
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 21 May 2024
Sec. Radiation Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1413936
Purpose: The purpose of this study was to provide advice for the indication of regional nodal irradiation (RNI) in patients with one to two positive sentinel lymph nodes (SLNs) without axillary lymph node dissection (ALND).
Methods: We conducted a retrospective study in Shandong Cancer Hospital, Fudan University Shanghai Cancer Center, and West China Hospital. Logistic analysis was performed in order to explore the influencing factors of positive non-SLNs (NSLNs) and >3 positive nodes among patients with one to two SLNs+. Then, nomograms were constructed.
Results: Between May 2010 and 2020, among the 2,845 patients with one to two SLNs+ undergoing ALND (1,992 patients in the training set and 853 patients in the validation set), there were 34.3% harbored NSLNs+ and 15.6% harbored >3 positive nodes. Multivariate analysis showed that cN stage, the number of positive/negative SLN, pathological tumor stage, lympho-vascular invasion (LVI), multicenter, and molecular subtypes were significantly associated with NSLN metastasis. Similarly, multivariate analysis also showed that cN stage, the number of positive/negative SLNs, pathological tumor stage, and LVI could be independent predictors of >3 positive nodes. Then, nomograms for NSLN metastasis and >3 positive nodes were constructed using these parameters, respectively.
Conclusions: The nomograms will be useful in estimating positive NSLNs and >3 positive nodes, and they might provide advice for the optimization of RNI.
The health burden of cancer is increasing in China, with more than 1.6 million people being diagnosed and 1.2 million people dying of the disease each year. As in most other countries, breast cancer is now the most common cancer in Chinese women (1). In the past, the regional nodal irradiation (RNI) was based on axillary tumor burden information after axillary lymph node dissection (ALND) in breast cancer. The evidence-based medical evidence has supported that sentinel lymph node biopsy (SLNB) followed by RNI could replace safely ALND in patients with limited sentinel lymph node (SLN) involvement. The optimization of RNI fields should also take into account this newer approach in clinical practice (2). The Early Breast Cancer Trialists' Collaborative Group (EBCTCG) meta-analysis showed that RNI in patients with positive axillary lymph nodes (ALNs) could improve survival, even after ALND (3). However, there was still no related evidence to design the optimal RNI fields in patients with one to two SLNs+ without ALND.
There were 15.9%–38.6% of patients with positive non-SLNs (NSLNs) when detected one to two SLNs+ (4–7); in other words, there might be one-third of patients with one to two SLNs+ without ALND that have additional axilla up-stage (8). So, the RNI fields of these patients should not be smaller than patients with pN1 after ALND (8).
The purpose of this study was to identify the predict factors of axilla residual tumor burden in patients with one to two SLNs+ based on multicenter population data. Then, we created nomograms that could predict axilla residual tumor burden in patients with one to two SLNs+ without ALND, in order to provide advice for the optimization of RNI, including internal mammary nodal irradiation (IMNI).
Between May 2010 and 2020, we enrolled patients with breast cancer in Shandong Cancer Hospital, Fudan University Shanghai Cancer Center, and West China Hospital of Sichuan University. The inclusion criteria include the following: 1) histologically confirmed invasive breast cancer; 2) cN0 on physical examination or imaging abnormal with/without confirmatory biopsy. According to the National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) guidelines, patients with imaging abnormal disease can be offered SLNB as first-line axillary staging (1, 9); 3) undergone SLNB followed by ALND; and 4) had one to two SLNs+. The exclusion criteria include the following: 1) T3–4 primary tumor; 2) bilateral breast cancer; 3) undergone neoadjuvant therapy; and 4) received axillary surgery or radiotherapy. We collected the clinico-pathological data of enrolled patients.
The informed consent had been agreed by the ethical committee (No. SDTHEC20220324) of Shandong Cancer Hospital. The study protocol was approved by the Institutional Review Board of the Shandong Cancer Hospital, and the study was performed in accordance with the principles of the Declaration of Helsinki.
