- 1Department of Radiation Therapy & Oncology, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan
- 2Institute of Veterinary Clinical Science, School of Veterinary Medicine, National Taiwan University, Taipei, Taiwan
- 3Department of General Surgery, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan
- 4Department of Research, Shin-Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan
Background: Intraoperative radiation therapy (IORT) and whole breast irradiation (WBI) are both effective adjuvant radiotherapy methods for ductal carcinoma in situ (DCIS) or early-stage breast cancer (BC) patients undergoing breast-conserving surgery (BCS). We aim to evaluate the long-term oncological efficacy and refine patient selection criteria based on our findings.
Methods: Female patients who underwent either IORT or WBI from January 2016 to December 2019, with a minimum follow-up of 12 months were collected. IORT was administered as a single fraction of 20 Gray (Gy) to the lumpectomy cavity using the Axxent electronic brachytherapy system, while WBI consisted of a standard fractionation of 50 Gy in 25 fractions, along with a reduced boost of 10 Gy. The clinicopathologic characteristics and oncological outcomes were retrospectively analyzed.
Results: A total of 247 patients were enrolled, comprising 164 with BC and 83 with DCIS. Among them, 112 underwent IORT, and 135 received WBI after BCS. The median age was 62.2 years, with median tumor sizes of 1.5 cm for BC and 1.2 cm for DCIS. At a median follow-up of 64.6 months, IORT demonstrated 11 locoregional recurrences (LRR), 1 metastasis, and 1 death, compared to 4 LRR, 5 metastases, and 2 deaths in the WBI group. WBI yielded significantly higher locoregional control (97.0% vs. 90.2%, p = 0.033), although metastasis-free (96.3% vs. 99.1%, p = 0.166) and overall survival rates (98.4% vs. 99%, p = 0.688) did not differ. The LRR rate was significantly higher in the IORT group among the DCIS or BC patients (p = 0.043). The hazard ratio for locoregional recurrence significantly increased in estrogen-receptor-negative (ER-) patients in both univariate analysis (HR = 4.98, 95% CI = 1.76-14.09, p = 0.002) and multivariate analysis (HR = 40.88, 95% CI = 1.29-1297.84, p = 0.035). Additionally, IORT was associated with increased LRR in the multivariate analysis (HR = 4.71, 95% CI = 1.16-19.06, p = 0.030).
Conclusion: At a long-term follow-up, the LRR rate was higher in the BCS followed by IORT, without significant differences in metastasis-free or overall survival rates. Our data confirmed the importance of exclusion ER- patients for IORT.
Introduction
Treatment for early-stage breast cancer (BC) or ductal carcinoma in situ (DCIS) involving breast conservation surgery (BCS) followed with adjuvant radiotherapy (RT) or modified radical mastectomy (1, 2). Adjuvant RT halve the 10-year disease recurrence rate and decrease the 15-year BC death rates (3). Published literatures even suggests a survival benefit of breast conserving therapy over mastectomy in early-stage patients (4, 5). However, a conventional 4- to 6-week RT regimen can be challenging for some patients, leading to the exploration of accelerated irradiation options, such as ultra-hypofractionation (26-28.5 Gy in 5 fractions) or intraoperative radiation therapy (IORT) (6–10). A 1-week ultra-hypofractionation course is non-inferior to the standard 3-week schedule in terms of 5-year tumor recurrence (9, 10). Nonetheless, the single-session treatment by IORT may offer a more convenient and less disruptive alternative to whole breast irradiation (WBI).
IORT delivered either by electron or photon beams, administers a singular high-dose radiation to a reduced breast volume during BCS. Rigorous accelerated partial breast irradiation (APBI) criteria, endorsed by the American Society for Radiation Oncology (ASTRO) and the European Society for Radiotherapy & Oncology (ESTRO), support its application in highly selected early-stage BC (11–13). IORT has demonstrated favorable cosmetic outcomes, likely due to the small treatment volumes and complete skin sparing (12). A meta-analysis by Zhang et al. emphasized fewer side effects, better cosmetic outcomes, and similar mortality rates with IORT compared to whole breast irradiation (WBI) (14). The long-term findings from two pivotal trials, the TARGIT-A (photon IORT) and ELIOT (electron IORT), have been published. The TARGIT-A trial showed similar outcomes between IORT and (WBI) (15, 16), while the ELIOT trial found a higher 10-year local recurrence rate of 8.1% in the IORT group compared to 1.1% in the WBI group (17). Extended follow-up in other reports generally reported a higher locoregional recurrence (LRR) rate with IORT (18–21). Considering increased patient convenience and preference, even with the potential for a higher LRR risk, it may be reasonable to conduct IORT in a prospective clinical trial or multi-institutional registry (13).
Based upon our prior data, we have demonstrated a 1.9% recurrence rate by IORT with Axxent electronic brachytherapy system (Xoft/iCad, Inc., San Jose, CA) in a 31.1-month follow-up (22). This article aims to contribute a long-term follow-up of the oncological outcome in comparison with WBI, and optimization the patient selection criteria for IORT.
Patients and methods
Patient selection
From January 2016 to January 2020, patients with stage I, II BC or DCIS who underwent BCS followed by either IORT or WBI were retrospectively reviewed. Collected data included the patient’s clinical pathological status and oncological outcomes within a minimum follow-up of 12 months. Each patient is required for preoperative mammography and breast sonography. The institute adjusted its IORT inclusion criteria based on the suitable and cautionary groups outlined in the ASTRO consensus guideline for APBI (11). These require patients older than 45 years old, with unifocal DCIS or BC less than 4 cm, without lymph node involvement on preoperative images, and a negative sentinel lymph node biopsy (SLNB). Criteria for WBI in this study required DCIS or BC less than 4 cm, with RT targeting only the breast. Although all the IORT cases qualified for standard WBI, potential candidates were informed of both treatment modalities by the surgeon or radiation oncologist prior to surgery, with the understanding that IORT would not be administered if SLNB was positive. This study was approved by the Institutional Review Board.
