- 1Department of Pain and Rehabilitation, Xinqiao Hospital, Army Medical University, Chongqing, China
- 2Department of Clinical Laboratory, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Department of Spine Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 4Department of Emergency Medicine Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
To date, despite extensive research, the prognosis of advanced osteosarcoma has not improved significantly. Thus, patients experience a reduced survival rate, suggesting that a reevaluation of current treatment strategies is required. Recently, in addition to routine surgery, chemotherapy and radiotherapy, researchers have explored more effective and safer treatments, including targeted therapy, immunotherapy, anti-angiogenesis therapy, metabolic targets therapy, and nanomedicine therapy. The tumorigenesis and development of osteosarcoma is closely related to angiogenesis. Thus, anti-angiogenesis therapy is crucial to treat osteosarcoma; however, recent clinical trials found that it has insufficient efficacy. To solve this problem, the causes of treatment failure and improve treatment strategies should be investigated. This review focuses on summarizing the pathophysiological mechanisms of angiogenesis in osteosarcoma and recent advances in anti-angiogenesis treatment of osteosarcoma. We also discuss some clinical studies, with the aim of providing new ideas to improve treatment strategies for osteosarcoma and the prognosis of patients.
1 Introduction
Osteosarcoma is a bone-derived primary malignant tumor that is characterized by malignant proliferation and the production of bone-like tissue and matrix (1, 2). Epidemiological investigations showed that the age distribution of patients with osteosarcoma was bimodal. It mainly occurs in children and young adults with rapid bone growth between 10 and 30 years old, and in certain people over 65 years old (3–5). The first peak group coincides with the peak of adolescent growth, and the second is thought to be secondary to long-term Paget’s disease and radiation therapy (6–9). Osteosarcoma is considered to be concealed, malignant, and aggressive. Approximately 25% of patients with osteosarcoma present with metastasis at the time of initial diagnosis and experience recurrence during the treatment (10, 11). Osteosarcoma mainly occurs at the epiphyseal end of the long leg bone, for example, around the knee and near the humerus. Patients often experience pain, swelling, restricted limb activity, and accessible clumps at the lesion site (2). Diagnosis depends mainly on pathological biopsy. Osteosarcoma can be classified into eight categories according to fifth edition of the 2020 World Health Organization (WHO) classification of tumors of soft tissue and bone tumors, including low-grade central osteosarcoma, conventional osteosarcoma, telangiectatic osteosarcoma, small cell osteosarcoma, parosteal osteosarcoma, periosteal osteosarcoma, high-grade surface osteosarcoma, and secondary osteosarcoma (12). According to the degree of malignancy, osteosarcoma can be classified into low and high grade. Low-grade osteosarcoma, which consists of low-grade central osteosarcoma and cortical osteosarcoma, is less malignant, and can usually be treated using surgery alone. However, high-grade osteosarcoma is one of the most malignant tumors, and is often accompanied by lung metastases. High-grade osteosarcoma always requires surgery combined with neoadjuvant chemotherapy and postoperative chemotherapy, frequently requiring other comprehensive therapies. Unfortunately, the prognosis of patients with osteosarcoma has not improved significantly after nearly 40 years of treatment using the multi-mode combination strategy of surgical and neoadjuvant chemotherapy or postoperative chemotherapy (13–15). According to statistics, the 5-year survival rate of primary osteosarcoma can reach about 65–70% (16, 17). However, for advanced osteosarcoma, which almost always involves lung metastasis, the 5-year survival rate is only approximately 20% (18–21). It is believed that high invasiveness and resistance are the main causes of poor treatment efficacy; therefore, new treatment strategies are urgently required (22–24).
Tumor growth and progression are intricately linked to angiogenesis, which is essential for the provision of nutrients. Moreover, the formation of new blood vessels facilitates the metastatic dissemination of cancer cells into circulation and subsequent establishment of metastases (3, 20). Thus, anti-angiogenesis treatment has become an important therapeutic strategy and comprehensive therapy for advanced tumors, aiming to limit the growth and metastasis of tumors by inhibiting neoangiogenesis and normalizing tumor vessels (25). For example, the classic anti-angiogenesis drug, Bevacizumab, has been approved to treat lung cancer, hepatocellular carcinoma, glioma, and colorectal cancer, and has demonstrated desirable clinical efficacy (26).
Advanced osteosarcoma, namely with metastatic, recurrent or unresectable diseases, is a highly vascularized tumor, and the progression of advanced osteosarcoma is closely related to angiogenesis (27). Angiogenesis not only plays a pivotal role in the metastasis of osteosarcoma, but also facilitates the colonization of tumor cells at secondary sites (1, 3). Therefore, inhibiting angiogenesis appears to be beneficial for preventing advanced progression of osteosarcoma. Additionally, studies have shown that the microvascular density of osteosarcoma correlates positively with tumor prognosis, and the expression of vascular endothelial growth factor (VEGF), induced by angiogenesis, has been used as an important method to evaluate the prognosis of osteosarcoma (20). Although anti-angiogenesis therapy represents a promising strategy to treat advanced osteosarcoma, currently, the therapeutic effect of anti-angiogenesis treatment in osteosarcoma remains controversial (25). Thus, this review aims to summarize the molecular mechanism of angiogenesis and the current role of anti-angiogenesis therapy in advanced osteosarcoma to provide new ideas for its effective treatment.
2 The mechanism of angiogenesis in osteosarcoma
Angiogenesis is a complex and dynamic process that is regulated by the homeostasis of angiogenic and anti-angiogenic factors in the physiological state (28, 29). In the tumor microenvironment (TME), angiogenic homeostasis is disrupted, resulting in excessive angiogenesis in the lesion (30), which provides nutritional support for the tumor, thereby promoting the growth, invasion, and metastasis of osteosarcoma (31, 32). The expansion of osteosarcoma relies on neovascularization to maintain oxygen and nutrient supplies (33), and the rapid growth of osteosarcoma subsequently opens the “angiogenesis switch” caused by the higher oxygen demand in the high metabolic TME, forming a vicious circle. Large amounts of neoangiogenesis lead to disordered tumor vessels, neovascular dysfunction, and low perfusion within the TME (34), thus promoting the growth, invasion, immunosuppression, and distant metastasis of osteosarcoma (35).
2.1 Angiogenesis patterns in tumors
Tumors, including osteosarcoma, demonstrate several patterns of angiogenesis (Figure 1), such as sprouting angiogenesis (36, 37), intussusception angiogenesis (38), vasculogenesis (39), vessel mimicry (40, 41), trans-differentiation of tumor stem cells (26, 42, 43), and vessel co-option (44). Sprouting angiogenesis involves the formation of a sprouting bud based on existing blood vessels and is the main pattern of physiological and pathological angiogenesis (45) (Figure 1A). Under hypoxia, quiescent endothelial cells (ECs) are activated by angiogenic stimulators, such as VEGF, and are converted into tip cells or stalk cells. Tip cells are characterized by their location at the tip of the sprouting buds, which contain many filopodia to sense the VEGF concentration gradient. They guide the direction of angiogenesis, but lack proliferative activity (46). Stalk cells are located behind the tip cells. They are highly proliferative and mainly form the lumen to elongate the vascular buds under tip cell guidance (47). Vasculogenesis is the process by which the bone marrow-derived endothelial progenitor cells (EPCs) differentiate into ECs and migrate inside the tumor to form new vessels (48) (Figure 1B). Intussusception angiogenesis is a process that inserts mesenchymal structures into the interior of a pre-existing vessel, splitting the vessel into two vessels, which is considered an important complementary modality to sprouting angiogenesis (49, 50) (Figure 1C). Vessel mimicry involves the formation of a “microvascular channel” by EC-like tumor cells with the extracellular matrix (ECM), which exposes tumor stem cells to the blood stream, thereby facilitating metastasis (40, 51) (Figure 1D). Tumor stem cells can participate in angiogenesis inside tumors by transforming into EC-like cells (52) (Figure 1E). Finally, vessel co-option is not a true angiogenesis, but comprises a pattern in which tumor cells colonize around existing blood vessels to encapsulate them inside the tumor (44) (Figure 1F).
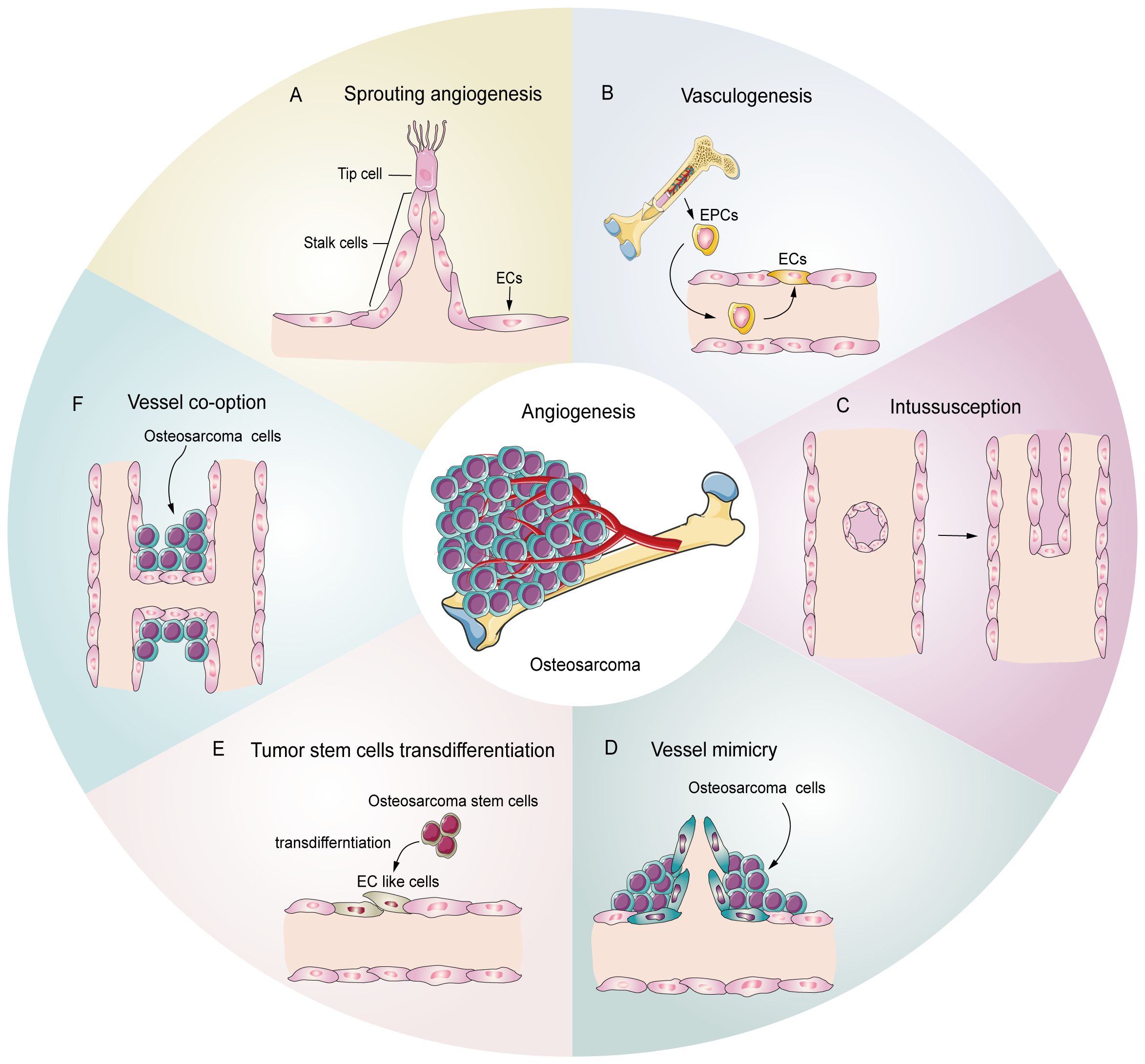
Figure 1. Potential schematic diagram of osteosarcoma angiogenesis patterns. (A) Sprouting angiogenesis: the main pattern in osteosarcoma, which is induced by tip cells and stalk cells. (B) Vasculogenesis: EPCs are recruited and differentiate into ECs to participate in angiogenesis (C) Intussusception: an existing vessel is split into two vessels through EC reorganization. (D) Vessel mimicry: tumor cells form tubular structures to sustain tumor perfusion. (E) Tumor stem cell transdifferentiation: osteosarcoma stem cells differentiate into EC like cells and participate in angiogenesis. (F) Vessel co-option: osteosarcoma cells colonize around the existing vessels.
