- 1Department of Pathology, Xi’an No.3 Hospital, the Affiliated Hospital of Northwest University, Xi’an, Shaanxi, China
- 2Department of Intensive Care Unit, Xi’an No.3 Hospital, the Affiliated Hospital of Northwest University, Xi’an, Shaanxi, China
Background: Acute appendicitis (AA) is one of the most prevalent acute abdominal diseases and appendectomy is the definitive treatment of appendicitis. However, whether appendicitis and appendectomy cause colorectal cancer (CRC) is controversial. The results of observational studies are contradictory, but randomized controlled trials (RCT) cannot be conducted.
Methods: Data of appendectomy, AA, and CRC were obtained from the IEU Open GWAS project. We selected several Genome-wide association studies (GWAS) summary statistics for CRC: statistics for colon cancer (CC) were obtained from MRC-IEU and Neale lab, respectively; statistics for rectum cancer (RC) were obtained from MRC-IEU and FinnGen, respectively; statistics for CRC were provided by Sakaue S et al. Mendelian randomization (MR) was used to evaluate the causal relationships between exposure and outcomes. Inverse variance weighting (IVW) was the most important analysis method. Meta-analysis was used to summarize the results of IVW to increase the reliability and sensitivity analysis was used to evaluate the robustness of the results.
Results: According to the results of IVW, appendectomy did not increase risk of CC: MRC-IEU (OR:1.009, 95%CI:0.984-1.035, P=0.494), Neale lab (OR:1.016, 95%CI:0.993-1.040, P=0.174); Appendectomy also did not increase risk of RC: MRC-IEU(OR:0.994, 95%CI:0.974-1.014, P=0.538), FinnGen(OR:2.791, 95%CI:0.013-580.763, P=0.706); Appendectomy also did not increase risk of CRC: Sakaue S(OR:1.382, 95%CI:0.301-6.352, P=0.678). Appendicitis did not increase risk of CC: MRC-IEU(OR:1.000, 95%CI:0.999-1.001, P=0.641), Neale lab(OR:1.000, 95%CI:1.000-1.001, P=0.319); Appendicitis also did not increase risk of RC: MRC-IEU(OR:1.000, 95%CI:0.999-1.000, P=0.361), FinnGen(OR:0.903, 95%CI:0.737-1.105, P=0.321); Appendicitis also did not increase risk of CRC: Sakaue S (OR:1.018, 95%CI:0.950-1.091, P=0.609). The results of Meta-analysis also showed appendectomy (P=0.459) and appendicitis (P=0.999) did not increase the risk of CRC.
Conclusions: Appendectomy and appendicitis do not increase the risk of colorectal cancer. More clinical trials are needed in the future to verify the causal relationships.
1 Introduction
Acute appendicitis (AA) is one of the most prevalent acute abdominal diseases (1, 2). According to epidemiological data from different countries, lifetime incidence rate of AA can be as high as close to 20% (1–4), and the global incidence rate of AA has increased by more than 60% in the past 30 years (5). Appendicitis is often treated by appendectomy (6, 7), which is the most common emergency abdominal surgery in western countries, with an average of 100 cases per 100,000 person (8). However, many observational studies have revealed that appendectomy may increase the risk of colorectal cancer(CRC): results from Shi et al. showed a 73.0% increase in CRC risk among appendectomy cases throughout 20 years follow-up (SHR:1.73, 95% CI:1.49-2.01, P < 0.001) (9) and results from Chen et al. showed appendectomy was independent risk factors for CRC (OR: 9.10; 95%CI:1.83–50.02, P=0.0055) (10)], AA itself may be a precursor of CRC, which is based on the results of several retrospective studies with follow-up over 5 years: (HR = 4.67; 95% CI: 3.51-6.21, P=0.0011) (11, 12). Other studies have shown that appendectomy can reduce the risk of CRC (HR = 0.90, 95% CI = 0.81‐0.99) (13, 14). Therefore, it is still controversial whether appendicitis and appendectomy are the etiology of(CRC). Due to the limitation of ethics, the relevant randomized controlled trial (RCT) cannot be carried out.
Mendelian randomization (MR) facilitates the study of the causal relationships between exposure factors and outcomes (15, 16). In MR, the single nucleotide polymorphisms (SNPs) from genome-wide association studies (GWASs) were used as instrumental variables (IVs), and the causality was analyzed by IVs instead of exposure factors (15, 16). The time sequence of exposure and outcomes in MR is reasonable, which avoids reverse causality (15, 16). MR is not subject to ethical constraints, and its level of evidence is equivalent to RCT (17). Therefore, this study intends to explore whether appendicitis and appendectomy can cause CRC by MR.
2 Materials and methods
2.1 Study design
Figure 1 showed three key assumptions of this MR study: ① SNPs are strongly associated with appendectomy or appendicitis; ②SNPs are independent of known confounders; ③SNPs only affect CRC via appendectomy or appendicitis.
