- 1School of Basic Medicine, Tianjin Medical University, Tianjin, China
- 2National Population Health Data Center, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 3School of Medicine, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 4School of Food Science and Engineering, Tianjin University of Science and Technology, Tianjin, China
Objective: The lymphocyte-to-C-reactive protein (LCR) ratio, an immune-inflammatory marker, shows prognostic potential in various cancers. However, its utility in gastrointestinal malignancies remains uncertain due to inconsistent findings. This systematic review and meta-analysis synthesizes recent evidence to elucidate the association between LCR and prognosis in gastrointestinal cancer patients, aiming to clarify LCR’s potential role as a prognostic biomarker.
Methods: We searched PubMed, Embase, Cochrane, and Web of Science databases up to May 2024 to evaluate the association between LCR and prognosis in gastrointestinal cancer patients. The main outcomes included overall survival (OS), recurrence-free survival (RFS), and disease-free survival (DFS). We also analyzed secondary parameters such as geographical region, study duration, sample size, LCR threshold, and patient characteristics (age, gender, tumor location, and TNM stage).
Results: This meta-analysis of 21 cohort studies (n=9,131) finds a significant association between reduced LCR levels and poor prognosis in gastrointestinal cancer. Lower LCR levels were associated with worse overall survival (HR=2.01, 95% CI=1.75-2.31, P<0.001), recurrence-free survival (HR=1.90, 95% CI=1.32-2.76, P<0.001), and disease-free survival (HR=1.76, 95% CI=1.45-2.13, P<0.001). Subgroup analyses by cancer type, timing, and LCR threshold consistently confirmed this relationship (P<0.05).
Conclusion: LCR may serve as a prognostic marker in gastrointestinal cancer patients, with lower LCR levels associated with poorer prognosis. However, more high-quality studies are needed to validate these findings, considering the limitations of the current evidence.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023486858.
1 Introduction
Gastrointestinal cancer, which includes esophageal cancer (EC), gastric cancer (GC), and colorectal cancer (CRC), is a major contributor to global cancer-related mortality. In 2020, gastrointestinal cancer accounted for 2,228,749 deaths worldwide (1). Among gastrointestinal cancers, CRC was the second most prevalent at 9.4%, followed by GC (7.7%) and EC (5.5%). The incidence of gastrointestinal cancers is expected to continue increasing over the next decade. In Asia, the incidence of GC is projected to reach approximately 20 cases per 100,000 individuals (2). Moreover, the global burden of GC is expected to increase by 62% by 2040 (3), while CRC incidence is projected to reach 3.2 million new cases, with 1.6 million deaths by 2040 (4). Despite advancements in multidisciplinary treatments, gastrointestinal cancer mortality remains high. Prognostic biomarkers are needed to identify high-risk patients and enable personalized therapy, potentially improving prognosis.
Cancer-associated inflammation is linked to intratumoral immunosuppression and cancer progression. Systemic inflammation is associated with reduced survival in various malignancies due to mucosal injury and DNA damage (5, 6). Numerous studies have shown that systemic inflammation predicts tumor recurrence and survival in various cancers, including gastrointestinal cancer. Parameters such as the preoperative neutrophil-to-lymphocyte ratio (NLR) (7), platelet-to-lymphocyte ratio (PLR) (8), Prognostic Index (PI) (9), Prognostic Nutritional Index (PNI) (10), Glasgow Prognostic Score (GPS) (11), and Modified Glasgow Prognostic Score (mGPS) (12) have been identified. CRP and lymphocyte levels are reliable indicators of post-operative infections and immune status. Lymphocytes have high specificity but low sensitivity, while CRP has low specificity but high sensitivity (13). Thus, lymphocyte-to-CRP ratio (LCR) may better reflect inflammatory status (14). Recent studies have highlighted LCR’s potential in gastrointestinal cancer (15–37). However, LCR’s role is not fully understood, and most meta-analyses have focused on specific cancers. This study conducts a comprehensive meta-analysis across gastrointestinal cancers to explore the correlation between LCR and prognosis.
2 Methods
2.1 Literature search
We conducted a comprehensive literature search of PubMed, Embase, Cochrane, and Web of Science databases up to May 2024 to identify English studies investigating the association between LCR and prognosis in gastrointestinal cancer. The search terms included: “lymphocyte”, “C-reactive protein”, “ratio”, “gastrointestinal cancer”, “colorectal cancer”, “gastric cancer”, and “esophageal cancer” (detailed search strategy in Supplementary Table 1). Reference lists of eligible studies were manually scrutinized. Two investigators (XML and JCZ) independently conducted the search and study selection, with discrepancies resolved through consensus. This study was preregistered in PROSPERO (CRD42023486858). Studies were included if they met the following criteria: P: patients diagnosed with gastrointestinal cancer; I: low LCR; C: high LCR; O: at least one survival outcome, such as overall survival (OS), disease-free survival (DFS), or recurrence-free survival (RFS); S: cohort studies. Exclusions comprised reviews, letters, editorials, case reports, conference abstracts, inadequate data, duplicate literature, and non-English articles.
2.2 Data extraction
Data extraction was conducted independently by two investigators (XML, JCZ), with any disparities resolved by a third investigator (HYA) to achieve consensus. The extracted information from the studies included essential details such as the first author, publication year, study period, country, design, cancer type, sample size, age, gender, body mass index (BMI), timing, tumor size, TNM stage, LCR threshold, and relevant outcome measures.
2.3 Quality evaluation
The Newcastle–Ottawa Scale (NOS) was employed to assess study quality, which including representativeness, selection of non-exposed, ascertainment of exposure, outcome not present at start, comparability on most important factors, comparability on other risk factors, assessment of outcome, long enough follow-up (median≥1 year) and adequacy (completeness) of follow-up. Studies scoring seven to nine points were considered high quality (38), as per the scale’s criteria. Two investigators independently evaluated the quality and level of evidence for eligible studies, and any discrepancy was resolved through discussion.
2.4 Statistical analysis
The study utilized Hazard Ratios (HR) with a 95% Confidence Interval to assess the correlation between Lymphocyte-to-CRP Ratio (LCR) and prognosis (OS, DFS, RFS, etc.) in gastrointestinal cancer patients. Subgroup analyses were conducted based on LCR thresholds, cancer types, and timing to explore its impact on prognosis. Heterogeneity was assessed using the I2 statistic and Q test, considering significance at P<0.1 and/or I2>50%. Meta-analysis employed a random-effects model for significant heterogeneity; otherwise, a fixed-effects model was used. Sensitivity analyses were performed for indicators with all included studies. Funnel plot and Egger’s test were used for outcomes that included all literature to evaluate publication bias with a P-value of 0.05. All statistical analyses were performed using Review Manager 5.4 versions and STATA 15.0.
