- 1Section of Medical Oncology, Department of Precision Medicine in Medical, Surgical and Critical Care (Me.Pre.C.C.), University of Palermo, Palermo, Italy
- 2U.O. Oncologia, Fondazione Istituto G. Giglio, Cefalù, Palermo, Italy
- 3Division of General and Oncological Surgery, Department of Precision Medicine in Medical, Surgical and Critical Care (Me.Pre.C.C.), University of Palermo, Palermo, Italy
- 4Department of Biomedicine, Neuroscience and Advanced Diagnostics, University of Palermo, Palermo, Italy
Introduction: Although hereditary male neoplasms are quite rare, individuals harbouring germline BRCA1/2 pathogenic variants (PVs) may have a risk of developing tumours associated with Hereditary Breast and Ovarian Cancer (HBOC) syndrome, including male breast (MBC), prostate (PCa) and pancreatic (PC) cancers, and melanoma. Women and men showed a comparable genetic architecture of cancer susceptibility, but there are some gender-specific features. Since little is known about cancer genetic susceptibility in male population, our study was aimed at investigating the frequency of BRCA1/2 PVs in men with HBOC syndrome-associated tumors, in order to understand whether differences in gender may reflect in the prevalence and spectrum of germline alterations.
Patients and methods: We retrospectively collected and analysed clinical information of 352 HBOC-associated male cancer patients genetically tested for germline BRCA1/2 PVs by Next-Generation Sequencing analysis, enrolled, from February 2018 to January 2024, at the “Regional Center for the prevention, diagnosis and treatment of rare and heredo-familial tumors of adults” of the University-Hospital Policlinico “P. Giaccone” of Palermo (Italy).
Results: Our investigation revealed that 7.4% of patients was carrier of a germline BRCA PV, with an almost total prevalence of BRCA2 alterations. In particular, 65.4% of BRCA-positive patients developed MBC, 19.2% had PC, 11.6% developed PCa, and only 3.8% had melanoma. Specifically, MBC individuals showed a BRCA-associated genetic predisposition in 17% of cases, whereas patients with PCa or PC exhibited a lower frequency of BRCA2 PVs, taking into account the current national criteria for access to germline genetic testing.
Discussion: Our study showed a high heterogeneity in prevalence of germline BRCA2 PVs among men which could reflect a potential gender-specific genetic heterogeneity. Therefore, BRCA-associated male tumours could be due to BRCA2 PVs different from those usually detected in women. In the event that it is demonstrated, in future, that male cancers are genetically distinct entities from those female this could improve personalized risk evaluation and guide therapeutic choices for patients of both sexes, in order to obtain a gender equality in cancer care.
1 Introduction
Germline pathogenic and likely pathogenic variants (herein called pathogenic variants, PVs) in the tumour suppressor genes BRCA1 and BRCA2 are predominantly associated with a significantly increased risk of breast and ovarian cancer in women, breast cancer (BC) and prostate cancer (PCa) in men, and pancreatic cancer (PC) and melanoma in both genders, with different risk rates (1). These BRCA-related tumours are associated with the hereditary breast and ovarian cancer (HBOC) syndrome, an inherited disorder which follows an autosomal dominant transmission mode (2).
Male breast cancer (MBC) is rare, accounting for about 1% of all BC diagnoses worldwide and less than 1% of cancers detected among men, with an increasing incidence (3, 4). In the general male population, the lifetime risk of developing BC is 0.1%, increasing up to 1.2% and 6.8% in the carriers of germline PVs in BRCA1 and BRCA2, respectively (5). Due to the extremely low incidence rate and the few conducted large-scale studies, current knowledge about MBC is low and treatment recommendations for male patients have largely followed those for postmenopausal women, even though MBC is a distinct tumour with different molecular and clinico-pathological features (6). Approximately 10% of all MBCs are hereditary tumours caused by germline alterations in well-defined BC susceptibility genes (7). Among these, BRCA1, and most commonly BRCA2, represent the most frequently involved high-penetrance genes, whose alterations are responsible for about 10–16% and 60–76% of hereditary MBC cases, respectively (8). The first multicenter study carried out in Italy showed a frequency of BRCA1/2 PVs of about 13% (9).
BRCA-related MBCs have been shown to have specific molecular phenotypes and clinical characteristics (10). In particular, most BRCA1-related MBCs are grade 3, HER2-negative (HER2-) tumours with high proliferative activity, whereas BRCA2-associated MBCs are high-grade, HER2-positive (HER2+) tumours and show absence of progesterone receptor (PR) expression (7, 9).
PCa is the second most diagnosed cancer in men and the fifth leading cause of male cancer death, globally accounting for about 7% of newly diagnosed cases (11). Approximately 85% of new diagnoses involves individuals over 60 years of age (12).
PCa is a complex and heterogeneous neoplasm, classified as aggressive or non-aggressive, high-grade or low-grade, or early-onset (if occurring before age 55) or indolent (13). The PCa risk is 6% in the general population, while it rises up to about 9% in individuals harbouring BRCA1 germline mutations and 15% in carriers of BRCA2 PVs (5). PCa is diagnosed based on histologic subtype, location and Gleason score, which is currently the strongest prognostic factor (13).
Age, family history and genetic predisposition associated with germline BRCA1/2 PVs have also been identified as important risk factors (14). In fact, the incidence of alterations in DNA damage repair defect (DDR) genes accounts for about 25% (15), with a higher prevalence in metastatic than localized disease, at about 12–16% and 5%, respectively (14). Inherited BRCA2 PVs, most frequently detected in PCa, were found in about 3% of patients and were associated with early-onset, high-grade, greater aggressiveness and worse outcomes (15, 16).
PC is the 12th most frequent tumour and the sixth leading cause of death among men, with an incidence rate increasing by 0.5–1% per year and a death-to-incidence ratio of approximately 94% (11, 17). Most PCs are ductal adenocarcinomas (PDACs) of the exocrine pancreatic glands, accounting for about 85% of cases, frequently located in the head of the pancreas (18). Although they are predominantly of sporadic nature, a small percentage of PDAC is of familial origin and exhibits PVs which increase cancer genetic susceptibility. These alterations occur in BRCA1/2 genes, predominantly in BRCA2, but also in mismatch repair genes, such as MLH1, MSH2, MSH6 and PMS2, responsible for Lynch syndrome (19, 20).
In individuals with family history of cancer, 5–10% carries PVs in BRCA2 gene and 1% in BRCA1 with a risk of developing PC of 5 and 10%, respectively (16).
Another tumour which has been shown to be associated with germline BRCA alterations is melanoma. Although studies assessing the association between melanoma and BRCA are restricted and often inconclusive, BRCA2 mutation carriers have been shown to have an increased risk of melanoma (21). The Breast Cancer Linkage Consortium study showed that carriers of BRCA2 PVs were 2.5 times more likely to develop melanoma compared to the general population (22). Furthermore, also the studies by Moran et al. (23) and Johannsson et al. (24) confirmed an increased risk of melanoma in the presence of germline BRCA2 PVs.
Several studies reported a significant heterogeneity in the prevalence of PVs across different populations. Because germline BRCA PVs confer an increased risk of different types of cancer and the prevalence of genetic alterations differs by ethnicity, race, gender, and different geographic location (25, 26), it could be interesting to investigate the prevalence of germline variants in Sicilian male patients affected by tumours included in the spectrum of the HBOC syndrome. For this purpose, in this work, we genetically tested Sicilian male patients with MBC, PCa, PC and melanoma for germline BRCA1/2 PVs, in order to assess the type and prevalence of these high-risk susceptibility variants in individuals from the southernmost region of Italy.