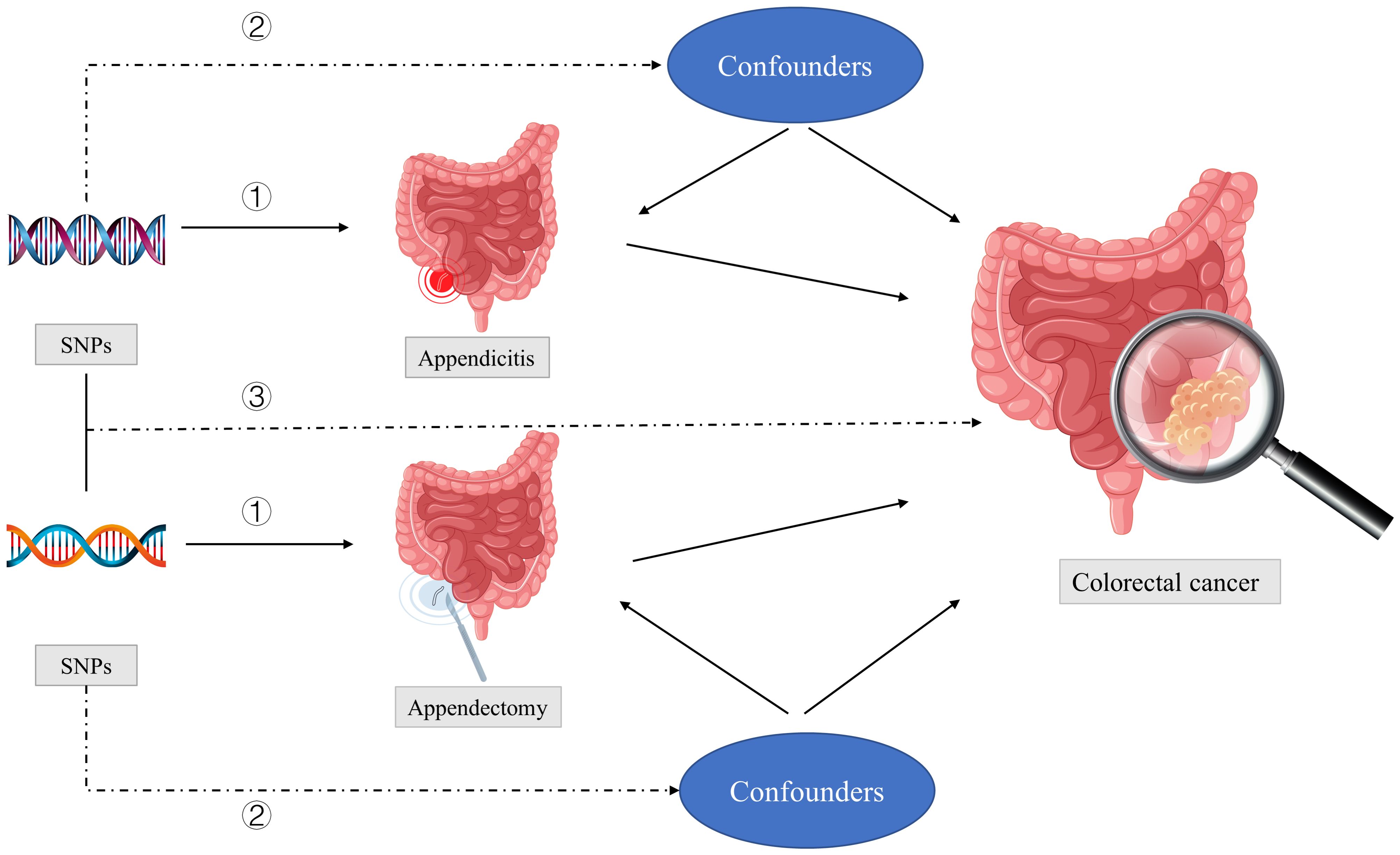
Figure 1 Three key assumptions of this MR study: ① SNPs are strongly associated with appendectomy or appendicitis; ②SNPs are independent of known confounders; ③SNPs only affect colorectal cancer via appendectomy or appendicitis. SNPs, single-nucleotide polymorphisms.
2.2 Data sources
The data used in this study were all from the public GWASs and all from Europeans. We obtained the data from the IEU Open GWAS (https://gwas.mrcieu.ac.uk/); GWAS summary statistics for appendicectomy (SNPs= 9,851,867) were obtained from MRC-IEU; GWAS summary statistics for appendicitis (SNPs= 12,243,521) were obtained from UK Biobank; GWAS summary statistics for colon cancer (CC) were obtained from MRC-IEU (SNPs= 9,851,867) and Neale lab (SNPs= 10,833,390) respectively; GWAS summary statistics for rectum cancer (RC) were obtained from MRC-IEU (SNPs= 9,851,867) and FinnGen (SNPs= 16,380,466) respectively; GWAS summary statistics for CRC were obtained from the data reported by Sakaue S et al. (SNPs= 24,182,361). Supplementary Table 1 showed the baseline characteristics of selected GWAS summary statistics. We deleted SNPs related to confounders and outcomes variables through the PhenoScanner database (http://www.phenoscanner.medschl.cam.ac.uk/). There was no requirement for further ethical clearance because we used publicly available GWAS data.
2.3 Selection of SNPs
The selection of SNPs must meet three requirements (18, 19): First, we selected SNPs associated with appendicectomy at the genome-wide significance threshold with p < 5 × 10−8 and we adjusted the significance threshold of appendicitis to 1× 10−5 to obtain enough SNPs; Second, in order to ensure the independence of selected SNPs, we need to eliminate the linkage disequilibrium (r2 > 0.001, clumping window =10,000 kb); Third, we selected strong IVs according to F-statistics (F=β2/SE2). When F > 10, SNP was considered a strong instrumental variable. Before performing the MR analysis, we also conducted data-harmonization steps, as the effects of an SNP on the exposure and the outcome had to correspond to the same allele.
2.4 MR analysis
Inverse variance weighted (IVW)was the most important analysis method. Sensitivity analysis was used to evaluate the robustness of selected SNPs. To increase the reliability of the results, we chose several GWAS summary statistics of outcome variables. We then employed Meta-analysis to compile the IVW method’s results in order to further increase the reliability.
Pleiotropy, MR-Egger and MR-PRESSO were used to evaluate pleiotropy of selected SNPs. The weighted-median method can provide valid estimates if more than 50% of information comes from valid IVs (20); Cochrane’s Q-value was used to evaluate the heterogeneity of SNPs: When there was heterogeneity, random effect model was used, otherwise, common effect model was used. A significance level of P < 0.05 was considered statistically significant. The analysis was performed by “TwoSampleMR” packages in R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Selection of SNPs
Appendectomy: First, after significance test and removal of linkage disequilibrium, there were 18 SNPs related to appendectomy; Then, 4 SNPs related to confounders were excluded (rs34236350, rs2524069, rs224029, rs10849448). Supplementary Table 2 showed the final SNPs after data-harmonization step.
Appendicitis: After significance testing and removal of linkage disequilibrium, there were 19 SNPs related to appendicitis. Supplementary Table 2 showed the final SNPs after data-harmonization step.
3.2 Results of MR
Table 1 showed results of MR and Table 2 showed results of sensitivity analysis.
