- 1Department of Genetics, Institut Curie, Paris, France
- 2Department of Medical Oncology, Saint Quentin Hospital, Saint Quentin, France
- 3Department of Radiology, Saint Quentin Hospital, Saint Quentin, France
- 4Department of Digestive Surgery, Saint Quentin Hospital, Saint Quentin, France
- 5Department of Pneumology, Saint Quentin Hospital, Saint Quentin, France
Malignant peritoneal mesothelioma (MPM) is a rare tumor associated with a poor prognosis and a lack of consensus regarding treatment strategies. While the Checkmate 743 trial demonstrated the superiority of first-line nivolumab and ipilimumab over chemotherapy in malignant pleural mesothelioma (MPlM), few studies have assessed the effectiveness of immunotherapy against MPM, due to its rarity. Here, we report a major and sustained 12-month response in a 74-year-old female patient who received the anti-PD-1 nivolumab and the anti-CTLA4 ipilimumab as first-line therapy for diffuse MPM. PD-L1 was expressed and BAP1 expression was lost, as shown by immunohistochemistry, however the BAP1 gene was not mutated. Our findings suggest a role for ICI in non-resectable diffuse MPM exhibiting PD-L1 overexpression and loss of BAP1 expression, and instill new hope in their treatment. To our knowledge, this is the second reported case of dual immunotherapy used as first-line in MPM with a major clinical response. To investigate the clinical outcome, we conducted additional molecular analyses of the MPM tumor and we reviewed the literature on immunotherapy in MPM to discuss the role of PD-L1 and BAP1.
1 Introduction
Malignant peritoneal mesothelioma (MPM) is a rare tumor with a poor prognosis that develops from the parietal cells of the peritoneum. Its main risk factors are prolonged asbestos exposure and germline pathogenic variants in the BAP1 tumor suppressor gene (1, 2).
There is no consensus on the management of MPM. When the tumor is resectable, the treatment approach usually relies on cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) (3). For advanced or unresectable MPM, systemic chemotherapy with cisplatin and pemetrexed (+/- bevacizumab) is the predominant treatment, as was the case for malignant pleural mesothelioma (MPlM) until 2021 (1, 2, 4). Since then, the combination of nivolumab and ipilimumab has become the standard first-line treatment for MPlM. Indeed, the Checkmate 743 trial demonstrated the superiority of the combination of the two immune checkpoint inhibitors (ICI) anti-PD-1 nivolumab and anti-CTLA4 ipilimumab in the efficacy on overall survival, compared to first-line chemotherapy with cisplatin-pemetrexed in patients with unresectable MPlM (5).
However, despite some studies reporting encouraging results, the efficacy of ICI in MPM has not been formally demonstrated due to the low number of MPM cases in clinical trials, which predominantly include MPlM. The molecular heterogeneity between MPM and MPlM also complicates the interpretation and extrapolation of results (1, 2, 6, 7). Predictive biomarkers for response to immunotherapy in MPM are poorly defined. About 47 to 60% of MPM cases exhibit loss-of-function mutations in BAP1, and nearly 50% show overexpression of the PD-L1 protein (6).
