- 1Department of Thyroid and Breast Surgery, Clinical Medical College and The First Affiliated Hospital of Chengdu Medical College, Chengdu, Sichuan, China
- 2The First Department of Breast Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin, China
- 3Department of Breast Surgery, Mianyang Central Hospital, Mianyang, Sichuan, China
Background: The combination of CDK4/6 inhibitors (CDK4/6i) and endocrine therapy (ET) is currently the standard first-line treatment for patients with metastatic hormone receptor positive (HR+), and HER2-negative (HER2-) breast cancer. However, the impact of HER2 status on the prognosis of patients receiving CDK4/6i and ET remains unclear. The meta-analysis was conducted to evaluate different outcomes between HER2-low and HER2-zero patients in advanced HR+ breast cancer receiving CDK4/6i and ET.
Methods: A systematic search was performed in PubMed and EMBASE databases for relevant published literature. Objective response rate (ORR), overall survival (OS), and progression-free survival (PFS) were pooled by fixed or random effects models.
Results: Overall, 12 studies with 3567 patients were eligible for analysis. The pooled analysis suggested that no significant differences were observed in terms of ORR and OS between HER2-low and HER2-zero patients who underwent CDK4/6i and ET. Similarly, no significant difference in PFS was found between HER2-low and HER2-zero patients who underwent post-line CDK4/6i and ET or first-line Palbociclib and ET. However, in patients who received mixed-line (not a single treatment line) or first-line CDK4/6i and ET, the PFS was significantly shorter in the HER2-low subgroup than in the HER2-zero subgroup (mixed-line: HR = 1.36; 95% CI = 1.11–1.65; P = 0.002; first-line: HR = 1.14; 95% CI = 1.01–1.28; P = 0.04). A similar phenomenon was observed in patients who received mixed-line or post-line Palbociclib and ET (mixed-line: HR = 1.60; 95% CI = 1.09–2.34; P = 0.02; post-line: HR = 1.43; 95% CI = 1.03–2.00; P = 0.03).
Conclusion: These results indicated that HER2-low status did not have a significant association with ORR and OS, but it may have a worse impact on PFS in patients who received mixed-line or first-line CDK4/6i and ET, as well as mixed-line or post-line palbociclib plus ET.
Introduction
In recent years, new antibody-drug conjugate (ADC) drugs have been continuously developed. Destiny-Breast 04 has proved that new ADC are not only effective in HER2-positive breast cancer but also have good anti-tumor effects in HER2-low breast cancer (1, 2). This led to the concept of HER2-low breast cancer and aroused the research interest of scholars. Currently, HER2-low breast cancer is defined as tumors with an immunohistochemical (IHC) score of 1+ or 2+ and negative in situ hybridization (ISH) results, accounting for approximately 45%-55% of all breast cancers (3, 4).
CDK4/6i combined with ET can significantly improve the prognosis of patients with HR+/HER2- metastatic breast cancer (MBC) and has become the standard treatment for such patients (5–7). Previous studies have shown bidirectional crosstalk between ER and HER2 pathways. HER2 over-expression can reduce ER expression, regulate ER transcriptional activity, and cause ET resistance and drug resistance (8, 9). It is unclear whether HER2-low could cause endocrine resistance and drug resistance. Recent evidence shows that the expression of HER2-low is significantly higher in HR+ breast cancer compared with HR-negative breast cancer (10, 11). Moreover, Several studies have shown that HER2-low breast cancer has unique clinical biological characteristics. Compared with HER2-zero breast cancer, it has a lower Ki-67 score, lower histological grade, and less axillary lymph node metastasis (12–14). Therefore, it is particularly important to know whether HER2-low affects the prognosis of patients receiving CDK4/6i combined with ET. Some studies believe that HER2-low does not affect the survival outcomes of MBC patients treated with CDK4/6i plus ET (15–18). Other studies have suggested that HER2-low is associated with worse survival outcomes in MBC patients treated with CDK4/6i and ET (19–22). Given the conflicting results, we conducted this study to evaluate whether outcomes differ between HR+ MBC patients with HER2-low and HER2-zero treated with CDK4/6i and ET.
Materials and methods
Search strategy
The meta-analysis was conducted according to the PRISMA statement (23). The PRISMA checklist is provided in Supplementary Table 1. A systematic search was conducted in the PubMed and Embase databases for studies published by January 2024. It was performed using the following keywords: “HER2-low” OR “ERBB2 low” AND “CDK 4/6” OR “Palbociclib” OR “Ribociclib” OR “Abemaciclib”. The details of the search strategy are provided in Supplementary Table 2. The study was registered in PROSPERO (CRD42024521829).
Inclusion and exclusion criteria
Publications were considered eligible if they met all the following inclusion criteria; 1) patients diagnosed with HR+/HER2- MBC; 2) patients who received CDK4/6i plus ET (aromatase inhibitor or fulvestrant); 3) the study compared the survival outcomes in terms of OS and/or PFS between HER2-low and HER2-zero. Exclusion criteria were as follows; 1) review articles, meta-analysis, case reports or letters; 2) patients with other malignant tumors; 3) HRs and 95% CIs cannot be extracted.
Data extraction and quality assessment
Data were collected from the eligible studies independently by two investigators. The relevant information was extracted such as the first author’s name, nationality, publication year, study type, the number of participants, median age, median follow-up time, treatment line, metastatic sites type, CDK4/6i, treatment manner, ORR, OS, PFS.
The non-randomized experimental research-MINORS scale was used to assess the study quality of the included studies (24). Eight items needed to be evaluated. Each item was scored as 0, 1, or 2, corresponding to not reported, inadequately reported, or adequately reported. Studies with scores of 8 or more were rated as high-quality (See Supplementary Table 3).
Summary measures and statistical analysis
The Meta-analysis was performed using RevMan version 5.3 (RevMan, version 5.3 for Windows; Cochrane Collaboration, Oxford, UK). The hazard ratios (HRs) and 95% CIs were directly extracted from reported data or calculated from Kaplan-Meier survival curves. Statistical heterogeneity within studies was detected with Chi-squared and I2. When I2 > 50%, the heterogeneity was significant and the random-effects model was used; otherwise, there was no heterogeneity and the fixed-effects model was used (25). Funnel plot and Begg’s test were performed with the Stata 11.0 (Stata Corporation, College Station, TX, USA) to assess the potential publication bias (26). All tests were two-sided. P < 0.05 was considered statistically significant.
Results
Study selection and characteristics
147 articles were identified from the two databases, of which 50 were duplications. After screening the titles and abstracts of the remaining 97 studies, 58 articles were removed. Another 27 articles were excluded when the full texts were examined. Finally, 12 studies with 3567 patients met the qualifying criteria were included (15–22, 27–30). Figure 1 shows the flowchart of the literature selection.