2.2 Molecular mechanism of angiogenesis
In osteosarcoma, the fast-growing tumor and the lagging angiogenesis result in a long-term hypoxic TME. The main targets affecting angiogenesis are VEGF, hypoxia inducible factors (HIFs), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), insulin like growth factor (IGF), transforming growth factor-β (TGF-β), and angiopoietins (ANGs) (53, 54). HIFs accumulate inside the tumor during hypoxia and subsequently cause high expression of VEGF, which rapidly stimulates ECs (55, 56). The VEGF family includes VEGF-A\B\C\D\E\F and placental growth factor (PLGF). VEGF-A and vascular endothelial growth factor receptor 2 (VEGFR2) are the main inducers of angiogenesis and the major targets of anti-angiogenesis therapy. VEGF-A activates downstream phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (AKT), P38, and extracellular regulated kinase (ERK)/mitogen activated protein kinase (MAPK) pathways after binding with VEGFR2, which then promote the proliferation and migration of ECs, resulting in angiogenesis (57–59). In addition, studies have shown that growth factors, such as FGF and PDGF, also promote tumor angiogenesis through VEGF/VEGFR pathways (41, 43, 60). PDGF, TGF-β, angiopoietins/TEK receptor tyrosine kinase (TIE) are also active in the maturation of new blood vessels. ECs attract PDGFR-β+ pericytes to complete the vascular barrier by releasing PDGF-β. Suppressing PDGFR-β signals can lead to a decrease in pericellular coverage and pericellular detachment, resulting in vascular dysfunction and inhibition of tumor growth (61). In the physiological state, angiopoietin 1 (ANG-1) is mainly expressed around ECs, where it activates TIE-2 to mediate vascular maturation. ANG-2 is released by tip cells and mainly has anti-ANG-1 functions, guiding vascular degeneration (62, 63). In the TME, the abundant tip cells secrete excess ANG-2, resulting in disturbance of ANG-1 and ANG-2 homeostasis, thus leading to immature neovascularization (30). The Notch and Wnt signaling pathways also participate in tumor angiogenesis. The Notch signaling pathway is involved in the dynamic regulation of tip cells and stalk cells. Suppression of Notch signaling can lead to a tip cell phenotype (47), and activating Notch signaling leads to a stalk cell phenotype and activates the Wnt pathway, which facilitates the proliferation of the stalk cell phenotype (42), thus promoting vascular bud formation. Integrins are transmembrane receptors that mediate adhesion between cells and extracellular matrices. They promote growth factors like VEGF and FGFs, and enhance ANG-1 binding with its receptors VEGFR-2 and FGFRs. Integrins can also promote the maturation of neovascular tissue and regulate the connection of ECs with the ECM (64). The molecular mechanism of angiogenesis in osteosarcoma is summarized in Figure 2.
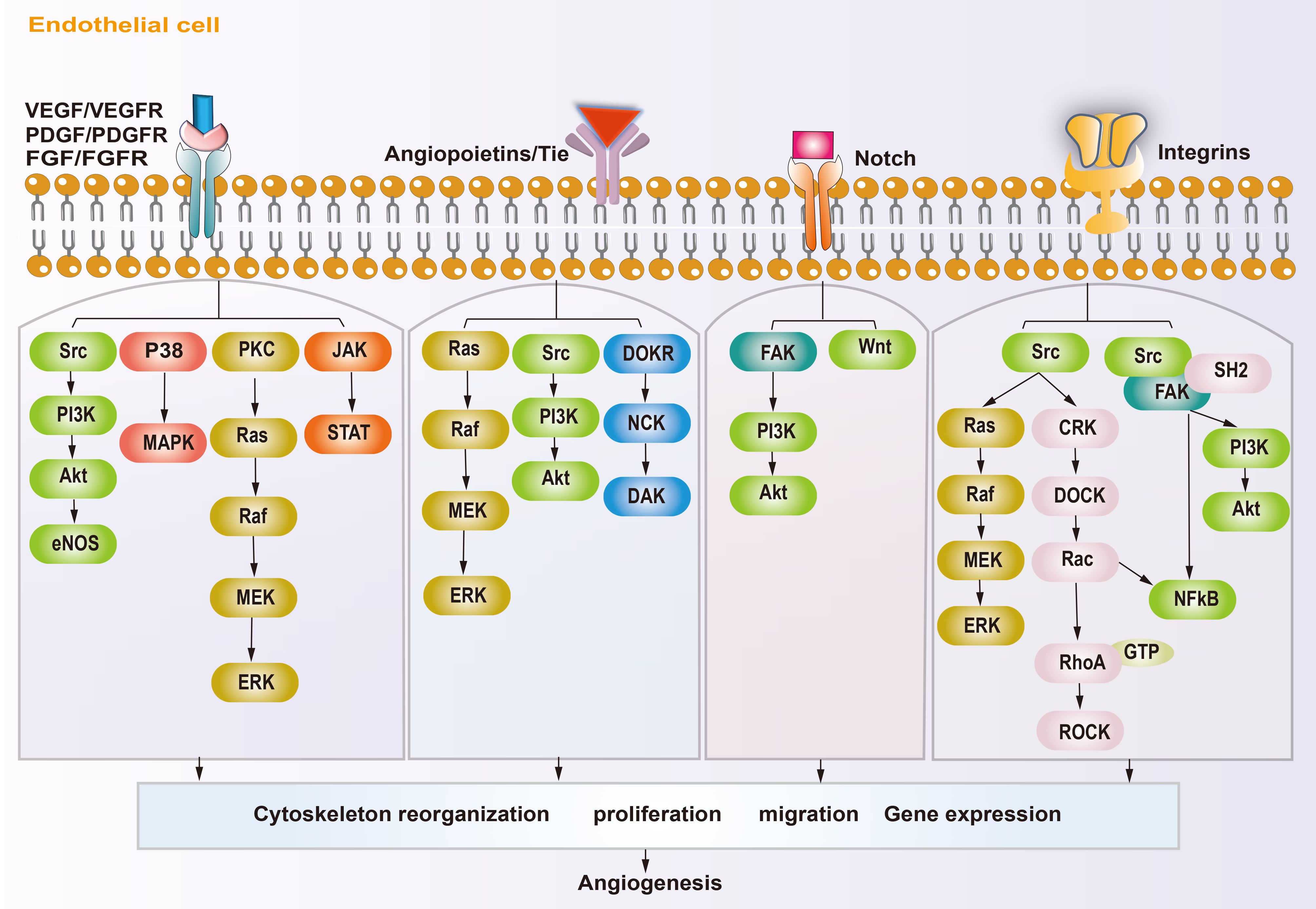
Figure 2. Schematic diagram showing the angiogenic mechanism of osteosarcoma. Angiogenesis-promoting factors interact with receptors on endothelial cell, triggering specific signaling pathways, subsequently reorganization cytoskeleton, proliferation, migration and gene expression, ultimately influence angiogenesis.
2.3 Other potential proangiogenic factors in osteosarcoma
There are some other potential proangiogenic factors and cytokines that participate in osteosarcoma angiogenesis, which could be promising targets for anti-angiogenesis therapy in osteosarcoma. Human interleukin (IL) family members have been shown to play important roles in regulating the immune and inflammatory responses (65, 66). Recently, several IL family members were found to participate in the regulation of the angiogenesis in osteosarcoma. Tzeng et al. reported that IL-6 could upregulate VEGF expression through the apoptosis signal-regulating kinase 1 (ASK1) and P38 pathways, and induced angiogenesis in osteosarcoma (67). IL-34 is associated with the progression of osteosarcoma and an increase in neo-angiogenesis (68). Moreover, some evidence suggests that IL-1 and IL-8 also show certain proangiogenic effects in osteosarcoma (69–71). In addition, the expression of IL-17A, IL-1β and IL-10 was proven to be involved in osteosarcoma carcinogenesis (72–74). Moreover, the latest research indicates that IL-17A, IL-1β, and IL-10 might be related to angiogenesis in other types of cancer (75–77). Thus, targeting them might be a promising strategy to induce anti-angiogenesis effects in osteosarcoma.
Tumor necrosis factor alpha (TNF-α) is involved in many tumor cell pathological cellular pathways, including tumor invasion, epithelial-mesenchymal transition, vascular invasion, and the destruction of tumor vasculature (78, 79). Ségaliny et al. reported that TNF-α could stimulate osteosarcoma cells to secrete IL-34 and increased their angiogenesis (68). Moreover, recent evidence suggested that nuclear factor kappa B (NF-κB) and HIF-1α pathways might be potential downstream targets to regulate angiogenesis in tumors (79). However, further study is needed to confirm this molecular mechanism in osteosarcoma.
Endothelial-specific molecule 1 (ESM1) is thought to be a tip cell marker, and was found to be significantly related to angiogenesis (80). Angiopoietin-like proteins (ANGPTLs) are similar to angiopoietin and also promote angiogenesis; however, they do not bind to the TIE family of angiopoietin receptors (81). In recent years, these two types of proteins have gained increasing significance as their proangiogenic effect in cancers have been explored (80, 82, 83). Recently, a study found that ANGPTL2 could enhance angiogenesis in osteosarcoma by upregulating the expression of hexokinase 2 (HK2) and VEGF (81). However, so far, there has been little research on the roles of these proteins in osteosarcoma. A more detailed understanding of how these proteins function in osteosarcoma angiogenesis is required to develop targeted therapy.
2.4 Metabolism regulates tumor angiogenesis
Current anti-angiogenic therapy targeting VEGF and its related pathways has not achieved the desired results in osteosarcoma, which has forced scientists to explore new anti-angiogenic strategies (3, 16, 25). Although little research has focused on targeting EC metabolism in the anti-angiogenic therapy of osteosarcoma, it has received increased research attention (84), and might represent an effective way to inhibit osteosarcoma angiogenesis.
Unique metabolic fluxes and metabolic patterns are closely related to survival, phenotypic transformation, migration, and proliferation. Studies have shown that EC metabolic reprogramming regulates angiogenesis through energy supply, biosynthesis, signaling transduction, and epigenetic remodeling (Figure 3). Unlike other cells, ECs rely mainly on aerobic glycolysis for energy, although they are directly exposed to the hyperoxic, high-sugar blood environment, which is similar to the Warburg effect of cancer cells (85, 86). In the physiological state, phalanx ECs attach to the intravascular surface in a hibernation state, maintaining the endothelial barrier via a low aerobic glycolysis flux. When ECs are awakened by angiogenic factors, the transcription factors forkhead box O1 (FOXO1) and Kruppel-like factor 2 (KLF2) are activated, and the quiescent cells transform into tip cells or stalk cells, which is accompanied by a change in cell metabolic patterns (87, 88). ECs obtain a tip cell phenotype via high expression VEGFR2. VEGF upregulates the expression of fructose 6-phosphate-2-kinase/fructose-26-diphosphatase 3 (PFKFB3) and HK2 inside tip cells by binding to VEGFR2, and then increases internal glycolysis levels to meet the high energy demands of tip cells during pathfinding (89). At this point, tip cells activate the Notch signaling pathway in adjacent ECs through lateral inhibition mechanisms, which forces the neighboring cells to transform into stalk cells (47). At the same time, activated Notch downregulates PFKFB3 expression to reduce glycolysis levels, followed by activation of mitochondrial fatty acid oxidation, which promotes the synthesis of nucleotides, endowing the stalk cells with a high proliferation status, thus facilitating angiogenesis (90). In addition to promoting angiogenesis in ECs, PFKFB3 and HK2 are also crucially involved in the progression of osteosarcoma by regulating aerobic glycolysis (91, 92). Therefore, targeting glycolytic enzymes PFKFB3 and HK2 might be a promising therapy for osteosarcoma.