2 Patients and methods
2.1 Study cohort
This retrospective cohort study was carried out from February 2018 to January 2024 at the “Sicilian Regional Centre for the Prevention, Diagnosis and Treatment of Rare and Hereditary Tumours” of the Medical Oncology Section of the “P. Giaccone” University Hospital in Palermo (Italy). The study involved 352 male cancer patients, including 100 consecutively recruited subjects with BC, 59 with PCa, 95 with PC and 98 with melanoma, taking into account the current national criteria for access to germline genetic testing. All patients, who had previously signed and accepted a written informed consent to study, were genetically tested for BRCA1/2 PVs. The study (Protocol ‘G-Land 2017’) has been approved by the ethical committee (Comitato Etico Palermo 1; approval number: 0103–2017) of the university-affiliated hospital AOUP ‘P. Giaccone’ of Palermo. All relevant personal, family and clinical history information, including age at cancer diagnosis, histological subtype and stage of disease, was acquired and recorded anonymously for all patients, during a genetic counselling involving the presence of a multidisciplinary team made up of a geneticist, an oncologist, and a psychologist. The data on cancer diagnosis and histological subtype was recovered by pathology reports.
Following the genetic counselling for assessment of risk of HBOC syndrome, the patients were selected for germline BRCA1/2 mutational screening based on probability rate of harbouring a PV, evaluated through the BRCAPRO genetic risk prediction model (27) and according to the criteria established by the Italian Association of Medical Oncology (AIOM) (https://www.aiom.it/raccomandazioni-per-limplementazione-del-test-brca-predittivo-e-preventivo-nei-tumori-della-mammella-dellovaio-del-pancreas-e-della-prostata/ and https://www.aiom.it/linee-guida-aiom-2023-melanoma/). These criteria, need to identify subjects at high risk of carrying a PV in the HBOC syndrome predisposition genes, rely on personal and family history of cancer and/or age at diagnosis. Specifically, on the basis of the different tumours included in the study, the selection of patients to be genetically tested for the search for germline BRCA1/2 PVs was carried out using the following criteria: 1) MBC patients, regardless age of onset and family history of cancer; 2) metastatic PCa individuals, regardless age of onset and family history of cancer, and non-metastatic PCa patients with family history of no-Grade Group 1 PCa (according to the International Society of Urological Pathologists) diagnosed in at least one first-degree relative below age 60 years or in at least two family members below age 50 years (28); 3) metastatic/locally advanced PC individuals, regardless age at diagnosis and family history of cancer; 4) patients with personal history of synchronous/metachronous multiple melanoma (even in the absence of family history), melanoma patients with at least one affected first-degree relative in the same branch of the family, regardless age at diagnosis, and melanoma individuals with personal and/or family history of pancreatic adenocarcinoma.
The germline test result was considered informative when a pathogenic variant (PV) or likely pathogenic variant (LPV) was identified. Conversely, the test result was considered non-informative when no PV/LPV was identified, but its presence could not be excluded, or a variant of uncertain significance (VUS) was detected to which a risk value could not be attributed (29).
Carriers of a germline BRCA1/2 PV have been subjected to intensive surveillance programs drawn up by an oncologist with expertise in cancer genetics. Targeted BRCA1/2 testing has been proposed and extended to the first-degree family members of BRCA-mutated patients, after providing informed consent.
2.2 Sample collection and BRCA1/2 genetic testing
Peripheral blood was collected from all patients included in the study. Genomic DNA was extracted from the peripheral leucocytes, using the DNeasy® Blood Kit (QIAGEN, Hilden, Germany), and quantified by Qubit®3.0 fluorometer (Thermo Fisher Scientific, Waltham, MA). Its quality was assessed using 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). The genetic analysis for identifying the BRCA1/2 variants was carried out through next-generation sequencing (NGS) as previously described (26, 30). Furthermore, a Multiplex Ligation-dependent Probe Amplification (MLPA) analysis, performed as previously described (26, 30), was used to eventually detect the presence of large genomic rearrangements (LGRs) in BRCA1 and BRCA2 genes.
2.3 DNA Sanger sequencing
BRCA1/2 PVs/LPVs were confirmed by Sanger sequencing using a BigDye Therminator 3.1 Cycle Sequencing Kit (Life Technologies, Carlsbad, CA, USA) and read through the 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA), according to the manufacturers’ protocols.
2.4 Genetic variant classification
The detected genetic variants were classified based on the criteria developed by the Evidence-based Network for the Interpretation of Germline Mutant Alleles (ENIGMA) consortium (https://enigmaconsortium.org/) and according to the recommendations of the International Agency for Research on Cancer (IARC) (31). The classification involves a five-class division system: class I (benign variant), class II (probably benign variant), class III (VUS), class IV (probably PV), class V (PV). Several databases were used for the identification and classification of genetic variants, such as ClinVar, BRCA Exchange, LOVD and Varsome. The variants detected were denominated according to the recommendations for the description of sequence variants provided by the Human Genome Variation Society (HGVS). The HGVS nomenclature has been endorsed by HGVS, the Human Variome Project (HVP) and the Human Genome Organisation (HUGO) (32).
The localization of the germline variants on BRCA1 and BRCA2 genes detected in genetically tested patients was obtained and graphically represented using the informatic tool Mutation Mapper-cBioPortal for Cancer Genomics (33, 34).
3 Results
3.1 Clinico-pathological features of male cancer patients undergoing BRCA1/2 genetic testing
Three hundred and fifty-two male cancer patients affected by MBC, PCa, PC and melanoma were recruited and studied over a period ranging from February 2018 to January 2024 at the “Regional Center for the prevention, diagnosis and treatment of rare and heredo-familial tumors of adults” of the Section of Medical Oncology of the University Hospital Policlinico “P. Giaccone” of Palermo (Italy). Among 352 recruited male individuals, 100 (28.4%) were affected by MBC, 59 (16.8%) by PCa, 95 (27%) by PC and 98 (27.8%) by melanoma.
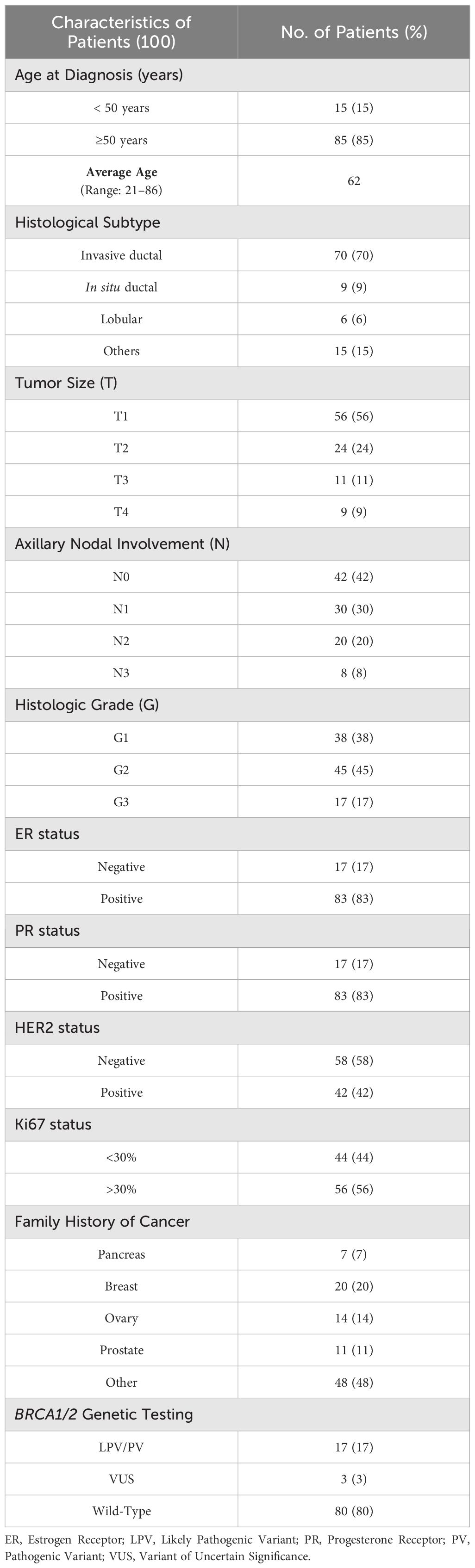
One hundred MBC patients showed an average age at the diagnosis of 62 years. Considering the histological subtype, 9 (9%) out of 100 patients had in situ ductal carcinoma (DCIS), 70 (70%) invasive ductal carcinoma (IDC), 6 (6%) invasive lobular carcinoma (ILC) and, finally, 15 patients showed other types of BC. The most representative molecular phenotypes of MBC were luminal B/HER2- and luminal A (63% and 33%, respectively). Considering the family history, 20 patients (20%) had two or more family members affected by BC, 14 and 11 patients (14% and 11%, respectively) had a family history of ovarian cancer and PCa, respectively, and only 7 patients (7%) had one relative affected by PC. The clinical-pathological features of 100 MBC patients are summarized in Table 1.