Surgical treatment
BCS was carried down to the level of the pectoralis fascia, and an intraoperative frozen section for margin status was done for both DCIS and BC. SLNB with frozen-section diagnoses was done for BC. For IORT patients, only a negative SLNB would proceed to treatment. Per institutional protocol, a negative microscopic margin was highly recommended, and re-excision was strongly suggested for positive margins.
Radiotherapy
IORT was carried out by Axxent electronic brachytherapy system. The Axxent system uses a miniature X-ray source of 50kVp to deliver a single dose of 20 grays (Gy) by a balloon applicator. A balloon-to-tissue apposition was done by retention sutures to maintain a minimum 10mm balloon-to-skin distance. A flexible lead shield was placed for radioprotection. Before beam-on, the balloon will be checked for its reproducibility. The lead shield, applicator balloon, and retention sutures were removed after IORT.
WBI was administered using either the Elekta Synergy (Elekta, Stockholm, Sweden) with image-guided, volumetric modulated arc therapy or TomoTherapy (Accuray, Sunnyvale, CA, USA), both employing standard immobilization devices. Target volumes were defined according to the Radiation Therapy Oncology Group contouring atlas (23). For patients with left-sided DCIS or BC, a deep-inspiration breath-hold technique was recommended for cardiac protection. By institute’s protocol, all patients received standard fractionation of 50 Gy in 25 fractions with a 10 Gy boost to the primary tumor bed. The regional node was not irradiated due to node negativity.
Adjuvant treatment and follow-up
Postoperatively, all patients with positive hormone receptors received endocrine therapy. Adjuvant chemotherapy was recommended for BC patients with tumors larger than 1 cm in diameter or positive nodal metastases on final pathology. Adjuvant trastuzumab was suggested for positive HER-2 BC patients according to guidelines. Oncotype Dx stratification was not performed at the time of analysis. If nodal metastases were identified in the final pathology, an axillary nodal dissection was performed. Adjuvant WBI and regional nodal irradiation were mandatory for patients with four or more positive nodes and strongly recommended for those with one to three positive nodes following systemic treatment. After treatment, patients attend regular clinical check-ups every 3 months, breast sonography every 3 to 6 months, and yearly mammography. For recurrent or metastatic disease, the standard salvage treatments were prescribed.
Endpoints
The primary endpoint was the LRR rate between the two treatment groups. Recurrence in the lumpectomy site was defined as a true local recurrence, recurrences within the ipsilateral breast and the lumpectomy field was defined as secondary local recurrence, and ipsilateral regional nodal recurrences were defined as regional recurrence. The secondary endpoints include metastatic-free survival, overall survival, and treatment-related side effects.
Statistical analysis
The Statistical analysis was conducted using SPSS for Windows version 26.0 (SPSS Inc., Chicago, IL) software. The continuous variables of the clinical-pathological status were compared by t-test and Mann-Whitney U test. The categorical variables were compared by the Chi-squared test or Fisher’s exact test. Kaplan-Meier analysis was used to measure the cumulative risks of recurrences and survival between two groups and the log-rank test was used to examine the two curves. A Cox proportional hazard model was used to investigate the associations between the two groups. A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 249 patients were included in the study, with 166 diagnosed with BC and 83 with DCIS. Among the 114 patients in the IORT group, 2 were lost to follow-up, while the WBI group comprised 135 patients, resulting in a final analyzed patient count of 247. The demographics and clinical characteristics are detailed in Table 1. The median age was 62.2 years (range: 46–81 years), with median tumor sizes of 1.5 cm for IDC and 1.2 cm for DCIS. Ki-67 levels and lymphovascular invasion data were not mandatory on the pathology report before 2019, leading to insufficient data for univariate and multivariate analysis. As this is a retrospective study, some patients fell outside the ASTRO-defined “suitable” category for IORT. Of the IORT group, 17 patients exhibited a single positive nodal involvement on final pathology, categorized as N0 i+ (1 patient), N1mi (3 patients), and N1a (13 patients).
Oncological outcomes
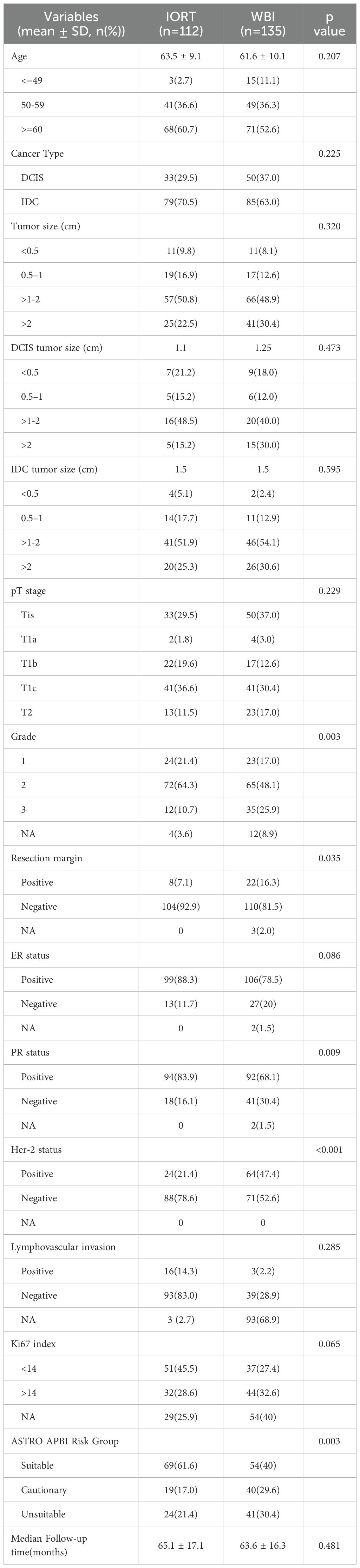
The median follow-up was 64.6 months (range: 13.6 - 94.2 months, mean of 64.3 months). In the IORT group, there were 11 LRR, 1 metastasis, and 1 death, compared to 4 LRR, 5 metastases, and 2 deaths in the WBI group. Locoregional control rate was significantly higher in the WBI group (97.0% vs. 90.2%, p = 0.033), as depicted in Figure 1. Figure 2 demonstrated no significant differences in metastasis-free survival (96.3% vs. 99.1%, p = 0.166) or overall survival rates (98.4% vs. 99%, p = 0.688) between WBI and IORT, respectively.
