- 1Department of Paediatrics, Shanxi Medical University, Taiyuan, China
- 2Department of Second Clinical Medical College, Shanxi Medical University, Taiyuan, China
- 3Department of Radiology, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China
Purpose: This meta-analysis is conducted to evaluate the comparative diagnostic efficacy of 68Ga-PSMA PET vs. mpMRI in detecting local staging of prostate cancer(PCa).
Methods: A comprehensive search was conducted in the PubMed and Embase databases to identify publications up to February 2024. The analysis included studies that evaluated the direct comparison of 68Ga-PSMA PET and mpMRI for local staging of prostate cancer. The reliability of the analyzed studies was evaluated using the QUADAS-2 tool.
Results: The meta-analysis included 10 articles involving 505 patients, which revealed that both 68Ga-PSMA PET and mpMRI had similar sensitivities and specificities in detecting extracapsular extension(ECE) and seminal vesicle invasion(SVI). The sensitivities for ECE were 0.56 (95% CI: 0.41-0.71) for 68Ga-PSMA PET and 0.57 (95% CI: 0.43-0.71) for mpMRI, and specificities were both 0.84 (68Ga-PSMA PET 95% CI: 0.75-0.91, mpMRI 95% CI: 0.76-0.91).For SVI, sensitivities were 0.57 (95% CI: 0.46-0.68) for 68Ga-PSMA PET and 0.70 (95% CI: 0.60-0.80) for mpMRI, with specificities of 0.92 (95% CI: 0.86-0.96) for 68Ga-PSMA PET and 0.94 (95% CI: 0.89-0.98) for mpMRI. There were no notable variations in sensitivity or specificity between the two methods for detecting ECE and SVI (P = 0.89 and 0.93 for ECE, 0.09 and 0.57 for SVI).
Conclusions: This meta-analysis indicates that 68Ga-PSMA PET has similar sensitivity and specificity to mpMRI in local prostate cancer staging. Nevertheless, the limited study sample size calls for further, larger prospective studies to validate these findings.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=522438, identifier CRD42024522438.
1 Introduction
Prostate cancer is a major public health issue, being among the most prevalent cancers in males globally (1).Around 30% of people diagnosed with prostate cancer undergo curative treatment, yet between 20-50% encounter biochemical recurrence within ten years (2). Timely diagnosis is crucial for improving survival rates by extending life expectancy, especially in detecting extracapsular extension and seminal vesicle invasion (3).
Traditional diagnostic tools for PCa, including computed tomography (CT), ultrasound, and histopathological biopsy, have been the mainstay in clinical practice (4, 5). However, these modalities present limitations in sensitivity and specificity, particularly in detecting extracapsular extension and seminal vesicle invasion (6). CT and ultrasonography demonstrate constrained resolution capabilities when identifying minuscule anatomical structures and discerning subtle tissue contrasts, frequently resulting in the oversight of incipient stages of ECE and SVI in prostate cancer (7). Conversely, while pathology biopsies provide a more direct approach, they may overlook areas of ECE/SVI that are not included in the sample and are susceptible to errors inherent in the sampling process (8). Their efficacy in accurately staging PCa, especially in advanced cases, is often constrained, leading to potential underestimation of the disease (4).
Prostate-Specific Membrane Antigen(PSMA), a protein found in abundance on the exterior of prostate cancer cells, is a crucial focus for both diagnosing and treating prostate cancer (9, 10). PET scans that target PSMA, particularly those utilizing the 68Ga-labeled PSMA ligand, are frequently used in prostate cancer imaging (11). 68Ga-PSMA PET is known for its high sensitivity in detecting PCa cells, whereas mpMRI excels in detailed soft-tissue characterization (12, 13). Despite their individual strengths, a comparative analysis of their effectiveness in the local staging of PCa, especially in discerning ECE and SVI, is not well-established due to a lack of comprehensive head-to-head studies (14).
To fill this knowledge void, a systematic review and meta-analysis will be conducted to compare the diagnostic precision of 68Ga-PSMA PET and mpMRI for local staging of PCa. The goal is to assess their effectiveness in identifying ECE and SVI, offering a detailed insight into their contributions to PCa treatment and assisting healthcare providers in selecting the optimal diagnostic approach.
2 Methods
The meta-analysis adhered to the PRISMA-DTA guidelines for reporting systematic reviews and meta-analyses of diagnostic test accuracy (15). The protocol for this meta-analysis has been registered with PROSPERO under registration number CRD42024522438.
2.1 Search strategy
A thorough investigation was carried out in the PubMed and Embase repositories to locate existing publications until February 2024.The search utilized the keywords ‘68Ga-PSMA PET’, ‘mpMRI’, ‘PSMA’, and ‘Prostate Cancer’. Additional information can be found in Supplementary Table 1. The identification of relevant articles was done through manual searches of the reference lists in the included studies.
2.2 Inclusion and exclusion criteria
For studies to be eligible for inclusion in this meta-analysis, they needed to evaluate the diagnostic accuracy of 68Ga-PSMA PET and mpMRI in determining the local staging of prostate cancer. The included studies had to meet specific criteria: (1) population: patients with prostate cancer undergoing local staging; (2) interventions: use of 68Ga-PSMA PET; (3) comparators: use of mpMRI; (4) outcomes: sensitivity and specificity; and (5) study type: retrospective studies and prospective studies.
Articles that were duplicates, lacked full texts, consisted of editorials, letters, case reports, reviews, meta-analyses, items with irrelevant titles or abstracts, and publications in languages other than English were excluded. Additionally, studies without enough data to calculate sensitivity or specificity of the imaging technique being studied were also excluded.
2.3 Quality assessment
Two researchers assessed the studies’ quality using the QUADAS-2 tool (16, 17), which evaluates diagnostic performance in four main areas: patient selection, index test, reference standard, and flow and timing. Each study was categorized as having high, low, or unclear bias risk.
2.4 Data extraction
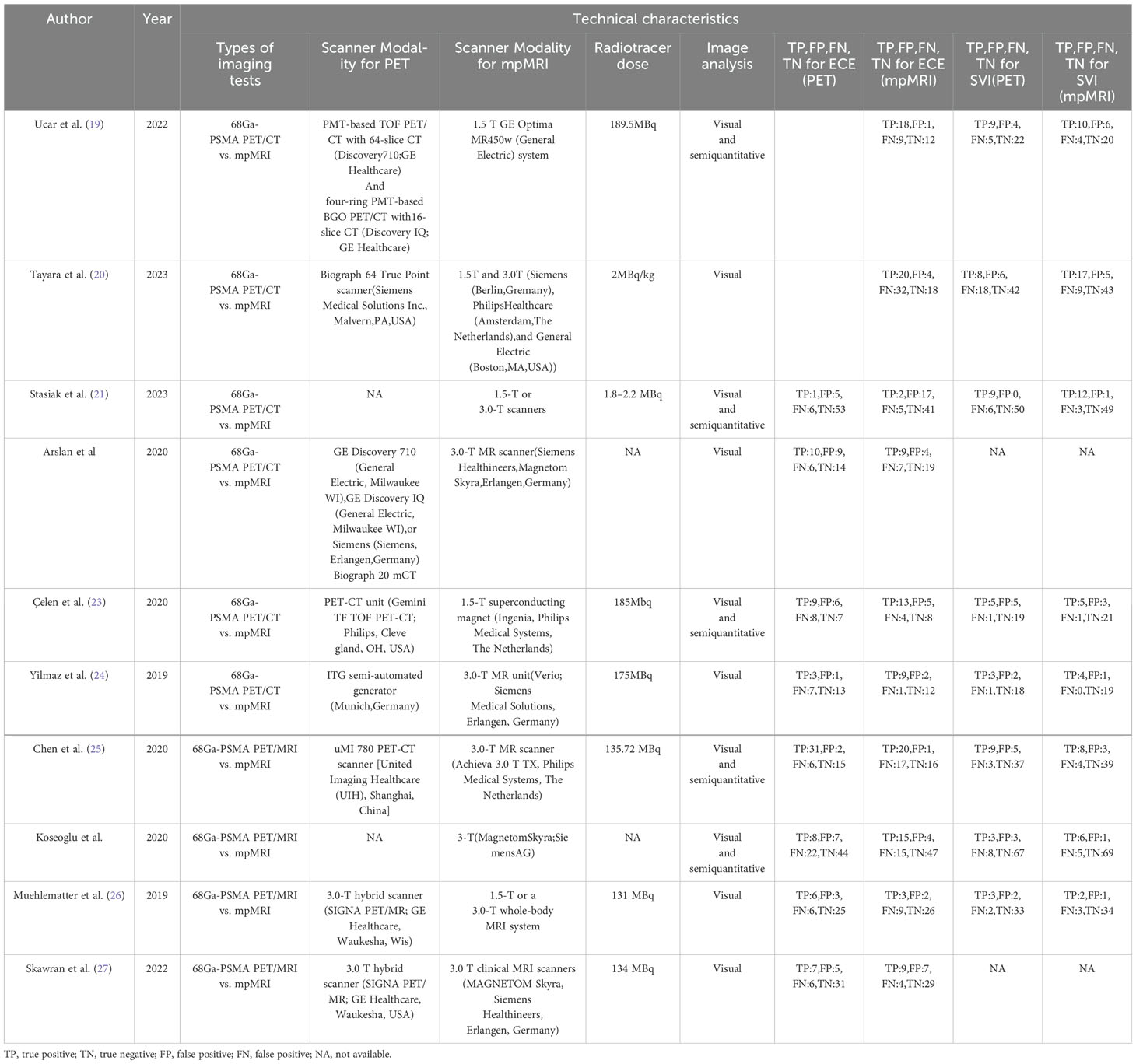
Data extraction for all included papers was carried out independently by two researchers. The data that were extracted included: (1) the author; (2) year of publication; (3) study characteristics including country, study design, analysis, outcome; (4) patient characteristics including number of patients, PSA level, mean/median age, Gleason score, reference standard; (5) technical characteristics including types of imaging tests, scanner modality for PET, scanner modality for mpMRI, radiotracer dose, image analysis, image analysis, TP, FP, FN, TN for ECE(PET), TP, FP, FN, TN for ECE(mpMRI), TP, FP, FN, TN for SVI(PET), TP, FP, FN, TN for SVI (mpMRI).