3 Results
3.1 Literature search and study characteristics
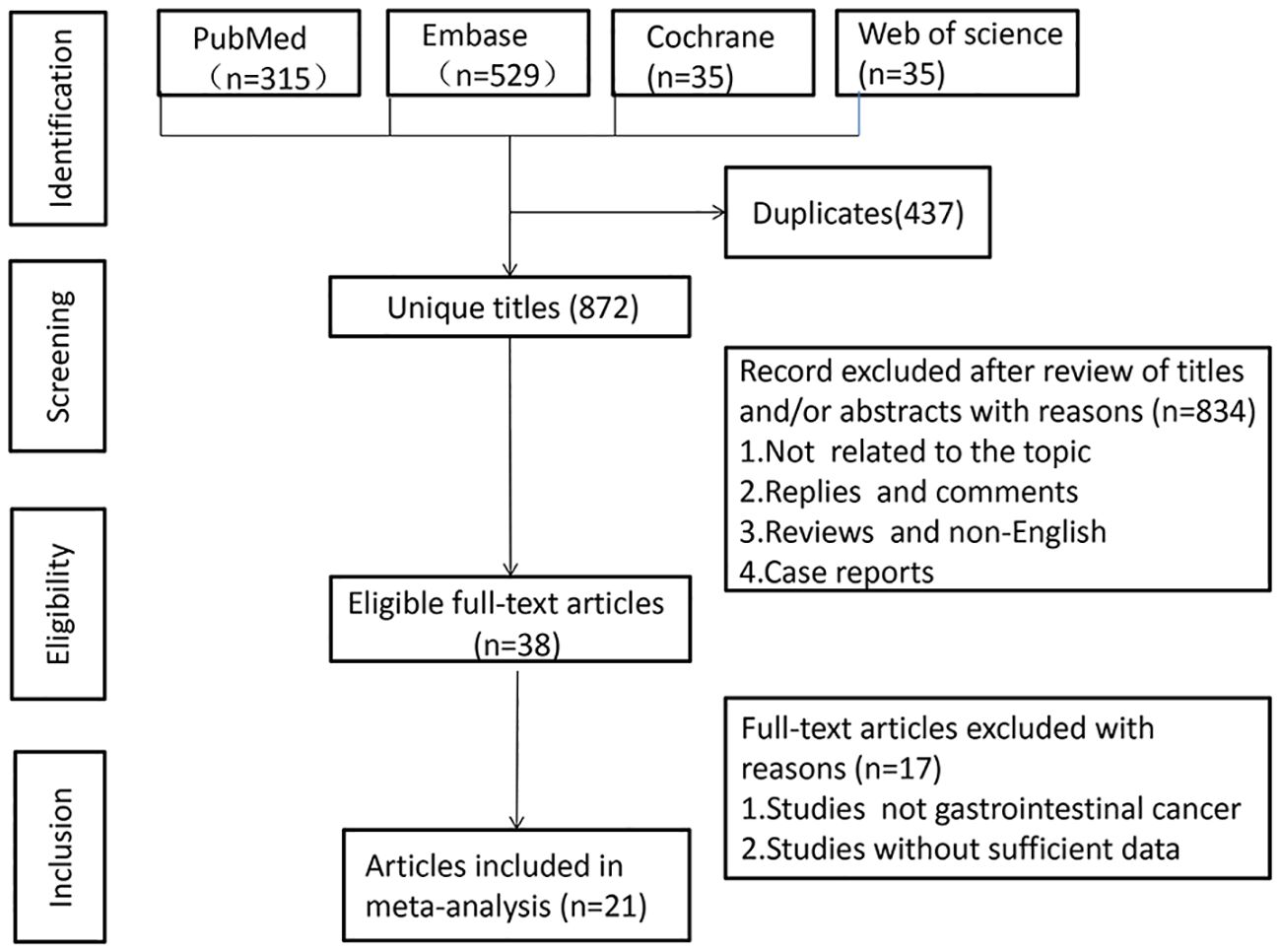
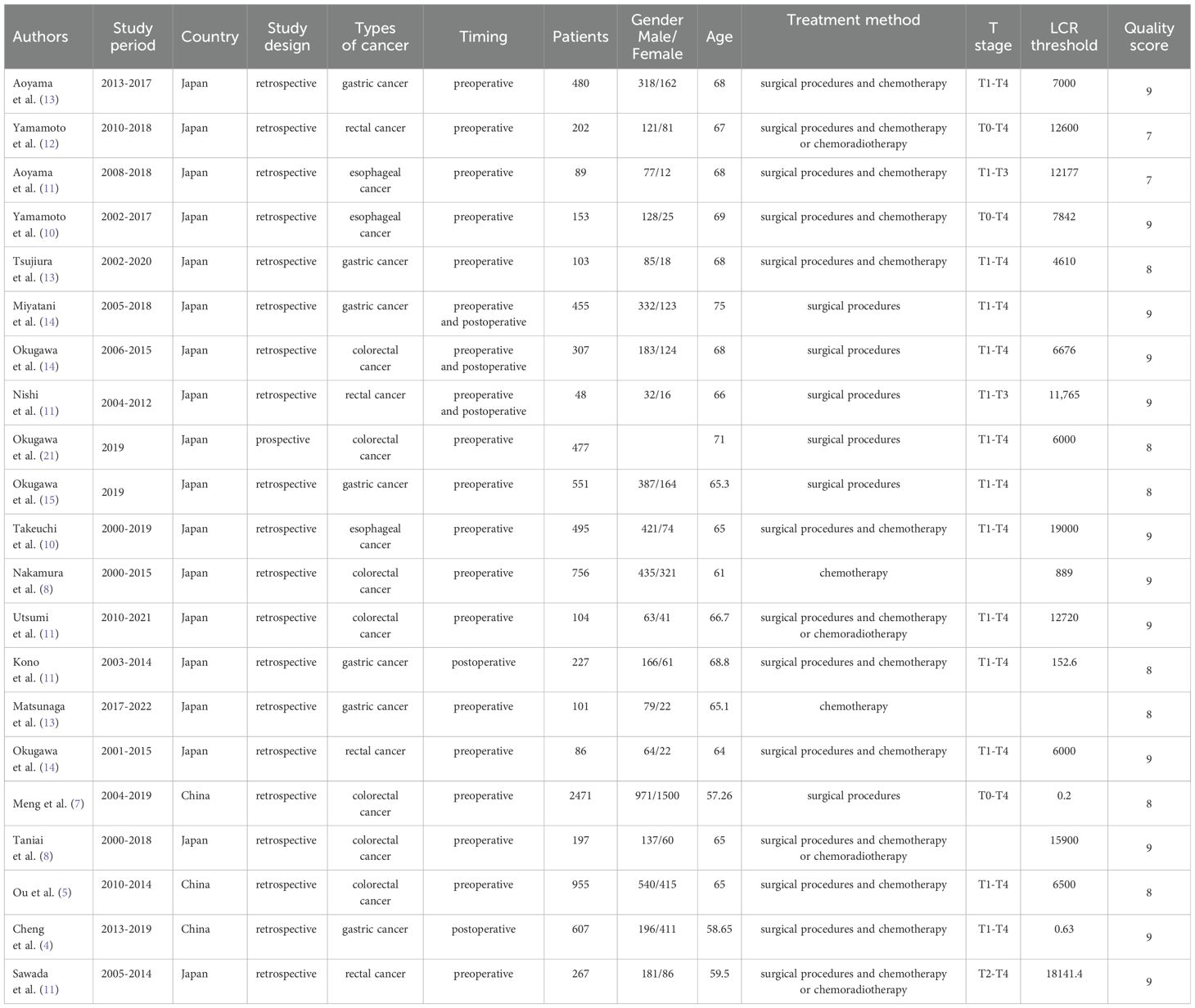
A literature search across PubMed (n = 315), Embase (n = 529), Cochrane (n = 35), and Web of Science (n = 430) yielded 1,309 articles (Figure 1). After removing duplicates, 872 titles and abstracts were reviewed. Finally, 21 articles, involving 9,131 patients, were included. One study was a prospective cohort (23), while 20 were retrospective cohorts. The studies, published between 2019-2023, included 18 from Japan and 3 from China. Twenty studies examined the association between LCR and OS, six explored LCR and DFS, and eight investigated LCR and RFS. LCR was defined as lymphocytes-to-C-reactive protein ratio in 19 studies, while three used the CLR (28, 31, 32). Table 1 summarizes the characteristics and quality scores (median: 9). Supplementary Table 2 provides quality assessment details.
