
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 11 July 2024
Sec. Molecular and Cellular Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1416241
 Yong Li1,2,3*
Yong Li1,2,3* Jinpeng Huang1,2,3
Jinpeng Huang1,2,3 Xian Chen1,2
Xian Chen1,2 Yongsong Ye4
Yongsong Ye4 Xiaohua Du5
Xiaohua Du5 Ioannis A. Voutsadakis6,7
Ioannis A. Voutsadakis6,7 Mahesh Seetharam8
Mahesh Seetharam8 Haibo Zhang1,2
Haibo Zhang1,2 Min Lu1,2,3*
Min Lu1,2,3*Background: Undifferentiated small round-cell sarcomas (uSRCSs) are a subgroup of sarcomas that are difficult to diagnose. Some uSRCSs have specific gene re-arrangements, but others do not. Currently, there is no specific treatments for advanced uSRCSs, and its treatment is largely based on general experience with sarcomas, which includes chemotherapy, targeted therapy, and immunotherapy. In this article, we report a patient with uSRCS who responded to treatment with anti-VEGF inhibitor surufatinib and anti-PD-1 inhibitor camrelizumab after progression on first-line chemotherapy, second-line anlotinib combined with immunotherapy, and third-line chemotherapy.
Case description: In July 2020, a 37-year-old female patient was diagnosed with advanced uSRCS. Results for the Ewing sarcoma RNA binding protein 1 and Wilms tumor suppressor (EWSR1/WT1) fusion gene were negative. The patient was also negative with BCOR (BCL6 co-repressor) and CIC (capicua transcriptional repressor) fusion gene. The next-generation sequencing results revealed point mutations on Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Beta (PIK3CB), Transcription Factor Binding To IGHM Enhancer 3 (TFE3), Mucin 16 (MUC16), and AXL (Axl, also called UFO, ARK, and Tyro7, is part of a family of receptor tyrosine kinases). The patient received 4 cycles of the Ifosfamide and epirubicin hydrochloride regimen, and her best objective response was stable disease. On November 3, 2020, a computed tomography (CT) scan revealed progressive disease (PD). Two cycles of camrelizumab (a programmed death-1 inhibitor) plus anlotinib (an anti- vascular endothelial growth factor drug) were administered, but PD was again observed. Thus, a regimen of gemcitabine plus docetaxel was adopted. Unfortunately, the disease progressed once again after two cycles of the treatment. On February 4, 2021, the patient began to receive targeted therapy with surufatinib combined with camrelizumab. A CT scan showed that the tumor achieved a partial response. As of April 2023, the patient had a progression-free survival time of 26 months.
Conclusions: Surufatinib in combination with camrelizumab could be effective in the treatment of advanced uSRCSs.
Undifferentiated small round-cell sarcomas (uSRCSs) are a subgroup of sarcomas and are defined by the World Health Organization (WHO, 2020) as soft tissue and bone tumors (1). USRCSs are difficult to identify because of their undifferentiated round cytomorphology and scant stromal changes, and are mostly high-grade tumors. Ewing sarcoma RNA binding protein 1 (EWSR1) gene fusion, Capicua transcriptional repressor gene(CIC)-rearranged genes, and BCL6 corepressor (BCOR)-rearranged genes are usually tested to clarify their genetic types, which include Ewing sarcomas, round-cell sarcomas with EWSR1 gene fusion and non-ETS fusion, CIC-rearranged sarcomas, and BCOR-rearranged sarcomas. However, some uSRCS do not show specific gene fusions.
Advanced uSRCSs comprise a heterogeneous group of highly aggressive tumors associated with a poor prognosis, especially in metastatic disease which are treated according to the conventional recommended regimens for sarcomas, which include chemotherapy using anthracyclines, alkylating agents, topoisomerase inhibitors, gemcitabine and docetaxel (2, 3). Novel targeted drugs have been approved, including pazopanib, regorafenib, and anlotinib (which was approved in China) for second or later line therapies (4–6). Although it has not yet been approved for this indication, immunotherapy has shown promise in some subtypes of sarcoma (7). However, currently there is a paucity of efficacious regimens after the third-line treatment.
Surufatinib is a multi-target drug that has the potential to reverse resistance to the dominant single anti-VEGF anlotinib. In this article, we report a case of a patient with uSRCS who responded to treatment with surufatinib and camrelizumab after resistance to first-line chemotherapy, second-line anlotinib combined with immunotherapy, and third-line chemotherapy. It is the first case report in which surufatinib was shown to be effective in treating uSRCS. Based on our results, we suggested that surufatinib could be effective in the treatment of some advanced uSRCSs in combination with immunotherapy.
In July 2020, a 37 years-old woman with no relevant past medical history, presented with left lumbar spine pain accompanied by mobility limitations including local edema and claudication for more than half a year, worsening over the last 2 months with analgesics. A positron emission tomography–computed tomography (PET-CT) scan was performed and showed multiple enlarged retroperitoneal lymph nodes and multiple masses in the vicinity of the left ischial tuberosity, left pubic bone, and left iliac acetabulum. Magnetic resonance imaging (MRI) showed left acetabular bone destruction which were considered bone metastases. A left inguinal lymph node biopsy was performed on July 15 2020. The pathological diagnosis was uSRCS and the immunohistochemical results were as follows: Vimentin (Vim) (+++), B-cell lymphoma 2(Bcl-2) (++), Desmin (+), Neuron-specific enolase (NSE) (++), Cluster of Differentiation 56(CD56) (+++), Synaptophysin (Syn) (+), Chromogranin A (CgA) (–), Cytokeratin AE1/AE3(CK AE1/AE3) (+), CK8 (positive in few cells), Cytokeratin 18(CK18) (positive in few cells), Myogenic differentiation 1(MyoD1) (positive in few cells), Myogenin (–), S100 protein (S100) (–), Human Melanoma Black 45(HMB45) (–), Melan-A (–), Cluster of Differentiation 20(CD20) (–), Cluster of Differentiation 3 (CD3) (–), Cluster of Differentiation 21(CD21) (–), P40 protein (P40) (–), Protein 16(P16) (–), Epithelial membrane antigen(EMA) (–), Thyroid transcription factor 1(TTF-1) (–), Cluster of Differentiation 99(CD99) (–), Friend leukemia integration 1(Fli-1) (–), Cluster of Differentiation 30(CD30) (–), Integrase interactor 1(INI-1) (+), and Ki67 antigen (Ki67) (90%+) (Figure 1). The Epstein-Barr virus-encoded small RNA (EBER) in-situ hybridization result was negative. PD-L1 staining was negative. A diagnosis of desmoplastic small round-cell tumor was excluded because the fluorescence in-situ hybridization assay result for the EWSR1/WT1 fusion gene was negative. The next-generation sequencing (NGS) results revealed PIK3CB, TFE3, MUC16 and AXL point mutations, but no other mutations were found. NGS of RNAseq fusion genes, including BCOR and CIC fusion gene, were negative (Figure 1).