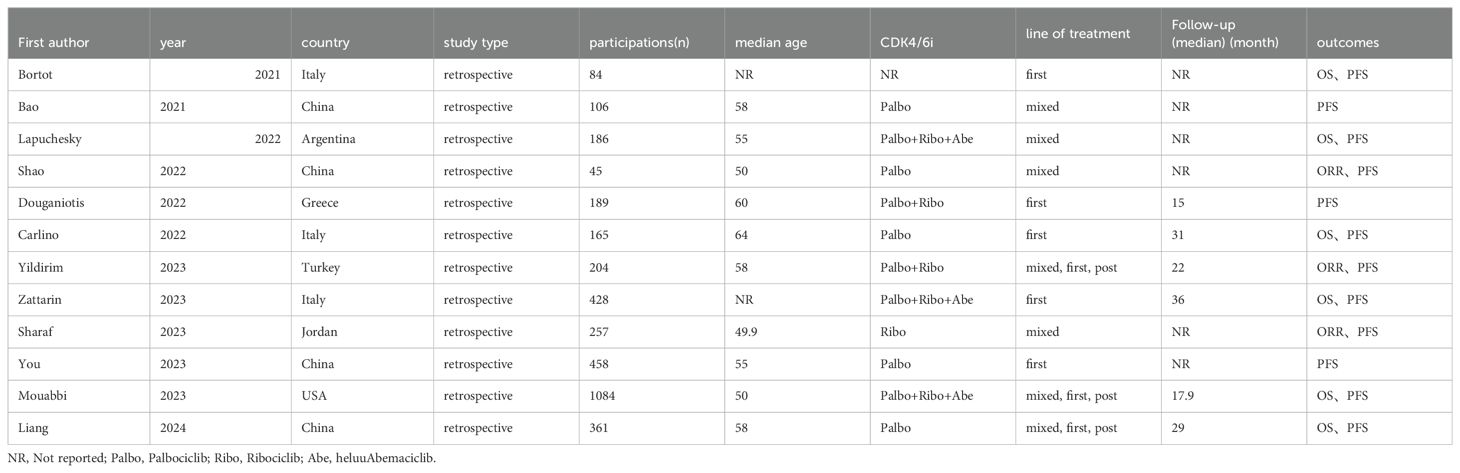
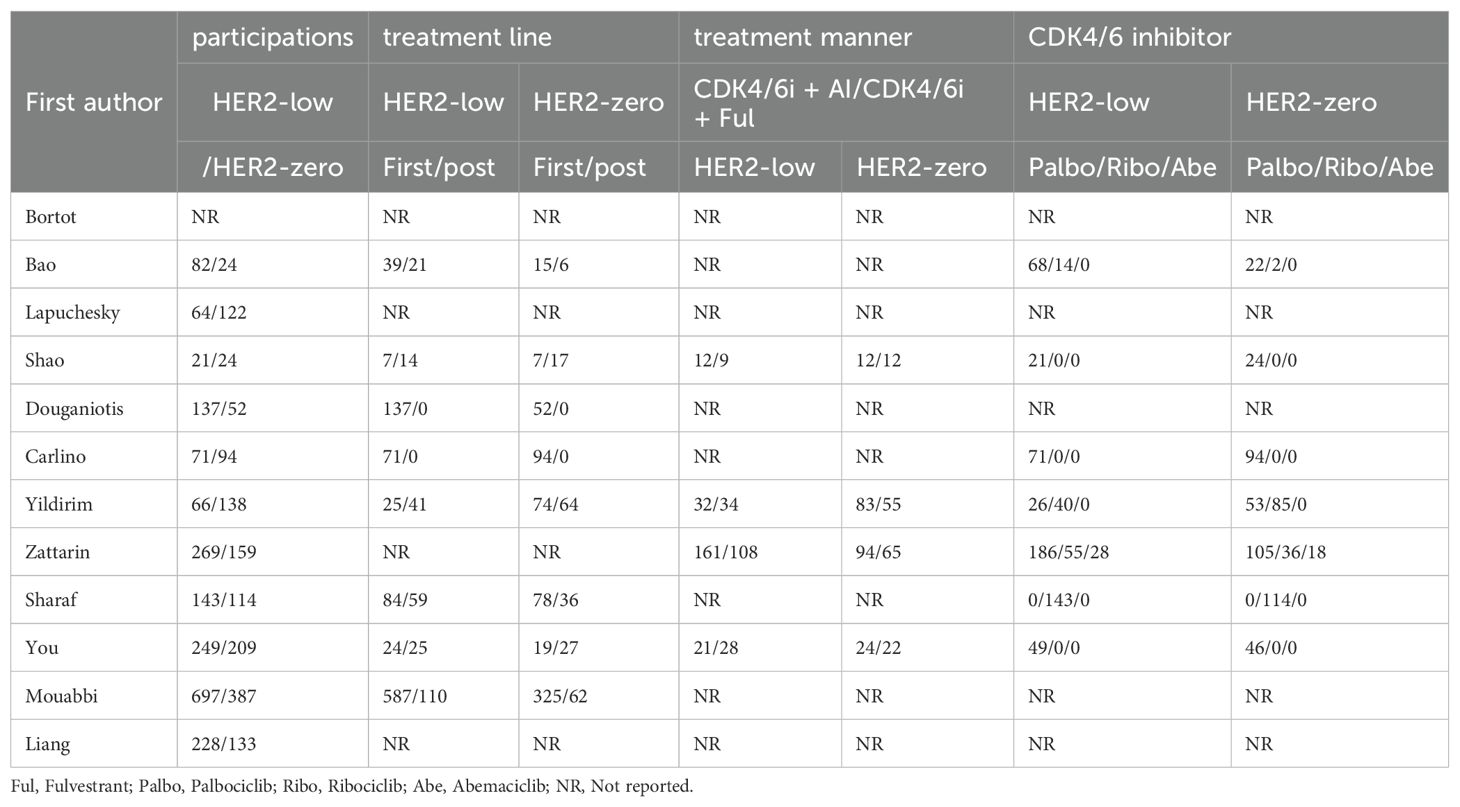
All the studies were retrospective studies from 8 countries such as Italy, Argentina, China, Greece, and so on. The median age of patients ranges from 48.3 to 63. Median follow-up time ranges from 15 to 36. The follow up time was adequate for most of the studies. 11 studies reported the number of patients with HER2-low and HER2-zero respectively. Among 3359 patients, there were 1903 (56.65%) HER2-low patients and 1456 (43.35%) HER2-zero patients. In terms of CDK4/6i, Palbociclib was the most commonly used among patients included in the study. In 5 studies, patients were treated with CDK4/6i and ET as first-line treatment, while the remaining 7 studies also used it as second-line or third-line treatment. Tables 1, 2 show the study characteristics.
