, particularly concerning immune-related adverse effects, which instilled considerable confidence in the outcomes (26, 37).
However, it is important to note that this study was subject to several limitations. Firstly, the sample sizes of the included studies were relatively small, warranting larger sample sizes randomized controlled studies in this area. Furthermore, there was presently a lack of direct comparisons regarding the efficacy of two distinct neoadjuvant immunotherapy groups, rendering head-to-head comparisons unfeasible. Consequently, this article employed a meta-analysis of rates. We anticipated the publication of additional clinical studies in the future, which would enable us to derive more reliable conclusions through evidence-based medicine. Additionally, the majority of studies were conducted in a single center, highlighting the need for large-scale, multicenter prospective randomized controlled trials to validate the long-term efficacy and safety of the treatment approach. Moreover, variations in neoadjuvant treatments among patients may have influenced the results, underscoring the necessity for the exploration of more effective treatment regimens tailored to individual patients. Furthermore, the majority of the articles had a limited follow-up period and did not provide data on progression-free survival and overall survival. Only one article presented relevant data in this regard. There is a need for future studies to report on long-term survival outcomes.
Conclusion
The initial findings indicated that the neoadjuvant therapy utilizing the PD-1 inhibitor showed promise in terms of effectiveness and safety for LACRC patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YY: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. LH: Formal analysis, Methodology, Software, Validation, Writing – review & editing. RY: Conceptualization, Methodology, Project administration, Resources, Software, Writing – original draft. MJ: Formal analysis, Resources, Software, Writing – review & editing. S-JL: Data curation, Software, Visualization, Writing – review & editing. W-DF: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Lu L, Mullins CS, Schafmayer C, Zeißig S, Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond). (2021) 41:1137–51. doi: 10.1002/cac2.12220
3. Araghi M, Soerjomataram I, Jenkins M, Brierley J, Morris E, Bray F, et al. Global trends in colorectal cancer mortality: projections to the year 2035. Int J Cancer. (2019) 144:2992–3000. doi: 10.1002/ijc.32055
4. Shi JF, Wang L, Ran JC, Wang H, Liu CC, Zhang HZ, et al. Clinical characteristics, medical service utilization, and expenditure for colorectal cancer in china, 2005 to 2014: Overall design and results from a multicenter retrospective epidemiologic survey. Cancer. (2021) 127:1880–93. doi: 10.1002/cncr.33445
5. Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:1139–67. doi: 10.6004/jnccn.2022.0051
6. Wang PH, Yang ST, Liu CH. Neoadjuvant therapy. J Chin Med Assoc. (2023) 86:133–4. doi: 10.1097/JCMA.0000000000000855
7. Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang M, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. (2022) 7:38–48. doi: 10.1016/S2468-1253(21)00348-4
8. Chalabi M, Fanchi LF, Dijkstra KK, Van Den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. (2020) 26:566–76. doi: 10.1038/s41591-020-0805-8
9. Zhang X, Yang R, Wu T, Cai X, Li G, Yu K, et al. Efficacy and safety of neoadjuvant monoimmunotherapy with PD-1 inhibitor for dMMR/MSI⁃H locally advanced colorectal cancer: A single-center real-world study. Front Immunol. (2022) 13:913483. doi: 10.3389/fimmu.2022.913483
10. Wang R, Lian J, Wang X, Pang X, Xu B, Tang S, et al. Survival rate of colorectal cancer in china: A systematic review and meta-analysis. Front Oncol. (2023) 13:1033154. doi: 10.3389/fonc.2023.1033154
11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. (2009) 339:b2700. doi: 10.1136/bmj.b2700
12. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj. (2011) 343:d5928. doi: 10.1136/bmj.d5928
13. The Ottawa Hospital Research Institute, NOS, Wells GA, Shea B, O’Connell D, Peterson J, et al. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2021). Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed May 3, 2021).
14. Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol. (2023) 8:422–31. doi: 10.1016/S2468-1253(22)00439-3
15. Shamseddine A, Zeidan YH, El Husseini Z, Kreidieh M, Al Darazi M, Turfa R, et al. Efficacy and safety-in analysis of short-course radiation followed by mFOLFOX-6 plus avelumab for locally advanced rectal adenocarcinoma. Radiat Oncol. (2020) 15:233. doi: 10.1186/s13014-020-01673-6
16. Fox DA, Bhamidipati D, Konishi T, Kaur H, You N, Raghav KPS, et al. Endoscopic and imaging outcomes of PD-1 therapy in localised dMMR colorectal cancer. Eur J Cancer. (2023) 194:113356. doi: 10.1016/j.ejca.2023.113356
17. Huang J, Pei F, He W, Duan Y, Shi T, Zhao Y, et al. PD-1 blockade boosted chemo-target therapy in cT4NxM0 pMMR/MSS colorectal cancer. J Clin Oncol. (2022) 40(16_suppl):e15606. doi: 10.1200/JCO.2022.40.16_suppl.e15606
18. Li YJ, Zhang L, Dong QS, Cai Y, Zhang YZ, Wang L, et al. Short-term outcome of programmed cell death protein1 (PD-1) antibody combined with total neoadjuvant chemoradiotherapy in the treatment of locally advanced middle-low rectal cancer with high risk factors. Chin J Gastrointestinal Surg. (2021) 24:998–1007. doi: 10.3760/cma.j.cn441530-20210927-00386
19. Lin Z, Cai M, Zhang P, Li G, Liu T, Li X, et al. Single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J Immunother Cancer. (2021) 9:e003554. doi: 10.1136/jitc-2021-003554
20. Liu XZ, Xiong Z, Xiao BY, Yu GY, Li YJ, Yao YF, et al. Multicenter real-world study on safety and efficacy of neoadjuvant therapy in combination with immunotherapy for colorectal cancer. Chin J Gastrointestinal Surg. (2022) 25:219–27. doi: 10.3760/cma.j.cn441530-20220228-00070
21. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair–deficient, locally advanced rectal cancer. N Engl J Med. (2022) 386:2363–76. doi: 10.1056/NEJMoa2201445
22. Michael M, Wong R, Gill SS, Goldstein D, Ngan S, Heriot AG, et al. Phase II trial PD-L1/PD-1 blockade avelumab with chemoradiotherapy for locally advanced resectable T3B-4/N1-2 rectal cancer: The ave-rec trial. J Clin Oncol. (2019) 37. doi: 10.1200/JCO.2019.37.15-suppl.TPS3622
23. Peng Z, Weizhen L, Xin C, Zhenyu L, Ming Y, Lan Z, et al. Short-term efficacy of laparoscopic surgery after short-course radiotherapy followed by sequential chemotherapy combined with anti-PD-1 antibody therapy for locally advanced rectal cancer: a prospective study. Chin J Digestive Surg. (2022) 21:766–72. doi: 10.3760/cma.j.cn115610-20220323-00159
24. Salvatore L, Bensi M, Corallo S, Bergamo F, Pellegrini I, Rasola C, et al. O-12 phase II study of preoperative chemoradiotherapy plus avelumab in patients with locally advanced rectal cancer: The AVANA study. Ann Oncol. (2021) 32:S223. doi: 10.1016/j.annonc.2021.05.016
25. Wang Q, Xiao B, Jiang W, Steele S, Cai J, Pan Z, et al. P-187 watch-and-wait strategy for DNA mismatch repair-deficient/microsatellite instability-high rectal cancer with a clinical complete response after neoadjuvant immunotherapy: An observational cohort study. Ann Oncol. (2021) 32:163–64. doi: 10.1016/j.annonc.2021.05.242
26. Wang Y, Shen L, Wan J, Zhang H, Wu R, Wang J, et al. Short-course radiotherapy based total neoadjuvant therapy combined with PD-1 inhibitor for locally advanced rectal cancer: Preliminary findings of TORCH. BMC Cancer. (2022) 22:274. doi: 10.1186/s12885-022-09348-z
27. Wang Y, Shen L, Wan J, Zhang H, Wu R, Wu X, et al. Short-course radiotherapy combined with CAPOX and toripalimab for the total neoadjuvant therapy of locally advanced rectal cancer: a randomized, prospective, multicentre, double-arm, phase II trial (TORCH). Zhonghua Wei Chang Wai Ke Za Zhi. (2023) 26:448–58. doi: 10.3760/cma.j.cn441530-20230107-00010