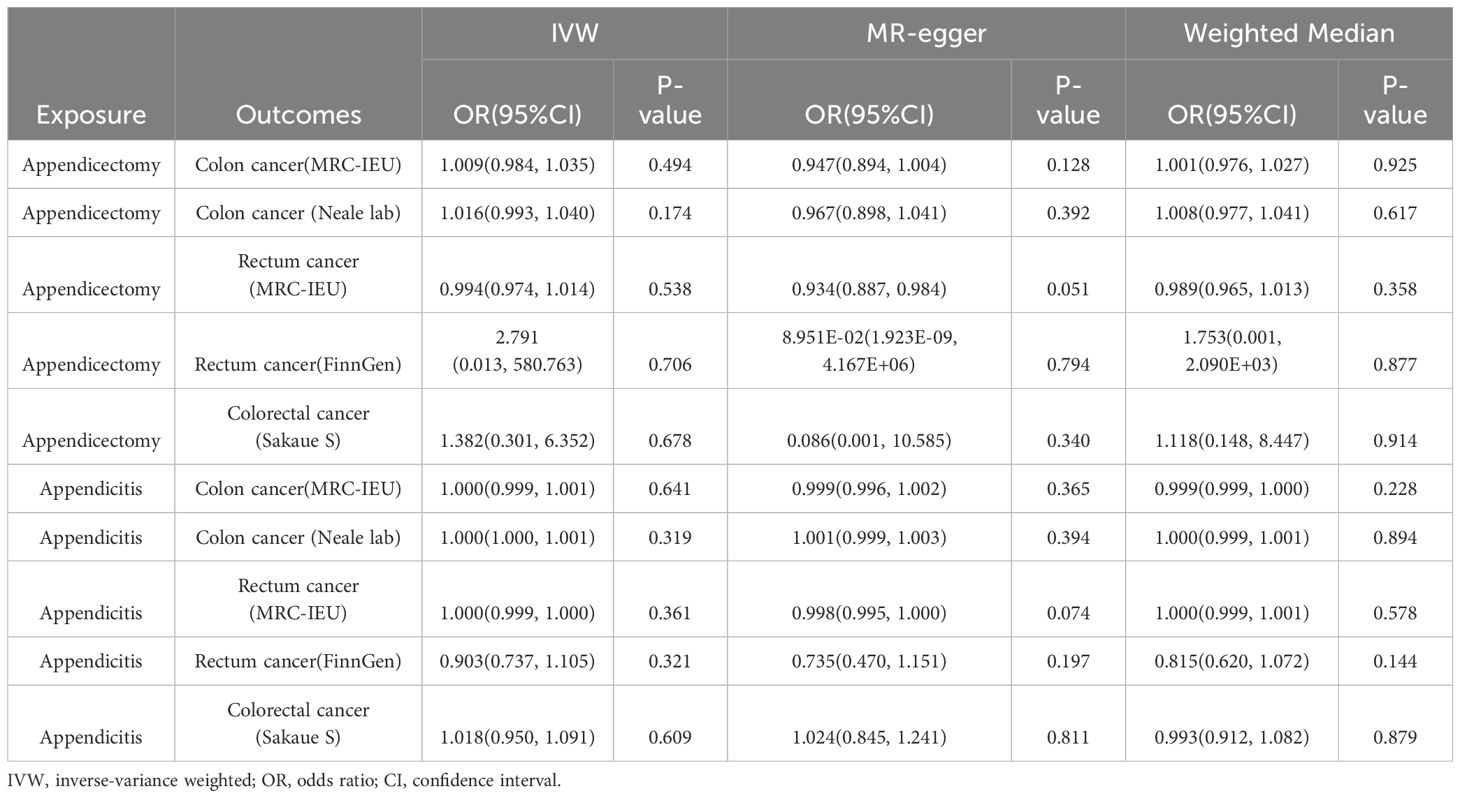
Table 1 Associations between genetically predicted exposure and outcomes using the Mendelian randomization.
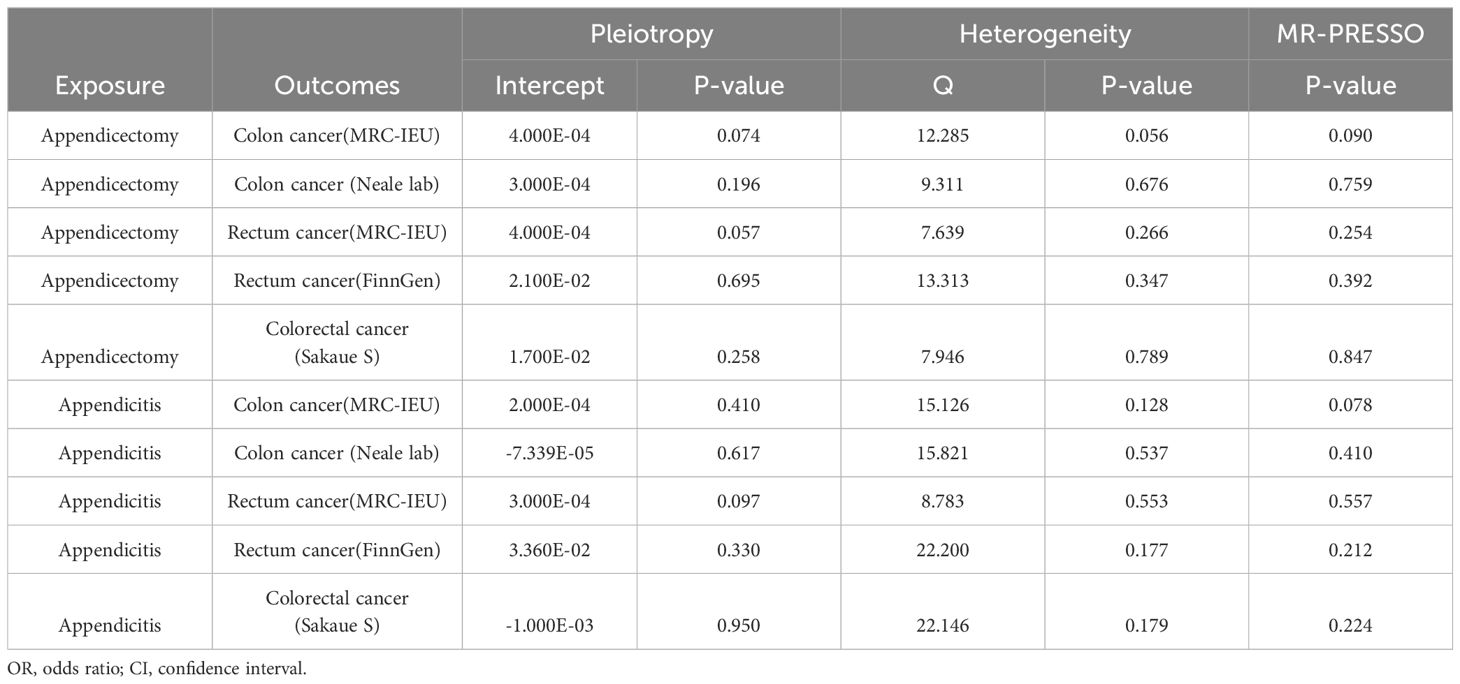
Table 2 Mendelian randomization in sensitivity analyses predicts causal relationships of exposure and outcomes.