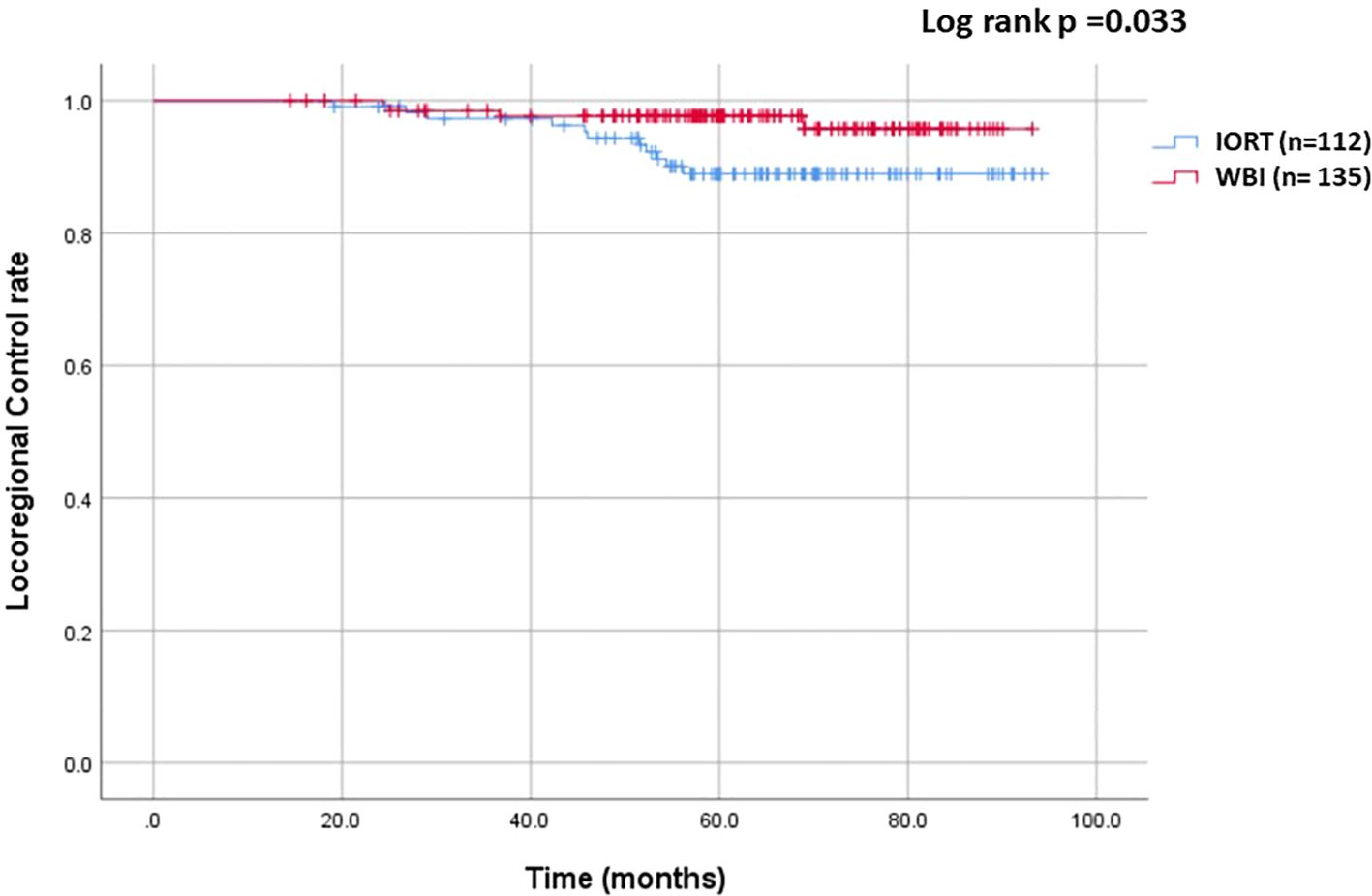
Figure 1. Cumulative incidence of locoregional control in the IORT and WBI groups. The locoregional control rate was significantly higher with WBI than with IORT (97.0% vs. 90.2%, p = 0.033).
