
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 June 2024
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1415345
This article is part of the Research Topic Newest Challenges and Advances in the Treatment of Colorectal Disorders; From Predictive Biomarkers to Minimally Invasive Techniques View all 23 articles
Background: Endoscopic obstruction (eOB) is associated with a poor prognosis in colorectal cancer (CRC). Our study aimed to investigate the association between tumor location and eOB, as well as the prognostic differences among non-endoscopic obstruction (N-eOB), eOB with tumor size ≤ 5 cm, and eOB with tumor size > 5 cm in non-elderly patients.
Methods: We retrospectively reviewed the clinicopathological variables of 230 patients with CRC who underwent curative surgery. The multivariable logistic regression model was used to identify risk factors for eOB. The association between eOB with tumor size ≤ 5 cm and disease-free survival (DFS) was evaluated using multivariate cox regression analysis.
Results: A total of 87 patients had eOB while 143 had N-eOB. In multivariate analysis, preoperative carcinoembryonic antigen (p = 0.014), tumor size (p = 0.010), tumor location (left-side colon; p = 0.033; rectum; p < 0.001), and pT stage (T3, p = 0.009; T4, p < 0.001) were significant factors of eOB. The DFS rate for eOB with tumor size ≤ 5 cm was significantly lower (p < 0.001) in survival analysis. The eOB with tumor size ≤ 5 cm (p = 0.012) was an unfavorable independent factor for DFS.
Conclusions: The patients with eOB were significantly associated with right-side colon cancer as opposed to left-side colon cancer and rectal cancer. The eOB with tumor size ≤ 5 cm was an independent poor prognostic factor. Further studies are needed to target these high-risk groups.
Colorectal cancer (CRC), with an estimated 1.9 million new cases and 935,000 deaths globally in 2020, stands as the third most prevalent cancer and the second leading cause of cancer-related mortality, contributing to approximately one in 10 cancer cases and deaths (1). Since circa 2010, the incidence of regional-stage and distant-stage disease has increased by about 3% per year in people younger than 50 years and by 2% and 0.5% per year, respectively, in people aged 50–64 years, while rates in people aged 65 years and older have stabilized since about 2015 after a decade of steep decline (2). This trend may necessitate a revision of current screening programs (3).
Endoscopy, particularly colonoscopy, has evolved to be an integral component of the preoperative assessment in patients with colorectal cancer, not just for its diagnostic capabilities but also for its prognostic implications. The occurrence of endoscopic obstruction (eOB) in individuals with colorectal cancer is not uncommon (4), and eOB is defined as the inability of standard colonoscopy to penetrate the tumor, regardless of clinical signs of intestinal obstruction (abdominal distention, peristalsis abdominal pain, nausea, or vomiting) or imaging findings of intestinal obstruction (dilated intestinal loops). Meanwhile, recent studies indicate that eOB is a marker of poor prognosis in patients with stage II colon cancer and stage III rectal cancer following curative surgery (5, 6). Therefore, early identification of eOB is critical for the patient’s treatment. Colorectal obstruction caused by colorectal cancer occurs in 7%–29% of all patients with colorectal cancers (7, 8). Yang et al. indicated that left-side colon cancer was more common than right-side colon cancer in the complete obstructive colorectal cancer compared to the non-obstructive colorectal cancer (9). However, the association between eOB and tumor location has not been investigated. Beyond the influence of tumor location, a distinct variation in tumor size distribution has been noted between eOB and N-eOB. Chalieopanyarwong et al. observed that CRC with N-eOB had a significantly smaller size (4). Smaller tumor size has been reported to be associated with good survival and oncological prognosis in CRC (10). However, several recent studies have reported that tumor size is not associated with survival (11, 12), while others have shown that a smaller size is associated with poorer survival (13, 14). Based on these recent findings, the association between tumor size and oncological prognosis is controversial.
The objectives of this study were to conduct a population-based analysis evaluating the association between primary tumor location and eOB. Additionally, the study aimed to determine the impact of eOB with tumor size ≤ 5 cm on oncological prognosis.
In this single-center retrospective study, patients with histologically confirmed stage I-III colorectal cancer were included. The patient records were maintained in the colorectal tumor database of Longyan First Hospital Affiliated to Fujian Medical University (Fujian, China) between January 2015 and December 2018. The access and use of clinical data were approved by the Institutional Review Board of Longyan First Hospital Affiliated to Fujian Medical University.