3.2.1 Effect of appendectomy on CRC
According to the results of IVW, appendectomy did not increase risk of CC: Colon cancer(MRC-IEU)(OR:1.009, 95%CI:0.984-1.035, P=0.494), Colon cancer(Neale lab)(OR:1.016, 95%CI:0.993-1.040, P=0.174); Appendectomy also did not increase risk of RC: Rectum cancer(MRC-IEU)(OR:0.994, 95%CI:0.974-1.014, P=0.538), Rectum cancer(FinnGen)(OR:2.791, 95%CI:0.013-580.763, P=0.706); Appendectomy did not increase risk of CRC: Colorectal cancer (Sakaue S): (OR:1.382, 95%CI:0.301-6.352, P=0.678). Weighted Median method also showed appendectomy did not increase risk of CRC (P were all greater than 0.05). The results of heterogeneity test showed that the common effect model should be selected (P were all greater than 0.05). The results of Pleiotropy, MR-Egger and MR-PRESSO showed that there were no horizontal pleiotropy (P were all greater than 0.05). Figure 2 showed the main results of MR analysis. Leave-one-out analysis in Supplementary Figures 1A–E showed that there were no SNPs which significantly affected the result. Supplementary Figures 2A–E and Supplementary Figures 3A–E showed forest plot and scatter plot of the association between appendectomy and CRC, respectively, which were also consistent with the IVW results. The funnel plot in Supplementary Figures 4A–E showed that the selection of SNPs was reasonable and the analysis result was robust.
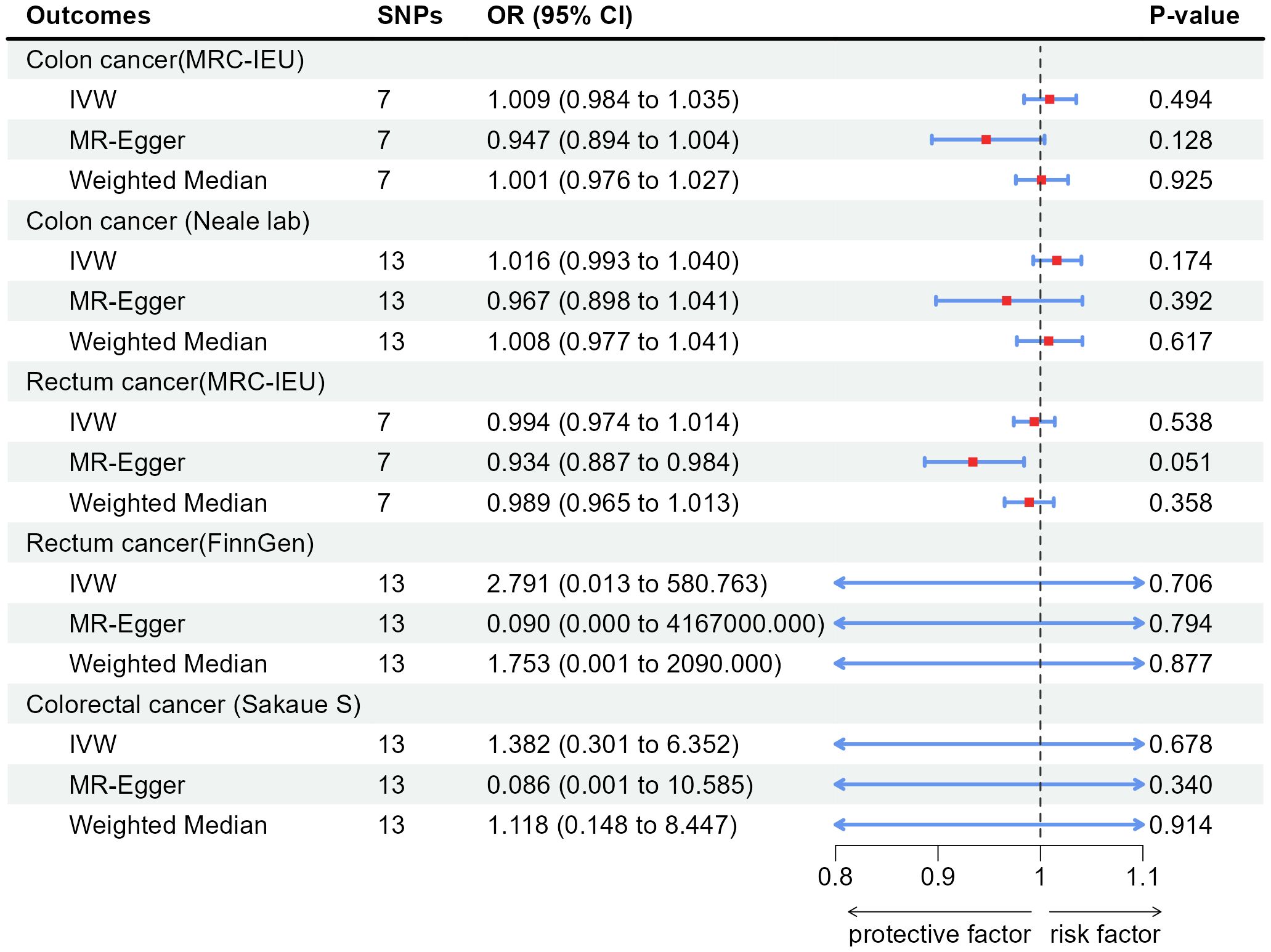
Figure 2 Associations of genetically predicted appendectomy with colorectal cancer by IVW, MR-Egger and Weighted Median. SNPs, single-nucleotide polymorphisms; OR, odds ratio; CI, confidence interval; IVW, inverse-variance weighted method.
3.2.2 Effect of appendicitis on CRC
According to the results of IVW, appendicitis did not increase risk of CC: Colon cancer(MRC-IEU)(OR:1.000, 95%CI:0.999-1.001, P=0.641), Colon cancer(Neale lab)(OR:1.000, 95%CI:1.000-1.001, P=0.319); Appendicitis did not increase risk of RC: Rectum cancer(MRC-IEU)(OR:1.000, 95%CI:0.999-1.000, P=0.361), Rectum cancer(FinnGen)(OR:0.903, 95%CI:0.737-1.105, P=0.321); Appendicitis also did not increase risk of CRC: Colorectal cancer (Sakaue S): (OR:1.018, 95%CI:0.950-1.091, P=0.609). Weighted Median method also showed appendicitis did not increase risk of CRC (P were all greater than 0.05). The results of heterogeneity test showed that the common effect model should be selected (P were all greater than 0.05). The results of Pleiotropy, MR-Egger and MR-PRESSO showed that there were no horizontal pleiotropy (P > 0.05). Figure 3 showed the main results of MR analysis. Leave-one-out analysis in Supplementary Figures 1F–J showed that there were no SNPs which significantly affected the result. Supplementary Figures 2F–J and Supplementary Figures 3F–J showed forest plot and scatter plot of the association between appendicitis and CRC, respectively, which were also consistent with the IVW results. The funnel plot in Supplementary Figures 4F–J showed that the selection of SNPs was reasonable and the analysis result was robust.