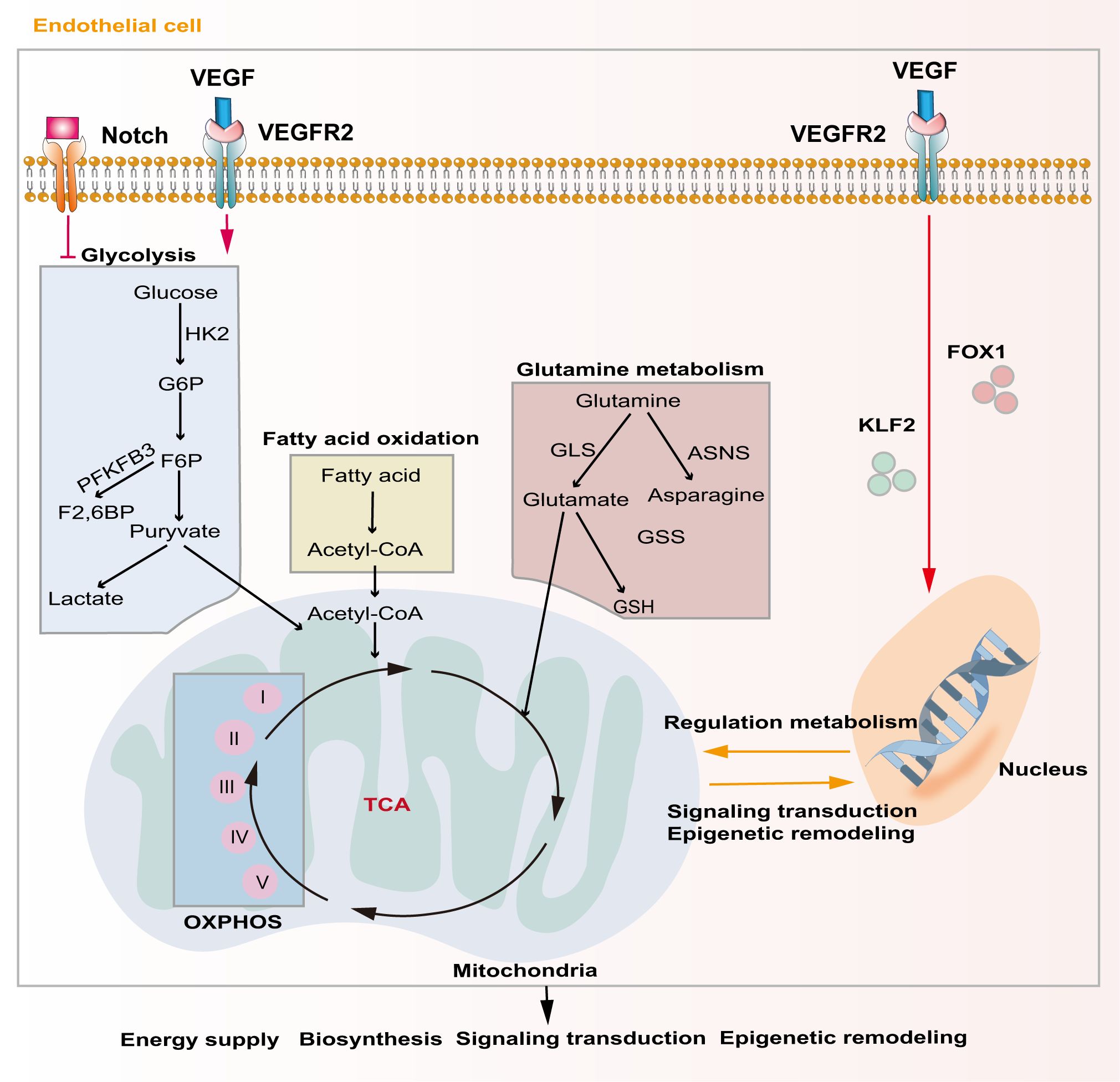
Figure 3. Major metabolic pathways of endothelial cells during angiogenesis. Activation of the Notch signaling pathway inhibits intercellular glycolytic level. VEGF binds to VEGFR2 and enhances downstream glycolysis flux. Additionally, VEGF regulates gene expression and metabolism in endothelial cells via activating of FOXO1 and KLF2. Metabolic products like acetyl-CoA and glutamate act in energy supply, biosynthesis, signal transduction and epigenetic remodeling through mitochondrial-related oxidative phosphorylation pathways.
Although ECs mainly rely on glycolysis to maintain their physiological activity, mitochondria also play a crucial role in ECs. Mitochondria-related oxidative phosphorylation (OXPHOS) plays an indispensable role in providing substrates and maintaining the NAD+/NADH ratio in ECs (93). Tricarboxylic acid cycle (TCA)-related products are important substrates for EC biosynthesis and mitochondrial complex I is extremely important for EC proliferation in tumors (94). Indeed, blocking mitochondrial complexes I and III inhibits the proliferation of ECs and causes pathological angiogenesis (95).
Lipid metabolism is also essential to maintain EC structure and function. During angiogenesis, VEGF-B promotes the uptake of fatty acids by upregulating the expression of fatty acid transport protein 3 (FATP3) and fatty acid transport protein 4 (FATP4) (96). Inhibiting fatty acid synthesis reduces the proliferation and migration of ECs by preventing post-translational modification of proteins and the mechanistic target of rapamycin kinase (mTOR) pathways, ultimately resulting in sprouting angiogenesis (97). Fatty acid oxidation (FAO) maintains EC proliferation by increasing the synthesis of aspartate through mitochondria (98). Another major substrate of mitochondrial respiration in ECs is glutamine, which plays important roles not only in biosynthesis, but also in replenishing the TCA cycle as a carbon source (99). Moreover, the integrity of glutamine metabolic pathways is closely related to the migration of tip cells and the proliferation of stalk cells. Glutathione, a downstream product of glutamine, also helps to maintain EC redox homeostasis. Blocking glutamine metabolism pathways by silencing ASNS (encoding asparagine synthase) inhibited angiogenesis by stopping tip cell migration and stalk cell proliferation, resulting in suppression of sprouting angiogenesis (100). Recent evidence shows that dihydroartemisinin interferes with lipid metabolism in osteosarcoma cells, especially the FAO process, and impedes antiangiogenic drug resistance (101). However, there is still lack of studies exploring EC lipid metabolism in osteosarcoma.
3 Anti-angiogenesis therapy in osteosarcoma
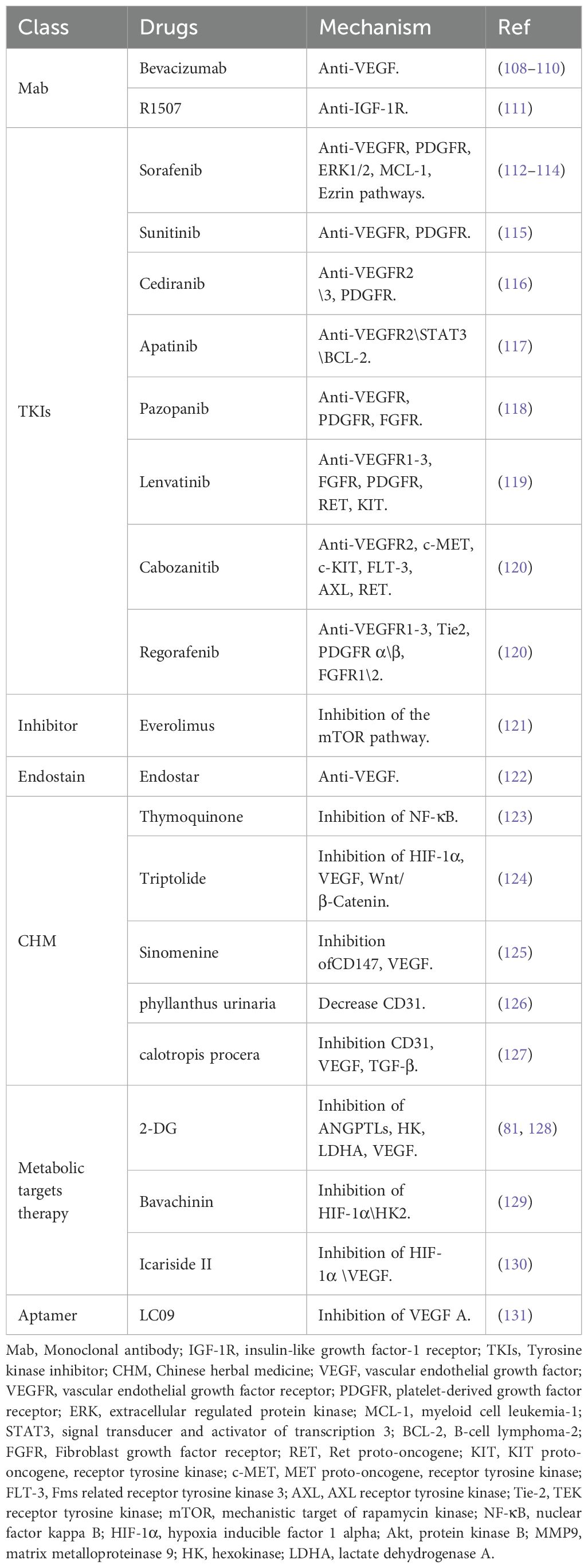
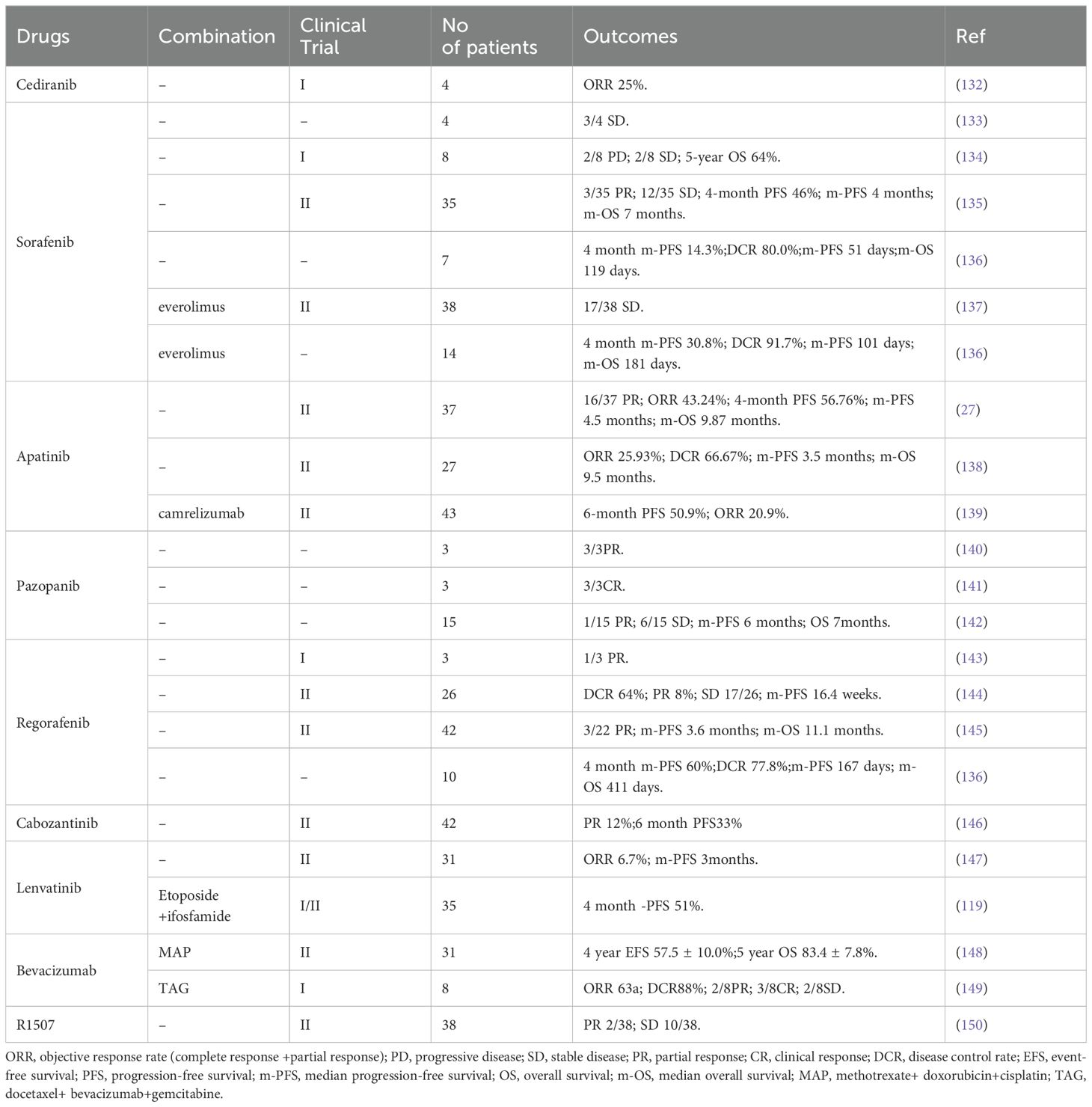
The treatment of osteosarcoma mainly relies on surgery and chemotherapy. As an effective supplement to routine treatment, recently, anti-angiogenesis therapy has received increased attention. Anti-angiogenesis therapy aims to block the nutrient supply of tumors and impede the TME in the following ways: destruction of tumor blood vessels by inducing apoptosis (102); inhibiting angiogenesis by limiting cell proliferation and migration, and promoting the normalization of blood vessels in tumors (103–105); and restoring the perfusion inside tumors (106), thereby altering the TME (43, 89, 90, 107). The current anti-angiogenic treatments for osteosarcoma can be classified into the following categories: Monoclonal antibodies (Mabs), tyrosine kinase inhibitors (TKIs), small molecule inhibitors, Chinese herbal medicine, aptamers, and nano-particles (NPs). The targets and mechanisms of these drugs are shown in Table 1, and the clinical trials involving these drugs are listed in Table 2.