2.5 Statistical analysis
The DerSimonian and Laird method was utilized for random-effects meta-analysis to evaluate sensitivities and specificities, accounting for variability between studies. Sensitivities and specificities were subsequently converted using the Freeman-Tukey double inverse sine transformation to stabilize the variance of proportion data and improve the reliability of pooled estimates (18). Confidence intervals were determined using the Jackson technique. Heterogeneity within and across groups was assessed utilizing the Cochrane Q and I² statistics. If significant diversity was observed among the studies (P<0.10 or I2 > 50%), a sensitivity analysis was performed to determine the reasons for the discrepancies.
Funnel plots and Egger’s test were used to evaluate publication bias. Statistical tests had to reach a significance level of P < 0.05. Statistical analysis and graphical representation were conducted using R software version 4.3.2.
3 Results
3.1 Study selection
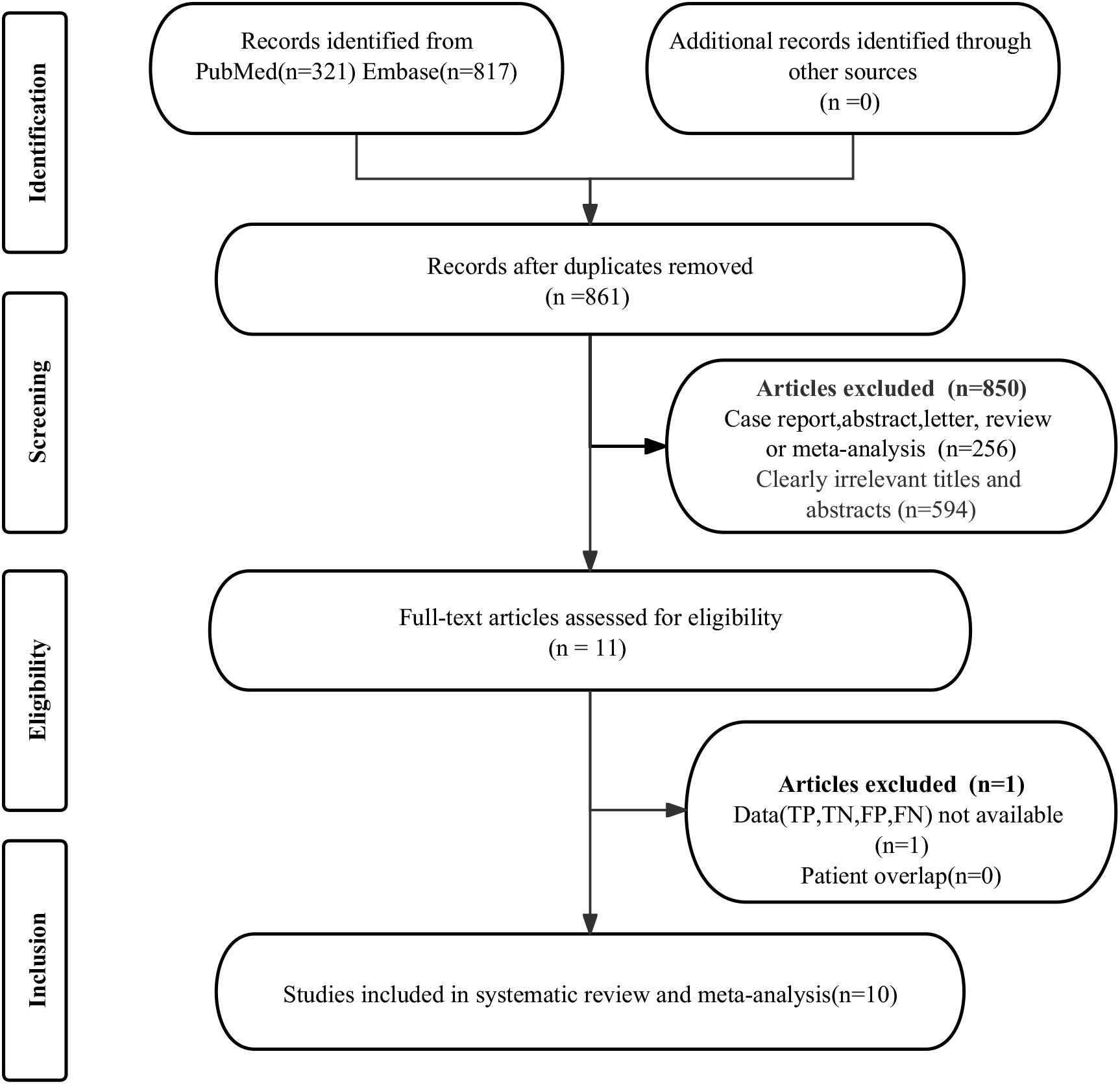
The initial search found a total of 1138 published works. However, 277 studies were duplicates, and 850 were considered ineligible and removed from further review. After evaluating the full manuscripts of the remaining 11 studies, one was disqualified for missing critical data (TP, FP, FN, and TN). Ultimately, the meta-analysis included 10 studies (19–28) that evaluated the diagnostic efficacy of 68Ga-PSMA PET and mpMRI. The article selection process, as detailed in the PRISMA flowchart (29), is depicted in Figure 1.