3.2 Meta-analysis results
3.2.1 The relationship between LCR and OS
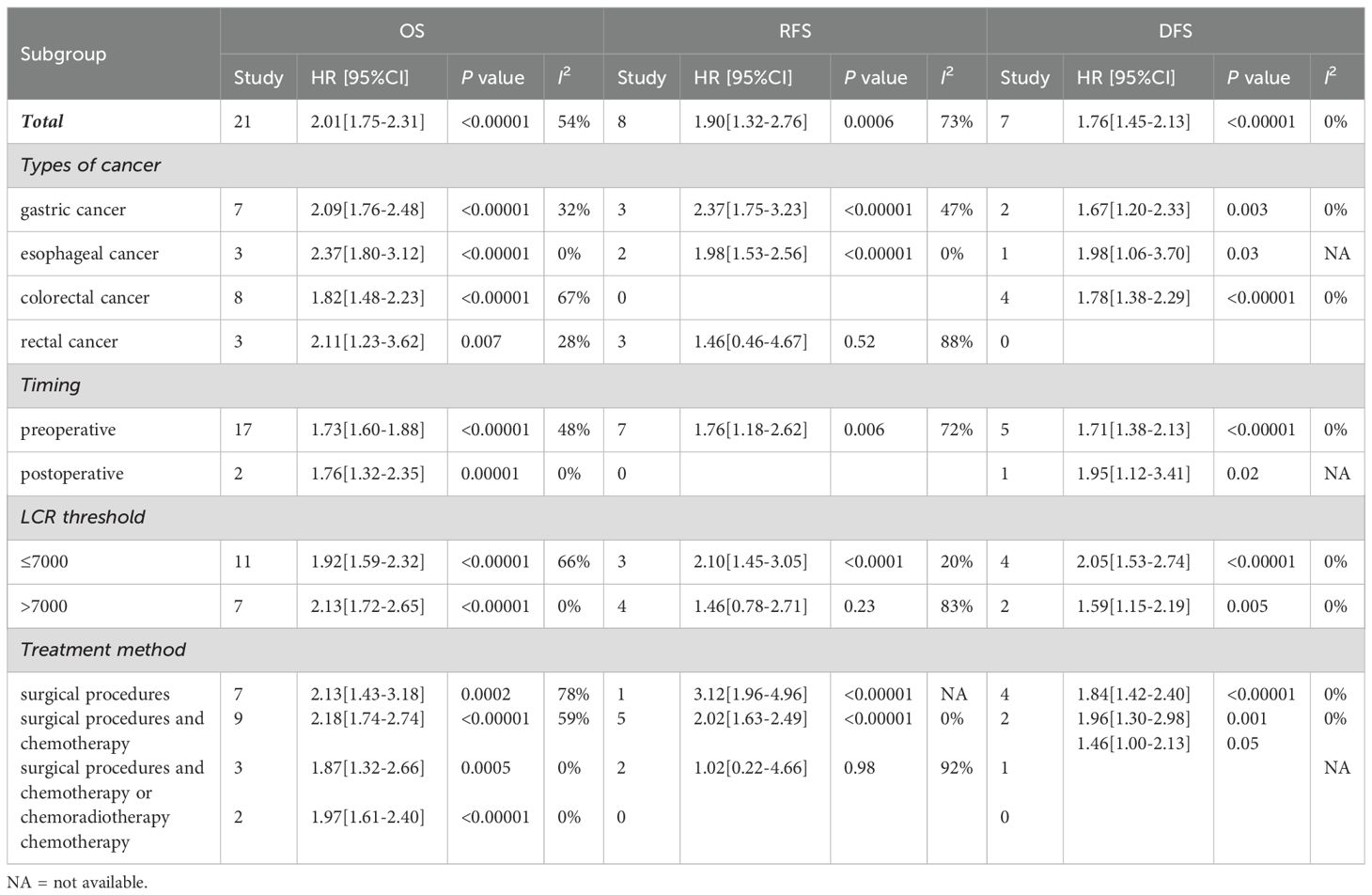
The association between LCR and overall survival (OS) was investigated in 20 studies, revealing significant heterogeneity (I2 = 54%, P = 0.002). Using a random-effects model, our meta-analysis showed that patients with lower LCR levels had significantly worse OS (HR = 2.01, 95% CI = 1.75-2.31, P < 0.00001) (Figure 2A). Subgroup analyses (Table 2), including different cancer types, timing, LCR thresholds, and treatment method, maintained predictive significance (P < 0.05). Heterogeneity decreased with preoperative (I2 = 48%, P < 0.00001) and postoperative timing (I2 = 0%, P = 0.00001), suggesting timing contributed to heterogeneity.