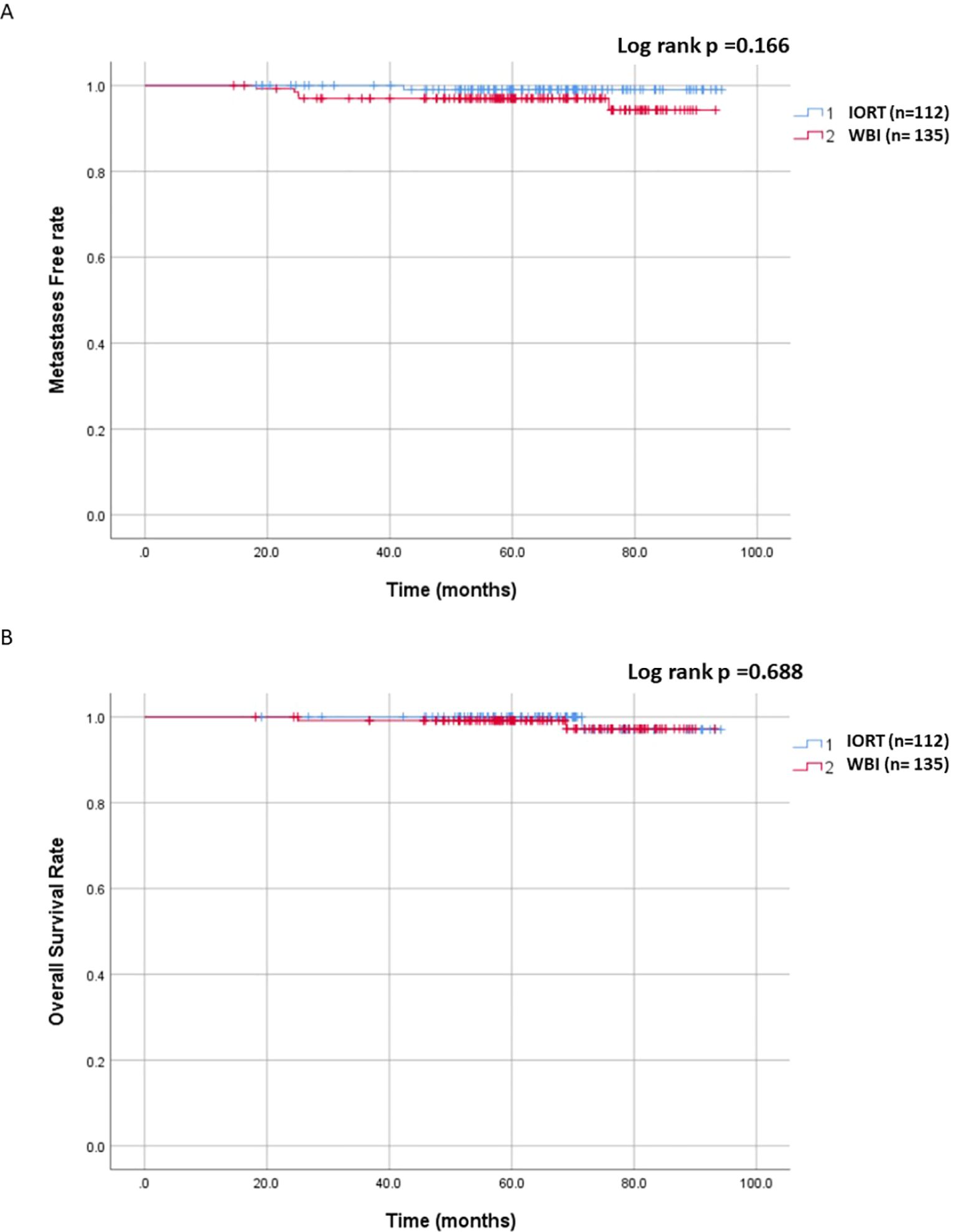
Figure 2. Cumulative incidence of metastasis-free and overall survival in the IORT and WBI groups. (A) No significant differences were observed in metastasis-free survival between WBI and IORT (96.3% vs. 99.1%, p = 0.166). (B) No significant differences were observed in overall survival rates between WBI and IORT (98.4% vs. 99%, p = 0.688).
Stratified analysis showed a notably higher LRR in the IORT group among DCIS and BC patients (p= 0.043). Locoregional control rates were 95.2% (80 out of 83) for DCIS patients, with 93.9% (31 out of 33) in IORT and 98% (49 out of 50) in WBI. Among all BC patients, the locoregional control rate stood at 92.7% (152 out of 164), with 88.6% (70 out of 79) in the IORT group and 96.5% (82 out of 85) in the WBI group.
When stratifying LRR into local or regional recurrences, there were no significant differences between IORT and WBI for local recurrence (p = 0.235). However, the regional recurrence rate was statistically higher with IORT (p = 0.03). In the IORT group, one DCIS and five BC patients experienced true local recurrences, and one DCIS patient had a secondary local recurrence; all were successfully salvaged with surgery. Among the 17 patients with single nodal metastases, all received adjuvant chemotherapy, resulting in only one local recurrence, which was successfully treated with surgery. Two patients also received adjuvant radiotherapy after chemotherapy; one received radiation to the breast and regional nodes, while the other received only regional nodal irradiation. In the WBI group, true local recurrence occurred in 1 DCIS case and 3 BC cases and were managed surgically.
A statistically significant difference was observed in the locoregional control rate when stratified by the ASTRO APBI risk group (p= 0.038). Within the suitable subgroup, the control rate was 95.1% (6 recurrences out of 123 patients), with 91.3% (6/69) in the IORT group and 100% (0/54) in the WBI group. For the cautionary subgroup, the control rate stood at 89.8% (6/59), with 78.9% (4/19) in the IORT group and 95% (2/40) in the WBI group. In the unsuitable subgroup, the control rate reached 95.3% (3/65), with 95.8% (1/24) in the IORT group and 95.1% (2/41) in the WBI group.
In the IORT group, one local recurrent patient developed lung metastases one year later after salvage surgery and WBI. The metastatic lesion was surgically salvaged, and she is currently undergoing chemotherapy. The single mortality case in the IORT group was not related to BC. In the WBI group, there were four cases of metastasis, in which 2 were from the 3 cases with local recurrence. The remaining two cases had no LRR, which resulted in one cancer-related death. The other fatality was not related to BC.