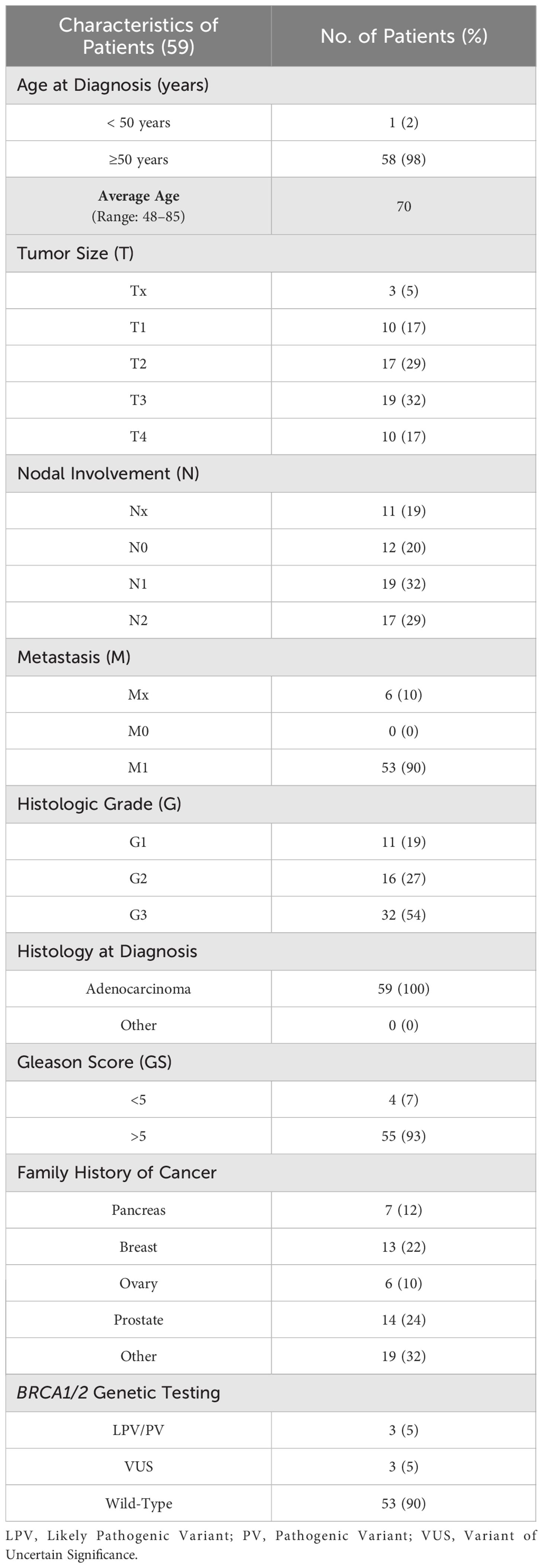
Considering the fifty-nine (16.8%) patients affected by PCa, the average age at diagnosis was 70 years, according to literature data (35). All patients presented a histological diagnosis of adenocarcinoma, poorly differentiated (G3) in 54% of cases, with a Gleason Score (GS) > 5 in 93% of individuals. Fourteen (24%) out of 59 patients showed a family history of PCa, whereas 13 (22%) subjects had at least one relative affected by BC (Table 2).
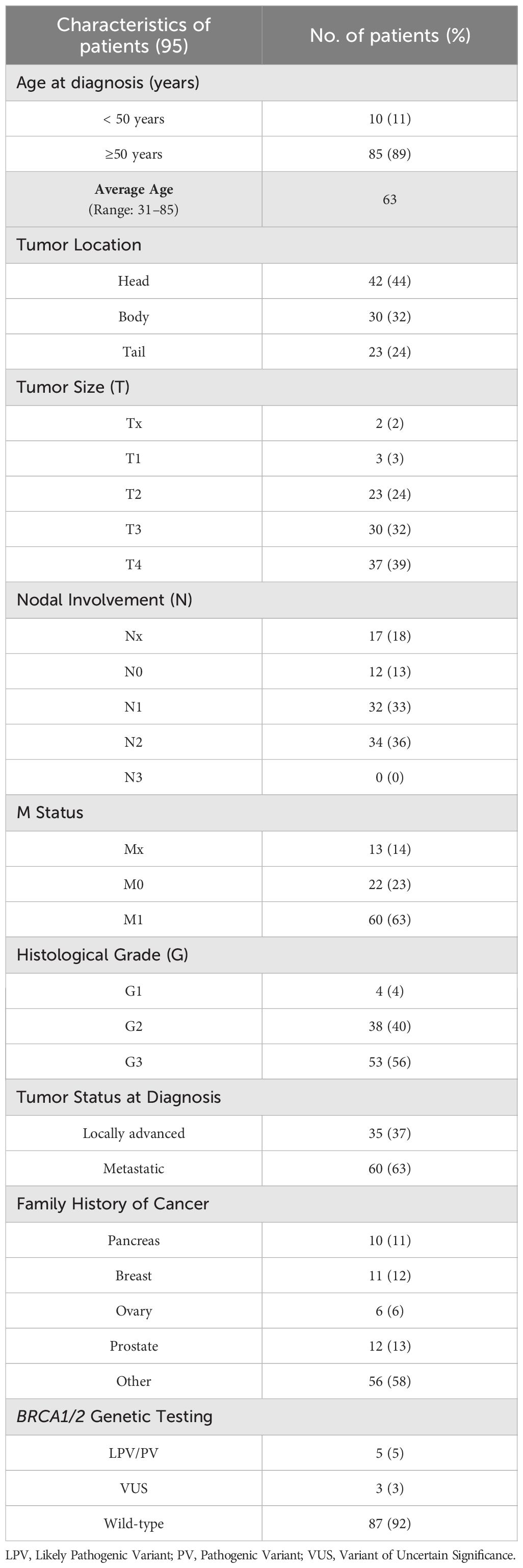
The average age at diagnosis of 95 patients with PC was 63 years. In 42 (44%) individuals the tumor was localized in the pancreas head, whereas 30 (32%) and 23 (24%) patients had a tumor in the body and tail, respectively. At diagnosis time, 60 (63%) patients had a metastatic tumor and 35 (37%) a locally advanced cancer. Ten (11%) out of 95 patients had a family history of PC, 11 (12%) subjects had at least a relative with BC, and 12 (13%) showed a positive family history for PCa (Table 3).
