- 1Department of Pharmacy, Gifu University Hospital, Gifu, Japan
- 2Innovative and Clinical Research Promotion Center, Gifu University Hospital, Gifu, Japan
- 3Patient Safety Division, Gifu University Hospital, Gifu, Japan
- 4Laboratory of Pharmacy Practice and Social Science, Gifu Pharmaceutical University, Gifu, Japan
- 5Laboratory of Advanced Medical Pharmacy, Gifu Pharmaceutical University, Gifu, Japan
- 6Department of Breast Surgery, Gifu University Hospital, Gifu, Japan
- 7Department of Emergency and Disaster Medicine, Gifu University Graduate School of Medicine, Gifu, Japan
- 8Department of Gastroenterology, Gifu University Graduate School of Medicine, Gifu, Japan
Background: The effectiveness of a dexamethasone-sparing strategy in the treatment of breast cancer with anthracycline-cyclophosphamide therapy when combined with first-generation 5-HT3 receptor antagonists (RAs) and neurokinin-1 RAs is unclear. This is attributable to a lack of evidence from direct comparison of multiple doses of DEX to a single dose of DEX in combination with first-generation 5-HT3 RAs in anthracycline-cyclophosphamide therapy. Our goal was to clarify the impact of dexamethasone-sparing strategies that involve both first-generation 5-HT3 RAs and palonosetron when combined with neurokinin-1 RAs, using a network meta-analysis.
Materials and methods: A literature search was conducted on PubMed/Medline for articles published up to July 4, 2023. We included randomized controlled trials which assessed the efficacy of antiemetic regimens which combined 5-HT3 RAs and dexamethasone, with or without neurokinin-1 RAs, for the initial dose in anthracycline-cyclophosphamide therapy for patients with breast cancer. The primary outcome was the proportion of patients achieving a complete response during the delayed phase (CR-DP).
Results: The difference in the proportion of patients achieving CR-DP between multiple and single doses of dexamethasone was 0.1% (95%CI: -12.4 to 12.5) with palonosetron and neurokinin-1 RAs, compared to 5.3% (95%CI: -13.4 to 23.0) with a single dose of a first-generation 5-HT3 receptor antagonist. Additionally, the difference was 12.7% (95% CI: -2.8 to 28.2) when comparing palonosetron against first-generation 5-HT3 RAs in combination with a single dose of dexamethasone and neurokinin-1 RAs.
Conclusion: Palonosetron is recommended rather than a single dose of first-generation 5-HT3 RAs in dexamethasone-sparing strategies for anthracycline-cyclophosphamide therapy.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) greatly affects patients’ quality of life, treatment adherence, and therapy effectiveness, and ranks as the second-most aversion condition after death among those receiving chemotherapy (1). Reducing CINV is crucial for improving patient well-being and maintaining chemotherapy continuity.
Anthracycline-cyclophosphamide (AC) therapy, commonly used for breast cancer, often causes severe nausea and vomiting and is classified as highly emetogenic. A triplet antiemetic regimen of dexamethasone (DEX), 5-HT3 receptor antagonists (5HT3 RAs), and neurokinin-1 receptor antagonists (NK1 RAs) effectively reduces CINV, as evidenced by several phase III studies (2–8), and is recommended in global guidelines (9, 10).
Palonosetron is a second-generation 5HT3 RA that has a longer half-life and higher binding affinity compared to first-generation 5HT3 RAs (1st 5HT3 RAs) (11, 12). Palonosetron has been demonstrated to be more effective at preventing CINV than 1st 5HT3 RAs in patients receiving AC therapy and moderately emetogenic chemotherapy (13–15). It allows the DEX-sparing strategy, which limits dosing to a single dose of DEX rather than multiple doses to minimize corticosteroids side effects (16–18). The DEX-sparing strategy alongside palonosetron has been shown to be as effective as multiple DEX doses in the prophylaxis of CINV (19–25). In AC therapy, the DEX-sparing strategy in combination with palonosetron and an NK1 RA demonstrated acceptable differences in the complete response rate during both the overall phase (-1.1; 95% CI: -12.0 to 9.8) and the delayed phase (-3.3; 95% CI: -14.4 to 7.8) (26). Consequently, the DEX-sparing strategy has become widely adopted in AC therapy (9, 27).
Even though the DEX-sparing strategy has been established in combination with palonosetron, global guidelines have not specified the type of 5HT3 RA to be used for DEX-sparing in AC therapy (9, 10, 27). Using DEX-sparing with 1st 5HT3 RAs might be inadequate in preventing delayed-onset CINV associated with AC therapy, as symptoms persist beyond the first day (28).
The impact of the DEX-sparing strategy with 1st 5HT3 RAs on AC therapy is unknown. This network meta-analysis aimed to compare the efficacy of a single dose of DEX with that of multiple doses of DEX when combined with 1st 5HT3 RAs based on data from randomized control trials.
Materials and methods
Search strategy and eligibility criteria
We included randomized controlled trials which evaluated the effectiveness of antiemetic regimens that combined 5HT3RA and DEX, with or without NK1RA, for the initial dose in AC-based regimens for patients with breast cancer. Crossover studies were eligible only if they offered data for the first cycle. If over 5% of participants were given a non-AC regimen, studies were included only if they presented outcome data specifically for patients who received AC therapy, such as through subgroup analysis. Our selection was limited to studies written in English.
We classified each antiemetic regimen based on the duration of DEX administration, the type and duration of 5HT3 RA used, and concomitant NK1 RA. We divided DEX into two categories: a single dose of DEX, given only on the first day, and multiple doses of DEX, administered from the second day onwards. Similarly, we categorized 5HT3 RA into three groups: a single dose of a 1st generation 5HT3 RA, comprising ondansetron, granisetron, or ramosetron, administered on the first day only; multiple doses of 1st 5HT3 RA, given from the second day onwards; and palonosetron. NK1 RAs, including aprepitant, fosaprepitant, casopitant, rolapitant, netupitant, and fosnetupitant, were regarded as equivalent. Different dosages or routes of administration of the same agent were regarded as equivalent. An overview of all experimental antiemetic regimens included in our analysis is available in Supplementary Table 1.
This study followed the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) extension statement for the reporting of systematic reviews incorporating network meta-analyses (29, 30). The protocol for this review was previously published in the PROSPERO database (CRD42024511693).
Information sources
We conducted a systematic search for eligible studies published up to July 4, 2023, utilizing PubMed/Medline databases. Additionally, we performed a manual search through the reference lists of pertinent reviews and meta-analyses. The specific search terms employed are detailed in Supplementary Table 2.
Study selection and data extraction
Two reviewers (DW and HI) independently screened all relevant studies in duplicate to confirm their eligibility and extracted the following information: author, title, publication source, date of publication, and language; factors contributing to risk of bias evaluation (randomization methods, allocation concealment, blinding method, handling of incomplete outcome data, selective outcome reporting, and identification of other potential biases); study characteristics (trial design, participant source, inclusion and exclusion criteria, subgroup analyses, adherence to allocated intervention); participant details (demographics such as age and sex/gender, total number recruited, allocated, and assessed, cancer type, antineoplastic treatments, known CINV risk factors); intervention and comparator specifics (antiemetic agents, dosages, prophylaxis duration); and outcomes. All outcome measures were extracted from the first planned chemotherapy cycle. Discrepancies between reviewers were resolved through discussion with a third author (RK) to achieve consensus.
Outcome measures
The primary outcome was a complete response during the delayed phase (CR-DP), defined as the absence of emesis and no use of rescue medication from 24 to 120 hours post-chemotherapy initiation. The results were reported as the proportion of patients achieving CR-DP. For comparisons between antiemetic regimens, both the risk difference and risk ratio are presented.