3.2 Assessment of study description and quality
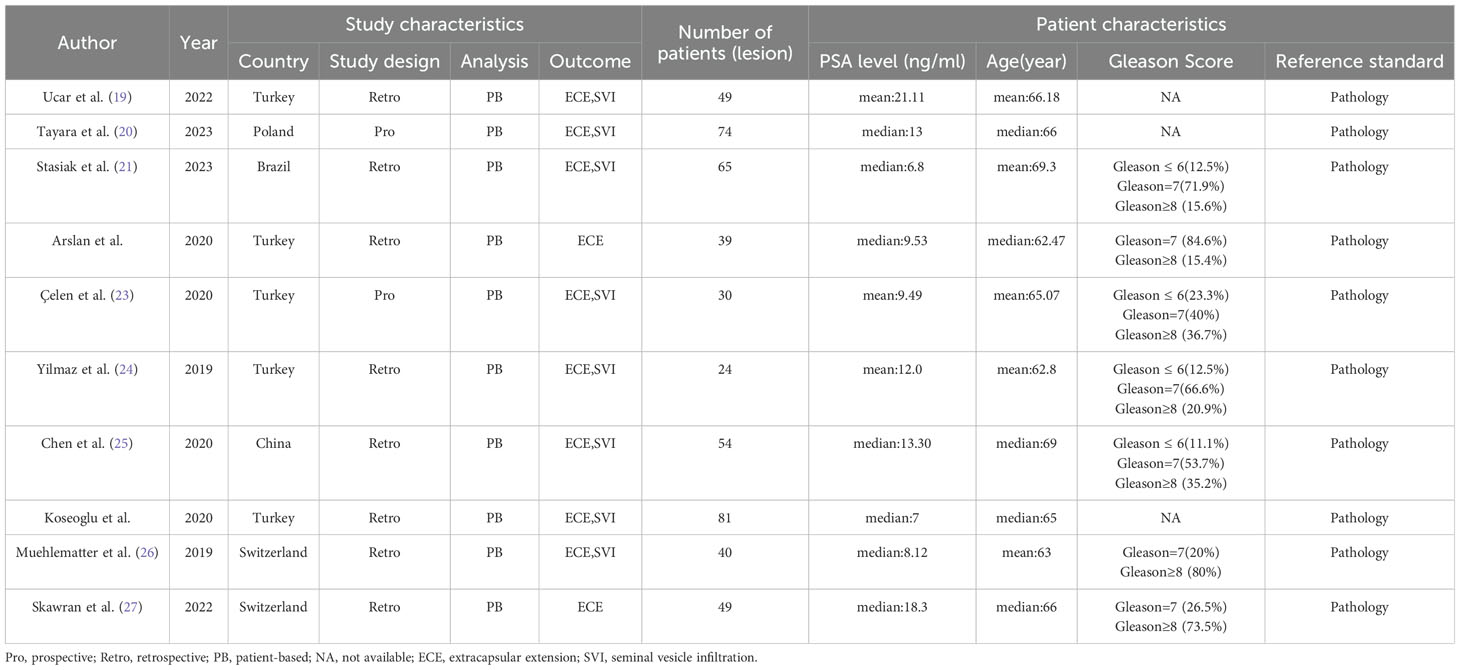
The 10 qualifying studies included 505 patients diagnosed with prostate cancer, aged between 24 and 81. Out of these studies, 8 were retrospective (19, 21, 22, 24–28), while 2 were prospective (20, 23). All 10 studies used patient-based analyses, consistently referring to pathology as the standard for analysis. 8 studies (21–27) provided data on the sensitivity and specificity of two diagnostic tools for extracapsular extension. Furthermore, 8 studies included statistics on the sensitivity and specificity for seminal vesicle invasion (19–21, 23–26, 28). The details of the study and methodology for 68Ga-PSMA PET and mpMRI were outlined in Tables 1, 2.
Figure 2 displays the potential bias in each study as determined by the QUADAS-2 tool. Eight studies were deemed ‘unclear’ in terms of patient selection due to a lack of information on the inclusion of consecutive patients, indicating potential selection bias. All 10 studies received an ‘unclear’ rating for the index test because visual grading thresholds could not be identified, raising concerns about detection bias. Regarding the reference standard, all 10 studies were also rated as ‘unclear’ due to uncertainty about whether the final diagnosis was independently made by two or more doctors, which may impact the reliability of the reference standard. Seven studies were classified as ‘unclear’ in terms of flow and timing criteria due to uncertainty about the correct timing between the diagnostic test and the gold standard evaluation, which could introduce variability in diagnostic accuracy. Overall, while these potential biases may have some impact, the quality of the included literature is generally acceptable.
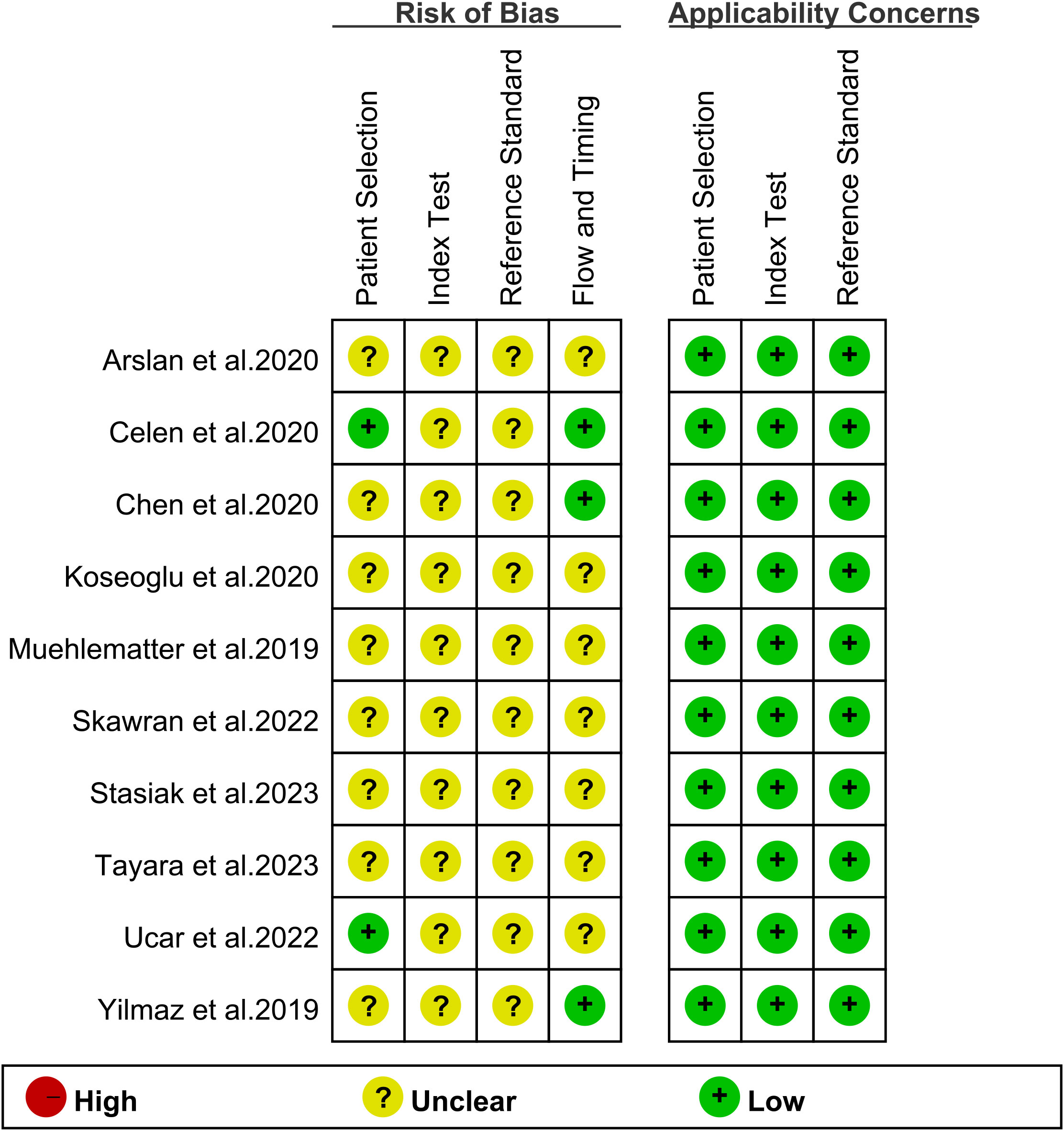
Figure 2. Evaluation of study reliability and relevance using QUADAS-2 for diagnostic accuracy assessments.
3.3 Comparing the sensitivity of 68Ga-PSMA PET and mpMRI in identifying extracapsular extension in prostate cancer
The evaluation comprised 8 research studies, with a sensitivity of 0.56 (95% CI:0.41-0.71) in identifying ECE of prostate cancer through 68Ga-PSMA PET, whereas mpMRI demonstrated an overall sensitivity of 0.57 (95% CI:0.43-0.71) (Figure 3). There was no notable significant difference in sensitivity between 68Ga-PSMA PET and mpMRI (P = 0.89) (Figure 3).