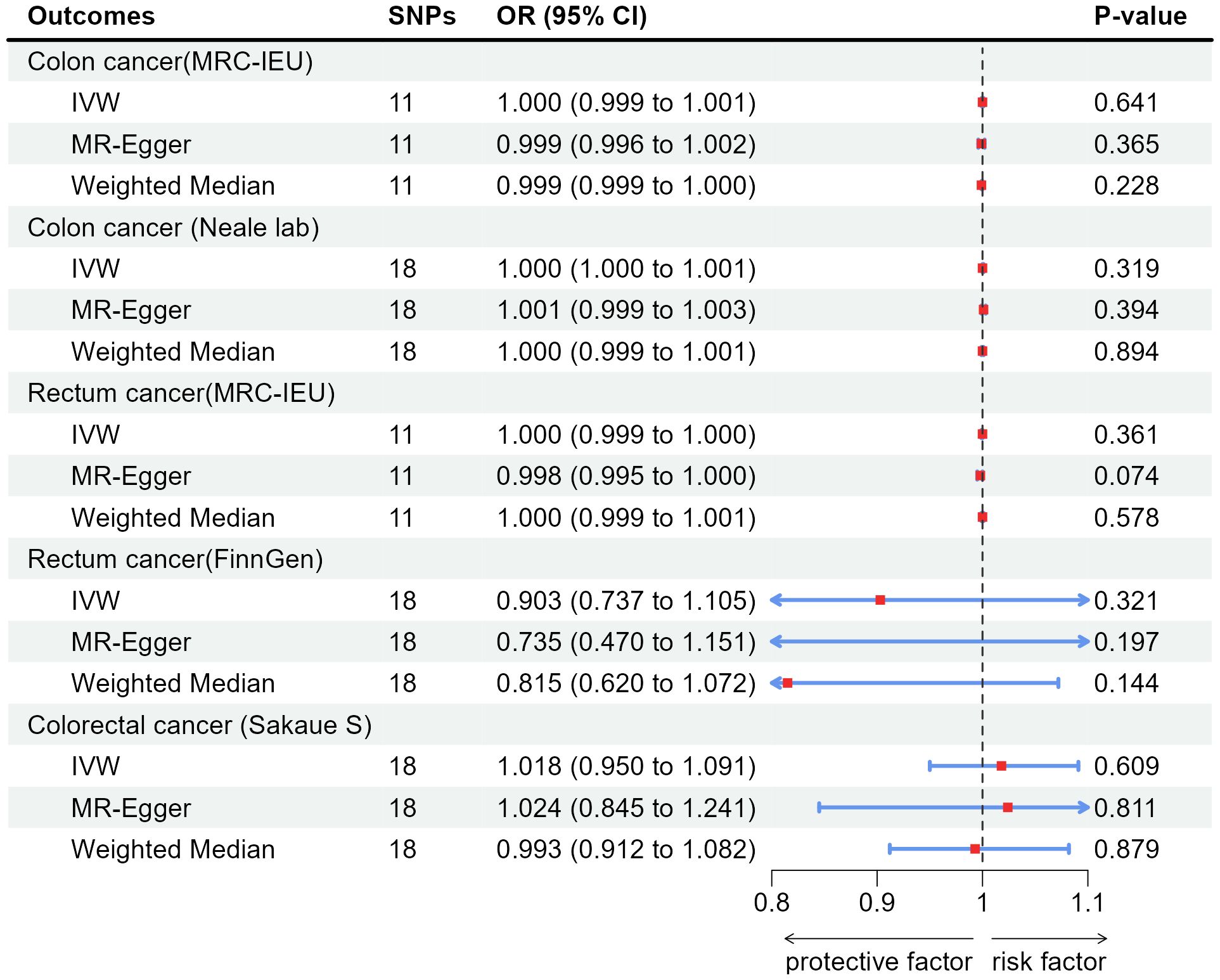
Figure 3 Associations of genetically predicted appendicitis with colorectal cancer by IVW, MR-Egger and Weighted Median. SNPs, single-nucleotide polymorphisms; OR, odds ratio; CI, confidence interval; IVW, inverse-variance weighted method.
3.3 Results of Meta-analysis
3.3.1 Meta-analysis to evaluate effect of appendectomy on CRC
Figure 4 showed results of Meta-analysis, which evaluated effect of appendectomy on CRC. The results of heterogeneity test showed that the common effect model should be selected (P were all greater than 0.05). Figure 4A showed appendectomy did not increase risk of CC(OR:1.013, 95%CI:0.996-1.030, P=0.144); Figure 4B showed appendectomy did not increase risk of RC(OR:0.994, 95%CI:0.974-1.014, P=0.550); Figure 4C showed appendectomy did not increase risk of CRC(OR:1.005, 95%CI:0.992-1.018, P=0.459).