Risk of bias assessment
Two reviewers (DW and HI) independently assessed the risk of bias due to the randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, selection of the reported results, and other biases of the included studies using the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) by The Cochrane Collaboration (http://www.cochrane.de). Any disagreements were resolved through discussion with a third reviewer (RK) to achieve consensus.
Statistical analysis
An arm-based network meta-analysis using Bayesian methods was conducted to compare the CR-DP rates of multiple antiemetic strategies. Network meta-analysis enables both the direct comparison of treatments in individual trials and indirect comparison across trials (31). Notably, an arm-based approach estimates population-averaged, treatment-specific event rates. The CR-DP rate for each antiemetic regimen was aggregated using the nma.ab.bin function within the R package pcnetmeta (32). This model accounts for heterogeneity in the variance of random effects and the correlation between different treatments within each cohort. Final estimation routines involved 3 chains, each with 50,000 burn-in iterations, followed by 100,000 estimation iterations without thinning, resulting in a total of 150,000 iterations for analysis. The results of the network meta-analysis were reported as the posterior median with corresponding 95% credible intervals (CIs). Statistical significance was assessed using 95% CIs. We also performed standard random effects and fixed effect meta-analysis to aggregate the proportion of patients achieving CR-DP with each antiemetic regimen using the metaprop function in the R package meta (33, 34). All analyses were performed in R, version 4.3.2.
Results
Eligible studies and characteristics
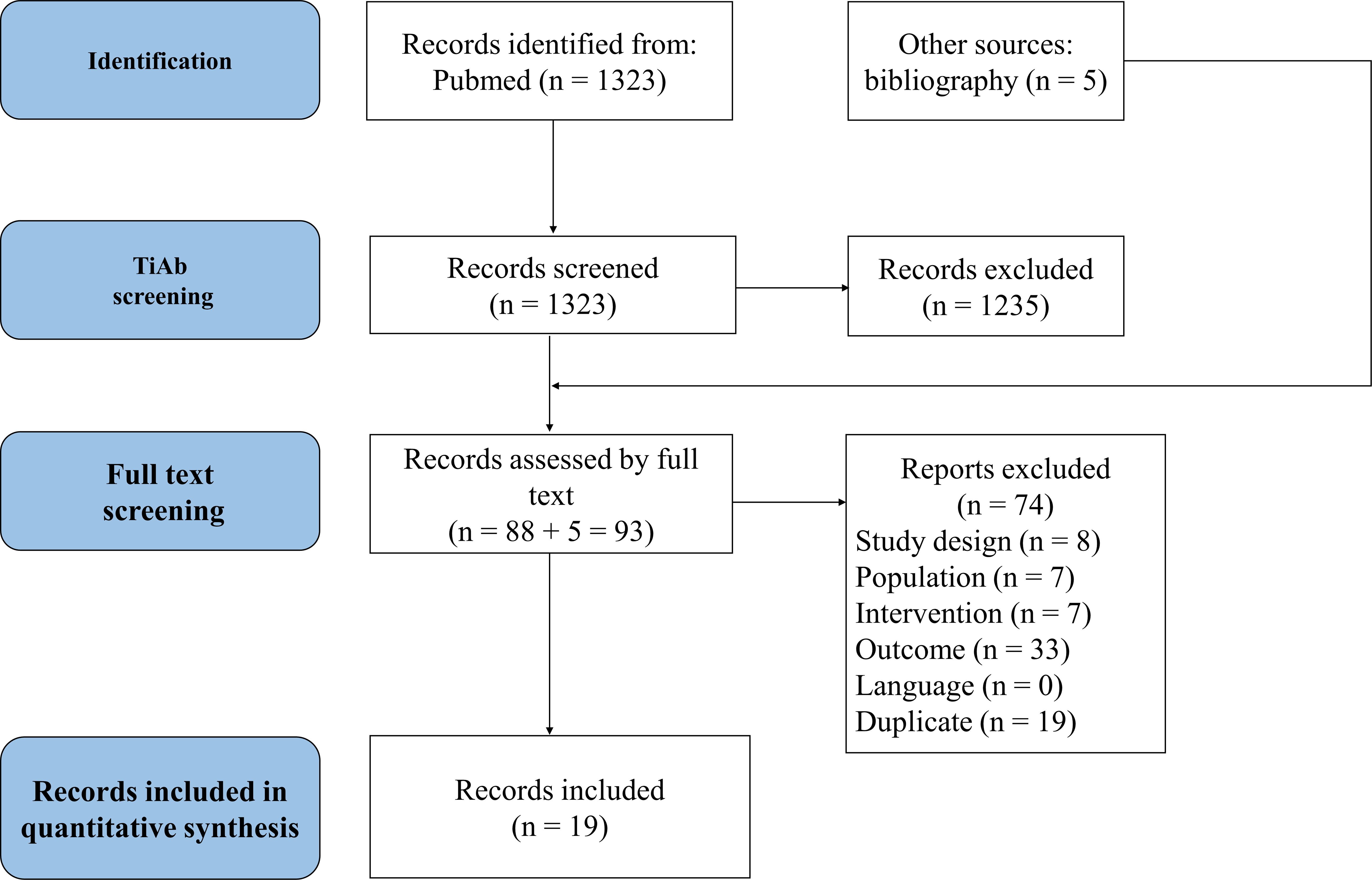
Through literature research, we identified 1,323 potentially relevant references. After reviewing titles and abstracts, 88 studies were selected. An additional 5 studies were included via manual search, resulting in 93 studies undergoing full-text review. Ultimately, 21 studies met our inclusion criteria; however, two were excluded from analysis due to the absence of CR-DP data. These exclusions included a study on patients who had previously undergone chemotherapy (2) and another which compared fosnetupitant with fosaprepitant (35). As a result, 19 studies were included in our network meta-analysis, as depicted in the Prisma flow diagram in Figure 1.
Supplementary Table 3 presents the key characteristics of the included studies, with antiemetic regimens organized into 10 categories by the antiemetic agents used. Supplementary Table 4 offers detailed information on the agents included in each antiemetic regimen. Risk of bias tables for the included studies are shown in Supplementary Figures 1 and 2.
A total of 9,108 patients across 19 studies were included in our network meta-analysis (3–8, 21, 23, 26, 36–45). These studies were published between 2005 and 2021, of which 14 (73.6%) were double-blind randomized controlled trials (3–8, 26, 36, 38–42, 45), and sample size ranged from 40 to 1,917 patients. Almost all participants were women with breast cancer who were naïve to emetogenic chemotherapy and received an AC-based regimen.
To summarize treatments involving 6,187 patients with NK1 RA, 51.7% received aprepitant/fosaprepitant, 23.2% casopitant, 19.5% netupitant, and 5.6% rolapitant. Regarding the administration of DEX, among 2,158 patients given multiple doses of DEX, 78.0% took 8 mg and 22.0% took 4 mg. Additionally, 61.5% received 3-day doses and 38.5% received 4-day doses. Regarding the administration of palonosetron, among 3,475 patients given palonosetron, 28.8% received 0.75 mg, 51.5% received 0.5 mg, 19.0% received 0.25 mg, and 0.7% received 0.075 mg.
The antiemetic regimens that were directly compared for CR-DP in each study were as follows: Four studies compared multiple doses of DEX with a single dose, in combination with palonosetron (21, 23, 26, 38). Three of these studies also used a NK1RA (23, 26, 38). Six studies compared the use of NK1RA with its absence, in combination with DEX and 5HT3 RA (3–8). Six studies compared palonosetron with 1st 5HT3 RAs (36, 37, 39, 43–45). Additionally, three studies compared different dosages or methods of administering 5HT3 RAs (40–42). Notably, none of the studies directly compared multiple doses of DEX with a single dose when combined with a 1st 5HT3 RA and NK1RA. The network plot of CR-DP is shown in Figure 2.