BAP1 (BRCA1-Associated Protein 1) is an ubiquitin hydrolase enzyme involved in various pathways including chromatin remodeling and genome integrity maintenance through homologous recombination DNA repair (8, 9). Haploinsufficiency of the BAP1 gene in MPM has also been associated with strong immunogenicity of the tumor microenvironment and hyperactivation of immune checkpoint receptors PD-1, PD-L1 and CTLA4. Therefore, new studies are crucial to assess whether loss of BAP1 expression and overexpression of PD-L1 are biomarkers for MPM response to ICI (10, 11).
Here, we present the case of a 74-year-old woman diagnosed with diffuse epithelioid-type MPM infiltrating the ileocecal and uterine regions. First-line dual immunotherapy using nivolumab and ipilimumab was used by analogy to MPlM and was efficient.
2 Case report
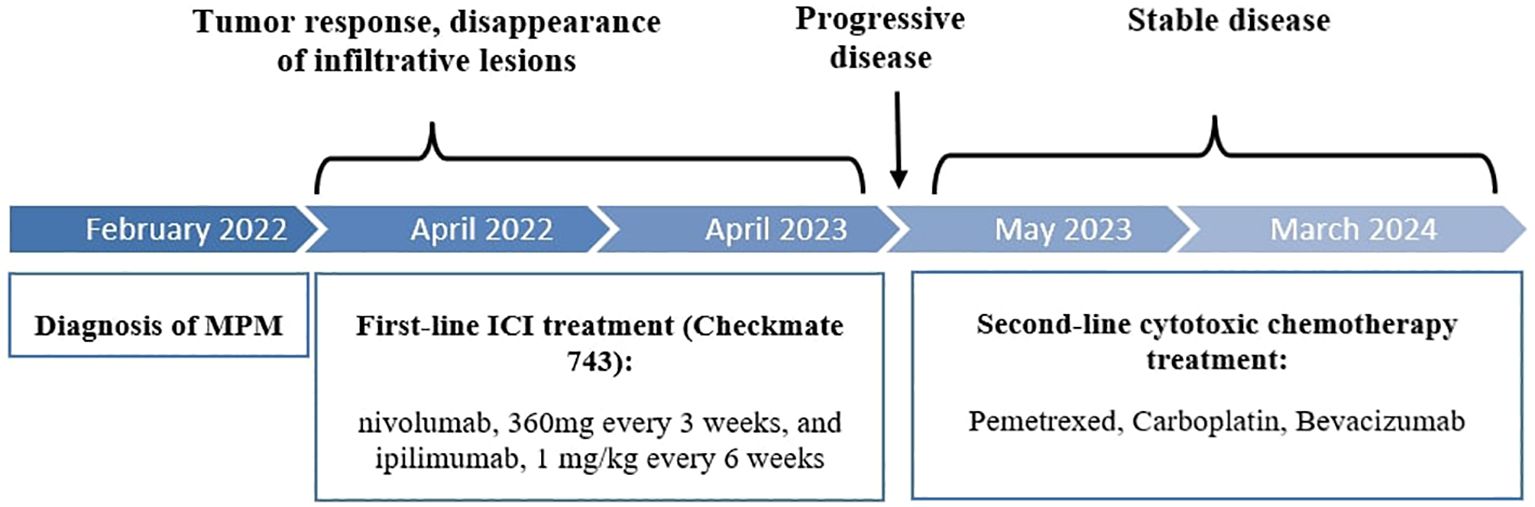
A 74 year-old female patient was referred in February 2022 for weight loss and abdominal pain in the right iliac fossa. Performance status was altered (WHO score 3). Medical history revealed hypertension and a surgically treated syndrome of the pyelo-ureteral junction. There was no personal or family history of cancer, nor any history of exposure to asbestos. Colonoscopy and gastroscopy yielded normal results. Contrast-enhanced CT scan revealed thickening of the peritoneum adjacent to the right colon and cecum, along with infiltration of the right lateral-uterine region and small right pleural effusion (Figures 1, 2A). A laparotomy was conducted, during which biopsies revealed a diffuse infiltrating epithelioid-type malignant peritoneal mesothelioma without possibility of complete resection. Additionally, the surgical treatment was contraindicated due to pleural metastatic involvement. Diagnosis was confirmed by the French National Reference Network MESOPATH. Immunohistochemical staining (IHC) showed the loss of expression of the BAP1 protein and expression of the PD-L1 protein in tumor-infiltrating lymphocytes (TILs) and tumor cells, with a combined positive score (CPS) of 10. Ki67 proliferation index was 5%. Tumor mutational burden (TMB) was low. The treatment decision was based on the first-line treatment of unresectable MPlM, and considering the expression of the PD-L1 protein. After providing clear information to the patient and her husband regarding the off-label use of this treatment for MPM, she consented and signed an informed consent. From April 2022 to April 2023, she received 10 injections of nivolumab, 360 mg every 3 weeks, and ipilimumab, 1 mg/kg every 6 weeks, following the regimen outlined in the Checkmate 743 trial for MPlM. Abdominal pain disappeared and performance status improved, without any significant toxicity. Subsequent CT scans showed a reduction in peritoneal infiltration (Figure 2B). However, in May 2023, the patient experienced renewed pain in the right hypochondria. A CT scan revealed new supra-centimetric nodules in the peritoneum, located in the perihepatic and infrahepatic regions, in contact with the right diaphragm, in the pelvis and in the right pleura (Figure 2C). Immunotherapy was discontinued and a regimen of pemetrexed, carboplatin and bevacizumab was initiated and led to a sustained major response. In March 2024, 24 months following the initial diagnosis, the patient remained well while receiving a pemetrexed and bevacizumab maintenance therapy.
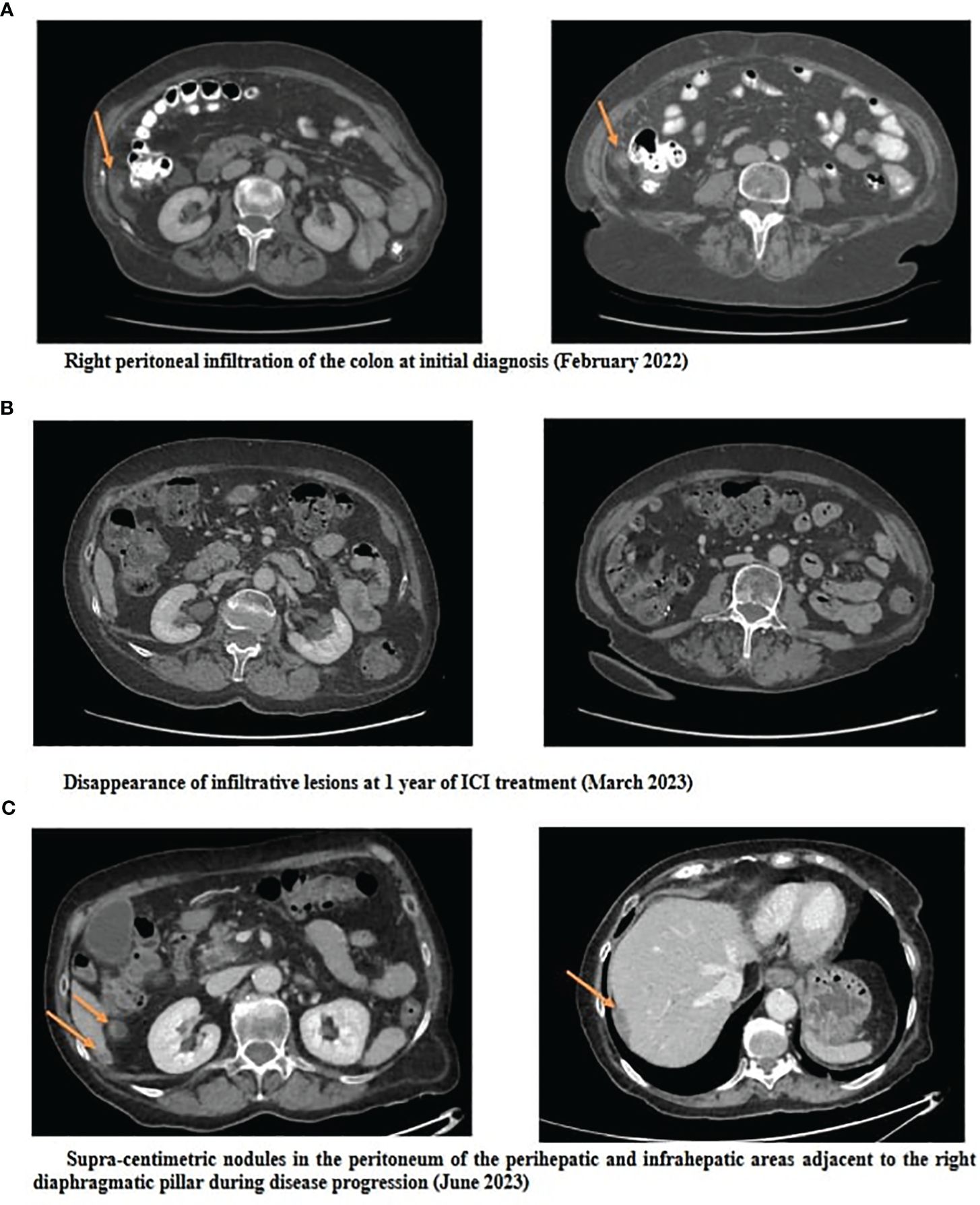
Figure 2 Abdominopelvic CT scans at initial diagnosis (A), at 1 year of ICI treatment (B) and at progression (C).
