- 1Department of Medicine, King Edward Medical University, Lahore, Pakistan
- 2Department of Research and Education, Medical Research Circle, Goma, Democratic Republic of Congo
Myelofibrosis (MF) is a rare BCR-ABL negative myeloproliferative neoplasm characterized by clonal proliferation of stem cells, with mutations in JAK2, CALR, or MPL genes. MF presents in primary and secondary forms, with common symptoms including splenomegaly, anemia, and thrombocytopenia. Diagnostic criteria involve bone marrow examination and mutation studies. Current treatments are limited, with allogeneic stem cell transplant as the only curative option. Recent FDA approval of Momelotinib (MMB) offers new promise for MF patients with anemia. MMB, a JAK1/2 and ACVR1 inhibitor, effectively reduces spleen size, improves hemoglobin levels, and decreases transfusion dependency. The MOMENTUM trial compared MMB to danazol in JAK inhibitor-treated MF patients with anemia, showing MMB’s superior symptom relief and transfusion independence rates. Additionally, the SIMPLIFY-1 and SIMPLIFY-2 trials evaluated MMB in JAK inhibitor-naïve and experienced patients, respectively, confirming MMB’s non-inferiority to ruxolitinib in spleen volume reduction and highlighting its benefits in transfusion requirements. MMB’s unique dual inhibition mechanism addresses anemia by suppressing hepcidin production, thus enhancing erythropoiesis. These trials collectively suggest MMB as an effective treatment for MF, improving quality of life and offering a survival advantage for patients with anemia. Despite challenges, such as trial design limitations and adverse events, MMB represents a significant advancement in MF management, providing a new therapeutic option for a previously underserved patient population.
Introduction
Myelofibrosis (MF) is a rare disease with an annual incidence rate of about 0.5–1.5 cases per 100,000 individuals in the United States, although increased prevalence is observed among the Ashkenazi Jews (1). MF is a breakpoint cluster region-Abelson (BCR-ABL) negative myeloproliferative neoplasm (2). This condition primarily affects the myeloid cell lineage, resulting in reduced production of red blood cells leading to anemia and platelets leading to thrombocytopenia. It can be categorized into two forms: primary MF and secondary MF, which includes cases arising after polycythemia vera and essential thrombocythemia. Primary myelofibrosis (PMF) is a type of myeloproliferative neoplasm characterized by the clonal proliferation of stem cells, often accompanied by mutations in Janus kinase 2 (JAK2), calreticulin (CALR), or myeloproliferative leukemia (MPL) genes (3). Bone marrow examination, including cytogenetic and mutation studies, is used to diagnose PMF. While mutations in JAK2, CALR, or MPL are typically expected, they are not always required (3). Although, over 80% of PMF patients have specific driver mutations, including JAK2 V617F (50–60%), CALR exon 9 mutations (20–25%), and MPL exon 10 mutations (6–7%), 10–15% of PMF patients lack these common driver mutations, a condition known as triple-negative PMF (4). The International Consensus Classification distinguishes between “prefibrotic” and “overtly fibrotic” PMF, with the former potentially resembling essential thrombocythemia (ET) in its presentation (3). Furthermore, approximately 15% of individuals diagnosed with ET or polycythemia vera (PV) eventually acquire a myelofibrosis-like manifestation, known as secondary myelofibrosis (SMF), post-ET myelofibrosis, or post-PV myelofibrosis. These conditions share comparable treatment approaches and outcomes with PMF (5).
MF is associated with splenomegaly, severely low blood counts, anemia and thrombocytopenia, debilitating symptoms, and an ability to develop vascular complications and a blast phase (persistent elevation in peripheral blood or bone marrow blasts of 20% or more) (6). Anemia in MF is a complex condition resulting from factors such as displacement of erythropoietic tissue by fibrotic stroma, suboptimal environments in extramedullary sites, and splenomegaly-induced RBC sequestration. Abnormal cytokine expression and inflammation in the bone marrow further disrupt erythropoiesis. An element of anemia in MF can occasionally be attributed to the effects of treatment (7).
Pathogenesis of MF
Most cases of this disease are attributed to the mutation in the JAK2, MPL, and CALR genes. The proteins produced by JAK2 and MPL genes are an integral part of the Janus kinase/signal transducer and activator of transcription (JAK-STAT) pathway, essential for regulating the proliferation and differentiation of the megakaryocytes (8). The low level of these proteins induces the proliferation of cells, while the high level of these proteins leads to the differentiation of the cells (9).
MPLW515K/L/A/R and S505N mutations, along with JAK2, are believed to activate JAK2-STAT, leading to cytokine-independent myeloproliferation. Mutant CALR may induce myeloproliferation by binding to MPL in the endoplasmic reticulum. Phenotypic differences exist between JAK2V617F and CALR mutations, with distinct clinical characteristics. Other high risk molecular mutations, including ASXL1, SRSF2, U2AF1, and EZH2 are associated with inferior survival (10). Mutations such as IDH1/IDH2, TP53, DNMT3A, and LNK are more frequent in blast phase MPN (11). The pathogenetic role of these mutations involves disruption of epigenetic, RNA splicing, or transcriptional regulation. The stromal changes in MF, particularly intense in MF compared to PV and ET, involve abnormal proliferation of fibroblasts, endothelial, and mesenchymal cells. The role of fibroblasts in bone marrow fibrosis is supported by their resemblance to hematopoietic stem cells and monocytes (11).
The disruption of the JAK-STAT pathway is the hallmark of MF. This ultimately results in abnormalities related to the proliferation of megakaryocytes, which leads to the failure of the hematopoietic transcription factor GATA-binding factor 1 expression and affects granulocytes (basophils, neutrophils, and eosinophils) (12). Consequently, there is an increased release of inflammatory cytokines (e.g., TGF-β), causing myelo-proliferation with increased activity of fibroblasts leading to bone marrow fibrosis and the development of extramedullary hematopoiesis (13). In addition to this pathway, there are other pathways involved in MF, some of which are potential targets for drug treatment. About two-thirds of PMF patients carry the JAK2V617F mutation, while one-quarter have CALR gene mutations, and 10% each have MPL mutations or a “triple-negative” status (12). Furthermore, approximately 80% of patients also have additional variants in myeloid genes. Almost all of the patients develop anemia along the course of the disease.