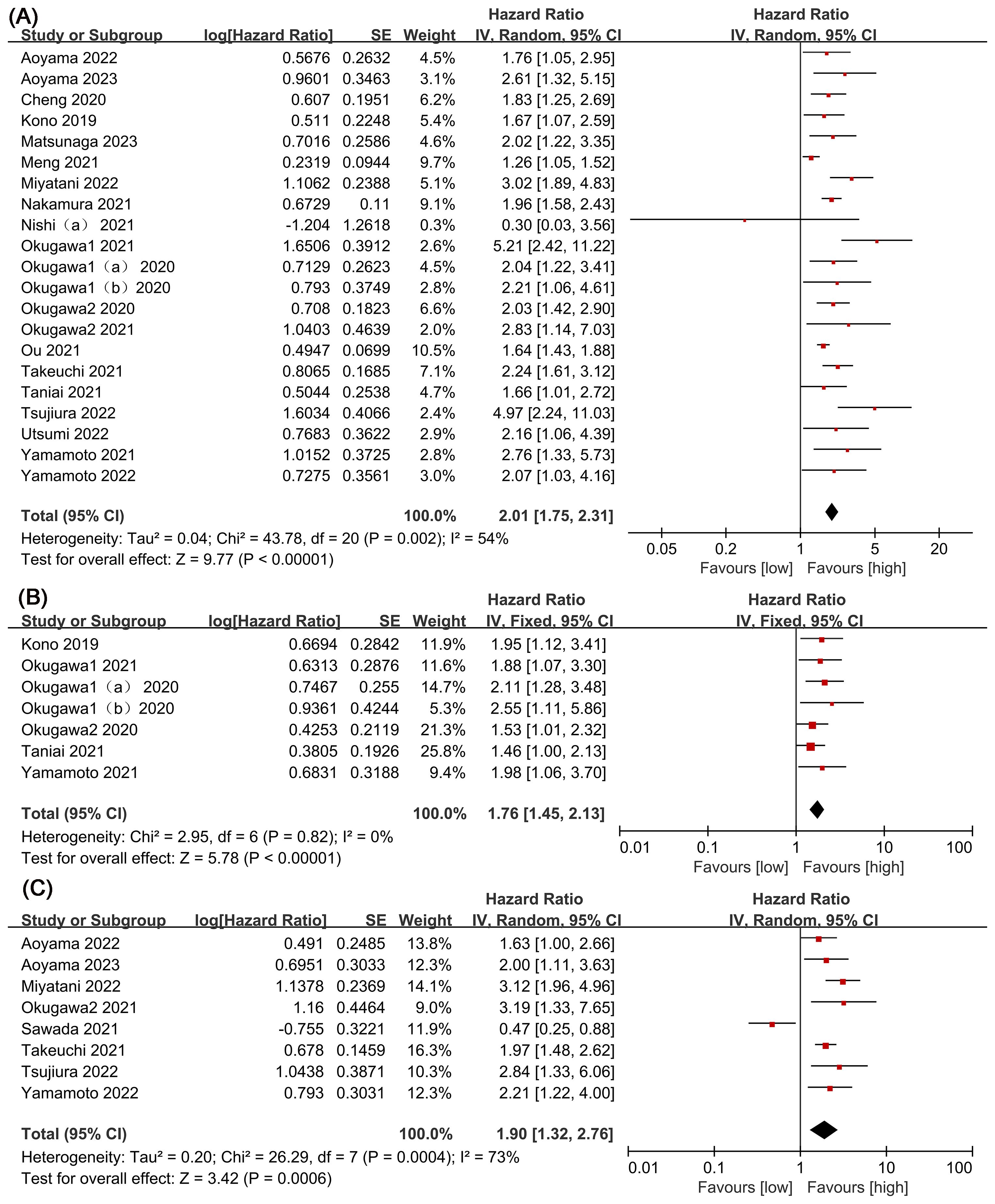
Figure 2. Forest plots of perioperative outcomes: (A) overall survival (OS), (B) disease-free survival (DFS), (C) recurrence-free survival (RFS).
3.2.2 Relationship between LCR and DFS
Six studies were examined to assess the correlation between LCR and DFS, revealing no significant heterogeneity (I2 = 0%, P = 0.82). Using a fixed-effect model, our meta-analysis demonstrated a strong association between lower LCR and worse DFS (HR = 1.76, 95% CI = 1.45-2.13, P < 0.00001) (Figure 2B). Subgroup analysis (Table 2) identified significant differences in cancer types, timing, LCR threshold and treatment method (P < 0.05).
3.2.3 Relationship between LCR and RFS
Eight studies explored the association between LCR and RFS, unveiling notable heterogeneity (I2= 73%, P=0.0004). Employing a random-effects model to address this heterogeneity, the meta-analysis revealed a significant correlation: lower LCR levels associated with markedly diminished RFS (HR=1.90, 95%CI=1.32-2.76, P=0.0006) (Figure 2C). Subgroup analysis (Table 2) indicated persistent significance in studies stratified by cancer type and timing (P<0.05), while significance diminished in rectal cancer studies (P=0.52), LCR threshold>7000 studies (P=0.23) and surgical procedures and chemotherapy or chemoradiotherapy studies (P=0.98). The potential prognostic value of LCR for rectal cancer and LCR threshold>7000 warrants further investigation. Notably, timing emerged as a major source of heterogeneity (I2 = 72, P=0.006), suggesting its pivotal role in influencing study outcomes.
3.2.4 Publication bias
Publication bias in LCR’s OS prediction was identified through Egger’s test (t=2.93, P=0.0009) and funnel plot analysis (Figure 3A). Likewise, potential publication bias in LCR’s DFS prediction was observed using Egger’s test (t=4.46, P=0.007) and funnel plot (Figure 3B). However, no significant publication bias for LCR’s RFS prediction was detected by Egger’s test (t=-0.12, P=0.909) and funnel plot analysis (Figure 3C).