3 Molecular analysis
To investigate the hypothesis explaining the notable clinical response, genomic analyses were conducted on the tumor tissue. Specifically, we focused on commonly mutated tumor suppressor genes in MPM, including NF2, CDKN2A, CDKN2B, PBRM1, TP53, SETD2 and SETDB1 (12–14). Our examination of these genes in the patient’s tumor did not identify any mutations. Additionally, we sought mutations in oncogenes, such as KRAS, EGFR, FGFR3, ALK, which have been documented in rare cases of MPM (7, 13). However, our analysis of these oncogenes in the patient’s tumor did not uncover any mutations.
The gene sequencing of the tumor unveiled the absence of alterations in the BAP1 gene despite the loss of protein expression in IHC. Moreover, no modifications were detected in the gene transcript (Figure 3).
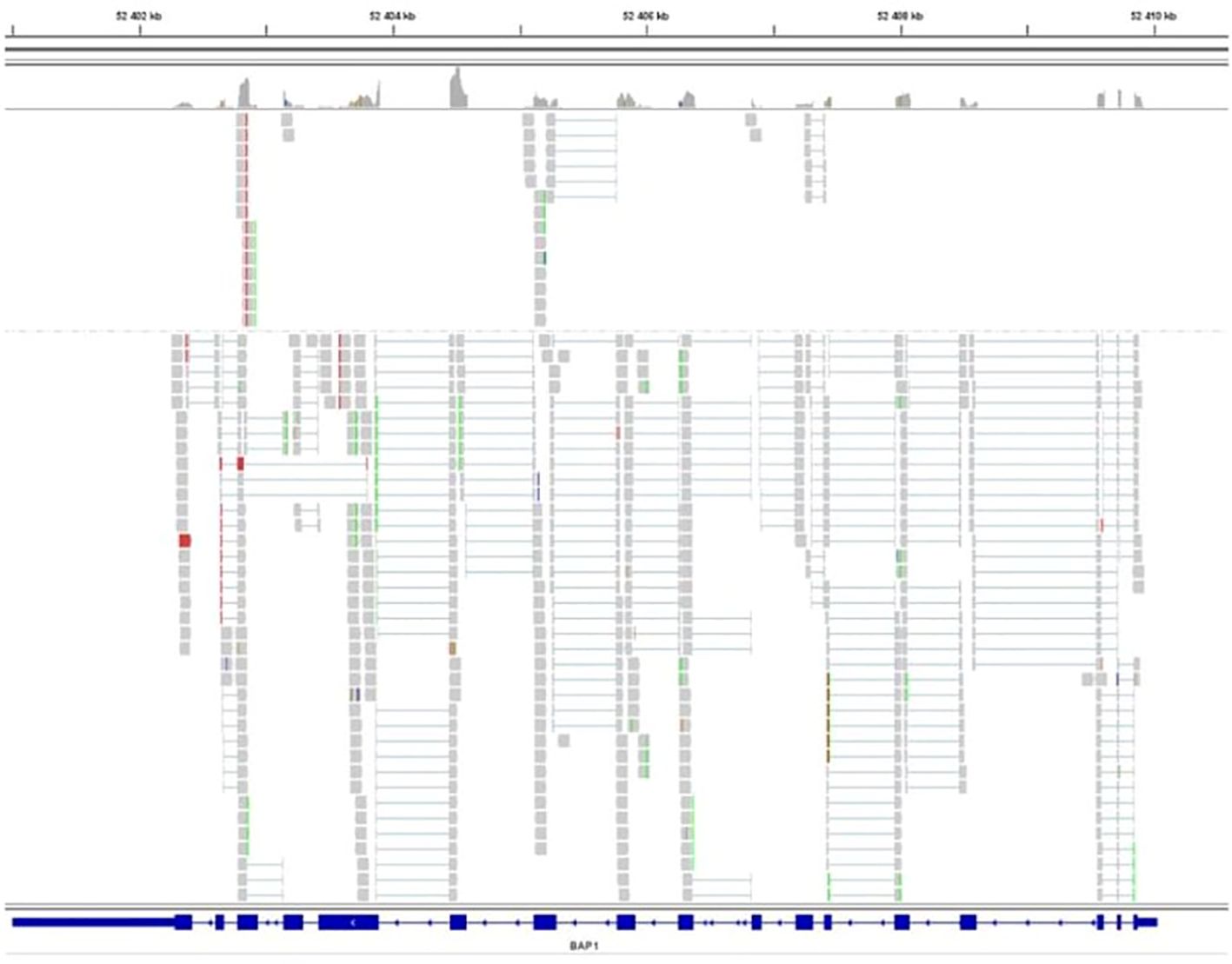
Figure 3 Sequencing of the BAP1 transcript. BAP1 exons are represented as solid rectangles. RNA sequence reads are shown in grey and exhibit homology to the reference sequence, indicating no splicing alteration.