Objective response rate
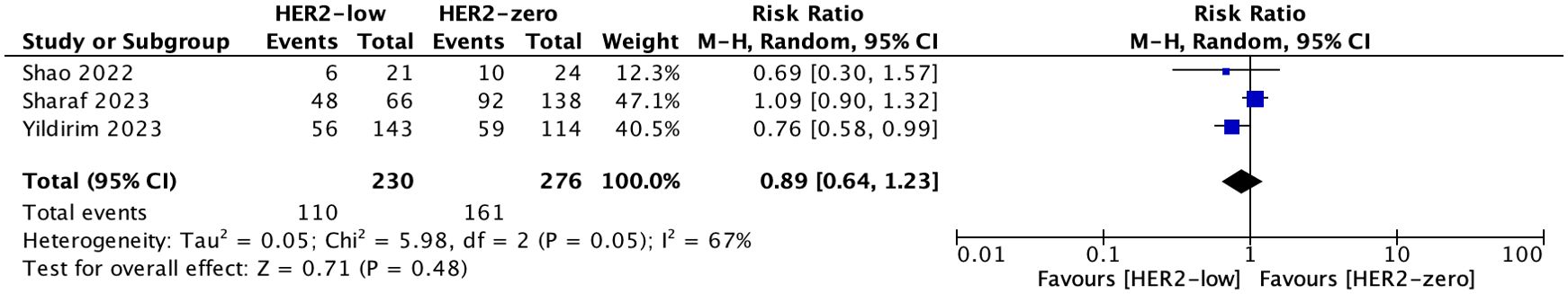
3 studies with 506 patients reported ORR. The ORR was 47.83%(110/230) in patients with HER2-low and 58.33%(161/276) in patients with HER2-zero. There was no significant difference in ORR between HER2-low and HER2-zero patients (HR = 0.89; 95% CI = 0.64–1.23; P = 0.48). Heterogeneity was not detected among the included studies (I2 = 67%, P = 0.05). As shown in Figure 2.
Overall survival
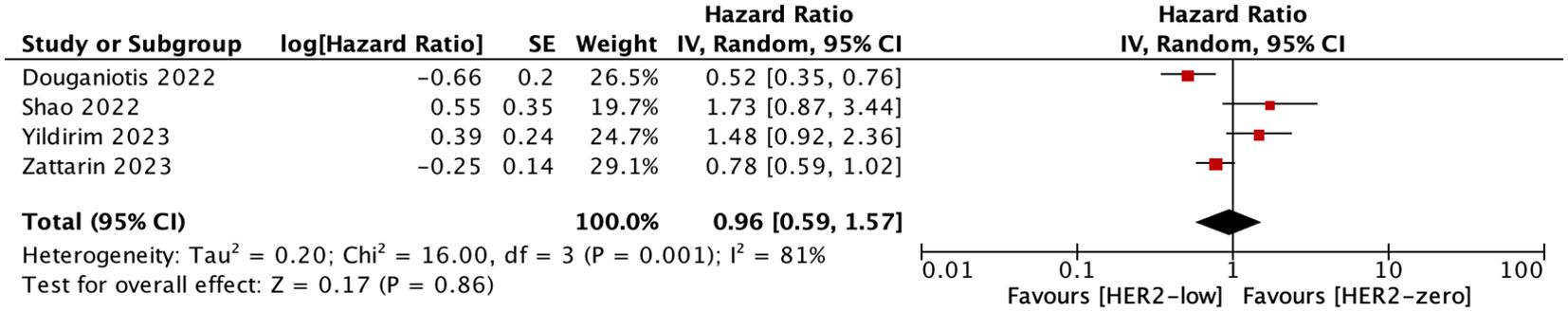
Six studies involving 2306 patients reported OS. The OS of HER2-low VS. HER2-zero in patients who received CDK4/6i plus ET as mixed-line therapy was evaluated in the Lapuchesky and Liang trials. The results revealed there was no statistically significant difference in OS between the two groups (HR = 1.00; 95% CI = 0.67–1.49; P = 0.99). 4 studies reported the OS in patients who were treated with first-line and only one study reported those treated with post-line. The OS of HER2-low VS. HER2-zero patients was similar in both of the two subgroups (first-line: HR = 1.07; 95% CI = 0.76–1.52; P = 0.69; post-line: HR = 1.25; 95% CI = 0.81–1.92; P = 0.32). No heterogeneity was detected within the included studies (mixed-line: I2 = 0%, P = 0.89; first-line: I2 = 62%, P = 0.05). As shown in Figure 3.
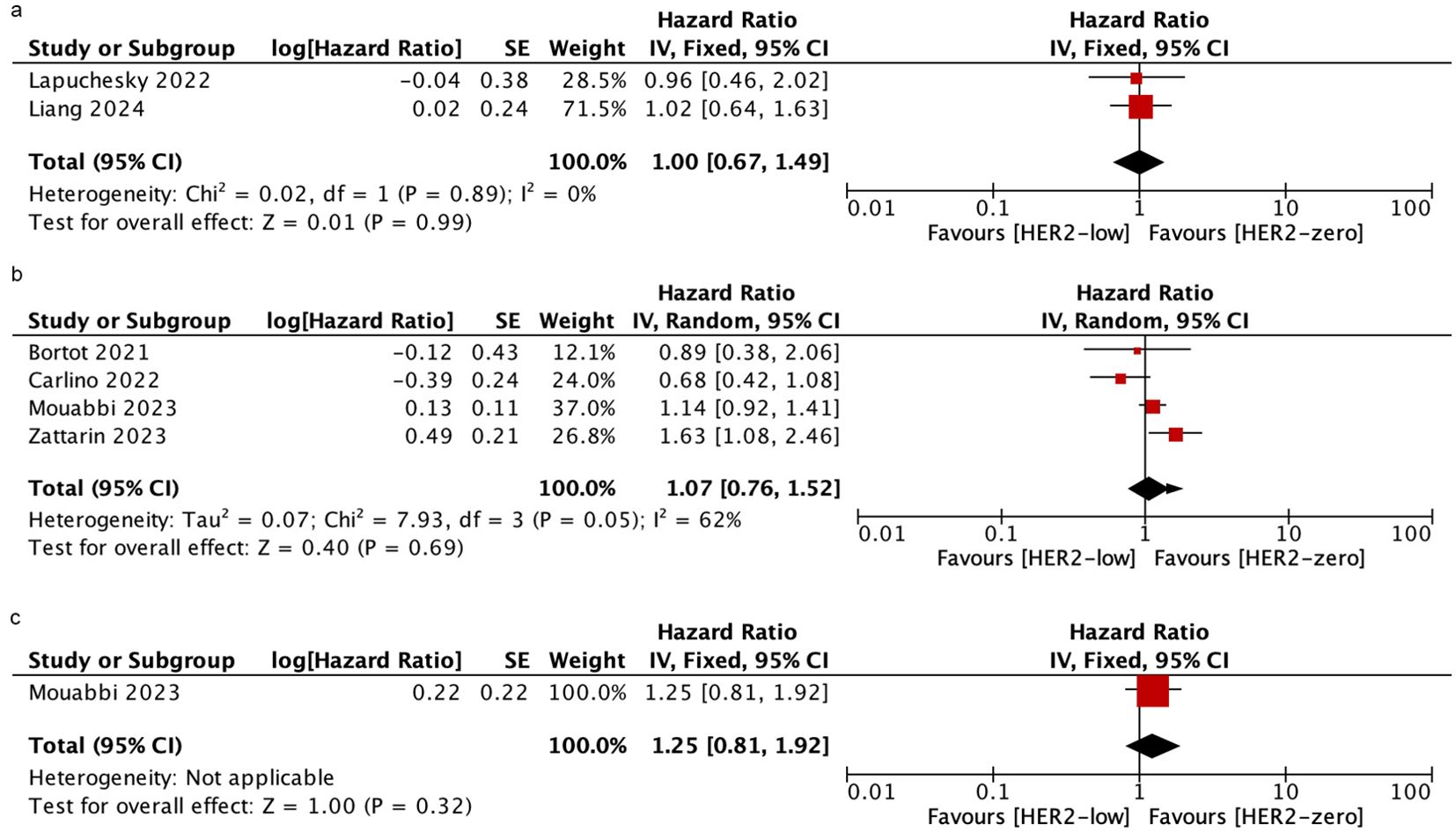
Figure 3. Forest plot of the HR for OS of HER2-low breast cancer vs. HER2-zero breast cancer in mixed-line (A), first-line (B), post-line (C).