All patients who underwent consecutive curative treatments for colorectal cancer were included in this study, with surgical procedures being carried out by a specialized team. Our treatment policy was curative resection of the primary lesion with sufficient margin and appropriate lymph node dissection, followed by observation or adjuvant chemotherapy based on individual risk features. Exclusion criteria were age of 65 years or older, history of neoadjuvant therapy, diagnosis of multiple primary colorectal cancers, other history of malignant tumor, familial adenomatous polyposis, undergoing emergent surgery, preoperative stent insertion, receiving colonoscopy from another medical institution, missing data, and being lost to follow-up post-operation (Figure 1).
Tumor stages were coded as described by the 8th edition of the AJCC tumor-node-metastasis grading system (15). The tumor location was classified as the right-side colon (including the cecum, the ascending colon, the hepatic flexure and the transverse colon), the left-side colon (including the splenic flexure and the descending, sigmoid colons and rectosigmoid junction) and rectum (16). The surgical techniques include laparoscopic surgery and open surgery, with the latter encompassing conversion to open surgery. Tumor size was categorized into two groups: ≤ 5 cm and > 5 cm (9). Based on this, the eOB-size group was categorized as N-eOB, eOB with tumor size ≤ 5 cm and eOB with tumor size > 5 cm.
The chi-square test and Fisher’s exact probability test were used to compare categorical data. In order to screen the final predictors of endoscopic obstruction, all candidate predictors with p < 0.05 in the univariate analysis were included in the multivariate logistic regression model. In the multivariate analysis, variables with p < 0.05 were considered as independent predictors. The Kaplan–Meier method was used to calculate overall survival (OS) and disease-free survival (DFS). And the log-rank test was used to evaluate the difference in survival rate. Univariate and multivariate Cox regression analyses were performed to determine variables related to survival. OS was defined as the time between the date of surgery and the date of death. DFS was defined as the time between the date of surgery and the date of first recurrence of the disease or death. All statistical analyses were carried out using R software (version 4.2.2), and a two-sided p-value below 0.05 was considered statistically significant.
A total of 87 (37.8%) patients were eOB and 143 (62.2%) patients were N-eOB in the study cohort. 56 (24.3%) patients were below 50 years of age. The collected clinical and pathological characteristics of the patients were subjected to univariate analysis (Table 1). Sex, BMI, history of smoking, history of drinking, diabetes mellitus, hypertension, history of abdominal surgery, preoperative carbohydrate antigen 19–9 (CA19–9), differentiation, vascular invasion, perineural invasion and pN stage of eOB patients were similar to those of N-eOB patients. Regarding the patient characteristics, patients with younger age or higher preoperative carcinoembryonic antigen (CEA) had a higher risk for eOB (32.2 vs. 19.6%; p = 0.045 and 54.0 vs. 25.9%; p < 0.001, respectively). Additionally, the patients who experienced eOB had significantly larger number of harvested lymph nodes (98.9 vs. 88.8%; p = 0.010) compared with the patients who experienced N-eOB. Regarding tumor characteristics, the highest rate of right-side colon (51.7%) was observed for patients with eOB. Patients with larger tumor size, higher pT stage and pTNM stage had a higher associated risk of eOB in our study (p < 0.001, respectively). For treatment factors, including surgical technique, and adjuvant chemotherapy, there were significant differences between eOB patients and N-eOB patients.