Each center detected SLNs according to the same method. The SLNB was done with technetium-99m colloid and blue dye. All radioactive or blue-stained ALNs were detected as SLNs. The ALND was defined as a dissection of at least ten nodes from anatomical levels I and II (10). Each SLN was examined at multiple histologic levels (11).
Before treatment, all patients were biopsied by ultrasound. The pathological evaluation including Hematoxylin-Eosin (HE) and Immunohistochemical (IHC) staining. Positive hormone receptor (HR) status was defined as at least 1% of tumor cells expressing the receptor. HER-2 status was determined on the basis of the ASCO/College of American Pathologists guidelines. To accurately evaluate the effect of molecular subtypes, we classified patients into three subtypes: HR-positive/HER-2–negative (HR+/HER2−), triple-negative (TNBC), and HER-2–positive (HER2+) subtype.
We analyzed the correlation between clinic-pathological factors and ALN status. Univariate analysis was performed using the Pearson chi-square or Fisher exact test. Multivariable logistic regression analysis was performed using backward stepwise analysis. Then, nomogram was constructed by “rms” package for R. We used SPSS software (statistics 22.0) and R software (version 3.3.3) to perform statistical analysis. A p < 0.05 was considered statistically significant.
Figure 1 shows the consort diagram of this study. Between May 2010 and 2020, a total of 18,600 patients with breast cancer who underwent SLNB were identified on the basis of the database of the three institutions. After excluding cases having negative SLNs or lacking medical examination data, 2,845 patients with one to two SLNs+ followed by ALND were finally enrolled in Shandong Cancer Hospital (n = 556), Fudan University Shanghai Cancer Center (n = 1,800), and West China Hospital (n = 489).
We used computer to create a unique, random number for each enrolled patient. Patients were classified in line with the random numbers. Finally, 1,992 patients were designated as the training set, and the other 853 patients were designated as the validation set. Table 1 shows the clinical-pathologic characteristics of enrolled patients. The median age was 48 years (range of 21–80 years). Notably, there were 84.2%, 11.8%, and 4.0% of patients had one to three positive nodes (pN1), four to nine positive nodes (pN2), and more than nine positive nodes (pN3), respectively.
The number of positive SLNs and NSLNs is summarized in Table 2. Among the 2,845 patients with one to two SLNs+, 34.3% (975) of them had positive NSLNs, whereas the remaining 65.7% had negative pathological NSLNs. Out of 2,845 patients with one to two SLNs+, 15.8% (449) of them had >3 metastatic ALNs, whereas the remaining 84.2% had ≤3 positive ALNs.
The logistic regression analysis results are shown in Table 3. The multivariate analysis indicated that the independent predictors of positive NSLNs including clinical nodal stage (OR = 2.841; 95% CI, 1.487–5.430; p = 0.002), the number of positive SLNs (OR = 1.737; 95% CI, 1.451–2.079; p < 0.001), the number of negative SLNs (OR = 0.722; 95% CI, 0.678–0.770; p < 0.001), pT stage (OR = 1.204; 95% CI, 1.017–1.425, p = 0.031), multicenter (OR = 1.636; 95% CI, 1.195–2.239; p = 0.002), lympho-vascular invasion (LVI; OR = 3.564; 95% CI, 3.000–4.234; p = 0.004), and molecular subtypes (OR = 0.826; 95% CI, 0.726–0.940, p = 0.004).
According to results of multivariate analysis, a nomogram was constructed to predict positive NSLNs in patients with one to two SLNs+ (Figure 2A). The prediction accuracy of different cutoff point is shown in Supplementary Table 1. In the training set, the area under the curve (AUC) value was 0.766 (95% CI, 0.735–0.794) (Figure 2B). In the external validation set, the AUC value was 0.751 (95% CI, 0.715–0.786), showing a good discriminatory ability (Figure 2C). The difference between the two AUCs was not statistically significant (p = 0.423). The calibration curve showed a satisfactory fit between the observed and predicted outcomes in the training sets (Figure 2D) and validation sets (Figure 2E).