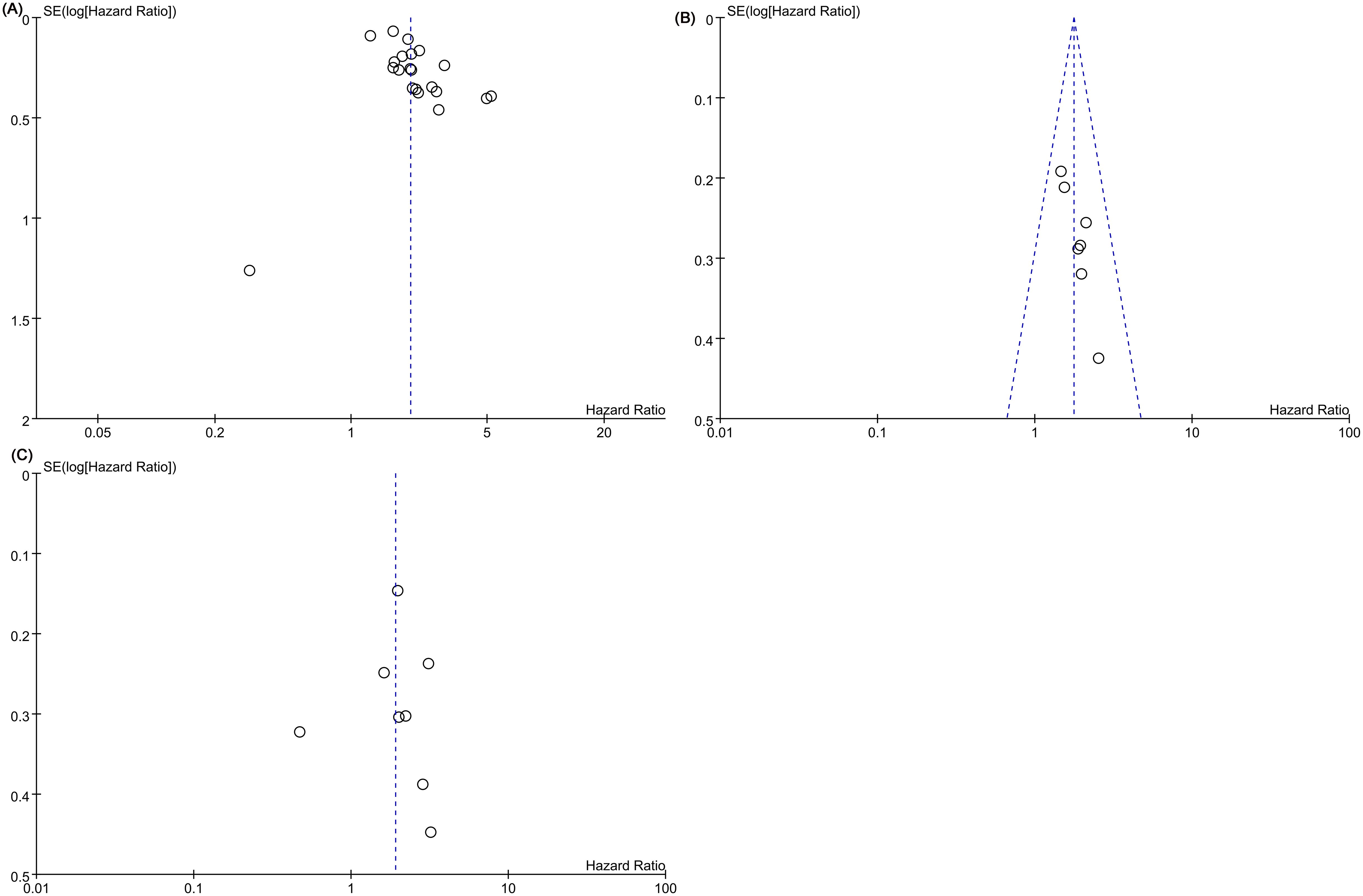
Figure 3. Funnel plots of (A) overall survival (OS), (B) disease-free survival (DFS), (C) recurrence-free survival (RFS).
3.2.5 Sensitivity analysis
Figure 4 illustrates sensitivity analyses examining the influence of excluding certain literature on overall survival (OS) (Figure 4A), recurrence-free survival (RFS) (Figure 4B) and disease-free survival (DFS) (Figure 4C) significance levels. The results indicate that excluding any literature did not alter the significance of OS, RFS and DFS, underscoring the stability of the relationship between LCR and all outcomes. Notably, upon excluding Sawada 2021, RFS heterogeneity decreased from 73% to 0%. The observed heterogeneity in this study may be attributed to variations in testing methods and statistical approaches. Similarly, heterogeneity in OS could be associated with differences in sample sizes and study designs across populations.
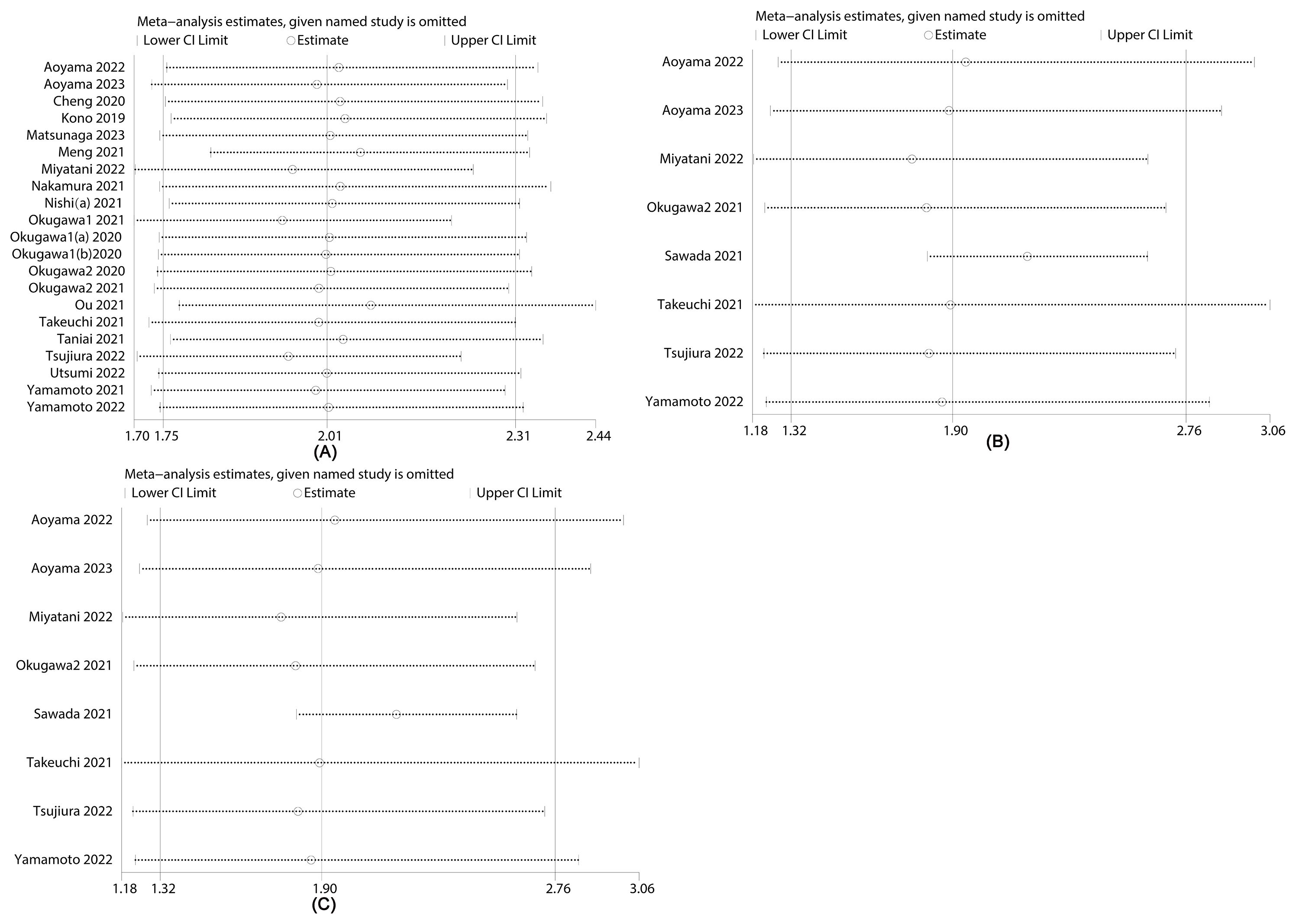
Figure 4. Sensitivity analysis of (A) overall survival (OS), (B) recurrence-free survival (RFS) and (C) disease-free survival (DFS).