Risk factors of locoregional recurrence
In the univariate analysis, estrogen-receptor negative (ER-) patients showed a significantly elevated hazard ratio for LRR (HR = 4.98, 95% CI=1.76 -14.09, p = 0.002). In the multivariate analysis, IORT (HR = 4.71, 95% CI = 1.16 -19.06, p = 0.030) and ER- (HR = 40.88, 95% CI = 1.29 -1297.84, p = 0.035) were associated with increased LRR. There were no statistical differences based on age, tumor size, margins, HER-2 subtype, or ASTRO APBI risk groups in both univariate and multivariate analyses for both IORT and WBI, as shown in Table 2.
Side Effects
After treatment, both groups experienced no side effects beyond grade 2 dermatitis. Throughout the follow-up period, there were no occurrences of rib fractures, wound infections, or fat necrosis. Among the two patients in the IORT group who underwent salvage RT for LRR, a grade 1 dermatitis and grade 1 esophagitis were documented during treatment and resolved within one-month post-RT.
Discussion
This study represents the largest comparison between IORT and WBI conducted at a single institute in Taiwan. Our prior findings showed a 1.9% rate of locoregional recurrence (LRR) for IORT, with a mean follow-up duration of 31.1 months (22). Subsequent observations during extended follow-up revealed an increased LRR rate of 9.8% over a mean follow-up period of 64.3 months, providing long-term insights into oncological outcomes.
The treatment landscape for early-stage BC emphasizes minimizing treatment intensity, making IORT an appealing option (7, 24, 25). Its popularity surged tenfold in the USA from 2010 to 2013, highlighting its attraction for a shorter treatment course (25). IORT offers advantages in avoiding geographical and temporal misses, along with a radiobiological advantage of single high-dose treatment, while reducing radiation exposure to the internal organs (26, 27). It ensures timely RT completion while reducing risks of viral exposure, which is crucial during the COVID pandemic (28). Economically, IORT enhance patients’ quality of life while conserving healthcare resources (29, 30). Notably, the Taiwan IORT Study Cooperative Group pointed out patients’ preference toward IORT are often due to work-related considerations (31). This was also evident form our analysis, with 44.9% of all treated early BC patients (62 out of 138) choosing IORT from the period of January 2018 to December 2019.
An additive benefit of IORT for local control could be expected. Preclinical studies have shown that IORT influences wound response by downregulating miR-223, which in turn reduces the activation of the epidermal growth factor receptor and disrupts the tumor growth-stimulating loop (32). Furthermore, analysis of surgical fluids from IORT-treated patients has indicated inhibition of breast cancer cell growth and motility through alterations in cytokine expression and intracellular signaling pathways (33).
Under a stringent institutional selection criterion, the majority IORT participants were over 60 years old, had tumors less than 2cm in size, had negative margins, and belonged to the ASTRO suitable risk profile. In line with the published data (17–19), although the overall survival and metastasis-free rate were both high and comparable, the LRR was higher after IORT. Meta-analysis also reported a higher recurrence rate over a median follow-up of 8.6 years in the IORT group (34), concluding that IORT is suitable for the selected low-risk BCs, consistent with the ASTRO and ESTRO guidelines (11, 12). The updated TARGIT-A and ELIOT trials similarly highlight an increased LRR rate following IORT in patients within the ASTRO unsuitable or cautionary risk group (16, 17). A study by Daphne et al. comparing APBI with external beam radiation to IORT reported a local recurrence rate of 10.6% in the IORT group compared to 3.7% with external beam at 5 years, with no significant differences in distant recurrence or overall survival (35). Consistent with our findings, these studies highlight the critical role of patient selection and treatment protocols in shaping outcomes.
The significance of excluding ER- patients for IORT was supported by statistically significant risk of LRR in the post hoc analysis. Patients with a negative hormone receptor have an increased risk of recurrence within the first 5 years (36). In a study by Cannon et al., which included 277 breast cancer cases treated with APBI using high dose rate brachytherapy, an ER- status was strongly linked to LRR in the multivariate analysis (37). Likewise, another study involving 147 patients treated with IORT found correlations between negative hormone receptors, axillary node involvement, positive margins, and lymphovascular invasion with LRR (38).
While the local control rates were similar, the IORT group experienced significantly more regional recurrences. Nodal metastases was a notable risk factor for LRR (39). Despite the institute’s protocol mandating a negative SLNB for IORT eligibility, 15.2% of patients exhibited a single nodal metastasis upon the final pathology, surpassing the rates reported in other studies (31, 40–45). Although the TARGIT-A and ELIOT trials indicated that lymph node involvement of less than three does not necessary contraindicate IORT, patients with positive nodes typically require more aggressive treatment (40, 41, 46). Consistent with this, among the 17 patients with positive nodal involvement in the analysis, all underwent salvage surgery and chemotherapy, with two also received irradiation, resulting in only one case of local recurrence.
Given the unknown final histopathology at the time of IORT, a risk adapted IORT protocol may be crucial for the high-risk patients. Considering a higher LRR associated with IORT, the 2024 ASTRO APBI guideline does not recommend kV IORT alone (without WBI) or electron IORT for early-stage BC receiving PBI, unless part of a clinical trial or multi-institutional registry (13). While awaiting the results of the TARGIT-B trial (NCT01792726), the European group of the International Society of Intraoperative Radiation Therapy has integrated IORT as tumor bed boost administered before WBI, particularly in Grade 3 tumor or triple-negative BCs. The study demonstrated excellent long-term tumor control rates of 95% (12). Silverstein et al. had reported on a series of 1600 cases treated with IORT, of which 207 high-risk patients received additional WBI. This resulted in only two local recurrences, with a 5-year LRR rate of 0.5% (47). In another study, Stoian et al. compared IORT at 20 Gy followed by WBI or simultaneous integrated boost with WBI (SIBRT) in high-risk patients, also reported comparable 5-year local control rates (93% with IORT, 98% with SIBRT) (48). Additionally, for patients with DCIS who underwent IORT and had positive margins, both salvage mastectomy and adjuvant WBI achieved a favorable 3-year local control rate of 94.3% (49).