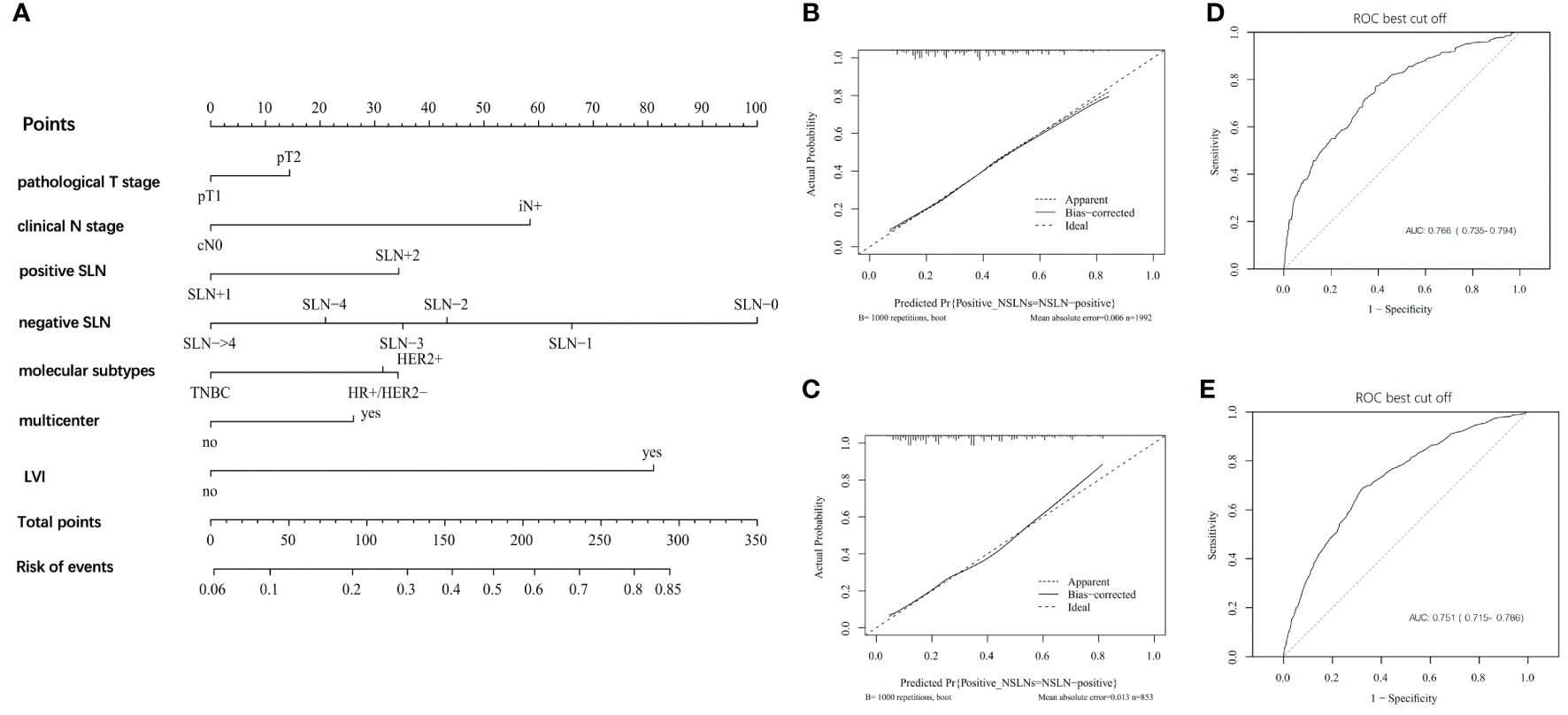
Figure 2 The development and validation of nomogram to predict patients with positive NSLNs. (A) The nomogram to predict patients with positive NSLNs in population with one to two positive SLNs. To calculate the probability of positive NSLNs, the scores for each factor were summed up. In addition, the total scores and bottom risk scale were referenced. (B) The receiver operating characteristic (ROC) curve in the training cohort indicates an AUC value of 0.766. (C) In the validation cohort, the ROC curve indicates an AUC of 0.751. (D) The calibration curve showed a satisfactory fit between the observed and predicted outcomes in the training cohorts. (E) The calibration curve in the validation cohorts.