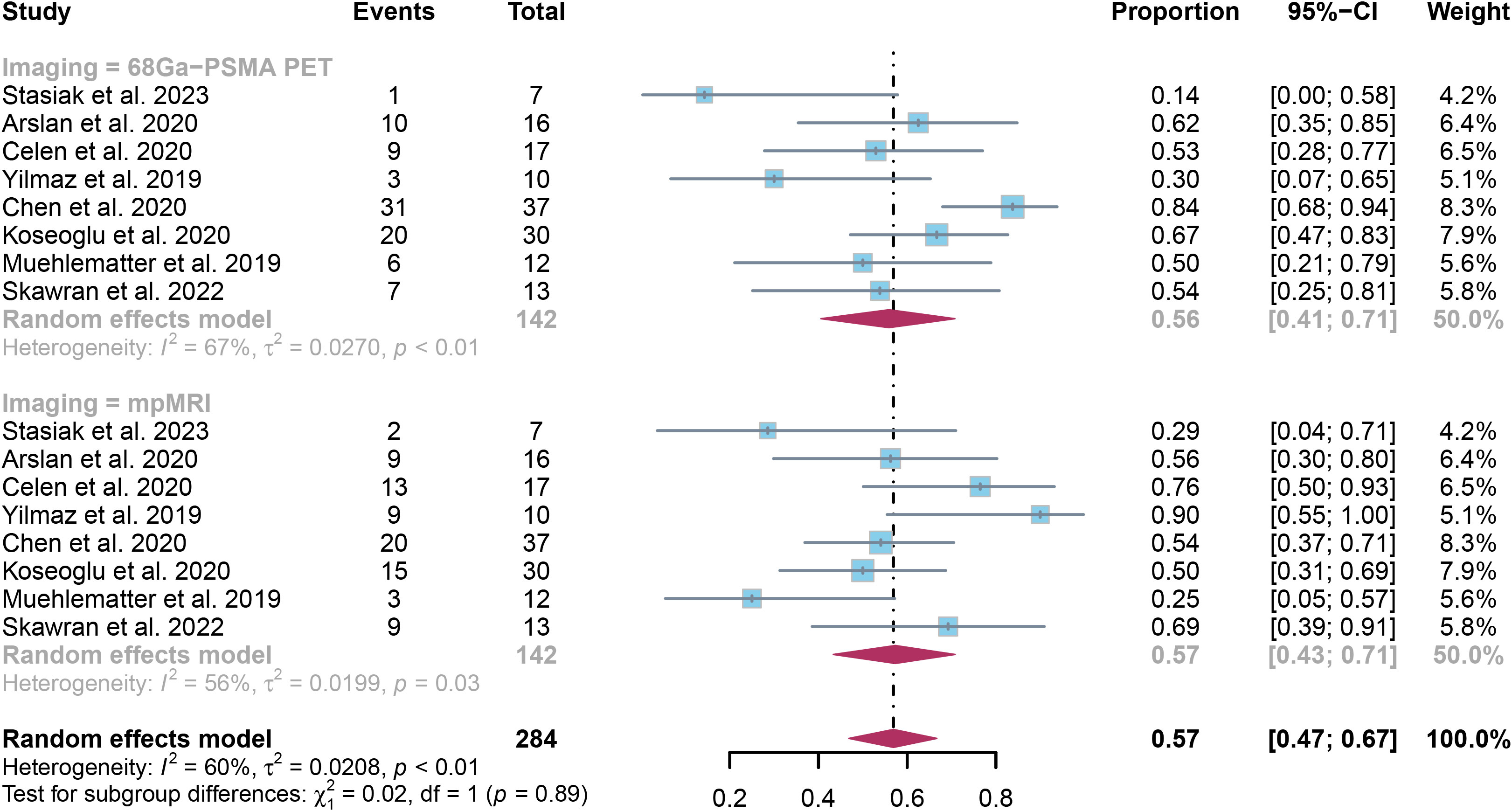
Figure 3. Forest plot showing the pooled sensitivities of 68Ga-PSMA PET and mpMRI in extracapsular extension of prostate cancer patients.
The overall sensitivity of 68Ga-PSMA PET and mpMRI demonstrated I2 percentages of 67% and 56% correspondingly. Upon leave-one-out sensitivity analysis, the I2 percentage for 68Ga-PSMA PET dropped to 33% when Chen’s study was omitted, indicating it may have caused heterogeneity. Likewise, excluding Yilmaz’s or Muehlematter’s study led to reduced I2 percentages for mpMRI, reaching 42% and 44% respectively, suggesting each study could have contributed to heterogeneity (Supplementary Figures 1, 2).
3.4 Comparing the specificity of 68Ga-PSMA PET and mpMRI in detecting extracapsular extension of prostate cancer
The examination revealed that the specificity was 0.84 (95% CI:0.75-0.91), with mpMRI demonstrating a comparable specificity of 0.84 (95% CI:0.76-0.91). There was no notable difference in overall specificity between 68Ga-PSMA PET and mpMRI (P = 0.93) (Figure 4).
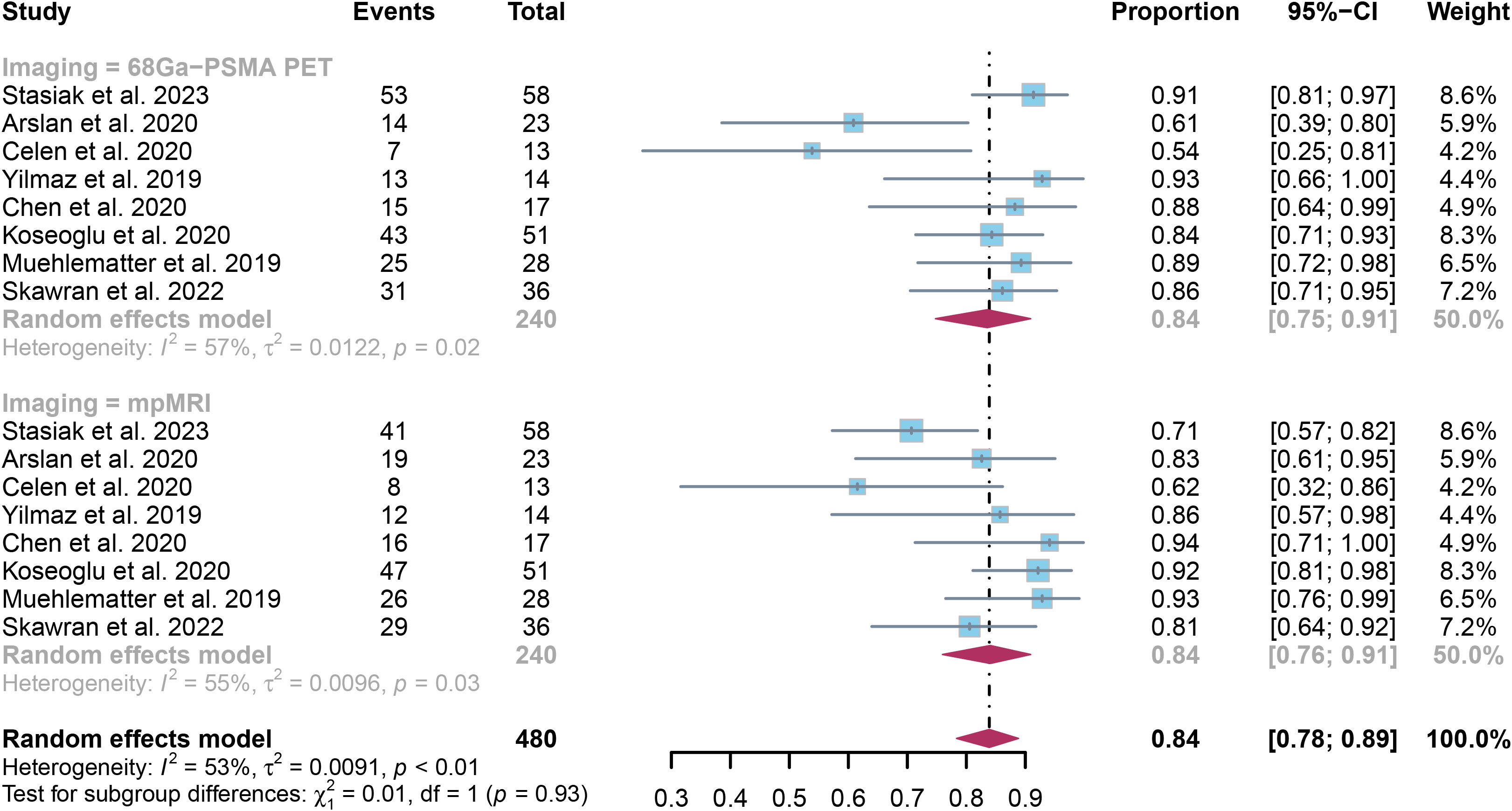
Figure 4. Forest plot showing the pooled specificities of 68Ga-PSMA PET and mpMRI in extracapsular extension of prostate cancer patients.