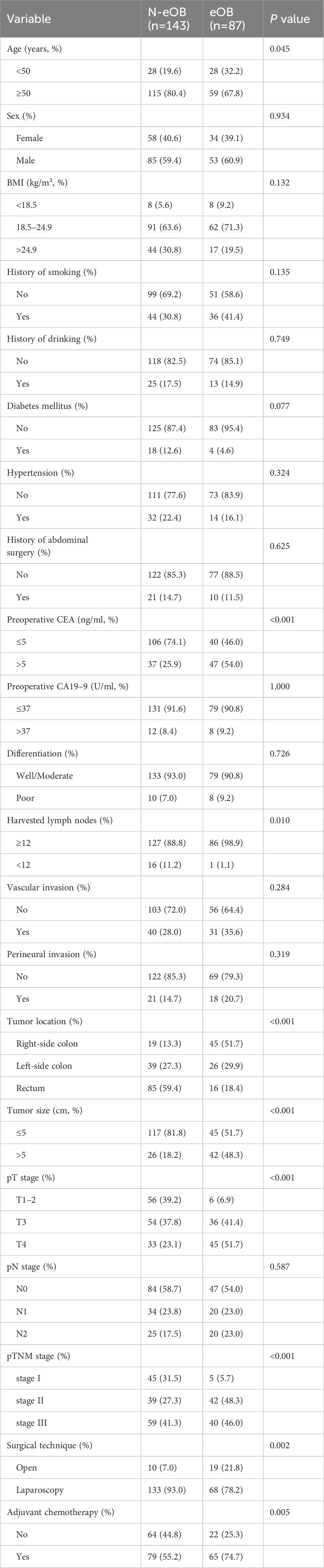
Table 1 Clinicopathological characteristics of patients with endoscopic obstruction and non-endoscopic obstruction.
In the categorization of patients with eOB, we divided them into two groups based on tumor size: eOB with tumor size ≤ 5 cm and eOB with tumor size > 5 cm. Among the 230 patients reviewed, as illustrated in Table 2, 143 (62.2%) were classified as N-eOB, 45 (19.6%) had eOB with a tumor size of ≤ 5 cm, and 42 (18.3%) had eOB with a tumor size of > 5 cm. Follow-up periods were between 10 and 88 months (median, 58 months). A total number of 45 (19.6%) patients had nodal involvement of eOB with tumor size ≤ 5 cm. Age, sex, history of smoking, history of drinking, hypertension, diabetes mellitus, history of abdominal surgery, preoperative CA19–9, differentiation, vascular invasion, perineural invasion, and pN stage were comparable among patients with N-eOB, eOB with tumor size ≤ 5cm, and eOB with tumor size > 5cm. Statistical differences were observed in the population concerning BMI, preoperative CEA, harvested lymph nodes, tumor location, pT stage, pTNM stage, surgical technique, and adjuvant chemotherapy.
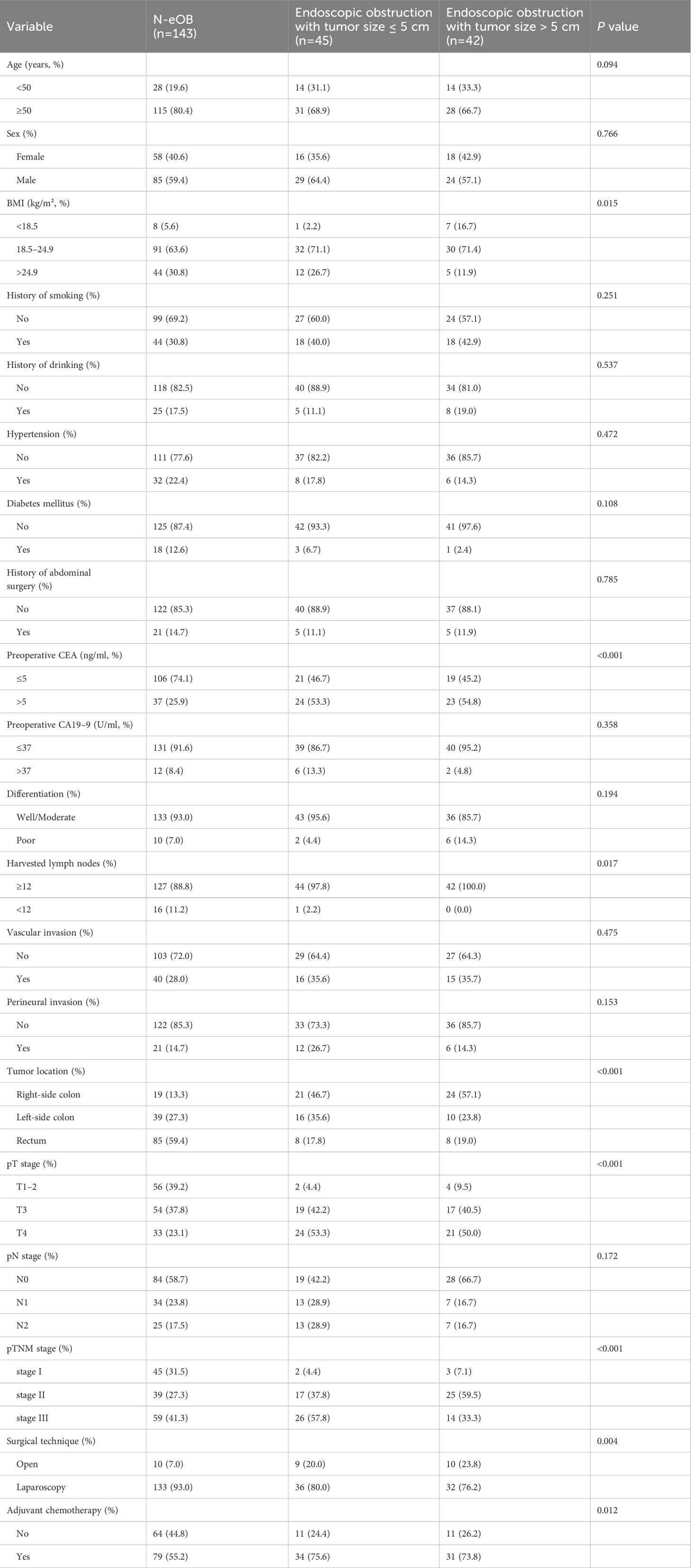
Table 2 Clinicopathological characteristics of patients with non-endoscopic obstruction, endoscopic obstruction with tumor size ≤ 5 cm and endoscopic obstruction with tumor size > 5 cm.