Table 3 also shows the logistic regression analysis of >3 positive nodes. The multivariate analysis indicated that the independent predictors of >3 positive ALNs including the number of SLNs+ (OR = 3.077; 95% CI, 2.460–3.849; p < 0.001), the number of negative SLNs (OR = 0.614; 95% CI, 0.561–0.672; p < 0.001), pT stage (OR = 1.317; 95% CI, 1.052–1.648; p = 0.016), LVI (OR = 4.078; 95% CI, 3.208–5.184; p < 0.001), and cN stage (OR = 3.366; 95% CI, 1.639–6.911; p = 0.001). Similarly, a nomogram was also created to predict >3 positive ALNs in patients with one to two SLNs+ (Figure 3A). The prediction accuracy of different cutoff point was shown in Supplementary Table 2. The nomogram had an AUC value of 0.814 (95% CI, 0.784–0.825) in the training set (Figure 3B) and 0.804 (95% CI, 0.768–0.820) in the validation set (Figure 3C). The calibration curve showed a satisfactory fit between the observed and predicted outcomes in the training sets (Figure 3D) and the validation sets (Figure 3E).
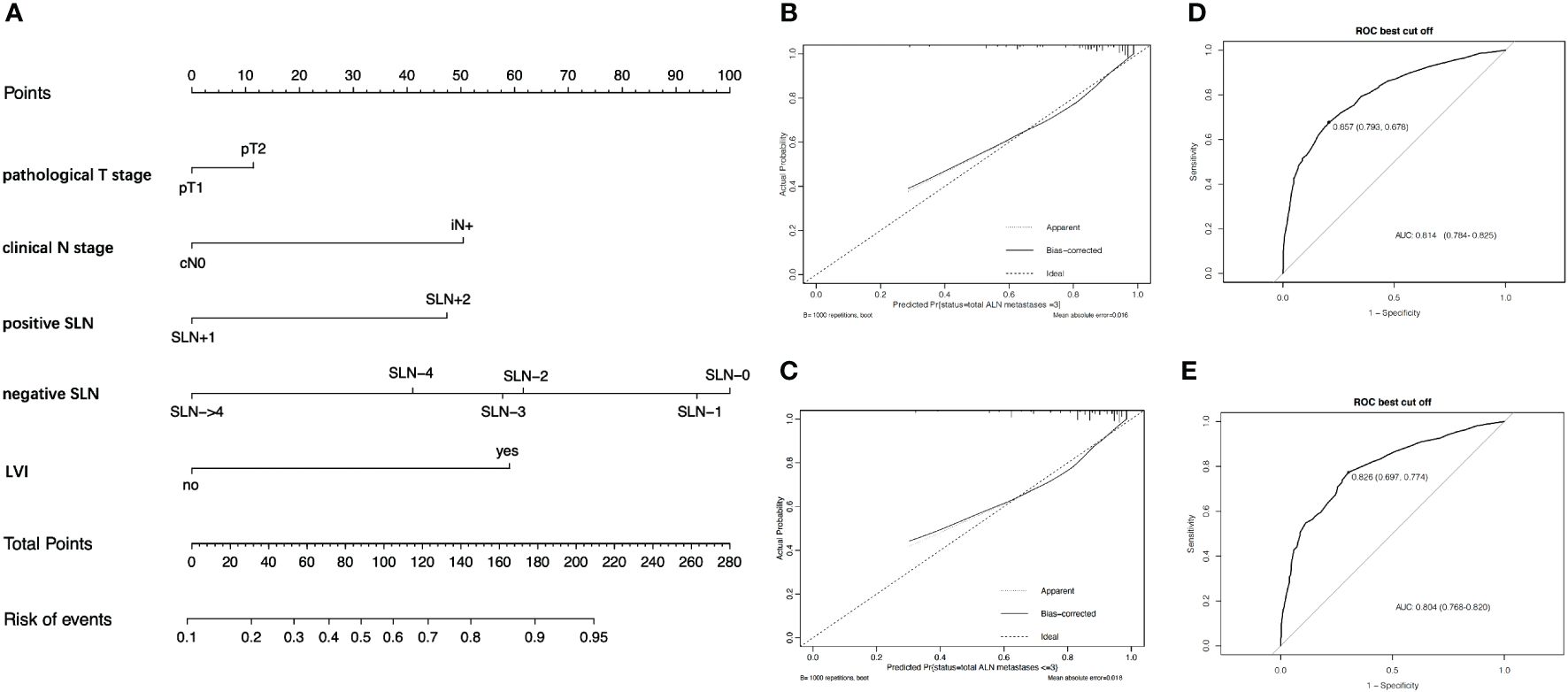
Figure 3 The development and validation of nomogram to predict patients with >3 positive ALNs. (A) The nomogram to predict patients with >3 positive ALNs in population with one to two positive SLNs. To calculate the probability of >3 positive ALNs, the scores for each factor were summed up. In addition, the total scores and bottom risk scale were referenced. (B) The ROC curve in the training cohort indicates an AUC value of 0.814. (C) In the validation cohort, the ROC curve indicates an AUC of 0.804. (D) The calibration curve showed a satisfactory fit between the observed and predicted outcomes in the training cohorts. (E) The calibration curve in the validation cohorts.