4 Discussion
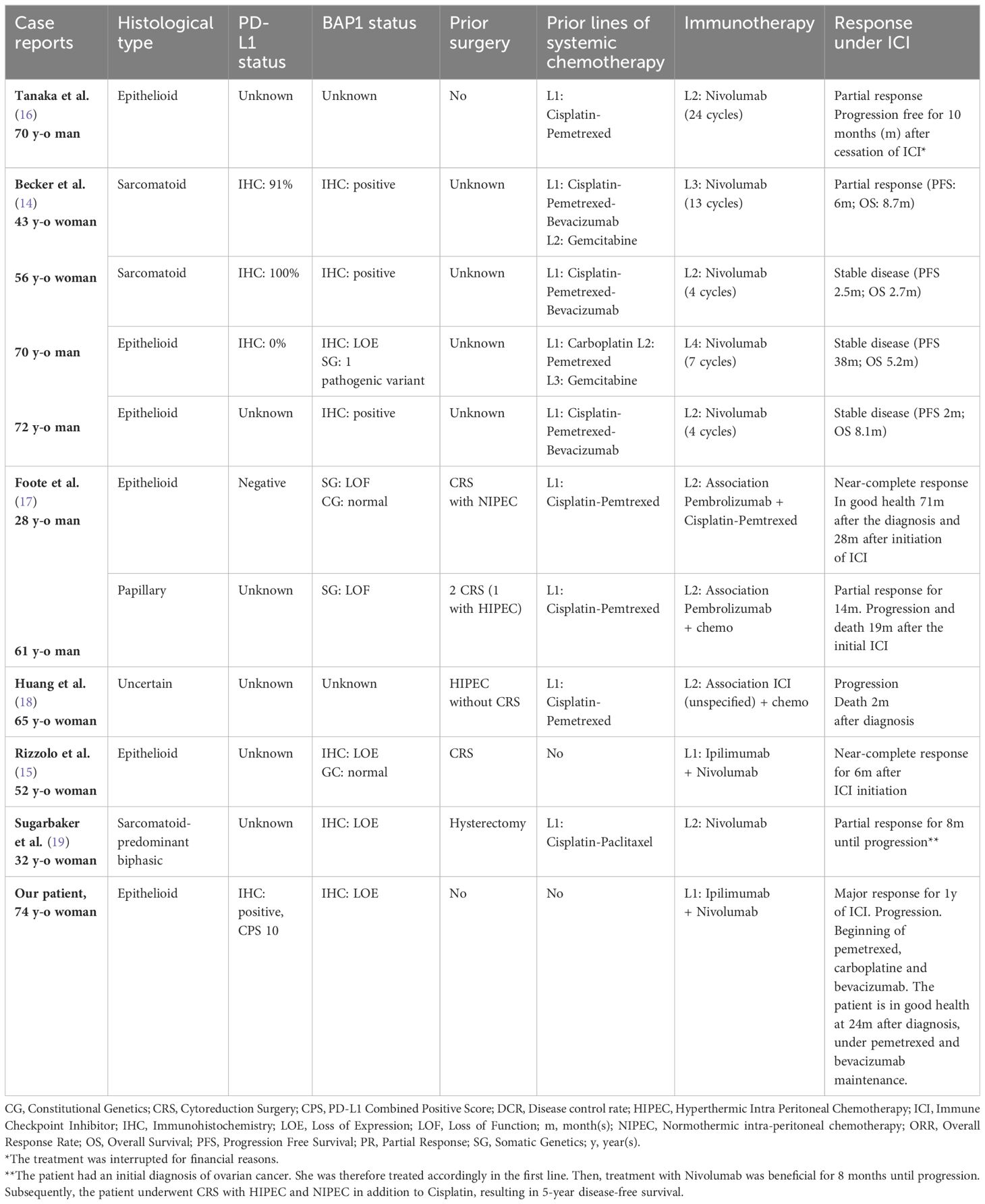
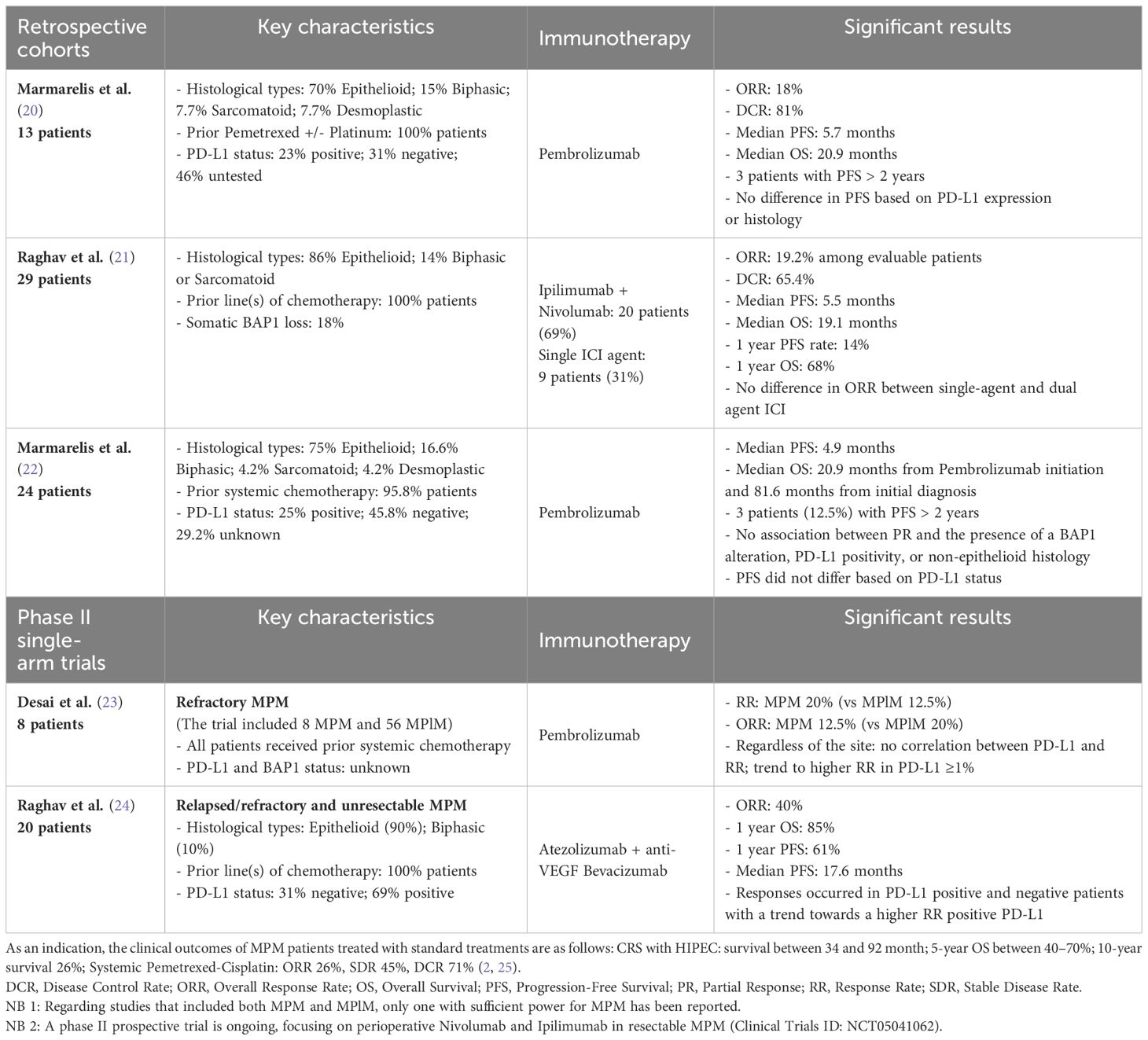
Here, we present a major and sustained 12-month response of a patient with MPM treated in the first line with nivolumab and ipilumumab. In 2022, Rizzolo et al. reported a near-complete response in a patient with resectable MPM treated in the first line with the combination of nivolumab and ipilimumab before and after surgery, suggesting a role for perioperative use of ICI in operable MPM cases (15). To our knowledge, we present the second reported case of dual immunotherapy used in first line in MPM with a major clinical response. Several studies have reported promising outcomes with the use of ICI in MPM following initial treatment. Table 1 provides an overview of these studies, confronting the main histological and molecular characteristics, including BAP1 and PD-L1 expression status when available, with clinical outcomes. Currently, a phase II prospective trial is ongoing, focusing on perioperative Nivolumab and Ipilimumab in resectable MPM (Clinical Trials ID: NCT05041062). Interestingly, our patient also presented an ongoing 10 month response to a subsequent chemotherapy regimen by pemetrexed, carboplatin and bevacizumab. The biological basis of this second major response is unknown, but a similar observation has been reported for paclitaxel and platinum salt after ICI failure in head and neck carcinoma (26).
In some solid tumors, such as advanced clear cell renal cell carcinoma, BAP1 alterations have been reported as a significant predictor of the immune microenvironment and have been associated with a longer progression-free survival (PFS) in patients when treated with ICI (27). BAP1-altered MPM are associated with a more inflammatory tumor microenvironment and seem to constitute a distinct immunogenic class, with possible implications for immunotherapeutic response (10, 11, 28, 29). In their study, Shrestha et al. found that BAP1-altered MPM had higher levels of immune checkpoint receptors (PD-1, PD-L1, and CTLA4) with higher cytokine secretion and an increased recruitment of T lymphocytes, leading to genomic instability and a DNA repair defect, compared to wild-type BAP1 MPM (10). In the reported case, the loss of BAP1 expression in IHC was identified, with no corresponding alteration observed in the BAP1 gene or its transcript. The absence of identified mutations in the BAP1 gene despite the loss of the protein may be explained by alterations in the BAP1 regulatory regions, such as the promoter and introns, through complex structural variants like promoter deletions that were not detected by the panel. Inactivation of BAP1 expression by BAP1 