Progression free survival
Six studies were included to assess the PFS in patients who received mixed-line treatment. The results revealed that the PFS was shorter in the HER2-low subgroup than in the HER2-zero subgroup (HR = 1.36; 95% CI = 1.11–1.65; P = 0.002). Similarly, in patients who were treated with first-line, HER2-low patients had shorter PFS than HER2-zero patients based on nine studies (HR = 1.14; 95% CI = 1.01–1.28; P = 0.04). However, data obtained from three studies demonstrated there was no significant difference in PFS between HER2-low and HER2-zero patients who were treated with post-line (HR = 1.22; 95% CI = 0.98–1.52; P = 0.08). There was no significant heterogeneity among the studies. As shown in Figure 4.
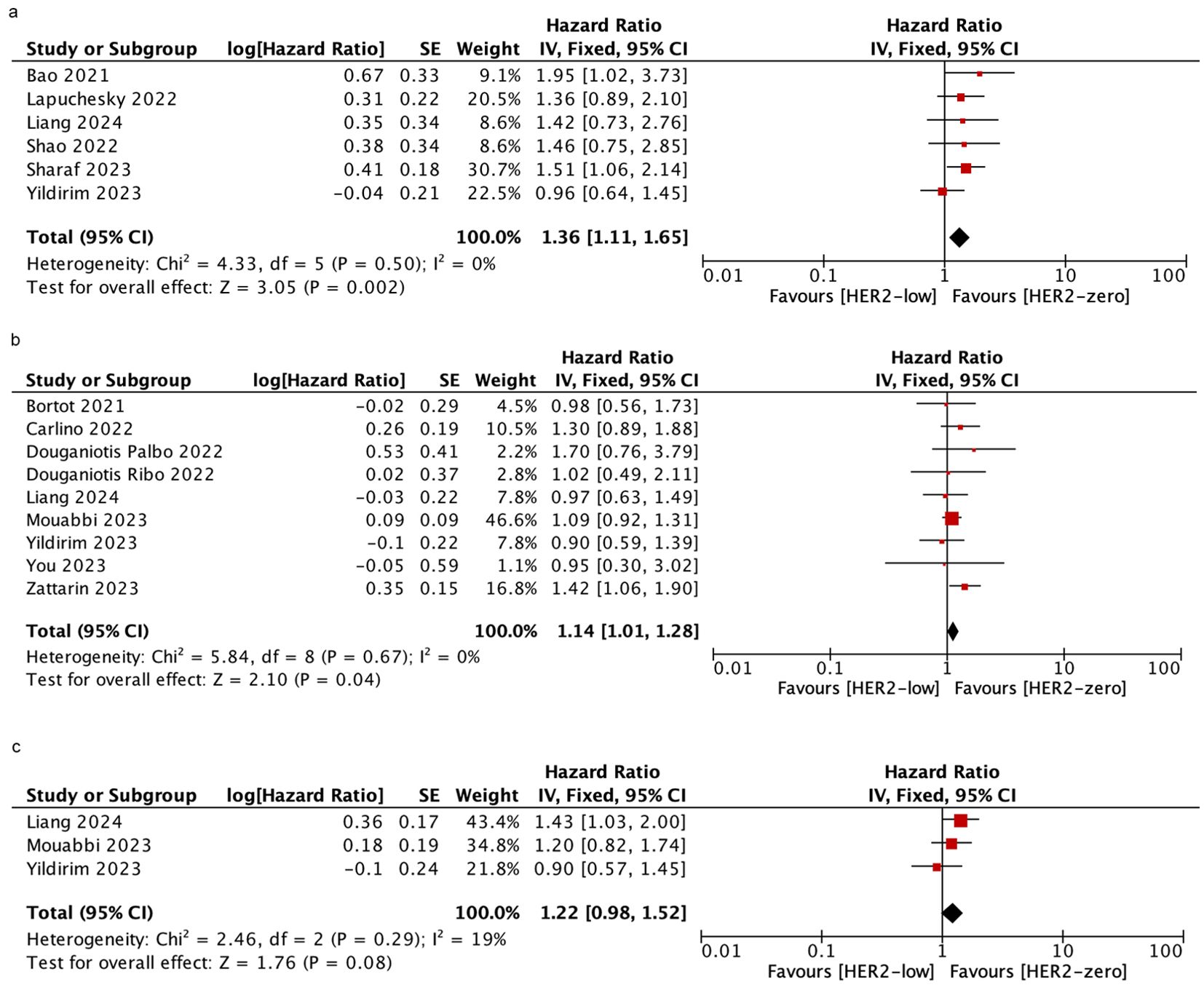
Figure 4. Forest plot of the HR for PFS of HER2-low breast cancer vs. HER2-zero breast cancer in mixed-line (A), first-line (B), post-line (C).
Palbociclib is the most commonly used CDK4/6i in the included studies. To determine whether HER2-low was associated with PFS in patients who were treated with palbociclib plus ET, further analyses were performed. we assessed three studies to evaluate the PFS in patients who received mixed-line treatment. The results showed that HER2-low patients had shorter PFS than HER2-zero patients (HR = 1.60; 95% CI = 1.09–2.34; P = 0.02). Furthermore, 4 studies and 1 study were included to assess the PFS in patients who received first-line treatment and post-line treatment, respectively. There was no significant difference in PFS between HER2-low and HER2-zero patients during the first-line treatment (HR = 1.18; 95% CI = 0.91–1.53; P = 0.2), while a significantly poor PFS was observed in HER2-low patients compared to those with HER2-zero in the post-line treatment subgroup (HR = 1.43; 95% CI = 1.03–2.00; P = 0.03). No heterogeneity was detected within the included studies. As shown in Figure 5.