In the era of SLNB, SLNB combined with radiotherapy replaced ALND might be the preferred strategy in patients with one to two SLNs+. In our study, we also observed that there were 19.3% of patients with one to two SLNs+ who did not receive ALND, and this trend will be greater. The changing perceptions of axilla treatment make it impossible to fully assess the overall ALNs metastases status (12). Moreover, omitting ALND created a new area of uncertainty for RNI in patients with one to two SLNs+ (8, 12). In the era of SLNB, RNI fields need to be designed in the case of limited nodal tumor information. Therefore, the strength of our study was that the nomograms could help to select precisely populations with one or two positive SLNs that would have positive NSLNs or >3 positive nodes on final pathology. They could provide advice for the optimization of RNI in Chinese patients, and it will be of value to surgeons, medical oncologists, radiation oncologist, and patients in discussing treatment options and their potential outcomes. RNI is strongly recommended to reduce the risk of LRR when the risk of NSLN metastasis >30%. Similarly, IMNI is strongly recommended to be performed when the risk of >3 ALN+ exceeds 20%.
At present, the nomograms have been confirmed to predict axilla metastasis status, combined with actual clinical situations (13–17). Based on the multicenter population data, we developed the nomogram to predict NSLN metastasis in patients with one to two SLNs+ without ALND. In patients with high risk of NSLN metastasis, the risk of recurrence is also high. If no further axilla surgery is performed, then the infra/supraclavicular regions irradiation should be performed to further reduce the risk of regional recurrence. This treatment strategy might the safest approach in the era of limited data concerning outcome after SLNB without ALND (12).
Meanwhile, we found that molecular subtype was also an important factor for predicting NSLN metastasis. Compared with patients with TNBC and HR+/HER2−, patients with HER2+ had a higher probability of NSLN metastasis in patients with one to two SLNs+. This observation is consistent with several published series relating these characteristics to molecular subtype. Crabb et al. (18) demonstrated in a retrospective analysis of 3,441 early-stage breast cancers that subtype as approximated by ER, PR, and HER-2 was predictive of nodal involvement, independent of grade and tumor size. The TNBC subtype had the lowest odds of having ALN involvement, with an OR of 0.53 (95% CI, 0.41–0.6; p < 0.0001) relative to the HR+/HER2− subtype. Ugras et al. (19) also found that HR+/HER2− and TNBC tumors were also less likely to have high-volume lymph node involvement (≥4 nodes involved) than HER2+ tumors. It is suggested that molecular subtype could be used as a predictive factor of lymph node metastasis.
IMNI is one of the main managements of internal mammary region (20–22). However, the benefits of IMNI may also be diluted to some extent by increasing local control when systemic therapy is effective. So, it is very important to grasp indication of IMNI accurately (22). At present, number of positive axillary nodes are still the main indication of IMNI, as it is associated with internal mammary node metastasis. The NCCN guidelines recommend that IMNI should be performed with >3 positive ALNs (category 1) and strongly suggest IMNI to patients with 1 to 3 positive ALNs (category 2A) (9). With more patients received SLNB omitting ALND, IMNI must also be completed without axilla tumor burden.