Standard therapies for MF
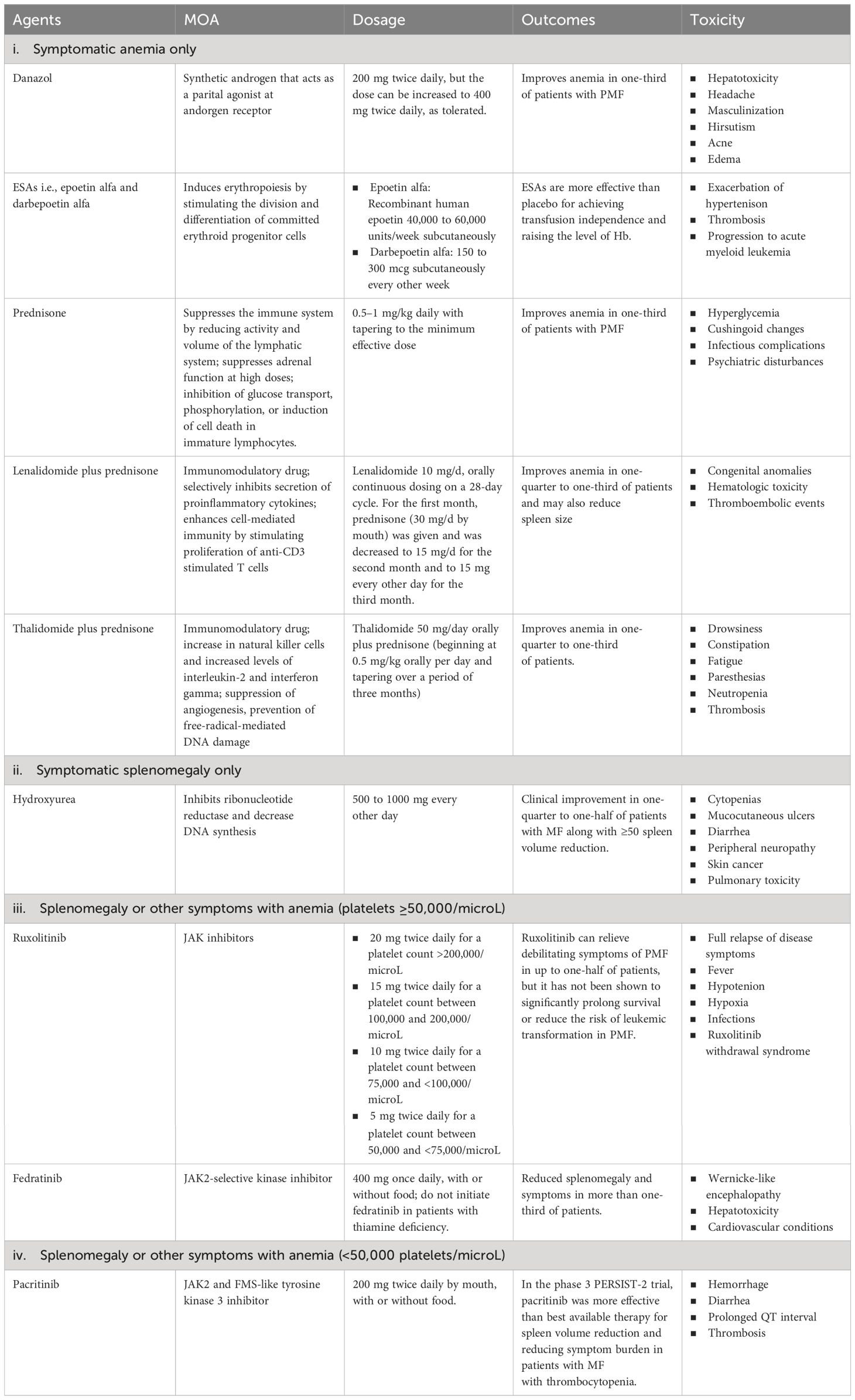
Current therapies for myelofibrosis are only partially effective, with allogeneic stem cell transplant being the only curative option in transplant-eligible patients despite the advancements in the availability of novel therapeutic agents (14). There is also an emerging practice of using ruxolitinib before a stem cell transplant to decrease spleen size, which has shown promise in improving the outcomes of the transplantation process by supporting quicker bone marrow recovery (15). Management strategies for patients with MF who are not candidates for transplantation are tailored based on their specific symptoms and their severity. In cases where patients exhibit no significant symptoms i.e., who do not display significant anemia (hemoglobin < 10 g/dl), splenomegaly (palpable spleen size > 10 cm), leukocytosis (leukocyte count > 25 × 109/l), or marked thrombocytosis (platelet count > 1000 × 109/l), an observational approach is recommended, with regular monitoring to track the emergence of any symptoms (16). However, the available treatments for PMF typically include corticosteroids, androgens, erythropoietin-stimulating agents (ESAs), and immunomodulatory drugs for managing MF-related anemia; hydroxyurea for managing symptomatic splenomegaly; and JAK inhibitors (such as ruxolitinib, fedratinib, and pacritinib) for managing splenomegaly or other symptoms with anemia. For those patients who are experiencing anemia but do not have splenomegaly or systemic symptoms (like fever or weight loss), the preferred treatment approach is to manage the anemia through red blood cell (RBC) transfusions (17). Supportive treatments aimed at reducing the frequency of transfusions are utilized alongside transfusions. While there is no universally agreed-upon protocol, options such as danazol (18) or ESAs are generally considered suitable (19). If a patient’s anemia and the need for frequent transfusions are not effectively managed with danazol or ESAs, alternative treatments might be considered. These include the combination of lenalidomide with prednisone (20) or thalidomide with prednisone (21), which can offer some benefits. Managing anemia and the side effects of repeated blood transfusions, such as iron buildup in the body, is critical and is often addressed through effective iron chelation treatments. Iron chelation therapy, such as deferasirox, maybe a feasible treatment option for iron overload in MF patients, including those who develop iron overload due to anemia worsening during JAK inhibitor treatment, particularly with ruxolitinib; the ‘RUX-IOL’ study demonstrated the safety and efficacy of combining ruxolitinib and deferasirox in MF patients. In a significant number of MF patients, the combination of ruxolitinib and deferasirox resulted in effective iron chelation and erythroid responses, leading to improved clinical outcomes without unexpected side effects (22). In cases where the patient suffers from noticeable splenomegaly but does not have anemia, the cytoreductive treatment i.e., hydroxyurea, demonstrates effectiveness in approximately 40% of individuals, although with frequently transient outcomes (23). The treatment strategy becomes more nuanced when patients exhibit both symptomatic splenomegaly and anemia. The choice of treatment in such scenarios is influenced significantly by the patient’s platelet count. For patients with a platelet count of 50,000/microL or higher, ruxolitinib is typically recommended (24). Conversely, for those with a platelet count below 50,000/microL, pacritinib is advised as it is deemed more suitable due to its safety profile and effectiveness (25). These standard therapies for patients not eligible for stem cell transplants are summarized in Table 1.
Most MF patients experience anemia during the progression of the disease, resulting in the discontinuation of the ongoing treatment. These patients then need blood transfusions (7). However, the United States Food and Drug Administration (US-FDA) approved Momelotinib (MMB) on 15 September 2023 for treating intermediate or high-risk myelofibrosis in adults with anemia (26). This is a landmark step in managing MF as it is the first and only approved treatment for newly diagnosed and previously treated myelofibrosis patients who are experiencing anemia (27).
Mechanism of action of momelotinib
MMB is a small adenosine triphosphate (ATP)-a competitive molecule that effectively inhibits JAK1 and JAK2 in their typical forms and the mutant JAK2V617F variant (28). MMB and its primary metabolite in the human body, called M21, demonstrate a more substantial inhibitory effect on JAK2 when compared to JAK3 and tyrosine kinase 2 (TYK2) (17). The beneficial effects of MMB in addressing anemia and reducing the need for blood transfusions are associated with its ability to suppress the production of hepcidin mediated by Activin A receptor type I (ACVR1) also known as activin receptor-like kinase-2 (ALK2). ACVR1 plays a vital role in the production of blood cells and the development of anemia by influencing the BMP6/ACVR1/SMAD pathway, which regulates the expression of hepcidin, a key controller of iron balance in the body. In MF patients, hepcidin levels are elevated due to excessive activation of BMP6-stimulated ACVR1/ALK2 signaling and increased IL-6-driven inflammatory cytokine signaling. Suppressing liver hepcidin expression raises circulating iron and hemoglobin levels, thereby enhancing erythropoiesis in the bone marrow (29). Higher levels of hepcidin are linked to lower levels of iron in the blood, and when hepcidin is consistently high, it can lead to anemia due to insufficient iron. Anemia and the need for blood transfusions are linked to poor survival outcomes in MF patients (17). Research by Mora et al., which largely included patients who were not exposed to JAK inhibitors, found anemia correlated with higher prognostic risk categories, cytopenic phenotype and higher incidence of evolution into blast phase (BP) (30), while Palandri et al. study, primarily involving ruxolitinib-treated patients, showed that baseline or treatment-emergent anemia increased the risk of BP development and significantly worsened BP-free survival (31). Among the JAK inhibitors approved for treating MF MMB and pacritinib is often preferred for patients with low blood cell counts. Both MMB and pacritinib are potent inhibitors of ACVR1, effectively reducing hepcidin expression via the BMP6/ACVR1/SMAD pathway and restoring the balance of iron in the body, thereby aiding in the production of red blood cells although the benefits of pacritinib versus anemia are not yet well defined (32). The mechanism is well explained in the Figure 1.