3.1 Monoclonal antibodies
VEGFA/VEGFR are the main targets for anti-angiogenic therapy; therefore, Mabs targeting VEGF are also widely used to treat solid tumors, especially osteosarcoma. Bevacizumab was the first Mab to be approved as an angiogenesis inhibitor for solid tumors. Its main anti-angiogenic function is binding to all VEGFA subtypes in the circulation and preventing them from activating VEGFR (108). In a pre-clinical study, Zhao et al. reported that intraperitoneal Bevacizumab injection exhibited strong anti-tumor growth and anti-angiogenesis activity toward osteosarcoma in a nude mouse model. However, the authors claimed that Bevacizumab did not influence the incidence of mouse lung metastasis (109). As mentioned above, surgery combined with chemotherapy is still the first-line treatment for the advanced osteosarcoma. To date, in clinical trials, Bevacizumab has been used as an adjuvant therapy in combination with multiple chemotherapy drugs after surgery, if required. In 2017, Navid et al. reported the results of a phase II trial to evaluate the feasibility and efficacy of combining Bevacizumab with methotrexate, doxorubicin, and cisplatin (MAP) in patients with localized and resectable osteosarcoma (Table 2) (148). They claimed that the addition of Bevacizumab to MAP for osteosarcoma was tolerated, with low toxicity, except for frequent wound complications. However, the addition of Bevacizumab did not significantly improve the outcome of patients with localized osteosarcoma. In another clinical trial, Kuo et al. (149) reported that combining Bevacizumab with docetaxel and gemcitabine was well tolerated and had activity to treat relapsed or metastatic high-grade sarcomas (including eight patients with osteosarcoma) (Table 2). Collectively, the addition of Bevacizumab to multiple chemotherapy drugs to treat osteosarcoma induces no additional toxicity. However, the survival benefit is still unclear and large scale trials are needed.
R1507 is a monoclonal antibody recognizing the insulin-like growth factor-1 receptor (IGF-1R) (111), a receptor that plays an important role in tumor proliferation, apoptosis, angiogenesis, and metastasis (151). Thus, theoretically, R1507 should show some anti-angiogenic effect. In fact, R1507 has been proven to delay tumor growth in osteosarcoma mice xenograft tumors (152). A phase 2 trial also showed that R1507 is safe and well-tolerated in patients with osteosarcoma (150). However, the anti-angiogenic effect of R1507 in the clinic is still unclear. Despite the strong targeting and demonstrated efficacy in combination therapy for osteosarcoma, Mabs targeting VEGF or other proangiogenic factors face limitations in clinical application due to their high cost, uncertain survival benefit, and side effects (148–150). Therefore, extensive large scale clinical trials and more detailed research on the underlying molecular mechanism of Mabs targeting angiogenesis in osteosarcoma will be the potential research hotspots.
3.2 Tyrosine kinase inhibitors
TKIs are the most widely used angiogenesis inhibitors, being mainly used to block multiple signals downstream of VEGFR. Other targets include, PDGFR, Fms-like tyrosine kinase (FLT3), recombinant activated factor (RAF), and c-kit (a receptor tyrosine kinase, also called CD117 and stem cell factor receptor) (43). Research has shown that TKIs, such as Sorafenib, Sunitinib, Cediranib, Apatinib, Lenvatinib, Cabozantinib, and Regorafenib, show certain effects in the treatment of advanced osteosarcoma (Table 2). In particular, a phase 1/2 trial in 2021 showed that Lenvatinib combined with etoposide and ifosfamide could exert promising anti-tumor activity in patients with relapsed or refractory osteosarcoma; however, a high rate of treatment emergent adverse events was observed (119). The main concern related to TKIs is their poor organ targeting and short half-life inside the tumor, leading to limited efficacy and excessive side effects (119, 147). Treatment with TKIs causes secondary activation of the mTOR pathway in osteosarcoma (153). Small molecular inhibitors targeting the mTOR pathway, such as Everolimus, can effectively inhibit the proliferation and migration of ECs (153). Therefore, it was suggested that Everolimus could be used as a supplement to TKI therapy. However, clinicians should consider the adverse effects caused by mTOR inhibitors, such as high blood lipids, high blood sugar, mucositis, gastrointestinal reactions, and rashes, when formulating a treatment strategy (137).
3.3 Chinese herbal medicine
Chinese herbal medicine is believed to exert anti-angiogenesis effects via multiple targets and pathways, such as PI3K/AKT, Wnt/β-catenin, Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3), Notch, NF-κB, and MAPK (154, 155). Moreover, many constituents of herbs have shown significant efficacy in anti-tumor and anti-angiogenesis therapeutics, with lower rates of adverse events (156, 157). For example, thymoquinone inhibits angiogenesis in osteosarcoma by inhibiting the NF-κB pathway (123). Triptolide can also inhibit angiogenesis in osteosarcoma through the HIF-1α, VEGF, and Wnt/β-Catenin pathways (124). Xie et al. reported that sinomenine, an active natural product derived from the plant Sinomenium acutum Rehd. et Wils, can effectively reduce CD147 and VEGF expression in OS cells through the C-X-C motif chemokine receptor 4 (CXCR4)-STAT3 pathway, thus inhibiting angiogenesis (125). Moreover, Phyllanthus urinaria, a widely used folk medicine in cancer treatment, could decrease the microvessel density and CD31 expression of osteosarcoma mouse xenografts, suggesting its potential anti-angiogenic effect (126). In addition, a recent study by Rabelo et al. reported that an extract of Calotropis procera could reduce angiogenesis and tumor progression in canine osteosarcoma cells by suppressing the expression of CD31, VEGF, osteopontin, and TGF-β (127). Collectively, these studies demonstrated that Chinese herbal medicine might be a promising adjunct treatment for advanced osteosarcoma via its anti-angiogenesis effects. However, to date, all the research on the use of herbal medicine in osteosarcoma has been carried out at using cellular and animal models and there remains a significant challenge to evaluate their safety in the clinic (157).
3.4 Potential metabolic antiangiogenic therapies in osteosarcoma
Osteosarcoma cells and ECs have similar metabolic characteristics, and regulating the metabolism of tumor cells mainly involves inhibiting nucleotide synthesis, inhibiting energy metabolism (e.g., glycolysis), and regulating redox metabolism and other metabolic pathways (158). These metabolic pathways also affect EC-guided angiogenesis in osteosarcoma (84). A study showed that 2-Deoxy-D-glucose (2-DG) could reduce osteosarcoma growth by inhibiting HK and lactate dehydrogenase A (LDHA), which are the key enzymes involved in anaerobic glycolysis (128). This signaling pathway also plays a key role in osteosarcoma angiogenesis. Bavachinin reduced osteosarcoma angiogenesis by inhibiting glycolysis via targeting HIF-1α and HK2 (129). Another study showed that Icariside II inhibits glucose metabolism and reduces HIF-1α-induced VEGF expression in human osteosarcoma cells, while simultaneously suppressing angiogenesis (130). Recently, Wang et al. reported that ANGPTL2 enhanced angiogenesis in osteosarcoma by upregulating the expression of HK2 and VEGF, and treatment with 2-DG could reversed this outcome (81) (Table 1). However, whether these drugs also act on similar metabolic targets in ECs and inhibit osteosarcoma angiogenesis requires further study.
3.5 Aptamers in osteosarcoma angiogenesis
An aptamer is an RNA or single stranded DNA with a specific three-dimensional structure, which acts as a starter or inhibitor trough that combines with specific molecules via Van der Waals forces or hydrogen bonds (159–161). Recent research has demonstrated that aptamers can act as tumor suppressors by impeding tumor angiogenesis, with advantages such as low immunogenicity, reversibility, wide target range, and short preparation cycle (162–164). Aptamers can be modified for different purposes. Modifications using fluorescein, biotin, and magnetic beads can amplify the transduction signals (165); adding polyethylene glycol (PEG) will increase their circulating time in the body; and binding them to drugs and NPs enable targeted delivery and controlled drug release (131, 162, 166–168). Liang et al. reported the construction of a specific aptamer (LC09) of osteosarcoma cells that was conjugated to a PEG-polyethylenimine (PEI) Cholesterol (PPC) lipopolymer, followed by combination with clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated protein 9 (Cas9) plasmids encoding VEGFA (131). They found that this system could inhibit osteosarcoma growth, lung metastasis, and angiogenesis in vitro and in osteosarcoma mouse models. However, further investigative studies are required to determine its clinical efficacy.
3.6 Nanoparticles in anti-vascular treatment
Nanotechnology is gradually being used in anti-vascular treatment of tumors, including osteosarcoma (131, 169). Utilizing flexible surface modification, researchers can promote drug targeting and prolong the half-life of nanotechnology-based drugs in tumors. Such NPs can release the drug precisely according to the specific microenvironment of the tumor, enhance efficacy, and reduce drug resistance and adverse reactions (170, 171). Nanotechnology can also overcome the shortcomings of traditional anti-tumor therapy and have a large capacity to combine anti-vascular therapy with chemotherapy and radiotherapy (170, 172). The development of nanopharmaceuticals has gone through three generations: The first generation was organ-targeted and aimed to increase the concentration of nanopharmaceuticals in solid tumors and reduce drug-induced off-target effects (173, 174). The second generation were cell-targeted, mainly focusing on surface modification of NPs to deliver drugs to specific tumor cells (175). The third generation are designed to deliver drugs to specific organelles, such as mitochondria, the endoplasmic reticulum, lysosomes, and nuclei (176–181).
In anti-vascularization therapy, many studies have focused on selectively delivering NP-loaded drugs to the tumor’s vascular system. Researchers modified short peptides on NPs to ensure their specific binding with integrin αvβ3 on the surface of tumor vascular ECs (182). Nano-particle platforms include liposomes, albumin NPs, polymer micelles, gold NPs, and mesoporous silica (183). Studies have placed effectors, such as small interfering RNAs (siRNAs) or other small molecule drugs (e.g., Rapamycin), on the surface of, or inside, NPs (169), thus allowing the successful delivery of the effectors into tumor ECs to exert a therapeutic function. Meanwhile, some studies focused on the combination of aptamers and NPs. As mentioned in the aptamers section, the osteosarcoma cell-targeted aptamer LC09 could be loaded into special NPs, which exert an anti-vascular effect with reduced adverse reactions (131). In addition, researchers assembled gold nanoclusters (GNCs) with Sgc8, an aptamer targeting TKIs, to form the GNCs@aptamer. This not only improved drug targeting, but also increased the duration of the drug’s residence in the tumor (184). Although there is still lack of research focusing on the anti-angiogenic effect of aptamers or NPs on osteosarcoma, this research direction might provide valuable new perspectives on anti-vascular treatment.
4 Challenges and perspectives
Multi-mode therapy, including surgery, chemotherapy, immunotherapy, radiotherapy, gene therapy, or other targeted drugs, can help to improve the therapeutic effect against osteosarcoma compared with any single method (185, 186). Anti-angiogenesis therapy is mainly used to inhibit the formation of new blood vessels, not to destroy existing vessels. Therefore, we still need to consider the coincidental destruction of existing blood vessels, because this might inhibit the delivery of drugs to the tumor. Traditional anti-angiogenic drugs, such as Everolimus, have many shortcomings in the treatment solid tumors, such as drug resistance, unexpected adverse reactions, poor targeting, and short tumor residency time.
In recent years, the role of metabolism in tumor anti-angiogenesis therapy has been widely recognized. Although limited research has been conducted on targeting EC metabolism in the anti-angiogenic therapy of osteosarcoma, recently it has attracted considerable attention (84, 91, 92). Consequently, we anticipate that metabolic regulation will emerge as a promising therapeutic strategy for advanced osteosarcoma in the future. However, several challenges still need to be addressed, including the identifying unique metabolic patterns specific to osteosarcoma, developing targeted drugs, and rigorously evaluating the safety and efficacy of metabolic regulation therapy. Therefore, further research and clinical exploration in related fields are imperative to propel the advancement of metabolic regulation in osteosarcoma treatment.