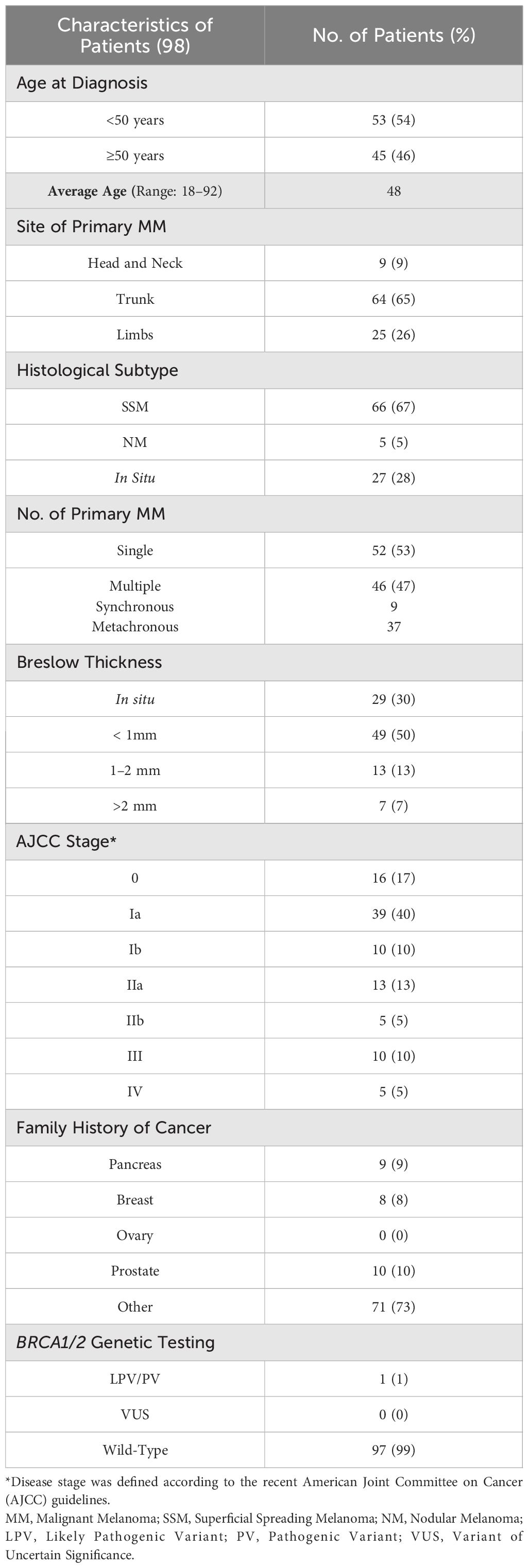
Finally, the average age at diagnosis of 98 investigated patients with melanoma was 48 years. In 64 (65%) out of 98 patients the primary tumors were mainly located in the trunk, whereas 25 (26%) individuals presented melanomas in limbs and 9 (9%) patients in head and neck region. The most frequent histological tumor subtypes observed in patients enrolled in this study were superficial spreading melanoma (67%) followed by in situ and nodular melanoma (28% and 5%, respectively). Ten patients (10%) had a family history of PCa, whereas 9 (9%) and 8 (8%) patients had a positive family history for PC and BC, respectively (Table 4).
3.2 Germline BRCA1/2 pathogenic variants in male patients with HBOC syndrome-associated tumours
All 352 male probands with HBOC syndrome-associated tumours, after appropriate genetic counselling, aimed at ascertaining the criteria concerning personal and family history of cancer recommended by the AIOM national guidelines, were genetically tested for germline variants in BRCA1 and BRCA2 genes. The mutational screening of the investigated study population showed that 26 (7.4%) out of 352 patients with MBC, PCa, PC or melanoma harboured germline BRCA PVs (BRCA-positive), whereas 9 (2.6%) probands were carriers of germline BRCA1/2 VUS (class III), and 317 (90%) subjects carried a germline BRCA1/2 benign/likely benign variants (BRCA-w.t.) (Figure 1). No LGR in BRCA1/2 genes was detected in examined study cohort.
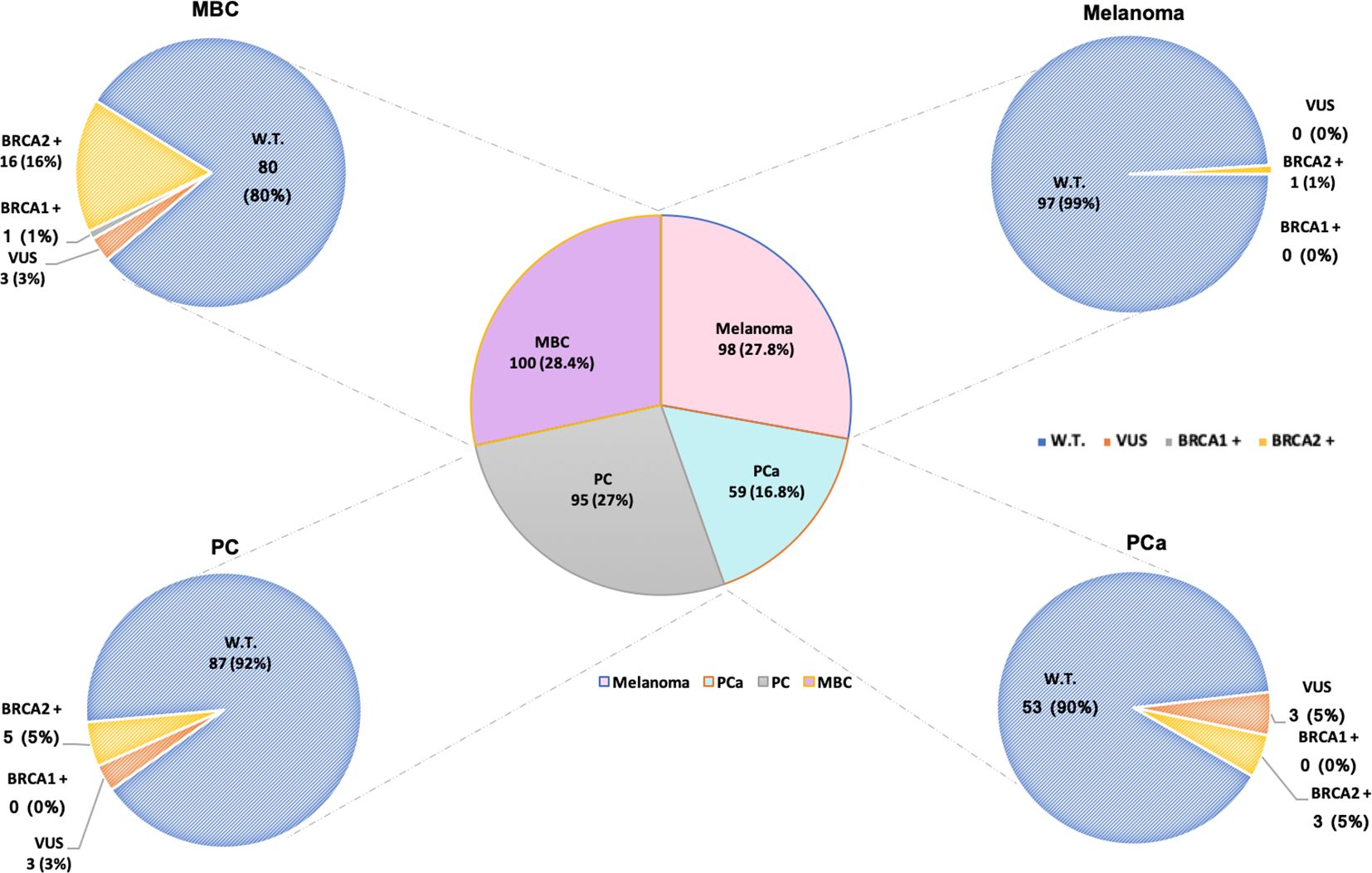
Figure 1 Number of male patients affected by HBOC syndrome-associated tumors genetically tested for germline BRCA1/2 variants. Total number of analyzed patients, on the basis of the different tumors, is divided into carriers of BRCA1/2 wild-type (blue), variants of uncertain significance (VUS) in BRCA1/2 (orange), and pathogenic/likely pathogenic variants (PVs/LPVs) in BRCA1 (grey) and BRCA2 (yellow) genes, respectively. Patients harboring benign/likely benign variants (BVs/LBVs) are considered carriers of BRCA1/2 wild-type.