The specificity of PET and mpMRI demonstrated I² percentages of 57% and 55%, respectively. Upon further leave-one-out sensitivity analysis, it was found that excluding either Arslan’s or Celen’s research decreased the I² value for 68Ga-PSMA PET to 34% and 40%, respectively, indicating they may be sources of heterogeneity. Similarly, the removal of Stasiak’s or Koseoglu’s study led to a reduction in I² values for mpMRI to 34% and 45%, respectively, highlighting their potential impact on heterogeneity. (Supplementary Figures 3, 4).
3.5 Comparing the sensitivity of 68Ga-PSMA PET and mpMRI in identifying seminal vesicle invasion in prostate cancer
The analysis of 8 research studies revealed that 68Ga-PSMA PET had a sensitivity of 0.57 (95% CI:0.46-0.68) for detecting SVI in prostate cancer, while mpMRI had a sensitivity of 0.70 (95% CI:0.60-0.80), as shown in Figure 5. There was no significant difference in sensitivity between the two imaging methods (P = 0.09) (Figure 5).
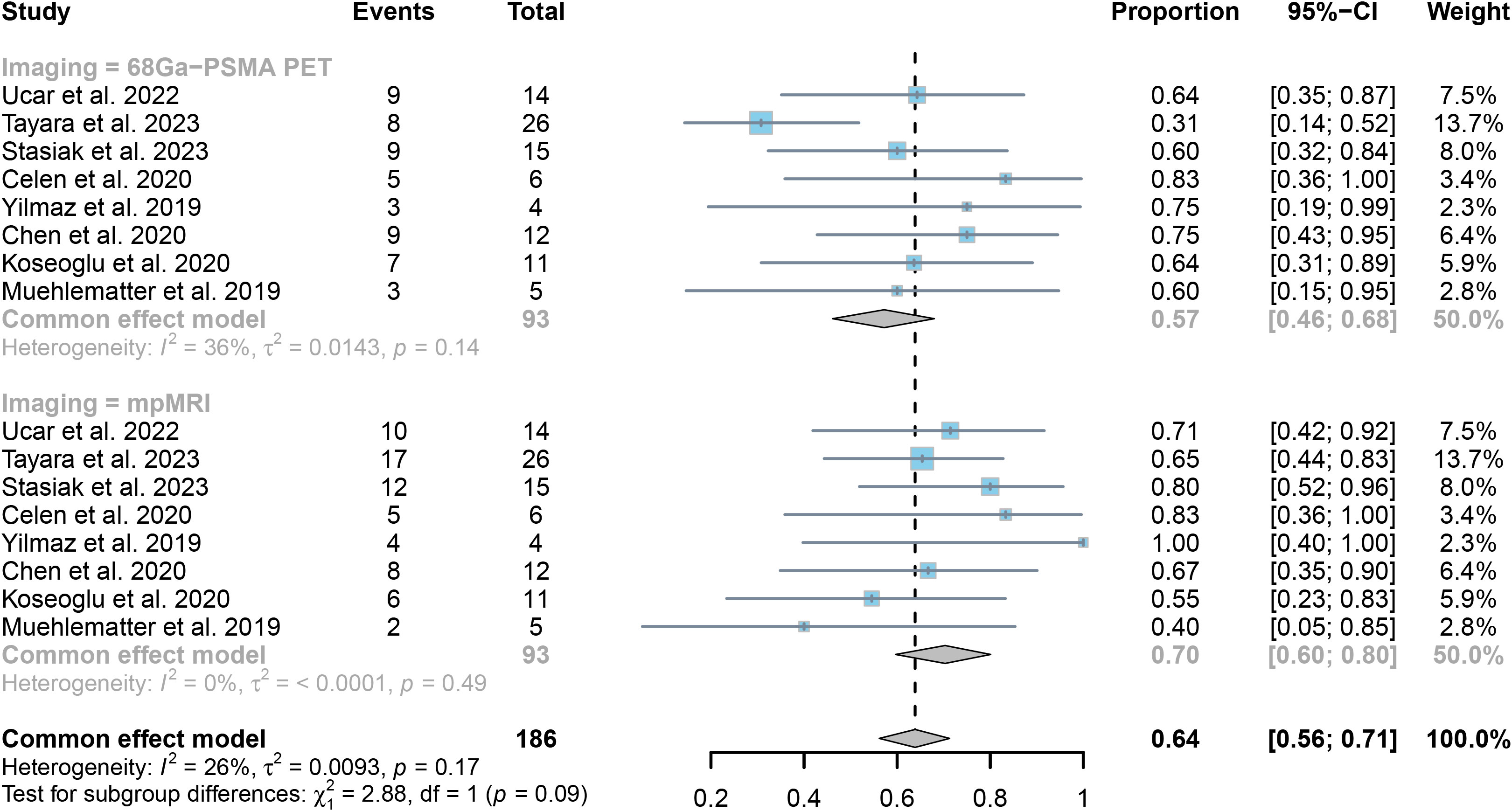
Figure 5. Forest plot showing the pooled sensitivities of 68Ga-PSMA PET and mpMRI in seminal vesicle invasion of prostate cancer patients.
The collective responsiveness of PET and mpMRI indicated I2 values of 36% and 0%, respectively, indicating that both techniques exhibit acceptable heterogeneity in identifying SVI.
3.6 Comparing the specificity of 68Ga-PSMA PET and mpMRI in detecting seminal vesicle invasion in prostate cancer patients
Analysis of 8 studies with 417 patients found that 68Ga-PSMA PET had an specificity of 0.92 (95% CI:0.86-0.96) in detecting SVI in prostate cancer, while mpMRI had an specificity of 0.94 (95% CI:0.89-0.98), as illustrated in Figure 6. The methods did not show any significant difference in specificity, with a P value of 0.57.
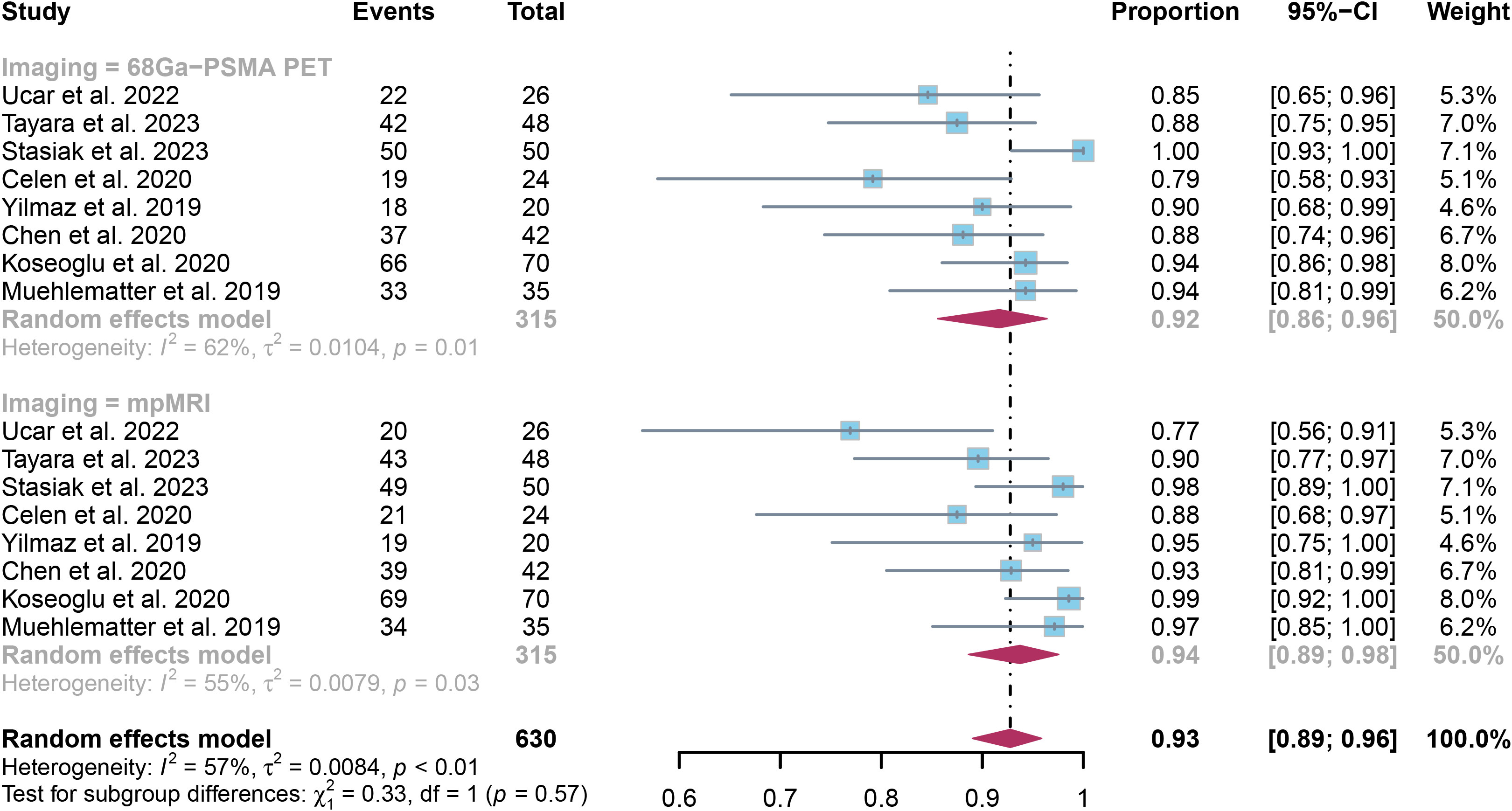
Figure 6. Forest plot showing the pooled specificities of 68Ga-PSMA PET and mpMRI in seminal vesicle invasion of prostate cancer patients.