Additionally, the TME maintains the endothelium in a highly proliferative and metabolic state through various mechanisms. Studies have shown that the secondary hypoxia environment within the tumor after anti-angiogenesis treatment will stimulate a new pathway of angiogenesis, which might lead to drug resistance or tumor recurrence, and thus poor anti-tumor efficacy (187–189). Therefore, we anticipate that anti-angiogenic therapy targeting the TME will hold great promise for preventing drug resistance and tumor recurrence in the treatment of advanced osteosarcoma in the future.
Exploring new multi-targeted anti-vascular drugs or improving the pharmacokinetics of current drugs will also be helpful in future treatment strategies (190, 191). The ultimate aim is to induce a series of intracellular cascade reactions through multi-target synergies, exert a full range of anti-angiogenesis effects, maintain more stable pharmacodynamics, and avoid side effects and drug interactions (26, 192). In addition, the development of nanomedicine can help with the targeted delivery of drugs, improve their bio-availability, and reduce adverse reactions, thereby playing an innovative role in anti-tumor treatment (183, 193, 194). Comprehensive treatment based on nanotechnology can also combine chemotherapy, radiotherapy, anti-vascular therapy, and immunotherapy (170), thus exerting synergistic anti-tumor effects (105). However, many nanopharmaceuticals failed in phase II or III clinical trials, which was mainly ascribed to poor treatment effects (195). Therefore, while comprehensive treatment based on nanotechnology might be a potential strategy in future therapy, it requires improvement and optimization.
5 Conclusion
Angiogenesis is crucial for the growth, expansion, and metastasis of osteosarcoma. Therapy combining anti-angiogenic treatments and other methods has gradually emerged as the anti-tumor strategy with the most potential to treat advanced osteosarcoma. However, there are still some limitations that require optimization, such as an insufficient therapeutic effect, drug resistance, and side effects. Developing new angiogenesis inhibitors, exploring the possibility of multi-target drug combinations and sequential therapy, and combining classical drugs with NPs to improve delivery and reduce off-target side effects, will help to break the current bottleneck of treatment, thus providing more benefits to patients and improving their outcomes.
Author contributions
QZ: Data curation, Resources, Software, Writing – original draft. YX: Writing – review & editing. LW: Writing – review & editing. YW: Validation, Writing – review & editing. YB: Validation, Visualization, Writing – review & editing. G-SZ: Funding acquisition, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (NSFC) (grant number 82303465), the Natural Science Foundation of Chongqing, China (grant number CSTB2022NSCQ-MSX0103), the Special Financial Aid to the Post doctor Research Project of Chongqing (grant number 2022CQBSHTB3079), and the China Postdoctoral Science Foundation (grant number 2023MD734132).
Acknowledgments
The authors thank the native English speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Anwar MA, El-Baba C, Elnaggar MH, Elkholy YO, Mottawea M, Johar D, et al. Novel therapeutic strategies for spinal osteosarcomas. Semin Cancer Biol. (2020) 64:83–92. doi: 10.1016/j.semcancer.2019.05.018
2. McGuire J, Utset-Ward TJ, Reed DR, Lynch CC. Re-calculating! Navigating through the osteosarcoma treatment roadblock. Pharmacol Res. (2017) 117:54–64. doi: 10.1016/j.phrs.2016.12.004
3. Xie L, Ji T, Guo W. Anti-angiogenesis target therapy for advanced osteosarcoma (review). Oncol Rep. (2017) 38:625–36. doi: 10.3892/or.2017.5735
4. Durfee RA, Mohammed M, Luu HH. Review of osteosarcoma and current management. Rheumatol Ther. (2016) 3:221–43. doi: 10.1007/s40744-016-0046-y
5. Meltzer PS, Helman LJ. New horizons in the treatment of osteosarcoma. N Engl J Med. (2021) 385:2066–76. doi: 10.1056/NEJMra2103423
6. Sadykova LR, Ntekim AI, Muyangwa-Semenova M, Rutland CS, Jeyapalan JN, Blatt N, et al. Epidemiology and risk factors of osteosarcoma. Cancer Invest. (2020) 38:259–69. doi: 10.1080/07357907.2020.1768401
7. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. (2009) 152:3–13. doi: 10.1007/978-1-4419-0284-9_1
8. Linabery AM, Ross JA. Trends in childhood cancer incidence in the u.s. (1992-2004). Cancer. (2008) 112:416–32. doi: 10.1002/cncr.23169
9. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004 data from the surveillance, epidemiology, and end results program. Cancer. (2009) 115:1531–43. doi: 10.1002/cncr.24121
10. Miller BJ, Cram P, Lynch CF, Buckwalter JA. Risk factors for metastatic disease at presentation with osteosarcoma: an analysis of the seer database. J Bone Joint Surg Am. (2013) 95:e89. doi: 10.2106/JBJS.L.01189
11. Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma - connecting aetiology, biology and therapy. Nat Rev Endocrinol. (2017) 13:480–91. doi: 10.1038/nrendo.2017.16
12. Anderson WJ, Doyle LA. Updates from the 2020 world health organization classification of soft tissue and bone tumours. Histopathology. (2021) 78:644–57. doi: 10.1111/his.14265
13. Zavros Y, Merchant JL. The immune microenvironment in gastric adenocarcinoma. Nat Rev Gastroenterol Hepatol. (2022) 19:451–67. doi: 10.1038/s41575-022-00591-0
14. Ghafouri-Fard S, Shirvani-Farsani Z, Hussen BM, Taheri M. The critical roles of lncrnas in the development of osteosarcoma. BioMed Pharmacother. (2021) 135:111217. doi: 10.1016/j.biopha.2021.111217
15. Beird HC, Bielack SS, Flanagan AM, Gill J, Heymann D, Janeway KA, et al. Osteosarcoma (vol 8, 77, 2022). Nat Rev Dis Primers. (2022) 8. doi: 10.1038/s41572-022-00416-z
16. Gill J, Gorlick R. Advancing therapy for osteosarcoma. Nat Rev Clin Oncol. (2021) 18:609–24. doi: 10.1038/s41571-021-00519-8
17. Paulussen M, Bielack S, Jurgens H, Casali PG. Ewing's sarcoma of the bone: esmo clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. (2009) 20 Suppl 4:140–42. doi: 10.1093/annonc/mdp155
18. Li X, Moretti VM, Ashana AO, Lackman RD. Impact of close surgical margin on local recurrence and survival in osteosarcoma. Int Orthop. (2012) 36:131–37. doi: 10.1007/s00264-011-1230-x
19. Harris MA, Hawkins CJ. Recent and ongoing research into metastatic osteosarcoma treatments. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23073817
20. Assi T, Watson S, Samra B, Rassy E, Le Cesne A, Italiano A, et al. Targeting the vegf pathway in osteosarcoma. Cells. (2021) 10. doi: 10.3390/cells10051240
21. Zhu J, Simayi N, Wan R, Huang W. Car t targets and microenvironmental barriers of osteosarcoma. Cytotherapy. (2022) 24:567–76. doi: 10.1016/j.jcyt.2021.12.010
22. Zhu T, Han J, Yang L, Cai Z, Sun W, Hua Y, et al. Immune microenvironment in osteosarcoma: components, therapeutic strategies and clinical applications. Front Immunol. (2022) 13:907550. doi: 10.3389/fimmu.2022.907550
23. Piperno-Neumann S, Ray-Coquard I, Occean BV, Laurence V, Cupissol D, Perrin C, et al. Results of API-AI based regimen in osteosarcoma adult patients included in the french Os2006/Sarcome-09 study. Int J Cancer. (2020) 146:413–23. doi: 10.1002/ijc.32526
24. Li S, Zhang H, Shang G. Current status and future challenges of CAR-T cell therapy for osteosarcoma. Front Immunol. (2023) 14:1290762. doi: 10.3389/fimmu.2023.1290762
25. Versleijen-Jonkers YM, Vlenterie M, van de Luijtgaarden AC, van der Graaf WT. Anti-angiogenic therapy, a new player in the field of sarcoma treatment. Crit Rev Oncol Hematol. (2014) 91:172–85. doi: 10.1016/j.critrevonc.2014.02.001
26. Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. (2023) 8:198. doi: 10.1038/s41392-023-01460-1
27. Xie L, Xu J, Sun X, Tang X, Yan T, Yang R, et al. Apatinib for advanced osteosarcoma after failure of standard multimodal therapy: an open label phase ii clinical trial. Oncologist. (2019) 24:e542–50. doi: 10.1634/theoncologist.2018-0542
28. Siemerink MJ, Klaassen I, Van Noorden CJ, Schlingemann RO. Endothelial tip cells in ocular angiogenesis: potential target for anti-angiogenesis therapy. J Histochem Cytochem. (2013) 61:101–15. doi: 10.1369/0022155412467635
29. Yetkin-Arik B, Kastelein AW, Klaassen I, Jansen C, Latul YP, Vittori M, et al. Angiogenesis in gynecological cancers and the options for anti-angiogenesis therapy. Biochim Biophys Acta Rev Cancer. (2021) 1875:188446. doi: 10.1016/j.bbcan.2020.188446
30. Parmar D, Apte M. Angiopoietin inhibitors: a review on targeting tumor angiogenesis. Eur J Pharmacol. (2021) 899:174021. doi: 10.1016/j.ejphar.2021.174021
31. Folkman J. Angiogenesis. Annu Rev Med. (2006) 57:1–18. doi: 10.1146/annurev.med.57.121304.131306
32. Wunder JS, Nielsen TO, Maki RG, O'Sullivan B, Alman BA. Opportunities for improving the therapeutic ratio for patients with sarcoma. Lancet Oncol. (2007) 8:513–24. doi: 10.1016/S1470-2045(07)70169-9
33. Gorlick R, Anderson P, Andrulis I, Arndt C, Beardsley GP, Bernstein M, et al. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary. Clin Cancer Res. (2003) 9:5442–53.
34. Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. (2003) 3:401–10. doi: 10.1038/nrc1093
35. Maiborodin I, Mansurova A, Chernyavskiy A, Romanov A, Voitcitctkii V, Kedrova A, et al. Cancer angiogenesis and opportunity of influence on tumor by changing vascularization. J Pers Med. (2022) 12. doi: 10.3390/jpm12030327
36. Li X, Carmeliet P. Targeting angiogenic metabolism in disease. Science. (2018) 359:1335–36. doi: 10.1126/science.aar5557
37. Herkenne S, Ek O, Zamberlan M, Pellattiero A, Chergova M, Chivite I, et al. Developmental and tumor angiogenesis requires the mitochondria-shaping protein opa1. Cell Metab. (2020) 31:987–1003. doi: 10.1016/j.cmet.2020.04.007
38. Burri PH, Djonov V. Intussusceptive angiogenesis–the alternative to capillary sprouting. Mol Aspects Med. (2002) 23:S1–27. doi: 10.1016/s0098-2997(02)00096-1
39. Ratajska A, Jankowska-Steifer E, Czarnowska E, Olkowski R, Gula G, Niderla-Bielinska J, et al. Vasculogenesis and its cellular therapeutic applications. Cells Tissues Organs. (2017) 203:141–52. doi: 10.1159/000448551
40. Wei X, Chen Y, Jiang X, Peng M, Liu Y, Mo Y, et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer. (2021) 20:7. doi: 10.1186/s12943-020-01288-1
41. Thijssen VL, Paulis YW, Nowak-Sliwinska P, Deumelandt KL, Hosaka K, Soetekouw PM, et al. Targeting pdgf-mediated recruitment of pericytes blocks vascular mimicry and tumor growth. J Pathol. (2018) 246:447–58. doi: 10.1002/path.5152
42. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. (2011) 473:298–307. doi: 10.1038/nature10144
43. Tang B, Ma W, Lin Y. Emerging applications of anti-angiogenic nanomaterials in oncotherapy. J Control Release. (2023) 364:61–78. doi: 10.1016/j.jconrel.2023.10.022
44. Teuwen LA, De Rooij L, Cuypers A, Rohlenova K, Dumas SJ, Garcia-Caballero M, et al. Tumor vessel co-option probed by single-cell analysis. Cell Rep. (2021) 35:109253. doi: 10.1016/j.celrep.2021.109253
45. Korbel C, Gerstner MD, Menger MD, Laschke MW. Notch signaling controls sprouting angiogenesis of endometriotic lesions. Angiogenesis. (2018) 21:37–46. doi: 10.1007/s10456-017-9580-7
46. Fukumoto M, Kondo K, Uni K, Ishiguro T, Hayashi M, Ueda S, et al. Tip-cell behavior is regulated by transcription factor foxo1 under hypoxic conditions in developing mouse retinas. Angiogenesis. (2018) 21:203–14. doi: 10.1007/s10456-017-9588-z
47. Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. (2013) 3:a6569. doi: 10.1101/cshperspect.a006569
48. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. (2019) 176:1248–64. doi: 10.1016/j.cell.2019.01.021
49. Diaz-Flores L, Gutierrez R, Gayoso S, Garcia MP, Gonzalez-Gomez M, Diaz-Flores L, et al. Intussusceptive angiogenesis and its counterpart intussusceptive lymphangiogenesis. Histol Histopathol. (2020) 35:1083–103. doi: 10.14670/HH-18-222
50. Saravanan S, Vimalraj S, Pavani K, Nikarika R, Sumantran VN. Intussusceptive angiogenesis as a key therapeutic target for cancer therapy. Life Sci. (2020) 252. doi: 10.1016/j.lfs.2020.117670
51. Luo Q, Wang J, Zhao W, Peng Z, Liu X, Li B, et al. Vasculogenic mimicry in carcinogenesis and clinical applications. J Hematol Oncol. (2020) 13. doi: 10.1186/s13045-020-00858-6
52. Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. (2010) 468:829–33. doi: 10.1038/nature09624
53. Egners A, Rezaei M, Kuzmanov A, Poitz DM, Streichert D, Mueller-Reichert T, et al. PHD3 acts as tumor suppressor in mouse osteosarcoma and influences tumor vascularization via PDGF-C signaling. Cancers (Basel). (2018) 10. doi: 10.3390/cancers10120496
54. Liu Y, Huang N, Liao S, Rothzerg E, Yao F, Li Y, et al. Current research progress in targeted anti-angiogenesis therapy for osteosarcoma. Cell Prolif. (2021) 54:e13102. doi: 10.1111/cpr.13102
55. Jaszai J, Schmidt MHH. Trends and challenges in tumor anti-angiogenic therapies. Cells. (2019) 8. doi: 10.3390/cells8091102
56. Corrado C, Fontana S. Hypoxia and HIF signaling: one axis with divergent effects. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21165611
57. Uemura A, Fruttiger M, D'Amore PA, De Falco S, Joussen AM, Sennlaub F, et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog Retin Eye Res. (2021) 84. doi: 10.1016/j.preteyeres.2021.100954
58. Katayama Y, Uchino J, Chihara Y, Tamiya N, Kaneko Y, Yamada T, et al. Tumor neovascularization and developments in therapeutics. Cancers (Basel). (2019) 11. doi: 10.3390/cancers11030316
59. Zhao M, Guan P, Xu S, Lu H, Liu Z. Molecularly imprinted nanomedicine for anti-angiogenic cancer therapy via blocking vascular endothelial growth factor signaling. Nano Lett. (2023) 23:8674–82. doi: 10.1021/acs.nanolett.3c02514
60. Cao Y, Cao R, Hedlund EM. Regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways. J Mol Med (Berl). (2008) 86:785–89. doi: 10.1007/s00109-008-0337-z
61. Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. (2009) 29:630–38. doi: 10.1161/ATVBAHA.107.161521
62. Yu X, Ye F. Role of angiopoietins in development of cancer and neoplasia associated with viral infection. Cells. (2020) 9. doi: 10.3390/cells9020457
63. Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. (2013) 328:18–26. doi: 10.1016/j.canlet.2012.08.018
64. Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. (2010) 10:9–22. doi: 10.1038/nrc2748
65. Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. (2013) 39:1003–18. doi: 10.1016/j.immuni.2013.11.010
66. Rose-John S. Interleukin-6 family cytokines. Cold Spring Harb Perspect Biol. (2018) 10. doi: 10.1101/cshperspect.a028415
67. Tzeng HE, Tsai CH, Chang ZL, Su CM, Wang SW, Hwang WL, et al. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem Pharmacol. (2013) 85:531–40. doi: 10.1016/j.bcp.2012.11.021
68. Segaliny AI, Mohamadi A, Dizier B, Lokajczyk A, Brion R, Lanel R, et al. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int J Cancer. (2015) 137:73–85. doi: 10.1002/ijc.29376
69. Cam M, Gardner HL, Roberts RD, Fenger JM, Guttridge DC, London CA, et al. ΔNp63 mediates cellular survival and metastasis in canine osteosarcoma. Oncotarget. (2016) 7:48533–46. doi: 10.18632/oncotarget.10406
70. Tan PH, Chia SS, Toh SL, Goh JC, Nathan SS. The dominant role of IL-8 as an angiogenic driver in a three-dimensional physiological tumor construct for drug testing. Tissue Eng Part A. (2014) 20:1758–66. doi: 10.1089/ten.TEA.2013.0245
71. Honicke AS, Ender SA, Radons J. Combined administration of EGCG and IL-1 receptor antagonist efficiently downregulates IL-1-induced tumorigenic factors in U-2 OS human osteosarcoma cells. Int J Oncol. (2012) 41:753–58. doi: 10.3892/ijo.2012.1498
72. Liang X, Guo W, Ren T, Huang Y, Sun K, Zhang H, et al. Macrophages reduce the sensitivity of osteosarcoma to neoadjuvant chemotherapy drugs by secreting interleukin-1 beta. Cancer Lett. (2020) 480:4–14. doi: 10.1016/j.canlet.2020.03.019
73. Wang M, Wang L, Ren T, Xu L, Wen Z. IL-17A/IL-17RA interaction promoted metastasis of osteosarcoma cells. Cancer Biol Ther. (2013) 14:155–63. doi: 10.4161/cbt.22955
74. Nastasi N, Pasha A, Bruno G, Subbiani A, Pietrovito L, Leo A, et al. Blockade of IL-10 signaling ensures mifamurtide efficacy in metastatic osteosarcoma. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15194744
75. Moadab A, Valizadeh MR, Nazari A, Khorramdelazad H. Association of interleukin-17A and chemokine/vascular endothelial growth factor-induced angiogenesis in newly diagnosed patients with bladder cancer. BMC Immunol. (2024) 25:20. doi: 10.1186/s12865-024-00612-4
76. Yan D, Cui D, Zhu Y, Chan C, Choi C, Liu T, et al. Serglycin-induced interleukin-1β from oesophageal cancer cells upregulate hepatocyte growth factor in fibroblasts to promote tumour angiogenesis and growth. Clin Transl Med. (2022) 12:e1031. doi: 10.1002/ctm2.1031
77. Yu Z, Zhang Q, Wei S, Zhang Y, Zhou T, Zhang Q, et al. Cd146+CAFs promote progression of endometrial cancer by inducing angiogenesis and vasculogenic mimicry via IL-10/JAK1/STAT3 pathway. Cell Commun Signal. (2024) 22:170. doi: 10.1186/s12964-024-01550-9
78. Mahdavi SP, Jabbari P, Razi S, Keshavarz-Fathi M, Rezaei N. Importance of TNF-alpha and its alterations in the development of cancers. Cytokine. (2020) 130:155066. doi: 10.1016/j.cyto.2020.155066
79. Liu BX, Xie Y, Zhang J, Zeng S, Li J, Tao Q, et al. SERPINB5 promotes colorectal cancer invasion and migration by promoting EMT and angiogenesis via the TNF-α/NF-κB pathway. Int Immunopharmacol. (2024) 131:111759. doi: 10.1016/j.intimp.2024.111759
80. Yang J, Shu G, Chen T, Dong A, Dong C, Li W, et al. ESM1 interacts with c-Met to promote gastric cancer peritoneal metastasis by inducing angiogenesis. Cancers (Basel). (2023) 16. doi: 10.3390/cancers16010194
81. Wang X, Hu Z, Wang Z, Cui Y, Cui X. Angiopoietin-like protein 2 is an important facilitator of tumor proliferation, metastasis, angiogenesis and glycolysis in osteosarcoma. Am J Transl Res. (2019) 11:6341–55.
82. Li YK, Zeng T, Guan Y, Liu J, Liao NC, Wang MJ, et al. Validation of ESM1 Related to Ovarian Cancer and the Biological Function and Prognostic Significance. Int J Biol Sci. (2023) 19:258–80. doi: 10.7150/ijbs.66839
83. Li YK, Gao AB, Zeng T, Liu D, Zhang QF, Ran XM, et al. ANGPTL4 accelerates ovarian serous cystadenocarcinoma carcinogenesis and angiogenesis in the tumor microenvironment by activating the JAK2/STAT3 pathway and interacting with ESM1. J Transl Med. (2024) 22:46. doi: 10.1186/s12967-023-04819-8
84. García-Caballero M, Sokol L, Cuypers A, Carmeliet P. Metabolic reprogramming in tumor endothelial cells. Int J Mol Sci. (2022) 23:11052. doi: 10.3390/ijms231911052
85. Zhu X, Wang Y, Soaita I, Lee HW, Bae H, Boutagy N, et al. Acetate controls endothelial-to-mesenchymal transition. Cell Metab. (2023) 35:1163–78. doi: 10.1016/j.cmet.2023.05.010
86. Abdali A, Baci D, Damiani I, Belloni F, De Dominicis C, Gelmi ML, et al. In vitro angiogenesis inhibition with selective compounds targeting the key glycolytic enzyme PFKFB3. Pharmacol Res. (2021) 168:105592. doi: 10.1016/j.phrs.2021.105592
87. Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R, et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. (2016) 529:216–20. doi: 10.1038/nature16498
88. Yu P, Wilhelm K, Dubrac A, Tung JK, Alves TC, Fang JS, et al. FGF-dependent metabolic control of vascular development. Nature. (2017) 545:224–28. doi: 10.1038/nature22322
89. Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, Pircher A, et al. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell. (2016) 30:968–85. doi: 10.1016/j.ccell.2016.10.006
90. Wang R, Lou X, Feng G, Chen J, Zhu L, Liu X, et al. IL-17A-stimulated endothelial fatty acid beta-oxidation promotes tumor angiogenesis. Life Sci. (2019) 229:46–56. doi: 10.1016/j.lfs.2019.05.030
91. Zhou B, Wang N, Chen Q, Ren J, Fu X, Cheng X. Deubiquitinase USP33 promotes the glycolysis and growth of osteosarcoma by modifying PFKFB3 ubiquitination and degradation. Am J Cancer Res. (2023) 13:922–35.