methylation, as has been observed in some uveal melanoma cases, could also be a contributing factor (30, 31). Other epigenomic alterations have been suggested to contribute to carcinogenesis in MPM (13). Indeed, Bozzi et al. have described alterations of epigenetic regulator genes that may affect BAP1 expression, such as EZH2, reporting a correlation between strong expression of EZH2 and the loss of BAP1 in MPM samples (13, 32). EZH2 mutations were specifically investigated in our patient’s tumor and were not detected. However, for investigating a potential correlation between EZH2 and BAP1 expression, analyzing EZH2 protein levels would provide more informative insights, given that its overexpression is primarily influenced by epigenetic, transcriptional, and post-transcriptional alterations. Loss of the BAP1 protein, despite the absence of alterations in the BAP1 gene and its transcript, has previously been reported in mesotheliomas and other BAP1-related neoplasms, including clear cell renal carcinoma and uveal melanoma. Proportions of IHC-negative cases associated with a wild-type BAP1 gene have ranged from 11% to 25% among the samples (33–35). Bott et al. identified such MPlM cases, characterized by normal BAP1 mRNA expression, suggesting the possibility of post-translational dysregulations leading to its loss-of-function (33, 36). Dysregulation of ubiquitination, which plays a role in the metabolic reprogramming of cancer cells, might be one such mechanism and has also been reported in certain cases of MPM (13). Regarding the genomic profile of our patient’s tumor, the absence of identified mutations in BAP1, coupled with the patient’s solitary neoplasm and the absence of a family history of BAP1-related tumors, make it highly unlikely that the origin of her tumor was constitutional. It is more probable that it resulted from acquired genomic and/or post-translational events leading to the loss of BAP1 expression, subsequent DNA repair defects, and genomic instability leading to a robust immune response in the microenvironment through the recruitment of cytokines and T lymphocytes. In this context, our patient’s MPM might align with a specific subgroup identified by Hiltbrunner et al. (BAP1 alteration without CDKN2A/B alteration) associated with a better prognosis (7). These findings are also consistent with the results of Osmanbeyoglu et al., who identified a distinct subgroup of MPlM characterized by specific immunogenicity, a PD-L1 response signature, and longer survival in patients with altered BAP1 alone, without abnormalities in CDKN2A/B or NF2 genes, suggesting that BAP1 loss alone could serve as a candidate marker for ICI therapy (29).