The overall specificity of 68Ga-PSMA PET and mpMRI showed I² values of 62% and 55%, in that order. Upon leave-one-out sensitivity analysis, the I2 value for 68Ga-PSMA PET dropped to 0% when excluding Stasiak’s study, indicating it may be a significant factor contributing to heterogeneity. For mpMRI, after omitting Ucar’s study or Koseoglu’s study, the I2 value reduced to 27% and 44%, respectively.(Supplementary Figures 5, 6)
3.7 Examining the sensitivity and specificity of various 68Ga-PSMA PET imaging techniques (PET/CT and PET/MRI) and mpMRI in identifying extracapsular extension and seminal vesicle invasion in cases of prostate cancer
The information in Table 3 outlines the effectiveness of 68Ga-PSMA PET/CT and mpMRI in detecting ECE in prostate cancer, with a collective sensitivity of 0.43 (95% CI:0.24-0.64) and 0.66 (95% CI:0.41-0.87) respectively. There was no significant difference in sensitivity between these methods (P = 0.17). Additionally, 68Ga-PSMA PET/MRI demonstrated a combined sensitivity of 0.67 (95% CI:0.50-0.82), while mpMRI had a sensitivity of 0.51 (95% CI:0.40-0.62). This difference in sensitivity was also not significant (P = 0.12).
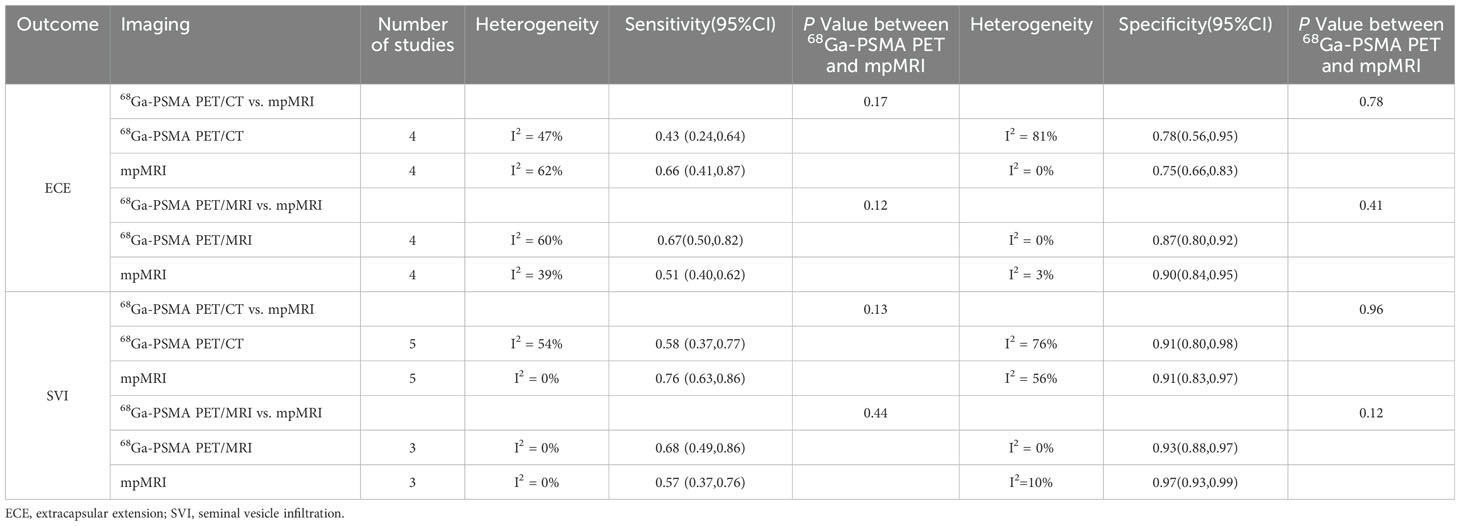
Table 3. Subgroup analysis based on 68Ga-PSMA PET/CT vs. mpMRI and 68Ga-PSMA PET/MRI vs. mpMRI in detecting local staging for prostate cancer.
It is demonstrated that 68Ga-PSMA PET/CT had a combined specificity of 0.78 (95% CI:0.56-0.95) for assessing ECE specificity, while mpMRI had a specificity of 0.75 (95% CI:0.66-0.83). The specificity difference between these techniques was not statistically significant (P = 0.78). Additionally, 68Ga-PSMA PET/MRI exhibited a combined specificity of 0.87 (95% CI:0.80-0.92), compared to 0.90 (95% CI: 0.84-0.95) for mpMRI. This specificity difference was also not statistically significant (P = 0.41).
We found that 68Ga-PSMA PET/CT had a sensitivity of 0.58 (95% CI:0.37-0.77) for detecting SVI in prostate cancer, while mpMRI had a sensitivity of 0.76 (95% CI:0.63-0.86). The difference in sensitivity was not considered statistically significant (P = 0.13). Additionally, the sensitivity of 68Ga-PSMA PET/MRI was 0.68 (95% CI:0.49-0.86) compared to mpMRI’s 0.57 (95% CI:0.37-0.76), with no significant difference observed (P = 0.44).
Our analysis of SVI specificity revealed that 68Ga-PSMA PET/CT and mpMRI both had a specificity of 0.91 (95% CI:0.80-0.98 and 0.83-0.97, respectively). The difference in specificity between these imaging techniques was not significant (P = 0.96). Additionally, 68Ga-PSMA PET/MRI had a specificity of 0.93 (95% CI: 0.88-0.97) while mpMRI had a specificity of 0.97 (95% CI:0.93-0.99), with no statistically significant difference in specificity (P = 0.12). All data are presented in Table 3.
3.8 Publication bias in 68Ga-PSMA PET and mpMRI in the identification of extracapsular extension and seminal vesicle invasion in cases of prostate cancer
The analysis of funnel plot asymmetry showed that there was no significant publication bias detected for the majority of outcomes, as all Egger’s test findings were above 0.05.Nevertheless, there were signs of bias in the publication of data regarding the sensitivity of 68Ga-PSMA PET in identifying ECE and SVI, as shown by Egger’s test results of below 0.001 and 0.05, respectively (refer to Supplementary Figures 7-14).
4 Discussion
In the context of early prostate cancer diagnosis according to the NCCN guidelines, the importance of using multiparametric MRI (mpMRI) in clinical decision-making before biopsy is emphasized (30). However, there’s conflicting evidence in the literature exists regarding the accuracy of mpMRI versus 68Ga-PSMA PET/CT in identifying extraprostatic extension. Yilmaz et al. (24) found that mpMRI is more accurate than 68Ga-PSMA PET/CT in detecting extraprostatic extension, with a detection rate of 87.5% compared to 66.7%. Conversely, Chen et al. (25)’s study suggests a different outcome. mpMRI showed a lower sensitivity compared to 68Ga-PSMA PET/CT for ECE, with rates of 54% and 78% respectively. These contentious conclusions have sparked our research interest in this subject.
In our meta-analysis examining the diagnostic performance of mpMRI and 68Ga-PSMA PET in detecting ECE and SVI in primary prostate cancer, we found comparable efficacy between these imaging modalities. In 2023, a comparison study was carried out by Wang et al. (31) on PSMA PET/CT and mpMRI in patients with localized prostate cancer, with reported results consistent with our own findings. Additionally, Kalapara et al. (32) and Ren et al. (33) also supports these conclusions. It is shown that there is no notable distinction between the two diagnostic methods in identifying or pinpointing primary prostate tumors.