92. Yang F, Liu Y, Xiao J, Li B, Chen Y, Hu A, et al. Circ-CTNNB1 drives aerobic glycolysis and osteosarcoma progression via m6A modification through interacting with RBM15. Cell Prolif. (2023) 56:e13344. doi: 10.1111/cpr.13344
93. Falkenberg KD, Rohlenova K, Luo Y, Carmeliet P. The metabolic engine of endothelial cells. Nat Metab. (2019) 1:937–46. doi: 10.1038/s42255-019-0117-9
94. Schiffmann LM, Werthenbach JP, Heintges-Kleinhofer F, Seeger JM, Fritsch M, Gunther SD, et al. Mitochondrial respiration controls neoangiogenesis during wound healing and tumour growth. Nat Commun. (2020) 11:3653. doi: 10.1038/s41467-020-17472-2
95. Diebold LP, Gil HJ, Gao P, Martinez CA, Weinberg SE, Chandel NS. Mitochondrial complex III is necessary for endothelial cell proliferation during angiogenesis. Nat Metab. (2019) 1:158–71. doi: 10.1038/s42255-018-0011-x
96. Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. (2010) 464:917–21. doi: 10.1038/nature08945
97. Bruning U, Morales-Rodriguez F, Kalucka J, Goveia J, Taverna F, Queiroz K, et al. Impairment of angiogenesis by fatty acid synthase inhibition involves mTOR malonylation. Cell Metab. (2018) 28:866–80. doi: 10.1016/j.cmet.2018.07.019
98. Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I, et al. Fatty acid carbon is essential for DNTP synthesis in endothelial cells. Nature. (2015) 520:192–97. doi: 10.1038/nature14362
99. Oberkersch RE, Pontarin G, Astone M, Spizzotin M, Arslanbaeva L, Tosi G, et al. Aspartate metabolism in endothelial cells activates the mTORC1 pathway to initiate translation during angiogenesis. Dev Cell. (2022) 57:1241–56. doi: 10.1016/j.devcel.2022.04.018
100. Huang H, Vandekeere S, Kalucka J, Bierhansl L, Zecchin A, Bruning U, et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. (2017) 36:2334–52. doi: 10.15252/embj.201695518
101. Ding X, Zhang Y, Liang J, Li Q, Hu H, Zhou Y, et al. Dihydroartemisinin potentiates VEGFR-TKIS antitumorigenic effect on osteosarcoma by regulating Loxl2/VEGFA expression and lipid metabolism pathway. J Cancer. (2023) 14:809–20. doi: 10.7150/jca.81623
102. Smolarczyk R, Czapla J, Jarosz-Biej M, Czerwinski K, Cichon T. Vascular disrupting agents in cancer therapy. Eur J Pharmacol. (2021) 891:173692. doi: 10.1016/j.ejphar.2020.173692
103. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. (2005) 307:58–62. doi: 10.1126/science.1104819
104. Martin JD, Seano G, Jain RK. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu Rev Physiol. (2019) 81:505–34. doi: 10.1146/annurev-physiol-020518-114700
105. Martin JD, Cabral H, Stylianopoulos T, Jain RK. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. (2020) 17:251–66. doi: 10.1038/s41571-019-0308-z
106. Viallard C, Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. (2017) 20:409–26. doi: 10.1007/s10456-017-9562-9
107. De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. (2013) 154:651–63. doi: 10.1016/j.cell.2013.06.037
108. Garcia J, Hurwitz H, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. (2020) 86. doi: 10.1016/j.ctrv.2020.102017
109. Zhao ZX, Li X, Liu WD, Liu XZ, Wu SJ, Hu XH. Inhibition of growth and metastasis of tumor in nude mice after intraperitoneal injection of bevacizumab. Orthop Surg. (2016) 8:234–40. doi: 10.1111/os.12236
110. Scharf VE, Farese JP, Coomer AR, Milner RJ, Taylor DP, Salute ME, et al. Effect of bevacizumab on angiogenesis and growth of canine osteosarcoma cells xenografted in athymic mice. Am J Vet Res. (2013) 74:771–78. doi: 10.2460/ajvr.74.5.771
111. Ferté C, Loriot Y, Clémenson C, Commo F, Gombos A, Bibault J, et al. IGF-1R targeting increases the antitumor effects of DNA-damaging agents in SCLC model: an opportunity to increase the efficacy of standard therapy. Mol Cancer Ther. (2013) 12:1213–22. doi: 10.1158/1535-7163.MCT-12-1067
112. Pignochino Y, Grignani G, Cavalloni G, Motta M, Tapparo M, Bruno S, et al. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol Cancer. (2009) 8. doi: 10.1186/1476-4598-8-118
113. Mei J, Zhu X, Wang Z, Wang Z. VEGFR. RET, and RAF/MEK/ERK pathway take part in the inhibition of osteosarcoma MG63 cells with sorafenib treatment. Cell Biochem Biophys. (2014) 69:151–56. doi: 10.1007/s12013-013-9781-7
114. Wolfesberger B, Tonar Z, Gerner W, Skalicky M, Heiduschka G, Egerbacher M, et al. The tyrosine kinase inhibitor sorafenib decreases cell number and induces apoptosis in a canine osteosarcoma cell line. Res Vet Sci. (2010) 88:94–100. doi: 10.1016/j.rvsc.2009.06.009
115. Kumar RMR, Arlt MJE, Kuzmanov A, Born W, Fuchs B. Sunitinib malate (SU-11248) reduces tumour burden and lung metastasis in an intratibial human xenograft osteosarcoma mouse model. Am J Cancer Res. (2015) 5:2156.
116. Brave SR, Ratcliffe K, Wilson Z, James NH, Ashton S, Wainwright A, et al. Assessing the activity of cediranib, a VEGFR-2/3 tyrosine kinase inhibitor, against VEGFR-1 and members of the structurally related PDGFR family. Mol Cancer Ther. (2011) 10:861–73. doi: 10.1158/1535-7163.MCT-10-0976
117. Liu K, Ren T, Huang Y, Sun K, Bao X, Wang S, et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis. (2017) 8. doi: 10.1038/cddis.2017.422
118. Kumar S, Mokhtari RB, Sheikh R, Wu B, Zhang L, Xu P, et al. Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumor. Clin Cancer Res. (2011) 17:5656–67. doi: 10.1158/1078-0432.CCR-11-0078
119. Gaspar N, Venkatramani R, Hecker-Nolting S, Melcon SG, Locatelli F, Bautista F, et al. Lenvatinib with etoposide plus ifosfamide in patients with refractory or relapsed osteosarcoma (ITCC-050): a multicentre, open-label, multicohort, phase 1/2 study. Lancet Oncol. (2021) 22:1312–21. doi: 10.1016/S1470-2045(21)00387-9
120. Fleuren E, Vlenterie M, van der Graaf W. Recent advances on anti-angiogenic multi-receptor tyrosine kinase inhibitors in osteosarcoma and ewing sarcoma. Front Oncol. (2023) 13:1013359. doi: 10.3389/fonc.2023.1013359
121. Chen Y, Liu R, Wang W, Wang C, Zhang N, Shao X, et al. Advances in targeted therapy for osteosarcoma based on molecular classification. Pharmacol Res. (2021) 169:105684. doi: 10.1016/j.phrs.2021.105684
122. Xu M, Xu CX, Bi WZ, Song ZG, Jia JP, Chai W, et al. Effects of endostar combined multidrug chemotherapy in osteosarcoma. Bone. (2013) 57:111–15. doi: 10.1016/j.bone.2013.07.035
123. Peng L, Liu A, Shen Y, Xu H, Yang S, Ying X, et al. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncol Rep. (2013) 29:571–78. doi: 10.3892/or.2012.2165
124. Li X, Lu Q, Xie W, Wang Y, Wang G. Anti-tumor effects of triptolide on angiogenesis and cell apoptosis in osteosarcoma cells by inducing autophagy via repressing WNT/β-catenin signaling. Biochem Biophys Res Commun. (2018) 496:443–49. doi: 10.1016/j.bbrc.2018.01.052
125. Xie T, Ren H, Lin H, Mao J, Zhu T, Wang S, et al. Sinomenine prevents metastasis of human osteosarcoma cells via S phase arrest and suppression of tumor-related neovascularization and osteolysis through the CXCR4-STAT3 pathway. Int J Oncol. (2016) 48:2098–112. doi: 10.3892/ijo.2016.3416
126. Huang ST, Huang CC, Sheen JM, Lin TK, Liao PL, Huang WL, et al. Phyllanthus urinaria's inhibition of human osteosarcoma xenografts growth in mice is associated with modulation of mitochondrial fission/fusion machinery. Am J Chin Med. (2016) 44:1507–23. doi: 10.1142/S0192415X16500841
127. Rabelo AC, Borghesi J, Carreira A, Hayashi RG, Bessa F, Barreto R, et al. Calotropis procera (aiton) dryand (apocynaceae) as an anti-cancer agent against canine mammary tumor and osteosarcoma cells. Res Vet Sci. (2021) 138:79–89. doi: 10.1016/j.rvsc.2021.06.005
128. Gao S, Tu DN, Li H, Jiang JX, Cao X, You JB, et al. Pharmacological or genetic inhibition of LDHA reverses tumor progression of pediatric osteosarcoma. BioMed Pharmacother. (2016) 81:388–93. doi: 10.1016/j.biopha.2016.04.029
129. Nepal M, Jung Choi H, Choi B, Lim Kim S, Ryu J, Hee Kim D, et al. Anti-angiogenic and anti-tumor activity of bavachinin by targeting hypoxia-inducible factor-1α. Eur J Pharmacol. (2012) 691:28–37. doi: 10.1016/j.ejphar.2012.06.028
130. Choi HJ, Eun J, Kim DK, Li RH, Shin T, Park H, et al. Icariside II from epimedium koreanum inhibits hypoxia-inducible factor-1α in human osteosarcoma cells. Eur J Pharmacol. (2008) 579:58–65. doi: 10.1016/j.ejphar.2007.10.010
131. Liang C, Li F, Wang L, Zhang ZK, Wang C, He B, et al. Tumor cell-targeted delivery of CRISPR/CAS9 by aptamer-functionalized lipopolymer for therapeutic genome editing of VEGFA in osteosarcoma. Biomaterials. (2017) 147:68–85. doi: 10.1016/j.biomaterials.2017.09.015
132. Fox E, Aplenc R, Bagatell R, Chuk MK, Dombi E, Goodspeed W, et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol. (2010) 28:5174–81. doi: 10.1200/JCO.2010.30.9674
133. Penel-Page M, Ray-Coquard I, Larcade J, Girodet M, Bouclier L, Rogasik M, et al. Off-label use of targeted therapies in osteosarcomas: data from the french registry outc's (observatoire de l'utilisation des therapies ciblees dans les sarcomas). BMC Cancer. (2015) 15. doi: 10.1186/s12885-015-1894-5
134. Raciborska A, Bilska K. Sorafenib in patients with progressed and refractory bone tumors. Med Oncol. (2018) 35. doi: 10.1007/s12032-018-1180-x
135. Grignani G, Palmerini E, Dileo P, Asaftei SD, D'Ambrosio L, Pignochino Y, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an italian sarcoma group study. Ann Oncol. (2012) 23:508–16. doi: 10.1093/annonc/mdr151
136. Sugiyama M, Arakawa A, Shirakawa N, Tao K, Tanimura K, Nakajima M, et al. Safety and efficacy of multiple tyrosine kinase inhibitors in pediatric/adolescent and young adult patients with relapsed or refractory osteosarcomas: a single-institution retrospective analysis. Pediatr Blood Cancer. (2023) 70:e30360. doi: 10.1002/pbc.30360
137. Grignani G, Palmerini E, Ferraresi V, D'Ambrosio L, Bertulli R, Asaftei SD, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol. (2015) 16:98–107. doi: 10.1016/S1470-2045(14)71136-2
138. Tian Z, Gu Z, Wang X, Liu Z, Yao W, Wang J, et al. Efficacy and safety of apatinib in treatment of osteosarcoma after failed standard multimodal therapy: an observational study. Med (Baltimore). (2019) 98:e15650. doi: 10.1097/MD.0000000000015650
139. Xie L, Xu J, Sun X, Guo W, Gu J, Liu K, et al. Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: a single-arm, open-label, phase 2 trial. J Immunother Cancer. (2020) 8. doi: 10.1136/jitc-2020-000798
140. Umeda K, Kato I, Saida S, Okamoto T, Adachi S. Pazopanib for second recurrence of osteosarcoma in pediatric patients. Pediatr Int. (2017) 59:937–38. doi: 10.1111/ped.13307
141. Safwat A, Boysen A, Lucke A, Rossen P. Pazopanib in metastatic osteosarcoma: significant clinical response in three consecutive patients. Acta Oncol. (2014) 53:1451–54. doi: 10.3109/0284186X.2014.948062
142. Longhi A, Paioli A, Palmerini E, Cesari M, Abate ME, Setola E, et al. Pazopanib in relapsed osteosarcoma patients: report on 15 cases. Acta Oncol. (2019) 58:124–28. doi: 10.1080/0284186X.2018.1503714
143. Mross K, Frost A, Steinbild S, Hedbom S, Buechert M, Fasol U, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. (2012) 18:2658–67. doi: 10.1158/1078-0432.CCR-11-1900
144. Duffaud F, Mir O, Boudou-Rouquette P, Piperno-Neumann S, Penel N, Bompas E, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. (2019) 20:120–33. doi: 10.1016/S1470-2045(18)30742-3