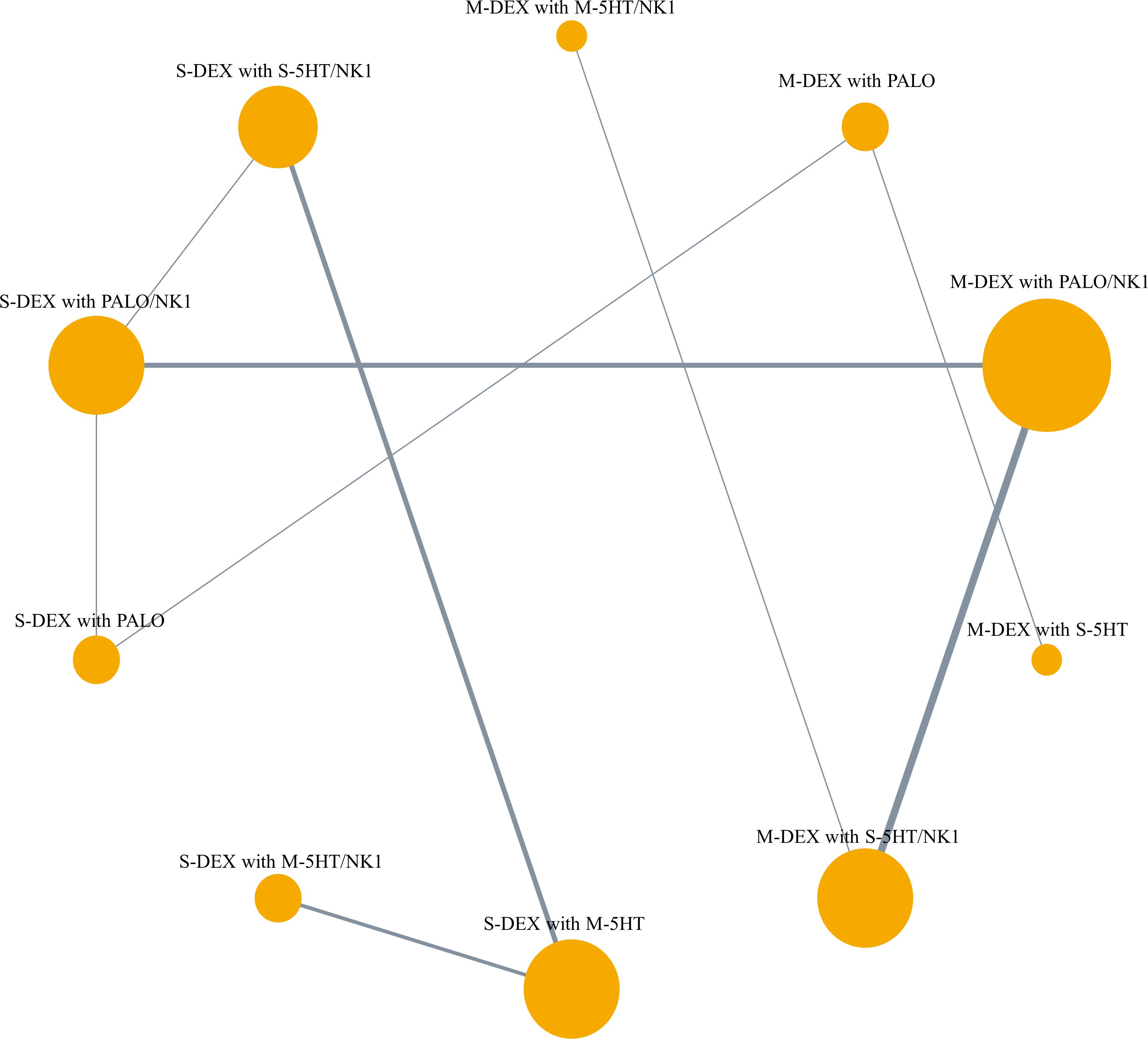
Figure 2 Network plot of primary outcome, complete response during the delayed phase. The lines represent direct comparisons between treatments in trials. Line thickness indicates the number of trials evaluated in each comparison. Node size indicates the number of participants assigned to each treatment. M-DEX with M-5HT/NK1, multiple doses of dexamethasone in combination with multiple doses of a first-generation 5-HT3 receptor antagonist and neurokinin-1 receptor antagonist; M-DEX with PALO, multiple doses of dexamethasone in combination with palonosetron; M-DEX with PALO/NK1, multiple doses of dexamethasone in combination with palonosetron and neurokinin-1 receptor antagonist; M-DEX with S-5HT, multiple doses of dexamethasone in combination with a single dose of a first-generation 5-HT3 receptor antagonist; M-DEX with S-5HT/NK1, multiple doses of dexamethasone in combination with a single dose of a first-generation 5-HT3 receptor antagonist and neurokinin-1 receptor antagonist; S-DEX with M-5HT, single dose of dexamethasone in combination with multiple doses of a first-generation 5-HT3 receptor antagonist; S-DEX with M-5HT/NK1, single dose of dexamethasone in combination with multiple doses of a first-generation 5-HT3 receptor antagonist and neurokinin-1 receptor antagonist; S-DEX with PALO, single dose of dexamethasone in combination with palonosetron; S-DEX with PALO/NK1, single dose of dexamethasone in combination with palonosetron and neurokinin-1 receptor antagonist; S-DEX with S-5HT/NK1, single dose of dexamethasone in combination with a single dose of a first-generation 5-HT3 receptor antagonist and neurokinin-1 receptor antagonist.
Complete response rate during the delayed phase in arm-based network meta-analysis
Our primary outcome, CR-DP rate, is presented for each eligible study in Supplementary Table 5, and the pooled CR-DP rate at the cohort level (by classified antiemetic regimen) in the arm-based network meta-analysis is presented in Table 1. Cohorts with multiple or single doses of DEX combined with palonosetron and NK1 RA accounted for seven and six studies, respectively. Both groups showed a CR-DP rate of 72.3% (95%CI: 61.2 to 81.1) for multiple and 72.3% (95%CI: 60.4 to 81.0) for single doses of DEX. When combined with single doses of 1st 5HT3 RA and NK1 RA, the studies included five for multiple and four for single doses of DEX, respectively. CR-DP rates were 64.7% (95%CI: 50.5 to 76.3) for multiple and 59.3% (95%CI: 46.3 to 71.0) for single doses of DEX. When combined with multiple doses of 1st 5HT3 RA and NK1 RA, only a few studies were available: one for multiple and two for single doses of DEX. CR-DP rates were 62.6% (95%CI: 34.2 to 85.0) for multiple and 69.0% (95%CI: 49.9 to 84.3) for single doses of DEX. The results of aggregated proportion and heterogeneity of CR-DP in each antiemetic regimen using meta-analysis of proportions are shown in Supplementary Table 6.