Our patient’s tumor had a low TMB, which is common in MPM (6). However, high TMB in some solid cancers (e.g., melanoma) is a biomarker of a favorable response to ICI through accumulation of neo-antigens from tumor mutations (37). Tumors with a low TMB can still trigger robust immune responses if their mutations lead to the expression of immunogenic neo-antigens. Solid tumor immunogenicity also relies on factors independent of mutational burden, including T-cell migration, PD-1 expression, cytokine balance, metabolic regulation, and BAP1 gene mutations in MPM (38–40). Hence, when considering ICI treatment for MPM, the presence of a low TMB should not preclude the assessment of PD-L1 and BAP1 expression status, which seem to be better indicators of lymphocyte infiltration than TMB. Moreover, in the absence of an increase in TMB, mutations of the SETDB1 gene in MPM (which have not been found in our patient’s tumor) also appear to be a potential marker of sensitivity to ICI, as observed in the patients reported by Becker et al. (14).
Significant clinical responses have been observed in patients with BAP1 loss and negative PD-L1 expression, as well as vice versa. Foote et al. reported a quasi-complete response in a 28-year-old MPM patient treated with Pembrolizumab and Cisplatin-Pemetrexed, despite negative PD-L1 expression (17). Phase II single-arm trials by Raghav et al. and Desai et al. also showed significant clinical responses to PD-L1 or PD-1 inhibitors, regardless of PD-L1 status, although PD-L1 positive patients tended to have a better response rate (21, 23). Similarly, retrospective cohort studies by Marmarelis et al. found ICI responses across various PD-L1 statuses (20, 22). Checkmate 743 trial results in MPlM did not find a significant difference in survival based on PD-L1 status in patients treated with nivolumab-ipilimumab (5). The Raghav et al. trial showed promising results with the Atezolizumab-Bevacizumab “AtezoBev” combination therapy in both PD-L1 positive and negative patients, with slightly higher response rates in PD-L1 positive patients (24). Ongoing phase II multi-arm stratified trial MiST (Mesothelioma Stratified Therapy, ClinicalTrials.gov Identifier: NCT03654833) is currently investigating the AtezoBev combination in MPlM with PD-L1 overexpression. These observations led to the hypothesis that a significant immune response may be triggered by anti-PD-1/anti-PD-L1 agents, even in cases with PD-L1-negative tumors. In this regard, Foote et al. documented the emergence of significant tumor immune mobilization mediated by CD4 and CD8+ T lymphocytes following the initiation of Pembrolizumab in their patient, resulting in near-complete response of their MPM, while maintaining a negative PD-L1 status (17).
Taken together, these observations underscore the importance of integrating multiple biomarkers to refine the predictions of response to ICI and to refine molecular signatures associated with an ICI response in MPM. In a 3-year follow-up study of the Checkmate 743 trial, Peters et al. tested a four-gene inflammatory signature (based on mRNA expressions of CD8A, STAT1, LAG3 and PD-L1), with a high score correlating with improved survival in patients treated with the nivolumab-ipilimumab combination (41–44). Further studies are needed to determine whether this signature could serve as a biomarker for ICI response in MPM. In the ongoing multi-arm stratified phase II trial MiST (ClinicalTrials.gov Identifier: NCT03654833) focusing on MPlM, patients with BRCA1 or BAP1 alterations receive a Poly (ADP-ribose) polymerase (PARP) inhibitor, while patients with positive PD-L1 expression receive the “AtezoBev” combination therapy. However, in their phase II trial, Ghafoor et al. found only limited efficacy of olaparib in 23 patients with MPlM and MPM harboring a BAP1 mutation (45). The effectiveness of anti-PARP therapy in BAP1-mutated MPM remains uncertain, and further studies involving a larger cohort of patients are warranted.
5 Conclusion
In conclusion, we present a major and sustained 1-year response in a non-resectable diffuse MPM treated with first-line dual ipilimumab and nivolumab. Our case suggests a potential role for ICI in non-resectable diffuse MPM cases exhibiting PD-L1 overexpression and loss of BAP1 expression, and instills new hope in their treatment. However, a cautious interpretation of these findings is needed, and response rates should not be extrapolated from this case report, as there is significant publication bias in this field. Refinement of molecular classification and identification of potential biomarkers of ICI response in MPM, including PD-L1 and BAP1 status, are imperative for patient stratification and to guide therapeutic decision-making.