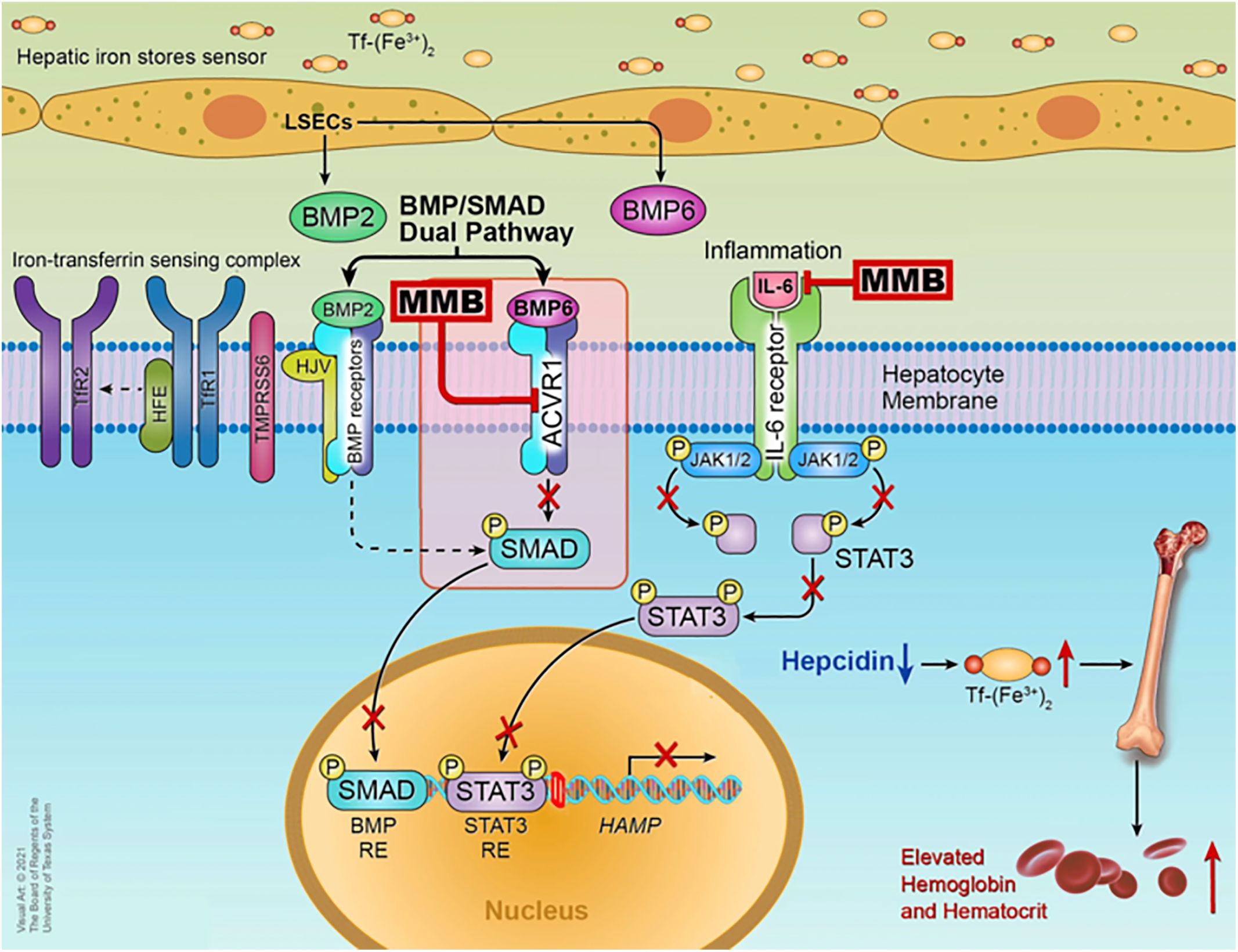
Figure 1 Mechanism of action of MMB © Chifotides HT, Bose P, Verstovsek S. CC BY 4.0 (29).
Importantly, elevated hepcidin levels are significantly linked to shorter overall survival (OS) in patients with myelofibrosis (33). The potential therapeutic targeting of ACVR1 raises promising prospects for MMB in treating other myeloid neoplasms characterized by ineffective erythropoiesis, such as myelodysplastic syndromes involving ring sideroblasts or the Splicing Factor 3B Subunit 1A (SF3B1) mutation, particularly when these conditions are co-expressed with a JAK2 mutation and thrombocytosis (34). The FDA approval of MMB is backed by the pivotal MOMENTUM trial (NCT04173494), and a subgroup of the patients experiencing anemia with MF symptoms participated in the SIMPLIFY-1 phase 3 trial (35). The primary outcome of these trials was to establish the efficacy and safety of MMB in MF patients.
MOMENTUM trial
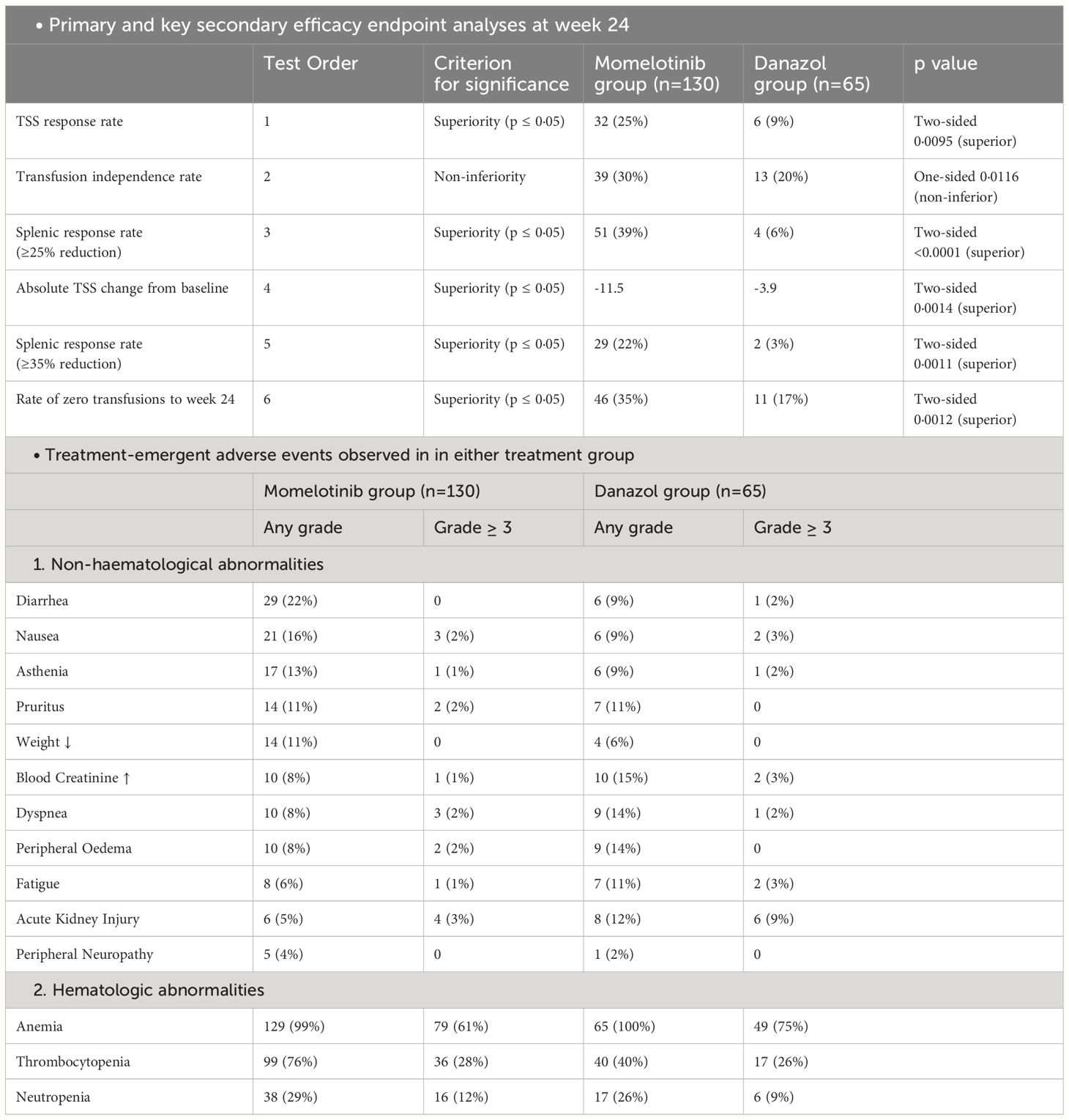
The MOMENTUM trial was a double-blind, active-controlled study that used a 2:1 randomized design to compare the efficacy of MMB against danazol in treating individuals with MF-related symptoms and anemia. This trial enrolled 195 patients who had previously been treated with JAK inhibitors. Before starting the trial, patients tapered off their existing JAK inhibitor treatment over more than a week and then underwent a non-treatment phase of at least two weeks. After completing a week-long baseline assessment period, participants were randomized using a biased coin minimization procedure to either the MMB group or the danazol group (33). This randomization aimed to minimize imbalances between the two groups based on factors such as Myelofibrosis Symptom Assessment Form (MFSAF; version 4.0) Total Symptom Score (TSS) (<22 vs ≥22), baseline palpable spleen length below the left costal margin (<12 cm vs ≥12 cm), baseline red blood cell or whole blood units transfused in the 8 weeks before randomization (0 units vs 1–4 units vs ≥5 units), and investigational sites (33). During the 24-week randomized treatment, patients in the MMB group received 200 mg orally once daily with danazol placebo, while the danazol group received 300 mg orally twice daily with MMB placebo. Both MMB and danazol doses were progressively reduced. MMB decreased by 50 mg increments, while danazol dropped by 200 mg initially and 100 mg subsequently, with minimum allowed doses of 50 mg for MMB and 200 mg for danazol (33). The trial’s primary objective was to assess the MFSAF TSS response rate at the end of the 24 weeks (33). Efficacy analyses followed the intention-to-treat principle, incorporating data from all randomly assigned patients, and the intention-to-treat and safety populations were identical. To control the overall type I error in the study, the evaluation of five key secondary endpoints was planned in hierarchical order. This assessment was to occur only if the primary endpoint demonstrated significance (two-sided p ≤ 0·05) in favor of MMB. The hypothesis test for transfusion independence rate at week 24 was focused on non-inferiority within the hierarchy, while superiority was tested within the hierarchy for all other endpoints (33). MMB demonstrated superiority over danazol, as evidenced by the mean TSS change from baseline at week 24 (−11·5 vs −3·9; least squares mean difference −6·2 [95% CI −10·0 to −2·4]; p=0·0014). Additionally, the rate of zero transfusions up to week 24 was higher in the MMB group (46 [35%; 95% CI 27–44] of 130) as compared to the danazol group (11 [17%; 9–28] of 65) (Table 2). The trial findings also revealed a significantly higher percentage of patients in the MMB group reporting a 50% or greater reduction in their TSS compared to the danazol group (32 [25%] of 130 vs. 6 [9%] of 65; proportion difference 16% [95% CI 6–26], p=0·0095) (33).