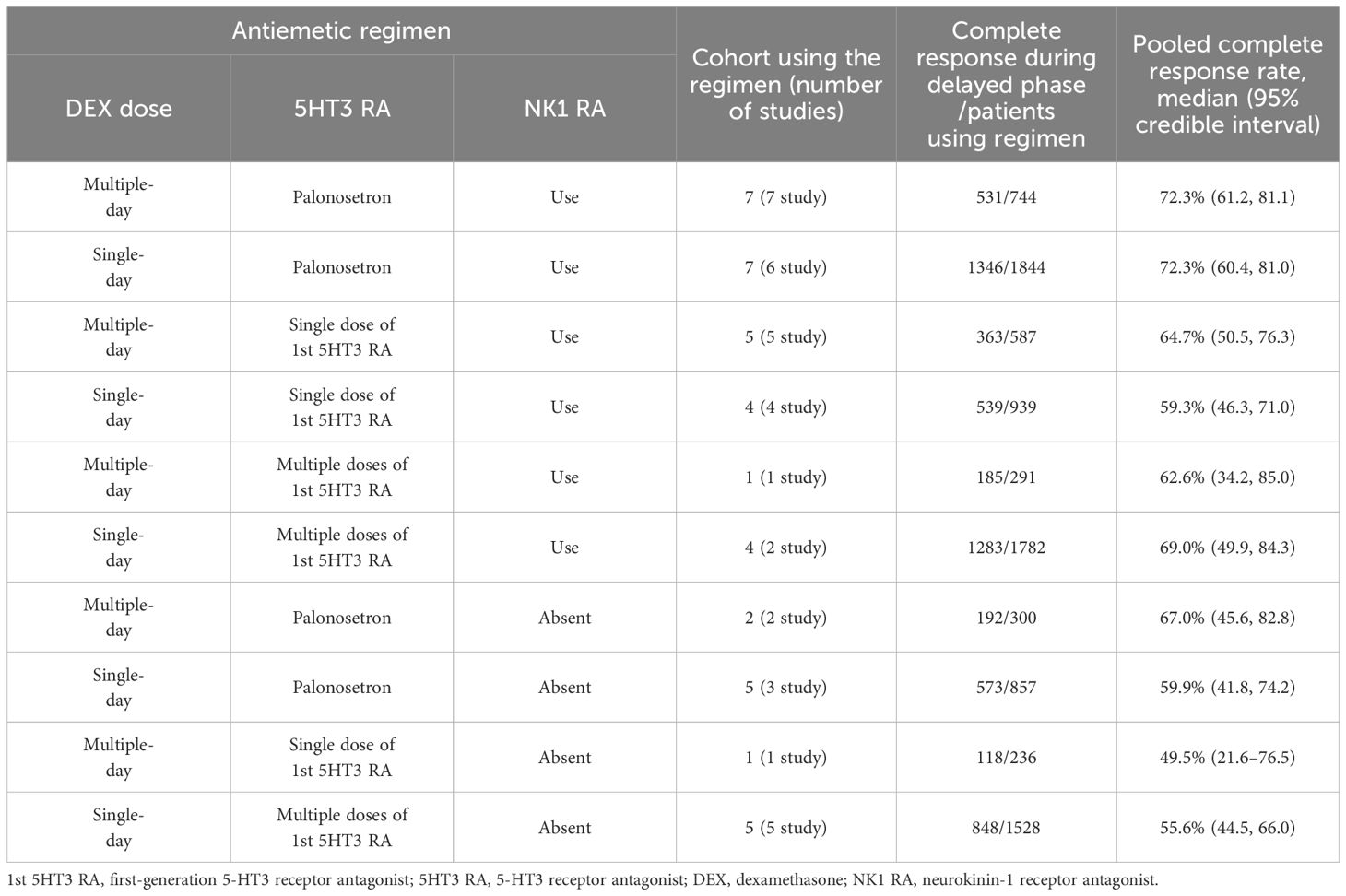
Table 1 Pooled proportions of patients achieving complete response during the delayed phase for each antiemetic regimen using arm-based network meta-analysis.
Figure 3 shows the risk differences in complete response during the delayed phase. The difference for DEX in multiple versus single doses, when used with palonosetron and NK1 RA, was 0.1% (95% CI: -12.4 to 12.5). In contrast, this difference increased to 5.3% (95% CI: -13.4 to 23.0) on comparison of DEX doses with 1st 5HT3 RA and NK1 RA, and additionally 12.7% (95% CI: -2.8 to 28.2) when comparing palonosetron against 1st 5HT3 RAs with a single dose of DEX or NK1 RA. All pairwise comparisons are shown in Figure 4.
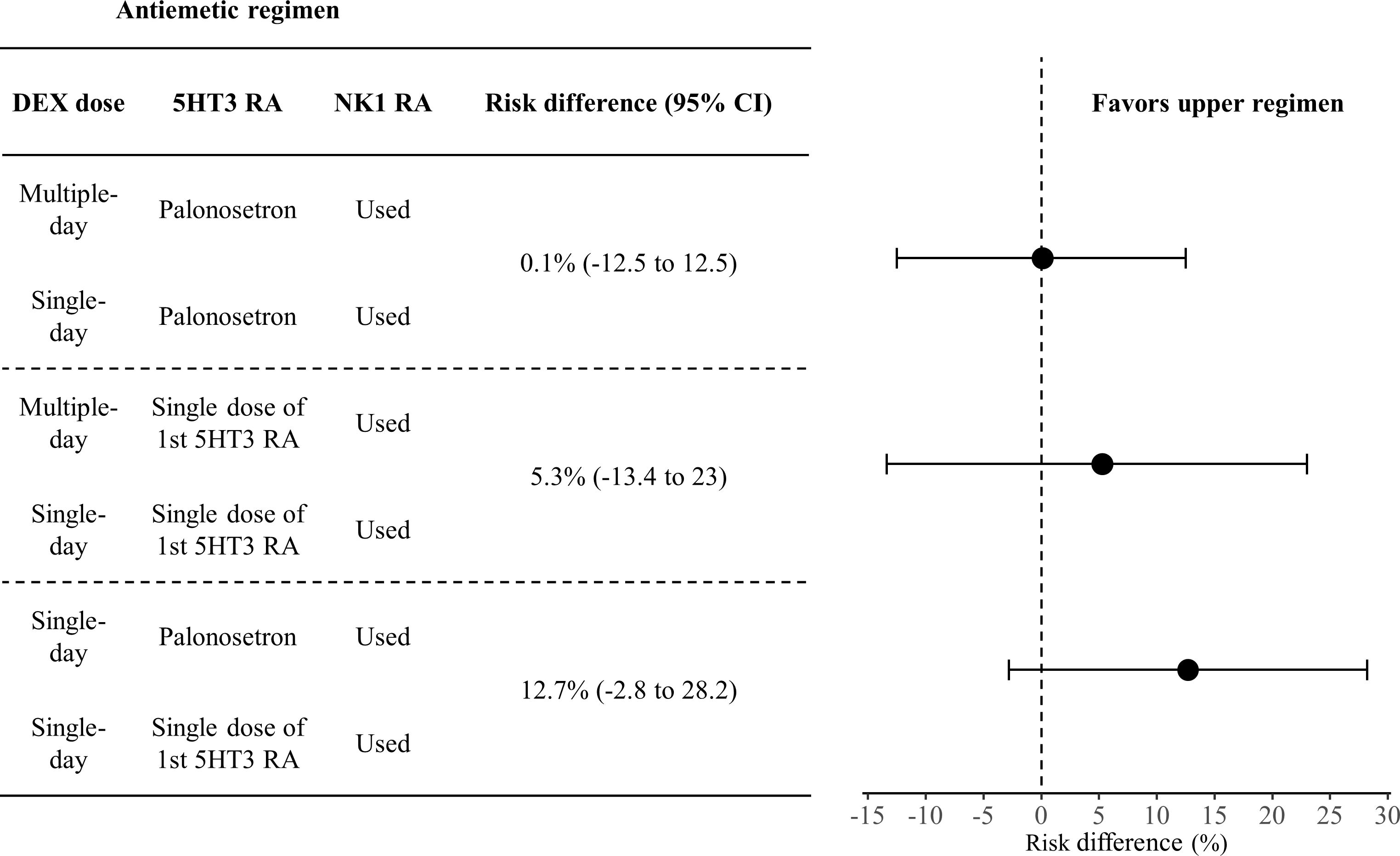
Figure 3 Forest plot of risk difference in complete response during the delayed phase. Effect sizes are from the network meta-analysis.