While our experience with IORT yielded an acceptable locoregional control rate during a 5-year follow-up period, several limitations warrant consideration. Firstly, despite employing stringent criteria for patient selection in our retrospective data collection, a selection bias exists. Secondly, the analysis was confined to a single institution, and the WBI regimen administered followed only by the conventional fractionation. Thirdly, the substantial amount of missing data regarding ki67 levels and lymphovascular invasion in the WBI group necessitated their exclusion from the post hoc analysis. Lastly, despite requiring a negative SLNB for IORT, seventeen patients were found to have single-node involvement on final pathology, placing them in the unsuitable risk group. In this group, 15 patients did not receive additional RT. Although the LRR at the follow-up time was comparable between IORT group (95.8%) and WBI group (95.1%), a longer follow-up is needed to fully assess outcomes.
In conclusion, while the rate of LRR is higher with IORT compared to WBI, the rates of metastasis and mortality are similar. IORT could be considered a viable alternative to WBI, particularly with careful patient selection, thorough counseling, and the exclusion of ER- patients. Additionally, IORT may serve as a beneficial tumor bed boost option for the high-risk patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional review board of Shin Kong Wu Ho Su Memorial Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The study was a retrospective analysis involving more than 200 cases, and an informed consent for each patient was not required by the IRB committee.
Author contributions
M-SC: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Investigation. H-LK: Data curation, Writing – original draft, Writing – review & editing. T-LY: Writing – review & editing, Methodology. Y-FL: Formal analysis, Writing – review & editing, Methodology. K-HC: Conceptualization, Writing – review & editing, Supervision. FC: Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. (2002) 347:1227–32. doi: 10.1056/NEJMoa020989
2. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. (2002) 347:1233–41. doi: 10.1056/NEJMoa022152
3. Early Breast Cancer Trialists' Collaborative G, Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. (2011) 378:1707–16. doi: 10.1016/S0140-6736(11)61629-2
4. Almahariq MF, Quinn TJ, Siddiqui Z, Jawad MS, Chen PY, Gustafson GS, et al. Breast conserving therapy is associated with improved overall survival compared to mastectomy in early-stage, lymph node-negative breast cancer. Radiother Oncol. (2020) 142:186–94. doi: 10.1016/j.radonc.2019.09.018
5. Wrubel E, Natwick R, Wright GP. Breast-conserving therapy is associated with improved survival compared with mastectomy for early-stage breast cancer: A propensity score matched comparison using the national cancer database. Ann Surg Oncol. (2021) 28:914–9. doi: 10.1245/s10434-020-08829-4
6. Goyal S, Chandwani S, Haffty BG, Demissie K. Effect of travel distance and time to radiotherapy on likelihood of receiving mastectomy. Ann Surg Oncol. (2015) 22:1095–101. doi: 10.1245/s10434-014-4093-8
7. Hoopes DJ, Kaziska D, Chapin P, Weed D, Smith BD, Hale ER, et al. Patient preferences and physician practice patterns regarding breast radiotherapy. Int J Radiat Oncol Biol Phys. (2012) 82:674–81. doi: 10.1016/j.ijrobp.2010.11.077
8. Vaidya JS, Vaidya UJ, Baum M, Bulsara MK, Joseph D, Tobias JS. Global adoption of single-shot targeted intraoperative radiotherapy (TARGIT-IORT) for breast cancer-better for patients, better for healthcare systems. Front Oncol. (2022) 12:786515. doi: 10.3389/fonc.2022.786515
9. Murray Brunt A, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfield DJ, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. (2020) 395:1613–26. doi: 10.1016/S0140-6736(20)30932-6
10. Brunt AM, Haviland JS, Sydenham M, Agrawal RK, Algurafi H, Alhasso A, et al. Ten-year results of FAST: A randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol. (2020) 38:3261–72. doi: 10.1200/JCO.19.02750
11. Correa C, Harris EE, Leonardi MC, Smith BD, Taghian AG, Thompson AM, et al. Accelerated Partial Breast Irradiation: Executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract Radiat Oncol. (2017) 7:73–9. doi: 10.1016/j.prro.2016.09.007
12. Fastner G, Gaisberger C, Kaiser J, Scherer P, Ciabattoni A, Petoukhova A, et al. ESTRO IORT Task Force/ACROP recommendations for intraoperative radiation therapy with electrons (IOERT) in breast cancer. Radiother Oncol. (2020) 149:150–7. doi: 10.1016/j.radonc.2020.04.059
13. Shaitelman SF, Anderson BM, Arthur DW, Bazan JG, Bellon JR, Bradfield L, et al. Partial breast irradiation for patients with early-Stage invasive breast cancer or ductal carcinoma in situ: an ASTRO clinical practice guideline. Pract Radiat Oncol. (2024) 14:112–32. doi: 10.1016/j.prro.2023.11.001