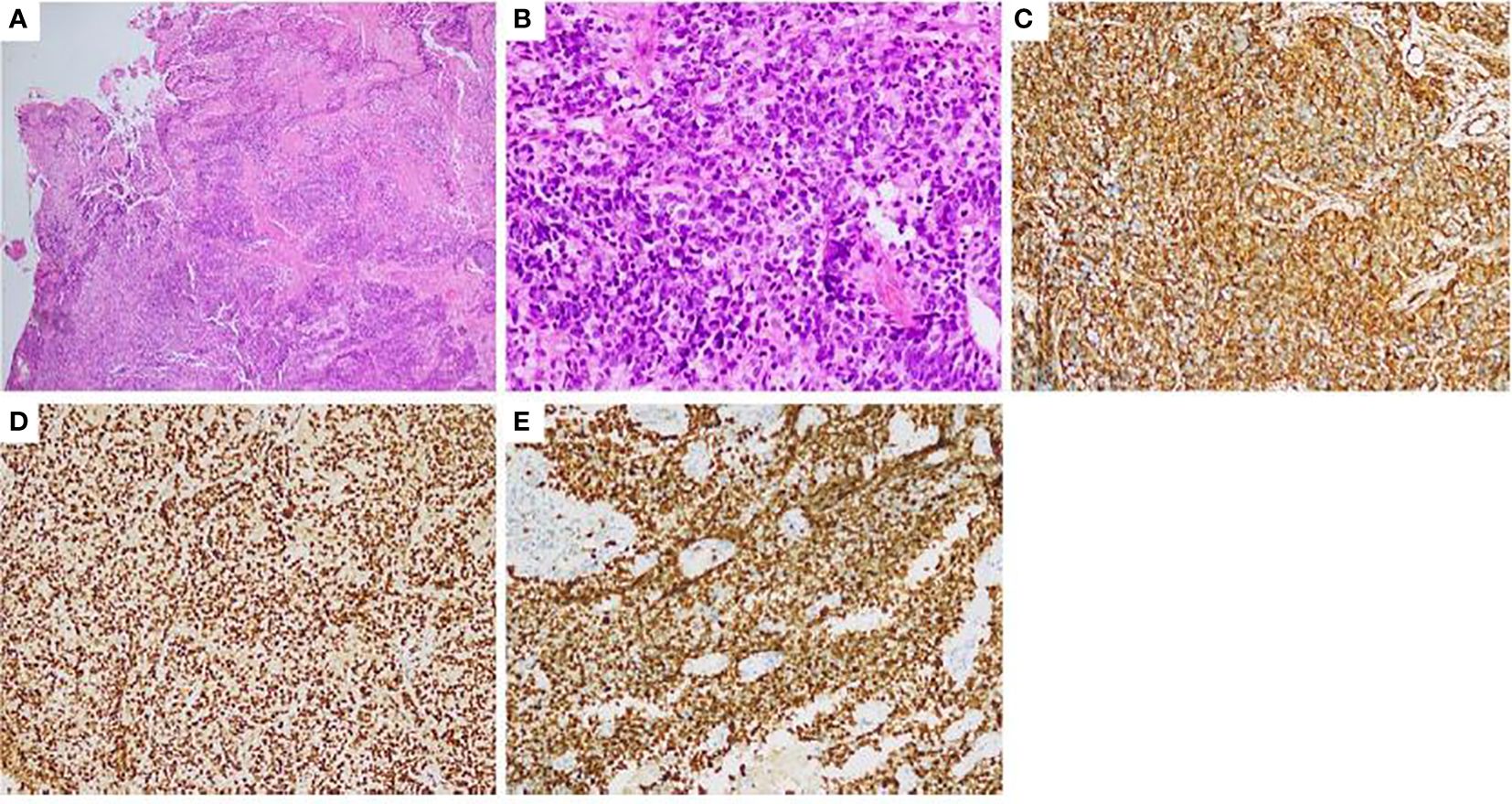
Figure 1 Pathology and immunohistochemistry of a left inguinal lymph node biopsy (A) HE 40x:the tumors appear to be arranged in sheets or nests; (B) HE 400x:The cytoplasm of tumor cells is sparse, the cell boundary is unclear. The round or oval nuclei and nuclear hyperchromatism and fission can be seen. immunohistochemical staining: (C) Vimentin: Diffuse cytoplasmic positive of tumor cells (HE 400x.); (D) INI-1: Nucleus positive (HE 400x.); (E) Ki67: It shows high proliferation of the tumor (400x.). HE, Hematoxylin-eosin stain.
The tumor stage was T4N1M1 with bone metastasis. The patient received 4 cycles of the IA regimen (ifosfamide 2g/m2, and epirubicin hydrochloride 30mg/m2 d1-d3) from August 5 to October 13, 2020. In September 2020, a CT examination revealed tumor shrinkage, which was consistent with stable disease. The pain, edema and claudication were relieved. On November 3, 2020, a CT scan revealed tumor regrowth. On November 4, 2020, the patient started 2 cycles of camrelizumab (a programmed death-1 inhibitor,200mg,q3w) plus anlotinib [an anti-vascular endothelial growth factor (VEGF) drug, 12mg,qd,2 weeks on/1 week off], but her disease progressed. Thus, a regimen of gemcitabine (900mg/m2, d1,d8,q3w) plus docetaxel (75mg/m2,d1,q3w) was administered on December 24, 2020 and January 14, 2021, but the tumor further progressed. Considering that the patient has already received third-line treatment and there is no standard treatment regimen, this patient can enter a clinical study or receive a new treatment. On February 4, 2021, after providing informed consent for experimental therapy, the patient began another line of targeted treatment with surufatinib(300mg,qd.po) combined with camrelizumab(200mg,q3w). A CT scan performed on December 24, 2021 showed a partial response. As of April 2023, the patient had a progression-free survival time of 26 months based on the last examination (Figures 2–11). No specific adverse effects were noted.