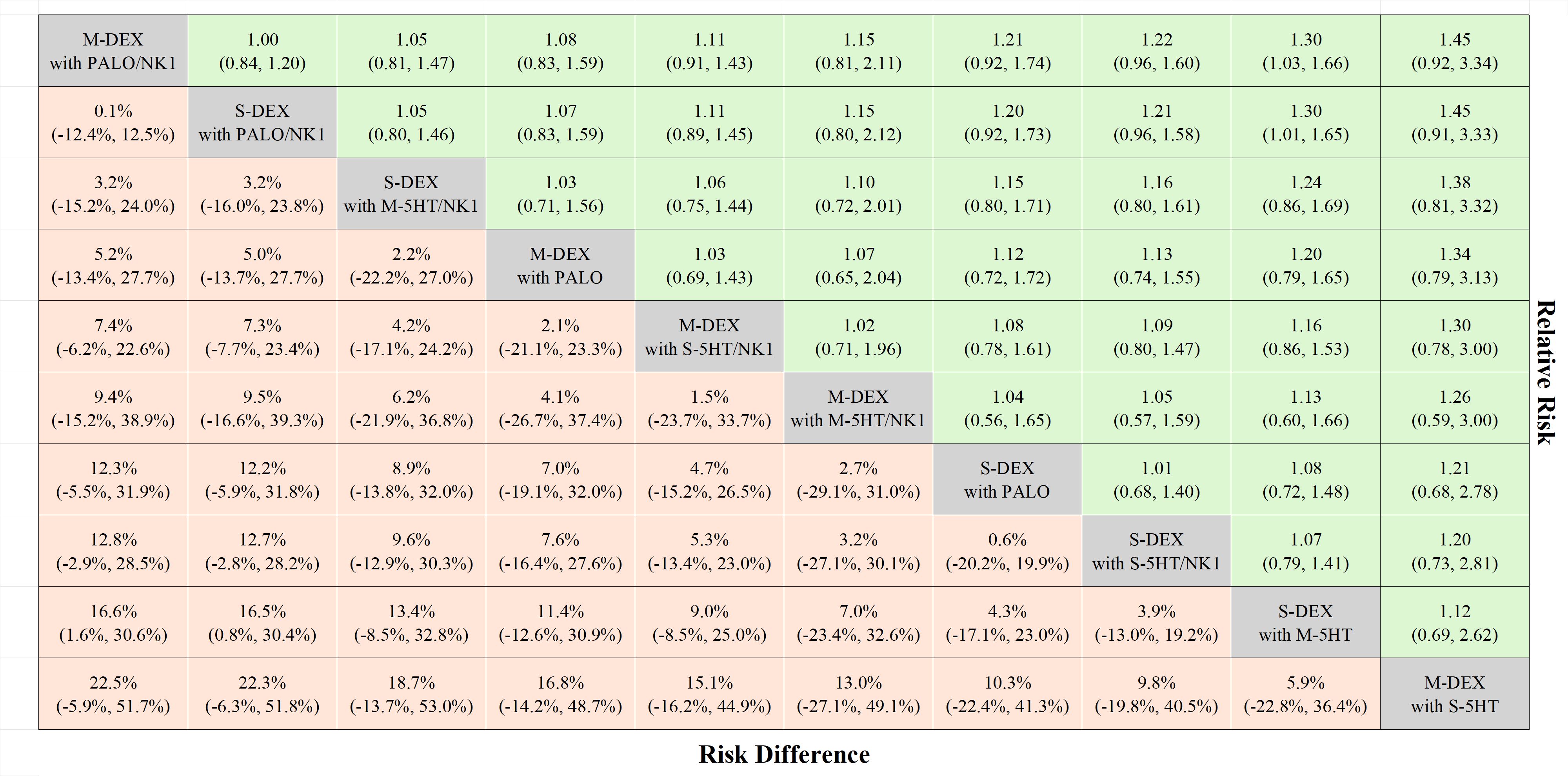
Figure 4 League table complete responses during the delayed phase. The treatments are listed according to the highest complete response rate during the delayed phase. Each cell presents the median risk difference and its associated 95% credible interval for comparison (treatment in the column versus treatment in the row), along with the median risk ratio and its 95% credible interval for reverse comparison (treatment in the row versus treatment in the column). M-DEX with M-5HT/NK1, multiple doses of dexamethasone in combination with multiple doses of a first-generation 5-HT3 receptor antagonist and neurokinin-1 receptor antagonist; M-DEX with PALO, multiple doses of dexamethasone in combination with palonosetron; M-DEX with PALO/NK1, multiple doses of dexamethasone in combination with palonosetron and neurokinin-1 receptor antagonist; M-DEX with S-5HT, multiple doses of dexamethasone in combination with a single dose of a first-generation 5-HT3 receptor antagonist; M-DEX with S-5HT/NK1, multiple doses of dexamethasone in combination with a single dose of a first-generation 5-HT3 receptor antagonist and neurokinin-1 receptor antagonist; S-DEX with M-5HT, single dose of dexamethasone in combination with multiple doses of a first-generation 5-HT3 receptor antagonist; S-DEX with M-5HT/NK1, single dose of dexamethasone in combination with multiple doses of a first-generation 5-HT3 receptor antagonist and neurokinin-1 receptor antagonist; S-DEX with PALO, single dose of dexamethasone in combination with palonosetron; S-DEX with PALO/NK1, single dose of dexamethasone in combination with palonosetron and neurokinin-1 receptor antagonist; S-DEX with S-5HT/NK1, single dose of dexamethasone in combination with a single dose of a first-generation 5-HT3 receptor antagonist and neurokinin-1 receptor antagonist.
Discussion
Our network meta-analysis showed a minimal difference of 0.1% in CR-DP rates with a DEX-sparing regimen combined with palonosetron and NK1 RA. This finding supports previous evidence (23, 26, 38). However, this gap widened to 5.3% when DEX-sparing was combined with 1st 5HT3 RA and NK1 RA. Additionally, the difference was 12.7% (95% CI: -2.8 to 28.2) on comparison of palonosetron against 1st 5HT3 RAs with a single dose of DEX and NK1 RA, marking a clinically meaningful difference of 10%, as deemed by the MASCC/ESMO guideline (46). Based on these findings, the concurrent use of palonosetron is recommended.
CINV related to AC therapy persists beyond the first day (28). Effective prevention in the initial cycle is vital for subsequent cycle management success. Conversely, a prolonged CINV duration correlates with minimal improvement in subsequent cycles (47), highlighting the importance of early CINV control. For managing delayed-phase CINV, palonosetron has shown a better control rate than 1st 5HT3 receptor antagonists when combined with DEX and NK1 RAs (36, 37, 43, 45). Additionally, a meta-analysis comparing 1st 5HT3 RAs with palonosetron revealed significant benefits associated with palonosetron (15). Our present results also indicate an improved CR-DP rate with palonosetron usage compared to a single dose of 5HT3 RA. Consequently, incorporating palonosetron from the first cycle of AC therapy is advisable for comprehensive CINV management throughout the entire course of AC therapy.