The trial also indicated that a significant number of patients initially required blood transfusions. At the beginning of the study, 20% of the patients in the MMB group (26 out of 130) and 17% in the danazol group (11 out of 65) did not need any red blood cell (RBC) transfusions for 28 days. As the trial progressed, the effectiveness of the MMB treatment in reducing the need for RBC transfusions became evident (36). During the treatment phase, 35% of the patients in the MMB group (46 out of 130) did not require any transfusions, compared to only 17% in the danazol group (11 out of 65). Notably, among those who did not need transfusions at the start, a higher percentage of patients treated with MMB (92%) continued to require no transfusions during the treatment phase, compared to 64% in the danazol group. Moreover, there was a noticeable decrease in the average number of RBC units needed per patient every 28 days (36). In the MMB group, this number dropped by 0.86 units (SD=1.748), while in the danazol group, the decrease was 0.28 units (SD=1.584). The trial also categorized changes in transfusion needs into different levels. In the MMB group, a substantial 85% of patients either maintained (19.2%) or improved (65.4%) their transfusion requirements compared to the baseline. In contrast, in the danazol group, 63% of patients either maintained (10.8%) or improved (52.3%) their transfusion requirements (36).
A post hoc time-dependent analysis of the MOMENTUM trial also investigated the prognostic influence of RBC transfusion status over time and other covariates on OS. RBC transfusion status was the consistent prognostic variable while only RBC transfusion status was significantly associated with survival. The study found a compelling correlation between transfusion status and overall survival. Specifically, the data revealed that patients who were non transfusion-independent had a five times higher risk of all-cause mortality compared to those who were transfusion-independent (HR, 5.18; 95% CI, 1.86–14.47; P=.0017) (37). Hence, MMB group had greater number of patients with lower risk of all-cause mortality. Another study described the descriptive responder, longitudinal responder, and time-to-event analyses that supported the primary endpoint of the MOMENTUM trial. The results indicate that MMB led to more consistent and significant symptom relief compared to danazol. Patients treated with MMB experienced early and ongoing reductions in symptoms such as night sweats, abdominal discomfort, rib pain, and bone pain, with notable improvements starting as early as day 29. Additionally, MMB was more effective in reducing both disease-related and cancer-related fatigue and enhancing physical functioning. These findings collectively suggest that MMB offers a rapid, progressive, and sustained benefit, enhancing the quality of life for patients with MF (38).
The most common treatment-emergent adverse effects are also summarized in Table 2. The most commonly reported hematological abnormalities based on laboratory values for both MMB and danazol were treatment-related anemia, which occurred in 79 (61%) out of 130 patients in the MMB group and 49 (75%) out of 65 patients in the danazol group, as well as thrombocytopenia, affecting 36 (28%) out of 130 patients in the MMB group and 17 (26%) out of 65 patients in the danazol group. Regarding non-hematological grade 3 or higher treatment-emergent AEs, MMB and danazol had different profiles (33). MMB resulted in acute kidney injury in four patients (3%) out of 130 and pneumonia in three patients (2%) out of 130. In comparison, danazol led to acute kidney injury in six patients (9%) out of 65 and pneumonia in six patients (9%) out of 65. Additionally, peripheral neuropathy (grade ≤2) occurred in five patients (4%) receiving MMB and one patient (2%) receiving danazol, with no study drug discontinuations reported (33).