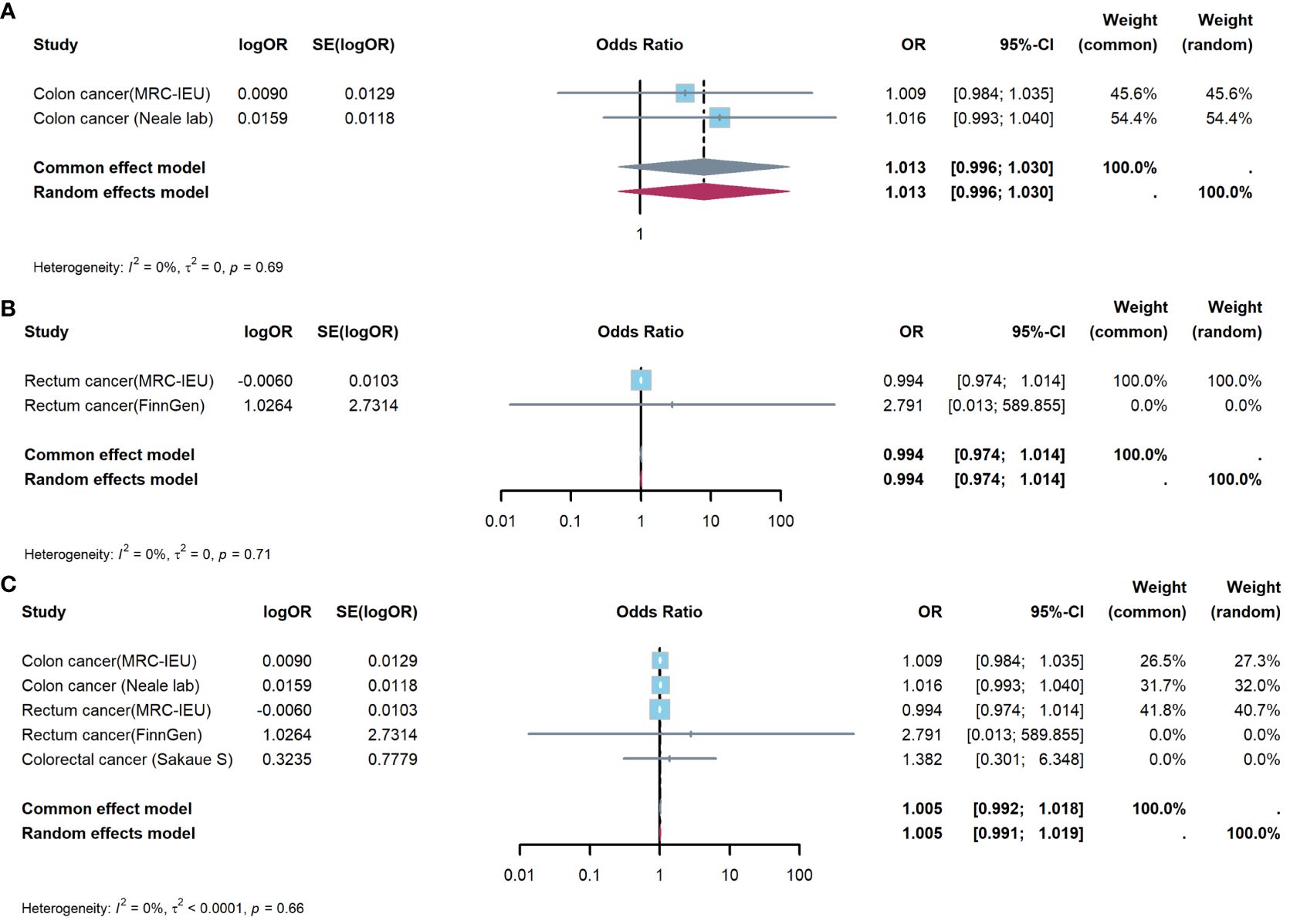
Figure 4 Meta-analysis of IVW results to predict associations of appendectomy with colorectal cancer. (A) appendectomy and colon cancer; (B) appendectomy and rectum cancer; (C) appendectomy and colorectal cancer. IVW, inverse-variance weighted method; OR, odds ratio; CI, confidence interval.
3.3.2 Meta-analysis to evaluate effect of appendicitis on CRC
Figure 5 showed results of Meta-analysis, which evaluated effect of appendicitis on CRC. The results of heterogeneity test showed that the common effect model should be selected (P were all greater than 0.05). Figure 5A showed appendicitis did not increase risk of CC(OR:1.000, 95%CI:1.000-1.000, P=1.000); Figure 5B showed appendicitis did not increase risk of RC(OR:1.000, 95%CI:0.999-1.000, P=0.998); Figure 5C showed appendicitis did not increase risk of CRC(OR:1.000, 95%CI:1.000-1.000, P=0.999).
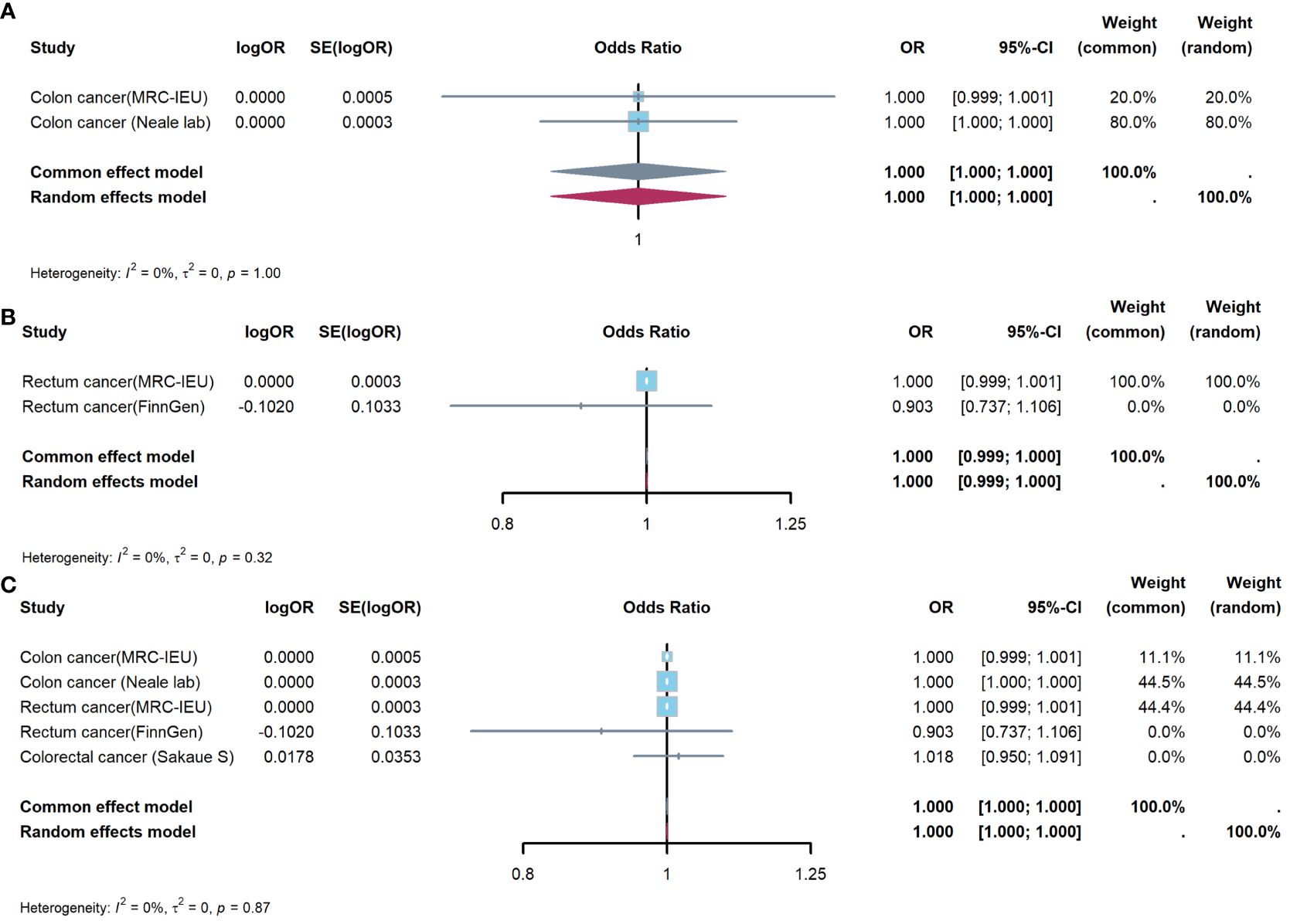
Figure 5 Meta-analysis of IVW results to predict associations of appendicitis with colorectal cancer. (A) appendicitis and colon cancer; (B) appendicitis and rectum cancer; (C) appendicitis and colorectal cancer. IVW, inverse-variance weighted method; OR, odds ratio; CI, confidence interval.