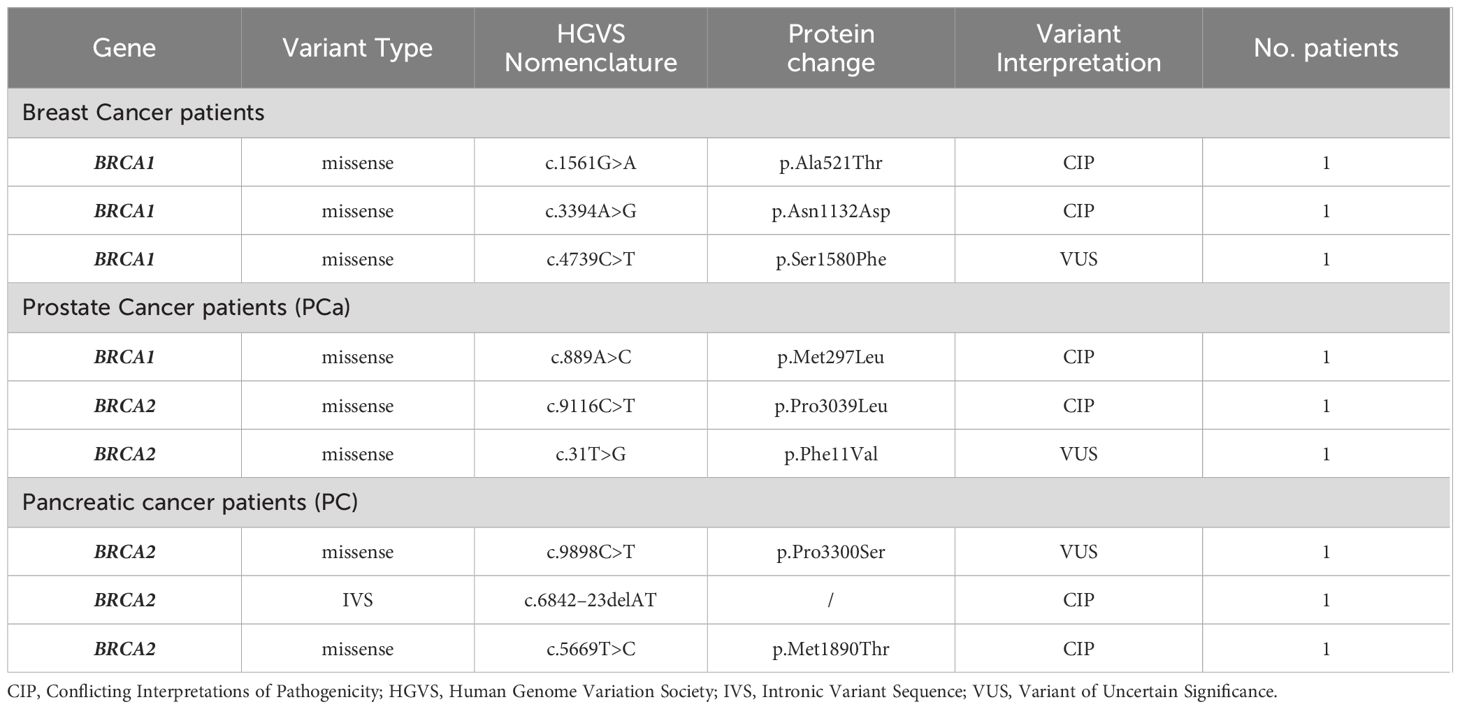
In particular, our genetic analysis showed that 17 (65.4%) out of 26 male individuals positively tested for BRCA PVs had MBC, whereas 5 (19.2%) were affected by PC, 3 (11.6%) showed PCa and only 1 (3.8%) had melanoma (Table 5). Based on the classification criteria developed by the ENIGMA consortium and according to the IARC recommendations (31), the mutational analysis revealed the presence of 20 different BRCA PVs, 19 of which in BRCA2 and only one in BRCA1, detected in 26 tested patients. The BRCA2 variant named c.4284dup (p.Gln1429fs) has been observed in both melanoma and MBC, whereas another BRCA2 variant named c.7681C>T (p.Gln2561Ter) was present in one individual with MBC and one with PC (Table 5). Both BRCA2 variants have been previously detected with low frequency rates (0.82% and 1.03%, respectively) in women of the same population affected by breast and/or ovarian cancer (26).
However, no variant in particular has shown high prevalence within the examined male population.
As regards the typology of germline BRCA PVs observed in our study population, approximately two-third of the variants were frameshift (7) and nonsense (7), whereas about one-third were intronic variant sequences (IVS, 3) and missense (3). All IVSs were detected only in men affected by PCa, while almost all found frameshift variants were present in MBC patients (Table 5).
Furthermore, we investigated the presence of VUS and other variants with conflicting interpretations of pathogenicity (CIP) in BRCA1/2 genes of 352 individuals, identifying 9 different VUS/CIP (4 in BRCA1 and 5 in BRCA2) in 9 patients, 3 of which had MBC, 3 showed PCa, and 3 were affected by PC. No germline BRCA1/2 VUS/CIP was detected in melanoma patients (Table 6).
Considering a distinction for neoplasm, among the 100 MBC patients, 16 were carriers of germline PVs in BRCA2 and only one in BRCA1, whereas 80 were BRCA1/2-wild-type (Figure 1). Specifically, 12 different PVs have been identified in BRCA2 gene and only one in BRCA1 gene. The BRCA2 variants named c.6078_6079del (p.Glu2028fs), c.9026_9030del (p.Tyr3009fs), c.1842dup (p.Asn615Ter) and c.1238del (p.Leu413Hisfs) have been detected each in two different MBC patients (Table 5).
Among 59 PCa patients, 53 (90%) showed no genetic susceptibility related to BRCA alterations (BRCA1/2-wild-type), whereas three men (5%) have been shown to be carriers of a germline PV in BRCA2 gene (Figure 1). All three BRCA2-mutated patients showed advanced disease and a significant family history of cancers associated with the HBOC syndrome. In particular, one of them had two first-degree relatives affected by PCa, whereas the other two showed a family history of female BC in three first-degree relatives (mother and sisters). No germline alteration was detected in BRCA1 gene. Likewise, in almost all men affected by PC (87/95, 92%) no BRCA-associated genetic predisposition was found, except in 5 (5%) patients with family history of cancer who harboured germline PVs only in BRCA2 gene. As regards the individuals with melanoma, only one out of 98 analyzed patients was carrier of a germline BRCA2 PV (Figure 1), showing a family history of female BC in two first-degree relatives (mother and sister).
Finally, our study also analyzed gene location of the germline BRCA PVs, in order to investigate eventual associations between specific variants and tumor phenotype, since several studies suggested a strong correlation between specific BRCA1/2 variants and changes in breast and ovarian cancer relative risk, by identifying specific putative Breast Cancer Cluster Regions (BCCRs) and Ovarian Cancer Cluster Regions (OCCRs), located on the coding DNA sequences of BRCA1 and BRCA2 genes (36–39). All BRCA2 variants detected in male cancer patients have been observed to be distributed along the entire BRCA2 gene sequence (Figure 2). However, in the case of MBC patients, half of these alterations (6/12) was mainly localized inside two putative cluster regions, BCCR1’ and BCCR2, present in the BRCA2 protein structure, near the N-terminus and C-terminus, respectively. Specifically, three BRCA2 variants were located in the N-terminal BCCR1’ region (nucleotides: 1238–1842; codons: 413–615), included inside the exon 10, whereas four variants (nucleotides: 7681–9030; codons: 2561–3009) were detected in the DNA sequence corresponding to the C-terminal DNA-binding domain (CTD), which includes the putative BCCR2 region. Additionally, other three BRCA2 PVs were located in the DNA sequence corresponding to the “BRC repeats” domain (nucleotides: 4284–6086; codons: 1429–2028), included within the exon 11 (Figure 2). Therefore, a correlation between the variant localization in the BCCRs of BRCA2 and type of tumor was observed in 50% (8/16) of BRCA2-positive MBC patients.