The current treatment of myelofibrosis is constrained by the myelosuppressive effects of approved JAK inhibitors. However, MMB presents dual JAK1/2 and ACVR1 inhibition, which provides enhanced therapeutic efficacy in MF. The study demonstrates rapid and sustained improvements in hemoglobin concentrations, non-inferior transfusion-independent rates, a superior rate of zero transfusions, and fewer transfusions compared to danazol (33). MMB’s unique ability to maintain higher doses due to reduced myelosuppressive activity is highlighted. Safety profiles and efficacy in subgroups with thrombocytopenia are consistent, supporting its use in patients with low platelet counts. Despite the challenges posed by the COVID-19 pandemic, the MOMENTUM study, completed within the planned timeframe, shows a trend towards improved OS for the MMB group, with ongoing patient follow-up for long-term survival analyses (33).
The MOMENTUM study faced limitations with its week 24 crossover design, preventing a direct, prolonged survival comparison between treatment groups. There was a potential for bias as patients and investigators might have anticipated treatment assignments, but danazol-treated individuals exhibited advantages in key efficacy measures, mitigating this concern. Although the possibility of early study discontinuation was recognized, a majority of patients with advanced myelofibrosis in both groups completed the randomized treatment phase. The observed higher rate of transfusion independence in MMB-randomized splenic responders at week 24 is confounded by the disproportionate assessment availability, as early discontinuations were deemed non-responders (33).
SIMPLIFY-1 and SIMPLIFY-2 trial
The SIMPLIFY-1 trial was a phase 3 study that included 432 patients with primary myelofibrosis (PMF), post-polycythemia vera, or post-essential thrombocythemia. These patients were JAK inhibitor-naïve and classified as intermediate- or high-risk. The study aimed to compare the efficacy of two treatments, MMB and ruxolitinib, over a 24-week period. Participants were randomly assigned to receive either MMB or ruxolitinib, with the MMB group taking 200 mg once daily and the ruxolitinib group taking 5–20 mg twice daily. In total, 430 subjects received at least one dose of the study treatment, and at week 24, 91.2% (197 out of 216) of ruxolitinib-randomized patients transitioned to MMB treatment (39). Out of these 432 patients, a subgroup of 181 had anemia and MF symptoms at the time of entry (35).
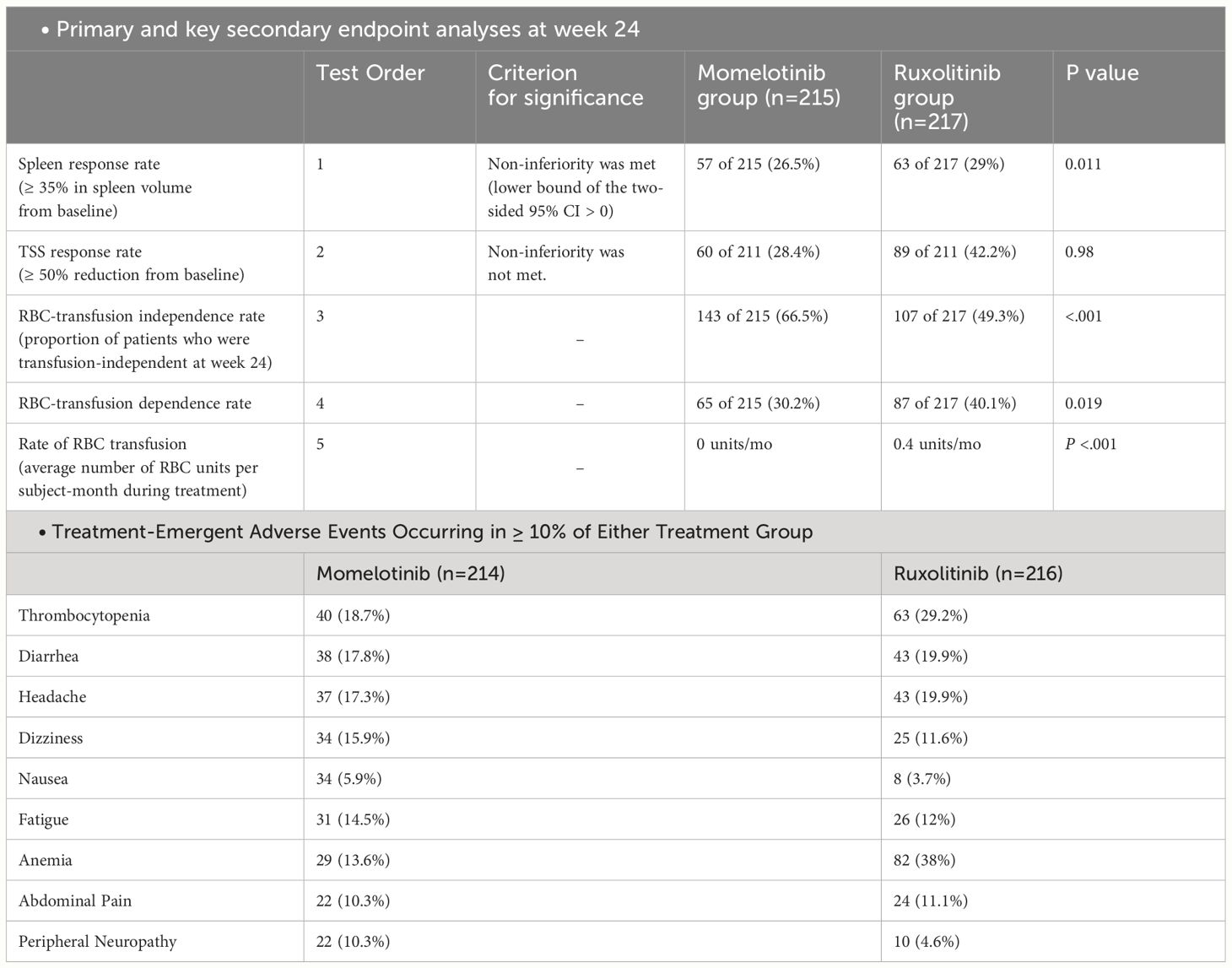
The main goal of the study was to observe if there was a reduction of at least 35% in spleen size after 24 weeks of treatment. Secondary end points were rates of symptom response and effects on RBC transfusion requirements (39). At the 24-week mark, approximately the same percentage of patients in both the momelotinib and ruxolitinib groups saw a ≥ 35% reduction in spleen volume, with 26.5% in the momelotinib group and 29% in the ruxolitinib group, demonstrating that momelotinib was not inferior to ruxolitinib (noninferior; P = .011). However, when it came to reducing the total symptom score by 50% or more, 28.4% of patients on momelotinib and 42.2% on ruxolitinib achieved this, meaning momelotinib did not meet the noninferiority standard for symptom reduction (P = .98). Nonetheless, momelotinib showed better outcomes in terms of reducing the need for transfusions, achieving transfusion independence, and reducing transfusion dependence, all with statistically significant improvements (nominal P ≤.019) (39).
RBC transfusion needs in patients treated with MMB versus ruxolitinib was also evaluated in a study (36). Initially, 70% (150 of 213) of the MMB group and 76% (163 of 216) of the ruxolitinib group did not require transfusions. Over 24 weeks, 95% (142 of 150) of these patients in the MMB group remained transfusion-free, compared to 57% (93 of 163) in the ruxolitinib group. The MMB group experienced a slight reduction in RBC units needed per 28 days (average decrease of 0.10 units), while the ruxolitinib group saw an increase (average rise of 0.39 units). Additionally, 87% of MMB patients maintained or improved (144 [67.6%]) or improved (41 [19.2%]) their transfusion status, compared to 54% (maintained, 94 [43.5%]; improved, 23 [10.6%]) in the ruxolitinib group (36). Another study indicated that age, platelet count, and initial spleen volume were significantly linked to OS in SIMPLIFY-1 trial. When adjusting for differences in prognostic factors, effect modifiers, and changes in transfusion status over time, the findings from SIMPLIFY-1 showed a strong and significant correlation between transfusion status and overall survival (HR, 3.32; 95% CI, 2.31–4.78; P<.0001). This indicates that patients who were not transfusion-independent had a more than threefold increased risk of all-cause mortality compared to those who were transfusion-independent (37).