28. Wu A, Li Y, Ji D, Zhang L, Zhang X, Cai Y, et al. Total neoadjuvant chemoradiation combined with neoadjuvant PD-1 blockade for patients with pMMR, high-risk, and locally advanced middle to low rectal cancer. J Clin Oncol. (2022) 40. doi: 10.1200/JCO.2022.40.16_suppl.3611
29. Xie Y, Lin J, Zhang N, Wang X, Wang P, Peng S, et al. Prevalent pseudoprogression and pseudoresidue in patients with rectal cancer treated with neoadjuvant immune checkpoint inhibitors. J Natl Compr Canc Netw. (2023) 21:133–42.e3. doi: 10.6004/jnccn.2022.7071
30. Yang R, Wu T, Yu J, Cai X, Li G, Li X, et al. Locally advanced rectal cancer with dMMR/MSI-h may be excused from surgery after neoadjuvant anti-PD-1 monotherapy: a multiple-center, cohort study. Front Immunol. (2023) 14:1182299. doi: 10.3389/fimmu.2023.1182299
31. Yang Z, Zhang X, Zhang J, Gao J, Bai Z, Deng W, et al. Rationale and design of a prospective, multicenter, phase II clinical trial of safety and efficacy evaluation of long course neoadjuvant chemoradiotherapy plus tislelizumab followed by total mesorectal excision for locally advanced rectal cancer (NCRT-PD1-LARC trial). BMC Cancer. (2022) 22:462. doi: 10.1186/s12885-022-09554-9
32. Zhou L, Yu G, Shen Y, Ding H, Zheng K, Wen R, et al. The clinical efficacy and safety of neoadjuvant chemoradiation therapy with immunotherapy for the organ preservation of ultra low rectal cancer: A single arm and open label exploratory study. J Clin Oncol. (2022) 40:e15603. doi: 10.1200/JCO.2022.40.16_suppl.e15603
33. Han K, Tang JH, Liao LE, Jiang W, Sui QQ, Xiao BY, et al. Neoadjuvant immune checkpoint inhibition improves organ preservation in T4bM0 colorectal cancer with mismatch repair deficiency: A retrospective observational study. Dis Colon Rectum. (2023) 66:e996–e1005. doi: 10.1097/dcr.0000000000002466
34. Cercek A, Lumish MA, Sinopoli JC, Weiss JA, Shia J, Stadler ZK, et al. Single agent PD-1 blockade as curative-intent treatment in mismatch repair deficient locally advanced rectal cancer. J Clin Oncol. (2022) 40:LBA5. doi: 10.1200/JCO.2022.40.17_suppl.LBA5
35. Huang MY, Jiang XM, Wang BL, Sun Y, Lu JJ. Combination therapy with PD-1/PD-L1 blockade in non-small cell lung cancer: strategies and mechanisms. Pharmacol Ther. (2021) 219:107694. doi: 10.1016/j.pharmthera.2020.107694
36. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
37. Salvatore L, Bensi M, Corallo S, Bergamo F, Pellegrini I, Rasola C, et al. Phase II study of preoperative chemoradiotherapy 855 plus avelumab in patients with locally advanced rectal cancer: the AVANA study. San 856 Francisco: ASCO GI (2021).
38. Diaz LA, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. (2022) 23:659–70. doi: 10.1016/s1470-2045(22)00197-8
Keywords: programmed cell death protein-1, PD-1, locally advanced colorectal cancer, neoadjuvant therapy, meta-analysis
Citation: Yu Y, Huang L, Yan R, Jiang M, Li S-J and Fan W-D (2024) The efficacy and safety of neoadjuvant treatment with the PD-1 inhibitor for locally advanced colorectal cancer: a meta-analysis. Front. Oncol. 14:1416943. doi: 10.3389/fonc.2024.1416943
Received: 13 April 2024; Accepted: 05 November 2024;
Published: 02 December 2024.
Edited by:
Jimmy Hwang, Atrium Healthcare, United StatesReviewed by:
Vita Golubovskaya, ProMab Biotechnologies, United StatesWenQing Yang, ClinBridge Biotech Co. Ltd, China
Copyright © 2024 Yu, Huang, Yan, Jiang, Li and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang-Dong Fan, WTE3NzQ4NDk1NTk2QDE2My5jb20=
 Yan Yu
Yan Yu Lin Huang1,2
Lin Huang1,2