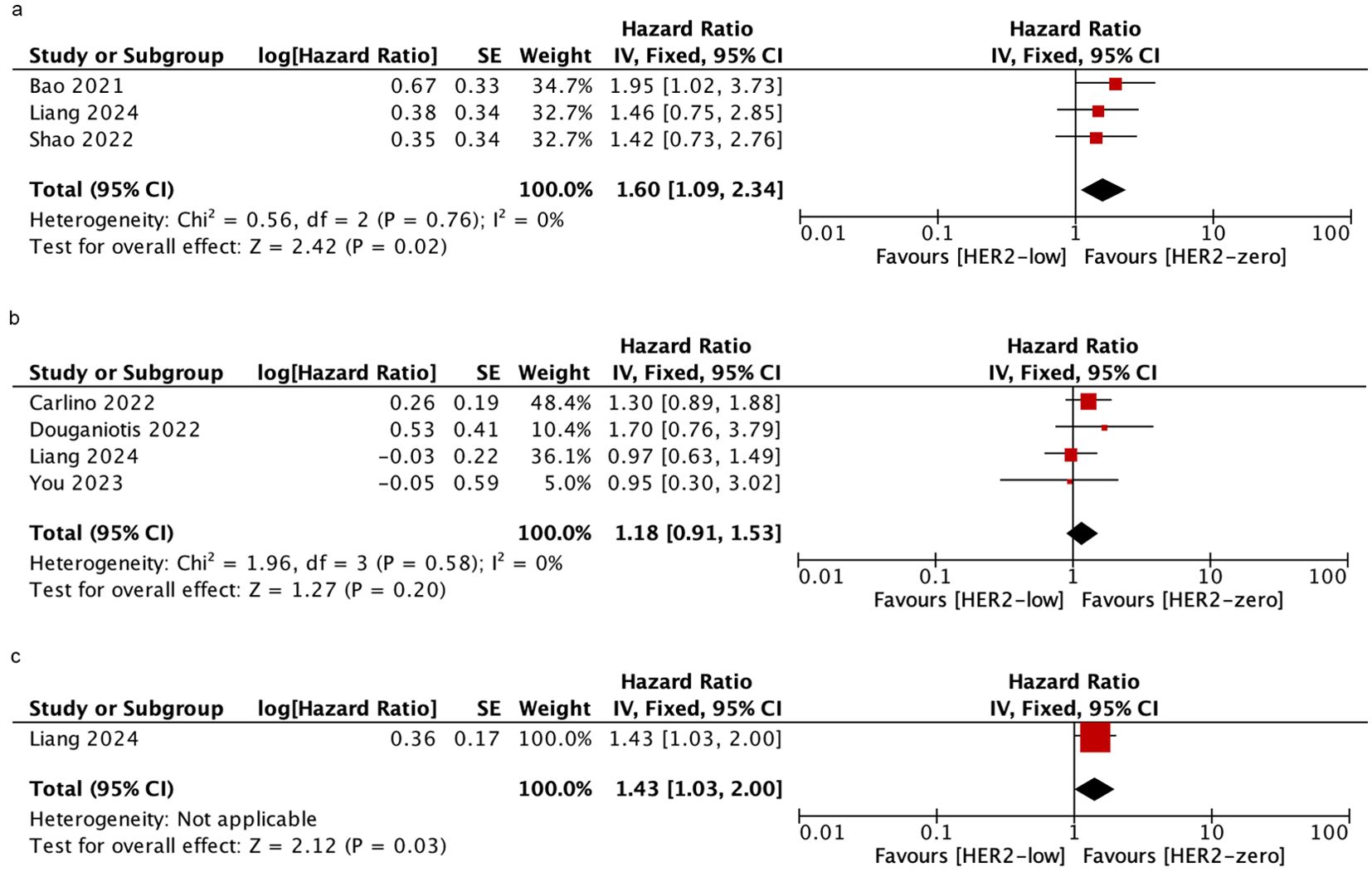
Figure 5. Forest plot of the HR for PFS (palbociclib plus ET) of HER2-low breast cancer vs. HER2-zero breast cancer in mixed-line (A), first-line (B), post-line (C).
Four studies included in our research compared the PFS in patients treated with CDK4/6i+AIs versus CDK4/6i+fulvestrant. We conducted a summary analysis, concluding that there was no difference in PFS between the patients who received CDK4/6i+AIs versus CDK4/6i+fulvestrant (HR = 0.96; 95% CI = 0.59–1.57; P = 0.86). As shown in Figure 6.
The funnel plots and Begg’s test were conducted to evaluate the publication bias (See Supplementary Figure 1). All P values were > 0.05, which revealed there was no significant publication bias found in ORR, OS, and PFS (See Supplementary Table 4).
Discussion
CDK4/6i and ET are the standard treatments for the vast majority of HR+/HER2- MBC patients. At present, studies on the effect of HER2-low on treatment outcomes in patients with HR+ MBC receiving CDK 4/6i + ET are quite limited and the results are contradictory. In this study, we investigated the association between HER2 status (low vs. zero) and prognosis in HR+/HER2- MBC patients treated with CDK4/6i and ET through a pooled analysis of 12 studies. The results showed that HER2-low was not associated with ORR and OS, yet it was significantly linked to worse PFS in certain treatment lines. In addition, Douganiotis et al. also pointed out that “Median PFS was numerically different among the different expression level of Her2(0 VS.1+, 0 VS. 2+), but this difference did not reach statistical significance (P=0.477) (28).
New antibody-targeting drugs have drawn our attention to HER2-low breast cancer. More and more studies have begun to compare the difference between HER2-zero breast cancer and HER2-low breast cancer. Studies have proven that histological grade 2 tumors are more common in HER2-low breast cancer patients, and the expression level of Ki67 is significantly lower than that in HER2-zero patients (13, 31). In terms of gene expression, compared with HER2-zero, HER2-low breast cancer has fewer TP53 mutations, proliferation-related genes are significantly down-regulated, and lumen-related genes are significantly up-regulated (11, 32). Therefore, scholars speculate that HER2-low is related to a good prognosis. However, studies have produced conflicting results. Many studies have reported that the prognosis of HER2-low breast cancer is better than that of HER2-zero breast cancer (33–35), but some studies have reported that the prognosis of the two groups is the same (36–38). Conflicting results were also obtained on the efficacy of neoadjuvant chemotherapy in the two groups. Some studies have shown that HER2-low is associated with lower pathological complete response (pCR) rates (39–41). Another part of the study showed that there was no significant difference in pCR rate between the two groups (42–44). Therefore, HER2-low as a unique biological subtype of breast cancer is still controversial.