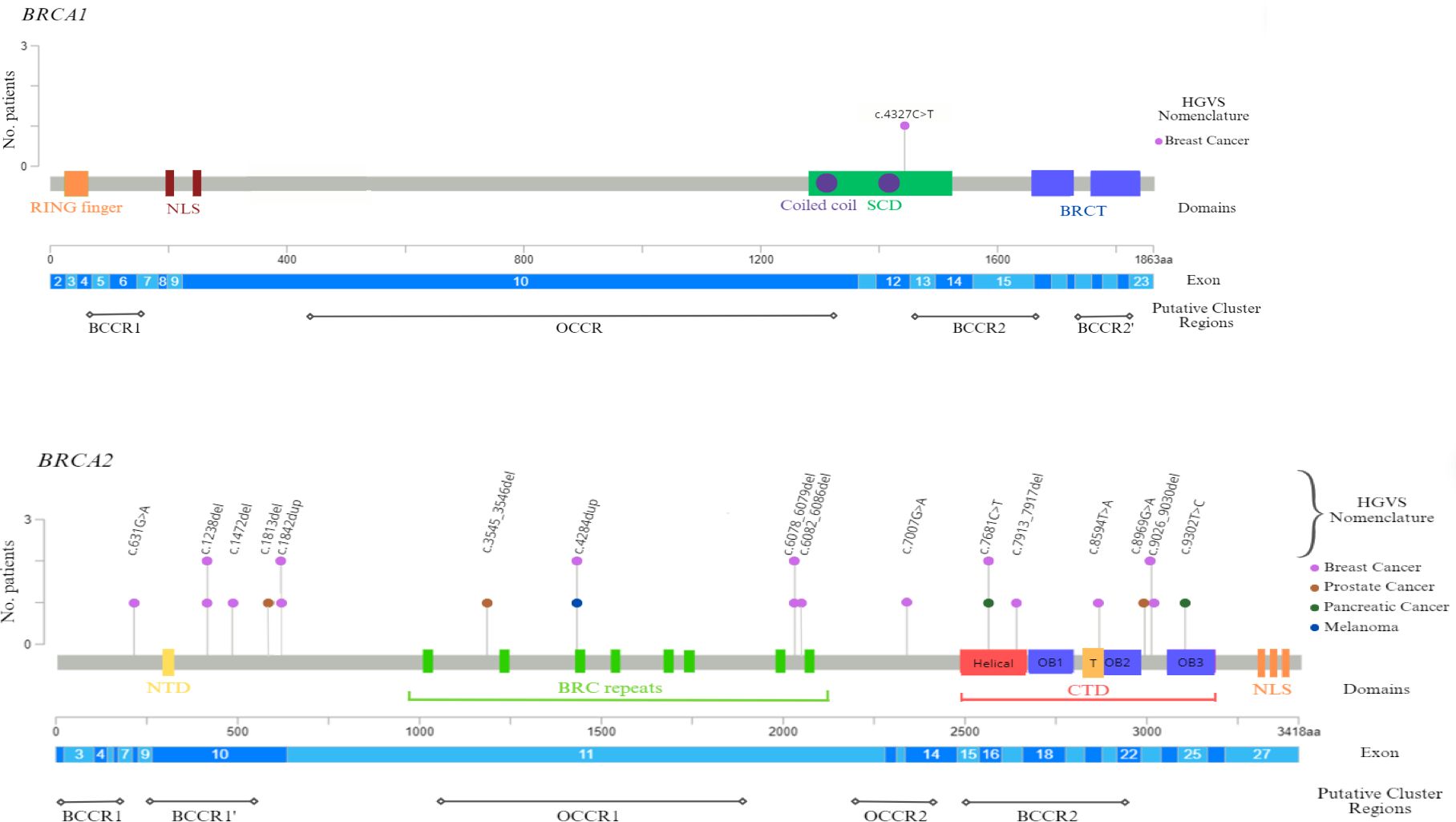
Figure 2 BRCA1 and BRCA2 functional domains and gene location of germline BRCA1/2 pathogenic variants detected in male cancer patients. The lollipop plots show the distribution and frequency of BRCA1/2 PVs identified in study population. The plots were obtained by the informatic tool Mutation Mapper-cBioPortal for Cancer Genomics (GenBank Reference BRCA1: NM_007294 and GenBank Reference BRCA2: NM_000059). The lollipop height indicates the frequency of BRCA1/2 PVs. BCCR, Breast Cancer Cluster Region; BRCT, BRCA1 C-terminus domain; CTD, C-terminal DNA-binding domain; HGVS, Human Genome Variation Society; NLS, nuclear localization sequence; NTD, N-terminal DNA-binding domain; OB, oligonucleotide binding; OCCR, Ovarian Cancer Cluster Region; SCD, serine cluster domain; T, Tower region.
4 Discussion
To date, extensive research on the risk of hereditary cancer and prevalence of BRCA1/2 PVs in women was performed, whereas little is known about this in men harboring germline BRCA1/2 alterations. Although women and men share a similar genetic architecture of cancer susceptibility, there are some sex-specific pathological features observed in BC (40, 41) (42) and potential additional differences that are currently being investigated by the CONFLUENCE project. Therefore, considering male cancers as genetically distinct entities from those female could improve the evaluation of personalized risk and drive therapeutic choices of patients of both sexes, with the purpose to obtain a gender equality in cancer care (43). Beyond the preventive significance for probands and family members, the BRCA1/2 genetic testing has recently assumed also a predictive value of response to specific biological therapies in patients affected by PCa or PC (44).
Previous results (26) regarding women from the same geographical area affected by breast and/or ovarian cancers showed a relatively lower frequency rate of BRCA1/2 PVs (14.8%, 200 out of 1346 probands) compared to males with BC investigated in our study (17%, 17 out of 100 probands). However, this data regards population cohorts different in numerical terms and by neoplasm, as ovarian cancer women have been also included in the studied population.
A significant heterogeneity in the prevalence of BRCA PVs across different populations was reported by several studies. Based on these data, the inherited cancer risk estimate could also be influenced by race and ethnic origin, beside gender (26, 43, 45). As already reported in other studies, the elaboration of new population-based genetic approaches may help to identify the 50% more BRCA PV carriers than those detected by conventional clinical and familial criteria. Therefore, population-based genetic information, in the future, could be useful to improve the detection strategies of BRCA1/2 PV carriers and maximize prevention paths, with significant implications for clinical management and surveillance of male cancer patients and their family members (46).
Since little is known about genetic susceptibility to cancer in Sicilian male population and data on germline variant frequency is limited or conflicting, our study was aimed at investigating the prevalence and type of inherited BRCA1/2 PVs in 352 men from a specific geographical area of Southern Italy. Our results showed that about 7% of men who met the current criteria for genetic testing were BRCA PV carriers. The majority of these were BRCA2 mutations (25 out of 26 patients), which is consistent with previous reported data (1, 47). Whereas different population-based studies often reported variable and non-uniform data about the frequency of BRCA2 PVs in MBC cases, showing percentages ranging between 6.8% and 16% (40, 42, 47), compared to the rest of the Italian population, our results support a higher BRCA mutation rate in Sicilian men. In contrast, both PCa and PC had similar mutation rates to those reported in the literature (15, 16). As recently highlighted by Li and colleagues (44), our analysis showed a very weak association between melanoma risk and germline BRCA2 alterations (only one individual out of 98 patients). In contrast, other studies either found no association between BRCA2 PV and melanoma risk or showed a moderate increase in risk compared with the general population (48–51). Globally, our results showed that there is a high heterogeneity of germline BRCA2 PVs among individuals of our study cohort, as only a very few specific variants were shared between patients.
No variant in particular has been observed with high prevalence within the examined male population. The most common Sicilian founder mutation named BRCA1–5083del19 (HGVS nomenclature: c.4964_4982del; p.Ser1655fs) (26, 52, 53), usually detected with high frequency in women with HBOC syndrome, was not found in the examined male population affected by HBOC syndrome-associated tumours. In addition, the most recurrent BRCA2 PV in Sicilian female population with HBOC (26), named 1466delT (HGVS nomenclature: c.1238del; p.Leu413fs), has been observed with very low frequency in men affected by MBC, PCa, PC or melanoma. The heterogeneous distribution of germline BRCA PVs and the lack of a specific territorial prevalence of these variants could reflect the genetic heterogeneity of the populations belonging to regions of Southern Italy and their historical background due to the different colonisations as well as to the crucial geographical localization of Sicily in the centre of Mediterranean Sea, crossroads of several ethnicities and cultures throughout history (54, 55). We previously observed a higher prevalence of some germline BRCA PVs in the Sicilian female population affected by hereditary breast or ovarian cancers which suggested the possibility of a population-specific genetic signature (26). This study, instead, showed the absence of specific genetic features in male population affected by BRCA-related tumors. As soon as the data from the CONFLUENCE project become available, probably it will be possible to better deep this hypothesis. A higher frequency of some germline BRCA1/2 PVs may be associated, not only with a particular ethnicity, but also with a specific gender. This suggests that those variants may be female-specific.