To identify predictors of eOB, multivariate analysis was carried out for sample using variables that were available for clinical and pathologic characteristics, including age, preoperative CEA, tumor size, tumor location, and pT stage. Among these factors, preoperative CEA (OR = 2.37; 95% CI: 1.19-4.71, p = 0.014), tumor size (OR = 2.56; 95% CI: 1.25-5.24, p = 0.010), tumor location (left-side colon; OR = 0.40; 95% CI: 0.17-0.93, p = 0.033; rectum; OR = 0.11; 95% CI: 0.05-0.26, p < 0.001), and pT stage (T3; OR = 4.07; 95% CI: 1.43-11.61, p = 0.009; T4, OR = 7.45; 95% CI: 2.58-21.55, p < 0.001) were found to be independently and significantly correlated with the development of eOB (Table 3).
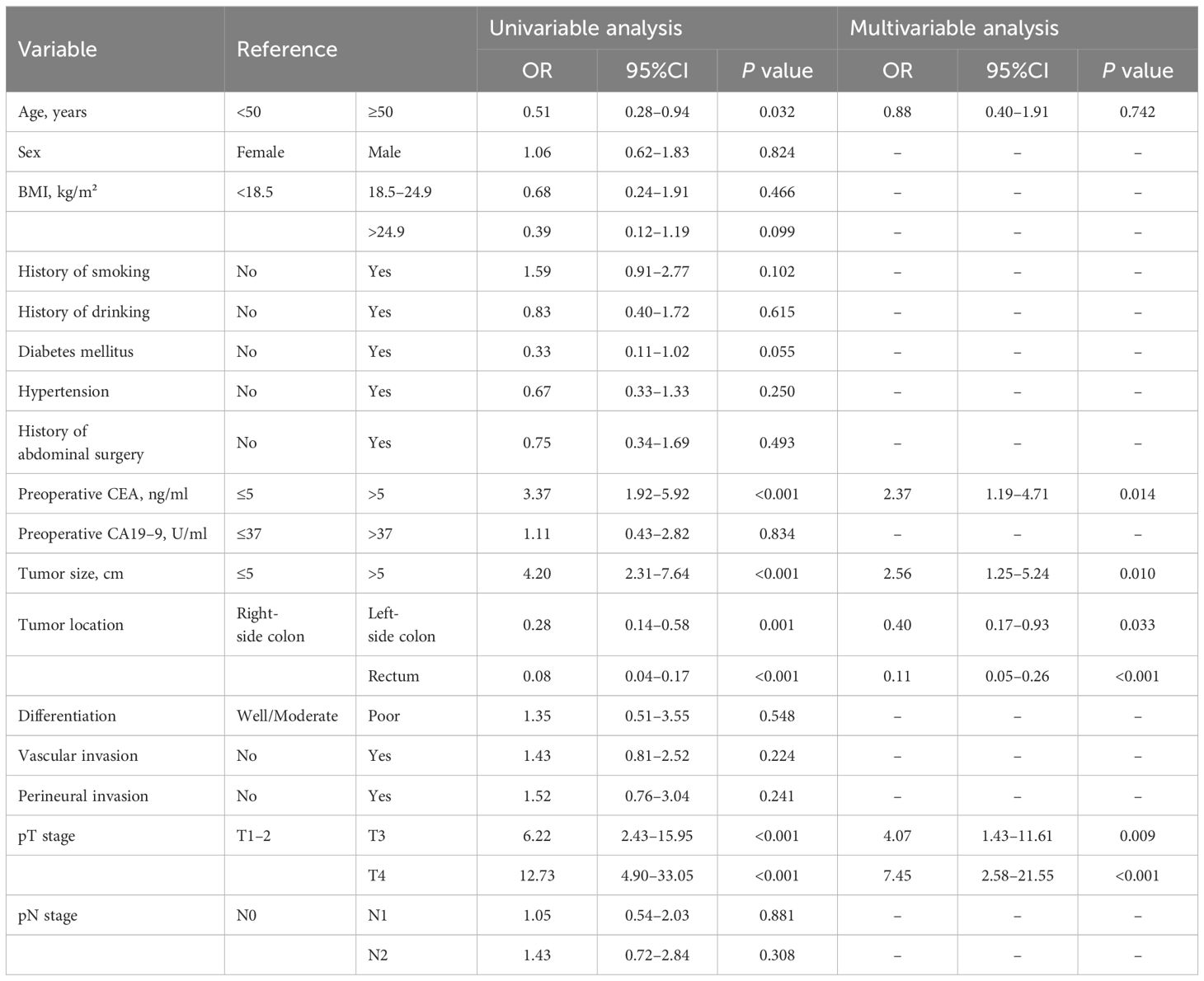
Table 3 Logistic regression analysis of clinical and pathological predictors for endoscopic obstruction.
There were no significant differences in OS among the three groups: eOB with tumor size ≤ 5 cm, eOB with tumor size > 5 cm and N-eOB (78.8% vs. 88.1% vs. 89.2%, p = 0.055) (Figure 2A). The DFS rate for eOB with tumor size ≤ 5 cm was significantly lower compared to that of eOB with tumor size > 5 cm and N-eOB (57.8% vs. 75.5% vs. 84.9%, p < 0.001) (Figure 2B).