Resistance to ET often occurs, and studies have shown that this is related to the over-activity of the RTK signaling pathway, in which overexpressed HER2 plays an important role (45). The HER family comprises 4 RTKs (HER1-4) and several of their ligands (46). The activation of HER2 can induce the transphosphorylation of ERBB dimer partners and stimulate multiple intracellular pathways, such as RAS/RAF/MEK/ERK, PI3K/AKT/TOR, Src kinase, and STAT transcription factors. The activation of these pathways can significantly interfere with ER transcriptional activity, which may lead to endocrine resistance (47). Recent studies have also demonstrated that members of the HER signaling pathway can reduce ER expression at both mRNA and protein levels, leading to decreased endocrine sensitivity (48). Several studies have shown that the estrogen receptor positive (ER+) breast cancer with HER2 over-expression shows resistance to ET (49–51). Compared with simple ET, the addition of anti-HER2 treatment into ET can also significantly improve the efficacy of HR+/HER2+ advanced breast cancer patients (52). It is unclear whether HER2-low expression contributes to resistance to ET. Wang et al. analyzed the prognosis of 72 HR+/HER2- advanced breast cancer patients who received first-line ET, and the study showed that the OS and PFS of HER2-low patients were lower than those of HER2-zero patients (53). Wu et al. analyzed 233 women with HR+/HER2- MBC who received ET with or without CDK4/6 inhibitors (54). The results showed that HER2-low subgroup showed a significantly shorter median PFS compared to the HER2-zero subgroup in the ET alone cohort (5.6 VS. 17.0 months; P = 0.0044). These seem to indicate that HER2-low patients are more likely to develop endocrine resistance. Collins et al. conducted a study on the ER+/HER2-low human breast cancer mouse xenotransplantation model, and the results showed that adding ET to the combination of anti-HER3 and anti-HER2 drugs could further improve the efficacy of tumor regression (55). In addition, in patients with ER+/HER2-low breast cancer who failed ET, the combination of lumretuzumab and pertuzumab treatment could prolong clinical response. These results confirm that there is also direct crosstalk between HER2 and ER in HER2-low breast cancer.
HR+/HER2- advanced breast cancer patients have poor chemo-sensitivity and poor prognosis after endocrine resistance. The development of CDK4/6i has brought new hope to such patients. Several large clinical studies have shown that compared with ET treatment alone, combined with CDK4/6i can achieve a higher ORR for patients and can significantly improve the PFS and OS of patients. Therefore, several authoritative guidelines unanimously recommend that CDK4/6i combined with ET is currently the standard treatment for patients with HR+/HER2- MBC (56–58). However, in clinical practice, there are still people who progress rapidly after first-line CDK4/6i + ET treatment, and the treatment effect is limited. There are currently no clear molecular markers for predicting therapeutic effects. Our research shows that HER2-low may have a worse impact on PFS in patients who received CDK4/6i and ET. Given this, we may need to improve the prognosis of HR+/HER2-low breast cancer patients who receive CDK4/6i and ET. Nearly 70% of HR+/HER2-low breast cancer patients in the DESTINY-Breast 04 study had previously received CDK4/6i treatment. Results showed that among these patients, the mPFS of the trastuzumab deruxtecan (T-DXd) group was twice that of the control chemotherapy group, significantly reducing the risk of disease progression or death (1). Based on this, we speculate whether CDK4/6i +T-DXd treatment can improve the prognosis of HR+/HER2-low patients, but there is currently no relevant research. The 2023 SABCS conference updated the results of the DESTINY-Breast 08 study. The ORR of the T-DXd combined with anastrozole group can reach 70%, and the median PFS reaches 13.4 months; the ORR of T-DXd combined with fulvestrant is 40%, and the median PFS was not reached. Studies have shown that the combination of T-DXd with anastrozole or fulvestrant has positive significance in the first or second-line treatment of HER2-low expression HR+ MBC patients (59). We look forward to future studies on T-DXd combined with CDK4/6i.
Our meta-analysis has a few limitations. Firstly, all the included studies are retrospective, which may have heterogenous in terms of menopausal status, levels of ER/PgR, analysis of HER2 on primary or metastases, intra- and inter-heterogeneity. Secondly, some subgroup analyses only included a few studies, We should interpret their results with caution. Thirdly, there are some confounding factors such as different CDK4/6i and different hormonal therapy companions (AIs and fulvestrant) were used in these studies. We were not able to perform an analysis by different CDK4/6i because no data was available for that. Finally, there may be some differences between the actual HR and that extracted from the survival curve, which can affect the final result we calculate.
Conclusion
In conclusion, our meta-analysis suggested that HR+ MBC patients with HER2-low and HER2-zero have similar ORR and OS when receiving CDK4/6i and ET treatment, but HER2-low may have a worse impact on PFS in patients who received mixed-line or first-line CDK4/6i and ET, as well as mixed-line or post-line palbociclib plus ET. Further large prospective studies are needed to confirm this result.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
LX: Data curation, Project administration, Supervision, Writing – original draft, Writing – review & editing. XC: Project administration, Writing – original draft, Writing – review & editing. QH: Data curation, Formal Analysis, Methodology, Software, Writing – review & editing. WX: Data curation, Formal Analysis, Methodology, Software, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1413674/full#supplementary-material
Supplementary Figure 1 | Funnel plot of HER2-low breast cancer vs. HER2-zero breast cancer. RR for the ORR (A); HR for OS: mixed-line (B), first-line (C); HR for PFS: mixed-line (D), first-line (E), post-line (F); HR for PFS (palbociclib plus ET): mixed-line (G), first-line (H); HR for PFS (CDK4/6i+AIs vs. CDK4/6i+fulvestrant) (I)
Abbreviations
CDK4/6i, CDK4/6 inhibitor; ET, endocrine therapy; HR+, hormone receptor positive; HER2-, HER2-negative; ORR, Objective response rate; OS, overall survival; PFS, progression-free survival; ADC, antibody-drug conjugates; IHC, immunohistochemical; ISH, in situ hybridization; MBC, metastatic breast cancer; HRs, hazard ratios; pCR, pathological complete response; ER+, estrogen receptor positive; T-DXd, trastuzumab deruxtecan.