Knowing the genetic background underlying the phenotype of each tumor may have not only prognostic, but also preventive and therapeutic implications.
Data availability statement
All relevant data is contained within the article. The datasets presented in this article are not readily available because of ethical and privacy reasons. Requests to access the datasets should be directed to antonio.russo@usa.net and viviana.bazan@unipa.it.
Ethics statement
The studies involving humans were approved by Comitato Etico Palermo 1 (approval number: 0103-2017) of the University-affiliated Hospital A.O.U.P. ‘P. Giaccone’ of Palermo. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. LC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. CB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. UR: Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. MB: Data curation, Methodology, Writing – review & editing. EP: Data curation, Methodology, Writing – review & editing. AP: Data curation, Methodology, Writing – review & editing. RS: Data curation, Formal analysis, Writing – review & editing. DC: Data curation, Methodology, Writing – review & editing. PP: Writing – review & editing, Data curation, Formal analysis. AG: Data curation, Methodology, Writing – review & editing. TB: Data curation, Methodology, Writing – review & editing. PF: Data curation, Methodology, Writing – review & editing. GR: Methodology, Writing – review & editing. VS: Data curation, Methodology, Writing – review & editing. VG: Data curation, Formal analysis, Methodology, Writing – review & editing. GFP: Formal analysis, Methodology, Writing – review & editing. SV: Data curation, Methodology, Validation, Writing – review & editing. GP: Data curation, Methodology, Validation, Writing – review & editing. AR: Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. VB: Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. SiciliAn MicronanOTecH Research And Innovation CEnter “SAMOTHRACE” (MUR, PNRR-M4C2, ECS_00000022), spoke 3: Università degli Studi di Palermo, “S2-COMMs - Micro and Nanotechnologies for Smart & Sustainable Communities”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ibrahim M, Yadav S, Ogunleye F, Zakalik D. Male BRCA mutation carriers: clinical characteristics and cancer spectrum. BMC Canc. (2018) 18:179. doi: 10.1186/s12885-018-4098-y
2. Bono M, Fanale D, Incorvaia L, Cancelliere D, Fiorino A, Calò V, et al. Impact of deleterious variants in other genes beyond BRCA1/2 detected in breast/ovarian and pancreatic cancer patients by NGS-based multi-gene panel testing: looking over the hedge. ESMO Open. (2021) 6:100235. doi: 10.1016/j.esmoop.2021.100235
3. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: A Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
4. Gucalp A, Traina TA, Eisner JR, Parker JS, Selitsky SR, Park BH, et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res Treat. (2018) 173:37–48. doi: 10.1007/s10549-018-4921-9
5. Annoni AM, Longhini C. Investigating men’s motivations to engage in genetic screening for BRCA1 and BRCA2 mutations. PloS One. (2022) 17:e0265387. doi: 10.1371/journal.pone.0265387
6. Chen Z, Xu L, Shi W, Zeng F, Zhuo R, Hao X, et al. Trends of female and male breast cancer incidence at the global, regional, and national levels, 1990–2017. Breast Cancer Res Treat. (2020) 180:481–90. doi: 10.1007/s10549-020-05561-1
7. Rizzolo P, Silvestri V, Tommasi S, Pinto R, Danza K, Falchetti M, et al. Male breast cancer: genetics, epigenetics, and ethical aspects. Ann Oncol. (2013) 24:viii75–82. doi: 10.1093/annonc/mdt316
8. Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet. (1998) 62:676–89. doi: 10.1086/301749
9. Ottini L, Silvestri V, Rizzolo P, Falchetti M, Zanna I, Saieva C, et al. Clinical and pathologic characteristics of BRCA-positive and BRCA-negative male breast cancer patients: results from a collaborative multicenter study in Italy. Breast Cancer Res Treat. (2012) 134:411–8. doi: 10.1007/s10549-012-2062-0
10. Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. (2002) 20:1480–90. doi: 10.1200/JCO.2002.20.6.1480
11. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
12. Sekhoacha M, Riet K, Motloung P, Gumenku L, Adegoke A, Mashele S. Prostate cancer review: genetics, diagnosis, treatment options, and alternative approaches. Molecules. (2022) 27:5730. doi: 10.3390/molecules27175730
13. Nguyen-Nielsen M, Borre M. Diagnostic and therapeutic strategies for prostate cancer. Semin Nucl Med. (2016) 46:484–90. doi: 10.1053/j.semnuclmed.2016.07.002
14. McNevin CS, Cadoo K, Baird A-M, Murchan P, Sheils O, McDermott R, et al. Pathogenic BRCA variants as biomarkers for risk in prostate cancer. Cancers. (2021) 13:5697. doi: 10.3390/cancers13225697
15. Messina C, Cattrini C, Soldato D, Vallome G, Caffo O, Castro E, et al. BRCA mutations in prostate cancer: prognostic and predictive implications. J Oncol. (2020) 2020:1–7. doi: 10.1155/2020/4986365
16. Pilarski R. The role of BRCA testing in hereditary pancreatic and prostate cancer families. Am Soc Clin Oncol Educ Book. (2019) 39):79–86. doi: 10.1200/EDBK_238977
17. Park W, Chawla A, O’Reilly EM. Pancreatic cancer. Jama. (2021) 326:851. doi: 10.1001/jama.2021.13027
18. Niger M, Prisciandaro M, Antista M, Monica MAT, Cattaneo L, Prinzi N, et al. One size does not fit all for pancreatic cancers: A review on rare histologies and therapeutic approaches. World J Gastrointestinal Oncol. (2020) 12:833–49. doi: 10.4251/wjgo.v12.i8.833
19. Rosen MN, Goodwin RA, Vickers MM. BRCA mutated pancreatic cancer: A change is coming. World J Gastroenterol. (2021) 27:1943–58. doi: 10.3748/wjg.v27.i17.1943
20. Fanale D, Corsini LR, Brando C, Dimino A, Filorizzo C, Magrin L, et al. Impact of different selection approaches for identifying lynch syndrome-related colorectal cancer patients: unity is strength. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.827822
21. Gumaste PV, Penn LA, Cymerman RM, Kirchhoff T, Polsky D, McLellan B. Skin cancer risk inBRCA1/2mutation carriers. Br J Dermatol. (2015) 172:1498–506. doi: 10.1111/bjd.2015.172.issue-6
22. Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. JNCI J Natl Cancer Institute. (1999) 91:1310–6. doi: 10.1093/jnci/91.15.1310
23. Moran A, O’Hara C, Khan S, Shack L, Woodward E, Maher ER, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Familial Canc. (2011) 11:235–42. doi: 10.1007/s10689-011-9506-2
24. Johannsson O, Loman N, Möller T, Kristoffersson U, Borg Å, Olsson H. Incidence of Malignant tumours in relatives of BRCA1 and BRCA2 germline mutation carriers. Eur J Canc. (1999) 35:1248–57. doi: 10.1016/S0959-8049(99)00135-5
25. Choi MC, Bae J-S, Jung SG, Park H, Joo WD, Song SH, et al. Prevalence of germline BRCA mutations among women with carcinoma of the peritoneum or fallopian tube. J Gynecol Oncol. (2018) 29:e80. doi: 10.3802/jgo.2018.29.e80
26. Incorvaia L, Fanale D, Badalamenti G, Bono M, Calò V, Cancelliere D, et al. Hereditary breast and ovarian cancer in families from southern Italy (Sicily)—Prevalence and geographic distribution of pathogenic variants in BRCA1/2 genes. Cancers. (2020) 12:1158. doi: 10.3390/cancers12051158