4 Discussion
The incidence of appendicitis is extremely high in the world, and appendectomy is the definitive treatment of appendicitis (1, 2, 6–8). However, whether appendicitis and appendectomy increase the risk of long-term CRC has been controversial (9–14). One of the reasons for the controversy is that retrospective studies and animal experiments show opposite results, while RCTs cannot be carried out (9–14). The evidence of MR is equivalent to that of RCT, which provides a possible way to resolve this controversy (17).
We selected several GWAS summary data for CRC, and IVW results showed that appendicitis and appendectomy did not increase the risk of CRC (P were all greater than 0.05). In order to further increased the robustness of the results, we used Meta-analysis to summarize the results of IVW analysis. The results of Meta-analysis showed appendectomy (P=0.459) and appendicitis (P=0.999) did not increase the risk of CRC, which were consistent with results of IVW. Our analysis confirms previous findings from Song and Mándi et al. (21, 22).
Meta-analysis from Joseph et al. showed appendectomy was associated with a lower risk of CC (HR = 0.90, 95% CI = 0.81‐0.99) and distal CC (HR = 0.77, 95% CI = 0.65‐0.90) (13). Research results of van et al. showed appendectomy was associated with a lower risk of gastrointestinal cancer (HR 0.75, 95% CI 0.56-0.99), in particular CC (HR 0.65, 95% 0.43-0.97) (14). A possible explanation is that the appendix is the germinal center of lymphoid follicles, and removing the appendix can help reduce intestinal inflammation (13, 23). It is well known that long-term chronic inflammation of gut is one of the causes of CRC; Secondly, the occurrence and progression of CRC are related to imbalance of gut microbes (13, 24). There are opportunistic pathogenic bacteria in the appendix cavity, and removal of the appendix reduces the possibility of opportunistic pathogenic bacteria entering the colorectum (24). However, other studies found that appendectomy increased the risk of CRC, and even appendicitis itself was a precursor to colorectal cancer (9–12, 25–27):results from Shi et al. showed a 73.0% increase in CRC risk among appendectomy cases throughout 20 years follow-up (SHR:1.73, 95% CI:1.49-2.01, P < 0.001) (9) and results from Lai et al. showed the odds ratio of CC incidence had a 38.5-fold increase among patients older than 40 with AA (25). The CRC risk of appendectomy and appendicitis may be due to the following reasons: first, the appendix is closely related to colorectal immunity, and removal of the appendix will reduce the colorectal immune surveillance function (28, 29); Secondly, the appendix regulates the gut microbes in the colorectum, and removal of the appendix and appendicitis can aggravate gut microbes disorders (24, 30, 31). The reason for these differences may be due to the limitations of observational studies, and the evidence of this study is equivalent to RCT, which is more convincing.
Several limitations to this study deserve our attention: First, this study mainly included GWAS summary data of Europeans, and the results may not be extended to other ethnic groups; Second, subgroup analysis was not possible in this study. Stratification based on age, comorbidities, etc. may have different results; Finally, selection of SNPs is difficult. There may be confounders that cannot be identified with current knowledge.
5 Conclusions
Appendectomy and appendicitis do not increase the risk of colorectal cancer. More clinical trials are needed in the future to verify the causal relationships.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
WW: Data curation, Methodology, Writing – original draft. JW: Formal analysis, Funding acquisition, Writing – original draft. DY: Formal analysis, Writing – review & editing. WL: Writing – review & editing. LZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. General Program of Xi’an Health Commission 2020ms04.