References
1. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. DESTINY-Breast04 Trial Investigators Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. (2022) 387:9–20. doi: 10.1056/NEJMoa2203690
2. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. (2020) 382:610–21. doi: 10.1056/NEJMoa1914510
3. Agostinetto E, Rediti M, Fimereli D, Debien V, Piccart M, Aftimos P, et al. HER2-low breast cancer: Molecular characteristics and prognosis. Cancers (Basel). (2021) 13:2824. doi: 10.3390/cancers13112824
4. Wei T, Wang DY, Gao SL, Wang X, Yue J, Kang YK, et al. Clinicopathologic characteristics and prognostic significance of HER2-low expression in patients with early breast cancer: A systematic review and meta-analysis. Front Oncol. (2023) 13:1100332. doi: 10.3389/fonc.2023.1100332
5. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. (2018) 36:2465–72. doi: 10.1200/JCO.2018.78.9909
6. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. (2016) 17:425–39. doi: 10.1016/S1470-2045(15)00613-0
7. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. (2017) 35:2875–84. doi: 10.1200/JCO.2017.73.7585
8. Giuliano M, Trivedi MV, Schiff R. Bidirectional crosstalk between the estrogen receptor and human epidermal growth factor receptor 2 signaling pathways in breast cancer: Molecular basis and clinical implications. Breast Care. (2013) 8:256–62. doi: 10.1159/000354253
9. Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: Molecular mechanism and clinical implications for endocrine therapy resistance. Endocrine Rev. (2008) 29:217–33. doi: 10.1210/er.2006-0045
10. Zhang H, Katerji H, Turner BM, Audeh W, Hicks DG. HER2-low breast cancers: Incidence, HER2 staining patterns, clinicopathologic features, MammaPrint and BluePrint genomic profiles. Mod Pathol. (2022) 35:1075–82. doi: 10.1038/s41379-022-01019-5
11. Schettini F, Chic N, Brasó-Maristany F, Paré L, Pascual T, Conte B, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. (2021) 7:1. doi: 10.1038/s41523-020-00208-2
12. Zhang G, Ren C, Li C, Wang Y, Cheng B, Wen L, et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. (2022) 20:142. doi: 10.1186/s12916-022-02346-9
13. Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. (2021) 22:1151–61. doi: 10.1016/S1470-2045(21)00301-6
14. Dehghani M, Keshavarz P, Talei A, Akrami M, Tahmasebi S, Safaie A, et al. The effects of low HER2/neu expression on the clinicopathological characteristics of triple-negative breast cancer patients. Asian Pac J Cancer Prev. (2020) 21:3027–32. doi: 10.31557/APJCP.2020.21.10.3027
15. Bortot L, Basile D, Targato G, Zara D, Palmero L, Alberti M, et al. Clinical characterization and outcome of a HER2-low metastatic breast cancer (mBC) cohort receiving frst-line treatment (1L) with ET +/- CDK 4/6 inhibitor (CDKi). Ann Oncol. (2021) 32:S493–3. doi: 10.1016/j.annonc.2021.08.578
16. Shao Y, Luo Z, Yu Y, Chen Q, He Y, Liu C, et al. HER2-low expression does not affect the clinical outcomes of metastatic breast cancer treated with CDK4/6 inhibitor: a real-world study. Front Endocrinol (Lausanne). (2022) 13:1000704. doi: 10.3389/fendo.2022.1000704
17. Carlino F, Diana A, Ventriglia A, Piccolo A, Mocerino C, Riccardi F, et al. HER2-Low status does not affect survival outcomes of patients with metastatic breast cancer (MBC) undergoing first-line treatment with endocrine therapy plus palbociclib: results of a multicenter, retrospective cohort study. Cancers. (2022) 14:4981. doi: 10.3390/cancers14204981
18. Yildirim EC, Atag E, Coban E, Unal OU, Celebi A, Keser M, et al. The effect of low HER2 expression on treatment outcomes in metastatic hormone receptor positive breast cancer patients treated with a combination of a CDK4/6 inhibitor and endocrine therapy: A multicentric retrospective study. Breast. (2023) 70:56–62. doi: 10.1016/j.breast.2023.06.006
19. Bao KKH, Sutanto L, Tse SSW, Man Cheung K, Chan JCH. The association of ERBB2-low expression with the efficacy of cyclin-dependent kinase 4/6 inhibitor in hormone receptor-positive, ERBB2-negative metastatic breast cancer. JAMA Netw Open. (2021) 4:e2133132. doi: 10.1001/jamanetworkopen.2021.33132
20. Zattarin E, Presti D, Mariani L, Sposetti C, Leporati R, Menichetti A, et al. Prognostic significance of HER2-low status in HR-positive/HER2-negative advanced breast cancer treated with CDK4/6 inhibitors. NPJ Breast Cancer. (2023) 9:27. doi: 10.1038/s41523-023-00534-1
21. Sharaf B, Abu-Fares H, Tamimi F, Al-Sawajneh S, Salama O, Daoud R, et al. Differences in treatment outcomes between patients with HER2-low versus HER2-zero, hormone receptor-positive advanced-stage breast cancer treated with ribociclib. Breast Cancer. (2023) 15:541–8. doi: 10.2147/BCTT. S415432
22. Liang X, Zhang L, Gui X, Di L, Li H, Song G. Real-world study of palbociclib combined with endocrine therapy for patients with metastatic breast cancer: A comparison of subsequent treatment patterns and HER2 expression analysis. Cancer. (2024) 130:1476–87. doi: 10.1002/cncr.35174
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
24. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J, et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
25. Armitage P, Berry G, Matthews J. Analysing means and proportions. Statistical Methods in Medical Research. Oxford: Blackwell Science (2002) p. 83–146.
26. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088e101. doi: 10.2307/2533446
27. Lapuchesky LS, Bortz M, Waisberg F, Enrico DH, Bruno LI, Ostinelli CA, et al. CDK4/6 inhibitors outcomes in patients with advanced breast cancer based on HER2-low expression. J Clin Oncol. (2022) 40:1056. doi: 10.1200/JCO.2022.40.16_suppl.1056
28. Douganiotis G, Kesisis G, Lalla E, Korantzis I, Boukovinas I, Papazisis K. Prognostic significance of low HER2 expression in patients with metastatic hormone receptor-positive breast cancer treated with first line cdk4/6 inhibitors: a greek multicenter real-world data analysis. Cancer Diagn Progn. (2022) 2:585–91. doi: 10.21873/cdp.