27. Mazzola E, Blackford A, Parmigiani G, Biswas S. Recent enhancements to the genetic risk prediction model BRCAPRO. Cancer Inf. (2015) 14s2:CIN.S17292. doi: 10.4137/CIN.S17292
28. Schaeffer EM, Srinivas S, Adra N, An Y, Barocas D, Bitting R, et al. Prostate cancer, version 4.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network. (2023) 21:1067–96. doi: 10.6004/jnccn.2023.0050
29. Fanale D, Pivetti A, Cancelliere D, Spera A, Bono M, Fiorino A, et al. BRCA1/2 variants of unknown significance in hereditary breast and ovarian cancer (HBOC) syndrome: Looking for the hidden meaning. Crit Rev Oncol/Hematol. (2022) 172:103626. doi: 10.1016/j.critrevonc.2022.103626
30. Incorvaia L, Fanale D, Bono M, Calò V, Fiorino A, Brando C, et al. BRCA1/2 pathogenic variants in triple-negative versus luminal-like breast cancers: genotype–phenotype correlation in a cohort of 531 patients. Ther Adv Med Oncol. (2020) 12:175883592097532. doi: 10.1177/1758835920975326
31. Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS, et al. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutation. (2008) 29:1282–91. doi: 10.1002/humu.v29:11
32. den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum Mutation. (2016) 37:564–9. doi: 10.1002/humu.22981
33. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. (2012) 2:401–4. doi: 10.1158/2159-8290.CD-12-0095
34. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signaling. (2013) 6:pl1. doi: 10.1126/scisignal.2004088
35. Siegel DA, O’Neil ME, Richards TB, Dowling NF, Weir HK. Prostate cancer incidence and survival, by stage and race/ethnicity — United states, 2001–2017. MMWR Morbidity Mortality Weekly Rep. (2020) 69:1473–80. doi: 10.15585/mmwr.mm6941a1
36. Thompson D, Easton D. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. (2001) 68:410–9. doi: 10.1086/318181
37. Rebbeck TR, Mitra N, Wan F, Sinilnikova OM, Healey S, McGuffog L, et al. Association of type and location ofBRCA1andBRCA2Mutations with risk of breast and ovarian cancer. Jama. (2015) 313:1347. doi: 10.1001/jama.2014.5985
38. Teixeira N, van der Hout A, Oosterwijk JC, Vos JR, Devilee P, van Engelen K, et al. The association between cancer family history and ovarian cancer risk in BRCA1/2 mutation carriers: can it be explained by the mutation position? Eur J Hum Genet. (2018) 26:848–57. doi: 10.1038/s41431-018-0111-9
39. Lubinski J, Phelan CM, Ghadirian P, Lynch HT, Garber J, Weber B, et al. Cancer variation associated with the position of the mutation in the BRCA2 gene. Familial Canc. (2002) 3:1–10. doi: 10.1023/B:FAME.0000026816.32400.45
40. Fentiman IS. Male breast cancer is not congruent with the female disease. Crit Rev Oncol/Hematol. (2016) 101:119–24. doi: 10.1016/j.critrevonc.2016.02.017
41. Maguire S, Perraki E, Tomczyk K, Jones ME, Fletcher O, Pugh M, et al. Common susceptibility loci for male breast cancer. JNCI: J Natl Cancer Institute. (2021) 113:453–61. doi: 10.1093/jnci/djaa101
42. Giordano SH, Longo DL. Breast cancer in men. N Engl J Med. (2018) 378:2311–20. doi: 10.1056/NEJMra1707939
43. Valentini V, Bucalo A, Conti G, Celli L, Porzio V, Capalbo C, et al. Gender-specific genetic predisposition to breast cancer: BRCA genes and beyond. Cancers. (2024) 16:579. doi: 10.3390/cancers16030579
44. Li S, Silvestri V, Leslie G, Rebbeck TR, Neuhausen SL, Hopper JL, et al. Cancer risks associated with BRCA1 and BRCA2 pathogenic variants. J Clin Oncol. (2022) 40:1529–41. doi: 10.1200/JCO.21.02112
45. Rebbeck TR, Friebel TM, Friedman E, Hamann U, Huo D, Kwong A, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutation. (2018) 39:593–620. doi: 10.1002/humu.23406
46. Manchanda R, Gaba F. Population based testing for primary prevention: A systematic review. Cancers. (2018) 10:424. doi: 10.3390/cancers10110424
47. Silvestri V, Leslie G, Barnes DR, Agnarsson BA, Aittomäki K, Alducci E, et al. Characterization of the cancer spectrum in men with germline BRCA1 and BRCA2 Pathogenic variants. JAMA Oncol. (2020) 6:1218. doi: 10.1001/jamaoncol.2020.0388
48. Easton DF, Steele L, Fields P, Ormiston W, Averill D, Daly PA, et al. Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12-13. Am J Hum Genet. (1997) 61:120–8. doi: 10.1086/513891
49. van Asperen CJ. Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet. (2005) 42:711–9. doi: 10.1136/jmg.2004.028829
50. Kadouri L, Temper M, Grenader T, Abeliovich D, Hamburger T, Peretz T, et al. Absence of founder BRCA1 and BRCA2 mutations in coetaneous Malignant melanoma patients of Ashkenazi origin. Familial Canc. (2008) 8:29–32. doi: 10.1007/s10689-008-9206-8
51. Landi MT. Genetic susceptibility in familial melanoma from northeastern Italy. J Med Genet. (2004) 41:557–66. doi: 10.1136/jmg.2003.016907
52. Fanale D, Incorvaia L, Filorizzo C, Bono M, Fiorino A, Calò V, et al. Detection of germline mutations in a cohort of 139 patients with bilateral breast cancer by multi-gene panel testing: impact of pathogenic variants in other genes beyond BRCA1/2. Cancers. (2020) 12:2415. doi: 10.3390/cancers12092415
53. Russo A, Calò V, Bruno L, Schirò V, Agnese V, Cascio S, et al. Is BRCA1-5083del19, identified in breast cancer patients of Sicilian origin, a Calabrian founder mutation? Breast Cancer Res Treat. (2008) 113:67–70. doi: 10.1007/s10549-008-9906-7
54. Fanale D, Incorvaia L, Bono M, Calò V, Cancelliere D, Fiorino A, et al. 247P Population-based testing for hereditary breast and ovarian cancer in a cohort of 1,346 patients from Southern Italy (Sicily): When historical background affects genetics. Ann Oncol. (2020) 31:S338–S9. doi: 10.1016/j.annonc.2020.08.368
Keywords: BRCA1, BRCA2, germline pathogenic variants, HBOC, male breast cancer, melanoma, pancreatic cancer, prostate cancer
Citation: Fanale D, Corsini LR, Brando C, Randazzo U, Bono M, Pedone E, Perez A, Sciacchitano R, Cancelliere D, Piraino P, Giurintano A, Bazan Russo TD, Ferraro P, Rinaldi G, Spinnato V, Gennusa V, Pernice G, Vieni S, Pantuso G, Russo A and Bazan V (2024) BRCA-associated hereditary male cancers: can gender affect the prevalence and spectrum of germline pathogenic variants? Front. Oncol. 14:1414343. doi: 10.3389/fonc.2024.1414343
Received: 08 April 2024; Accepted: 07 June 2024;
Published: 21 June 2024.
Edited by:
Daniele Vergara, University of Salento, ItalyReviewed by:
Dongbo Yang, The University of Chicago, United StatesTeresa Ramon Y. Cajal, Hospital de la Santa Creu i Sant Pau, Spain
Copyright © 2024 Fanale, Corsini, Brando, Randazzo, Bono, Pedone, Perez, Sciacchitano, Cancelliere, Piraino, Giurintano, Bazan Russo, Ferraro, Rinaldi, Spinnato, Gennusa, Pernice, Vieni, Pantuso, Russo and Bazan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Viviana Bazan, dml2aWFuYS5iYXphbkB1bmlwYS5pdA==; Antonio Russo, YW50b25pby5ydXNzb0B1c2EubmV0
†These authors have contributed equally to this work
‡These authors share last authorship
§ORCID: Antonio Russo, orcid.org/0000-0002-4370-2008
 Daniele Fanale
Daniele Fanale Lidia Rita Corsini1†
Lidia Rita Corsini1† Chiara Brando
Chiara Brando Marco Bono
Marco Bono Alessandro Perez
Alessandro Perez Tancredi Didier Bazan Russo
Tancredi Didier Bazan Russo Valeria Spinnato
Valeria Spinnato Salvatore Vieni
Salvatore Vieni Antonio Russo
Antonio Russo Viviana Bazan
Viviana Bazan