In the univariate analysis, preoperative CEA, preoperative CA19–9, eOB-size group, differentiation, vascular invasion, perineural invasion, pTNM stage, and adjuvant chemotherapy were all associated with predicting development of DFS (Table 4). These variables were included in a multivariable Cox regression analysis. Preoperative CA19–9 (HR = 2.38, p = 0.026), eOB-size group (eOB with tumor size ≤ 5 cm vs. N-eOB, HR = 2.32, p = 0.012), perineural invasion (HR = 1.97, p = 0.040), and pTNM stage (stage III vs. stage I, HR = 6.06, p = 0.032) remained significantly associated with DFS. While we did not find that preoperative CEA was statistically significant in the multivariate analysis. Differentiation, vascular invasion and adjuvant chemotherapy were also not found to be significantly associated with worse DFS.
Few studies have focused on identifying the factors associated with the emergence of eOB, as well as conducting analyses to evaluate the prognostic implications of eOB with different tumor sizes. This study demonstrated that the incidence of eOB is predominantly higher in the right-side colon compared to the left-side colon in patients diagnosed with non-elderly colorectal cancer. Furthermore, it was noted that eOB with tumor size ≤ 5 cm was associated with a poor survival compared to N-eOB. Conversely, this correlation was not observed for eOB with tumor size > 5 cm.