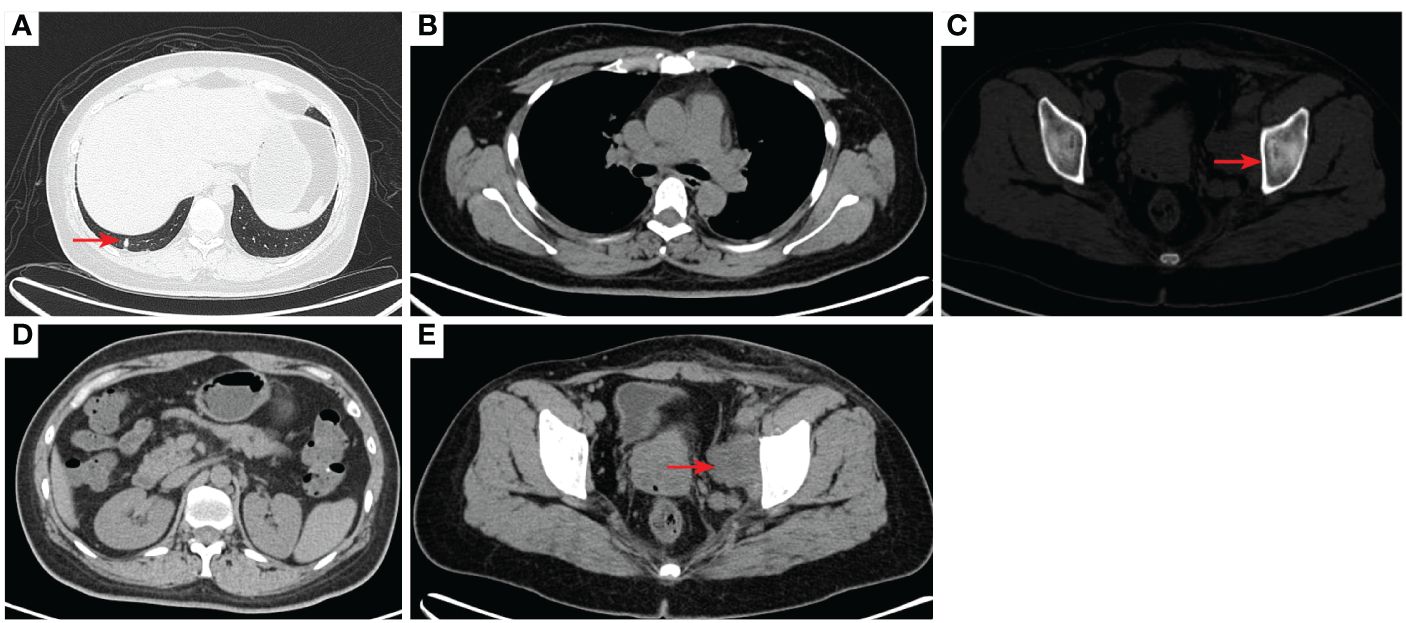
Figure 2 2020–7-10 CT scan: the metastatic tumor in the right lower lobe of the lung and the long diameter was 0.69 cm [red arrow, (A)]. Mediastinal lymph nodes were not significantly enlarged (B). Left acetabular metastases [red arrow, (C)]. Abdominal and abdominal lymph nodes were not significantly enlarged (D) No enlarged lymph nodes were found in the right hilum and the retroperitoneal area. The left pelvic lymph nodes were found and the short diameter was 4.05cm [red arrow, (E)].
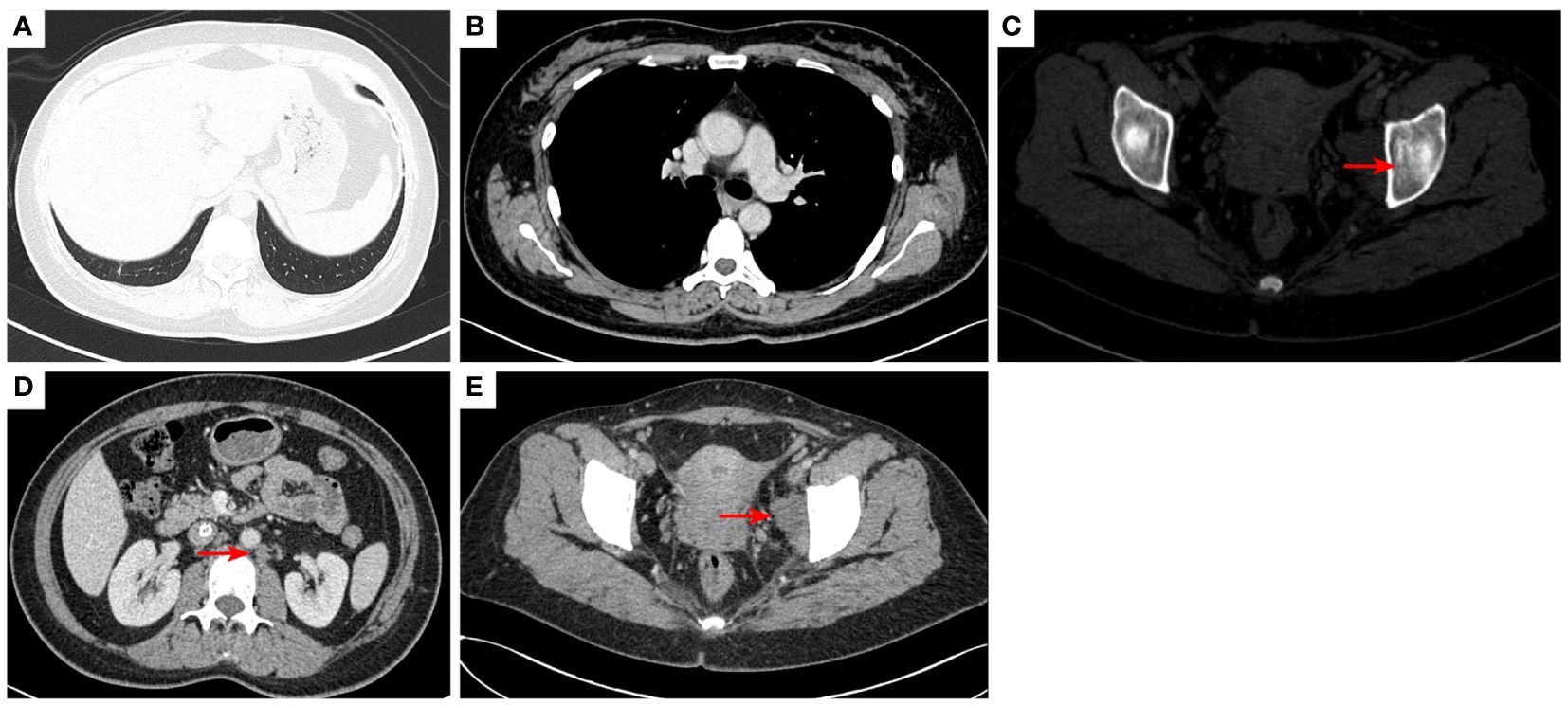
Figure 3 2020–9-16 CT scan: The original metastatic tumor in the right lower lobe of the lung disappeared (A); no enlarged lymph nodes were found in the right hilum (B). Left acetabular metastases [red arrow, (C)]. The short diameter of retroperitoneal lymph nodes was 0.91 cm which was a non-confirmed metastases [red arrow, (D)], and the short diameter of left pelvic lymph nodes was 2.98 cm [red arrow, (E)]. The efficacy was SD. SD, stable disease.