The MMB and ruxolitinib groups both showed similar overall improvements in symptoms, with a TSS of 17.4 in the MMB group and 16.4 in the ruxolitinib group, with a difference of less than 1.5 points (40). The survival outcomes did not significantly differ between JAK inhibitor-naïve patients randomly assigned to MMB and those initially given ruxolitinib followed by MMB in the SIMPLIFY-1 study, as indicated by the similar survival distributions (Overall Survival Hazard Ratio [OS HR] = 1.02 [95% CI: 0.73, 1.43]; Leukemia-Free Survival Hazard Ratio [LFS HR] = 1.08 [0.78, 1.50]) (41).
In this trial, 35.5% of patients receiving MMB experienced Grade 3 or higher AEs, compared to 43.5% of those on ruxolitib (39). The most frequently reported grade 3 or 4 AEs with MMB included thrombocytopenia (7.0%), anemia (5.6%), diarrhea, hypertension, and neutropenia (2.8% each). For ruxolitinib, the most commonly reported grade 3 or 4 AEs were anemia (23.1%), neutropenia and thrombocytopenia (4.6% each), and hypertension (4.2%). Moreover, peripheral neuropathy (all grade ≤ 2) occurred in 22 patients (10.3%) receiving MMB and 10 patients (4.6%) receiving ruxolitinib (all grade ≤ 3) (39). Serious AEs were noted in 22.9% of MMB-treated patients and 18.1% of ruxolitinib-treated patients (Table 3). AEs leading to discontinuation of the study drugs occurred in 13.1% of patients taking MMB and 5.6% of patients taking ruxolitinib. Additionally, AEs resulting in dose reduction or temporary interruption of the study drugs were reported by 17.8% of patients receiving MMB and 36.6% of patients receiving Ruxolitinib (11).
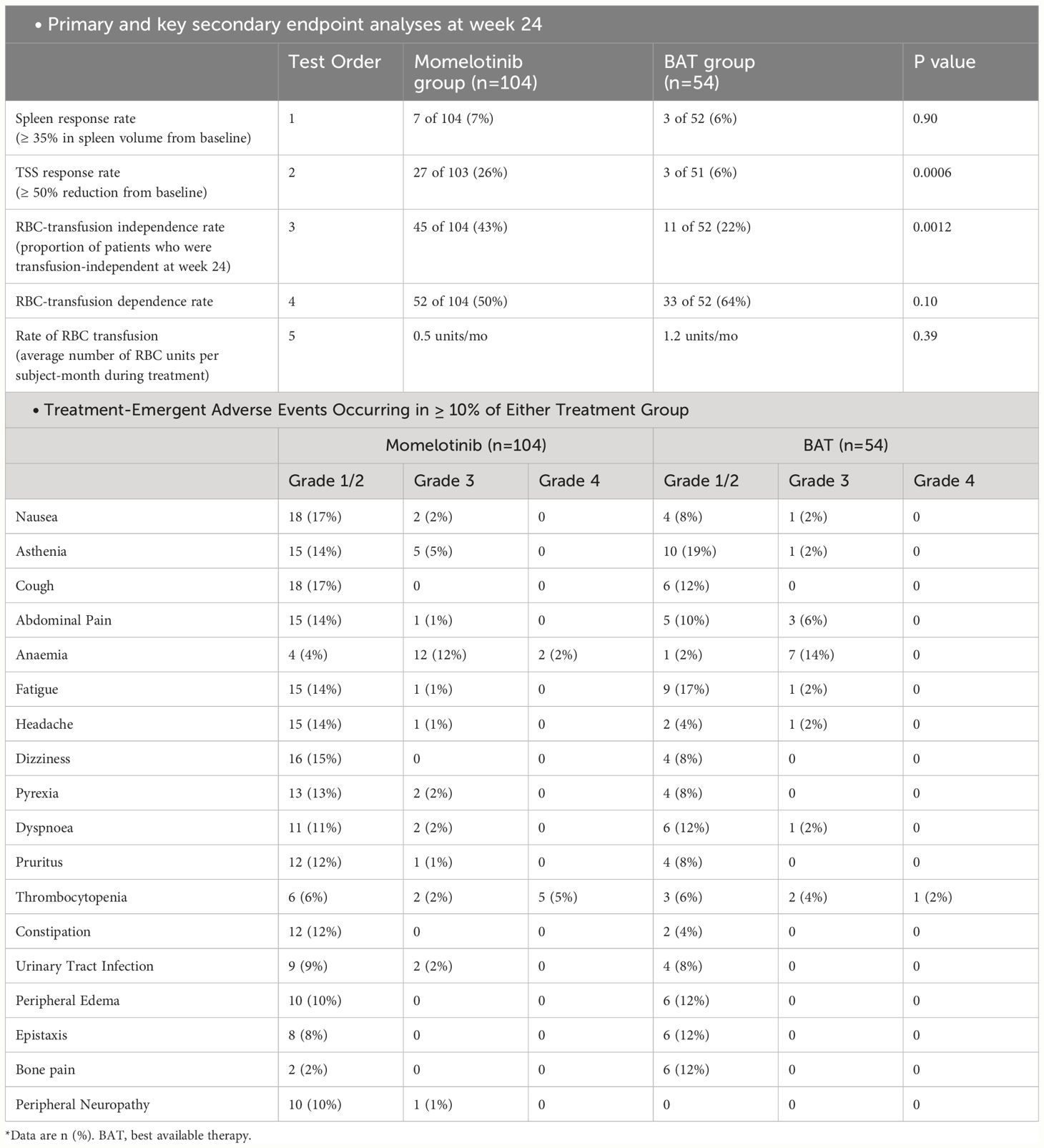
The SIMPLIFY-2 trial included the MF patients already treated with JAK inhibitors. It was a phase 3 study with a 2:1 randomization, conducted internationally in an open-label manner. The study aimed to establish the superiority of MMB over the best available therapy in individuals with PMF, post-polycythemia vera myelofibrosis, or post-essential thrombocythemia myelofibrosis who had previously undergone treatment with ruxolitinib and experienced hematologic toxicity (n=156) (40). All 156 subjects received the designated study treatment, and among the best available therapy/ruxolitinib randomized patients, 76.9% (40 out of 52) switched to MMB treatment at week 24.
The primary endpoint for both trials was the reduction of spleen volume by at least 35% from baseline at the 24-week mark. Secondary endpoints included response rates for TSS and red blood cell transfusion independence at 24 weeks, along with OS and LFS. The results of this trial indicated improvement in TSS in the MMB group, which was in line with what was seen in the SIMPLIFY-1 trial (40). In SIMPLIFY-2, patients previously exposed to ruxolitinib showed a two-year OS of 65.8% and LFS of 64.2% with MMB, compared to 61.2% and 59.7% with the best available therapy followed by MMB (OS HR = 0.98 [0.59, 1.62]; LFS HR = 0.97 [0.59, 1.60]). The presence of baseline transfusion independence was linked to improved survival in both SIMPLIFY-1 (HR = 0.474, p = 0.0001) and SIMPLIFY-2 (HR = 0.226, p = 0.0005) studies (41). The likelihood of symptom improvement was higher in the MMB group (42). The results of SIMPLIFY-1 and SIMPLIFY-2 demonstrated that MMB effectively improves clinically related symptoms of MF patients irrespective of whether they had previously received any treatment. The results and the major AEs of both SIMPLIFY trials are summarized in the Tables 3, 4 (35).