This study did not distinguish between the administration of different dosages of the same antiemetics. Regarding DEX dosage, the complete protection ratio for both the acute and delayed phases were equivalent in comparing 24 mg and 8 mg doses on day 1 (48). On the other hand, in the delayed phase, no confirmatory trial has compared different DEX dosages. However, in our network meta-analysis, all patients who received a triplet antiemetic regimen of DEX, 5HT3 RAs, and NK1 RAs took 8 mg of DEX. Regarding palonosetron dosage, several phase III trials have shown that palonosetron 0.75 mg is as effective as 0.25 mg, suggesting that the 0.25 mg dose is sufficient to achieve efficacy (13, 14, 49). However, palonosetron 0.75 mg is predominantly used in some countries like Japan. This preference is based on a phase III trial conducted in Japan (39), which demonstrated that palonosetron 0.75 mg was superior to 1st 5HT3 RAs in achieving a higher CR-DP rate for highly emetogenic chemotherapy. Our network meta-analysis included patients receiving palonosetron doses of 0.75 mg, 0.5 mg, and 0.25 mg, comprising 28.8%, 51.5%, and 19.0% of the total population, respectively. This distribution indicates that the analysis was not heavily weighted towards any particular dose within the 0.25–0.75 mg range. Therefore, it is reasonable to regard the different dosages of these antiemetics as equivalent.
The strength of this study stems from its strict inclusion of randomized controlled trials, which ensures a robust evidence base. Further, our network meta-analysis was limited to studies of patients with breast cancer undergoing AC therapy, ensuring consistency across key CINV risk factors such as chemotherapy regimen, patient age, sex, prior chemotherapy history, and dexamethasone dosages. This uniformity across trials facilitates the integration of various antiemetic regimens in a network meta-analysis. Additionally, we ascertained treatment-specific CR-DP rates and their differences across a range of antiemetic regimens through an arm-based network meta-analysis. Previous studies that compared different antiemetic regimens through contrast-based network meta-analyses (50–52) have commonly reported odds ratios only, potentially creating unnecessary obstacles for patients and clinicians in fully understanding and evaluating the efficacy of antiemetic treatments (31, 53–55).
We also acknowledge several limitations. First, comparisons between multiple doses and a single dose of DEX combined with 1st 5-HT3 RAs are based mainly on indirect comparisons, which cannot replace the direct comparisons obtained from randomized studies.
Second, the scope of our network meta-analysis was confined to the examination of antiemetic strategies, specifically those involving DEX, 5HT3 RAs, and NK1 RAs. Regarding olanzapine’s use for preventing CINV in AC therapy, our preliminary survey identified no randomized controlled trials of the efficacy of a DEX-sparing strategy for patients undergoing AC therapy. The number of trials that included olanzapine in at least one treatment arm was also limited (56–58). Other studies have noted concerns about undefined classification of 5-HT3 receptor antagonists (59) and a study design which was restricted to patients at high risk of CINV (60). Therefore, even if a network meta-analysis is performed, only an incomplete network can be formed, and it is not appropriate to indirectly compare the efficacy of DEX-sparing in olanzapine-combination regimens.
Third, our network meta-analysis lacks direct comparison of multiple versus single DEX doses in combination with multiple doses of 1st 5-HT3 RAs. Furthermore, only a few studies have incorporated the combination of multiple doses of 1st 5-HT3 RAs with DEX and NK1 RAs. Consequently, this has resulted in broad credible intervals for our estimates of the proportion of patients achieving CR-DP and its difference, which means in turn that we lack sufficient evidence to recommend a DEX-sparing approach combined with multiple doses of 1st 5-HT3 RAs.
Finally, our study’s evaluation focused exclusively on CR-DP outcomes. Other outcomes, such as the absence of nausea, did not allow the establishment of connections within the treatment network comparing multiple doses to a single dose of DEX when combined with 1st 5-HT3 RAs and NK1 RAs in the network meta-analysis.
Conclusions
For patients with breast cancer undergoing AC therapy, a DEX-sparing strategy that involves use of a single dose of a 1st 5-HT3 receptor antagonist is suggested to be inadequate. Consequently, based on current evidence, palonosetron is the preferred option.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
DW: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft. HI: Conceptualization, Supervision, Writing – review & editing. RK: Supervision, Writing – review & editing. HF: Supervision, Writing – review & editing. RM: Supervision, Writing – review & editing. KK: Supervision, Writing – review & editing. MS: Supervision, Writing – review & editing. MF: Supervision, Writing – review & editing. AS: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Gifu University Hospital Pharmacy Department Operation Grant.
Conflict of interest
DW reports honoraria from Chugai. HI reports receiving consulting fees from Eisai and Taiho; honoraria from Astellas, AstraZeneca, Chugai, Daiichi Sankyo, Eli Lilly, Nippon Kayaku, Ono, Sawai, Taiho, and Yakult. RK reports honoraria from Janssen. HF reports honoraria from Chugai, Daiichi Sankyo, Kyowa Kirin, Ono, Sanofi and Taiho. KK reports grants from Kyowa Kirin; honoraria from Tsumura Pharmaceuticals. MF reports honoraria from Daiichi Sankyo, Taiho, Chugai, Eisai, Lilly, and Nihon-Kayaku. AS reports institutional grants from Nippon Kayaku, Asahi Kasei Pharma, Chugai Pharm, Taiho Pharm, Daiichi Sankyo, Japan Blood Products Organization, Mochida Pharm, Sun Pharma; honoraria from Toa Eiyo, Asahi Kasei Pharma, Daiichi Sankyo, Pfizer, Eisai, Nippon Shinyaku, Kyowa Kirin, Tsumura, Towa Pharmaceutical, Nippon Kayaku, Mochida Pharmaceutical, EA Pharma, Yakult Honsha, Chugai Pharmaceutical.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1414037/full#supplementary-material
Abbreviations
1st 5HT3 RA, first-generation 5-HT3 receptor antagonist; 5HT3 RA, 5-HT3 receptor antagonist; AC, anthracycline-cyclophosphamide; CI, credible interval; CINV, chemotherapy-induced nausea and vomiting; CR, complete response; DP, delayed phase; DEX, dexamethasone; NK1 RA, neurokinin-1 receptor antagonist
References
1. Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW, et al. Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer. (2005) 13:219–27. doi: 10.1007/s00520-004-0710-6
2. Herrstedt J, Muss HB, Warr DG, Hesketh PJ, Eisenberg PD, Raftopoulos H, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. Cancer. (2005) 104:1548–55. doi: 10.1002/cncr.21343
3. Yeo W, Mo FKF, Suen JJS, Ho WM, Chan SL, Lau W, et al. A randomized study of aprepitant, ondansetron and dexamethasone for chemotherapy-induced nausea and vomiting in Chinese breast cancer patients receiving moderately emetogenic chemotherapy. Breast Cancer Res Treat. (2009) 113:529–35. doi: 10.1007/s10549-008-9957-9
4. Warr DG, Hesketh PJ, Gralla RJ, Muss HB, Herrstedt J, Eisenberg PD, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. (2005) 23:2822–30. doi: 10.1200/JCO.2005.09.050
5. Schwartzberg LS, Modiano MR, Rapoport BL, Chasen MR, Gridelli C, Urban L, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: a randomised, active-controlled, double-blind, phase 3 trial. Lancet Oncol. (2015) 16:1071–8. doi: 10.1016/S1470-2045(15)00034-0
6. Rapoport BL, Jordan K, Boice JA, Taylor A, Brown C, Hardwick JS, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support Care Cancer. (2010) 18:423–31. doi: 10.1007/s00520-009-0680-9
7. Herrstedt J, Apornwirat W, Shaharyar A, Aziz Z, Roila F, Van Belle S, et al. Phase III trial of casopitant, a novel neurokinin-1 receptor antagonist, for the prevention of nausea and vomiting in patients receiving moderately emetogenic chemotherapy. J Clin Oncol. (2009) 27:5363–9. doi: 10.1200/JCO.2009.21.8511
8. Aapro M, Rugo H, Rossi G, Rizzi G, Borroni ME, Bondarenko I, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. (2014) 25:1328–33. doi: 10.1093/annonc/mdu101
9. Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: ASCO guideline update. J Clin Oncol. (2020) 38:2782–97. doi: 10.1200/JCO.20.01296
10. National Comprehensive Cancer Network. Antiemesis. Version 1.2024. (2023). 3025 Chemical Road, Suite 100, Plymouth Meeting, PA 19462: NCCN. Available at: https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1415.