This study had some limitations. First, this retrospective database–based analysis may increase selection bias in the assignment of treatments. Second, our data are ethnically unique population, and the nomogram was not validated on external population. Third, the IMNI should base on the comprehensive judgement in clinical practice, such as tumor location, biological subtyping, and grade. In addition, we will further explore the indication of IMNI.
In summary, the nomogram will be useful in estimating the likelihood of having positive NSLNs and >3 positive nodes. Moreover, we hope our nomograms can provide advice for the optimization of RNI. In the era of SLNB, the benefits of systemic therapy and radiation therapy can be combined to narrow the scope of surgery and reduce complications, ultimately achieving a “net benefit” of breast cancer treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The informed consent had been agreed by the ethical committee (No. SDTHEC20220324) of Shandong Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
XW: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. ZB: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. JZ: Conceptualization, Formal analysis, Writing – review & editing. YW: Conceptualization, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the China Postdoctoral Science Foundation (grant no. 2022M721987) and Shanghai Cancer Prevention and Anti-cancer Development Foundation (CYBER-2022–002).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1413936/full#supplementary-material
1. Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol. (2014) 15:e279–89. doi: 10.1016/S1470-2045(13)70567-9
2. Costaz H, Boulle D, Bertaut A, Rouffiac M, Beltjens F, Desmoulins I, et al. Omitting axillary lymph node dissection after positive sentinel lymph node in the post-Z0011 era: Compliance with NCCN and ASCO clinical guidelines and Z0011 criteria in a large prospective cohort. Bull Cancer. (2022) 109:268–79. doi: 10.1016/j.bulcan.2021.09.018
3. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomized trials. Lancet. (2014) 383:2127–35. doi: 10.1016/S0140-6736(14)60488-8
4. Bartels SAL, Donker M, Poncet C, Sauvé N, Straver ME, van de Velde CJH, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-Year results of the randomized controlled EORTC 10981–22023 AMAROS Trial. J Clin Oncol. (2022) 41(12):2159–65. doi: 10.1200/JCO.22.01565
5. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomized phase 3 trial. Lancet Oncol. (2010) 11:927–33. doi: 10.1016/S1470-2045(10)70207-2
6. Yang ZB, Lan XW, Huang Z, Yang Y, Tang Y, Jing H, et al. Development and external validation of a nomogram to predict four or more positive nodes in breast cancer patients with one to three positive sentinel lymph nodes. Breast. (2020) 53:143–51. doi: 10.1016/j.breast.2020.08.001
7. Bi Z, Chen JJ, Liu PC, Chen P, Wang WL, Liu YB, et al. Candidates of genomic tests in HR+/HER2- breast cancer patients with 1–2 positive sentinel lymph node without axillary lymph node dissection: analysis from multicentric cohorts. Front Oncol. (2021) 11:722325. doi: 10.3389/fonc.2021.722325
8. Bi Z, Wang XE, Qiu PF, Chen P, Wang YS. Optimization of regional nodal irradiation in the era of sentinel lymph node biopsy. Cancer Biol Med. (2023) 20:89–92. doi: 10.20892/j.issn.2095-3941.2022.0625
9. Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:691–722. doi: 10.6004/jnccn.2022.0030
10. Gerber NK, Port E, Chadha M. The evolving and multidisciplinary considerations in nodal radiation in breast cancer. Semin Radiat Oncol. (2019) 29:150–7. doi: 10.1016/j.semradonc.2018.11.008
11. Wang Y-Z, Yin Y-M, Jiang Z-F. “Breast Cancer Diagnosis and Treatment Guidelines” update key points. Chinese Journal of Surgical Oncology. (2023) 15(03):209–13. Available at: https://kns.cnki.net/kcms2/article/abstract?v=vRpkk4QO0oiMEQNl-HhLbxK1RkKv3hLaHN27oFssWmmb7wyoOziMZ1yRJLI0_FSBfSbrruloPsJcagwPioGKB60QsEfNw_bRpx7fOCDaYoGz5AAwn-4iDeE6miWdj6TDsMQh131MoH8=&uniplatform=NZKPT&language=CHS.