Unplanned emergency surgeries for colorectal cancer are typically linked with a heightened risk of surgical complications, as well as increased mortality and morbidity rates in emergency scenarios (17–19). A recent study suggested that in instances of luminal obstruction, which is significant enough to impede the further advancement of a colonoscope, physicians should be prompted to contemplate the necessity of urgent surgical intervention, irrespective of the initial symptoms presented (4). Given the importance of preventive interventions, it is crucial to assess the risk factors associated with the emergence of eOB in patients with colorectal cancer. Previous studies presented inconsistent results regarding the relationship between bowel obstruction development and different tumor location. Theoretically, right-side colon cancers are usually ulcerative, and the stool in these regions tends to have a more liquid consistency. Conversely, left-side colon cancers are more likely to present with bowel obstruction, as proliferative lesions are common in this location and the stool is typically of a semisolid consistency. Xinger Lv et al. (20) and Phillips et al. (21) indicated that the left-side colon was more susceptible to bowel obstruction compared with the right-side colon. However, our results demonstrated an increased susceptibility of the right-side colon to eOB, in comparison with the left-side colon and the rectum. Similarly, Kumar et al. observed analogous finding that relatively younger patients present to health center with obstructive colorectal cancer with anatomical shift to the right-side colon (22). In their study, in 54% cases the lesion was in the proximal colon. It is highly plausible that this outcome is attributable to the screening policies. A study indicated that screening for colorectal cancer is associated with lower disease stage (23). Nevertheless, nearly 90% of colorectal cancer patients are diagnosed following the onset of symptoms, exhibiting more advanced disease stages compared to patients identified through asymptomatic screening (24). Furthermore, when presenting with obstruction symptoms, right-side colon cancers are generally at a more advanced stage of progression compared to left-side colon cancers. Relatedly, this study reveals a strong correlation between a more advanced pT stage and the emergence of eOB, which appears to support the hypothesis that eOB is more common in right-side colon cancers. In addition to the reasons previously mentioned, the inability of the colonoscope to smoothly navigate through the space-occupying lesions in the right-side colon, as compared to those in left-side colon and rectum, may also be attributed to discomfort experienced during the colonoscopy or to technical challenges encountered.
In this study, we observed that eOB with tumor size ≤ 5 cm exhibited a poorer DFS, compared to N-eOB. This relationship was present even when controlling for multiple patient-specific prognostic factors, such as preoperative CA19–9, perineural invasion, pTNM stage. Therefore, to give a potential explanation of our findings, we hypothesized that eOB with tumor size ≤ 5 cm may be a surrogate marker for biological aggressiveness resulting in inferior DFS, which indicated that initial biological heterogeneity of colorectal cancers determined their distinct growth pattern and different invasive and metastatic abilities. In this study, eOB with tumor size ≤ 5 cm was associated with higher pT stage, reflecting a vertical growth pattern. Tumors with a vertical growth pattern may have early acquired high metastatic potential which enable them to breach basal membrane, invading the surrounding tissue and finally disseminating to regional lymph nodes and distant metastasis (25). In contrast, tumors with a horizontal growth pattern reflected by eOB with larger tumors underline a biologically indolent disease and a lower metastatic ability. The fact that clinicians are more likely to treat large tumors more aggressively may also explain our results. Multivisceral resection (MVR) is associated with increased tumor size in locally advanced colorectal cancer (26). In addition, the larger tumor size often leads to more complete lymph node resection and evaluation in colorectal cancer (27). These more aggressive treatments may result in better survival rates. Therefore, in the diagnosis and treatment of colorectal cancer, the evaluation of eOB with smaller tumors should not be overlooked. In addition, preoperative CEA was not an independent prognostic biomarker according to our study. One study has shown that patients with elevated preoperative CEA that normalizes after surgery have a similar outcome to patients with normal preoperative CEA (28). This adequately demonstrates the limitations of preoperative CEA in predicting postoperative recurrence.
This study indeed has several limitations. (1) This study was conducted as a single-center retrospective analysis. Consequently, the number of patients was limited, and the selection process adhered to stringent inclusion and exclusion criteria. This approach may have introduced potential selection bias. (2) The stratification by tumor size and eOB led to relatively small subgroups, which reduced the statistical power to discriminate small differences. (3) Neoadjuvant chemotherapy, radiotherapy and laboratory examinations were not included in the present study. Further investigations of multi-center prospective study should be conducted and more baseline characteristics should be enrolled.