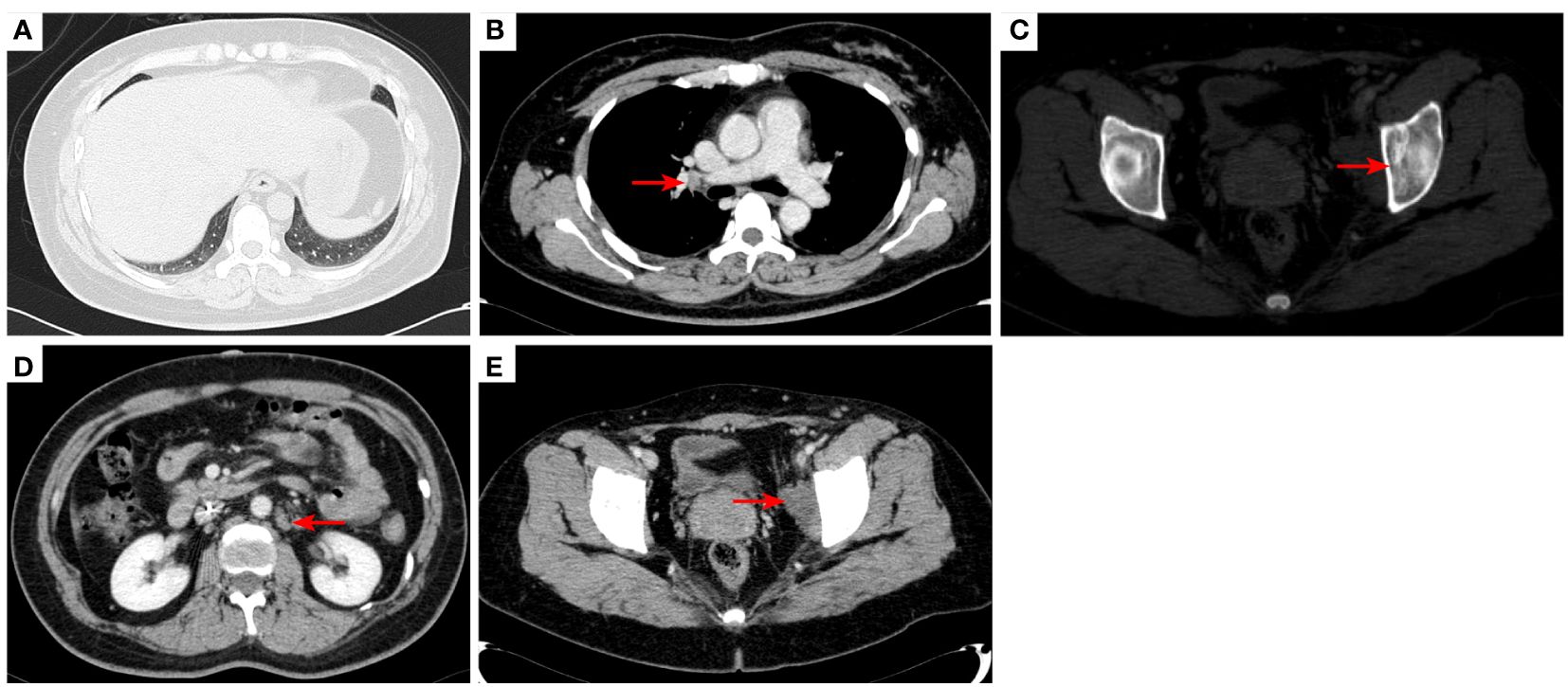
Figure 4 2020–11-3 CT scan: The original right lower lobe of the lung metastatic tumor disappeared (A); short diameter of new right hilar lymph node was 0.83 cm [red arrow, (B)]; Left acetabular metastases (red arrow, (C)). The short diameter of retroperitoneal lymph nodes was 1.01 cm which was bigger than before [red arrow, (D)], and the short diameter of left pelvic lymph nodes was 2.76 cm [red arrow, (E)]. The efficacy was PD. PD, progressive disease.
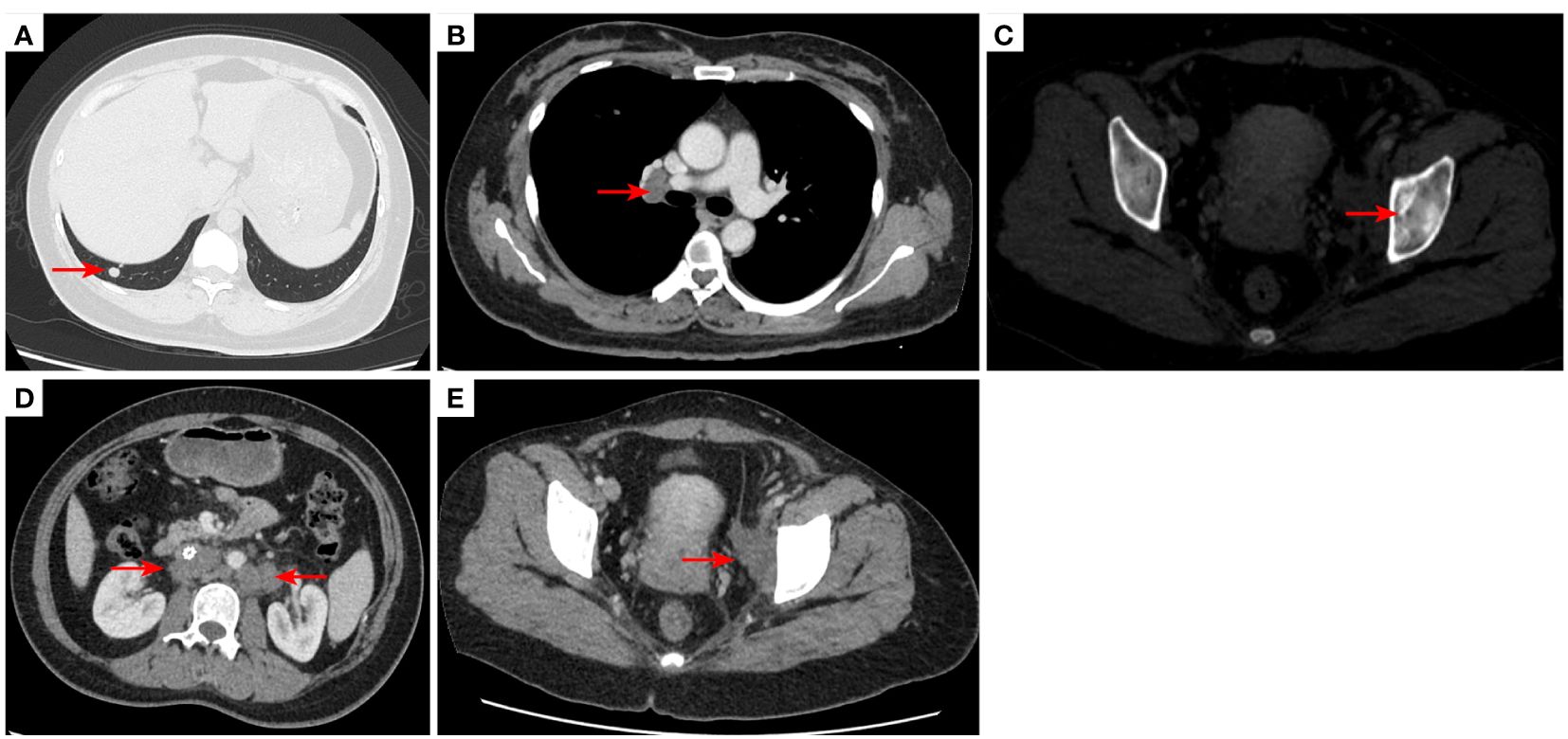
Figure 5 2020–12-22 CT scan: The right lower lobe of the lung metastatic tumor reappeared [red arrow, (A)] and the long diameter was 0.91 cm short diameter of right hilar lymph node was 1.66 cm [red arrow, (B)]; Left acetabular metastases [red arrow, (C)]. the short diameter of retroperitoneal lymph nodes was 1.66 cm, and the short diameter of left pelvic lymph nodes was 3.45 cm [red arrow, (D, E)]. The efficacy was PD. PD, progressive disease.