11. Rojas C, Thomas AG, Alt J, Stathis M, Zhang J, Rubenstein EB, et al. Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol. (2010) 626:193–9. doi: 10.1016/j.ejphar.2009.10.002
12. Eisenberg P, MacKintosh FR, Ritch P, Cornett PA, Macciocchi A. Efficacy, safety and pharmacokinetics of palonosetron in patients receiving highly emetogenic cisplatin-based chemotherapy: a dose-ranging clinical study. Ann Oncol. (2004) 15:330–7. doi: 10.1093/annonc/mdh047
13. Gralla R, Lichinitser M, van der Vegt S, Sleeboom H, Mezger J, Peschel C, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. (2003) 14:1570–7. doi: 10.1093/annonc/mdg417
14. Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. (2003) 98:2473–82. doi: 10.1002/cncr.11817
15. Hsu Y-C, Chen C-Y, Tam K-W, Hsu C-Y. Effectiveness of palonosetron versus granisetron in preventing chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis. Eur J Clin Pharmacol. (2021) 77:1597–609. doi: 10.1007/s00228-021-03157-2
16. Rowbottom L, Stinson J, McDonald R, Emmenegger U, Cheng S, Lowe J, et al. Retrospective review of the incidence of monitoring blood glucose levels in patients receiving corticosteroids with systemic anticancer therapy. Ann Palliat Med. (2015) 4:70–7. doi: 10.3978/j.issn.2224-5820.2015.04.07
17. Nakamura M, Ishiguro A, Muranaka T, Fukushima H, Yuki S, Ono K, et al. A prospective observational study on effect of short-term periodic steroid premedication on bone metabolism in gastrointestinal cancer (ESPRESSO-01). Oncologist. (2017) 22:592–600. doi: 10.1634/theoncologist.2016-0308
18. Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer. (2006) 94:1011–5. doi: 10.1038/sj.bjc.6603048
19. Aapro M, Fabi A, Nolè F, Medici M, Steger G, Bachmann C, et al. Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol. (2010) 21:1083–8. doi: 10.1093/annonc/mdp584
20. Komatsu Y, Okita K, Yuki S, Furuhata T, Fukushima H, Masuko H, et al. Open-label, randomized, comparative, phase III study on effects of reducing steroid use in combination with Palonosetron. Cancer Sci. (2015) 106:891–5. doi: 10.1111/cas.12675
21. Celio L, Frustaci S, Denaro A, Buonadonna A, Ardizzoia A, Piazza E, et al. Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multicenter, phase III trial. Support Care Cancer. (2011) 19:1217–25. doi: 10.1007/s00520-010-0941-7
22. Furukawa N, Kanayama S, Tanase Y, Ito F. Palonosetron in combination with 1-day versus 3-day dexamethasone to prevent nausea and vomiting in patients receiving paclitaxel and carboplatin. Support Care Cancer. (2015) 23:3317–22. doi: 10.1007/s00520-015-2760-3
23. Kosaka Y, Tanino H, Sengoku N, Minatani N, Kikuchi M, Nishimiya H, et al. Phase II randomized, controlled trial of 1 day versus 3 days of dexamethasone combined with palonosetron and aprepitant to prevent nausea and vomiting in Japanese breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer. (2016) 24:1405–11. doi: 10.1007/s00520-015-2905-4
24. Okada Y, Oba K, Furukawa N, Kosaka Y, Okita K, Yuki S, et al. One-day versus three-day dexamethasone in combination with palonosetron for the prevention of chemotherapy-induced nausea and vomiting: A systematic review and individual patient data-based meta-analysis. Oncologist. (2019) 24:1593–600. doi: 10.1634/theoncologist.2019-0133
25. Celio L, Bonizzoni E, Zattarin E, Codega P, de Braud F, Aapro M. Impact of dexamethasone-sparing regimens on delayed nausea caused by moderately or highly emetogenic chemotherapy: a meta-analysis of randomised evidence. BMC Cancer. (2019) 19:1268. doi: 10.1186/s12885-019-6454-y
26. Ito Y, Tsuda T, Minatogawa H, Kano S, Sakamaki K, Ando M, et al. Placebo-controlled, double-blinded phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin-1 receptor antagonist and palonosetron in high-emetogenic chemotherapy. J Clin Oncol. (2018) 36:1000–6. doi: 10.1200/JCO.2017.74.4375
27. Herrstedt J, Celio L, Hesketh PJ, Zhang L, Navari R, Chan A, et al. updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following high-emetic-risk antineoplastic agents. Support Care Cancer. (2023) 32:47. doi: 10.1007/s00520-023-08221-4
28. Tamura K, Aiba K, Saeki T, Nakanishi Y, Kamura T, Baba H, et al. Testing the effectiveness of antiemetic guidelines: results of a prospective registry by the CINV Study Group of Japan. Int J Clin Oncol. (2015) 20:855–65. doi: 10.1007/s10147-015-0786-7
29. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
30. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
31. Zhang J, Carlin BP, Neaton JD, Soon GG, Nie L, Kane R, et al. Network meta-analysis of randomized clinical trials: reporting the proper summaries. Clin Trials. (2014) 11:246–62. doi: 10.1177/1740774513498322
32. Lin L, Zhang J, Hodges JS, Chu H. Performing arm-based network meta-analysis in R with the pcnetmeta package. J Stat Softw. (2017). 80. doi: 10.18637/jss.v080.i05
33. Hamza TH, van Houwelingen HC, Stijnen T. The binomial distribution of meta-analysis was preferred to model within-study variability. J Clin Epidemiol. (2008) 61:41–51. doi: 10.1016/j.jclinepi.2007.03.016
35. Matsuura K, Tsurutani J, Inoue K, Tanabe Y, Taira T, Kubota K, et al. A phase 3 safety study of fosnetupitant as an antiemetic in patients receiving anthracycline and cyclophosphamide: CONSOLE-BC. Cancer. (2022) 128:1692–8. doi: 10.1002/cncr.34088
36. Matsumoto K, Takahashi M, Sato K, Osaki A, Takano T, Naito Y, et al. A double-blind, randomized, multicenter phase 3 study of palonosetron vs granisetron combined with dexamethasone and fosaprepitant to prevent chemotherapy-induced nausea and vomiting in patients with breast cancer receiving anthracycline and cyclophosphamide. Cancer Med. (2020) 9:3319–27. doi: 10.1002/cam4.2979
37. Ohzawa H, Miki A, Hozumi Y, Miyazaki C, Sagara Y, Tanaka Y, et al. Comparison between the antiemetic effects of palonosetron and granisetron in breast cancer patients treated with anthracycline-based regimens. Oncol Lett. (2015) 9:119–24. doi: 10.3892/ol.2014.2640
38. Roila F, Ruggeri B, Ballatori E, Del Favero A, Tonato M. Aprepitant versus dexamethasone for preventing chemotherapy-induced delayed emesis in patients with breast cancer: a randomized double-blind study. J Clin Oncol. (2014) 32:101–6. doi: 10.1200/JCO.2013.51.4547
39. Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. (2009) 10:115–24. doi: 10.1016/S1470-2045(08)70313-9