29. You S, Gong C, Li Y, Xie Y, Li Y, Zhao Y, et al. Clinicopathological characteristics, evolution, treatment pattern and outcomes of hormone-receptor-positive/HER2-low metastatic breast cancer. Front Endocrinol (Lausanne). (2023) 14:1270453. doi: 10.3389/fendo.2023.1270453
30. Mouabbi JA, Singareeka Raghavendra A, Bassett RL Jr, Hassan A, Tripathy D, Layman RM. Survival outcomes in patients with hormone receptorpositive metastatic breast cancer with low or no ERBB2 expression treated with targeted therapies plus endocrine therapy. JAMA Netw Open. (2023) 6:e2313017. doi: 10.1001/jamanetworkopen.2023.13017
31. Won HS, Ahn J, Kim Y, Kim JS, Song JY, Kim HK, et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean breast cancer society. Breast Cancer Res. (2022) 24:22. doi: 10.1186/s13058-022-01519-x
32. Bayona RS, Luna AM, Tolosa P, De Torre AS, Castelo A, Marín M, et al. HER2-low vs HER2-zero metastatic breast carcinoma: A clinical and genomic descriptive analysis. Ann Oncol. (2021) 32:S29–30. doi: 10.1016/j.annonc.2021.03.036
33. Li Y, Abudureheiyimu N, Mo H, Guan X, Lin S, Wang Z, et al. In real life, low-level HER2 expression may be associated with better outcome in HER2-negative breast cancer: A study of the national cancer center. China. Front Oncol. (2022) 11:774577. doi: 10.3389/fonc.2021.774577
34. Tan RSYC, Ong WS, Lee KH, Lim AH, Park S, Park YH, et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: An international multicentre cohort study and TCGA-METABRIC analysis. BMC Med. (2022) 20:105. doi: 10.1186/s12916-022-02284-6
35. Rosso C, Voutsadakis IA. Characteristics, clinical differences and outcomes of breast cancer patients with negative or low HER2 expression. Clin Breast Cancer. (2022) 22:391–7. doi: 10.1016/j.clbc.2022.02.008
36. Xu H, Han Y, Wu Y, Wang Y, Li Q, Zhang P, et al. Clinicopathological characteristics and prognosis of HER2-low early-stage breast cancer: A single-institution experience. Front Oncol. (2022) 12:906011. doi: 10.3389/fonc.2022.906011
37. Holthuis EI, Vondeling GT, Kuiper JG, Dezentjé V, Rosenlund M, Overbeek JA, et al. Real-world data of HER2-low metastatic breast cancer: A population based cohort study. Breast. (2022) 66:278–84. doi: 10.1016/j.breast.2022.11.003
38. Tarantino P, Jin Q, Tayob N, Jeselsohn RM, Schnitt SJ, Vincuilla J, et al. Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol. (2022) 8:1177–83. doi: 10.1001/jamaoncol.2022.2286
39. Peiffer DS, Zhao FY, Chen N, Hahn OM, Nanda R, Olopade OI, et al. Clinicopathologic characteristics and prognosis of ERBB2-low breast cancer among patients in the national cancer database. JAMA Oncol. (2023) 9:500–10. doi: 10.1001/jamaoncol.2022.7476
40. Kang S, Lee SH, Lee HJ, Jeong H, Jeong JH, Kim JE, et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur J cancer. (2022) 11:30–40. doi: 10.1016/j.ejca.2022.08.031
41. Di Cosimo S, La Rocca E, Ljevar S, De Santis MC, Bini M, Cappelletti V, et al. Moving HER2-low breast cancer predictive and prognostic data from clinical trials into the real world. Front Mol Biosci. (2022) 9:996434. doi: 10.3389/fmolb.2022.996434
42. Zhou SL, Liu T, Kuang XY, Zhen TT, Shi HJ, Lin Y, et al. Comparison of clinicopathological characteristics and response to neoadjuvant chemotherapy between HER2-low and HER2-zero breast cancer. Breast. (2023) 67:1–7. doi: 10.1016/j.breast.2022.12.006
43. Alves FR, Gil L, Vasconcelos de Matos L, Baleiras A, Vasques C, Neves MT, et al. Impact of human epidermal growth factor receptor 2 (HER2) low status in response to neoadjuvant chemotherapy in early breast cancer. Cureus. (2022) 14:e22330. doi: 10.7759/cureus.22330
44. Shao YB, Yu Y, Luo ZF, Guan HJ, Zhu FY, He YN, et al. Clinical, pathological complete response, and prognosis characteristics of HER2-low breast cancer in the neoadjuvant chemotherapy setting: A retrospective analysis. Ann Surg Oncol. (2022) 29:8026–34. doi: 10.1245/s10434-022-12369-4
45. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. (2011) 62:233–47. doi: 10.1146/annurev-med-070909-182917
46. Citri A, Yarden Y. EGF-erbB signalling: towards the systems level. Nat Rev Mol Cell Biol. (2006) 7:505–16. doi: 10.1038/nrm1962
47. Croessmann S, Formisano L, Kinch LN, Gonzalez-Ericsson PI, Sudhan DR, Nagy RJ, et al. Combined blockade of activating ERBB2 mutations and ER results in synthetic lethality of ER+/HER2 mutant breast cancer. Clin Cancer Res. (2019) 25:277–89. doi: 10.1158/1078-0432.CCR-18-1544
48. Guo S, Sonenshein GE. Forkhead box transcription factor FOXO3a regulates estrogen receptor alpha expression and is repressed by the HER-2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Mol Cell Biol. (2004) 24:8681–90. doi: 10.1128/MCB.24.19.8681-8690.2004
49. Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. (2004) 96:926–35. doi: 10.1093/jnci/djh166
50. De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. (2005) 11:4741–8. doi: 10.1158/1078-0432.CCR-04-2569
51. Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol. (2008) 26:1059–65. doi: 10.1200/JCO.2007.12.9437
52. Huober J, Fasching PA, Barsoum M, Petruzelka L, Wallwiener D, Thomssen C, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer-results of the eLEcTRA trial. Breast. (2012) 21:27–33. doi: 10.1016/j.breast.2011.07.006
53. Wang K, Du Q, Yu J, Li Y, Zhu X. Effect of HER2 expression status on the prognosis of patients with HR+/HER2- advanced breast cancer undergoing advanced first-line endocrine therapy. Oncol Lett. (2023) 26:299. doi: 10.3892/ol
54. Wu Y, Mo HN, Xu HC, Wang Y, Wang J, Ma F, et al. Impact of HER2-low expression on the efficacy of endocrine therapy with or without CDK4/6 inhibitor in HR-positive/HER2-negative metastatic breast cancer: A prospective study. Thorac Cancer. (2024) 15:965–73. doi: 10.1111/1759-7714.15282
55. Collins D, Jacob W, Cejalvo JM, Ceppi M, James I, Hasmann M, et al. Direct estrogen receptor (ER) / HER family crosstalk mediating sensitivity to lumretuzumab and pertuzumab in ER+ breast cancer. PLoS One. (2017) 12:e0177331. doi: 10.1371/journal.pone.0177331
56. Gradishar WJ, Moran MS, Abraham J, Abramson V, Aft R, Agnese D, et al. NCCN Guidelines™ Insights: Breast Cancer, Version 4.2023. Natl Compr Canc Netw. (2023) 21:594–608. doi: 10.6004/jnccn.2023.0031
57. Burstein HJ, Somerfield MR, Barton DL, Dorris A, Fallowfield LJ, Jain D, et al. Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. (2021) 39:3959–77. doi: 10.1200/JCO.21.01392
58. ESMO Metastatic Breast Cancer Living Guidelines,v1.1 (2023). Available online at: https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline/er-positive-her2-negative-breast-cancer. (accessed March 10,2024).
Keywords: breast cancer, HER2-low, CDK4/6 inhibitor, endocrine therapy, prognosis
Citation: Xia L-Y, Cao X-C, Hu Q-L and Xu W-Y (2024) Prognosis in HR-positive metastatic breast cancer with HER2-low versus HER2-zero treated with CDK4/6 inhibitor and endocrine therapy: a meta-analysis. Front. Oncol. 14:1413674. doi: 10.3389/fonc.2024.1413674
Received: 07 April 2024; Accepted: 31 July 2024;
Published: 29 August 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Claudia De Angelis, Careggi University Hospital, ItalyArya Mariam Roy, University at Buffalo, United States
Copyright © 2024 Xia, Cao, Hu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin-Yu Xia, Y2hhb2dlYmIxMjNAMTYzLmNvbQ==
 Lin-Yu Xia
Lin-Yu Xia Xu-Chen Cao2
Xu-Chen Cao2