145. Davis LE, Bolejack V, Ryan CW, Ganjoo KN, Loggers ET, Chawla S, et al. Randomized double-blind phase II study of regorafenib in patients with metastatic osteosarcoma. J Clin Oncol. (2019) 37:1424. doi: 10.1200/JCO.18.02374
146. Italiano A, Mir O, Mathoulin-Pelissier S, Penel N, Piperno-Neumann S, Bompas E, et al. Cabozantinib in patients with advanced ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. Lancet Oncol. (2020) 21:446–55. doi: 10.1016/S1470-2045(19)30825-3
147. Gaspar N, Campbell-Hewson Q, Gallego MS, Locatelli F, Venkatramani R, Hecker-Nolting S, et al. Phase I/II study of single-agent lenvatinib in children and adolescents with refractory or relapsed solid malignancies and young adults with osteosarcoma (ITCC-050)☆. Esmo Open. (2021) 6:100250. doi: 10.1016/j.esmoop.2021.100250
148. Navid F, Santana VM, Neel M, McCarville MB, Shulkin BL, Wu J, et al. A phase II trial evaluating the feasibility of adding bevacizumab to standard osteosarcoma therapy. Int J Cancer. (2017) 141:1469–77. doi: 10.1002/ijc.30841
149. Kuo C, Kent PM, Logan AD, Tamulonis KB, Dalton KL, Batus M, et al. Docetaxel, bevacizumab, and gemcitabine for very high risk sarcomas in adolescents and young adults: a single-center experience. Pediatr Blood Cancer. (2017) 64:e26265. doi: 10.1002/pbc.26265
150. Pappo AS, Vassal G, Crowley JJ, Bolejack V, Hogendoorn PC, Chugh R, et al. A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: results of a sarcoma alliance for research through collaboration study. Cancer. (2014) 120:2448–56. doi: 10.1002/cncr.28728
151. Zhou J, Ji Q, Li Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: underlying mechanisms and reversal strategies. J Exp Clin Cancer Res. (2021) 40:328. doi: 10.1186/s13046-021-02130-2
152. Kolb EA, Kamara D, Zhang W, Lin J, Hingorani P, Baker L, et al. R1507, a fully human monoclonal antibody targeting IGF-1R, is effective alone and in combination with rapamycin in inhibiting growth of osteosarcoma xenografts. Pediatr Blood Cancer. (2010) 55:67–75. doi: 10.1002/pbc.22479
153. Allen E, Mieville P, Warren CM, Saghafinia S, Li L, Peng MW, et al. Metabolic symbiosis enables adaptive resistance to anti-angiogenic therapy that is dependent on mTOR signaling. Cell Rep. (2016) 15:1144–60. doi: 10.1016/j.celrep.2016.04.029
154. Vaish V, Sanyal SN. Role of Sulindac and Celecoxib in the regulation of angiogenesis during the early neoplasm of colon: exploring PI3-K/PTEN/Akt pathway to the canonical Wnt/β-catenin signaling. BioMed Pharmacother. (2012) 66:354–67. doi: 10.1016/j.biopha.2012.01.004
155. Liu Y, Jiang B, Li Y, Zhang X, Wang L, Yao Y, et al. Effect of traditional Chinese medicine in osteosarcoma: cross-interference of signaling pathways and potential therapeutic targets. Med (Baltimore). (2024) 103:e36467. doi: 10.1097/MD.0000000000036467
156. Zhou J, Wang L, Peng C, Peng F. Co-targeting tumor angiogenesis and immunosuppressive tumor microenvironment: a perspective in ethnopharmacology. Front Pharmacol. (2022) 13:886198. doi: 10.3389/fphar.2022.886198
157. Su Q, Xu X, Wang J, Luan J, Ren X, Huang H, et al. Anticancer effects of constituents of herbs targeting osteosarcoma. Chin J Integr Med. (2019) 25:948–55. doi: 10.1007/s11655-019-2941-x
158. Xiao Y, Yu TJ, Xu Y, Ding R, Wang YP, Jiang YZ, et al. Emerging therapies in cancer metabolism. Cell Metab. (2023) 35:1283–303. doi: 10.1016/j.cmet.2023.07.006
159. Alhamhoom Y, Sobeai HMA, Alsanea S, Alhoshani A. Aptamer-based therapy for targeting key mediators of cancer metastasis (review). Int J Oncol. (2022) 60. doi: 10.3892/ijo.2022.5355
160. Mohammadinejad A, Gaman LE, Aleyaghoob G, Gaceu L, Mohajeri SA, Moga MA, et al. Aptamer-based targeting of cancer: a powerful tool for diagnostic and therapeutic aims. Biosensors (Basel). (2024) 14. doi: 10.3390/bios14020078
161. Tanno T, Zhang P, Bailey C, Wang Y, Ittiprasert W, Devenport M, et al. A novel aptamer-based small RNA delivery platform and its application to cancer therapy. Genes Dis. (2023) 10:1075–89. doi: 10.1016/j.gendis.2022.05.004
162. Narwade M, Shaikh A, Gajbhiye KR, Kesharwani P, Gajbhiye V. Advanced cancer targeting using aptamer functionalized nanocarriers for site-specific cargo delivery. Biomater Res. (2023) 27:42. doi: 10.1186/s40824-023-00365-y
163. Heo K, Min SW, Sung HJ, Kim HG, Kim HJ, Kim YH, et al. An aptamer-antibody complex (oligobody) as a novel delivery platform for targeted cancer therapies. J Control Release. (2016) 229:1–09. doi: 10.1016/j.jconrel.2016.03.006
164. Yang X, Zhao J, Duan S, Hou X, Li X, Hu Z, et al. Enhanced cytotoxic T lymphocytes recruitment targeting tumor vasculatures by endoglin aptamer and IP-10 plasmid presenting liposome-based nanocarriers. Theranostics. (2019) 9:4066–83. doi: 10.7150/thno.33383
165. Kulabhusan PK, Hussain B, Yuce M. Current perspectives on aptamers as diagnostic tools and therapeutic agents. Pharmaceutics. (2020) 12. doi: 10.3390/pharmaceutics12070646
166. Andersen VL, Vinther M, Kumar R, Ries A, Wengel J, Nielsen JS, et al. A self-assembled, modular nucleic acid-based nanoscaffold for multivalent theranostic medicine. Theranostics. (2019) 9:2662–77. doi: 10.7150/thno.32060
167. Omer M, Andersen VL, Nielsen JS, Wengel J, Kjems J. Improved cancer targeting by multimerizing aptamers on nanoscaffolds. Mol Ther Nucleic Acids. (2020) 22:994–1003. doi: 10.1016/j.omtn.2020.10.013
168. Guan B, Zhang X. Aptamers as versatile ligands for biomedical and pharmaceutical applications. Int J Nanomedicine. (2020) 15:1059–71. doi: 10.2147/IJN.S237544
169. Dahlman JE, Barnes C, Khan O, Thiriot A, Jhunjunwala S, Shaw TE, et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. (2014) 9:648–55. doi: 10.1038/nnano.2014.84
170. Zhu D, Li Y, Zhang Z, Xue Z, Hua Z, Luo X, et al. Recent advances of nanotechnology-based tumor vessel-targeting strategies. J Nanobiotechnology. (2021) 19:435. doi: 10.1186/s12951-021-01190-y
171. Sakurai Y, Akita H, Harashima H. Targeting tumor endothelial cells with nanoparticles. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20235819
172. Izci M, Maksoudian C, Manshian BB, Soenen SJ. The use of alternative strategies for enhanced nanoparticle delivery to solid tumors. Chem Rev. (2021) 121:1746–803. doi: 10.1021/acs.chemrev.0c00779
173. Youn YS, Bae YH. Perspectives on the past, present, and future of cancer nanomedicine. Adv Drug Delivery Rev. (2018) 130:3–11. doi: 10.1016/j.addr.2018.05.008
174. Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Delivery Rev. (2014) 66:2–25. doi: 10.1016/j.addr.2013.11.009
175. Chen L, Hong W, Ren W, Xu T, Qian Z, He Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct Target Ther. (2021) 6:225. doi: 10.1038/s41392-021-00631-2
176. Sakhrani NM, Padh H. Organelle targeting: third level of drug targeting. Drug Des Dev Ther. (2013) 7:585–99. doi: 10.2147/DDDT.S45614
177. Poon W, Kingston BR, Ouyang B, Ngo W, Chan W. A framework for designing delivery systems. Nat Nanotechnol. (2020) 15:819–29. doi: 10.1038/s41565-020-0759-5
178. Gao P, Pan W, Li N, Tang B. Boosting cancer therapy with organelle-targeted nanomaterials. ACS Appl Mater Interfaces. (2019) 11:26529–58. doi: 10.1021/acsami.9b01370
179. Chen WH, Luo GF, Zhang XZ. Recent advances in subcellular targeted cancer therapy based on functional materials. Adv Mater. (2019) 31:e1802725. doi: 10.1002/adma.201802725
180. Wang X, Qiu Y, Wang M, Zhang C, Zhang T, Zhou H, et al. Endocytosis and organelle targeting of nanomedicines in cancer therapy. Int J Nanomedicine. (2020) 15:9447–67. doi: 10.2147/IJN.S274289
181. Yang J, Griffin A, Qiang Z, Ren J. Organelle -targeted therapies: a comprehensive review on system design for enabling precision oncology. Signal Transduct Target Ther. (2022) 7. doi: 10.1038/s41392-022-01243-0
182. Zhao M, Ding J, Mao Q, Zhang Y, Gao Y, Ye S, et al. A novel αvβ3 integrin-targeted NIR-II nanoprobe for multimodal imaging-guided photothermal therapy of tumors. vivo. Nanoscale. (2020) 12:6953–58. doi: 10.1039/c9nr10720g
183. Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. (2017) 17:20–37. doi: 10.1038/nrc.2016.108
184. Xia F, He A, Zhao H, Sun Y, Duan Q, Abbas SJ, et al. Molecular engineering of aptamer self-assemblies increases in vivo stability and targeted recognition. ACS Nano. (2022) 16:169–79. doi: 10.1021/acsnano.1c05265
185. Zhao Y, Adjei AA. Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. Oncologist. (2015) 20:660–73. doi: 10.1634/theoncologist.2014-0465
186. Devisscher L, Vieri M, Logue SE, Panse J, Geerts A, van Vlierberghe H, et al. Targeting the angio-proteostasis network: combining the forces against cancer. Pharmacol Ther. (2016) 167:1–12. doi: 10.1016/j.pharmthera.2016.07.007
187. Lupo G, Caporarello N, Olivieri M, Cristaldi M, Motta C, Bramanti V, et al. Anti-angiogenic therapy in cancer: downsides and new pivots for precision medicine. Front Pharmacol. (2016) 7:519. doi: 10.3389/fphar.2016.00519
188. Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to anti-angiogenic therapy in cancer-alterations to anti-vegf pathway. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19041232
189. Ebos JML, Lee CR, Kerbel RS. Tumor and host-mediated pathways of resistance and disease progression in response to antiangiogenic therapy. Clin Cancer Res. (2009) 15:5020–25. doi: 10.1158/1078-0432.CCR-09-0095
190. Xiao L, Yan K, Yang Y, Chen N, Li Y, Deng X, et al. Anti-vascular endothelial growth factor treatment induces blood flow recovery through vascular remodeling in high-fat diet induced diabetic mice. Microvasc Res. (2016) 105:70–6. doi: 10.1016/j.mvr.2016.01.005
191. Piperigkou Z, Mohr B, Karamanos N, Gotte M. Shed proteoglycans in tumor stroma. Cell Tissue Res. (2016) 365:643–55. doi: 10.1007/s00441-016-2452-4
192. Broekman F, Giovannetti E, Peters GJ. Tyrosine kinase inhibitors: multi-targeted or single-targeted? World J Clin Oncol. (2011) 2:80–93. doi: 10.5306/wjco.v2.i2.80
193. Jahanban-Esfahlan R, Seidi K, Banimohamad-Shotorbani B, Jahanban-Esfahlan A, Yousefi B. Combination of nanotechnology with vascular targeting agents for effective cancer therapy. J Cell Physiol. (2018) 233:2982–92. doi: 10.1002/jcp.26051
194. Yu C, Jiang W, Li B, Hu Y, Liu D. The role of integrins for mediating nanodrugs to improve performance in tumor diagnosis and treatment. Nanomaterials (Basel). (2023) 13. doi: 10.3390/nano13111721
Keywords: advanced osteosarcoma, anti-angiogenesis therapy, mechanism of tumor angiogenesis, proangiogenic factors, comprehensive therapy
Citation: Zhang Q, Xia Y, Wang L, Wang Y, Bao Y and Zhao G-s (2024) Targeted anti-angiogenesis therapy for advanced osteosarcoma. Front. Oncol. 14:1413213. doi: 10.3389/fonc.2024.1413213
Received: 06 April 2024; Accepted: 08 August 2024;
Published: 26 August 2024.
Edited by:
Li Xiao, University of Virginia, United StatesReviewed by:
Xizhi Wen, Sun Yat-sen University Cancer Center (SYSUCC), ChinaYukun Li, Zhuzhou Central Hospital, China
Yu Kou, Yangzhou University, China
Shuqiang Liu, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2024 Zhang, Xia, Wang, Wang, Bao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-sheng Zhao, zhao_gs123@126.com; zhaoguosheng@cqmu.edu.cn; Yixi Bao, yixibao@cqmu.edu.cn
 Qiao Zhang
Qiao Zhang