4 Discussion
Currently, a growing body of evidence underscores the correlation between low lymphocyte-to-C-reactive protein ratio (LCR) and unfavorable survival outcomes among patients with gastrointestinal cancer. This meta-analysis, incorporating data from 21 studies encompassing 9,131 individuals with gastrointestinal cancer, aims to comprehensively evaluate the prognostic implications of LCR. The findings consistently demonstrate a significant association between diminished LCR levels and reduced overall survival (OS), disease-free survival (DFS), and recurrence-free survival (RFS) in patients with gastrointestinal cancers. Moreover, mounting research emphasizes the impact of systemic inflammation and nutritional status on the long-term prognosis of malignancies, including gastrointestinal cancers (39, 40). Tumor-infiltrating lymphocytes, recognized for their pivotal role in anti-tumor immunity, possess both prognostic and predictive value (41). C-reactive protein (CRP), capable of binding to diverse ligands on damaged cell membranes, strongly activates the classical complement pathway, potentially exacerbating tissue damage and contributing to more severe diseases (42). Elevated CRP levels within the inflammatory and tumor microenvironment promote various cancers (43). Furthermore, serum CRP levels correlate with tumor size, clinicopathological characteristics, and lymph node metastasis (44). Consequently, CRP stands as a crucial biomarker for tumor prognosis and treatment responses (43).
The current meta-analysis reveals that a diminished level of LCR is indicative of a poorer prognosis among patients with gastrointestinal cancer. This aligns with the evidence presented in the encompassed literature, highlighting substantial heterogeneity in OS and RFS. Subgroup analysis identifies cancer type, timing, LCR threshold and treatment method as sources of heterogeneity. Sensitivity analysis reinforces the stability of the meta-analysis, affirming the association between LCR and OS/RFS.
In recent investigations, numerous studies have underscored the superior accuracy of the LCR over other inflammation-related markers in predicting the prognosis of patients afflicted with gastrointestinal cancers. In a comprehensive meta-analysis and systematic review encompassing 2838 patients diagnosed with upper gastrointestinal cancer, Ye et al. elucidated that a diminished the LCR correlates with an unfavorable prognosis in upper gastrointestinal cancer cases (45). Despite these findings, the precise mechanisms underpinning the interplay between the LCR and gastrointestinal cancer prognosis remain elusive. A plausible explanation posited by researchers suggests a potential linkage between the LCR and the immune response among patients with gastrointestinal cancer (46). Lymphocytes consistently feature within the peritumoral inflammatory cell infiltrate, and their presence appears intricately tied to cancer cell activity, hinting at a relationship with tumor growth (47). Furthermore, the association between LCR and preoperative nutritional status, as well as surgical complications, merits attention. Okugawa observed a robust correlation between the LCR and preoperative nutritional status (23). In a study involving 607 patients with gastric cancer, Cheng et al. uncovered a statistically significant disparity in postoperative complications, with an incidence of 20.4% in the low LCR group versus 12.1% in the high LCR group (P = 0.006) (34). These findings underscore a noteworthy correlation between the LCR and postoperative surgical complications. Moreover, Okugawa et al. reported significantly elevated incidences of postoperative infectious complications, surgical site infections, and distant infections in the low LCR group compared to the high LCR group among 477 colorectal cancer patients (postoperative infectious complications: p = 0.0007, surgical site infections: p = 0.01, and distant infections: p = 0.021) (23). A low LCR emerges as an independent risk factor for postoperative complications in colorectal cancer, affirming the substantial association between LCR and postoperative surgical complications. In addition, Yasui et al. (48) found that the LCR and CAR inflammatory markers could accurately predict OS and recurrence‐free survival in patients with stage III CRC, which may identify predictive markers for treatment response and guide personalized therapeutic strategies in patients with gastrointestinal cancer. In conclusion, LCR stands out as the preeminent prognostic indicator among various inflammatory markers, as it is adept at identifying high-risk surgical patients and offering valuable guidance for treatment decisions.
We acknowledge several limitations in the current study. Primarily, the inclusion of mostly retrospective cohort studies introduces potential selection bias. To validate our findings, a prospective, multicenter randomized controlled trial is imperative. Additionally, the exclusively Asian population in our study raises concerns about generalizability; hence, further investigations encompassing diverse regions are warranted to authenticate and extend our discoveries. Moreover, the assessment of heterogeneity using P<0.1 and/or I2>50% alone may have certain limitations, this is also an unavoidable problem of meta-analysis; therefore, further studies are needed to confirm the findings of this article. Admittedly, The Newcastle–Ottawa Scale (NOS) was used to assess study quality, which is a widely accepted tool. However, the interpretation of NOS scores (seven to nine points indicating high quality) may be subjective. Future studies could provide a more detailed rationale for quality assessment criteria and scoring. Despite these limitations, our study boasts the largest sample size, encompassing all gastrointestinal cancers comprehensively. Our findings affirm that low lymphocyte-to-C-reactive protein ratio (LCR) holds prognostic value, advocating for the development of enhanced prognostic models based on LCR in clinical practice. Although high heterogeneity in overall survival (OS) and recurrence-free survival (RFS) may diminish confidence in our results, additional studies are indispensable to validate our inferences. Furthermore, since existing studies are predominantly retrospective, our study lays a foundation for guiding the design of future large prospective cohort studies. Overall, while the study provides valuable insights into the association between LCR and prognosis in gastrointestinal cancer patients, addressing the highlighted limitations could enhance the validity and generalizability of the findings. Future research could focus on addressing the identified limitations, including incorporating non-English studies, conducting comprehensive assessments of publication bias, exploring alternative approaches for assessing heterogeneity, refining quality assessment criteria, and conducting more extensive sensitivity analyses. Lastly, efforts should be made to translate research findings into clinical practice by conducting prospective validation studies and integrating LCR assessment into routine clinical care pathways. Collaborative efforts between researchers, clinicians, and policymakers are essential to facilitate the translation of biomarker discoveries into clinical utility. Future research could further advance our understanding of the prognostic significance of LCR in gastrointestinal cancer and its potential clinical implications.