40. Schnadig ID, Agajanian R, Dakhil C, Gabrail N, Vacirca J, Taylor C, et al. APF530 versus ondansetron, each in a guideline-recommended three-drug regimen, for the prevention of chemotherapy-induced nausea and vomiting due to anthracycline plus cyclophosphamide-based highly emetogenic chemotherapy regimens: a post hoc subgroup analysis of the Phase III randomized MAGIC trial. Cancer Manag Res. (2017) 9:179–87. doi: 10.2147/CMAR.S129059
41. Schwartzberg L, Navari R, Clark-Snow R, Arkania E, Radyukova I, Patel K, et al. Phase IIIb safety and efficacy of intravenous NEPA for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients with breast cancer receiving initial and repeat cycles of anthracycline and cyclophosphamide (AC) chemotherapy. Oncologist. (2020) 25:e589–97. doi: 10.1634/theoncologist.2019-0527
42. Segawa Y, Aogi K, Inoue K, Sano M, Sekine I, Tokuda Y, et al. A phase II dose-ranging study of palonosetron in Japanese patients receiving moderately emetogenic chemotherapy, including anthracycline and cyclophosphamide-based chemotherapy. Ann Oncol. (2009) 20:1874–80. doi: 10.1093/annonc/mdp243
43. Wenzell CM, Berger MJ, Blazer MA, Crawford BS, Griffith NL, Wesolowski R, et al. Pilot study on the efficacy of an ondansetron- versus palonosetron-containing antiemetic regimen prior to highly emetogenic chemotherapy. Support Care Cancer. (2013) 21:2845–51. doi: 10.1007/s00520-013-1865-9
44. Zelek L, Debourdeau P, Bourgeois H, Wagner JP, Brocard F, Lefeuvre-Plesse C, et al. A pragmatic study evaluating NEPA versus aprepitant for prevention of chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy. Oncologist. (2021) 26:e1870–9. doi: 10.1002/onco.13888
45. Ogata H, Saito M, Tsuneizumi M, Kutomi G, Hosoya K, Kawai Y, et al. Abstract P5-11-03: Difference between 1st and 2nd generation serotonin receptor antagonists in triplet antiemetic therapy for highly emetogenic chemotherapy in breast cancer patients – according to recent multi-institutional double-blind randomized clinical research on the AC regimen. Cancer Res. (2017) 77 doi: 10.1158/1538-7445.SABCS16-P5-11-03
46. Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. (2010) 21 Suppl 5:v232–43. doi: 10.1093/annonc/mdq194
47. Navari R, Binder G, Molasiotis A, Herrstedt J, Roeland EJ, Ruddy KJ, et al. Duration of chemotherapy-induced nausea and vomiting (CINV) as a predictor of recurrent CINV in later cycles. Oncologist. (2023) 28:208–13. doi: 10.1093/oncolo/oyac240
48. Italian Group For Antiemetic Research. Randomized, double-blind, dose-finding study of dexamethasone in preventing acute emesis induced by anthracyclines, carboplatin, or cyclophosphamide. J Clin Oncol. (2004) 22:725–9. doi: 10.1200/JCO.2004.09.040
49. Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, et al. double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. (2006) 17:1441–9. doi: 10.1093/annonc/mdl137
50. Filetti M, Lombardi P, Giusti R, Falcone R, Scotte F, Giannarelli D, et al. Efficacy and safety of antiemetic regimens for highly emetogenic chemotherapy-induced nausea and vomiting: A systematic review and network meta-analysis. Cancer Treat Rev. (2023) 115:102512. doi: 10.1016/j.ctrv.2023.102512
51. Zhang Y, Yang Y, Zhang Z, Fang W, Kang S, Luo Y, et al. Neurokinin-1 receptor antagonist-based triple regimens in preventing chemotherapy-induced nausea and vomiting: A network meta-analysis. J Natl Cancer Inst. (2017) 126:109. doi: 10.1093/jnci/djw217
52. Cheng J, Cai M, Shuai X, Gao J, Wang G, Tao K. Comparative efficacy and tolerability of antiemetic prophylaxis for adult highly emetogenic chemotherapy: A network meta-analysis of 143 randomized controlled trials. Int J Cancer. (2018) 142:1067–76. doi: 10.1002/ijc.31125
53. Grimes DA, Schulz KF. Making sense of odds and odds ratios. Obstet Gynecol. (2008) 111:423–6. doi: 10.1097/01.AOG.0000297304.32187.5d
54. Ho KM, Marshall RJ, Walters S. Use of odds ratios on anaesthesia related studies. Anaesth Intensive Care. (2003) 31:392–5. doi: 10.1177/0310057X0303100407
56. Yeo W, Lau TK, Li L, Lai KT, Pang E, Cheung M, et al. A randomized study of olanzapine-containing versus standard antiemetic regimens for the prevention of chemotherapy-induced nausea and vomiting in Chinese breast cancer patients. Breast. (2020) 50:30–8. doi: 10.1016/j.breast.2020.01.005
57. Tienchaiananda P, Nipondhkit W, Maneenil K, Sa-Nguansai S, Payapwattanawong S, Laohavinij S, et al. A randomized, double-blind, placebo-controlled study evaluating the efficacy of combination olanzapine, ondansetron and dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving doxorubicin plus cyclophosphamide. Ann Palliat Med. (2019) 8:372–80. doi: 10.21037/apm.2019.08.04
58. Maleki A, Ghadiyani M, Salamzadeh J, Salari S, Banihashem S, Tavakoli-Ardakani M. Comparison of mirtazapine and olanzapine on nausea and vomiting following anthracycline-cyclophosphamide chemotherapy regimen in patients with breast cancer. Iran J Pharm Res. (2020) 19:451–64. doi: 10.22037/ijpr.2020.113955.14584
59. Bajpai J, Kapu V, Rath S, Kumar S, Sekar A, Patil P, et al. Low-dose versus standard-dose olanzapine with triple antiemetic therapy for prevention of highly emetogenic chemotherapy-induced nausea and vomiting in patients with solid tumours: a single-centre, open-label, non-inferiority, randomised, controlled, phase 3 trial. Lancet Oncol. (2024) 25:246–54. doi: 10.1016/S1470-2045(23)00628-9
60. Clemons M, Dranitsaris G, Sienkiewicz M, Sehdev S, Ng T, Robinson A, et al. A randomized trial of individualized versus standard of care antiemetic therapy for breast cancer patients at high risk for chemotherapy-induced nausea and vomiting. Breast. (2020) 54:278–85. doi: 10.1016/j.breast.2020.11.002
Keywords: antiemetics, neurokinin-1 receptor antagonists, serotonin 5-HT3 receptor antagonists, dexamethasone, nausea, vomiting, anthracyclines, cyclophosphamide
Citation: Watanabe D, Iihara H, Kobayashi R, Fujii H, Mori R, Kumada K, Shimizu M, Futamura M and Suzuki A (2024) Dexamethasone-sparing strategies in anthracycline and cyclophosphamide-based chemotherapy with a focus on 5-HT3 receptor antagonists: a network meta-analysis. Front. Oncol. 14:1414037. doi: 10.3389/fonc.2024.1414037
Received: 08 April 2024; Accepted: 08 July 2024;
Published: 26 July 2024.
Edited by:
Giovanni Rosti, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
Shinya Suzuki, National Cancer Center Hospital East, JapanMarta Muzzana, University of Pavia, Italy
Copyright © 2024 Watanabe, Iihara, Kobayashi, Fujii, Mori, Kumada, Shimizu, Futamura and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hirotoshi Iihara, aWloYXJhLmhpcm90b3NoaS5wN0BmLmdpZnUtdS5hYy5qcA==
 Daichi Watanabe
Daichi Watanabe Hirotoshi Iihara
Hirotoshi Iihara Ryo Kobayashi1,5
Ryo Kobayashi1,5