14. Zhang L, Zhou Z, Mei X, Yang Z, Ma J, Chen X, et al. Intraoperative radiotherapy versus whole-breast external beam radiotherapy in early-stage breast cancer: A systematic review and meta-analysis. Med (Baltimore). (2015) 94:e1143. doi: 10.1097/MD.0000000000001143
15. Vaidya JS, Bulsara M, Baum M, Wenz F, Massarut S, Pigorsch S, et al. New clinical and biological insights from the international TARGIT-A randomised trial of targeted intraoperative radiotherapy during lumpectomy for breast cancer. Br J Cancer. (2021) 125:380–9. doi: 10.1038/s41416-021-01440-8
16. Vaidya JS, Bulsara M, Baum M, Wenz F, Massarut S, Pigorsch S, et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ. (2020) 370:m2836. doi: 10.1136/bmj.m2836
17. Orecchia R, Veronesi U, Maisonneuve P, Galimberti VE, Lazzari R, Veronesi P, et al. Intraoperative irradiation for early breast cancer (ELIOT): long-term recurrence and survival outcomes from a single-centre, randomised, phase 3 equivalence trial. Lancet Oncol. (2021) 22:597–608. doi: 10.1016/S1470-2045(21)00080-2
18. De Rose F, Mussari S, Di Brina L, Ravanelli D, Ziglio F, Menegotti L, et al. Whole breast irradiation versus intraoperative electron radiation therapy for breast conserving therapy: A large mature single institution matched-pair evaluation of true local relapse, progression free survival, and overall survival. Int J Radiat Oncol Biol Phys. (2023) 116:757–69. doi: 10.1016/j.ijrobp.2023.01.049
19. Yang HY, Tu CW, Chen CC, Lee CY, Hsu YC. Sole adjuvant intraoperative breast radiotherapy in Taiwan: a single-center experience. Breast Cancer Res. (2021) 23:43. doi: 10.1186/s13058-021-01421-y
20. He L, Zhou J, Qi Y, He D, Yuan C, Chang H, et al. Comparison of the oncological efficacy between intraoperative radiotherapy with whole-breast irradiation for early breast cancer: A meta-analysis. Front Oncol. (2021) 11:759903. doi: 10.3389/fonc.2021.759903
21. Wang L, Sun M, Yang S, Chen Y, Li T. Intraoperative radiotherapy is not a better alternative to whole breast radiotherapy as a therapeutic option for early-stage breast cancer. Front Oncol. (2021) 11:737982. doi: 10.3389/fonc.2021.737982
22. Chi MS, Ko HL, Chen CC, Hsu CH, Chen LK, Cheng FT. Single institute experience of intraoperative radiation therapy in early-stage breast cancer. Med (Baltimore). (2021) 100:e27842. doi: 10.1097/MD.0000000000027842
23. White J, Tai A, Arthur D, Buchholz T, Macdonald S, Marks L. (2009) Breast cancer atlas for radiation therapy planning: consensus definitions collaborators. Breast cancer atlas for radiation therapy planning, 73, 944-951. Available online at: https://www.rtog.org
24. Woodward SG, Varshney K, Anne PR, George BJ, Willis AI. Trends in use of hypofractionated whole breast radiation in breast cancer: an analysis of the national cancer database. Int J Radiat Oncol Biol Phys. (2021) 109:449–57. doi: 10.1016/j.ijrobp.2020.09.004
25. Lei J, Wang Y, Bi Z, Xue S, Ou B, Liu K. Intraoperative radiotherapy (IORT) versus whole-breast external beam radiotherapy (EBRT) in early stage breast cancer: results from SEER database. Jpn J Radiol. (2020) 38:85–92. doi: 10.1007/s11604-019-00891-7
26. Aziz MH, Schneider F, Clausen S, Blank E, Herskind C, Afzal M, et al. Can the risk of secondary cancer induction after breast conserving therapy be reduced using intraoperative radiotherapy (IORT) with low-energy x-rays? Radiat Oncol. (2011) 6:174. doi: 10.1186/1748-717X-6-174
27. Leonardi MC, Cormio CF, Frassoni S, Dicuonzo S, Fodor C, Intra M, et al. Ten-year results of hypofractionated whole breast radiotherapy and intraoperative electron boost in premenopausal women. Radiother Oncol. (2022) 177:71–80. doi: 10.1016/j.radonc.2022.10.025
28. Vavassori A, Tagliaferri L, Vicenzi L, D'Aviero A, Ciabattoni A, Gribaudo S, et al. Practical indications for management of patients candidate to Interventional and Intraoperative Radiotherapy (Brachytherapy, IORT) during COVID-19 pandemic - A document endorsed by AIRO (Italian Association of Radiotherapy and Clinical Oncology) Interventional Radiotherapy Working Group. Radiother Oncol. (2020) 149:73–7. doi: 10.1016/j.radonc.2020.04.040
29. Corica T, Nowak AK, Saunders CM, Bulsara M, Taylor M, Vaidya JS, et al. Cosmesis and breast-related quality of life outcomes after intraoperative radiation therapy for early breast cancer: A substudy of the TARGIT-A trial. Int J Radiat Oncol Biol Phys. (2016) 96:55–64. doi: 10.1016/j.ijrobp.2016.04.024
30. Vaidya JS, Tobias JS, Baum M, Keshtgar M, Joseph D, Wenz F, et al. Intraoperative radiotherapy for breast cancer. Lancet Oncol. (2004) 5:165–73. doi: 10.1016/S1470-2045(04)01412-3
31. Lai HW, Liu LC, Ouyang F, Yao CC, Jan HC, Chang YH, et al. Multi-center study on patient selection for and the oncologic safety of intraoperative radiotherapy (IORT) with the Xoft Axxent(R) eBx(R) System for the management of early stage breast cancer in Taiwan. PloS One. (2017) 12:e0185876. doi: 10.1371/journal.pone.0185876