The mature survival data from the phase 3 SIMPLIFY trials reveal that extended treatment with MMB demonstrates excellent OS and LFS in both JAK inhibitor-naïve and previously ruxolitinib-treated patients. The non-inferiority study, SIMPLIFY-1, shows nearly identical OS and LFS outcomes for patients initially randomized to MMB or ruxolitinib. While cross-study comparisons are challenging, the 5-year survival probability is approximately 55% in both arms of SIMPLIFY-1 (41). In SIMPLIFY-2, which involved ruxolitinib-exposed patients, extended MMB treatment exhibits a median OS of around 3 years and a 2-year survival rate of 61–66%, demonstrating promising survival post-ruxolitinib. MMB’s unique inhibition of JAK1, JAK2, and ACVR1/ALK2, a key player in iron homeostasis, leads to decreased hepcidin and increased serum iron availability, contributing to considerable anemia benefits. The study suggests that MMB, with its marked anemia and transfusion independence benefits, maybe a preferred treatment choice for specific subsets of myelofibrosis patients, potentially influencing future treatment decisions (41).
The week 24 crossover design of the SIMPLIFY trials introduces inherent limitations that may impact the OS findings in both SIMPLIFY-1 and SIMPLIFY-2 studies. Due to the crossover, the direct comparability of OS data between the MMB and control arms is compromised, making it challenging to accurately estimate the treatment effect of MMB. Although the studies aimed to provide 24-week comparative data, the survival outcomes are more descriptive for extended MMB treatment, as most control arm patients switched to MMB early in comparison to the median survival follow-up exceeding three years (41). Additionally, the SIMPLIFY-2 design lacked a washout period for prior JAK inhibitor therapy, potentially influencing the specificity of MMB effects and contributing to the non-significant association between week 24 clinical endpoints and OS in this study. Future research is necessary to identify factors beyond baseline hemoglobin and transfusion requirements that could predict which MMB-treated patients are likely to respond positively or negatively to transfusion independence (41).
Conclusion
Currently, allogeneic stem cell transplantation is the sole viable treatment for achieving long-term survival in MF, with a notable rise in the number of such transplants in recent years (43). For patients who are ineligible for transplantation, effective palliative care involves addressing key quality-of-life concerns, namely anemia, splenomegaly, and constitutional symptoms. MMB is considered a promising treatment for managing the adults with intermediate- or high-risk MF with anemia, particularly because it can help alleviate anemia and potentially enhance outcomes for patients with blood cancers. While this development is encouraging for those suffering from anemia related to MF, there are important considerations to keep in mind. Firstly, as a JAK inhibitor, MMB can have side effects such as immune suppression, making patients more susceptible to infections. Therefore, careful monitoring for opportunistic infections is essential. Additionally, patients need to be informed about the risk of drug-induced peripheral neuropathy and should be regularly assessed for this condition. On a positive note, using momelotinib in combination with other agents that stimulate red blood cell production or reduce disease burden holds promise for improving symptom relief in MF. However, it is important to note that, similar to other JAK inhibitors, momelotinib does not appear to reverse the structural or molecular characteristics of MF, nor does it seem to alter the disease’s progression. Though the statistical and clinical effectiveness of MMB has been established, the cost-effectiveness of MMB still needs to be established. Further studies are also required to assess the impact of this drug on other myeloid neoplasms associated with anemia such as myelodysplastic syndromes with ring sideroblasts or SF3B1 mutation, especially those with co-expression of a JAK2 mutation and thrombocytosis. We are optimistic that these recommendations could open a new paradigm for treating patients with coexisting anemia and myeloid neoplasms.
Author contributions
MF: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. MO: Data curation, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Primary Myelofibrosis: Practice Essentials, Pathophysiology, Etiology (2023). Available online at: https://emedicine.medscape.com/article/197954-overview?icd=login_success_email_match_norm#a6.
2. Mughal TI, Vaddi K, Sarlis NJ, Verstovsek S. Myelofibrosis-associated complications: pathogenesis, clinical manifestations, and effects on outcomes. Int J Gen Med. (2014) 7:89. doi: 10.2147/IJGM
3. Tefferi A. Primary myelofibrosis: 2023 update on diagnosis, risk-stratification, and management. Am J Hematology. (2023) 98:801–21. doi: 10.1002/ajh.26857
4. Al-Ghamdi YA, Lake J, Bagg A, Thakral B, Wang SA, Bueso-Ramos C, et al. Triple-negative primary myelofibrosis: A bone marrow pathology group study. Modern Pathol. (2023) 36(3)100016. doi: 10.1016/j.modpat.2022.100016
5. Tefferi A, Saeed L, Hanson CA, Ketterling RP, Pardanani A, Gangat N. Application of current prognostic models for primary myelofibrosis in the setting of post-polycythemia vera or post-essential thrombocythemia myelofibrosis. Leukemia. (2017) 31:2851–2. doi: 10.1038/leu.2017.268
7. Naymagon L, Mascarenhas J. Myelofibrosis-related anemia: Current and emerging therapeutic strategies. Hemasphere. (2017) 1:e1. doi: 10.1097/HS9.0000000000000001
8. Woods B, Chen W, Chiu S, Marinaccio C, Fu C, Gu L, et al. Activation of JAK/STAT signaling in megakaryocytes sustains myeloproliferation in vivo. Clin Cancer Res. (2019) 25:5901–12. doi: 10.1158/1078-0432.CCR-18-4089
9. Besancenot R, Roos-Weil D, Tonetti C, Abdelouahab H, Lacout C, Pasquier F, et al. JAK2 and MPL protein levels determine TPO-induced megakaryocyte proliferation vs differentiation. Blood. (2014) 124:2104–15. doi: 10.1182/blood-2014-03-559815
10. Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. (2013) 27:1861–9. doi: 10.1038/leu.2013.119
11. Tefferi A, Vannucchi AM, Barbui T. Essential thrombocythemia: 2024 update on diagnosis, risk stratification, and management. Am J Hematol (2024) 99(4):697–718. doi: 10.1002/ajh.27216
12. Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. (2017) 129:667–79. doi: 10.1182/blood-2016-10-695940
13. Zahr AA, Salama ME, Carreau N, Tremblay D, Verstovsek S, Mesa R, et al. Bone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategies. Haematologica. (2016) 101:660–71. doi: 10.3324/haematol.2015.141283
14. Choudhary D, Doval D, Khandelwal V, Setia R, Handoo A. Allogeneic stem cell transplant for myelofibrosis- A retrospective single-center study. Blood Cell Ther. (2023) 6:5–10. doi: 10.31547/bct-2022-003
15. Barraco D, Maffioli M, Passamonti F. Standard care and investigational drugs in the treatment of myelofibrosis. Drugs Context. (2019) 8:212603. doi: 10.7573/17404398
16. Barbui T, Tefferi A, Vannucchi AM, Passamonti F, Silver RT, Hoffman R, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. (2018) 32:1057–69. doi: 10.1038/s41375-018-0077-1
17. Gerds AT, Mesa RA, Tkacz J, Moore-Schiltz L, Schinkel J, Phiri K, et al. Anemia and transfusion dependency are important prognostic factors for overall survival in patients with myelofibrosis: Results of a real-world analysis of medicare patients. Blood. (2023) 142:6418. doi: 10.1182/blood-2023-178012
18. Shimoda K, Shide K, Kamezaki K, Okamura T, Harada N, Kinukawa N, et al. The effect of anabolic steroids on anemia in myelofibrosis with myeloid metaplasia: retrospective analysis of 39 patients in Japan. Int J Hematol. (2007) 85:338–43. doi: 10.1532/IJH97.06135
19. Cervantes F, Alvarez-Larrán A, Hernández-Boluda JC, Sureda A, Granell M, Vallansot R, et al. Darbepoetin-alpha for the anemia of myelofibrosis with myeloid metaplasia. Br J Haematol. (2006) 134:184–6. doi: 10.1111/j.1365-2141.2006.06142.x
20. Mesa RA, Yao X, Cripe LD, Li CY, Litzow M, Paietta E, et al. Lenalidomide and prednisone for myelofibrosis: Eastern Cooperative Oncology Group (ECOG) phase 2 trial E4903. Blood. (2010) 116:4436–8. doi: 10.1182/blood-2010-05-287417
21. Mesa RA, Elliott MA, Schroeder G, Tefferi A. Durable responses to thalidomide-based drug therapy for myelofibrosis with myeloid metaplasia. Mayo Clin Proc. (2004) 79:883–9. doi: 10.4065/79.7.883
22. Elli EM, Veroli AD, Bartoletti D, Iurlo A, Carmosino I, Benevolo G, et al. Deferasirox in the management of iron overload in patients with myelofibrosis treated with ruxolitinib: The multicentre retrospective RUX-IOL study. Br J Haematology. (2022) 197:190–200. doi: 10.1111/bjh.18057
23. Cervantes F. How I treat splenomegaly in myelofibrosis. Blood Cancer J. (2011) 1:e37. doi: 10.1038/bcj.2011.36
24. Grunwald MR, Spivak JL. Ruxolitinib enhances platelet production in patients with thrombocytopenic myelofibrosis. JCO. (2016) 34:e38–40. doi: 10.1200/JCO.2013.51.0057
25. Diaz AE, Mesa RA. Pacritinib and its use in the treatment of patients with myelofibrosis who have thrombocytopenia. Future Oncol. (2018) 14:797–807. doi: 10.2217/fon-2017-0494
26. FDA Approves Momelotinib for Myelofibrosis With Anemia (2023). Available online at: https://www.medscape.com/viewarticle/996531.