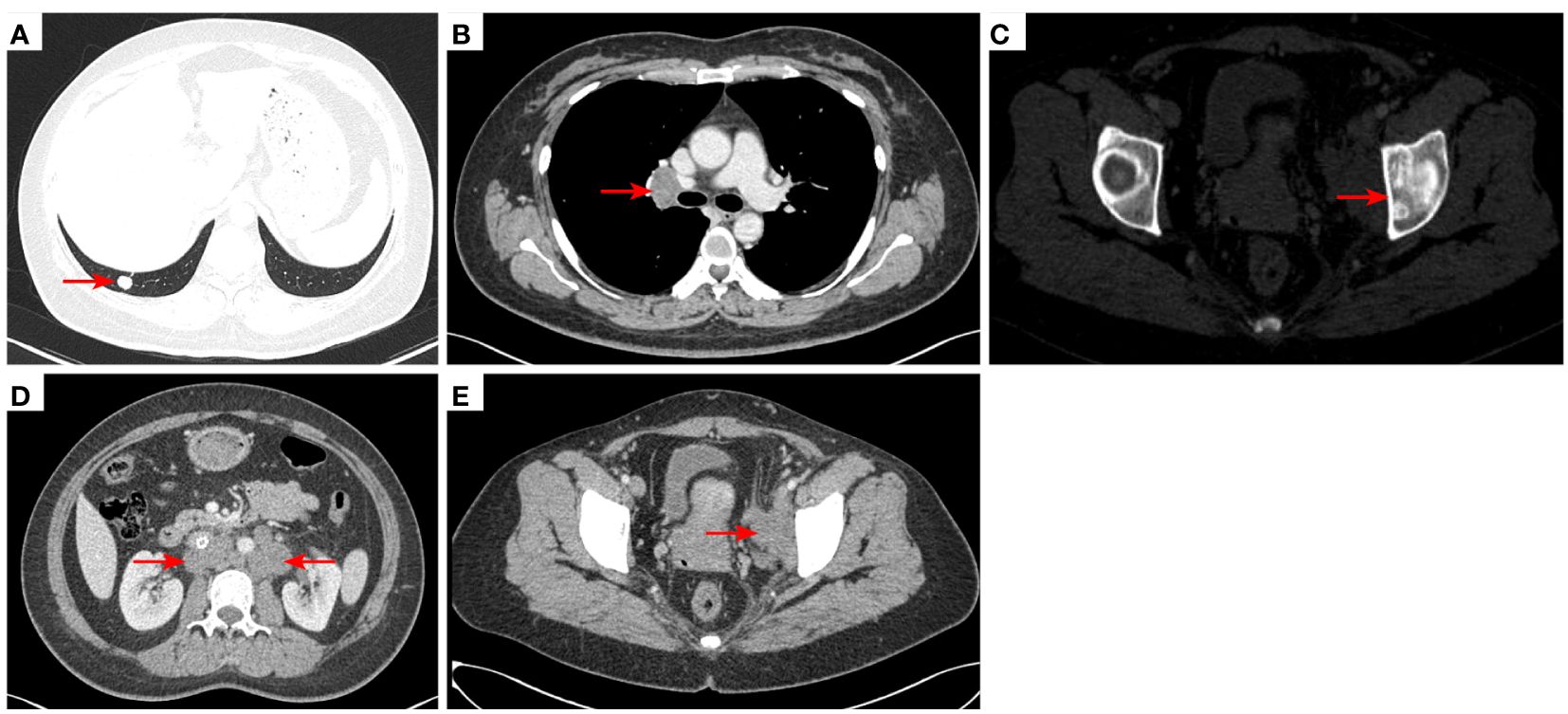
Figure 6 2021–2-3 CT scan: The right lower lung metastatic tumor was 1.28 cm [red arrow, (A)]; short diameter of right hilar lymph node 2.15 cm [red arrow, (B)]. Left acetabular metastases [red arrow, (C)]. The number of retroperitoneal lymph nodes increased and the short diameter was 1.61 cm [red arrow, (D)], and the left pelvic lymph nodes were 4.02 cm [red arrow, (E)]. The efficacy was PD. PD, progressive disease.
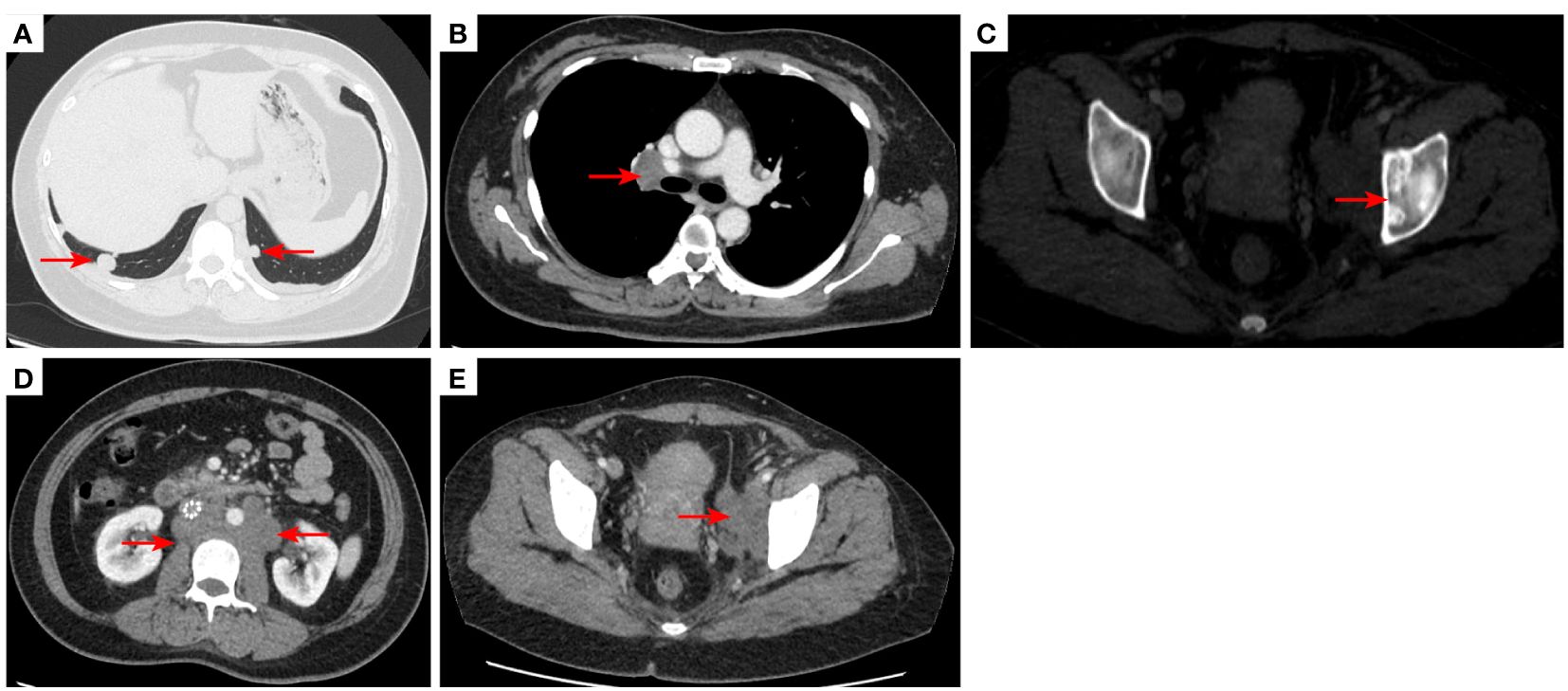
Figure 7 2021–3-9 CT scan: The number of lung metastases increased, and the length of the metastatic tumor in the right lower lobe of lung was 1.62 cm [red arrow, (A)], which was enlarged; short diameter of right hilar lymph node was 2.10 cm [red arrow, (B)]; Left acetabular metastases (C). the short diameter of retroperitoneal lymph nodes was 2.00 cm, and the short diameter of left pelvic lymph nodes was 4.14 cm [red arrow, (D, E)]. The efficacy was unconfirmed progressive disease (iUPD) according irRECIST. irRECIST, immune-related response evaluation criteria in solid tumors.