Data availability statement
The original contributions presented in the study are included in the article and/or can be inquiried about directly from the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics committee of the Saint Quentin Hospital, Saint Quentin, France. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
M-FR: Writing – original draft. JM-P: Writing – review & editing. MF: Writing – review & editing. AO: Writing – review & editing. BD: Writing – review & editing. ID: Writing – review & editing. CD: Writing – review & editing. LG: Writing – review & editing. BC: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1410322/full#supplementary-material
References
1. Gregory SN, Sarvestani AL, Blakely AM. Malignant peritoneal mesothelioma literature review: past, present, and future. Dig Med Res. (2022) 5:29. doi: 10.21037/dmr
2. Carbone M, Adusumilli PS, Alexander HR, Baas P, Bardelli F, Bononi A, et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin. (2019) 69:402–29. doi: 10.3322/caac.21572
3. Sugarbaker PH, Chang D. Cytoreductive surgery plus HIPEC with and without NIPEC for Malignant peritoneal mesothelioma: A propensity-matched analysis. Ann Surg Oncol. (2021) 28:7109–17. doi: 10.1245/s10434-021-10048-4
4. Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. (2016) 387:1405–14. doi: 10.1016/S0140-6736(15)01238-6
5. Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable Malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. (2021) 397:375–86. doi: 10.1016/S0140-6736(20)32714-8
6. Alaklabi S, Roy AM, Skitzki JJ, Iyer R. Immunotherapy in Malignant peritoneal mesothelioma (Review). Mol Clin Oncol. (2023) 18:31. doi: 10.3892/mco
7. Hiltbrunner S, Fleischmann Z, Sokol ES, Zoche M, Felley-Bosco E, Curioni-Fontecedro A. Genomic landscape of pleural and peritoneal mesothelioma tumours. Br J Cancer. (2022) 127:1997–2005. doi: 10.1038/s41416-022-01979-0
8. Louie BH, Kurzrock R. BAP1: not just a BRCA1-associated protein. Cancer Treat Rev. (2020) 90:102091. doi: 10.1016/j.ctrv.2020.102091
9. Caporali S, Butera A, Amelio I. BAP1 in cancer: epigenetic stability and genome integrity. Discov. Oncol. (2022) 13:117. doi: 10.1007/s12672-022-00579-x
10. Shrestha R, Nabavi N, Lin YY, Mo F, Anderson S, Volik S, et al. BAP1 haploinsufficiency predicts a distinct immunogenic class of Malignant peritoneal mesothelioma. Genome Med. (2019) 11:8. doi: 10.1186/s13073-019-0620-3
11. Ladanyi M, Sanchez Vega F, Zauderer M. Loss of BAP1 as a candidate predictive biomarker for immunotherapy of mesothelioma. Genome Med. (2019) 11:18. doi: 10.1186/s13073-019-0631-0
12. Dietz MV, van Kooten JP, Paats MS, Aerts JGVJ, Verhoef C, Madsen EVE, et al. Molecular alterations and potential actionable mutations in peritoneal mesothelioma: a scoping review of high-throughput sequencing studies. ESMO Open. (2023) 8:101600. doi: 10.1016/j.esmoop.2023.101600
13. Fortarezza F, Pezzuto F, Marzullo A, Cavone D, Romano DE, d’Amati A, et al. Molecular pathways in peritoneal mesothelioma: A minireview of new insights. Front Oncol. (2022) 12:823839. doi: 10.3389/fonc.2022.823839
14. Becker O, Beaulaton C, Masliah-Planchon J, Servois V, Watson S. Nivolumab activity in advanced refractory Malignant peritoneal mesothelioma. Eur J Cancer. (2021) 144:386–8. doi: 10.1016/j.ejca.2020.11.024
15. Rizzolo A, Ah-Lan KC, Nu TNT, Alcindor T. Response to ipilimumab and nivolumab in a patient with Malignant peritoneal mesothelioma. Clin Colorectal Cancer. (2022) 21:371–4. doi: 10.1016/j.clcc.2022.08.001
16. Tanaka T, Miyamoto Y, Sakai A, Fujimoto N. Nivolumab for Malignant peritoneal mesothelioma. BMJ Case Rep. (2020) 13:e237721. doi: 10.1136/bcr-2020-237721
17. Foote MB, Shia J, Zauderer MG, Nash GM, Cercek A. Treatment of platinum non-responsive metastatic Malignant peritoneal mesothelioma with combination chemoimmunotherapy. J Immunother. (2022) 45:100–3. doi: 10.1097/CJI.0000000000000399
18. Huang X, Hong Y, Xie SY, Liao HL, Huang HM, Liu JH, et al. Malignant peritoneal mesothelioma with massive ascites as the first symptom: A case report. World J Clin Cases. (2022) 10:10317–25. doi: 10.12998/wjcc.v10.i28.10317
19. Sugarbaker PH. Response to Nivolumab followed by complete cytoreductive surgery with HIPEC resulted in long-term survival in a patient with sarcomatoid-predominant biphasic peritoneal mesothelioma. A Case Rep Int J Surg Case Rep. (2023) 107:108359. doi: 10.1016/j.ijscr.2023.108359
20. Marmarelis ME, Wang X, Roshkovan L, Walker S, McNulty S, Ciunci CA, et al. Real-world outcomes of pembrolizumab in peritoneal mesothelioma. JCO. (2020) 38:e21094. doi: 10.1200/JCO.2020.38.15_suppl.e21094
21. Raghav K, Liu S, Overman M, Morani A, Willette A, Fournier K, et al. Clinical efficacy of immune checkpoint inhibitors in patients with advanced Malignant peritoneal mesothelioma. JAMA Netw Open. (2021) 4:e2119934. doi: 10.1001/jamanetworkopen.2021.19934
22. Marmarelis ME, Wang X, Roshkovan L, Grady CB, Miura JT, Ginsberg MS, et al. Clinical outcomes associated with pembrolizumab monotherapy among adults with diffuse Malignant peritoneal mesothelioma. JAMA Netw Open. (2023) 6:e232526. doi: 10.1001/jamanetworkopen.2023.2526
23. Desai A, Karrison T, Rose B, Tan Y, Hill B, Pemberton E, et al. OA08.03 phase II trial of pembrolizumab (NCT02399371) in previously-treated Malignant mesothelioma (MM): final analysis. J Thorac Oncol. (2018) 13:S339. doi: 10.1016/j.jtho.2018.08.277
24. Raghav K, Liu S, Overman MJ, Willett AF, Knafl M, Fu SC, et al. Efficacy, safety, and biomarker analysis of combined PD-L1 (Atezolizumab) and VEGF (Bevacizumab) blockade in advanced Malignant peritoneal mesothelioma. Cancer Discov. (2021) 11:2738–47. doi: 10.1158/2159-8290.CD-21-0331