32. Fabris L, Berton S, Citron F, D'Andrea S, Segatto I, Nicoloso MS, et al. Radiotherapy-induced miR-223 prevents relapse of breast cancer by targeting the EGF pathway. Oncogene. (2016) 35:4914–26. doi: 10.1038/onc.2016.23
33. Belletti B, Vaidya JS, D'Andrea S, Entschladen F, Roncadin M, Lovat F, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res. (2008) 14:1325–32. doi: 10.1158/1078-0432.CCR-07-4453
34. Goldberg M, Bridhikitti J, Khan AJ, McGale P, Whelan TJ. A meta-analysis of trials of partial breast irradiation. Int J Radiat Oncol Biol Phys. (2023) 115:60–72. doi: 10.1016/j.ijrobp.2022.09.062
35. Jacobs DHM, Mast ME, Horeweg N, Speijer G, Petoukhova AL, Straver M, et al. Accelerated partial breast irradiation using external beam or intraoperative electron radiation therapy: 5-year oncological outcomes of a prospective cohort study. Int J Radiat Oncol Biol Phys. (2022) 113:570–81. doi: 10.1016/j.ijrobp.2022.03.010
36. Esserman LJ, Moore DH, Tsing PJ, Chu PW, Yau C, Ozanne E, et al. Biologic markers determine both the risk and the timing of recurrence in breast cancer. Breast Cancer Res Treat. (2011) 129:607–16. doi: 10.1007/s10549-011-1564-5
37. Cannon DM, McHaffie DR, Patel RR, Adkison JB, Das RK, Anderson BD, et al. Locoregional recurrence following accelerated partial breast irradiation for early-stage invasive breast cancer: significance of estrogen receptor status and other pathological variables. Ann Surg Oncol. (2013) 20:3446–52. doi: 10.1245/s10434-013-3015-5
38. Gondim GRM, Makdissi FBA, Fogaroli RC, Collins JBD, Iyeyasu H, de Castro DG, et al. Intraoperative breast radiotherapy: survival, local control and risk factors for recurrence. Rep Pract Oncol radiotherapy: J GreatPoland Cancer Center Poznan Polish Soc Radiat Oncol. (2019) 24:551–5. doi: 10.1016/j.rpor.2019.09.004
39. Stankov A, Bargallo-Rocha JE, Silvio AN, Ramirez MT, Stankova-Ninova K, Meneses-Garcia A. Prognostic factors and recurrence in breast cancer: experience at the national cancer institute of Mexico. ISRN Oncol. (2012) 2012:825258. doi: 10.5402/2012/825258
40. Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. (2010) 376:91–102. doi: 10.1016/S0140-6736(10)60837-9
41. Veronesi U, Orecchia R, Maisonneuve P, Viale G, Rotmensz N, Sangalli C, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. (2013) 14:1269–77. doi: 10.1016/S1470-2045(13)70497-2
42. Ivanov O, Dickler A, Lum BY, Pellicane JV, Francescatti DS. Twelve-month follow-up results of a trial utilizing Axxent electronic brachytherapy to deliver intraoperative radiation therapy for early-stage breast cancer. Ann Surg Oncol. (2011) 18:453–8. doi: 10.1245/s10434-010-1283-x
43. Silverstein MJ, Epstein MS, Lin K, Chen P, Khan S, Snyder L, et al. Intraoperative radiation using low-kilovoltage X-rays for early breast cancer: A single site trial. Ann Surg Oncol. (2017) 24:3082–7. doi: 10.1245/s10434-017-5934-z
44. Silverstein MJ, Epstein M, Kim B, Lin K, Khan S, Snyder L, et al. Intraoperative radiation therapy (IORT): A series of 1000 tumors. Ann Surg Oncol. (2018) 25:2987–93. doi: 10.1245/s10434-018-6614-3
45. Kawamura M, Itoh Y, Kamomae T, Sawaki M, Kikumori T, Tsunoda N, et al. A phase I/II trial of intraoperative breast radiotherapy in an Asian population: 10-year results with critical evaluation. J Radiat Res. (2020) 61:602–7. doi: 10.1093/jrr/rraa029
46. Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. (2004) 9:606–16. doi: 10.1634/theoncologist.9-6-606
47. Silverstein MJ, Kim B, Lin K, Lloyd S, Snyder L, Khan S, et al. Risk-adapted intraoperative radiation therapy (IORT) for breast cancer: A novel analysis. Ann Surg Oncol. (2023) 30:6079–88. doi: 10.1245/s10434-023-13897-3
48. Stoian R, Exner JH, Gainey M, Erbes T, Gkika E, Popp I, et al. Comparison of intraoperative radiotherapy as a boost vs. simultaneously integrated boosts after breast-conserving therapy for breast cancer. Front Oncol. (2023) 13:1210879. doi: 10.3389/fonc.2023.1210879
Keywords: breast cancer, ductal carcinoma in situ, radiotherapy, intraoperative radiation therapy, whole breast irradiation
Citation: Chi M-S, Ko H-L, Yang T-L, Liu Y-F, Chi K-H and Cheng FT-F (2024) Comparative long-term oncological outcomes of intraoperative radiotherapy vs. whole-breast irradiation in early breast cancer: a single institute study. Front. Oncol. 14:1411598. doi: 10.3389/fonc.2024.1411598
Received: 03 April 2024; Accepted: 23 September 2024;
Published: 08 October 2024.
Edited by:
William Small, Jr., Loyola University Chicago, United StatesReviewed by:
Jana Fox, Albert Einstein College of Medicine, United StatesAnna L Petoukhova, Haaglanden Medical Center, Netherlands
Copyright © 2024 Chi, Ko, Yang, Liu, Chi and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fiona Tsui-Fen Cheng, c2d0dzg4QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Mau-Shin Chi
Mau-Shin Chi Hui-Ling Ko1†
Hui-Ling Ko1† Ya-Fang Liu
Ya-Fang Liu