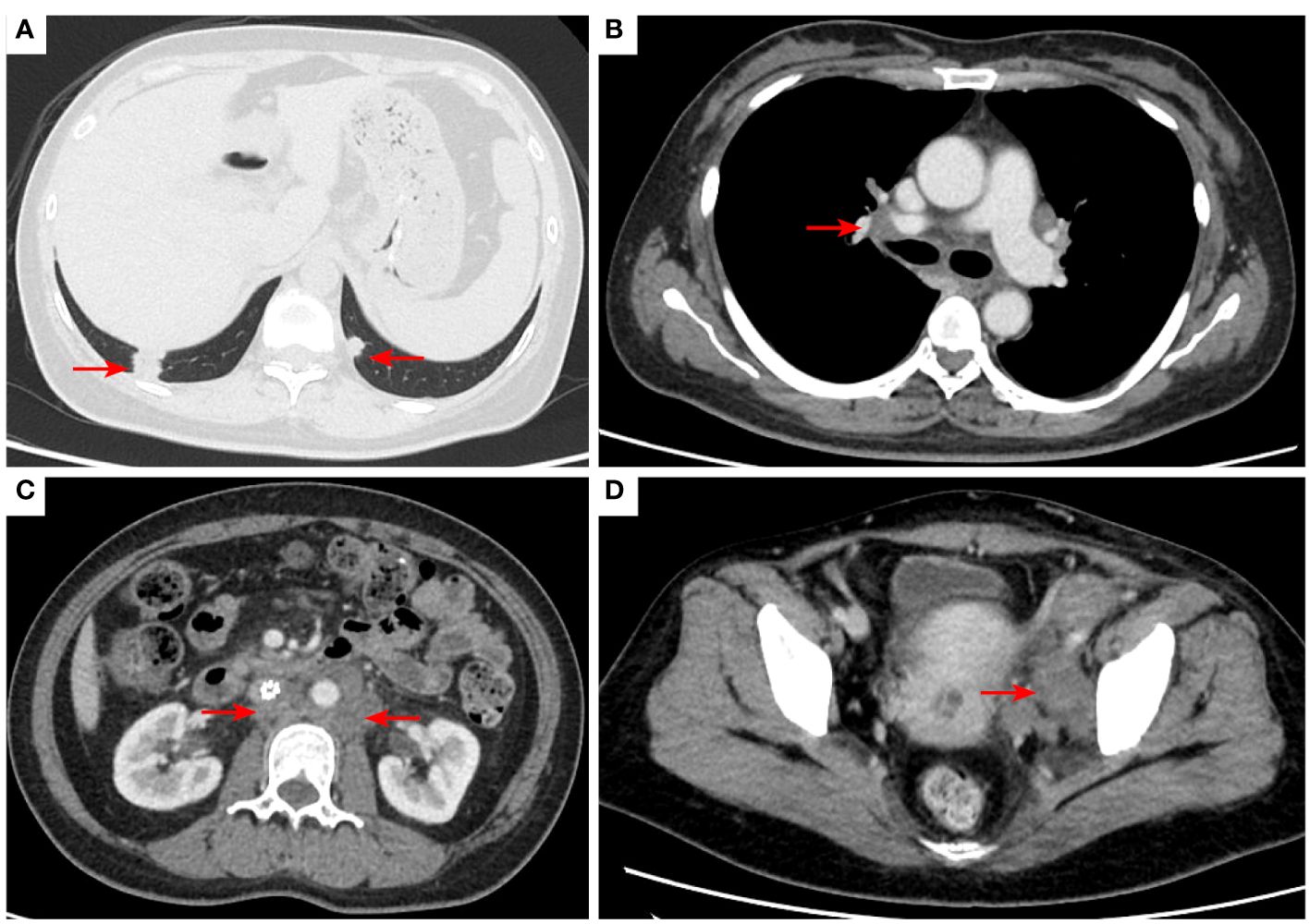
Figure 8 2021–8-24: Length of metastatic tumor in lower right lung 1.59 cm [red arrow, (A)]; short diameter of right hilar lymph node 1.53 cm [red arrow, (B)]; the short diameter of retroperitoneal lymph nodes was 1.61 cm, and the short diameter of left pelvic lymph nodes was 3.11 cm [red arrow, (C, D)]. The acetabulum was not scanned. The efficacy was SD. SD, stable disease.
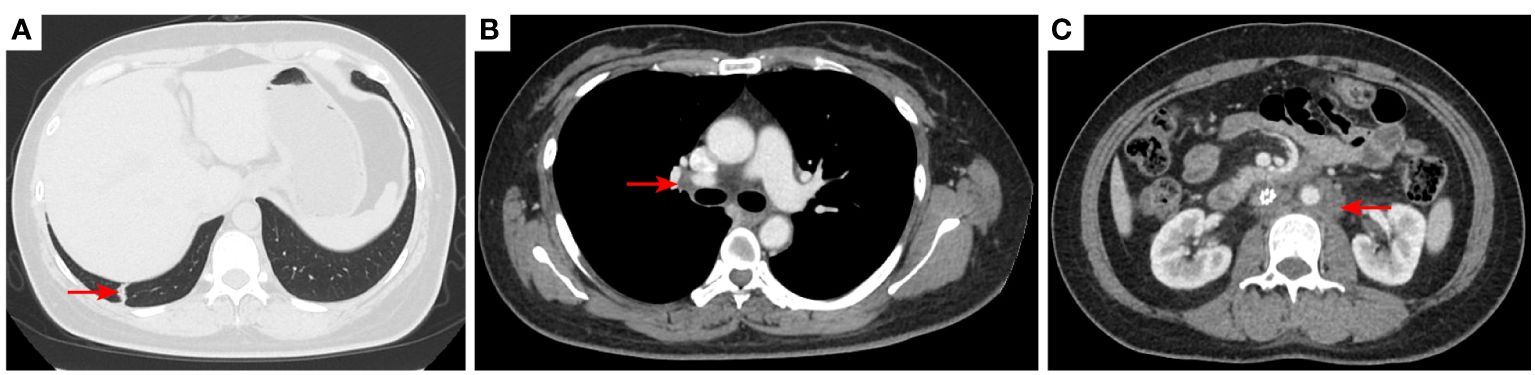
Figure 9 2021–12-15: Length of metastatic tumor in lower lobe of lung was 1.13 cm [red arrow, (A)]; short diameter of right hilar lymph node 1.26 cm [red arrow, (B)]; the short diameter of retroperitoneal lymph nodes was 1.56 cm [red arrow, (C)], and the pelvic cavity was not scanned. The efficacy was PR. PR, partial response.