Acknowledgments
We are indebted to all individuals who participated in or helped with this research project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1414946/full#supplementary-material
References
1. Stewart B, Khanduri P, McCord C, Ohene-Yeboah M, Uranues S, Vega Rivera F, et al. Global disease burden of conditions requiring emergency surgery. Br J Surg. (2014) 101:e9–e22. doi: 10.1002/bjs.9329
2. Lee JH, Park YS, Choi JS. The epidemiology of appendicitis and appendectomy in South Korea: national registry data. J Epidemiol. (2010) 20:97–105. doi: 10.2188/jea.JE20090011
3. Golz RA, Flum DR, Sanchez SE, Liu X, Donovan C, Drake FT. Geographic association between incidence of acute appendicitis and socioeconomic status. JAMA Surg. (2020) 155:330–8. doi: 10.1001/jamasurg.2019.6030
5. Yang Y, Guo C, Gu Z, Hua J, Zhang J, Qian S, et al. The global burden of appendicitis in 204 countries and territories from 1990 to 2019. Clin Epidemiol. (2022) 14:1487–99. doi: 10.2147/CLEP.S376665
6. Pátková B, Svenningsson A, Almström M, Svensson JF, Eriksson S, Wester T, et al. Long-term outcome of nonoperative treatment of appendicitis. JAMA Surg. (2023) 158:1105–6. doi: 10.1001/jamasurg.2023.2756
7. Talan DA. Diagnosis and management of acute appendicitis. JAMA. (2022) 327:1183. doi: 10.1001/jama.2022.1262
8. Blohs M, Mahnert A, Brunnader K, Flucher C, Castellani C, Till H, et al. Acute appendicitis manifests as two microbiome state types with oral pathogens influencing severity. Gut Microbes. (2023) 15:2145845. doi: 10.1080/19490976.2022.2145845
9. Shi F, Liu G, Lin Y, Guo CL, Han J, Chu ESH, et al. Altered gut microbiome composition by appendectomy contributes to colorectal cancer. Oncogene. (2023) 42:530–40. doi: 10.1038/s41388-022-02569-3
10. Chen L, Ma X, Dong H, Qu B, Yang T, Xu M, et al. Construction and assessment of a joint prediction model and nomogram for colorectal cancer. J Gastrointest Oncol. (2022) 13:2406–14. doi: 10.21037/jgo-22-917
11. Viennet M, Tapia S, Cottenet J, Bernard A, Ortega-Deballon P, Quantin C.. Increased risk of colon cancer after acute appendicitis: a nationwide, population-based study. EClinicalMedicine. (2023) 63:102196. doi: 10.1016/j.eclinm.2023.102196
12. Lee MS, Thomas A, Pearson JF, Purcell R, Frizelle F, Glyn T. Risk of colorectal cancer in patients with appendicitis over the age of 40 years. Colorectal Dis. (2023) 25:624–30. doi: 10.1111/codi.16429
13. Rothwell JA, Mori N, Artaud F, Fournier A, Conte M, Boutron-Ruault MC, et al. Colorectal cancer risk following appendectomy: a pooled analysis of three large prospective cohort studies. Cancer Commun (Lond). (2022) 42:486–9. doi: 10.1002/cac2.12265
14. van den Boom AL, Lavrijssen BDA, Fest J, Ikram MA, Stricker BH, van Eijck CHJ, et al. Appendectomy and the subsequent risk of cancer: A prospective population-based cohort study with long follow-up. Cancer Epidemiol. (2022) 77:102120. doi: 10.1016/j.canep.2022.102120
15. Larsson SC, Butterworth AS, Burgess S. Mendelian randomization for cardiovascular diseases: principles and applications. Eur Heart J. (2023) 44:4913–24. doi: 10.1093/eurheartj/ehad736
16. Gupta V, Walia GK, Sachdeva MP. 'Mendelian randomization': an approach for exploring causal relations in epidemiology. Public Health. (2017) 145:113–9. doi: 10.1016/j.puhe.2016.12.033
17. Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. (2021) 36:465–78. doi: 10.1007/s10654-021-00757-1
18. Perry BI, Upthegrove R, Kappelmann N, Jones PB, Burgess S, Khandaker GM. Associations of immunological proteins/traits with schizophrenia, major depression and bipolar disorder: A bi-directional two-sample mendelian randomization study. Brain Behav Immun. (2021) 97:176–85. doi: 10.1016/j.bbi.2021.07.009
19. Zhu S, Hu X, Fan Y. Association of triglyceride levels and prostate cancer: a Mendelian randomization study. BMC Urol. (2022) 22:167. doi: 10.1186/s12894-022-01120-6
20. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology. (2017) 28:30–42. doi: 10.1097/EDE.0000000000000559
21. Song H, Abnet CC, Andrén-Sandberg Å, Chaturvedi AK, Ye W. Risk of Gastrointestinal Cancers among Patients with Appendectomy: A Large-Scale Swedish Register-Based Cohort Study during 1970-2009. PloS One. (2016) 11:e0151262. doi: 10.1371/journal.pone.0151262
22. Mándi M, Keleti G, Juhász M. The role of appendectomy and cholecystectomy in the pathogenesis of colorectal carcinomas. Ann Med Surg (Lond). (2021) 72:102991. doi: 10.1016/j.amsu.2021.102991
23. Kooij IA, Sahami S, Meijer SL, Buskens CJ, Te Velde AA. The immunology of the vermiform appendix: a review of the literature. Clin Exp Immunol. (2016) 186:1–9. doi: 10.1111/cei.12821
24. Randal Bollinger R, Barbas AS, Bush EL, Bush EL, Lin SS, Parker W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J Theor Biol. (2007) 249:826–31. doi: 10.1016/j.jtbi.2007.08.032
25. Lai HW, Loong CC, Tai LC, Wu CW, Lui WY. Incidence and odds ratio of appendicitis as first manifestation of colon cancer: a retrospective analysis of 1873 patients. J Gastroenterol Hepatol. (2006) 21:1693–6. doi: 10.1111/j.1440-1746.2006.04426.x
26. Wu SC, Chen WT, Muo CH, Sung FC. Appendicitis as an early manifestation of subsequent Malignancy: an asian population study. PloS One. (2015) 10:e0122725. doi: 10.1371/journal.pone.0122725
27. Liu Z, Ma X, Zhu C, Fang JY. Risk of colorectal cancer after appendectomy: A systematic review and meta-analysis. J Gastroenterol Hepatol. (2023) 38:350–8. doi: 10.1111/jgh.16108
28. Collard MK, Tourneur-Marsille J, Uzzan M, Albuquerque M, Roy M, Dumay A, et al. The appendix orchestrates T-cell mediated immunosurveillance in colitis-associated cancer. Cell Mol Gastroenterol Hepatol. (2023) 15:665–87. doi: 10.1016/j.jcmgh.2022.10.016
29. Younginger BS, Mayba O, Reeder J, Nagarkar DR, Modrusan Z, Albert ML, et al. Enrichment of oral-derived bacteria in inflamed colorectal tumors and distinct associations of Fusobacterium in the mesenchymal subtype. Cell Rep Med. (2023) 4:100920. doi: 10.1016/j.xcrm.2023.100920
30. Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol. (2017) 32:43–53. doi: 10.1016/j.smim.2017.09.006
Keywords: appendectomy, appendicitis, colorectal cancer, Mendelian randomization, risk
Citation: Wei W, Wang J, Yu D, Liu W and Zong L (2024) Appendectomy and appendicitis do not increase colorectal cancer risk: evidence from Mendelian randomization. Front. Oncol. 14:1414946. doi: 10.3389/fonc.2024.1414946
Received: 09 April 2024; Accepted: 08 July 2024;
Published: 22 July 2024.
Edited by:
Gaetano Gallo, Sapienza University of Rome, ItalyReviewed by:
Sara Claudia Barone, Sapienza University of Rome, ItalyFlaminia Genualdo, Sapienza University of Rome, Italy
Copyright © 2024 Wei, Wang, Yu, Liu and Zong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zong, em9uZ2xlaTc5MTBAMTYzLmNvbQ==
 Wei Wei1
Wei Wei1 Daihua Yu
Daihua Yu Wei Liu
Wei Liu Lei Zong
Lei Zong