Among non-elderly patients, those with eOB were significantly associated with right-side colon cancer as opposed to left-side colon cancer and rectal cancer. The eOB with tumor size ≤ 5 cm was associated with lower DFS, while this association was not observed for eOB with tumor size > 5 cm. The observed shift in the incidence of eOB towards the right-side colon, coupled with the result that eOB with tumor size ≤ 5 cm may denote a more aggressive form of malignancy, highlights the imperative for comprehensive research.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Ethical Review Board of the Longyan First Affiliated Hospital of Fujian Medical University (approval number: LYREC2024-k024-01). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this study utilizes medical records obtained from previous research and meets all of the following conditions: Previous research has obtained written consent from the participants, allowing other research projects to use their medical records or specimens. This research complies with the permission conditions of the original informed consent. The confidentiality of the participants' privacy and identity information is ensured.
NY: Data curation, Methodology, Software, Writing – original draft. SL: Conceptualization, Data curation, Writing – original draft. XW: Writing – review & editing, Conceptualization. GH: Data curation, Methodology, Writing – original draft. RX: Data curation, Writing – original draft. ZQ: Data curation, Writing – original draft. RL: Data curation, Writing – original draft. DX: Conceptualization, Writing – review & editing, Data curation, Methodology.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank XW for his help in data interpretation and manuscript writing, which made this study possible.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. (2023) 73:233–54. doi: 10.3322/caac.21772
3. Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. (2015) 150:17–22. doi: 10.1001/jamasurg.2014.1756
4. Chalieopanyarwong V, Boonpipattanapong T, Prechawittayakul P, Sangkhathat S. Endoscopic obstruction is associated with higher risk of acute events requiring emergency operation in colorectal cancer patients. World J Emerg Surg. (2013) 8:34. doi: 10.1186/1749-7922-8-34
5. Hong KD, Um JW, Ji WB, Jung SY, Kang S, Lee SI, et al. Endoscopic obstruction in rectal cancers: survival and recurrence patterns following curative surgery. J Laparoendosc Adv Surg Tech A. (2015) 25:278–84. doi: 10.1089/lap.2014.0566
6. Mo TW, Zhang ZJ, Shi YQ, Xiang HD, Chen YL, Chen ZX, et al. Prognostic implications of endoscopic obstruction in patients with pathological stage II colon cancers: a single-center retrospective cohort study. J Gastrointest Surg. (2023) 27:141–51. doi: 10.1007/s11605-022-05516-5
7. Deans GT, Krukowski ZH, Irwin ST. Malignant obstruction of the left colon. Br J Surg. (1994) 81:1270–6. doi: 10.1002/bjs.1800810905
8. Smothers L, Hynan L, Fleming J, Turnage R, Simmang C, Anthony T. Emergency surgery for colon carcinoma. Dis Colon Rectum. (2003) 46:24–30. doi: 10.1007/s10350-004-6492-6
9. Yang Z, Wang L, Kang L, Xiang J, Peng J, Cui J, et al. Clinicopathologic characteristics and outcomes of patients with obstructive colorectal cancer. J Gastrointest Surg. (2011) 15:1213–22. doi: 10.1007/s11605-011-1563-1
10. Saha S, Shaik M, Johnston G, Saha SK, Berbiglia L, Hicks M, et al. Tumor size predicts long-term survival in colon cancer: an analysis of the National Cancer Data Base. Am J Surg. (2015) 209:570–4. doi: 10.1016/j.amjsurg.2014.12.008
11. Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer prognostic factors consensus conference. Cancer. (2000) 88:1739–57. doi: 10.1002/(ISSN)1097-0142
12. Crozier JE, McMillan DC, McArdle CS, Angerson WJ, Anderson JH, Horgan PG, et al. Tumor size is associated with the systemic inflammatory response but not survival in patients with primary operable colorectal cancer. J Gastroenterol Hepatol. (2007) 22:2288–91. doi: 10.1111/j.1440-1746.2006.04792.x
13. Santullo F, Biondi A, Cananzi FCM, Fico V, Tirelli F, Ricci R, et al. Tumor size as a prognostic factor in patients with stage IIa colon cancer. Am J Surg. (2018) 215:71–7. doi: 10.1016/j.amjsurg.2017.03.038
14. Huang B, Feng Y, Zhu L, Xu T, Huang L, Cai G. Smaller tumor size is associated with poor survival in stage II colon cancer: An analysis of 7,719 patients in the SEER database. Int J Surg. (2016) 33 Pt A:157–63. doi: 10.1016/j.ijsu.2016.07.073
15. Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. (2018) 25:1454–5. doi: 10.1245/s10434-018-6462-1
16. Lv Z, Liang Y, Liu H, Mo D. Association of chemotherapy with survival in stage II colon cancer patients who received radical surgery: a retrospective cohort study. BMC Cancer. (2021) 21:306. doi: 10.1186/s12885-021-08057-3
17. Wong SK, Jalaludin BB, Morgan MJ, Berthelsen AS, Morgan A, Gatenby AH, et al. Tumor pathology and long-term survival in emergency colorectal cancer. Dis Colon Rectum. (2008) 51:223–30. doi: 10.1007/s10350-007-9094-2
18. Diggs JC, Xu F, Diaz M, Cooper GS, Koroukian SM. Failure to screen: predictors and burden of emergency colorectal cancer resection. Am J Manag Care. (2007) 13:157–64.