12. Wang YJ, Chen JY. Radiotherapeutic strategies for breast cancer patients in accordance with ACOSOG Z0011 study criteria. Chin J Pract Surg. (2018) 38:1270–6. doi: 10.19538/j.cjps.issn1005-2208.2018.11.12
13. Katz A, Smith BL, Golshan M, Niemierko A, Kobayashi W, Raad RA, et al. Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer. J Clin Oncol. (2008) 26:2093–8. doi: 10.1200/JCO.2007.11.9479
14. Xie X, Tan W, Chen B, Huang X, Peng C, Yan S, et al. Preoperative prediction nomogram based on primary tumor miRNAs signature and clinical-related features for axillary lymph node metastasis in early-stage invasive breast cancer. Int J Cancer. (2018) 142:1901–10. doi: 10.1002/ijc.31208
15. Sa-Nguanraksa D, O-Charoenrat E, Kulprom A, Samarnthai N, Lohsiriwat V, Nimpoonsri K, et al. Nomogram to predict non-sentinel lymph node status using total tumor load determined by one-step nucleic acid amplification: first report from Thailand. Breast Cancer. (2019) 26:471–7. doi: 10.1007/s12282-019-00945-8
16. Unal B, Gur AS, Beriwal S, Tang G, Johnson R, Ahrendt G, et al. Predicting likelihood of having four or more positive nodes in patient with sentinel lymph node-positive breast cancer: a nomogram validation study. Int J Radiat Oncol Biol Phys. (2009) 75:1035–40. doi: 10.1016/j.ijrobp.2008.12.028
17. Kim I, Ryu JM, Kim JM, Choi HJ, Lee SK, Yu JH, et al. Development of a nomogram to predict N2 or N3 stage in T1–2 invasive breast cancer patients with No palpable lymphadenopathy. J Breast Cancer. (2017) 20:270–8. doi: 10.4048/jbc.2017.20.3.270
18. Crabb SJ, Cheang MC, Leung S, Immonen T, Nielsen TO, Huntsman DD, et al. Basal breast cancer molecular subtype predicts for lower incidence of axillary lymph node metastases in primary breast cancer. Clin Breast Cancer. (2008) 8:249–56. doi: 10.3816/CBC.2008.n.028
19. Ugras S, Stempel M, Patil S, Morrow M. Estrogen receptor, progesterone receptor, and HER2 status predict lymphovascular invasion and lymph node involvement. Ann Surg Oncol. (2014) 21:3780–6. doi: 10.1245/s10434-014-3851-y
20. Poortmans PM, Weltens C, Fortpied C, Kirkove C, Peignaux-Casasnovas K, Budach V, et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I-III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial. Lancet Oncol. (2020) 21:1602–10. doi: 10.1016/S1470-2045(20)30472-1
21. Thorsen LB, Offersen BV, Danø H, Berg M, Jensen I, Pedersen AN, et al. DBCG-IMN: A population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol. (2016) 34:314–20. doi: 10.1200/JCO.2015.63.6456
Keywords: breast cancer, sentinel lymph node biopsy, nomogram, internal mammary lymph node, regional nodal irradiation
Citation: Wang X-E, Bi Z, Zhang J and Wang Y-S (2024) Nomograms for metastasis of non-sentinel lymph nodes or more than three lymph nodes in patients with one or two positive sentinel lymph nodes. Front. Oncol. 14:1413936. doi: 10.3389/fonc.2024.1413936
Received: 08 April 2024; Accepted: 30 April 2024;
Published: 21 May 2024.
Edited by:
Jian-Guo Zhou, University of Erlangen Nuremberg, GermanyReviewed by:
Lei Fan, Fudan University, ChinaCopyright © 2024 Wang, Bi, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Sheng Wang, eXN3YW5nQHNkZm11LmVkdS5jbg==; Jin Zhang, emhhbmdqaW50am11Y2gxQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.