5 Conclusion
The findings of our investigation indicate that preoperative levels of lymphocyte-to-C-reactive protein ratio (LCR) serve as a valuable adjunctive prognostic marker for gastrointestinal cancer outcomes. Diminished LCR correlates with unfavorable survival rates, enabling identification of high-risk patients in clinical settings. However, heterogeneity exists among the included studies, necessitating further research for result validation.
Data availability statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
Author contributions
XL: Data curation, Formal analysis, Methodology, Resources, Software, Validation, Visualization, Writing – original draft. JZ: Formal analysis, Resources, Software, Writing – review & editing. HA: Formal analysis, Methodology, Software, Visualization, Writing – review & editing. WW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – review & editing. YZ: Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – review & editing. FW: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1407306/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Lin Y, Zheng Y, Wang HL, Wu J. Global patterns and trends in gastric cancer incidence rates (1988-2012) and predictions to 2030. Gastroenterology. (2021) 161:116–27.e8. doi: 10.1053/j.gastro.2021.03.023
3. Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. (2023) 20:338–49. doi: 10.1038/s41571-023-00747-0
4. Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. (2023) 72:338–44. doi: 10.1136/gutjnl-2022-327736
5. Radojicic J, Zaravinos A, Spandidos DA. HPV. KRAS mutations, alcohol consumption and tobacco smoking effects on esophageal squamous-cell carcinoma carcinogenesis. Int J Biol markers. (2012) 27:1–12. doi: 10.5301/JBM.2011.8737
6. Toh Y, Oki E, Ohgaki K, Sakamoto Y, Ito S, Egashira A, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: molecular mechanisms of carcinogenesis. Int J Clin Oncol. (2010) 15:135–44. doi: 10.1007/s10147-010-0057-6
7. Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du P, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. (2017) 58:1–13. doi: 10.1016/j.ctrv.2017.05.005
8. Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer epidemiol Biomarkers Prev. (2014) 23:1204–12. doi: 10.1158/1055-9965.EPI-14-0146
9. Kasymjanova G, MacDonald N, Agulnik JS, Cohen V, Pepe C, Kreisman H, et al. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol (Toronto Ont). (2010) 17:52–8. doi: 10.3747/co.v17i4.567
10. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J cancer. (2012) 106:1439–45. doi: 10.1038/bjc.2012.92
11. McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. (2013) 39:534–40. doi: 10.1016/j.ctrv.2012.08.003
12. Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer (Oxford England: 1990). (2011) 47:2633–41. doi: 10.1016/j.ejca.2011.03.028
13. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. (2002) 3:991–8. doi: 10.1038/ni1102-991
14. Wang L, Zhang YL, Jiang C, Duan FF, Yuan ZY, Huang JJ, et al. Novel signatures based on the lymphocyte-to-C-reactive protein ratio predict the prognosis of patients with early breast cancer: A retrospective study. J Inflamm Res. (2022) 15:3957–74. doi: 10.2147/JIR.S364284
15. Aoyama T, Nakazano M, Nagasawa S, Hara K, Komori K, Tamagawa H, et al. The association of the lymphocyte-to-C-reactive-protein ratio with gastric cancer patients who receive curative treatment. In Vivo (Athens Greece). (2022) 36:482–9. doi: 10.21873/invivo.12728
16. Yamamoto T, Fukuda M, Okuchi Y, Oshimo Y, Nishikawa Y, Hisano K, et al. Clinical impact of lymphocyte/C-reactive protein ratio on postoperative outcomes in patients with rectal cancer who underwent curative resection. Sci Rep. (2022) 12:17136. doi: 10.1038/s41598-022-21650-1
17. Aoyama T, Nagasawa S, Nakazono M, Segami K, Tamagawa H, Tamagawa A, et al. The clinical impacts of lymphocyte-to-C-reactive protein ratio for esophageal cancer patients who receive curative treatment. J Cancer Res Ther. (2023) 19:556–61. doi: 10.4103/jcrt.jcrt_139_21
18. Yamamoto A, Toiyama Y, Okugawa Y, Ichikawa T, Imaoka H, Yasuda H, et al. Clinical implications of the preoperative lymphocyte C-reactive protein ratio in esophageal cancer patients. Surg Today. (2021) 51:745–55. doi: 10.1007/s00595-020-02166-5
19. Tsujiura M, Yamamoto A, Imaoka H, Shimura T, Kitajima T, Morimoto Y, et al. Clinical utility of lymphocyte to C-reactive protein ratio in predicting survival and postoperative complication for esophago-gastric junction cancer. Surg Oncol. (2022) 44:101842. doi: 10.1016/j.suronc.2022.101842
20. Miyatani K, Sawata S, Makinoya M, Miyauchi W, Shimizu S, Shishido Y, et al. Combined analysis of preoperative and postoperative lymphocyte-C-reactive protein ratio precisely predicts outcomes of patients with gastric cancer. BMC cancer. (2022) 22:641. doi: 10.1186/s12885-022-09716-9
21. Okugawa Y, Fujikawa H, Omura Y, Yamamoto A, Kitajima T, Shimura T, et al. Cumurative periopertive lymphocyte/C-reactive protein ratio as a predictor of long-term outcomes in patients with colorectal cancer. Gastroenterology. (2021) 160:S–887. doi: 10.1016/S0016-5085(21)02849-3
22. Nishi M, Shimada M, Tokunaga T, Higashijima J, Yoshikawa K, Kashihara H, et al. Lymphocyte to C-reactive protein ratio predicts long-term outcomes for patients with lower rectal cancer. World J Surg Oncol. (2021) 19:201. doi: 10.1186/s12957-021-02319-x
23. Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ide S, Kitajima T, et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann surge. (2020) 272:342–51. doi: 10.1097/SLA.0000000000003239
24. Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ichikawa T, Yin C, et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin Nutr (Edinburgh Scotland). (2020) 39:1209–17. doi: 10.1016/j.clnu.2019.05.009