19. Bass G, Fleming C, Conneely J, Martin Z, Mealy K. Emergency first presentation of colorectal cancer predicts significantly poorer outcomes: a review of 356 consecutive Irish patients. Dis Colon Rectum. (2009) 52:678–84. doi: 10.1007/DCR.0b013e3181a1d8c9
20. Lv X, Yu H, Gao P, Song Y, Sun J, Chen X, et al. A nomogram for predicting bowel obstruction in preoperative colorectal cancer patients with clinical characteristics. World J Surg Oncol. (2019) 17:21. doi: 10.1186/s12957-019-1562-3
21. Phillips RK, Hittinger R, Fry JS, Fielding LP. Malignant large bowel obstruction. Br J Surg. (1985) 72:296–302. doi: 10.1002/bjs.1800720417
22. Kumar A, Dhungana SB, Gupta RK, Sah SP, Khanal B. Clinico-pathological characteristics of obstructing colorectal cancer and its management outcomes at a tertiary referral center of Eastern Nepal. BMC Gastroenterol. (2022) 22:303. doi: 10.1186/s12876-022-02380-0
23. Kubisch CH, Crispin A, Mansmann U, Goke B, Kolligs FT. Screening for colorectal cancer is associated with lower disease stage: A population-based study. Clin Gastroenterol Hepatol. (2016) 14:1612–8 e3. doi: 10.1016/j.cgh.2016.04.008
24. Moreno CC, Mittal PK, Sullivan PS, Rutherford R, Staley CA, Cardona K, et al. Colorectal cancer initial diagnosis: screening colonoscopy, diagnostic colonoscopy, or emergent surgery, and tumor stage and size at initial presentation. Clin Colorect Cancer. (2016) 15:67–73. doi: 10.1016/j.clcc.2015.07.004
25. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. (2011) 331:1559–64. doi: 10.1126/science.1203543
26. Nakafusa Y, Tanaka T, Tanaka M, Kitajima Y, Sato S, Miyazaki K. Comparison of multivisceral resection and standard operation for locally advanced colorectal cancer: analysis of prognostic factors for short-term and long-term outcome. Dis Colon Rectum. (2004) 47:2055–63. doi: 10.1007/s10350-004-0716-7
27. Chou JF, Row D, Gonen M, Liu YH, Schrag D, Weiser MR. Clinical and pathologic factors that predict lymph node yield from surgical specimens in colorectal cancer: a population-based study. Cancer. (2010) 116:2560–70. doi: 10.1002/cncr.25032
Keywords: colorectal cancer, endoscopic obstruction, tumor location, tumor size, disease-free survival
Citation: Yu N, Lin S, Wang X, Hu G, Xie R, Que Z, Lai R and Xu D (2024) Endoscopic obstruction predominantly occurs in right-side colon cancer and endoscopic obstruction with tumor size ≤ 5 cm seems poor prognosis in colorectal cancer. Front. Oncol. 14:1415345. doi: 10.3389/fonc.2024.1415345
Received: 10 April 2024; Accepted: 23 May 2024;
Published: 14 June 2024.
Edited by:
Francesk Mulita, General University Hospital of Patras, GreeceReviewed by:
Angelis Peteinaris, University of Patras, GreeceCopyright © 2024 Yu, Lin, Wang, Hu, Xie, Que, Lai and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongbo Xu, eGRiMjI5MjM4OEBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.