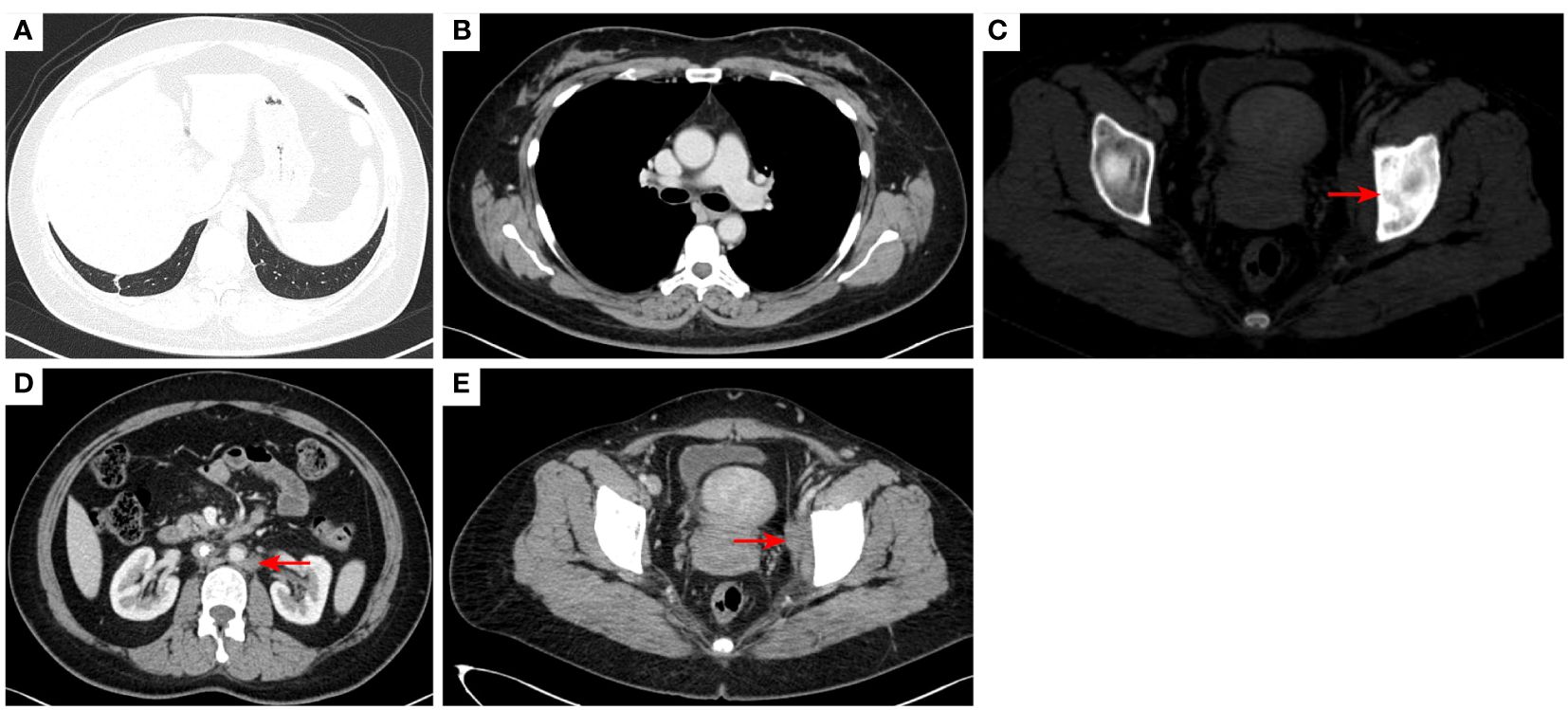
Figure 10 2023–4-13: The original right lower lobe of lung metastatic tumor disappeared (A); The original right hilar enlargement lymph node disappeared (B); Left acetabular metastases [red arrow, (C)]. The short diameter of retroperitoneal lymph nodes was 0.89cm [red arrow, (D)], and the short diameter of left pelvic lymph nodes was 2.13cm [red arrow, (E)].The efficacy was PR. PR, Partial response; SD, Stable disease; PD, Progressive disease; irRECIST, immune-related response evaluation criteria in solid tumors.
Soft-tissue sarcomas comprise a broad range of tumors. Undifferentiated round-cell sarcomas are listed separately by the WHO (2020), and are high-grade and aggressive sarcomas with a poor prognosis. Using traditional immunohistochemical ancillary techniques, about 5% of all sarcomas remain unclassified (8). In this case, the patient was diagnosed with uSRCS by morphology, immunohistochemistry, NGS, and EWSR1/WT1 fusion gene studies. The morphology on pathological examination identified small round cells with atypical immunohistochemistry. As both CD99 and EWSR1-WT-1 fusion genes were negative, the diagnoses of desmoplastic small round-cell tumor and Ewing sarcoma were excluded. The current diagnostic criteria requires testing as many fusion genes as possible by RNA-sequencing assays or other fusion gene methods for classification (9). Both NGS of RNAseq testing on fusion gene were negative in our case.
Under current guidelines, the treatments of advanced sarcomas include chemotherapy and targeted therapies, but no specific treatment strategy is detailed for uSRCSs (10). The usual chemotherapy regimens include ifosfamide/anthracyclines-based regimens, taxanes, or gemcitabine-containing treatments (2, 11). Targeted therapy for sarcomas is largely dominated by small-molecule, multi-target, anti-vascular drugs, including pazopanib and anlotinib, which have been approved for the treatment of different types of sarcomas (5, 6). Small round-cell sarcomas are heterogeneous, and some are resistant, while other are sensitive to chemotherapy (12). In this case, the patient was resistant to both chemotherapy regimens used.
Anti-angiogenic multi-kinase inhibitors can be used in patients with chemo-resistant tumors. Due to the low incidence rate of uSRCSs, few patients with uSRCS have been included in clinical trials (13). Anlotinib is a novel tyrosine kinase inhibitor that targets multiple receptors and inhibits VEGF/vascular endothelial growth factor receptor (VEGFR) signaling by selectively targeting VEGFR-2, and -3 and fibroblast growth factor receptor (FGFR)-1, -2, -3, and -4 (11). Anlotinib was approved for the treatment of different types of sarcomas, but there were no uSRCSs in the clinical trial for the drug (14).