25. Takeuchi M, Kawakubo H, Hoshino S, Matsuda S, Mayanagi S, Irino T, et al. Lymphocyte-to-C-reactive protein ratio as a novel marker for predicting oncological outcomes in patients with esophageal cancer. World J surge. (2021) 45:3370–7. doi: 10.1007/s00268-021-06269-z
26. Nakamura Y, Shida D, Boku N, Yoshida T, Tanabe T, Takamizawa Y, et al. Lymphocyte-to-C-reactive protein ratio is the most sensitive inflammation-based prognostic score in patients with unresectable metastatic colorectal cancer. Dis colon rectum. (2021) 64:1331–41. doi: 10.1097/DCR.0000000000002059
27. Utsumi M, Inagaki M, Kitada K, Tokunaga N, Kondo M, Yunoki K, et al. Lymphocyte-to-C-reactive protein ratio predicts prognosis in patients with colorectal liver metastases post-hepatic resection: A retrospective study. Anticancer Res. (2022) 42:4963–71. doi: 10.21873/anticanres.16003
28. Kono Y, Saito H, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, et al. Postoperative ratio of the maximum C-reactive protein level to the minimum peripheral lymphocyte count as a prognostic indicator for gastric cancer patients. Surg Today. (2019) 49:206–13. doi: 10.1007/s00595-018-1724-x
29. Matsunaga T, Saito H, Fukumoto Y, Kuroda H, Taniguchi K, Takahashi S, et al. The prognostic impact of the lymphocyte-to-C-reactive protein ratio in patients with unresectable or recurrent advanced gastric cancer treated with first- and second-line treatment. Surg Today. (2023) 53:940–8. doi: 10.1007/s00595-022-02638-w
30. Okugawa Y, Toiyama Y, Fujikawa H, Ide S, Yamamoto A, Omura Y, et al. Prognostic potential of lymphocyte-C-reactive protein ratio in patients with rectal cancer receiving preoperative chemoradiotherapy. J gastrointest Surg. (2021) 25:492–502. doi: 10.1007/s11605-019-04495-4
31. Meng Y, Long C, Huang X, Huang L, Liao L, Tang W, et al. Prognostic role and clinical significance of C-reactive protein-lymphocyte ratio in colorectal cancer. Bioengineered. (2021) 12:5138–48. doi: 10.1080/21655979.2021.1960768
32. Taniai T, Haruki K, Hamura R, Fujiwara Y, Furukawa K, Gocho T, et al. The prognostic significance of C-reactive protein-to-lymphocyte ratio in colorectal liver metastases. J Surg Res. (2021) 258:414–21. doi: 10.1016/j.jss.2020.08.059
33. Ou W, Zhou C, Zhu X, Lin L, Xu Q. Prognostic significance of preoperative lymphocyte-to-C-reactive protein ratio in patients with non-metastatic colorectal cancer. OncoTargets Ther. (2021) 14:337–46. doi: 10.2147/OTT.S290234
34. Cheng CB, Zhang QX, Zhuang LP, Sun JW. Prognostic value of lymphocyte-to-C-reactive protein ratio in patients with gastric cancer after surgery: a multicentre study. Japanese J Clin Oncol. (2020) 50:1141–9. doi: 10.1093/jjco/hyaa099
35. Sawada R, Akiyoshi T, Kitagawa Y, Hiyoshi Y, Mukai T, Nagasaki T, et al. Systemic inflammatory markers combined with tumor-infiltrating lymphocyte density for the improved prediction of response to neoadjuvant chemoradiotherapy in rectal cancer. Ann Surg Oncol. (2021) 28:6189–98. doi: 10.1245/s10434-021-09975-z
36. Iseda N, Iguchi T, Hirose K, Itoh S, Honboh T, Sadanaga N, et al. Prognostic impact of lymphocyte-to-C-reactive protein ratio in patients who underwent surgical resection for pancreatic cancer. Am surge. (2023) 89:4452–8. doi: 10.1177/00031348221117034
37. Feeney G, Waldron R, Miller N, Malone C, Sweeney K, McLaughlin R, et al. Association of clinical biomarkers and response to neoadjuvant therapy in breast cancer. Ir J Med Sci. (2023) 193(2):605–13. doi: 10.1007/s11845-023-03489-1
38. Kim SR, Kim K, Lee SA, Kwon SO, Lee JK, Keum N, et al. Effect of red, processed, and white meat consumption on the risk of gastric cancer: an overall and dose-Response meta-analysis. Nutrients. (2019) 11:826. doi: 10.3390/nu11040826
39. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. (2009) 12:223–6. doi: 10.1097/MCO.0b013e32832a7902
40. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
41. Brummel K, Eerkens AL, de Bruyn M, Nijman HW. Tumour-infiltrating lymphocytes: from prognosis to treatment selection. Br J cancer. (2023) 128:451–8. doi: 10.1038/s41416-022-02119-4
42. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. (2003) 111:1805–12. doi: 10.1172/JCI200318921
43. Kim ES, Kim SY, Moon A. C-reactive protein signaling pathways in tumor progression. Biomol Ther. (2023) 31:473–83. doi: 10.4062/biomolther.2023.132
44. Wang CS, Sun CF. C-reactive protein and Malignancy: clinico-pathological association and therapeutic implication. Chang Gung Med J. (2009) 32:471–82.
45. Ye Y, Wu G, Yuan H, Zheng Y, Wang Y, Guo Q. Prognostic role of preoperative lymphocyte/C-reactive protein associated with upper gastrointestinal cancer: a meta-analysis. Front Oncol. (2023) 13:1181649. doi: 10.3389/fonc.2023.1181649
46. Pirozzolo G, Gisbertz SS, Castoro C, van Berge Henegouwen MI, Scarpa M. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: a systematic review and meta-analysis. J Thorac dis. (2019) 11:3136–45. doi: 10.21037/jtd
47. Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. (2011) 71:2411–6. doi: 10.1158/0008-5472.CAN-10-2583
Keywords: lymphocyte-C-reactive, prognosis, gastrointestinal cancer, meta-analysis, systematic review
Citation: Liu X, Zhang J, An H, Wang W, Zheng Y and Wei F (2024) The role of lymphocyte-C-reactive protein ratio in the prognosis of gastrointestinal cancer: a systematic review and meta-analysis. Front. Oncol. 14:1407306. doi: 10.3389/fonc.2024.1407306
Received: 26 March 2024; Accepted: 05 August 2024;
Published: 29 August 2024.
Edited by:
Philip Rosenberg, National Cancer Institute (NIH), United StatesReviewed by:
Antonella Argentiero, National Cancer Institute Foundation (IRCCS), ItalyYin Huang, Sichuan University, China
Copyright © 2024 Liu, Zhang, An, Wang, Zheng and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: FengJiang Wei, d2ZqMTY2QHRtdS5lZHUuY24=
 XiaoMeng Liu
XiaoMeng Liu JingChen Zhang2
JingChen Zhang2 FengJiang Wei
FengJiang Wei