Interestingly, in the study by Chow et al. (34) the findings somewhat diverge from ours: PET/MRI demonstrated higher sensitivity in detecting ECE and SVI—78.7% for PET/MRI versus 52.9% for mpMRI in ECE detection, and 66.7% for PET/MRI versus 51.0% for mpMRI in SVI detection—yet exhibited slightly lower specificity than mpMRI (82.2% for PET/MRI versus 86.2% for mpMRI in ECE specificity). Compared to mpMRI, PET/CT seems to possess lower sensitivity in detecting ECE and SVI, with PET/CT at 51.5% versus 61.0% for mpMRI in ECE detection, and 44.9% for PET/CT versus 61.8% for mpMRI in SVI detection. The discrepancy in conclusions between our study and Chow et al.’ research may stem from the specific delineation of 68Ga-PSMA as the radiotracer in our study, whereas Chow’s paper defined PSMA as the contrast agent, leading to differences in the inclusion criteria. Additionally, due to the variation in search timelines, our paper incorporated the most recent research findings. Variations in patient age, risk profiles, imaging protocols, and interpretation criteria across studies, as well as the time interval between different imaging modalities, are also significant (35).
In our head-to-head meta-analysis, several strengths distinguish our study from previous researches. The primary benefit is the ability to perform a comprehensive and trustworthy direct comparison between 68Ga-PSMA PET and mpMRI. This approach minimizes biases associated with non-comparative studies. Our study effectively eliminates potential confounding effects by specifically concentrating on 68Ga-PSMA as the radiotracer, rather than using other tracers. Thirdly, we further subdivided the PET analysis into two subgroups: PET/CT and PET/MRI. By comparing each subgroup separately with mpMRI, we found no statistically significant differences in the diagnostic performance of both types of PET modalities compared to mpMRI. The subgroup analysis conducted in our study further refines the diagnostic performance of 68Ga-PSMA PET, thereby enhancing the interpretability of results and increasing the applicability of our conclusions.
In addition, the two different diagnostic tools each have their own advantages and disadvantages. 68Ga-PSMA PET is recognized for its high sensitivity in detecting prostate cancer cells, making it particularly useful in identifying metastatic or recurrent disease, while mpMRI excels in detailed soft-tissue characterization, which is crucial for local staging and guiding biopsy (12). However, both methods have limitations. The accuracy of mpMRI for diagnosis can be influenced by the subjective nature of the PI-RADS scoring system, which may lead to inter-observer variability (36). In contrast, 68Ga-PSMA PET can effectively distinguish ISUP grade groups using SUVmax values, although these values can vary between studies, potentially affecting consistency in clinical application (37). Practical factors like cost, availability, and accessibility also differ significantly. While 68Ga-PSMA PET is often pricier and less accessible than mpMRI, the latter may not always offer the necessary specificity in certain cases, particularly when evaluating extraprostatic extension (38). The findings suggest that a combined diagnostic approach using both 68Ga-PSMA PET and mpMRI may enhance accuracy and provide complementary information, leading to better decision-making in clinical practice (39).
Our thorough examination of 68Ga-PSMA PET and mpMRI in the setting of initial prostate cancer offers valuable insights, although it is important to recognize limitations due to the heterogeneity of studies, which could impact the relevance of our findings. Furthermore, 8 of the 10 included studies were retrospective, which may have introduced selection and recall biases and affected the reliability of the results. Additionally, our conclusions are based on a small sample size, with only 8 direct comparison studies for ECE and SVI, limiting the statistical power to detect significant differences. Therefore, more extensive and well-designed prospective studies are needed to confirm these findings and guide clinical implementation.
5 Conclusion
Our analysis of the data indicates that both mpMRI and 68Ga-PSMA PET exhibit comparable accuracy in detecting ECE and SVI in prostate cancer patients. Nevertheless, the limited study sample size calls for further, larger prospective studies to validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
XJ: Data curation, Formal analysis, Methodology, Software, Writing – original draft. YC: Data curation, Formal analysis, Visualization, Writing – original draft. XR: Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1410229/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Van den Broeck T, van den Bergh RCN, Arfi N, Gross T, Moris L, Briers E, et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: A systematic review. Eur Urol. (2019) 75:967–87. doi: 10.1016/j.eururo.2018.10.011
3. Caglic I, Sushentsev N, Shah N, Warren AY, Lamb BW, Barrett T. Comparison of biparametric versus multiparametric prostate MRI for the detection of extracapsular extension and seminal vesicle invasion in biopsy naïve patients. Eur J Radiol. (2021) 141:109804. doi: 10.1016/j.ejrad.2021.109804
4. Correas JM, Halpern EJ, Barr RG, Ghai S, Walz J, Bodard S, et al. Advanced ultrasound in the diagnosis of prostate cancer. World J Urol. (2021) 39:661–76. doi: 10.1007/s00345-020-03193-0
5. Streicher J, Meyerson BL, Karivedu V, Sidana A. A review of optimal prostate biopsy: indications and techniques. Ther Adv Urol. (2019) 11:1756287219870074. doi: 10.1177/1756287219870074
6. Guerra A, Flor-de-Lima B, Freire G, Lopes A, Cassis J. Radiologic-pathologic correlation of prostatic cancer extracapsular extension (ECE). Insights Imaging. (2023) 14:88. doi: 10.1186/s13244-023-01428-3
7. Zhang F, Liu CL, Chen Q, Shao SC, Chen SQ. Accuracy of multiparametric magnetic resonance imaging for detecting extracapsular extension in prostate cancer: a systematic review and meta-analysis. Br J Radiol. (2019) 92:20190480. doi: 10.1259/bjr.20190480
8. Ito K, Ichinose Y, Kubota Y, Imai K, Yamanaka H. Clinicopathological features of prostate cancer detected by transrectal ultrasonography-guided systematic six-sextant biopsy. Int J Urol. (1997) 4:474–9. doi: 10.1111/j.1442-2042.1997.tb00288.x
9. Liu FY, Sheng TW, Tseng JR, Yu KJ, Tsui KH, Pang ST, et al. Prostate-specific membrane antigen (PSMA) fusion imaging in prostate cancer: PET-CT vs PET-MRI. Br J Radiol. (2022) 95:20210728. doi: 10.1259/bjr.20210728
10. Roumeguère T, Aoun F, Albisinni S, Mjaess G. Antibodies targeting Prostate-Specific Membrane Antigen positive prostate cancer: from diagnostic imaging to theranostics. Curr Opin Oncol. (2021) 33:500–6. doi: 10.1097/cco.0000000000000767
11. Lenzo NP, Meyrick D, Turner JH. Review of gallium-68 PSMA PET/CT imaging in the management of prostate cancer. Diagnostics (Basel). (2018) 8. doi: 10.3390/diagnostics8010016
12. Chavoshi M, Mirshahvalad SA, Metser U, Veit-Haibach P. (68)Ga-PSMA PET in prostate cancer: a systematic review and meta-analysis of the observer agreement. Eur J Nucl Med Mol Imaging. (2022) 49:1021–9. doi: 10.1007/s00259-021-05616-5
13. Abghari-Gerst M, Armstrong WR, Nguyen K, Calais J, Czernin J, Lin D, et al. A comprehensive assessment of (68)Ga-PSMA-11 PET in biochemically recurrent prostate cancer: results from a prospective multicenter study on 2,005 patients. J Nucl Med. (2022) 63:567–72. doi: 10.2967/jnumed.121.262412
14. Zhou C, Tang Y, Deng Z, Yang J, Zhou M, Wang L, et al. Comparison of (68)Ga-PSMA PET/CT and multiparametric MRI for the detection of low- and intermediate-risk prostate cancer. EJNMMI Res. (2022) 12:10. doi: 10.1186/s13550-022-00881-3