25. Greenbaum A, Alexander HR. Peritoneal mesothelioma. Transl Lung Cancer Res. (2020) 9:S120–32. doi: 10.21037/tlcr
26. Tanaka H, Enokida T, Okano S, Fujisawa T, Tanaka N, Takeshita N, et al. Subsequent chemotherapy with paclitaxel plus cetuximab-based chemotherapy following immune checkpoint inhibitor in recurrent or metastatic squamous cell carcinoma of the head and neck. Front Oncol. (2023) 13:1221352. doi: 10.3389/fonc.2023.1221352
27. Liu K, Huang Y, Xu Y, Wang G, Cai S, Zhang X, et al. BAP1-related signature predicts benefits from immunotherapy over VEGFR/mTOR inhibitors in ccRCC: a retrospective analysis of JAVELIN Renal 101 and checkmate-009/010/025 trials. Cancer Immunol Immunother. (2023) 72:2557–72. doi: 10.1007/s00262-023-03424-4
28. Perrino M, De Vincenzo F, Cordua N, Borea F, Aliprandi M, Santoro A, et al. Immunotherapy with immune checkpoint inhibitors and predictive biomarkers in Malignant mesothelioma: Work still in progress. Front Immunol. (2023) 14:1121557. doi: 10.3389/fimmu.2023.1121557
29. Osmanbeyoglu HU, Palmer D, Sagan A, Sementino E, Becich MJ, Testa JR. Isolated BAP1 genomic alteration in Malignant pleural mesothelioma predicts distinct immunogenicity with implications for immunotherapeutic response. Cancers. (2022) 14:5626. doi: 10.3390/cancers14225626
30. Gentien D, Saberi-Ansari E, Servant N, Jolly A, de la Grange P, Némati F, et al. Multi-omics comparison of Malignant and normal uveal melanocytes reveals molecular features of uveal melanoma. Cell Rep. (2023) 42:113132. doi: 10.1016/j.celrep.2023.113132
31. Bakhoum MF, Curtis EJ, Goldbaum MH, Mischel PS. BAP1 methylation: a prognostic marker of uveal melanoma metastasis. NPJ Precis Oncol. (2021) 5:89. doi: 10.1038/s41698-021-00226-8
32. Bozzi F, Brich S, Dagrada GP, Negri T, Conca E, Cortelazzi B, et al. Epithelioid peritoneal mesothelioma: a hybrid phenotype within a mesenchymal-epithelial/epithelial-mesenchymal transition framework. Oncotarget. (2016) 7:75503–17. doi: 10.18632/oncotarget.v7i46
33. Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in Malignant pleural mesothelioma. Nat Genet. (2011) 43:668–72. doi: 10.1038/ng.855
34. Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, Leng N, Pavía-Jiménez A, Wang S, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. (2012) 44:751–9. doi: 10.1038/ng.2323
35. Farquhar N, Thornton S, Coupland SE, Coulson JM, Sacco JJ, Krishna Y, et al. Patterns of BAP1 protein expression provide insights into prognostic significance and the biology of uveal melanoma. J Pathol CR. (2018) 4:26–38. doi: 10.1002/cjp2.86
36. Masclef L, Ahmed O, Estavoyer B, Larrivée B, Labrecque N, Nijnik A, et al. Roles and mechanisms of BAP1 deubiquitinase in tumor suppression. Cell Death Differ. (2021) 28:606–25. doi: 10.1038/s41418-020-00709-4
37. Ning B, Liu Y, Wang M, Li Y, Xu T, Wei Y. The predictive value of tumor mutation burden on clinical efficacy of immune checkpoint inhibitors in melanoma: A systematic review and meta-analysis. Front Pharmacol. (2022) 13:748674. doi: 10.3389/fphar.2022.748674
38. Jardim DL, Goodman A, Gagliato D de M, Kurzrock R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell. (2021) 39:154–73. doi: 10.1016/j.ccell.2020.10.001
39. Vareki SM. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immunother Cancer. (2018) 6:157. doi: 10.1186/s40425-018-0479-7
40. Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. Cancer immunotherapy targets based on understanding the T cell-inflamed versus non-T cell-inflamed tumor microenvironment. Adv Exp Med Biol. (2017) 1036:19–31. doi: 10.1007/978-3-319-67577-0_2
41. Peters S, Scherpereel A, Cornelissen R, Oulkhouir Y, Greillier L, Kaplan MA, et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable Malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol. (2022) 33:488–99. doi: 10.1016/j.annonc.2022.01.074
42. Hodi FS, Wolchok JD, SChadendorf D, Larkin J, Long GV, Qian X, et al. TMB and inflammatory gene expression associated with clinical outcomes following immunotherapy in advanced melanoma. Cancer Immunol Res. (2021) 9:1202–13. doi: 10.1158/2326-6066.CIR-20-0983
43. Lei M, Siemers NO, Pandya D, Chang H, Sanchez T, Harbison C, et al. Analyses of PD-L1 and inflammatory gene expression association with efficacy of nivolumab ± Ipilimumab in gastric cancer/gastroesophageal junction cancer. Clin Cancer Res. (2021) 27:3926–35. doi: 10.1158/1078-0432.CCR-20-2790
44. Sangro B, Melero I, Wadhawan S, Finn RS, Abou-Alfa GK, Cheng AL, et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J Hepatol. (2020) 73:1460–9. doi: 10.1016/j.jhep.2020.07.026
Keywords: peritoneal mesothelioma, nivolumab, ipilimumab, BAP1, PD-L1, molecular stratification, immune checkpoint inhibitors
Citation: Reveneau M-F, Masliah-Planchon J, Fernandez M, Ouikene A, Dron B, Dadamessi I, Dayen C, Golmard L and Chauffert B (2024) Major response of a peritoneal mesothelioma to nivolumab and ipilimumab: a case report, molecular analysis and review of literature. Front. Oncol. 14:1410322. doi: 10.3389/fonc.2024.1410322
Received: 31 March 2024; Accepted: 05 July 2024;
Published: 18 July 2024.
Edited by:
Chiara Porta, University of Eastern Piedmont, ItalyReviewed by:
Michael G. White, University of Texas MD Anderson Cancer Center, United StatesDavid Morris, University of New South Wales, Australia
Copyright © 2024 Reveneau, Masliah-Planchon, Fernandez, Ouikene, Dron, Dadamessi, Dayen, Golmard and Chauffert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bruno Chauffert, Yi5jaGF1ZmZlcnRAY2gtc3RxdWVudGluLmZy
 Marie-Florence Reveneau
Marie-Florence Reveneau Julien Masliah-Planchon
Julien Masliah-Planchon Manuel Fernandez3
Manuel Fernandez3