27. Ojjaara (momelotinib) approved in the US as the first and only treatment indicated for myelofibrosis patients with anemia. London UK: GSK (2023). Available at: https://www.gsk.com/en-gb/media/press-releases/ojjaara-momelotinib-approved-in-the-us-as-the-first-and-only-treatment-indicated-for-myelofibrosis-patients-with-anaemia/.
28. Zheng J, Xin Y, Zhang J, Subramanian R, Murray BP, Whitney JA, et al. Pharmacokinetics and disposition of momelotinib revealed a disproportionate human metabolite-resolution for clinical development. Drug Metab Dispos. (2018) 46:237–47. doi: 10.1124/dmd.117.078899
29. Chifotides HT, Bose P, Verstovsek S. Momelotinib: an emerging treatment for myelofibrosis patients with anemia. J Hematol Oncol. (2022) 15:7. doi: 10.1186/s13045-021-01157-4
30. Mora B, Maffioli M, Rumi E, Guglielmelli P, Caramella M, Kuykendall A, et al. Incidence of blast phase in myelofibrosis according to anemia severity. EJHaem. (2023) 4:679–89. doi: 10.1002/jha2.745
31. Palandri F, Palumbo GA, Benevolo G, Iurlo A, Elli EM, Abruzzese E, et al. Incidence of blast phase in myelofibrosis patients according to anemia severity at ruxolitinib start and during therapy. Cancer. (2024) 130:1270–80. doi: 10.1002/cncr.35156
32. Duminuco A, Chifotides HT, Giallongo S, Giallongo C, Tibullo D, Palumbo GA. ACVR1: A novel therapeutic target to treat anemia in myelofibrosis. Cancers. (2024) 16:154. doi: 10.3390/cancers16010154
33. Verstovsek S, Gerds AT, Vannucchi AM, Al-Ali HK, Lavie D, Kuykendall AT, et al. Momelotinib versus danazol in symptomatic patients with anemia and myelofibrosis (MOMENTUM): results from an international, double-blind, randomised, controlled, phase 3 study. Lancet. (2023) 401:269–80. doi: 10.1016/S0140-6736(22)02036-0
34. Tefferi A, Pardanani A, Gangat N. Momelotinib (JAK1/JAK2/ACVR1 inhibitor): mechanism of action, clinical trial reports, and therapeutic prospects beyond myelofibrosis. Haematologica. (2023) 108:2919–32. doi: 10.3324/haematol.2022.282612
35. OJJAARA (momelotinib) tablets, for oral use. United States Food and Drug Administration (USA: FDA (2023) p. 01–23. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216873s000lbl.pdf.
36. Mesa RA, Perkins AC, Goh YT, Fox ML, McLornan DP, Palmer J, et al. Longitudinal assessment of transfusion intensity in patients with JAK inhibitor-naive or -experienced myelofibrosis treated with momelotinib in the phase 3 simplify-1 and momentum trials. Blood. (2023) 142:3182. doi: 10.1182/blood-2023-177774
37. Gupta V, Harrison CN, Gorsh B, Patel B, Wang Z, Purser M, et al. Red blood cell transfusion independence status is an independent predictor of survival: A post hoc time-dependent analysis of the phase 3 simplify-1, simplify-2, and momentum trials. Blood. (2023) 142:3188. doi: 10.1182/blood-2023-178772
38. Mesa RA, Harrison C, Palmer JM, Gupta V, McLornan DP, McMullin MF, et al. Patient-reported outcomes and quality of life in anemic and symptomatic patients with myelofibrosis: Results from the MOMENTUM study. Hemasphere. (2023) 7:e966. doi: 10.1097/HS9.0000000000000966
39. Mesa RA, Kiladjian JJ, Catalano JV, Devos T, Egyed M, Hellmann A, et al. SIMPLIFY-1: A phase III randomized trial of momelotinib versus ruxolitinib in janus kinase inhibitor–naïve patients with myelofibrosis. J Clin Oncol. (2017) 35:3844–50. doi: 10.1200/JCO.2017.73.4418
40. Mesa RA, Hudgens S, Floden L, Harrison CN, Palmer J, Gupta V, et al. Symptomatic benefit of momelotinib in patients with myelofibrosis: Results from the SIMPLIFY phase III studies. Cancer Med. (2023) 12:10612–24. doi: 10.1002/cam4.5799
41. Mesa R, Harrison C, Oh ST, Gerds AT, Gupta V, Catalano J, et al. Overall survival in the SIMPLIFY-1 and SIMPLIFY-2 phase 3 trials of momelotinib in patients with myelofibrosis. Leukemia. (2022) 36:2261–8. doi: 10.1038/s41375-022-01637-7
42. Harrison CN, Vannucchi AM, Platzbecker U, Cervantes F, Gupta V, Lavie D, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematology. (2018) 5:e73–81. doi: 10.1016/S2352-3026(17)30237-5
Keywords: myelofibrosis, JAKi inhibitors, momelotinib, MOMENTUM trial, SIMPLIFY trial, anemia
Citation: Furqan M and Oduoye MO (2024) Momelotinib – a promising advancement in the management of myelofibrosis in adults with anemia. Front. Oncol. 14:1411972. doi: 10.3389/fonc.2024.1411972
Received: 04 April 2024; Accepted: 12 June 2024;
Published: 25 June 2024.
Edited by:
Giuseppe Alberto Palumbo, University of Catania, ItalyReviewed by:
Andrea Duminuco, Gaspare Rodolico Ospedale, ItalyDaniele Cattaneo, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, Italy
Copyright © 2024 Furqan and Oduoye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Furqan, imfuqii@gmail.com
 Muhammad Furqan
Muhammad Furqan Malik O. Oduoye
Malik O. Oduoye