Surufatinib (Sulanda® in China) is an oral, small-molecule, anti-VEGFR kinase inhibitor that targets VEGFR-1, -2, and -3, FGFR-1, and colony-stimulating factor-1 receptor (CSF-1R) (15) and was approved in China for the treatment of pancreatic and non-pancreatic neuroendocrine tumors (16). Soft-tissue sarcomas have a “cold” immune phenotype which is used to refer to inflamed but non T cell-infiltrated, or non-inflamed tumors, reflecting well the lower immunoscore categories (17). The use of immune checkpoint blockade in combination with anti-angiogenic drugs has a synergistic effect in cancer treatment and may overcome resistance to checkpoint inhibitors (18).
CSF-1R blockade by surufutinib may provide a therapeutic advantage compared with other multi-kinase inhibitors that do not block this receptor, as CSF-1 is associated with immune microenvironment (19). Macrophage infiltration has been identified as an independent poor prognostic factor in several cancer types. CSF-1 is a major survival factor for tumor infiltrating macrophages. In solid tumors, such as those of the breast and pancreas, infiltrating Cluster of differentiation 68 (CD68)+ or Cluster of differentiation 163 (CD163)+ tumor-associated macrophages are correlated with poor outcomes and immunosuppression (20).
Studies have reported a synergistic effect with anti-CSF1 and immunotherapy (19). The dual action of surufatinib in targeting tumor angiogenesis and CSF-1R might enhance its antitumor activity, while also making it suitable for use in combination with immune checkpoint inhibitors against various types of cancers. The synergistic effect between immune checkpoint blockades and anti-CSF-1 therapy may explain why the combination of surufatinib and immunotherapy was more effective than anlotinib plus immunotherapy in this patient. Other possible explanations for the effectiveness of surufatinib might include pharmacokinetics and pharmacodynamic potency in the inhibition of targeted kinases. This could result in the drug being effective in patients who are resistant to anlotinib (21).
No data related to sarcomas and uSRCS have been reported for surufatinib. This case report suggests that surufatinib in combination with checkpoint immunotherapy could be effective for uSRCS. The result of this study indicates that surufatinib should be explored for the treatment of sarcomas in prospective studies.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The studies involving humans were approved by Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YL: Writing – original draft, Writing – review & editing. JH: Data curation, Writing – review & editing. XC: Investigation, Data curation, Writing – review & editing. YY: Investigation, Writing – original draft. XD: Investigation, Writing – review & editing. IV: Writing – review & editing. MS: Writing – review & editing. HZ: Writing – review & editing. ML: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. WHO Classification of Tumours Editorial Board. Press I: WHO classification of tumours editorial board. In: Soft Tissue and Bone Tumours, 5th edn, vol. 3. SWISS: World Health Organization.
2. Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2021) 32:1348–65. doi: 10.1016/j.annonc.2021.07.006
3. Seddon B, Strauss SJ, Whelan J, Leahy M, Woll PJ, Cowie F, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. (2017) 18:1397–410. doi: 10.1016/S1470-2045(17)30622-8
4. Mir O, Brodowicz T, Italiano A, Wallet J, Blay JY, Bertucci F, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. (2016) 17:1732–42. doi: 10.1016/S1470-2045(16)30507-1
5. van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. (2012) 379:1879–86. doi: 10.1016/S0140-6736(12)60651-5
6. Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res. (2018) 24:5233–8. doi: 10.1158/1078-0432.CCR-17-3766
7. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. (2017) 18:1493–501. doi: 10.1016/S1470-2045(17)30624-1
8. Pawel BR, Hamoudi AB, Asmar L, Newton WA Jr, Ruymann FB, Qualman SJ, et al. Undifferentiated sarcomas of children: pathology and clinical behavior–an Intergroup Rhabdomyosarcoma study. Med Pediatr Oncol. (1997) 29:170–80. doi: 10.1002/(ISSN)1096-911X
9. Pappo AS, Dirksen U. Rhabdomyosarcoma, ewing sarcoma, and other round-cell sarcomas. J Clin Oncol. (2018) 36:168–79. doi: 10.1200/JCO.2017.74.7402
10. von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, et al. Soft tissue sarcoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:815–33. doi: 10.6004/jnccn.2022.0035
11. Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol Oncol. (2008) 109:329–34. doi: 10.1016/j.ygyno.2008.03.010
12. Haidar A, Arekapudi S, DeMattia F, Abu-Isa E, Kraut M. High-grade undifferentiated small round-cell sarcoma with t(4;19)(q35;q13.1) CIC-DUX4 fusion: emerging entities of soft tissue tumors with unique histopathologic features–a case report and literature review. Am J Case Rep. (2015) 16:87–94.
13. Lessnick SL, Dei Tos AP, Sorensen PHB, Dileo P, Baker LH, Ferrari S, et al. Small round-cell sarcomas. Semin Oncol. (2009) 36:338–46. doi: 10.1053/j.seminoncol.2009.06.006
14. Sun Y, Niu W, Du F, Du C, Li S, Wang J, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. (2016) 9:105. doi: 10.1186/s13045-016-0332-8
15. Xu JM, Wang Y, Chen YL, Jia R, Li J, Gong JF, et al. Sulfatinib, a novel kinase inhibitor, in patients with advanced solid tumors: results from a phase I study. Oncotarget. (2017) 8:42076–86. doi: 10.18632/oncotarget.v8i26
16. Xu J, Shen L, Zhou Z, Li J, Bai C, Chi Y, et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. (2020) 21:1500–12. doi: 10.1016/S1470-2045(20)30496-4
17. Dancsok AR, Setsu N, Gao D, Blay JY, Thomas D, Maki RG, et al. Expression of lymphocyte immunoregulatory biomarkers in bone and soft-tissue sarcomas. Mod Pathol. (2019) 32:1772–85. doi: 10.1038/s41379-019-0312-y
18. Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. (2019) 18:60. doi: 10.1186/s12943-019-0974-6
19. Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. (2014) 25:846–59. doi: 10.1016/j.ccr.2014.05.016
20. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. (2013) 496:445–55. doi: 10.1038/nature12034
Keywords: undifferentiated small round-cell sarcoma, surufatinib, programmed death-1 inhibitor, target therapy, case report
Citation: Li Y, Huang J, Chen X, Ye Y, Du X, Voutsadakis IA, Seetharam M, Zhang H and Lu M (2024) Case report: Metastatic refractory undifferentiated small round-cell sarcoma successfully treated with surufatinib and camrelizumab. Front. Oncol. 14:1416241. doi: 10.3389/fonc.2024.1416241
Received: 12 April 2024; Accepted: 12 June 2024;
Published: 11 July 2024.
Edited by:
Dong-Hua Yang, New York College of Traditional Chinese Medicine, United StatesReviewed by:
Rongbo Lin, Fujian Provincial Cancer Hospital, ChinaCopyright © 2024 Li, Huang, Chen, Ye, Du, Voutsadakis, Seetharam, Zhang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Lu, ZG9jdG9ybHVAMTYzLmNvbQ==; Yong Li, eW9uZ2ppZTg4bmV0QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.