15. Kim W, Kim JH, Cha YK, Chong S, Kim TJ. Completeness of reporting of systematic reviews and meta-analysis of diagnostic test accuracy (DTA) of radiological articles based on the PRISMA-DTA reporting guideline. Acad Radiol. (2023) 30:258–75. doi: 10.1016/j.acra.2022.03.028
16. Lee J, Mulder F, Leeflang M, Wolff R, Whiting P, Bossuyt PM. QUAPAS: an adaptation of the QUADAS-2 tool to assess prognostic accuracy studies. Ann Intern Med. (2022) 175:1010–8. doi: 10.7326/m22-0276
17. Yang B, Mallett S, Takwoingi Y, Davenport CF, Hyde CJ, Whiting PF, et al. QUADAS-C: A tool for assessing risk of bias in comparative diagnostic accuracy studies. Ann Intern Med. (2021) 174:1592–9. doi: 10.7326/m21-2234
18. Chen Y, Chen D, Wang Y, Han Y. Using freeman-tukey double arcsine transformation in meta-analysis of single proportions. Aesthetic Plast Surg. (2023) 47:83–4. doi: 10.1007/s00266-022-02977-6
19. Ucar T, Gunduz N, Demirci E, Culpan M, Gunel H, Kir G, et al. Comparison of 68Ga-PSMA PET/CT and mp-MRI in regard to local staging for prostate cancer with histopathological results: A retrospective study. Prostate. (2022) 82:1462–8. doi: 10.1002/pros.24420
20. Tayara OM, Pełka K, Kunikowska J, Malewski W, Sklinda K, Kamecki H, et al. Comparison of multiparametric MRI, [(68)Ga]Ga-PSMA-11 PET-CT, and clinical nomograms for primary T and N staging of intermediate-to-high-risk prostate cancer. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15245838
21. Stasiak CES, Cardillo A, de Almeida SA, Rodrigues RS, de Castro PHR, Parente DB. Preoperative evaluation of prostate cancer by (68)Ga-PMSA positron emission tomography/computed tomography: comparison with magnetic resonance imaging and with histopathological findings. Radiol Bras. (2023) 56:171–8. doi: 10.1590/0100-3984.2022.0122-en
22. Arslan A, Karaarslan E, Güner AL, Sağlıcan Y, Tuna MB, Kural AR. Comparing the diagnostic performance of multiparametric prostate MRI versus 68Ga-PSMA PET-CT in the evaluation lymph node involvement and extraprostatic extension. Acad Radiol. (2022) 29:698–704. doi: 10.1016/j.acra.2020.07.011
23. Çelen S, Gültekin A, Özlülerden Y, Mete A, Sağtaş E, Ufuk F, et al. Comparison of 68Ga-PSMA-I/T PET-CT and multiparametric MRI for locoregional staging of prostate cancer patients: A pilot study. Urol Int. (2020) 104:684–91. doi: 10.1159/000509974
24. Yilmaz B, Turkay R, Colakoglu Y, Baytekin HF, Ergul N, Sahin S, et al. Comparison of preoperative locoregional Ga-68 PSMA-11 PET-CT and mp-MRI results with postoperative histopathology of prostate cancer. Prostate. (2019) 79:1007–17. doi: 10.1002/pros.23812
25. Chen M, Zhang Q, Zhang C, Zhou YH, Zhao X, Fu Y, et al. Comparison of (68)Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) and multi-parametric magnetic resonance imaging (MRI) in the evaluation of tumor extension of primary prostate cancer. Transl Androl Urol. (2020) 9:382–90. doi: 10.21037/tau.2020.03.06
26. Muehlematter UJ, Burger IA, Becker AS, Schawkat K, Hötker AM, Reiner CS, et al. Diagnostic accuracy of multiparametric MRI versus (68)Ga-PSMA-11 PET/MRI for extracapsular extension and seminal vesicle invasion in patients with prostate cancer. Radiology. (2019) 293:350–8. doi: 10.1148/radiol.2019190687
27. Skawran SM, Sanchez V, Ghafoor S, Hötker AM, Burger IA, Huellner MW, et al. Primary staging in patients with intermediate- and high-risk prostate cancer: Multiparametric MRI and (68)Ga-PSMA-PET/MRI - What is the value of quantitative data from multiparametric MRI alone or in conjunction with clinical information? Eur J Radiol. (2022) 146:110044. doi: 10.1016/j.ejrad.2021.110044
28. Koseoglu E, Kordan Y, Kilic M, Sal O, Seymen H, Kiremit MC, et al. Diagnostic ability of Ga-68 PSMA PET to detect dominant and non-dominant tumors, upgrading and adverse pathology in patients with PIRADS 4-5 index lesions undergoing radical prostatectomy. Prostate Cancer Prostatic Dis. (2021) 24:202–9. doi: 10.1038/s41391-020-00270-8
29. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
30. Moses KA, Sprenkle PC, Bahler C, Box G, Carlsson SV, Catalona WJ, et al. NCCN guidelines® Insights: prostate cancer early detection, version 1.2023. J Natl Compr Canc Netw. (2023) 21:236–46. doi: 10.6004/jnccn.2023.0014
31. Wang YF, Lo CY, Chen LY, Chang CW, Huang YT, Huang YY, et al. Comparing the detection performance between multiparametric magnetic resonance imaging and prostate-specific membrane antigen PET/CT in patients with localized prostate cancer: A systematic review and meta-analysis. Clin Nucl Med. (2023) 48:e321–e31. doi: 10.1097/rlu.0000000000004646
32. Kalapara AA, Nzenza T, Pan HYC, Ballok Z, Ramdave S, O’Sullivan R, et al. Detection and localisation of primary prostate cancer using (68) gallium prostate-specific membrane antigen positron emission tomography/computed tomography compared with multiparametric magnetic resonance imaging and radical prostatectomy specimen pathology. BJU Int. (2020) 126:83–90. doi: 10.1111/bju.14858
33. Ren X, Nur Salihin Yusoff M, Hartini Mohd Taib N, Zhang L, Wang K. (68)Ga-prostate specific membrane antigen-11 PET/CT versus multiparametric MRI in the detection of primary prostate cancer: A systematic review and head-to-head comparative meta-analysis. Eur J Radiol. (2024) 170:111274. doi: 10.1016/j.ejrad.2023.111274
34. Chow KM, So WZ, Lee HJ, Lee A, Yap DWT, Takwoingi Y, et al. Head-to-head comparison of the diagnostic accuracy of prostate-specific membrane antigen positron emission tomography and conventional imaging modalities for initial staging of intermediate- to high-risk prostate cancer: A systematic review and meta-analysis. Eur Urol. (2023) 84:36–48. doi: 10.1016/j.eururo.2023.03.001
35. Callender T, Emberton M, Morris S, Pharoah PDP, Pashayan N. Benefit, harm, and cost-effectiveness associated with magnetic resonance imaging before biopsy in age-based and risk-stratified screening for prostate cancer. JAMA Netw Open. (2021) 4:e2037657. doi: 10.1001/jamanetworkopen.2020.37657
36. Junker D, Quentin M, Nagele U, Edlinger M, Richenberg J, Schaefer G, et al. Evaluation of the PI-RADS scoring system for mpMRI of the prostate: a whole-mount step-section analysis. World J Urol. (2015) 33:1023–30. doi: 10.1007/s00345-014-1370-x
37. Stabile A, Giganti F, Kasivisvanathan V, Giannarini G, Moore CM, Padhani AR, et al. Factors influencing variability in the performance of multiparametric magnetic resonance imaging in detecting clinically significant prostate cancer: A systematic literature review. Eur Urol Oncol. (2020) 3:145–67. doi: 10.1016/j.euo.2020.02.005
38. Brown LC, Ahmed HU, Faria R, El-Shater Bosaily A, Gabe R, Kaplan RS, et al. Multiparametric MRI to improve detection of prostate cancer compared with transrectal ultrasound-guided prostate biopsy alone: the PROMIS study. Health Technol Assess. (2018) 22:1–176. doi: 10.3310/hta22390
Keywords: 68Ga-PSMA PET, mpMRI, local staging, prostate cancer, meta-analysis
Citation: Jin X, Cai Y and Ren X (2024) Comparison of 68Ga-PSMA PET and mpMRI for prostate cancer local staging: a comprehensive review and direct meta-analysis. Front. Oncol. 14:1410229. doi: 10.3389/fonc.2024.1410229
Received: 28 May 2024; Accepted: 14 October 2024;
Published: 01 November 2024.
Edited by:
Zhenghong Lee, Case Western Reserve University, United StatesReviewed by:
Bernhard Ralla, Charité University Medicine Berlin, GermanyEdel Noriega-Álvarez, University Hospital of Guadalajara, Spain
Copyright © 2024 Jin, Cai and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolu Ren, cmVueGlhb2x1MDJAZ21haWwuY29t
 Xinyu Jin1
Xinyu Jin1 Xiaolu Ren
Xiaolu Ren